Abstract
Objective
Although early trauma (trauma in childhood) has been linked to adult inflammation and adult disease of inflammatory origin, it remains unknown whether this relationship is due to long-term consequences of early life stress or other familial factors.
Methods
We examined 482 male middle aged twins (241 pairs) born between 1946 and 1956 from the Vietnam Era Twin Registry. Childhood traumatic experiences, before age 18, were measured retrospectively with the Early Trauma Inventory (ETI) and included physical, sexual, emotional abuse, and general trauma. Lifetime major depressive disorder and posttraumatic stress disorder were assessed with the Structured Clinical Interview for DSM IV. Traditional risk factors for cardiovascular disease were also assessed. Plasma C-reactive protein (CRP) and interleukin-6 (IL-6) were measured to determine levels of inflammation. Mixed-effect regression models with a random intercept for pair were used to separate between and within twin pair effects.
Results
When twins were analyzed as individuals, increasing levels of early trauma were positively related to CRP (p=0.03) but not IL-6 (p=0.12). When estimating within and between pair effects, only the between pair association of early trauma with the inflammatory markers remained significant.
Conclusion
The link between early trauma and inflammation is largely explained by familial factors shared by the twins, because levels of inflammation were highest when both twins were exposed to trauma. Exposure to early trauma may be a marker for an unhealthy familial environment. Clarification of familial factors associated with early stress and adult inflammation will be important to uncover correlates of stress and disease.
Keywords: childhood maltreatment, Interleukin-6, C-reactive protein, stress, risk factor
Introduction
Early trauma (severe stressful events experienced in childhood) is associated with increased risk of multiple age-related diseases (1-3). It is postulated that disruption of the physiological stress response due to exposure to early trauma may lead to dysregulation of the homeostatic balance of immune function. This leads to increased inflammation which may increase the susceptibility to chronic diseases in adulthood (4). In support of this notion, inflammatory biomarkers, such as C-reactive protein (CRP) and interleukin-6 (IL-6), have been linked to early trauma (5-8). The association was found to be dose dependent, and independent of cardiovascular risk factors.
Another explanation for the association between early trauma and inflammation is that exposure to early trauma may be a marker of an at-risk familial environment. This high-risk family environment may include, for example, parental/prenatal factors, lower socioeconomic status, and poor health behaviors in the family of origin. A study of early trauma experiences in twins raised together can address this question while controlling, by design, for other familial factors.
The purpose of this study was to assess the relationship between early trauma and adult inflammation, using a co-twin control design. Because both inflammation (9) and trauma exposure (10-12) have a genetic component, it is also important to take into account any potential shared genetic influences between early trauma and inflammation. Co-twin control studies allow tight control for shared genetic factors in addition to shared environment (13).
Methods
Subjects
The Emory Twin Studies include samples recruited in two companion studies: the Twins Heart Study (THS) and the Stress and Vascular Evaluation in Twins (SAVEIT). The studies address psychological, behavioral, and biological risk factors for subclinical cardiovascular disease using twins (14, 15). Both projects recruited middle-aged male monozygotic and dizygotic twin pairs from the Vietnam Era Twin (VET) Registry, one of the largest twin registries in the United States (16). Zygosity information was obtained from DNA samples using previously described methodology (17). Both studies followed identical procedures, measurements and protocols. THS enrolled 180 twin pairs between 2002 and 2006 (14) and SAVEIT included 82 twin pairs enrolled between 2005 and 2008. Twins included in the Emory Twin Studies were randomly selected from the VET Registry among those born between 1946 and 1956. In addition, a random sample of twin pairs discordant for major depressive disorder (MDD) was included in THS and a random sample of twin pairs discordant for posttraumatic stress disorder (PTSD) was included in SAVEIT. Twins were examined together at the Emory University General Clinical Research Center. Medical history was obtained at the time of examination. The study protocol was approved by the Institutional review board at Emory University, and informed consent was obtained from all subjects.
Measurement of Early Trauma
Childhood traumatic experiences, before age 18, were measured retrospectively with the Early Trauma Inventory (ETI) and included physical, sexual, emotional abuse, and general trauma (18). Briefly, physical abuse (9 items) was defined as physical contact, constraint, or confinement, with intent to hurt or injure. Sexual abuse (15 items) was defined as unwanted sexual contact performed solely for the gratification of the perpetrator or for the purposes of dominating or degrading the victim. Emotional abuse (7 items) captured verbal communication with the intention of humiliating or degrading the victim. General trauma (31) comprised a range of other stressful and traumatic events (i.e., natural disaster, family mental illness, separation of parents etc.). Exposure to each of these events was determined by a series of dichotomous (yes/no) questions. Frequency of abuse was also obtained on items where a positive response was received on the dichotomous exposure item. Values from each trauma domain were summed to obtain a total, continuous ETI score, an index of total early trauma exposure. Primary analysis was conducted on the continuous ETI scores. For descriptive purposes, dichotomous trauma variables were also calculated; cutoffs were defined as the mean ETI score in healthy individuals, without depression or PTSD, plus one standard deviation (18). Individuals above the cutoff were regarded as having early trauma, and those below the cutoff as not having early trauma.
Assessment of Lifetime Depression and PTSD
The Structured Clinical Interview for DSM IV (SCID), which yields a clinical diagnosis of MDD, was used to assess lifetime history of MDD (19). The SCID was also used to provide a diagnosis of PTSD.
Assessment of Inflammation
CRP and IL-6 were tested from plasma samples collected and frozen at −70°C for later analysis. Levels of CRP were measured with the high-sensitivity Beckman Coulter assay. High values of CRP are indicative of inflammation and values >3mg/L were used as a cutoff to identify at risk individuals. IL-6 was assessed using commercially available ELISA kits from R and D Systems. All biochemical assays for each twin pair were processed in the same analytical run.
Assessment of Socioeconomic Status
Socioeconomic status, or income, was considered in the analysis because it has previously been associated with early trauma exposure (20) and increased inflammation (6). Income data were used as a categorical variable: 1 = < $5000, 2 = $5000-$9999, 3 = $10,000-$14,999, 4 = $15,000-$19,999, 5 = $20,000-$24,999, 6 = $25,000-$29,999, 7 = $30,000-$34,999, 8 = $35,000-$39,999, 9 = $40,000-$49,999, 10 = $50,000+. This information was previously collected as part of the VET Registry Survey of Health questionnaire administered in 1985-1987 (21) in 10,979 participants. In our sample, data on income was available on 473 participants.
Other Measurements
All measurements were performed in the morning after an overnight fast, and both twins in a pair were tested at the same time. Medical history and physical evaluation were obtained from all twins by a research nurse. Traditional risk factors for cardiovascular disease (smoking, plasma cholesterol) were also assessed (see Table 1). Current medication use was obtained and included type, dosage, and frequency of use. Body mass index (BMI), calculated as weight in pounds divided by the square of the height in inches, was analyzed as a continuous variable. Obesity was defined as a BMI>30. Systolic and diastolic blood pressure was measured by a mercury sphygmomanometer on the right arm in sitting position after 10 minutes of rest. Two separate blood pressure measurements, taken 5 minutes apart, were averaged for analysis. Plasma fasting glucose, total, low, and high density cholesterol were measured from plasma samples collected after a 9-hour overnight fast. Total triglycerides were measured by enzymatic methods (Beckman Coulter Diagnostics, Fullerton, California). Direct high-density lipoprotein (HDL) and low-density lipoprotein (LDL) were measured with homogeneous assays (Equal Diagnostics, Exton, Pennsylvania). Smoking status was determined using standardized questionnaires from population studies (22). We classified participants: as never smoked - those who reported that they never smoked regularly, current smokers - those currently smoking cigarettes regularly, and past smokers - those who reported smoking more than 100 cigarettes in the past. For this study, we also classified participants into two groups: current smokers and non-smokers, the latter including never and past smokers. Total alcohol consumption (number of drinks within a typical week) was determined using a standardized questionnaire. Alcohol consumption was analyzed as a continuous variable and included number of alcoholic (wine, beer, or cocktail) beverages consumed per week (23). Current physical activity status was determined using the Baecke questionnaire, a 16-question instrument documenting level of physical activity at work, during sports and non-sports activities (24). The global physical activity score was used in the analysis. A history of coronary heart disease was defined as previously being diagnosed with myocardial infarction or angina pectoris or previous coronary revascularization procedures. Diabetes was defined as having a fasting plasma glucose >126 mg/dl or current treatment with anti diabetic medications. Demographic factors, such as education, marital and employment status were determined using a standardized questionnaire.
Table 1.
Demographic, lifestyle, clinical, hemodynamic and biochemical characteristics in individuals with early trauma and no trauma.
| No Trauma (n=237) |
Trauma (n=245) |
p-value | |
|---|---|---|---|
| Demographic Factors | |||
| Age | 55 (3) | 55 (3) | 0.23 |
| Greater than high school, n (%) | 84 (35) | 75 (31) | 0.16 |
| Body Mass Index, kg/m2 | 30 (5) | 30 (5) | 0.92 |
| Married, n (%) | 192 (81) | 179 (72) | 0.05 |
| Employed, n (%) | 190 (80) | 182 (74) | 0.13 |
| Socioeconomic Status | 7.4 (2.3) | 6.9 (2.3) | 0.62 |
| Hemodynamic Factors | |||
| Systolic blood pressure, mmHg | 129 (15) | 130 (17) | 0.75 |
| Diastolic blood pressure, mmHg | 81 (10) | 81 (11) | 0.92 |
| Biochemical Factors | |||
| Plasma glucose, mg/dL | 103 (18) | 100 (19) | 0.15 |
| HDL cholesterol, mmol/L | 39 (11) | 39 (11) | 0.33 |
| LDL cholesterol, mmol/L | 124 (35) | 120 (34) | 0.14 |
| Clinical Factors | |||
| Framingham Risk Score | 6.0 (2.1) | 6.0 (2.3) | 0.80 |
| Lifetime history of alcohol abuse or dependence, n (%) |
95 (40) | 145 (59) | <0.001 |
| Previous coronary heart disease, n (%) | 15 (6) | 36 (15) | 0.10 |
| Hypertension, n (%) | 69 (29) | 72 (29) | 0.20 |
| Diabetes mellitus, n (%) | 20 (8) | 30 (12) | 0.95 |
| Lifetime history of major depressive disorder, n (%) |
38 (16) | 77 (32) | 0.005 |
| Lifetime history of posttraumatic stress disorder, n (%) |
15 (6) | 39 (16) | <0.001 |
| Use of statins, n (%) | 52 (22) | 73 (30) | 0.08 |
| Use of aspirin, n (%) | 25 (11) | 38 (16) | 0.12 |
| Lifestyle Factors | |||
| Current Smoker, n (%) | 49 (21) | 71 (29) | 0.13 |
| Physical Activity | 7.3 (2) | 7.1 (2) | 0.15 |
| Number of alcoholic beverages in typical week |
4.9 (9.8) | 5.5 (10.0) | 0.56 |
| Inflammatory biomarkers | |||
| CRP (pg/mL) | 1.93 (2.0) | 3.0 (4.0) | 0.07 |
| IL-6 (pg/mL) | 1.8 (1.5) | 2.4 (2.4) | 0.07 |
| Zygosity | |||
| Monozygotic | 134 (56%) | 158 (64%) | 0.12 |
| Dizygotic | 103 (44%) | 87 (36%) |
Unless otherwise started, values presented are means (SD). P-values were calculated using mixed models for continuous variables or generalized estimating equations (GEE) for categorical variables.
Statistical Analysis
Statistical analysis was performed using SAS software version 9.2 (SAS Institute). Biomarkers were log-transformed to improve normality. Analyses were conducted using mixed model linear regression analysis for continuous variable and generalized estimating equation (GEE) models for categorical variables with a random intercept for each pair (25).
Initial descriptive analysis treating the twins as individuals, while accounting for clustering by twin pair, assessed the association between early trauma and each inflammatory biomarker.
The association between early trauma and inflammatory biomarkers, controlling for shared genetic and environmental factors, was examined using mixed effects models adapted for twin analyses (25). This model separately estimates the association between and within pairs. The within pair analysis is a matched-pair approach in which the influence of shared maternal factors, familial and childhood/adolescent environment are controlled. If the within pair effects are smaller than the effects seen when twins are analyzed as separate individuals, this is evidence that there is confounding by factors shared by co-twins. ETI scores were included as continuous variables in this analysis; effects were expressed as individual twin variation from their twin pair average, which corresponds to the absolute difference between the two brothers. Analyses were conducted before and after adjusting for factors that were a priori considered potentially important confounders or mediators of the relationship. These included income and education, behavioral factors (smoking, alcohol consumption, and physical activity), traditional cardiovascular risk factors (age, BMI, systolic blood pressure, diabetes, previous heart disease, total triglycerides, LDL and HDL cholesterol, statin medications, and anti-inflammatory medications) and psychiatric diagnoses (lifetime history of MDD and PTSD). These factors were sequentially added in groups, as specified above, as latter models that adjusted for earlier factors. Because obesity has been linked to inflammation (27, 28), a separate analysis comparing the effects of early trauma on inflammation was conducted after stratification by obesity, followed by a test of the interaction between early trauma and obesity on inflammation.
The within-pair analysis was further stratified by zygosity to determine whether the relationship between early trauma and inflammatory biomarkers was different between monozygotic and dizygotic twins. Monozygotic twins share 100% of their genes, whereas dizygotic twins only share on average 50% of their genes. Therefore, genetic factors are completely accounted for when comparing monozygotic twins with each other, but only partially accounted for when comparing dizygotic twins with each other. Thus, if the within-pair effect is smaller in monozygotic than in dizygotic twins, this is evidence that the association is confounded by genetic factors (29, 30). Intraclass coefficients were also calculated to determine whether genetic factors were involved in the traits under consideration. Significance level was set at 0.05, two sided.
Results
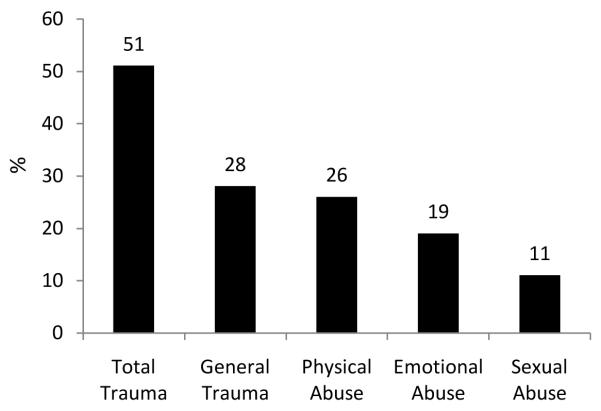
The study sample consisted of 237 individuals without early trauma and 245 with early trauma. There were 42 monozygotic and 27 dizygotic twin pairs discordant for early trauma. The remaining twin pairs consisted of 88 positive and 84 negative concordant twin pairs. General trauma was the most common source of early trauma followed by physical abuse, emotional abuse, and sexual abuse (Figure 1). Compared to individuals without early trauma (based on the cutoff for total trauma), those who were exposed to early trauma were less likely to be married, had higher rates of lifetime history of alcohol abuse or dependence, MDD, and PTSD, as well as higher CRP and IL-6 levels (Table 1). Intraclass coefficients for CRP, IL-6 and the total trauma score were higher in MZ compared to DZ twins, suggesting a genetic component in these traits (Table 2).
Figure 1.
Percentage of individuals with and without early traumatic events (defined as a dichotomous trauma variables). Data are shown for total trauma (total ETI score meeting the cutoff for dichotomous trauma exposure), and for trauma subtypes, derived from categorization of subscales of general trauma, physical abuse, emotional abuse, and sexual abuse.
Table 2.
Intraclass Correlation Coefficients
| Monozygotic | Dizygotic | |
|---|---|---|
| Continuous ETI score | 0.54 | 0.47 |
| CRP, mg/L | 0.59 | 0.31 |
| IL-6, pg/mL | 0.43 | 0.31 |
Calculated using linear mixed model with SAS
Overall Sample Comparisons
The relationship between each type of early trauma and inflammatory biomarkers in twins as individuals is shown in Table 3. Increasing levels of the total trauma score were positively related to CRP (β=0.03, p=0.03) but not IL-6 (β=0.01, p=0.12). Twins with early trauma (based on a total trauma score meeting the cutoff for dichotomous trauma exposure) had 22% higher CRP (p=0.07) and 13% higher IL-6 (p=0.07) compared to those without early trauma.
Table 3.
Unadjusted and adjusted relationships between the continuous ETI scores and inflammatory biomarkers, CRP and IL-6, treating twins as individuals.
| CRP |
IL-6 |
|||
|---|---|---|---|---|
| β | p-value | β | p-value | |
| Total Trauma | 0.03 | 0.03 | 0.01 | 0.12 |
| General Trauma | 0.04 | 0.07 | 0.02 | 0.25 |
| Physical Abuse | 0.05 | 0.20 | 0.04 | 0.24 |
| Emotional Abuse | 0.06 | 0.11 | 0.02 | 0.34 |
| Sexual Abuse | 0.02 | 0.81 | 0.05 | 0.35 |
| After Accounting for Income and Education Status | ||||
| Total Trauma | 0.03 | 0.03 | 0.01 | 0.11 |
| General Trauma | 0.05 | 0.05 | 0.02 | 0.22 |
| Physical Abuse | 0.06 | 0.23 | 0.04 | 0.23 |
| Emotional Abuse | 0.06 | 0.09 | 0.02 | 0.41 |
| Sexual Abuse | 0.02 | 0.77 | 0.06 | 0.31 |
| After Accounting for Behavioral Risk Factorsa | ||||
| Total Trauma | 0.02 | 0.09 | −0.001 | 0.88 |
| General Trauma | 0.04 | 0.10 | −0.004 | 0.82 |
| Physical Abuse | 0.04 | 0.33 | −0.004 | 0.87 |
| Emotional Abuse | 0.04 | 0.04 | −0.01 | 0.72 |
| Sexual Abuse | 0.001 | 0.99 | 0.05 | 0.34 |
| After Accounting for Traditional Risk Factorsb | ||||
| Total Trauma | 0.02 | 0.12 | −0.001 | 0.88 |
| General Trauma | 0.03 | 0.19 | −0.004 | 0.82 |
| Physical Abuse | 0.04 | 0.41 | −0.005 | 0.87 |
| Emotional Abuse | 0.04 | 0.23 | −0.01 | 0.72 |
| Sexual Abuse | 0.02 | 0.76 | 0.05 | 0.34 |
| After Accounting for Psychiatric Risk Factorsc | ||||
| Total Trauma | 0.02 | 0.17 | 0.001 | 0.98 |
| General Trauma | 0.03 | 0.25 | 0.001 | 0.98 |
| Physical Abuse | 0.03 | 0.49 | −0.003 | 0.90 |
| Emotional Abuse | 0.04 | 0.27 | −0.01 | 0.81 |
| Sexual Abuse | 0.03 | 0.73 | 0.04 | 0.41 |
CRP: C-reactive protein; IL-6: interleukin-6
Regression coefficient (β) and p values were derived from mixed models that included a random intercept for pair.
Adjusted for income, education, and behavioral risk factors: cigarette smoking, physical activity, and alcohol consumption
Adjusted for income, education, behavioral and coronary risk factors; including age, body mass index, previous history of coronary heart disease, plasma lipids (high, total, and low density lipoprotein cholesterol); use of aspirin and use of statins.
Adjusted for income, education, behavioral, cardiovascular, and psychiatric risk factors; including major depressive disorder and posttraumatic stress disorder.
The prevalence of a clinically significant CRP level (>3 mg/L) was 33% in those with early trauma (based on the total trauma dichotomous definition), and 20% in those without early trauma (p=0.02). The prevalence of a clinically significant CRP was also significantly associated with emotional abuse (p=0.04), and marginally associated with physical abuse (p=0.08). Of all the different types of trauma, emotional abuse was most strongly related to a clinically significant elevation in CRP.
After accounting for income and education, the total trauma score remained positively associated with CRP levels (Table 3). On the other hand, after accounting for behavioral, cardiovascular, and psychiatric risk factors, the relationship between total trauma and CRP levels was attenuated.
In our sample, 196 (41%) of the twins were obese. In non-obese individuals (BMI<30), total trauma was significantly related to CRP levels (β=0.04, p=0.046). On the other hand, in obese individuals (BMI>30) the relationship between total trauma and inflammation was attenuated (β=0.01, p=0.52). However, the interaction between total trauma and obesity on CRP levels was not significant (β= −0.12, p=0.54).
Within-pair Comparisons
In within-pair analyses, ETI scores as continuous variables were not associated with higher CRP or IL-6 levels, except for a positive association between physical abuse and IL-6 levels in the dizygotic twins (Table 4). This was generally true in both monozygotic and dizygotic twin pairs. On average, coefficients for IL-6 tended to be larger in dizygotic than monozygotic twins, but none of the interaction terms were significant.
Table 4.
Unadjusted relationship between the continuous ETI scores and inflammatory biomarkers, CRP and IL-6, within twin pairs.
| Overall Sample (241 Twin Pairs) | Monozygotic (146 Twin Pairs) | Dizygotic (95 Twin Pairs) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRP |
IL-6 |
CRP |
IL-6 |
CRP |
IL-6 |
|||||||
| β |
p- value |
β |
p- value |
β |
p- value |
β |
p- value |
β |
p- value |
β |
p- value |
|
| Within Twin Effects | ||||||||||||
| Total Trauma | 0.004 | 0.83 | −0.01 | 0.68 | −0.04 | 0.81 | −0.14 | 0.22 | −0.04 | 0.88 | 0.25 | 0.18 |
| General Trauma | 0.02 | 0.67 | −0.02 | 0.46 | −0.03 | 0.85 | −0.14 | 0.23 | 0.14 | 0.62 | 0.15 | 0.46 |
| Physical Abuse | −0.01 | 0.84 | 0.04 | 0.41 | −0.18 | 0.29 | −0.02 | 0.84 | −0.05 | 0.85 | 0.45 | 0.02 |
| Emotional Abuse | 0.02 | 0.73 | −0.01 | 0.69 | −0.17 | 0.37 | −0.10 | 0.44 | 0.32 | 0.26 | 0.29 | 0.17 |
| Sexual Abuse | −0.06 | 0.58 | −0.03 | 0.68 | 0.10 | 0.63 | 0.01 | 0.97 | −0.24 | 0.58 | 0.13 | 0.68 |
CRP: C-reactive protein; IL-6: interleukin-6
Regression coefficient (β) and p values were derived from mixed models for continuous variables that included a random intercept for pair. Effects are expressed as individual twin variation in ETI score points from their twin pair average, which corresponds to the absolute difference between the two brothers.
Post-hoc analysis revealed that our sample was adequately powered to detect a within twin effect of early trauma on IL-6 (p-value closer to significance) given β=0.25, and assuming a two tailed alpha of 0.05 and a statistical power of ≥ 0.80.
Between Pair Comparisons
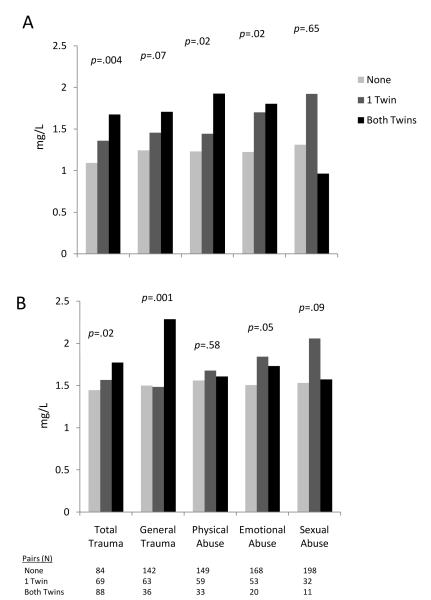
At the pair level, increasing levels of early trauma, using the total trauma score as a continuous variable were positively related to higher CRP (β=0.05, p=0.007) and higher IL-6 (β=0.03, p=0.02) (Table 5). The total trauma score remained associated with higher CRP levels after accounting for income, education, behavioral, cardiovascular, and psychiatric risk factors. Treating total trauma as a dichotomous variable, twin pairs where both members met the total trauma cutoff had 53% higher levels of CRP than pairs that did not meet the cutoff, with intermediate levels in twin pairs where only one member of the pair met the cutoff for early trauma (p for trend=0.004) (Figure 2a). These pair-level effects for total trauma and emotional trauma remained significant after accounting for income, education, behavioral, cardiovascular, and psychiatric risk factors. A similar pattern of results, albeit with less consistent dose response, was noted for IL-6 (Figure 2b). However, the pair-level association for IL-6 was attenuated and became nonsignificant for all trauma variables after accounting for other risk factors.
Table 5.
Unadjusted and adjusted relationship between the continuous ETI scores and inflammatory biomarkers, CRP and IL-6, between twin pairs.
|
CRP |
IL-6 |
|||
|---|---|---|---|---|
| β | p-value | β | p-value | |
| Total Trauma | 0.05 | 0.007 | 0.03 | 0.02 |
| General Trauma | 0.07 | 0.04 | 0.04 | 0.04 |
| Physical Abuse | 0.13 | 0.05 | 0.03 | 0.40 |
| Emotional Abuse | 0.10 | 0.05 | 0.05 | 0.11 |
| Sexual Abuse | 0.16 | 0.24 | 0.15 | 0.06 |
| After Accounting for Income and Education Status | ||||
| Total Trauma | 0.05 | 0.01 | 0.03 | 0.02 |
| General Trauma | 0.07 | 0.03 | 0.04 | 0.04 |
| Physical Abuse | 0.13 | 0.05 | 0.03 | 0.43 |
| Emotional Abuse | 0.10 | 0.07 | 0.05 | 0.13 |
| Sexual Abuse | 0.17 | 0.21 | 0.16 | 0.05 |
| After Accounting for Behavioral Risk Factorsa | ||||
| Total Trauma | 0.04 | 0.02 | 0.02 | 0.10 |
| General Trauma | 0.06 | 0.06 | 0.03 | 0.13 |
| Physical Abuse | 0.11 | 0.07 | 0.01 | 0.72 |
| Emotional Abuse | 0.07 | 0.17 | 0.04 | 0.25 |
| Sexual Abuse | 0.11 | 0.38 | 0.13 | 0.11 |
| After Accounting for Traditional Risk Factorsb | ||||
| Total Trauma | 0.04 | 0.02 | 0.01 | 0.54 |
| General Trauma | 0.06 | 0.08 | 0.01 | 0.58 |
| Physical Abuse | 0.11 | 0.05 | −0.02 | 0.54 |
| Emotional Abuse | 0.08 | 0.31 | 0.02 | 0.57 |
| Sexual Abuse | 0.16 | 0.20 | 0.15 | 0.06 |
| After Accounting for Psychiatric Risk Factorsc | ||||
| Total Trauma | 0.04 | 0.02 | −0.01 | 0.48 |
| General Trauma | 0.05 | 0.11 | 0.01 | 0.47 |
| Physical Abuse | 0.11 | 0.06 | −0.02 | 0.60 |
| Emotional Abuse | 0.07 | 0.10 | 0.02 | 0.52 |
| Sexual Abuse | 0.16 | 0.19 | 0.14 | 0.07 |
CRP: C-reactive protein; IL-6: interleukin-6
Regression coefficient (β) and p values were derived from mixed models that included a random intercept for pair.
Adjusted for income, education, and behavioral risk factors: cigarette smoking, physical activity, and alcohol consumption
Adjusted for income, education, behavioral and coronary risk factors; including age, body mass index, previous history of coronary heart disease, plasma lipids (high, total, and low density lipoprotein cholesterol); use of aspirin and use of statins.
Adjusted for income, education, behavioral, cardiovascular, and psychiatric risk factors; including major depressive disorder and posttraumatic stress disorder.
Figure 2.
Pair-level effects of (a) CRP and (b) IL-6 (unadjusted) according to the number of twins exposed to early trauma in a pair. Data are shown for total trauma (total ETI score meeting the cutoff for dichotomous trauma exposure), and for trauma subtypes, derived from categorization of subscales of general trauma, physical abuse, emotional abuse, and sexual abuse. P-values shown represent the trend.
Discussion
We found that trauma early in life is associated with increased inflammation as measured by CRP and IL-6 when twins are treated as individuals, but after separating within and between effects, significant effects were not observed within twin pairs, a comparison that effectively controls for familial factors. We only observed significant effects between twin pairs; in pairs where both members suffered early trauma inflammation was elevated the most. These results suggest that familial factors confound the relationship between early trauma and inflammation, or represent important antecedents to this association.
There are many ways through which familial factors may affect the relationship between early trauma and inflammation. First, there could be familial confounding factors. For example, individuals raised with an unfavorable socioeconomic status have shown a greater expression of pro-inflammatory genes, increased production of pro-inflammatory cytokines, and greater resistance to glucocorticoid signaling (31, 32). In turn, an unfavorable social environment may facilitate traumatic experiences. Glucocortioids are important for inhibiting inflammation, thereby controlling the hypothalamic-pituitary-adrenal axis response to stress (33, 34). These damaging effects of the social environment are irreversible even after upward social class mobility later in life, and remain after adjusting for life stress and poor health practices (i.e., smoking, alcohol consumption). In our study, socioeconomic status or education status did not attenuate the relationship between early trauma exposure and inflammation. This is most likely due to the use of adult socioeconomic status compared to income during childhood. Childhood income, although not available in our study, was accounted for in the within-pair analysis since twins were raised in the same family. Because the within-pair results were considerably attenuated compared with the between pair results, our results suggest that parental or other familial factors influencing trauma exposure, including income, may be important in the relationship between trauma and health. Furthermore, the influence of familial factors on trauma exposure is independent of behavioral, cardiovascular, and psychiatric disorders.
Familial factors may also be necessary antecedents to early stress, or more severe forms of early stress. Since twins are matched for parental and other familial factors, our within-pair comparisons excluded trauma exposures shared by both members of the pair. Pairs where both members were affected by early trauma may signal family environments at high risk for childhood abuse. For example, previous literature has shown that increased risk for childhood sexual abuse is observed when parental alcohol abuse was reported in one parent and even greater risk when both parents reported alcohol abuse (35). A meta-analysis of 166 studies reported that environmental factors such as low socioeconomic status, paternal unemployment, and living with one or neither parent was associated with increasing risk for sexual abuse (36). It seems plausible that shared familial factors play a role in the link between early trauma and adult inflammation.
Our study is in agreement with previous cross-sectional (6, 7) and longitudinal life-course reports (5, 8) that have observed a positive relationship between exposure to early trauma and adult inflammation in individuals exposed to various forms of abuse, neglect, and/or childhood maltreatment. Of these studies, only one was conducted in a U.S. population (6). The remaining studies were all based on the same New Zealand population where the oldest person was 32 (5, 7, 8). In contrast to a previous study (8), after accounting for behavioral, cardiovascular, and psychiatric risk factors, the relationship between early trauma and inflammation was attenuated; after accounting for familial factors, it was eliminated. In this previous study, the relationship between early trauma and adult inflammation was assessed in younger individuals that were approximately 32 years of age. On the other hand, our participants were older (average age of 55), so it is possible that the years of exposure to damaging risk factors had a larger impact on adult inflammation. Overall, the present study extends this literature by assessing a U.S. population of twins in middle age, a time when cardiovascular risk rises considerably. The advantage of studying twins is the ability to control for familial factors when examining the association of early trauma and inflammation.
Of the different forms of abuse, emotional abuse was most strongly linked to higher, clinically significant, CRP levels. Emotional abuse, specifically, has been shown to be highly associated with chronic stress or long-term psychopathology outcomes, such as PTSD (37-39). These long-term effects of emotional abuse may affect brain regions, such as the limbic system, which play a major role in emotions and memory (40). This in turn, could lead to greater dysfunction in the stress pathways of young children, subsequently altering inflammatory processes later on in life (8).
A significant relationship between trauma exposure and inflammation was observed in non-obese individuals but not in obese individuals. It is likely that obesity, which is associated with inflammation (41), may confound the relationship between early trauma and inflammation. However, in the absence of obesity, the relationship between early trauma and higher inflammation still exists.
The heritibability of inflammatory biomarkers such as IL-6 and CRP has been previously shown (9) and trauma exposure is also partially heritable (10-12), therefore a shared genetic component is theoretically possible. However, we found no significant within-pair association between trauma exposure and inflammation in either dizygotic or monozygotic twin pairs. Therefore it is unlikely that a genetic link between early trauma and inflammation exists.
This study has limitations that should be acknowledged. It has a cross-sectional design, and thus we are unable to address the temporal relationship between exposure to early trauma and inflammation. In addition, the inclusion of only middle aged males limits the generalizability of the results to women or individuals in other age groups. Nevertheless, our study is unique in that the twin study design allows for taking into account genetic and/or shared familial factors. In contrast to previous literature, we were able to uncover substantial familial confounding on this relationship. Lastly, because participants were asked to recall events that occurred in the distant past, recall bias may have been an issue in our sample. However, previous literature suggests that harmful memories are typically underreported rather than overreported (42).
Conclusions
Although we found an association between early trauma and adult inflammation, by using a twin study, we were able to determine that familial factors are likely a key element in this association. Based on these findings, self-reported early trauma is a marker for a stressful, or otherwise unhealthy, family environment that may potentially increase the risk of future disease. Our results emphasize the need for preventative efforts that begin in youth. Early intervention may allow children the chance to restore a sense of control and mastery early in life and decrease the likelihood of long-term effects of trauma on disease risk later in life.
Acknowledgments
The United States Department of Veterans Affairs has provided financial support for the development and maintenance of the VET Registry. Numerous organizations have provided invaluable assistance, including: VA Cooperative Study Program; Department of Defense; National Personnel Records Center, National Archives and Records Administration; the Internal Revenue Service; NIH; National Opinion Research Center; National Research Council, National Academy of Sciences; the Institute for Survey Research, Temple University. We gratefully acknowledge the continued cooperation and participation of the members of the Vietnam Era Twin Registry. Without their contribution this research would not have been possible.
Funding Sources: This study was supported by K24HL077506, R01 HL68630, R01 AG026255, and NIH/NIGMS IRACDA grant, 5K12 GM000680 from the National Institutes of Health; by the Emory University General Clinical Research Center MO1-RR00039 and by grant 0245115N from the American Heart Association.
Abbreviations
- BMI
body mass index
- CRP
C-reactive protein
- DSM IV
Diagnostic and Statistical Manual of Mental Disorders
- ELISA
Enzyme-Linked Immunosorbent Assay
- ETI
Early Trauma Inventory
- GEE
generalized estimating equation
- HDL
high density lipoprotein
- IL-6
interleukin-6
- LDL
low-density lipoprotein
- MDD
major depressive disorder
- PTSD
posttraumatic stress disorder
- SAVEIT
Stress and Vascular Evaluation in Twins
- SCID
Structured Clinical Interview for DSM IV
- THS
Twins Heart Study
- VET Registry
Vietnam Era Twin Registry
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Felitti VJ. Long-term medical consequences of incest, rape, and molestation. South Med J. 1991;84:328–31. doi: 10.1097/00007611-199103000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245–58. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 3.Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation. 2004;110:1761–6. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- 4.Shanks N, Lightman SL. The maternal-neonatal neuro-immune interface: are there long-term implications for inflammatory or stress-related disease? J Clin Invest. 2001;108:1567–73. doi: 10.1172/JCI14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, Poulton R, Caspi A. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med. 2009;163:1135–43. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the coronary artery risk development in young adults study. Biol Psychiatry. 2006;60:819–24. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry. 2008;65:409–15. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci U S A. 2007;104:1319–24. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su S, Miller AH, Snieder H, Bremner JD, Ritchie J, Maisano C, Jones L, Murrah NV, Goldberg J, Vaccarino V. Common genetic contributions to depressive symptoms and inflammatory markers in middle-aged men: the Twins Heart Study. Psychosom Med. 2009;71:152–8. doi: 10.1097/PSY.0b013e31819082ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stein MB, Jang KL, Taylor S, Vernon PA, Livesley WJ. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. Am J Psychiatry. 2002;159:1675–81. doi: 10.1176/appi.ajp.159.10.1675. [DOI] [PubMed] [Google Scholar]
- 11.Lyons MJ, Goldberg J, Eisen SA, True W, Tsuang MT, Meyer JM, Henderson WG. Do genes influence exposure to trauma? A twin study of combat. Am J Med Genet. 1993;48:22–7. doi: 10.1002/ajmg.1320480107. [DOI] [PubMed] [Google Scholar]
- 12.Koenen KC, Harley R, Lyons MJ, Wolfe J, Simpson JC, Goldberg J, Eisen SA, Tsuang M. A twin registry study of familial and individual risk factors for trauma exposure and posttraumatic stress disorder. J Nerv Ment Dis. 2002;190:209–18. doi: 10.1097/00005053-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 13.MacGregor AJ, Snieder H, Schork NJ, Spector TD. Twins. Novel uses to study complex traits and genetic diseases. Trends Genet. 2000;16:131–4. doi: 10.1016/s0168-9525(99)01946-0. [DOI] [PubMed] [Google Scholar]
- 14.Vaccarino V, Brennan ML, Miller AH, Bremner JD, Ritchie JC, Lindau F, Veledar E, Su S, Murrah NV, Jones L, Jawed F, Dai J, Goldberg J, Hazen SL. Association of major depressive disorder with serum myeloperoxidase and other markers of inflammation: a twin study. Biol Psychiatry. 2008;64:476–83. doi: 10.1016/j.biopsych.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaccarino V, Lampert R, Bremner JD, Lee F, Su S, Maisano C, Murrah NV, Jones L, Jawed F, Afzal N, Ashraf A, Goldberg J. Depressive symptoms and heart rate variability: evidence for a shared genetic substrate in a study of twins. Psychosom Med. 2008;70:628–36. doi: 10.1097/PSY.0b013e31817bcc9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldberg J, Curran B, Vitek ME, Henderson WG, Boyko EJ. The Vietnam Era Twin Registry. Twin Res. 2002;5:476–81. doi: 10.1375/136905202320906318. [DOI] [PubMed] [Google Scholar]
- 17.Forsberg CW, Goldberg J, Sporleder J, Smith NL. Determining zygosity in the Vietnam era twin registry: an update. Twin Res Hum Genet. 2010;13:461–4. doi: 10.1375/twin.13.5.461. [DOI] [PubMed] [Google Scholar]
- 18.Bremner JD, Bolus R, Mayer EA. Psychometric properties of the Early Trauma Inventory-Self Report. J Nerv Ment Dis. 2007;195:211–8. doi: 10.1097/01.nmd.0000243824.84651.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM IV - Patient Edition (SCID-P) American Psychiatric Press; Washington, D.C.: 1995. [Google Scholar]
- 20.Widom CS. The cycle of violence. Science. 1989;244:160–6. doi: 10.1126/science.2704995. [DOI] [PubMed] [Google Scholar]
- 21.Romeis JC, Scherrer JF, Xian H, Eisen SA, Bucholz K, Heath AC, Goldberg J, Lyons MJ, Henderson WG, True WR. Heritability of self-reported health. Health Serv Res. 2000;35:995–1010. [PMC free article] [PubMed] [Google Scholar]
- 22.Howard G, Wagenknecht LE, Burke GL, Diez-Roux A, Evans GW, McGovern P, Nieto FJ, Tell GS. Cigarette smoking and progression of atherosclerosis: The Atherosclerosis Risk in Communities (ARIC) Study. JAMA. 1998;279:119–24. doi: 10.1001/jama.279.2.119. [DOI] [PubMed] [Google Scholar]
- 23.Demirovic J, Nabulsi A, Folsom AR, Carpenter MA, Szklo M, Sorlie PD, Barnes RW. Alcohol consumption and ultrasonographically assessed carotid artery wall thickness and distensibility. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Circulation. 1993;88:2787–93. doi: 10.1161/01.cir.88.6.2787. [DOI] [PubMed] [Google Scholar]
- 24.Richardson MT, Ainsworth BE, Wu HC, Jacobs DR, Jr., Leon AS. Ability of the Atherosclerosis Risk in Communities (ARIC)/Baecke Questionnaire to assess leisure-time physical activity. Int J Epidemiol. 1995;24:685–93. doi: 10.1093/ije/24.4.685. [DOI] [PubMed] [Google Scholar]
- 25.Carlin JB, Gurrin LC, Sterne JA, Morley R, Dwyer T. Regression models for twin studies: a critical review. Int J Epidemiol. 2005;34:1089–99. doi: 10.1093/ije/dyi153. [DOI] [PubMed] [Google Scholar]
- 26.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 27.Florez H, Castillo-Florez S, Mendez A, Casanova-Romero P, Larreal-Urdaneta C, Lee D, Goldberg R. C-reactive protein is elevated in obese patients with the metabolic syndrome. Diabetes Res Clin Pract. 2006;71:92–100. doi: 10.1016/j.diabres.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Trayhurn P, Wood IS. Signalling role of adipose tissue: adipokines and inflammation in obesity. Biochem Soc Trans. 2005;33:1078–81. doi: 10.1042/BST0331078. [DOI] [PubMed] [Google Scholar]
- 29.McGue M. The end of behavioral genetics? 2008. Behav Genet. 2010;40:284–96. doi: 10.1007/s10519-010-9354-0. [DOI] [PubMed] [Google Scholar]
- 30.McGue M, Osler M, Christensen K. Causal Inference and Observational Research: The Utility of Twins. Perspect Psychol Sci. 2010;5:546–56. doi: 10.1177/1745691610383511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller G, Chen E. Unfavorable socioeconomic conditions in early life presage expression of proinflammatory phenotype in adolescence. Psychosom Med. 2007;69:402–9. doi: 10.1097/PSY.0b013e318068fcf9. [DOI] [PubMed] [Google Scholar]
- 32.Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, Cole S, Kobor MS. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci U S A. 2009;106:14716–21. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–62. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 34.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–9. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 35.Nelson EC, Lynskey MT, Heath AC, Madden PA, Martin NG. A family study of adult twins with and without a history of childhood abuse: stability of retrospective reports of maltreatment and associated family measures. Twin Res Hum Genet. 2010;13:121–30. doi: 10.1375/twin.13.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holmes WC, Slap GB. Sexual abuse of boys: definition, prevalence, correlates, sequelae, and management. JAMA. 1998;280:1855–62. doi: 10.1001/jama.280.21.1855. [DOI] [PubMed] [Google Scholar]
- 37.Yehuda R, Halligan SL, Grossman R. Childhood trauma and risk for PTSD: relationship to intergenerational effects of trauma, parental PTSD, and cortisol excretion. Dev Psychopathol. 2001;13:733–53. doi: 10.1017/s0954579401003170. [DOI] [PubMed] [Google Scholar]
- 38.Bremner JD, Vermetten E, Mazure CM. Development and preliminary psychometric properties of an instrument for the measurement of childhood trauma: the Early Trauma Inventory. Depress Anxiety. 2000;12:1–12. doi: 10.1002/1520-6394(2000)12:1<1::AID-DA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 39.Spertus IL, Yehuda R, Wong CM, Halligan S, Seremetis SV. Childhood emotional abuse and neglect as predictors of psychological and physical symptoms in women presenting to a primary care practice. Child Abuse Negl. 2003;27:1247–58. doi: 10.1016/j.chiabu.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Maclean PD. The limbic system (“visceral brain”) and emotional behavior. AMA Arch Neurol Psychiatry. 1955;73:130–4. doi: 10.1001/archneurpsyc.1955.02330080008004. [DOI] [PubMed] [Google Scholar]
- 41.Ferrante AW., Jr. Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med. 2007;262:408–14. doi: 10.1111/j.1365-2796.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- 42.Fergusson DM, Horwood LJ, Woodward LJ. The stability of child abuse reports: a longitudinal study of the reporting behaviour of young adults. Psychol Med. 2000;30:529–44. doi: 10.1017/s0033291799002111. [DOI] [PubMed] [Google Scholar]