Abstract
OBJECTIVE
Heart rate variability (HRV), a measure of autonomic function, has been associated with cognitive function, but studies are conflicting. Previous studies have also not controlled for familial and genetic influences.
METHODS
We performed power spectral analysis on 24-hour ambulatory ECG’s in 416 middle-aged male twins from the Vietnam Era Twin Registry. Memory and learning were measured by verbal and visual selective reminding tests (SRT). Mixed-effect regression models were used to calculate associations between and within twin pairs, while adjusting for covariates.
RESULTS
The mean age (SD) was 55 (2.9) years. A statistically significant positive association was found between measures of HRV and verbal, but not visual, SRT scores. The most statistically significant unadjusted association was found between very low frequency (VLF) HRV and verbal total recall SRT, such that each logarithm of increase in VLF was associated with an increased verbal SRT score of 4.85 points (p=0.002). The association persisted despite adjustment for demographic and cardiovascular risk factors, and after accounting for familial, and genetic factors by comparing twins within pairs. A significant interaction was found between post-traumatic stress disorder (PTSD) and HRV, such that total power and ultra low frequency were associated with SRT in twins (n=362) without PTSD, but not in those with PTSD.
CONCLUSION
In conclusion, lower frequency spectra of HRV are associated with verbal, but not visual, learning and memory, particularly in subjects without PTSD. This association may indicate that autonomic nervous system dysregulation plays a role in cognitive decline.
Keywords: memory, autonomic function, heart rate variability, cognitive function
Introduction
Heart rate variability (HRV), a measure of fluctuations in beat to beat intervals of the sinus node, is a non-invasive index of cardiac autonomic nervous system control. HRV predicts total mortality, sudden death, and cardiovascular disease risk, as well as other morbidities [1-5]. It is influenced by several factors, including age, hypertension [6], genetic factors [7], diet [8], and emotional factors such as depression [9] and anxiety [10]. Recent studies have suggested an association between HRV and cognitive performance [11]. Mild cognitive impairment and dementia affect over 20% of the US population over the age of 65 [12, 13], and this possible relationship may illuminate novel mechanisms of cognitive decline.
Data examining the relationship between HRV and cognitive performance (both memory and executive function tasks[14]), however, are conflicting; some have demonstrated associations [11, 15], but others have not [16, 17]. Few studies have examined the association between cognitive performance and 24-hour HRV [15], which provides more detailed physiologic information than short term measures [18-20], and may have the highest prognostic value [2, 21]. It is also not known to what extent the associations between HRV and cognitive function reflect potentially common psychological factors, such as depression, anxiety, and post-traumatic stress disorder, which may be involved in both cognitive dysfunction and reduced autonomic function [22-25].
Furthermore, studies have not examined the effects of genetic and familial factors. Although genes have been associated with both HRV [26] and cognitive performance [27], it is unclear if any genes are shared between them, and whether such genes may explain this association. For example, catechol O-methyltransferase (COMT), a dopamine gene which has been found to influence cognitive function [28], may also influence autonomic function [29]. The elucidation of genes such as COMT may provide valuable mechanistic insight into the mutual pathogenesis of cognitive and autonomic dysfunction. Because maternal factors, such as affection-giving and health behaviors during pregnancy, may also affect both HRV [30] and cognitive performance [31], familial associations may also be important considerations in examining this relationship.
We sought to examine the association between HRV and measures of cognitive performance in a cross-sectional study of middle-aged male monozygotic (MZ) and dizygotic (DZ) veteran twins. By analyzing the effects of HRV differences within pairs, we were able to examine the association with cognitive performance while controlling for genetic (100% genetic similarity for MZ and 50% for DZ twins), and early familial factors shared by the twins. If a significant association exists between HRV and cognitive performance that is independent of measured demographic, cardiovascular, and psychological factors, while also controlling for genetic and early familial factors, then a direct physiologic link between HRV and cognitive function is possible. Further elucidation of this relationship may be helpful in future preventive and treatment endeavors for both heart disease and cognitive decline.
Methods
Subjects
The Emory Twin Studies (ETS) includes samples recruited in two companion studies: the Twins Heart Study (THS) and the Stress and Vascular Evaluation in Twins (SAVEIT). The purpose of these studies was to elucidate the role of psychological, behavioral and biological risk factors for subclinical cardiovascular disease in twins. Both projects recruited middle-aged, male monozygotic and dizygotic twin pairs (who were raised in the same household) from the Vietnam Era Twin (VET) Registry, one of the largest twin registries in the United States [32]. Both studies followed identical procedures, measurements and protocols. THS enrolled 180 twin pairs between 2002 and 2006 [33] and SAVEIT included 82 twin pairs enrolled between 2005 and 2008. Twins included in ETS, most of whom were Caucasian, were randomly selected from the VET Registry among those born between 1946 and 1956. In addition, a random sample of twin pairs discordant for major depression was included in THS and a random sample of twin pairs discordant for posttraumatic stress disorder (PTSD) was included in SAVEIT. Pairs of twins were examined at the same time at the Emory University General Clinical Research Center, and all data collection, including ambulatory electrocardiogram (ECG) monitoring, occurred during a 24-hour admission under controlled conditions. Both studies were approved by the Emory Institutional Review Board, and all twins signed an informed consent.
The two twins maintained an identical schedule while in the study. Activity was limited to leisurely ambulation within the Emory facilities, and all assessment, including the ambulatory ECG monitoring, began and ended at the same time. Zygosity information by means of DNA typing was available for all twin pairs.
Measurement of HRV
Twins wore an ambulatory ECG (Holter) monitor (GE Marquette SEER digital system) for 24 hours. Both twins in a pair were studied at the same time and their recording times, schedule, and activity level during the recording were matched. Activity was restricted to quiet walking around the campus, and participants were instructed to refrain from smoking and drinking alcohol or coffee during the recording. HRV data were analyzed following published methodology [35, 36]. Each tape was manually processed, edited, and analyzed with customized software that used methods described in the literature [37, 38]. The heart rate spectrum was computed using a fast Fourier transform (FFT) with a Parzen window. Because long-term autonomic function was the goal of this study, the FFT was performed on the 24-hour R-R interval file. The power spectrum was integrated over four discrete frequency bands [36]: ultra low frequency (ULF) <0.0033 Hz; very low frequency (VLF) 0.0033 to <0.04 Hz; low frequency (LF) 0.04 to <0.15 Hz; and high frequency (HF) 0.15 to <0.40 Hz. Total power (TP), incorporating the full spectrum <0.40 Hz, was also measured. Twins whose recordings showed >20% interpolation or <18 recorded hours were excluded from the analysis. None of the twins were found to have atrial fibrillation or flutter.
Measurement of Cognitive Function
Verbal and visual learning and memory were assessed using the verbal Selective Reminding test (SRT) and the visual SRT [39]. In the verbal SRT, each twin was read a list of twelve unrelated words, and asked to immediately recall as many words as possible. Each twin was then reminded only of the words that were not recalled, and was asked to recall all twelve words again. Twelve total recall trials were conducted, except in cases with 2 consecutive trials of perfect recall. Total recall (TR) was defined as the total number of words recalled on all 12 trials, representing a sum of long-term and short-term storage and learning of words. The maximum score for total recall is 144, and the average score for ages 50-59 years is 121.6 ± 10.5 [40]. While other parameters were measured, including consistent long-term recall (CLTR) and delayed recall (DR), these were not included in the analysis because of the high degree of correlation with TR. The visual SRT was similar to the verbal test. Twelve designs were presented one at a time for 3 seconds each, followed by an opportunity to draw all from memory. Each design that was not accurately reproduced on a given trial was shown again until perfect recall was obtained or 12 trials.
Assessment of Depression, PTSD, and Other Psychiatric Diagnoses
We administered the Beck Depression Inventory-II (BDI-II) [41], a standardized scale providing a measure of depressive symptoms, with higher scores indicating more severe depression [42]. We also administered the Structured Clinical Interview for DSM IV (SCID) [43] to classify twins based on a lifetime history of major depressive disorder (MDD) and PTSD. The SCID also provided a diagnosis of other psychiatric disorders, including a lifetime history of alcohol and of drug abuse or dependence.
Other Measurements
A medical history and a physical exam were obtained by a research nurse. Abdominal and hip circumferences were measured to derive the waist to hip ratio. Hypertension was defined by a measured systolic blood pressure > 140 mm Hg or current treatment with anti-hypertensive medications. Diabetes was defined as having a fasting glucose level > 126 mg/dl or current treatment with anti-diabetic medications. Venous blood samples were drawn for the measurement of glucose and lipid profile after an overnight fast. Glucose was measured on the Beckman CX7 chemistry autoanalyzer. Direct high-density lipoprotein (HDL) and low density lipoprotein (LDL) cholesterol were measured with homogeneous assays (Equal Diagnostics, Exton, PA). Serum Vitamin 25(OH)D levels were measured by using an enzyme linked immunsorbent assay (IDS Inc, Foundations Hills, AZ). Dietary intake of omega 3 fatty acids, alcohol, and caffeine were measured using the Willett food frequency questionnaire [44]. Physical activity was assessed with a modified version of the Baecke Questionnaire of Habitual Physical Activity (used in the Atherosclerosis Risk in Communities (ARIC) Study [45]) that documented physical activity at work, during sports and non-sports activities. The global physical activity score was used in the analysis. Cigarette smoking was classified into current versus never or past smoker. A history of coronary heart disease was defined as a previous diagnosis of myocardial infarction or angina pectoris, or previous coronary revascularization procedures. Information on current use of other medications was also collected. Baseline intelligence, measured via the Armed Forces Qualification Test (AFQT), was abstracted from military records [34].
Statistical Analysis
Analysis was conducted using SAS 9.2 for Windows (©2008, Cary, NC). In initial analyses, we compared means and percents of study factors between twins with verbal/visual memory score above and below the median. Additional analyses were performed with verbal and visual memory on a continuous scale. P values were corrected for the correlation between co-twins using generalized estimating equations for categorical variables and mixed-models for continuous variables.
To examine the association between cognitive function and HRV, two sets of analyses were performed: 1) amongst individuals, in which all twins were eligible for inclusion regardless of whether their brother was available for analysis; and 2) between and within twin pairs, comparing one twin brother with the other (within-pair effect), as well as analyzing the association amongst pairs of brothers (between-pair effect). To do this, we used mixed effects regression models and accounted for the twin pairs using a random effect term for each pair. Verbal and visual selective reminding test scores were used as continuous outcomes, and log-transformed HRV measures were used as predictors. Statistical significance was determined if p<0.05, two-sided. For each unit increase in log (HRV), the β coefficient corresponds to the associated increase in cognitive function score. Model goodness-of-fit was assessed using the Akaike information criterion (AIC) before and after addition of HRV to the base model.
Multivariate Modeling
For each HRV variable, a core model was first built based on basic sociodemographic factors, including age and education, and traditional cardiovascular risk factors, including hypertension, LDL cholesterol, current/past smoking, and diabetes. Additional cardiovascular, sociodemographic, and psychological factors were deemed a-priori to be possible confounders, and where therefore added individually in a serial manner to the model to test for further confounding, which we defined as >10% change in the coefficient of the main predictor (HRV variables) [46]. These variables included beck depression score, lifetime/current depression, lifetime/current PTSD, AFQT score percentile (1990 baseline intelligence test), employment, body mass index, waist-hip ratio, fasting glucose, hemoglobin A1c, history of drug abuse, history of heart disease, medication use (antidepressants, beta-blockers, statins, aspirin, vitamin D, omega 3 consumption, alcohol intake, and caffeine intake. Only beck depression score was found to be a significant confounder. Given the known neurologic mechanisms linking affective disorders and cognitive function [14], we also tested for interaction with depression and PTSD, and in cases in which interaction was found, we conducted stratified analysis.
Within Pair Analysis
We performed within-pair analyses which examined the relationship of cognitive function with the difference in HRV between twins in a pair. The within-pair effects inherently control for demographic, shared familial and early environmental influences; in addition, daily activities and other environmental factors during the ambulatory ECG recording are controlled in this analysis since co-twins were examined at the same time and under nearly identical conditions. We fitted mixed effects models adapted for twin research [47], which allow for partitioning within and between pair differences in the dependent variable, cognitive function, as a function of the independent variables. This included the average HRV of the twin pair (“between effect”), as well as the difference in HRV between the individual twin and the pair average (“within effect”). This coefficient for the within effect is identical to the coefficient from a model that fits the absolute difference between the co-twins [47]. The analysis allowed the inclusion of unpaired twins, who contribute to the between-pair analysis, without substantially affecting the within-pair results, thus allowing full use of the dataset.
Genetic Influence
The last set of analyses involved testing for a possible genetic influence underlying the association between cognitive testing scores and HRV, by adding to the model a term for the interaction between zygosity and the within-pair difference in HRV. If the DZ effect is larger than the MZ effect and the interaction term is significant (p < .05), this may suggest a role for genetic factors in the association. If the MZ and DZ effects are not significantly different, then this suggests that genetics do not play a major role in the relationship.
Results
Group and Subset Characteristics
Of the 524 twins in the sample, 416 (79%) had usable HRV data, including 169 twin pairs (61% monozygotic) and 78 singletons. The twins excluded due to missing HRV data (n=108) were similar to the rest of the twins with respect to demographic, socioeconomic, psychiatric, and cardiovascular risk profiles. They had a similar verbal SRT score, and a slightly higher (131 vs. 127, p=0.04) visual SRT score than those with complete HRV data.
For the included subjects, the mean age was 55.1 (SD=2.9) years, 54 (13%) had a lifetime history of PTSD, 98 (24%) had a lifetime history of depression, and they completed on average 14 years of education. The median total power HRV was 12,441 ms2 (9.42 log ms2), with an interquartile range of 8,632 ms2 (9.06 log ms2) to 18,056 ms2 (9.8 log ms2). The median SRT total recall score was 114 of 144 maximum, with an interquartile range of 96 to 127 for the verbal test. For the visual SRT, the median score was 132 with an interquartile range of 125 to 137. The twins with higher verbal total recall were more educated and less likely to have a history of alcohol abuse (Table 1); otherwise, there were no statistically significant differences in cognitive function, physical health, mental health, and health behaviors between those above and below the median verbal SRT score. Associations between visual SRT and the background variables listed in Table 1 were also performed. Twins who scored above the median visual SRT score were found to have higher education (p=0.002), lower systolic blood pressure (p=0.03), and lower diastolic blood pressure (p=0.008), but otherwise were not different than the twins who scored below the median. Results were similar when examining verbal and visual total recall as continuous scores, with some exceptions; the associations of verbal SRT score with lifetime history of drug abuse (p=0.01), BDI-II score (p<0.01), and body mass index (p=0.049) became statistically significant, and the associations of visual SRT score with lifetime history of drug abuse (p=0.02) became significant as well.
Table 1.
Distribution of Demographic, Behavioral, and Coronary Risk Factors in Twins Stratified by Verbal Memory Score
| Below Median Verbal SRT Score (n=205) |
Above Median Verbal SRT Score (n=211) |
p value* |
|
|---|---|---|---|
| Age, years, mean (SD) | 55.2 (2.9) | 55.1 (2.8) | 0.59 |
| Greater than high school education, % | 20.0 | 46.0 | <0.001 |
| Mean AFQT score Percentile | 56.1 (1.9) | 65.4 (1.7) | <0.001 |
| Systolic blood pressure, mm Hg, mean (SD) | 129.4 (14.6) | 128.7 (16.6) | 0.75 |
| Diastolic blood pressure, mm Hg, mean (SD) | 81.2 (10.2) | 80.5 (10.7) | 0.59 |
| LDL-cholesterol, mg/dL, mean (SD) | 124.7 (35.8) | 119.5 (34.5) | 0.13 |
| HDL-cholesterol, mg/dL, mean (SD) | 38.3 (11.0) | 39.1 ( 9.9) | 0.41 |
| Diabetes, % | 13.1 | 10.4 | 0.41 |
| Body mass index, mean (SD) | 30.0 (5.4) | 29.2 (4.2) | 0.10 |
| Waist-to-hip ratio, mean (SD) | 0.95 (0.07) | 0.94 ( 0.06) | 0.06 |
| Physical activity (Baecke) score, mean (SD) | 7.1 (1.8) | 7.4 (1.8) | 0.08 |
| Prior coronary heart disease, % | 9.3 | 9.9 | 0.69 |
| Lifetime history of PTSD, % | 13.7 | 12.3 | 0.81 |
| Lifetime history of MDD, % | 25.4 | 21.8 | 0.41 |
| Beck Depression Inventory, mean (SD) | 6.5 (7.5) | 5.2 (7.2) | 0.12 |
| Lifetime history of alcohol abuse or dependence, % |
55.1 | 41.7 | 0.02 |
| Lifetime history of drug abuse or dependence, % | 23.9 | 17.5 | 0.24 |
| Taking antidepressant medications, % | 18.0 | 15.6 | 0.50 |
| Taking beta-blocker medications, % | 4.9 | 9.9 | 0.07 |
SRT: selective reminding test; AFQT=Armed Forces Qualification Test; SD=standard deviation; PTSD=post-traumatic stress disorder; MDD=major depression.
P values are obtained from mixed models for continuous variables or generalized estimating equations for categorical variables
Association of HRV and Selective Reminding Test Scores Amongst Twins
Overall, significant associations were found between HRV and verbal total recall both before and after multivariable adjustment for potential confounding factors, and the most statistically significant associations were found for twins without PTSD (Table 2). A significant interaction was found between TP and ULF HRV and lifetime PTSD. In the group without PTSD, each unit increase in log(TP) was associated with an adjusted increase in verbal total recall score of approximately 5 words (p=0.01). Similar adjusted associations were found with ULF and VLF HRV, although the effect sizes were not as large. With regards to model fit, the AIC decreased from 3917 to 3129 when log(TP) was added to the base multivariate model (p<0.001); similar model improvements were seen with the other frequency spectra as well. Although LF and HF HRV were also significantly associated with verbal memory in unadjusted analysis, the associations did not persist after multivariate adjustment. In the group with PTSD, no associations with HRV were found (not shown). Additionally, no associations were found in the entire sample between HRV and visual SRT, and no interaction with depression or PTSD was found (table 3).
Table 2.
Association of Verbal Selective Reminding Test Score with Heart Rate Variability (HRV) in Individual Twins
| HRV Frequency* |
Entire Sample (n=416) |
Twins without PTSD, adjusted (n=362)# |
Interaction between PTSD and HRV |
||||
|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted‡ | ||||||
| β † | p | β | p | β | p | p | |
| Total Power, ms2 | 5.14 | 0.01 | 3.72 | 0.048 | 4.93 | 0.01 | 0.04 |
| ULF, ms2 | 4.47 | 0.01 | 3.25 | 0.06 | 4.52 | 0.01 | 0.03 |
| VLF, ms2 | 4.85 | 0.002 | 3.65 | 0.02 | 3.91 | 0.01 | 0.32 |
| LF, ms2 | 3.16 | 0.01 | 2.23 | 0.07 | 2.01 | 0.12 | 0.91 |
| HF, ms2 | 2.23 | 0.05 | 1.76 | 0.11 | 2.15 | 0.05 | 0.44 |
ULF=ultra low frequency; VLF=very low frequency; LF=low frequency; HF=high frequency
each frequency domain measure was log-transformed.
β corresponds to increase in selective reminding test score per unit increase in log(HRV).
adjusted for age, hypertension, education, low density lipoprotein cholesterol, current/previous tobacco use, diabetes, and depressive symptoms.
No significant associations found in the group with PTSD
Table 3.
Association of Visual Selective Reminding Test Score with Heart Rate Variability (HRV) in Individual Twins
| Entire Sample (n=416) | ||||
|---|---|---|---|---|
| HRV Frequency | Unadjusted | Adjusted | ||
| β | p | β | p | |
| Total Power, ms2 | 1.12 | 0.45 | −0.04 | 0.98 |
| ULF, ms2 | 0.95 | 0.49 | 0.01 | 0.99 |
| VLF, ms2 | 1.03 | 0.40 | 0.01 | 1.00 |
| LF, ms2 | 0.43 | 0.66 | −0.34 | 0.75 |
| HF, ms2 | 0.93 | 0.30 | 0.73 | 0.42 |
ULF=ultra low frequency; VLF=very low frequency; LF=low frequency; HF=high frequency
each frequency domain measure was log-transformed.
β corresponds to increase in selective reminding test score per unit increase in log(HRV).
adjusted for age, hypertension, education, low density lipoprotein cholesterol, current/previous tobacco use, diabetes, and depressive symptoms
Association of HRV and Selective Reminding Test Scores Within Pairs
Within-pair analysis included 169 complete pairs and 78 unpaired twins. In general, adjusted within pair analysis showed similar results to adjusted analysis of individual twins (table 4). A significant interaction was found between TP and ULF spectra and PTSD, and the subgroup without PTSD showed the most significant associations between TP and ULF spectra and verbal total recall; each log(TP) increase in discordance between twins was positively associated with an approximately 7 word difference in verbal memory score. No associations were found between LF and HF spectra and verbal memory. The between pair coefficients, which represent the association of the average HRV of the pair and cognitive function, were less than the within pair coefficients, and were not statistically significant. No significant between and within-pair associations were found with visual total recall score, nor with verbal recall score in the PTSD subgroup. Also, no significant interaction was found with zygosity, meaning that the difference in the within-pair effect for verbal and visual SRT did not significantly differ between MZ and DZ pairs.
Table 4.
Adjusted* Between and Within Pair Associations of Verbal Selective Reminding Test Score and Heart Rate Variability (HRV)
| Including PTSD (169 pairs, 78 unpaired twins) |
Excluding PTSD (133 pairs, 96 unpaired twins)# |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| HRV Frequency† |
Between Pairs‡ |
Within Pairs | Between Pairs | Within Pairs | PTSD interaction |
||||
| β | p | β | p | β | p | β | p | p | |
| Total Power, ms2 | 3.58 | 0.12 | 3.98 | 0.19 | 3.71 | 0.11 | 7.20 | 0.02 | 0.01 |
| ULF, ms2 | 3.56 | 0.10 | 2.75 | 0.32 | 3.20 | 0.14 | 6.86 | 0.02 | <0.001 |
| VLF, ms2 | 2.64 | 0.16 | 5.44 | 0.03 | 3.20 | 0.09 | 5.02 | 0.05 | 0.72 |
| LF, ms2 | 1.37 | 0.37 | 3.66 | 0.06 | 1.41 | 0.35 | 2.64 | 0.18 | 0.11 |
| HF, ms2 | 1.56 | 0.22 | 2.22 | 0.25 | 1.88 | 0.14 | 2.18 | 0.26 | 0.67 |
ULF=ultra low frequency; VLF=very low frequency; LF=low frequency; HF=high frequency
Adjusted for age, hypertension, education, low density lipoprotein cholesterol, current/previous tobacco use, diabetes, and depressive symptoms.
Each frequency domain measure was log-transformed; for total power and ULF
β corresponds to increase in selective reminding test score per unit increase in log(HRV).
No significant associations found in the group with PTSD
Discussion
In this cross-sectional study of predominantly healthy, asymptomatic middle-aged men, we found a positive association between verbal, but not visual, memory performance and HRV. This association remained when controlling for cardiovascular risk factors, education, depressive symptoms, and familial factors. Because no significant zygosity-interaction was found, genetic confounding is also unlikely. Furthermore, HRV was selectively associated with verbal, and not visual, working memory, implying that the neurobiology of this association may be specific to verbal learning and memory only. Given the known association between poor performance on verbal SRT and dementia risk [48], these results suggest that autonomic dysfunction may be a marker of early cognitive decline, and it may also have direct physiologic effects on certain neural circuits.
Previous research has also shown an association between HRV and cognitive tasks that specifically involve executive function, which is also likely utilized during the verbal selective reminding task [49]. In a study of male sailors, Hansen et al. found that 5-minute supine HF HRV was positively associated with cognitive test scores that used executive function, but not with tests that did not use executive function [50]. Poor working memory and executive function may portend increased risk of later dementia [51], which several studies have also found to be associated with HRV [11, 15, 52, 53]. Our study expands upon the findings of previous smaller studies by finding an association between verbal learning and memory with more prognostic, long-term HRV measures that are independent of cardiovascular comorbidities, depression, familial factors, and genetic factors.
Visual memory was not associated with HRV. There are several possible explanations for this finding. First, the mean score was higher (127 vs. 110) and variance was lower (286 vs. 441) for visual SRT compared to verbal SRT, which may lead to a ceiling effect in our data and reduce our ability to detect an association. Additionally, there is debate as to whether visual memory utilizes the prefrontal cortex [54-56]. Many known regions involved in visual function, including parietal, temporal, and occipital lobes, are all outside of the central autonomic network [55, 57]. These other regions may help compensate for a possible loss of prefrontal function. Therefore, HRV may correlate with verbal, but not visual, working memory performance because verbal memory more specifically involves the central autonomic network than visual memory.
Not every study has found an association between HRV and cognitive function, however. Allan et al. found no association between short-term HRV parameters and Alzheimer’s dementia or vascular dementia compared to controls [16]. However, the sample size was small, and only a short-term supine measurement of HRV was examined, which may have limited the sensitivity in detecting autonomic dysfunction. Similarly, short-term measures (HF and LF) showed less association with cognitive function in our study. Britton and coworkers found no consistent association between short term HRV parameters and several cognitive tasks that involved memorization, knowledge, and reasoning [17]. While their sample size was large, they also only used supine short-term HRV measures. Our methodology differs from many previous studies because we used long term, 24-hour ambulatory ECG data that included VLF and ULF spectra.
Long term, 24-hour HRV indices best predict mortality after myocardial infarction [36]. In our study, these indices demonstrated the most significant and consistent association with verbal memory performance. The higher frequency bands (HF, LF) are known to vary with position [21], respiration [58], and blood pressure [59], while low frequency bands may vary with physical activity (ULF) [20], temperature regulation (ULF) [60], and hormonal activity in the renin-angiotensin-aldosterone system (RAAS) (VLF) [61]. Many of these factors were carefully controlled by restricting the twins to light physical activity, and matching activity status in twin pairs during recordings. However, respiration, which may affect HF and LF, was not controlled during the recording in our study. The confidence intervals for these HRV measures were wider than VLF and ULF for their association with memory performance. This suggests more random error, and this may help to explain their weaker association. Additionally, VLF may be related to cognitive function because of its link with RAAS function, which has been associated with cognitive function as well [62-65].
Possible Mechanisms
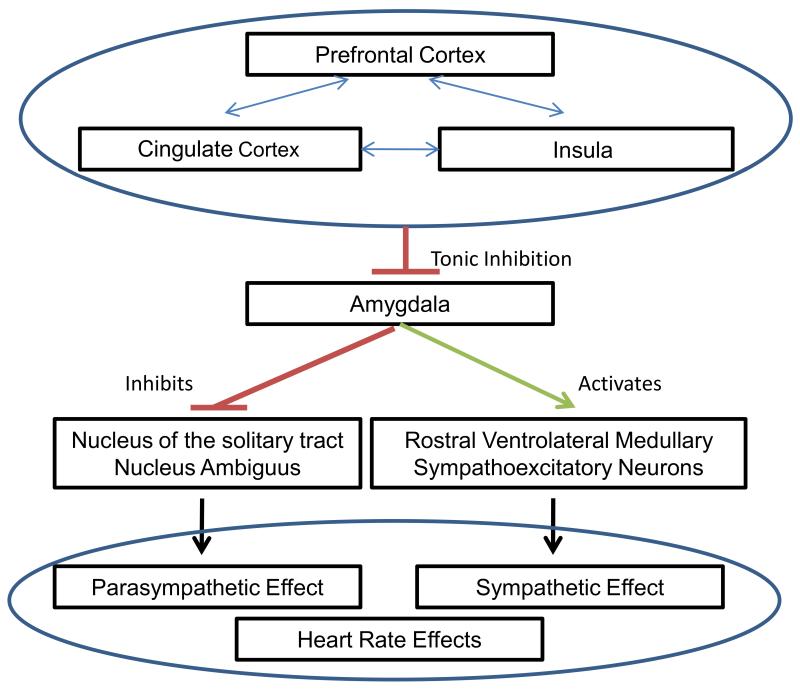
Thayer and Lane have described a neurovisceral integration theory that links together cognitive, affective, and autonomic neural networks (Figure 1) [66]. They propose a central autonomic network that involves structures such as the anterior cingulate, insular, and ventromedial prefrontal cortices (as well as multiple subcortical structures) that regulate visceromotor, neuroendocrine, and behavioral activity. Within this model, HRV could be a marker of both autonomic function and executive function, which are both regulated by the prefrontal cortex [67]. Thus, HRV could be a useful measure of the performance of this entire network.
Figure 1.
Association of Neural Structures involved in Neurovisceral Integration Theory Associating Executive Function and Autonomic Function
In addition to neurologic mechanisms, it is also possible that poor autonomic function has an effect on brain circulation. In particular, the baroreceptor reflex, which is an important component of the autonomic nervous system, is important for regulation of blood flow [68], and may be essential during executive function tasks involving mental effort [69]. Furthermore, poor regulation of blood flow may increase the risk of transient episodes of cerebral ischemia and neurologic damage during orthostasis, although this is subject to debate [70].
Interaction with PTSD
Subjects with PTSD were found not to have an association between TP and ULF HRV and verbal/visual SRT, which may be due to independent effects of PTSD on memory. In our sample, those with lifetime PTSD scored lower on the verbal SRT (105 vs. 111, p=0.20), although this was not statistically significant; no difference was found with visual SRT, p=0.99. PTSD is known to be associated with poor memory performance [22], which may be a consequence of damaging effects of traumatic stress on areas of the brain such as the hippocampus that are important for memory [71]. In subjects with PTSD, it is possible that stress-related changes to the hippocampus that reduce memory performance override any possible effects due to other risk factors for memory performance, or otherwise create a floor effect that limits the ability to find an association with HRV. Further research is needed to understand and confirm this unexpected interaction.
Implications
The present sample included primarily Caucasian middle-aged male twins from the US who were Vietnam War veterans, and our findings support a possible mechanistic link between autonomic and cognitive function, and provide important insight towards possible future interventions that may improve both. Exercise, for example, affects both autonomic function [20, 72] and cognitive status, particularly executive function [73-75]. In the study of sailors by Hansen and coworkers, those who underwent a 4-week exercise training program had higher HF HRV and better cognitive performance scores on working memory tests compared to sailors who did not undergo the training program [76]. Eisenberg et al. showed the efficacy of biofeedback on HRV and symptoms of attention-deficit-hyperactivity-disorder (ADHD) in children, and also found a positive correlation between HRV and ADHD improvement [77]. Multiple drug and non-drug interventions have been found to increase HRV, highlighting the possibility of developing interventions that may improve cognitive function through enhancement of the central autonomic network [72].
Limitations
Our population included primarily Caucasian middle-aged male veteran twins, and our findings may not generalize to other races, women, and other age groups. Performance on cognitive tests and HRV measures may have been subject to circumstances faced by individual twins, such as prolonged travel or undetected medical conditions. While such circumstances were not taken into account, outliers were carefully examined for extraneous circumstances that may have affected cognitive performance. Also, this is a cross-sectional study, and therefore direction of the association and causation cannot be proven. Unmeasured confounders, such as differences in quality of education environments, may play a role, although they were minimized by our within-pair analysis, in which we compared each twin to his brother, thus controlling for unmeasured common childhood environmental factors.
Conclusion
In a sample of middle-aged male veteran twins, we found an association between HRV and verbal (but not visual) memory performance that was independent of demographic and cardiovascular risk factors, as well as familial and genetic factors. This association was particularly robust for twins without PTSD. These findings indicate that cortical neurologic mechanisms relevant to cognitive performance are linked to autonomic regulation pathways, and therefore treatments, such as exercise, that improve autonomic function may also reduce the risk of cognitive impairment.
Acknowledgments
Funding Sources: This work was supported by the National Institutes of Health [K24HL077506, R01 HL68630 and R01 AG026255]; the Emory University General Clinical Research Center [MO1-RR00039] and by the American Heart Association [0245115N].
The United States Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. Numerous organizations have provided invaluable assistance, including: VA Cooperative Study Program; Department of Defense; National Personnel Records Center, National Archives and Records Administration; the Internal Revenue Service; NIH; National Opinion Research Center; National Research Council, National Academy of Sciences; the Institute for Survey Research, Temple University.
Most importantly, we acknowledge the continued cooperation and participation of the members of the Vietnam Era Twin Registry and their families; without their contribution, this research would not have been possible.
Additionally, we would like to thank the tireless staff at Emory, including Lucy Shallenberger, Linda Jones, and Nancy Murrah, who ensured the successful execution of this study.
Partial support from National Institutes of Health
Acronyms
- HRV
Heart Rate Variability
- SD
Standard Deviation
- SRT
Selective Reminding Test
- ECG
electrocardiogram
Footnotes
Disclosures: None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tsuji H, Venditti FJ, Jr., Manders ES, Evans JC, Larson MG, Feldman CL, Levy D. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation. 1994;90(2):878–83. doi: 10.1161/01.cir.90.2.878. [DOI] [PubMed] [Google Scholar]
- 2.Bigger JT, Jr., Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992;85(1):164–71. doi: 10.1161/01.cir.85.1.164. [DOI] [PubMed] [Google Scholar]
- 3.Dekker JM, Crow RS, Folsom AR, Hannan PJ, Liao D, Swenne CA, Schouten EG. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC Study. Atherosclerosis Risk In Communities. Circulation. 2000;102(11):1239–44. doi: 10.1161/01.cir.102.11.1239. [DOI] [PubMed] [Google Scholar]
- 4.Algra A, Tijssen JG, Roelandt JR, Pool J, Lubsen J. Heart rate variability from 24-hour electrocardiography and the 2-year risk for sudden death. Circulation. 1993;88(1):180–5. doi: 10.1161/01.cir.88.1.180. [DOI] [PubMed] [Google Scholar]
- 5.Liao D, Cai J, Rosamond WD, Barnes RW, Hutchinson RG, Whitsel EA, Rautaharju P, Heiss G. Cardiac autonomic function and incident coronary heart disease: a population-based case-cohort study. The ARIC Study. Atherosclerosis Risk in Communities Study. Am J Epidemiol. 1997;145(8):696–706. doi: 10.1093/aje/145.8.696. [DOI] [PubMed] [Google Scholar]
- 6.Tsuji H, Venditti FJ, Jr., Manders ES, Evans JC, Larson MG, Feldman CL, Levy D. Determinants of heart rate variability. J Am Coll Cardiol. 1996;28(6):1539–46. doi: 10.1016/s0735-1097(96)00342-7. [DOI] [PubMed] [Google Scholar]
- 7.Kupper NH, Willemsen G, van den Berg M, de Boer D, Posthuma D, Boomsma DI, de Geus EJ. Heritability of ambulatory heart rate variability. Circulation. 2004;110(18):2792–6. doi: 10.1161/01.CIR.0000146334.96820.6E. [DOI] [PubMed] [Google Scholar]
- 8.Mozaffarian D, Stein PK, Prineas RJ, Siscovick DS. Dietary fish and omega-3 fatty acid consumption and heart rate variability in US adults. Circulation. 2008;117(9):1130–7. doi: 10.1161/CIRCULATIONAHA.107.732826. [DOI] [PubMed] [Google Scholar]
- 9.Vaccarino V, Lampert R, Bremner JD, Lee F, Su S, Maisano C, Murrah NV, Jones L, Jawed F, Afzal N, Ashraf A, Goldberg J. Depressive symptoms and heart rate variability: evidence for a shared genetic substrate in a study of twins. Psychosom Med. 2008;70(6):628–36. doi: 10.1097/PSY.0b013e31817bcc9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedman BH, Thayer JF. Autonomic balance revisited: panic anxiety and heart rate variability. J Psychosom Res. 1998;44(1):133–51. doi: 10.1016/s0022-3999(97)00202-x. [DOI] [PubMed] [Google Scholar]
- 11.Kim DH, Lipsitz LA, Ferrucci L, Varadhan R, Guralnik JM, Carlson MC, Fleisher LA, Fried LP, Chaves PH. Association between reduced heart rate variability and cognitive impairment in older disabled women in the community: Women’s Health and Aging Study I. J Am Geriatr Soc. 2006;54(11):1751–7. doi: 10.1111/j.1532-5415.2006.00940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham JE, Rockwood K, Beattie BL, Eastwood R, Gauthier S, Tuokko H, McDowell I. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet. 1997;349(9068):1793–6. doi: 10.1016/S0140-6736(97)01007-6. [DOI] [PubMed] [Google Scholar]
- 13.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL, Steffens DC, McArdle JJ, Willis RJ, Wallace RB. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148(6):427–34. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neurosci Biobehav Rev. 2009;33(2):81–8. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Zulli R, Nicosia F, Borroni B, Agosti C, Prometti P, Donati P, De Vecchi M, Romanelli G, Grassi V, Padovani A. QT dispersion and heart rate variability abnormalities in Alzheimer’s disease and in mild cognitive impairment. J Am Geriatr Soc. 2005;53(12):2135–9. doi: 10.1111/j.1532-5415.2005.00508.x. [DOI] [PubMed] [Google Scholar]
- 16.Allan LM, Kerr SR, Ballard CG, Allen J, Murray A, McLaren AT, Kenny RA. Autonomic function assessed by heart rate variability is normal in Alzheimer’s disease and vascular dementia. Dement Geriatr Cogn Disord. 2005;19(2-3):140–4. doi: 10.1159/000082885. [DOI] [PubMed] [Google Scholar]
- 17.Britton A, Singh-Manoux A, Hnatkova K, Malik M, Marmot MG, Shipley M. The association between heart rate variability and cognitive impairment in middle-aged men and women. The Whitehall II cohort study. Neuroepidemiology. 2008;31(2):115–21. doi: 10.1159/000148257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furlan R, Guzzetti S, Crivellaro W, Dassi S, Tinelli M, Baselli G, Cerutti S, Lombardi F, Pagani M, Malliani A. Continuous 24-hour assessment of the neural regulation of systemic arterial pressure and RR variabilities in ambulant subjects. Circulation. 1990;81(2):537–47. doi: 10.1161/01.cir.81.2.537. [DOI] [PubMed] [Google Scholar]
- 19.Oinuma S, Kubo Y, Otsuka K, Yamanaka T, Murakami S, Matsuoka O, Ohkawa S, Cornelissen G, Weydahl A, Holmeslet B, Hall C, Halberg F. Graded response of heart rate variability, associated with an alteration of geomagnetic activity in a subarctic area. Biomed Pharmacother. 2002;56(Suppl 2):284s–288s. doi: 10.1016/s0753-3322(02)00303-7. [DOI] [PubMed] [Google Scholar]
- 20.Serrador JM, Finlayson HC, Hughson RL. Physical activity is a major contributor to the ultra low frequency components of heart rate variability. Heart. 1999;82(6):e9. doi: 10.1136/hrt.82.6.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17(3):354–81. [PubMed] [Google Scholar]
- 22.Bremner JD, Scott TM, Delaney RC, Southwick SM, Mason JW, Johnson DR, Innis RB, McCarthy G, Charney DS. Deficits in short-term memory in posttraumatic stress disorder. Am J Psychiatry. 1993;150(7):1015–9. doi: 10.1176/ajp.150.7.1015. [DOI] [PubMed] [Google Scholar]
- 23.Carney RM, Blumenthal JA, Freedland KE, Stein PK, Howells WB, Berkman LF, Watkins LL, Czajkowski SM, Hayano J, Domitrovich PP, Jaffe AS. Low heart rate variability and the effect of depression on post-myocardial infarction mortality. Arch Intern Med. 2005;165(13):1486–91. doi: 10.1001/archinte.165.13.1486. [DOI] [PubMed] [Google Scholar]
- 24.Cohen H, Benjamin J. Power spectrum analysis and cardiovascular morbidity in anxiety disorders. Auton Neurosci. 2006;128(1-2):1–8. doi: 10.1016/j.autneu.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Hickie I, Naismith S, Ward PB, Turner K, Scott E, Mitchell P, Wilhelm K, Parker G. Reduced hippocampal volumes and memory loss in patients with early- and late-onset depression. Br J Psychiatry. 2005;186:197–202. doi: 10.1192/bjp.186.3.197. [DOI] [PubMed] [Google Scholar]
- 26.Thayer JF, Merritt MM, Sollers JJ, 3rd, Zonderman AB, Evans MK, Yie S, Abernethy DR. Effect of angiotensin-converting enzyme insertion/deletion polymorphism DD genotype on high-frequency heart rate variability in African Americans. Am J Cardiol. 2003;92(12):1487–90. doi: 10.1016/j.amjcard.2003.08.069. [DOI] [PubMed] [Google Scholar]
- 27.Barnett JH, Jones PB, Robbins TW, Muller U. Effects of the catechol-O-methyltransferase Val158Met polymorphism on executive function: a meta-analysis of the Wisconsin Card Sort Test in schizophrenia and healthy controls. Mol Psychiatry. 2007;12(5):502–9. doi: 10.1038/sj.mp.4001973. [DOI] [PubMed] [Google Scholar]
- 28.Malhotra AK, Kestler LJ, Mazzanti C, Bates JA, Goldberg T, Goldman D. A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. Am J Psychiatry. 2002;159(4):652–4. doi: 10.1176/appi.ajp.159.4.652. [DOI] [PubMed] [Google Scholar]
- 29.Lindvall O, Bjorklund A, Skagerberg G. Dopamine-containing neurons in the spinal cord: anatomy and some functional aspects. Ann Neurol. 1983;14(3):255–60. doi: 10.1002/ana.410140302. [DOI] [PubMed] [Google Scholar]
- 30.May LE, Glaros A, Yeh HW, Clapp JF, 3rd, Gustafson KM. Aerobic exercise during pregnancy influences fetal cardiac autonomic control of heart rate and heart rate variability. Early Hum Dev. 2010;86(4):213–7. doi: 10.1016/j.earlhumdev.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Bernier A, Carlson SM, Whipple N. From external regulation to self-regulation: early parenting precursors of young children’s executive functioning. Child Dev. 2010;81(1):326–39. doi: 10.1111/j.1467-8624.2009.01397.x. [DOI] [PubMed] [Google Scholar]
- 32.Goldberg J, Curran B, Vitek ME, Henderson WG, Boyko EJ. The Vietnam Era Twin Registry. Twin Res. 2002;5(5):476–81. doi: 10.1375/136905202320906318. [DOI] [PubMed] [Google Scholar]
- 33.Vaccarino V, Brennan ML, Miller AH, Bremner JD, Ritchie JC, Lindau F, Veledar E, Su S, Murrah NV, Jones L, Jawed F, Dai J, Goldberg J, Hazen SL. Association of major depressive disorder with serum myeloperoxidase and other markers of inflammation: a twin study. Biol Psychiatry. 2008;64(6):476–83. doi: 10.1016/j.biopsych.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henderson WG, Eisen S, Goldberg J, True WR, Barnes JE, Vitek ME. The Vietnam Era Twin Registry: a resource for medical research. Public Health Rep. 1990;105(4):368–73. [PMC free article] [PubMed] [Google Scholar]
- 35.Lampert R, Ickovics JR, Viscoli CJ, Horwitz RI, Lee FA. Effects of propranolol on recovery of heart rate variability following acute myocardial infarction and relation to outcome in the Beta-Blocker Heart Attack Trial. American Journal of Cardiology. 2003;91(2):137–142. doi: 10.1016/s0002-9149(02)03098-9. [DOI] [PubMed] [Google Scholar]
- 36.Bigger J, Fleiss J, Steinman R, Rolnitsky L, Kleiger R, Rottman J. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992;85:164–171. doi: 10.1161/01.cir.85.1.164. [DOI] [PubMed] [Google Scholar]
- 37.Lampert R, Ickovics J, Horwitz R, Lee F. Depressed autonomic nervous system function in African Americans and individuals of lower social class: a potential mechanism of race- and class-related disparities in health outcomes. Am Heart J. 2005;150(1):153–60. doi: 10.1016/j.ahj.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 38.Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Dell’Orto S, Piccaluga E. Power spectral analysis of heart rate and arterial pressure variability as a marker of sympatho-vagal interaction in man and conscious dog. Circulation Research. 1986;59:178–193. doi: 10.1161/01.res.59.2.178. e al. [DOI] [PubMed] [Google Scholar]
- 39.Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24(11):1019–25. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- 40.Larrabee GJ, Trahan DE, Levin HS. Normative data for a six-trial administration of the Verbal Selective Reminding Test. Clin Neuropsychol. 2000;14(1):110–8. doi: 10.1076/1385-4046(200002)14:1;1-8;FT110. [DOI] [PubMed] [Google Scholar]
- 41.Beck AT, Steer RA, Brown GK. BDI-II. Beck Depression Inventory. Second Edition The Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- 42.Osman A, Kopper BA, Barrios F, Gutierrez PM, Bagge CL. Reliability and validity of the Beck depression inventory--II with adolescent psychiatric inpatients. Psychol Assess. 2004;16(2):120–32. doi: 10.1037/1040-3590.16.2.120. [DOI] [PubMed] [Google Scholar]
- 43.First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM IV-Patient Edition (SCID-P) American Psychiatric Press; Washington, D.C.: 1995. [Google Scholar]
- 44.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 45.Richardson MT, Ainsworth BE, Wu H, Jacobs DR, Leon AS. Ability of the Atherosclerosis Risk in Communities (ARIC)/Baecke Questionnaire to assess leisure-time physical activity. International Journal of Epidemiology. 1995;24:685–693. doi: 10.1093/ije/24.4.685. [DOI] [PubMed] [Google Scholar]
- 46.Kleinbaum DG. Applied regression analysis and other multivariable methods. 4th ed Brooks/Cole; Australia ; Belmont, CA: 2007. p. xxi.p. 906. [Google Scholar]
- 47.Carlin JB, Gurrin LC, Sterne JAC, Morley R, Dwyer T. Regression models for twin studies: a critical review. International Journal of Epidemiology. 2005;34(5):1089–1099. doi: 10.1093/ije/dyi153. [DOI] [PubMed] [Google Scholar]
- 48.Masur DM, Fuld PA, Blau AD, Crystal H, Aronson MK. Predicting development of dementia in the elderly with the Selective Reminding Test. J Clin Exp Neuropsychol. 1990;12(4):529–38. doi: 10.1080/01688639008400999. [DOI] [PubMed] [Google Scholar]
- 49.Turner MS, Cipolotti L, Yousry T, Shallice T. Qualitatively different memory impairments across frontal lobe subgroups. Neuropsychologia. 2007;45(7):1540–52. doi: 10.1016/j.neuropsychologia.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 50.Hansen AL, Johnsen BH, Thayer JF. Relationship between heart rate variability and cognitive function during threat of shock. Anxiety Stress Coping. 2009;22(1):77–89. doi: 10.1080/10615800802272251. [DOI] [PubMed] [Google Scholar]
- 51.Nakata E, Kasai M, Kasuya M, Akanuma K, Meguro M, Ishii H, Yamaguchi S, Meguro K. Combined memory and executive function tests can screen mild cognitive impairment and converters to dementia in a community: the Osaki-Tajiri project. Neuroepidemiology. 2009;33(2):103–10. doi: 10.1159/000222092. [DOI] [PubMed] [Google Scholar]
- 52.Giubilei F, Strano S, Imbimbo BP, Tisei P, Calcagnini G, Lino S, Frontoni M, Santini M, Fieschi C. Cardiac autonomic dysfunction in patients with Alzheimer disease: possible pathogenetic mechanisms. Alzheimer Dis Assoc Disord. 1998;12(4):356–61. doi: 10.1097/00002093-199812000-00017. [DOI] [PubMed] [Google Scholar]
- 53.Aharon-Peretz J, Harel T, Revach M, Ben-Haim SA. Increased sympathetic and decreased parasympathetic cardiac innervation in patients with Alzheimer’s disease. Arch Neurol. 1992;49(9):919–22. doi: 10.1001/archneur.1992.00530330041013. [DOI] [PubMed] [Google Scholar]
- 54.Desimone R. Neural mechanisms for visual memory and their role in attention. Proc Natl Acad Sci U S A. 1996;93(24):13494–9. doi: 10.1073/pnas.93.24.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pessoa L, Gutierrez E, Bandettini P, Ungerleider L. Neural correlates of visual working memory: fMRI amplitude predicts task performance. Neuron. 2002;35(5):975–87. doi: 10.1016/s0896-6273(02)00817-6. [DOI] [PubMed] [Google Scholar]
- 56.Baxter MG, Gaffan D, Kyriazis DA, Mitchell AS. Dorsolateral prefrontal lesions do not impair tests of scene learning and decision-making that require frontal-temporal interaction. Eur J Neurosci. 2008;28(3):491–9. doi: 10.1111/j.1460-9568.2008.06353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roland PE, Gulyas B. Visual imagery and visual representation. Trends Neurosci. 1994;17(7):281–7. doi: 10.1016/0166-2236(94)90057-4. discussion 294-7. [DOI] [PubMed] [Google Scholar]
- 58.Cooke WH, Cox JF, Diedrich AM, Taylor JA, Beightol LA, Ames J.E.t., Hoag JB, Seidel H, Eckberg DL. Controlled breathing protocols probe human autonomic cardiovascular rhythms. Am J Physiol. 1998;274(2 Pt 2):H709–18. doi: 10.1152/ajpheart.1998.274.2.h709. [DOI] [PubMed] [Google Scholar]
- 59.Taylor JA, Eckberg DL. Fundamental relations between short-term RR interval and arterial pressure oscillations in humans. Circulation. 1996;93(8):1527–32. doi: 10.1161/01.cir.93.8.1527. [DOI] [PubMed] [Google Scholar]
- 60.Fleisher LA, Frank SM, Sessler DI, Cheng C, Matsukawa T, Vannier CA. Thermoregulation and heart rate variability. Clin Sci (Lond) 1996;90(2):97–103. doi: 10.1042/cs0900097. [DOI] [PubMed] [Google Scholar]
- 61.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213(4504):220–2. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 62.Basso N, Paglia N, Stella I, de Cavanagh EM, Ferder L, del Rosario Lores Arnaiz M, Inserra F. Protective effect of the inhibition of the renin-angiotensin system on aging. Regul Pept. 2005;128(3):247–52. doi: 10.1016/j.regpep.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 63.Gard PR, Rusted JM. Angiotensin and Alzheimer’s disease: therapeutic prospects. Expert Rev Neurother. 2004;4(1):87–96. doi: 10.1586/14737175.4.1.87. [DOI] [PubMed] [Google Scholar]
- 64.Amouyel P, Richard F, Berr C, David-Fromentin I, Helbecque N. The renin angiotensin system and Alzheimer’s disease. Ann N Y Acad Sci. 2000;903:437–41. doi: 10.1111/j.1749-6632.2000.tb06395.x. [DOI] [PubMed] [Google Scholar]
- 65.Li NC, Lee A, Whitmer RA, Kivipelto M, Lawler E, Kazis LE, Wolozin B. Use of angiotensin receptor blockers and risk of dementia in a predominantly male population: prospective cohort analysis. BMJ. 2010;340:b5465. doi: 10.1136/bmj.b5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord. 2000;61(3):201–16. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- 67.Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Ann Behav Med. 2009;37(2):141–53. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- 68.Saul JP, Berger RD, Albrecht P, Stein SP, Chen MH, Cohen RJ. Transfer function analysis of the circulation: unique insights into cardiovascular regulation. Am J Physiol. 1991;261(4 Pt 2):H1231–45. doi: 10.1152/ajpheart.1991.261.4.H1231. [DOI] [PubMed] [Google Scholar]
- 69.Duschek S, Muckenthaler M, Werner N, del Paso GA. Relationships between features of autonomic cardiovascular control and cognitive performance. Biol Psychol. 2009;81(2):110–7. doi: 10.1016/j.biopsycho.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 70.RAPELA C, GREEN H, DENISON A., Jr Baroreceptor reflexes and autoregulation of cerebral blood flow in the dog. Circulation Research. 1967;21(4):559. doi: 10.1161/01.res.21.4.559. [DOI] [PubMed] [Google Scholar]
- 71.Bremner JD. Does stress damage the brain? Biol Psychiatry. 1999;45(7):797–805. doi: 10.1016/s0006-3223(99)00009-8. [DOI] [PubMed] [Google Scholar]
- 72.Nolan RP, Jong P, Barry-Bianchi SM, Tanaka TH, Floras JS. Effects of drug, biobehavioral and exercise therapies on heart rate variability in coronary artery disease: a systematic review. Eur J Cardiovasc Prev Rehabil. 2008;15(4):386–96. doi: 10.1097/HJR.0b013e3283030a97. [DOI] [PubMed] [Google Scholar]
- 73.Erickson KI, Kramer AF. Aerobic exercise effects on cognitive and neural plasticity in older adults. Br J Sports Med. 2009;43(1):22–4. doi: 10.1136/bjsm.2008.052498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aberg MA, Pedersen NL, Toren K, Svartengren M, Backstrand B, Johnsson T, Cooper-Kuhn CM, Aberg ND, Nilsson M, Kuhn HG. Cardiovascular fitness is associated with cognition in young adulthood. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0905307106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, Gandevia SC, Gomez-Pinilla F, Greenwood BN, Hillman CH, Kramer AF, Levin BE, Moran TH, Russo-Neustadt AA, Salamone JD, Van Hoomissen JD, Wade CE, York DA, Zigmond MJ. Neurobiology of exercise. Obesity (Silver Spring) 2006;14(3):345–56. doi: 10.1038/oby.2006.46. [DOI] [PubMed] [Google Scholar]
- 76.Hansen AL, Johnsen BH, Sollers JJ, 3rd, Stenvik K, Thayer JF. Heart rate variability and its relation to prefrontal cognitive function: the effects of training and detraining. Eur J Appl Physiol. 2004;93(3):263–72. doi: 10.1007/s00421-004-1208-0. [DOI] [PubMed] [Google Scholar]
- 77.Eisenberg J, Ben-Daniel N, Mei-Tal G, Wertman E. An autonomic nervous system biofeedback modality for the treatment of attention deficit hyperactivity disorder--an open pilot study. Isr J Psychiatry Relat Sci. 2004;41(1):45–53. [PubMed] [Google Scholar]