Abstract
The identity and behavior of a cell is shaped by the molecular and mechanical composition of its surroundings. Molecular cues have firmly established roles in guiding both neuronal fate decisions and the migration of cells and axons. However, there is growing evidence that topographical and rigidity cues in the extracellular environment act synergistically with these molecular cues. Like chemical cues, physical factors do not elicit a fixed response, but rather one that depends on the sensory makeup of the cell. Moreover, from developmental studies and the plasticity of neural tissue, it is evident that there is dynamic feedback between physical and chemical factors to produce the final morphology. Here we focus on our current understanding of how these physical cues shape cellular differentiation and migration, and discuss their relevance to repairing the injured nervous system.
INTRODUCTION
The properties of the extracellular environment can be divided into its chemical and physical components. The importance of chemical cues in cellular differentiation, cell migration, and axon guidance is well established. Proteins known as morphogens specify the fates of neural precursors (reviewed in Wen et al., 2009), and protein cues in the extracellular space guide cell migration and axon extension (reviewed in Raper and Mason, 2010).
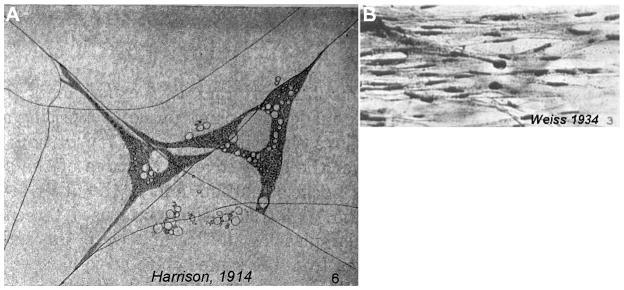
However, there is mounting evidence that the physical characteristics of the extracellular environment have important roles during development. These physical cues can be broadly divided into topography and rigidity. Interestingly, observations made in some of the earliest cell culture experiments suggested an important role for the mechanical properties of the substrate. Upon culturing embryonic frog cells on spider web filaments, Ross Granville Harrison noted the necessity of a solid substrate for cellular extension (Figure 1A) and remarked that “when the lymph clotted firmly the movement was nearly always active, while in the cases in which the medium remained fluid only rounded cells were seen and these failed to undergo characteristic changes of form and locomotion” (Harrison, 1914). Paul Weiss obtained early evidence that topographical features of the substrate might guide axons. Specifically he noticed that the trajectory of axons aligned to the parallel grooves generated by brushed clotting blood onto a glass coverslip (Figure 1B, Weiss, 1934). Despite these seemingly straightforward observations, a clear causal effect of the mechanical properties on cellular behavior in these studies became unclear. For one, the clotting process of blood was found to release chemical growth factors (Kohler and Lipton, 1974). As such, the rounded cells observed by Harrison may have been a consequence of a lower concentration of growth factors and Weiss’ painted grooves could have selectively deposited chemical factors.
Figure 1. Early observations of the importance of solid substrates and contact guidance of axons.
(A) Ross Granville Harrison cultured embryonic frog spinal neurons culture in a meshwork of spider web filaments and observed that these cells only extended along the solid support of these filaments. (B) When Paul Weiss cultured embryonic chicken spinal neurons on grooves generated by brushing clotting blood, he noted that axon tended to follow the direction of these channels. Panel A is reprinted, with permission, from the Journal of Experimental Zoology 1914 volume 17, issue 4, pages 521–44. Panel B is reprinted, with permission from the Journal of Experimental Zoology 1934 volume 68, issue 3, pages 393–448.
Recent studies have sought to clarify the role of physical parameters by carefully engineering substrates to selectively vary rigidity and topography, while maintaining a constant chemical composition. Here we will discuss these studies and present emerging evidence that mechanical factors influence the differentiation of precursor cells, the extension rate and branching of axons and the clustering of vesicles at pre-synaptic sites. We will also address recent findings of interplay between chemical and physical forces during development, including our finding that growth cones exert a traction force on the netrin-1 cue during chemoattraction. Finally, we will outline how a better understanding of cellular responses to physical cues will aid our ability to repair the damaged nervous system.
Topography of the nervous system
The nervous system is a complex three-dimensional environment whose topographical features likely span a large spectrum of morphologies and size scales. Unfortunately, there is very little detail on the topographical features of the nervous system. There have been recent attempts to address this deficit using atomic force microscopy (AFM). For instance, the filopodia of rat hippocampal neuron growth cones have been reported to have thicknesses in multiples of 60nm (Parpura et al., 1993). As well, the topography of fixed sciatic nerve axons have been examined from 6-month old wild type (myelinated) and trembler-mutant (unmyelinated) mice (Heredia et al., 2007). Although a quantitative account was not provided, unmyelinated axons were qualitatively reported to have a smoother surface. With the greater availability of tools for structural analyses and the growing appreciation of the role of topographical features on cellular behavior, precisely mapping the landscape of the nervous system will undoubtedly yield unexpected and stimulating findings.
Despite the absence of a clear picture of the nervous system’s topography, some useful inferences can be made. Notably, categorizing the size scale of topographical features emanating from cell bodies, axons and dendrites or the ECM. Cell bodies vary greatly in size between cell types, however in the vertebrate nervous system they typically have diameters in the tens of micrometers (10 to 50 μm), whereas axons and dendrites have diameters in the micrometer range (typically 0.2–3 μm) (Hollenbeck and Bamburg, 2003; Palay and Chan-Palay, 2010). ECM components like fibronectin, collagen and laminin have features at the level of the hundred nanometers and under. For instance, collagens have lengths of ~300nm; and when they form fibrils they are staggered ~65nm apart and the fibrils have diameters of 33–250nm (Amenta et al., 1986; Wess, 2005). Laminins have globular domains of approximately 4nm in diameter and fibronectin filaments have diameters of approximately 10nm (Beck et al., 1990; Pompe et al., 2005).
Effect of topography on migration
An in vivo context in which topography guides either cells or axons has been difficult to firmly establish. However, two contexts are particularly suggestive: during development of the cerebral cortex, cortical neurons migrate along radial glia (Rakic, 1972) and in the development of the cerebellum, neuroblasts migrate along aligned axon tracts (Hynes et al., 1986). Moreover, an elegant experiment showed that dorsal root ganglion axon trajectories were influenced when cultured on astrocytes that were previously aligned using an electric field (Alexander et al., 2006). However in each of these instances, because the topographical substrate is a living cell that can actively alter its chemical composition, it is difficult to dissociate chemical and mechanical influences.
As such, the best support for contact guidance of both cells and axons comes from extensive in vitro experiments using micro-fabricated substrates (reviewed in Hoffman-Kim et al., 2010). These experiments have shown that cells, axons and dendrites can be reoriented with feature dimensions down to a couple hundred nanometers – in other words, on the size scale of neurite diameters (see above, Clark et al., 1990; Fozdar et al., 2010; Hirono et al., 1988; Johansson et al., 2006). Whether ECM features that are on the order of tens of nanometers or less can influence cell and axon migration has yet to be firmly established. The best evidence that axons can sense ECM topography was obtained by culturing DRG explants in collagen gels where the fiber orientation was biased by tilting the culture dish during the polymerization process (Ebendal, 1976). These aligned fibers, with diameters of 50–100nm, were found to direct axon growth in the direction of their alignment. In terms of cell migration, it has been reported that 35nm diameter pits affect cytoskeletal organization and that surfaces with 10nm high bumps increase the number of leading edge filopodia in fibroblasts (Dalby et al., 2004a; Dalby et al., 2004b). A key challenge will be to use modern fabrication techniques to test whether features on the scale of tens of nanometers and under can guide axon and cell migration.
Effects of topography on neural precursor differentiation
Recent in vitro findings are providing tantalizing evidence that surface topography influences the cell fate decisions of neural progenitors. Specifically, substrate topography has been reported to guide the differentiation of precursors derived from either embryonic neural tissue or neural progenitor cell lines (e.g. H945RB.3) (Christopherson et al., 2009; Lee et al., 2010; Recknor et al., 2006; Tsuruma et al., 2008; Wu et al., 2010; Yim et al., 2007). Neuronal fates in these studies are typically determined based on immuno-detection of markers of undifferentiated cells (e.g. Nestin), neurons (e.g. β3-tubulin, MAP2) or glia (e.g. GFAP, CNPase); however up-regulation of multiple neural associated genes can be demonstrated by extensive profiling of mRNA expression levels (Yim et al., 2007). There is not yet a clear pattern defining which topographies promote a given lineage, although a unifying theme throughout these studies is that topographical complexity on scales between a few hundred nanometers and several microns promotes a faster differentiation than a flat substrate.
Topography Sensing Mechanisms
The topographic sensing mechanisms of cells and growth cones are poorly understood. At longer length scales, when features are above 10–20μm, the decision likely reflects intrinsic constraints on cellular probing distances. For instance, when embryonic mouse cortical neurons were plated on large polylysine-coated PDMS channels with heights 10 μm or less and covered with a gel composed of laminin and collagen (Matrigel) (Li and Folch, 2005), growth cones preferred the PDMS/polylysine surface to the gel. However, when the height of the channel exceeded 11 μm, axons extended straight into the laminin/collagen gel. One potential interpretation of this is that at distances above 11μm the surface below is no longer within reach of the growth cone’s filopodia. In this situation, the growth cone is therefore blind to the surface below and therefore continues straight into a less preferred environment.
To sense features under a micron, however, other mechanisms that have yet to be fully understood, must be at play. One possibility is that the curvature of the plasma membrane is responsible. Simulations and observations in rat hippocampal slices suggest that morphological perturbations of cells can directly influence intracellular signaling (Neves et al., 2008). However, this mechanism suggests that topographical sensing is passive, which is not always the case. This is best illustrated by the unique responses of different neuronal populations to topography. Notably, PNS neurons tend to migrate parallel to grooved surfaces, while CNS neuroblasts have been observed to migrate in both parallel and perpendicular directions on grooves that had depths between 0.3 μm and 0.8 μm and a width of 1 μm, which roughly mimics a tightly aligned neurite bundle (Nagata et al., 1993). As such, cells can control their response to the environment’s topography.
Active topography sensing might arise from membrane curvature sensing proteins. Three classes of proteins are recognized for their ability to sense membrane curvature: Bin-Amphiphysin-Rvs (BAR) domain containing proteins, amphipathic helices and certain membrane-anchored proteins (reviewed in Bhatia et al., 2010). Amphipathic helices and BAR-domain containing proteins accumulate at curved membranes through insertion of their amphipathic motifs into lipid packing defects (Bhatia et al., 2009; Hatzakis et al., 2009). Though primarily studied in the context of endocytosis, BAR-domain proteins can also regulate the actin cytoskeleton (Dawson et al., 2006). For instance, focal adhesion protein 52 (FAP52, also known as PACSINs or Syndapin) contains an amino terminal F-BAR domain and is known to regulate actin organization and to bind filamin (Nikki et al., 2002; Plomann et al., 2010). Membrane curvature can also regulate the enzymatic activity of membrane-associated proteins. For instance, the kinase activity of protein kinase C (PKC) is increased with membrane curvature (Slater et al., 1994). A key challenge of the future will be to determine the mechanism responsible for cellular substrate topography sensing.
Rigidity landscape of the nervous system
Rigidity is commonly reported as either shear (G) or elastic (E) moduli (Figure 2). These two measures are related by E=2G(1+ ν), where v is the Poisson ratio. When a material does not change volume upon deflection the Poisson ratio equals 0.5 and its shear modulus will therefore be a third of its elastic modulus. For simplicity, the values discussed here will be elastic moduli.
Figure 2. Shear and Elastic Moduli of Rigidity.
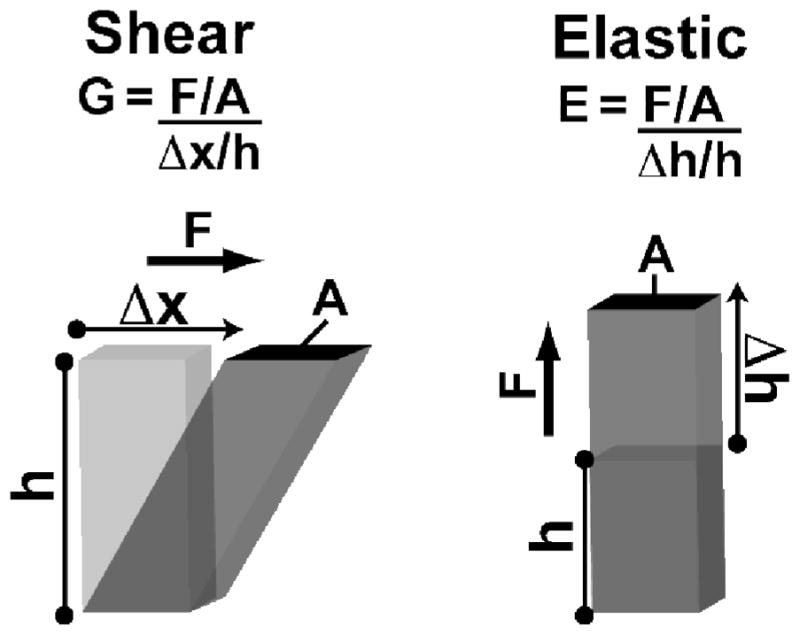
Both shear and elastic moduli are defined as the ratio of stress for a given strain. The key difference between the two is the direction of applied force. For shear modulus, the direction is perpendicular to the height, whereas for the elastic modulus it is parallel.
The central nervous system (CNS) is one of the softest tissues in our body with an elastic modulus falling between 0.1 and 10 nN/μm2 (equal to 0.1–10 kPa, Elkin et al., 2007; Gefen et al., 2003; Hirakawa et al., 1981; Metz et al., 1970). Comparatively, muscle (10 – 100 nN/μm2), connective tissue and arteries (100 – 1,000 nN/μm2) and bone (15,000,000 – 30,000,000 nN/μm2) are much more rigid (reviewed in Moore et al., 2010). Unfortunately, most studies of the nervous system’s rigidity have been performed in the context of injury models and the data is often incomplete and sometimes contradictory (reviewed in Cheng et al., 2008). For instance, the rigidity of CNS has been reported to both increase (Thibault and Margulies, 1998) and decrease with age (Gefen et al., 2003; Prange and Margulies, 2002). Discrepancies likely reflect measurement methods such as the rate, distance and surface area of indentation. Moreover, commonly used methods do not probe mechanics on the scales relevant to cells. Atomic force microscopy (AFM) and a technique known as laser-tracking microrheology (based on differences in Brownian motion) have been used to detect rigidity differences on a sub-cellular scale in cultured endothelial and epithelial cells (Roca-Cusachs et al., 2008; Yamada et al., 2000). These techniques have the potential to map rigidity at a resolution relevant to cells; in other words at a resolution of 1–5μm2 at rates between 0.1–1μm/s. AFM has been used to map the rigidity of a growth cone and the adult hippocampus. Regional rigidity differences have been reported in live Aplysia growth cones (Xiong et al., 2009). Specifically, the elastic modulus of the central domain (3–7 nN/μm2) was found to be softer than the transition zone (7–23 nN/μm2) and the peripheral domain (10–40 nN/μm2). For rigidity mapping of the adult mouse hippocampus, a 25μm diameter bead was attached to the AFM tip avoid detecting subcellular features (Elkin et al., 2007). At an indentation depth of 3μm, significant rigidity variations were detected between the CA1 (0.14 nN/μm2) and the CA3 (0.23 nN/μm2) pyramidal cell layers. The CA3 stratum radiatum (0.31 nN/μm2) was found to be stiffer than all other regions of the hippocampus examined.
Rigidity effects on cell and axon migration
Substrate rigidity can affect the branching and extension rates of neurons. This phenomenon has been particularly well documented for DRG axons in both 2D and 3D environments (Balgude et al., 2001; Norman and Aranda-Espinoza, 2010; Willits and Skornia, 2004). Increased extension rates have been observed on softer substrates when comparing rigidities ranging from 0.014 and 13 nN/μm2. This behavior is apparently reversed below rigidities of 0.003 nN/μm2; in other words, DRG axons extend slower when on substrates softer than 0.003 nN/μm2 (Willits and Skornia, 2004). However, the physiological relevance of this is questionable given that tissue rigidities below 0.01 nN/μm2 have not been reported. Increased axon extension rates on softer substrates have also been observed for hippocampal neurons on rigidities between 0.5 and 7.5 nN/μm2 (Kostic et al., 2007). Interestingly, embryonic spinal and cortical neuron axon extension rate appear insensitive to substrate stiffness (Flanagan et al., 2002; Norman and Aranda-Espinoza, 2010). This absence of cortical neuron rigidity sensitivity was particularly well characterized using several substrate rigidities (0.26, 0.87 and 13 nN/μm2) in the vicinity of the average, AFM measured cortical tissue rigidity (0.3 nN/μm2). Moreover, this same study reproduced the increased outgrowth of DRG axons on softer substrates. Thus there appears to be cell type specific, axon extension responses to substrate rigidity (see below).
Aside from axon extension, substrate stiffness can also affect cell migration and axonal branching. Embryonic spinal neurons have been reported to have a 5-fold increase in the number of branches per millimeter on softer substrates of 0.05 nN/μm2 compared to 0.55 nN/μm2 substrates (Flanagan et al., 2002). Although the effect of substrate rigidity on migration has not been directly examined with respect to neuronal cells, studies have been carried out on fibroblasts and leukocytes (Lo et al., 2000; Mandeville et al., 1997). The studies found that those cells migrated toward more rigid substrates, a phenomenon known as durotaxis.
Substrate rigidity effects on neural precursor differentiation
Cellular stiffness and contraction have important roles from the very early stages of embryogenesis to organogenesis (reviewed in Mammoto and Ingber, 2010). Cells that make up the extracellular environment can dynamically regulate ECM rigidity. For instance, surface tension of tissue is linearly related to the amount of cell surface cadherin receptor (Foty and Steinberg, 2005). When exposed to mechanical stress, undifferentiated mouse embryonic stem cells rapidly change their mechanics and lose pluripotency (as measured by the suppression of Oct3/4 gene expression) (Chowdhury et al., 2010). Orientation of the spindle axis in cell division is governed by the spatial distribution of ECM adhesions that resist cell traction forces, and not by chemical signals generated in response to ECM binding (Thery et al., 2005). Substrate rigidity can selectively promote the survival of neurons over glia (Georges et al., 2006). Specifically, plating dissociated embryonic rat cortices (embryonic day 17 to 19) on substrates with elastic moduli of ~ 0.75 nN/μm2 promoted survival of neurons, while substrates above 6 nN/μm2 promoted survival of glia. An important goal of future research will be to firmly establish whether substrate rigidity can influence the differentiation into specific neuronal fates, as has been reported using mesemchymal stem cells (Engler et al., 2006).
Rigidity Sensing Mechanisms
Exactly how cells and neurons sense rigidity is unclear. One possibility, that may be most relevant to cellular differentiation, is that mechanical factors directly influence gene expression. There is evidence that surface adhesion receptors, such as integrins and cadherins, are mechanically coupled to gene expression. Forces exerted on integrins can reshape the nucleus and chromosomes are mechanically interconnected (Maniotis et al., 1997a; Maniotis et al., 1997b). It has been demonstrated that the cytoskeleton and the nucleoskeleton are mechanically connected through a complex composed of Sun proteins and Nesprins (Crisp et al., 2006; Haque et al., 2006; Padmakumar et al., 2005). This LINC (Linker of Nucleoskeleton and Cytoskeleton) complex could potentially regulate gene expression through a variety of mechanisms, including: (1) unfolding DNA and therefore easing access to certain regions, (2) sequestration of transcription factors and (3) regulation of nuclear pore size (reviewed in Wang et al., 2009).
Another possibility is that rigidity sensing occurs through the traction forces generated by cells and growth cones (reviewed in Moore et al., 2010). Though this mechanism may be more relevant to effects on migration, intracellular cascades induced through mechano-transduction could equally influence cell fate decisions. In this model, cells sense rigidity by evaluating the amount of give (strain) for a given pulling force (stress, Figure 2). Both cells and growth cones actively pull on their environment (Bray, 1979; Harris et al., 1980). Cells have been reported to pull on their environment with up approximately 200 nN/μm2 (see Moore et al., 2010) and the retraction of individual vertebrate growth cone filopodia exerts approximately ten piconewtons (Hallstrom et al., 2010; Moore et al., 2009). Non-muscle myosin II is the motor protein responsible for cellular traction forces. Myosin II is organized as bipolar mini-filaments in cells and growth cones (Bridgman, 2002; Cai et al., 2006; Svitkina et al., 1989). Each double-headed myosin II generates between 1.3 and 3.7 picoNewtons (pN) (Finer et al., 1994; Guilford et al., 1997; Ishijima et al., 1994; Molloy et al., 1995; Tyska et al., 1999).
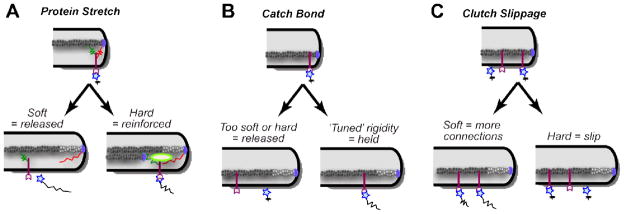
Three traction force dependent, rigidity-sensing mechanisms can be envisioned: ‘protein stretching’, ‘catch bond formation’ and ‘clutch slippage’ (Figure 3). The protein stretching mechanism is founded on the observation that mechanical tension felt on a protein can alter its activity through conformational changes. Examples include the exposure of phosphorylation sites within p130Cas (Sawada et al., 2006), the exposure of vinculin-binding sites within talin (del Rio et al., 2009), activation of titin kinase (Puchner et al., 2008) and the opening of ion channels (Sukharev and Corey, 2004). In its simplest form, this model predicts that stiffer substrates will better stretch proteins leading to greater reinforcement of the ECM-cytoskeletal link, and therefore predicts larger forces on stiffer substrates. The second ‘catch-bond formation’ mechanism is based on the observation that the strength of certain interactions can be increased under a given range of loading rates. A catch bond mechanism has been reported between integrins and fibronectin at forces of 10–30 pN (Kong et al., 2009). Similarly, a catch bond has been reported between myosin II and actin filaments at approximately 6 pN (Guo and Guilford, 2006). Because catch bonds have a ‘window’ of loading forces where binding strength is increased, this model would be consistent with a certain range of rigidities evoking larger traction forces. The third ‘clutch slippage’ mechanism is founded on the idea that the bridging of the extracellular environment to retrogradely flowing actin occurs at a constant rate (Chan and Odde, 2008). Because the loading rates on these linkages will rise much quicker on rigid substrates, fewer linkages form and smaller traction forces are generated. For growth cones, it has been proposed that there is a sharp boundary at an elastic modulus (E) of 1 nN/μm2 where traction forces are dramatically reduced and actin retrograde flow increases (Chan and Odde, 2008). Given that the nervous system has an E between 0.1–10 nN/μm2, this would indicate that the physiological rigidity of the nervous system profoundly influences a growth cone’s behavior. Moreover, it could explain why some neuronal populations have reduced axon extension rates on rigid substrates (see above). However, as mentioned above, not all neuronal populations are responsive to substrate rigidity. Further, this ‘clutch slippage’ model runs against observations in numerous other cell types, including epithelial, fibroblasts and mesenchymal stem cells, where there are increased traction forces with substrate rigidity (Choquet et al., 1997; Fu et al., 2010; Saez et al., 2005).
Figure 3. Three rigidity sensing mechanisms based on ECM coupling.
(A) On hard surfaces intracellular proteins that bridge the ECM to the cytoskeleton could be mechanically stretched leading to the exposure of cryptic functions (e.g. protein binding regions or kinase activity) that reinforce ECM linkages. (B) A catch bond between the receptor and the ECM could lead to a specific range of rigidities that lead to reinforcement. (C) According to the clutch slippage model, more rigid substrates result in fewer receptor/ECM linkages due to faster loading rates.
Clearly further work is required to characterize and understand the rigidity sensing mechanisms of cells. However given the obvious variability in the responses between populations, rigidity sensing decisions must be a product of neural identity. In other words, rigidity cues need to be viewed in a similar light as chemical ones where the response is not intrinsic to the cue, but rather depends on the makeup of the cell’s sensing apparatus.
At the interface: The physical side of molecular cues
There is an important reciprocal relationship between molecular ligands and mechanical forces. This well appreciated for classical ECM components (e.g. fibronectin, collagen, laminin) and adhesive receptors (e.g. Integrins, Cadherins and Ig-class receptors) (reviewed in Moore et al., 2010; Wang et al., 2009). However this relationship exists with several molecular cues involved in neuronal cell fate decisions and guidance. Nodal, a member of the TGFβ family that is important for specifying left-right asymmetry cell fates in numerous contexts, including certain regions of the brain (Roussigne et al., 2009), has been shown to regulate actomyosin-dependent cell-cortex tension (Krieg et al., 2008). Wnt and hedgehog proteins, which have been implicated in both cellular differentiation and guidance, promote cytoskeletal contraction (Corrigall et al., 2007; Lee et al., 2006). Similarly, regulation of myosin II contractility has been implicated in the response to several ‘classical’ axon guidance cues, including: ephrins, slits, netrins, semaphorins (Gallo, 2006; Murray et al., 2010; Yue et al., 2008).
There has also been a recent explosion of reports on the importance of mechanical forces in the function of chemical cues. In terms of cell fate, mechanical forces may be important for the function of Notch proteins. Notch proteins are a family of single-pass transmembrane receptors implicated in the differentiation of numerous cell types including neurons (Gaiano and Fishell, 2002). There is mounting evidence that Notch ligands activate Notch through mechanical stretching (Kopan and Ilagan, 2009). In terms of axon guidance cues, we have shown that the secreted netrin-1 cue experiences traction forces when it attracts the growth of spinal commissural neuron axons (Moore et al., 2009). Further, Ephrins signaling through their Eph receptors, known to guide both axon and cell migration during development (Pitulescu and Adams, 2010), have been shown to have important mechanical contributions (Salaita et al., 2010). Specifically, restricting the lateral diffusion of the EphA1 receptor within the membrane is required for numerous signaling events with GPI-linked Ephrin-A1.
Importance of Biophysical Interactions in Neural Repair
A better understanding of the substrate preferences of cells and axon will impact our ability to repair the injured nervous system. It has been reported that DRG axon extension can be arrested by discontinuities in substrate rigidity (Yu and Bellamkonda, 2001). This is particularly relevant for the design of scaffolds to bridge the injured nervous system. Attempts to use peripheral grafts in the CNS of animals have been met with limited success and currently grafting is only applied to injuries of the PNS in humans (David and Aguayo, 1981; Schlosshauer et al., 2006). Synthetic scaffolds are being developed to improve regrowth efficiency and to address situations where there is insufficient autologous graft material. These synthetic scaffolds can achieve regrowth that is comparable to autologous nerve grafts (Schlosshauer et al., 2006). There is hope that better-designed synthetic scaffolds, in terms of both their chemical and physical properties, could expand their effectiveness in the PNS and eventually be used to treat CNS injury (Madigan et al., 2009; Straley et al., 2010).
A better understanding of the biophysics of axon extension will also improve the development of pharmacological agents that promote axon regeneration. Several pharmacological manipulations have been identified that promote the ability of an axon to extend across inhibitory substrates. Notably, elevating intracellular cyclic AMP levels, stabilizing microtubules and inhibition of the RhoA Rho GTPase and non-muscle myosin II have been reported (Cai et al., 1999; Dergham et al., 2002; Hellal et al., 2011; Hur et al., 2011). With each of these pharmacological manipulations, however, it is important to consider their effect on the mechanical environment through their action on surrounding cells, particularly with respect to cytoskeleton. Although the effects of stabilizing microtubules and inhibition of myosin are self-evident, elevating cAMP can reduce actin polymerization (Lambrechts et al., 2000; Ohta et al., 1987) and inhibiting the RhoA pathway is known to inhibit myosin contractility that relaxes the cortical actin meshwork (Sandquist et al., 2006; Sullivan et al., 1999).
Biophysical considerations are also important for stem cell-based treatments. Clinical studies on humans are under way to test the safety and potential of mesenchymal stem cells (MSCs) to treat neurological disorders, including Parkinson’s and amyotrophic lateral sclerosis (Joyce et al., 2010; Miller et al., 2010). These studies intend to use MSCs to improve health through trophic support, however there is the potentially they could be used to replace lost neurons. Bone marrow derived MSCs have been reported to spontaneously express markers of differentiated neurons (βII-tubulin, NFM) and glia (S100β, GFAP) both in culture and when implanted into the lateral ventricle of the neonatal mouse brain (Deng et al., 2006). Importantly, it has been reported that substrate rigidity and topography can influence the differentiation of cells from these precursors (Engler et al., 2006; Yim et al., 2007).
CONCLUSION
From the above discussion, it is clear that rigidity and topography influence the migration and differentiation of cells within the nervous system, and that the mechanism by which cells sense the mechanical properties of their surrounding is a local and dynamic process. Although our understanding of this mechanical sensing is still in its infancy, its importance is beginning to emerge in unexpected physiological processes throughout the body. One notable example, relevant to neurobiology, is the recent implication of mechanical forces in synaptic function and remodeling. Tension along axons and its importance for neurite extension is well documented (Bray, 1979; Heidemann and Buxbaum, 1994). Recent findings using locust frontal ganglion neurons on patterned quartz surfaces proposes that tension is important for synaptic maintenance (Anava et al., 2009). Specifically, tension was estimated based on the branching angles of neurites and was correlated with the persistence of neurite connections. Similarly, in the developing Drosophila melanogaster embryo, applying tension to motor neuron axons is necessary for the accumulation of presynaptic vesicles at neuromuscular junctions (Siechen et al., 2009). Moreover, using a single silicone crystal that was calibrated using AFM, this study also measured the tension along a single motor axon to be approximately 1nN.
As our appreciation of mechano-chemical influences matures, it is likely that additional unforeseen roles will surface. Clearly, awareness of the collaborative nature of the mechanical and chemical forces is indispensable for a comprehensive understanding of the cellular processes that shape and regulate our bodies.
References
- Alexander JK, Fuss B, Colello RJ. Electric field-induced astrocyte alignment directs neurite outgrowth. Neuron Glia Biol. 2006;2:93–103. doi: 10.1017/S1740925X0600010X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amenta PS, Gay S, Vaheri A, Martinez-Hernandez A. The extracellular matrix is an integrated unit: ultrastructural localization of collagen types I, III, IV, V, VI, fibronectin, and laminin in human term placenta. Coll Relat Res. 1986;6:125–152. doi: 10.1016/s0174-173x(86)80021-8. [DOI] [PubMed] [Google Scholar]
- Anava S, Greenbaum A, Ben Jacob E, Hanein Y, Ayali A. The regulative role of neurite mechanical tension in network development. Biophysical Journal. 2009;96:1661–1670. doi: 10.1016/j.bpj.2008.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balgude AP, Yu X, Szymanski A, Bellamkonda RV. Agarose gel stiffness determines rate of DRG neurite extension in 3D cultures. Biomaterials. 2001;22:1077–1084. doi: 10.1016/s0142-9612(00)00350-1. [DOI] [PubMed] [Google Scholar]
- Beck K, Hunter I, Engel J. Structure and function of laminin: anatomy of a multidomain glycoprotein. FASEB J. 1990;4:148–160. doi: 10.1096/fasebj.4.2.2404817. [DOI] [PubMed] [Google Scholar]
- Bhatia VK, Hatzakis NS, Stamou D. A unifying mechanism accounts for sensing of membrane curvature by BAR domains, amphipathic helices and membrane-anchored proteins. Semin Cell Dev Biol. 2010;21:381–390. doi: 10.1016/j.semcdb.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Bhatia VK, Madsen KL, Bolinger PY, Kunding A, Hedegard P, Gether U, Stamou D. Amphipathic motifs in BAR domains are essential for membrane curvature sensing. The EMBO journal. 2009;28:3303–3314. doi: 10.1038/emboj.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray D. Mechanical tension produced by nerve cells in tissue culture. J Cell Sci. 1979;37:391–410. doi: 10.1242/jcs.37.1.391. [DOI] [PubMed] [Google Scholar]
- Bridgman PC. Growth cones contain myosin II bipolar filament arrays. Cell Motil Cytoskeleton. 2002;52:91–96. doi: 10.1002/cm.10038. [DOI] [PubMed] [Google Scholar]
- Cai D, Shen Y, De Bellard M, Tang S, Filbin MT. Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG and myelin via a cAMP-dependent mechanism. Neuron. 1999;22:89–101. doi: 10.1016/s0896-6273(00)80681-9. [DOI] [PubMed] [Google Scholar]
- Cai Y, Biais N, Giannone G, Tanase M, Jiang G, Hofman JM, Wiggins CH, Silberzan P, Buguin A, Ladoux B, Sheetz MP. Nonmuscle myosin IIA-dependent force inhibits cell spreading and drives F-actin flow. Biophys J. 2006;91:3907–3920. doi: 10.1529/biophysj.106.084806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CE, Odde DJ. Traction dynamics of filopodia on compliant substrates. Science. 2008;322:1687–1691. doi: 10.1126/science.1163595. [DOI] [PubMed] [Google Scholar]
- Cheng S, Clarke EC, Bilston LE. Rheological properties of the tissues of the central nervous system: a review. Med Eng Phys. 2008;30:1318–1337. doi: 10.1016/j.medengphy.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- Chowdhury F, Na S, Li D, Poh YC, Tanaka TS, Wang F, Wang N. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nature materials. 2010;9:82–88. doi: 10.1038/nmat2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson GT, Song H, Mao HQ. The influence of fiber diameter of electrospun substrates on neural stem cell differentiation and proliferation. Biomaterials. 2009;30:556–564. doi: 10.1016/j.biomaterials.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Clark P, Connolly P, Curtis AS, Dow JA, Wilkinson CD. Topographical control of cell behaviour: II. Multiple grooved substrata. Development. 1990;108:635–644. doi: 10.1242/dev.108.4.635. [DOI] [PubMed] [Google Scholar]
- Corrigall D, Walther RF, Rodriguez L, Fichelson P, Pichaud F. Hedgehog signaling is a principal inducer of Myosin-II-driven cell ingression in Drosophila epithelia. Developmental cell. 2007;13:730–742. doi: 10.1016/j.devcel.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, Stahl PD, Hodzic D. Coupling of the nucleus and cytoplasm: role of the LINC complex. The Journal of cell biology. 2006;172:41–53. doi: 10.1083/jcb.200509124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby MJ, Gadegaard N, Riehle MO, Wilkinson CD, Curtis AS. Investigating filopodia sensing using arrays of defined nano-pits down to 35 nm diameter in size. The international journal of biochemistry & cell biology. 2004a;36:2005–2015. doi: 10.1016/j.biocel.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Dalby MJ, Riehle MO, Johnstone H, Affrossman S, Curtis AS. Investigating the limits of filopodial sensing: a brief report using SEM to image the interaction between 10 nm high nano-topography and fibroblast filopodia. Cell Biol Int. 2004b;28:229–236. doi: 10.1016/j.cellbi.2003.12.004. [DOI] [PubMed] [Google Scholar]
- David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- Dawson JC, Legg JA, Machesky LM. Bar domain proteins: a role in tubulation, scission and actin assembly in clathrin-mediated endocytosis. Trends in cell biology. 2006;16:493–498. doi: 10.1016/j.tcb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323:638–641. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Petersen BE, Steindler DA, Jorgensen ML, Laywell ED. Mesenchymal stem cells spontaneously express neural proteins in culture and are neurogenic after transplantation. Stem Cells. 2006;24:1054–1064. doi: 10.1634/stemcells.2005-0370. [DOI] [PubMed] [Google Scholar]
- Dergham P, Ellezam B, Essagian C, Avedissian H, Lubell WD, McKerracher L. Rho signaling pathway targeted to promote spinal cord repair. J Neurosci. 2002;22:6570–6577. doi: 10.1523/JNEUROSCI.22-15-06570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebendal T. The relative roles of contact inhibition and contact guidance in orientation of axons extending on aligned collagen fibrils in vitro. Exp Cell Res. 1976;98:159–169. doi: 10.1016/0014-4827(76)90475-4. [DOI] [PubMed] [Google Scholar]
- Elkin BS, Azeloglu EU, Costa KD, Morrison B., 3rd Mechanical heterogeneity of the rat hippocampus measured by atomic force microscope indentation. J Neurotrauma. 2007;24:812–822. doi: 10.1089/neu.2006.0169. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Finer JT, Simmons RM, Spudich JA. Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature. 1994;368:113–119. doi: 10.1038/368113a0. [DOI] [PubMed] [Google Scholar]
- Flanagan LA, Ju YE, Marg B, Osterfield M, Janmey PA. Neurite branching on deformable substrates. Neuroreport. 2002;13:2411–2415. doi: 10.1097/01.wnr.0000048003.96487.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foty RA, Steinberg MS. The differential adhesion hypothesis: a direct evaluation. Developmental biology. 2005;278:255–263. doi: 10.1016/j.ydbio.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Fozdar DY, Lee JY, Schmidt CE, Chen S. Hippocampal neurons respond uniquely to topographies of various sizes and shapes. Biofabrication. 2010;2:035005. doi: 10.1088/1758-5082/2/3/035005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Wang YK, Yang MT, Desai RA, Yu X, Liu Z, Chen CS. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nature methods. 2010;7:733–736. doi: 10.1038/nmeth.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaiano N, Fishell G. The role of notch in promoting glial and neural stem cell fates. Annu Rev Neurosci. 2002;25:471–490. doi: 10.1146/annurev.neuro.25.030702.130823. [DOI] [PubMed] [Google Scholar]
- Gallo G. RhoA-kinase coordinates F-actin organization and myosin II activity during semaphorin-3A-induced axon retraction. Journal of cell science. 2006;119:3413–3423. doi: 10.1242/jcs.03084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefen A, Gefen N, Zhu Q, Raghupathi R, Margulies SS. Age-dependent changes in material properties of the brain and braincase of the rat. J Neurotrauma. 2003;20:1163–1177. doi: 10.1089/089771503770802853. [DOI] [PubMed] [Google Scholar]
- Georges PC, Miller WJ, Meaney DF, Sawyer ES, Janmey PA. Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys J. 2006;90:3012–3018. doi: 10.1529/biophysj.105.073114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez N, Chen S, Schmidt CE. Polarization of hippocampal neurons with competitive surface stimuli: contact guidance cues are preferred over chemical ligands. J R Soc Interface. 2007;4:223–233. doi: 10.1098/rsif.2006.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilford WH, Dupuis DE, Kennedy G, Wu J, Patlak JB, Warshaw DM. Smooth muscle and skeletal muscle myosins produce similar unitary forces and displacements in the laser trap. Biophys J. 1997;72:1006–1021. doi: 10.1016/S0006-3495(97)78753-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B, Guilford WH. Mechanics of actomyosin bonds in different nucleotide states are tuned to muscle contraction. Proc Natl Acad Sci U S A. 2006;103:9844–9849. doi: 10.1073/pnas.0601255103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallstrom W, Lexholm M, Suyatin DB, Hammarin G, Hessman D, Samuelson L, Montelius L, Kanje M, Prinz CN. Fifteen-piconewton force detection from neural growth cones using nanowire arrays. Nano Lett. 2010;10:782–787. doi: 10.1021/nl902675h. [DOI] [PubMed] [Google Scholar]
- Haque F, Lloyd DJ, Smallwood DT, Dent CL, Shanahan CM, Fry AM, Trembath RC, Shackleton S. SUN1 interacts with nuclear lamin A and cytoplasmic nesprins to provide a physical connection between the nuclear lamina and the cytoskeleton. Molecular and cellular biology. 2006;26:3738–3751. doi: 10.1128/MCB.26.10.3738-3751.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AK, Wild P, Stopak D. Silicone rubber substrata: a new wrinkle in the study of cell locomotion. Science. 1980;208:177–179. doi: 10.1126/science.6987736. [DOI] [PubMed] [Google Scholar]
- Harrison RG. The reaction of embryonic cells to solid structures. JExpZool. 1914;17:521–544. [Google Scholar]
- Hatzakis NS, Bhatia VK, Larsen J, Madsen KL, Bolinger PY, Kunding AH, Castillo J, Gether U, Hedegard P, Stamou D. How curved membranes recruit amphipathic helices and protein anchoring motifs. Nat Chem Biol. 2009;5:835–841. doi: 10.1038/nchembio.213. [DOI] [PubMed] [Google Scholar]
- Heidemann SR, Buxbaum RE. Mechanical tension as a regulator of axonal development. Neurotoxicology. 1994;15:95–107. [PubMed] [Google Scholar]
- Hellal F, Hurtado A, Ruschel J, Flynn KC, Laskowski CJ, Umlauf M, Kapitein LC, Strikis D, Lemmon V, Bixby J, et al. Microtubule Stabilization Reduces Scarring and Causes Axon Regeneration After Spinal Cord Injury. Science. 2011 doi: 10.1126/science.1201148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia A, Bui CC, Suter U, Young P, Schaffer TE. AFM combines functional and morphological analysis of peripheral myelinated and demyelinated nerve fibers. Neuroimage. 2007;37:1218–1226. doi: 10.1016/j.neuroimage.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Hirakawa K, Hashizume K, Hayashi T. Viscoelastic property of human brain -for the analysis of impact injury (author’s transl) No To Shinkei. 1981;33:1057–1065. [PubMed] [Google Scholar]
- Hirono T, Torimitsu K, Kawana A, Fukuda J. Recognition of artificial microstructures by sensory nerve fibers in culture. Brain Res. 1988;446:189–194. doi: 10.1016/0006-8993(88)91314-5. [DOI] [PubMed] [Google Scholar]
- Hoffman-Kim D, Mitchel JA, Bellamkonda RV. Topography, cell response, and nerve regeneration. Annu Rev Biomed Eng. 2010;12:203–231. doi: 10.1146/annurev-bioeng-070909-105351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbeck PJ, Bamburg JR. Comparing the properties of neuronal culture systems: a shopping guide for the cell biologist. Methods in cell biology. 2003;71:1–16. doi: 10.1016/s0091-679x(03)01001-x. [DOI] [PubMed] [Google Scholar]
- Hur EM, Yang IH, Kim DH, Byun J, Saijilafu Xu WL, Nicovich PR, Cheong R, Levchenko A, Thakor N, Zhou FQ. Engineering neuronal growth cones to promote axon regeneration over inhibitory molecules. Proceedings of the National Academy of Sciences of the United States of America; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO, Patel R, Miller RH. Migration of neuroblasts along preexisting axonal tracts during prenatal cerebellar development. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1986;6:867–876. doi: 10.1523/JNEUROSCI.06-03-00867.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishijima A, Harada Y, Kojima H, Funatsu T, Higuchi H, Yanagida T. Single-molecule analysis of the actomyosin motor using nano-manipulation. Biochem Biophys Res Commun. 1994;199:1057–1063. doi: 10.1006/bbrc.1994.1336. [DOI] [PubMed] [Google Scholar]
- Johansson F, Carlberg P, Danielsen N, Montelius L, Kanje M. Axonal outgrowth on nano-imprinted patterns. Biomaterials. 2006;27:1251–1258. doi: 10.1016/j.biomaterials.2005.07.047. [DOI] [PubMed] [Google Scholar]
- Joyce N, Annett G, Wirthlin L, Olson S, Bauer G, Nolta JA. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med. 2010;5:933–946. doi: 10.2217/rme.10.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler N, Lipton A. Platelets as a source of fibroblast growth-promoting activity. Experimental cell research. 1974;87:297–301. doi: 10.1016/0014-4827(74)90484-4. [DOI] [PubMed] [Google Scholar]
- Kong F, Garcia AJ, Mould AP, Humphries MJ, Zhu C. Demonstration of catch bonds between an integrin and its ligand. J Cell Biol. 2009;185:1275–1284. doi: 10.1083/jcb.200810002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostic A, Sap J, Sheetz MP. RPTPalpha is required for rigidity-dependent inhibition of extension and differentiation of hippocampal neurons. Journal of cell science. 2007;120:3895–3904. doi: 10.1242/jcs.009852. [DOI] [PubMed] [Google Scholar]
- Krieg M, Arboleda-Estudillo Y, Puech PH, Kafer J, Graner F, Muller DJ, Heisenberg CP. Tensile forces govern germ-layer organization in zebrafish. Nature cell biology. 2008;10:429–436. doi: 10.1038/ncb1705. [DOI] [PubMed] [Google Scholar]
- Lambrechts A, Kwiatkowski AV, Lanier LM, Bear JE, Vandekerckhove J, Ampe C, Gertler FB. cAMP-dependent protein kinase phosphorylation of EVL, a Mena/VASP relative, regulates its interaction with actin and SH3 domains. The Journal of biological chemistry. 2000;275:36143–36151. doi: 10.1074/jbc.M006274200. [DOI] [PubMed] [Google Scholar]
- Laney DE, Garcia RA, Parsons SM, Hansma HG. Changes in the elastic properties of cholinergic synaptic vesicles as measured by atomic force microscopy. Biophysical Journal. 1997;72:806–813. doi: 10.1016/s0006-3495(97)78714-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Marston DJ, Walston T, Hardin J, Halberstadt A, Goldstein B. Wnt/Frizzled signaling controls C. elegans gastrulation by activating actomyosin contractility. Current biology : CB. 2006;16:1986–1997. doi: 10.1016/j.cub.2006.08.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Kwon KW, Jung H, Kim HN, Suh KY, Kim K, Kim KS. Direct differentiation of human embryonic stem cells into selective neurons on nanoscale ridge/groove pattern arrays. Biomaterials. 2010;31:4360–4366. doi: 10.1016/j.biomaterials.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Li N, Folch A. Integration of topographical and biochemical cues by axons during growth on microfabricated 3-D substrates. Experimental cell research. 2005;311:307–316. doi: 10.1016/j.yexcr.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan NN, McMahon S, O’Brien T, Yaszemski MJ, Windebank AJ. Current tissue engineering and novel therapeutic approaches to axonal regeneration following spinal cord injury using polymer scaffolds. Respir Physiol Neurobiol. 2009;169:183–199. doi: 10.1016/j.resp.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammoto T, Ingber DE. Mechanical control of tissue and organ development. Development. 2010;137:1407–1420. doi: 10.1242/dev.024166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandeville JT, Lawson MA, Maxfield FR. Dynamic imaging of neutrophil migration in three dimensions: mechanical interactions between cells and matrix. J Leukoc Biol. 1997;61:188–200. doi: 10.1002/jlb.61.2.188. [DOI] [PubMed] [Google Scholar]
- Maniotis AJ, Bojanowski K, Ingber DE. Mechanical continuity and reversible chromosome disassembly within intact genomes removed from living cells. J Cell Biochem. 1997a;65:114–130. [PubMed] [Google Scholar]
- Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proceedings of the National Academy of Sciences of the United States of America. 1997b;94:849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz H, McElhaney J, Ommaya AK. A comparison of the elasticity of live, dead, and fixed brain tissue. J Biomech. 1970;3:453–458. doi: 10.1016/0021-9290(70)90017-5. [DOI] [PubMed] [Google Scholar]
- Miller RH, Bai L, Lennon DP, Caplan AI. The potential of mesenchymal stem cells for neural repair. Discov Med. 2010;9:236–242. [PubMed] [Google Scholar]
- Molloy JE, Burns JE, Kendrick-Jones J, Tregear RT, White DC. Movement and force produced by a single myosin head. Nature. 1995;378:209–212. doi: 10.1038/378209a0. [DOI] [PubMed] [Google Scholar]
- Moore SW, Biais N, Sheetz MP. Traction on immobilized netrin-1 is sufficient to reorient axons. Science. 2009;325:166. doi: 10.1126/science.1173851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SW, Roca-Cusachs P, Sheetz MP. Stretchy proteins on stretchy substrates: the important elements of integrin-mediated rigidity sensing. Dev Cell. 2010;19:194–206. doi: 10.1016/j.devcel.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A, Naeem A, Barnes SH, Drescher U, Guthrie S. Slit and Netrin-1 guide cranial motor axon pathfinding via Rho-kinase, myosin light chain kinase and myosin II. Neural Dev. 2010;5:16. doi: 10.1186/1749-8104-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata I, Kawana A, Nakatsuji N. Perpendicular contact guidance of CNS neuroblasts on artificial microstructures. Development. 1993;117:401–408. doi: 10.1242/dev.117.1.401. [DOI] [PubMed] [Google Scholar]
- Neves SR, Tsokas P, Sarkar A, Grace EA, Rangamani P, Taubenfeld SM, Alberini CM, Schaff JC, Blitzer RD, Moraru II, Iyengar R. Cell shape and negative links in regulatory motifs together control spatial information flow in signaling networks. Cell. 2008;133:666–680. doi: 10.1016/j.cell.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikki M, Merilainen J, Lehto VP. FAP52 regulates actin organization via binding to filamin. The Journal of biological chemistry. 2002;277:11432–11440. doi: 10.1074/jbc.M111753200. [DOI] [PubMed] [Google Scholar]
- Norman LL, Aranda-Espinoza H. Cortical Neuron Outgrowth is Insensitive to Substrate Stiffness. Cell Mol Bioeng. 2010;3:398–414. [Google Scholar]
- Ohta Y, Akiyama T, Nishida E, Sakai H. Protein kinase C and cAMP-dependent protein kinase induce opposite effects on actin polymerizability. FEBS letters. 1987;222:305–310. doi: 10.1016/0014-5793(87)80391-5. [DOI] [PubMed] [Google Scholar]
- Padmakumar VC, Libotte T, Lu W, Zaim H, Abraham S, Noegel AA, Gotzmann J, Foisner R, Karakesisoglou I. The inner nuclear membrane protein Sun1 mediates the anchorage of Nesprin-2 to the nuclear envelope. Journal of cell science. 2005;118:3419–3430. doi: 10.1242/jcs.02471. [DOI] [PubMed] [Google Scholar]
- Palay SL, Chan-Palay V. General Morphology of Neurons and Neuroglia. John Wiley & Sons, Inc; 2010. [Google Scholar]
- Parpura V, Haydon PG, Henderson E. Three-dimensional imaging of living neurons and glia with the atomic force microscope. Journal of cell science. 1993;104(Pt 2):427–432. doi: 10.1242/jcs.104.2.427. [DOI] [PubMed] [Google Scholar]
- Pitulescu ME, Adams RH. Eph/ephrin molecules--a hub for signaling and endocytosis. Genes Dev. 2010;24:2480–2492. doi: 10.1101/gad.1973910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomann M, Wittmann JG, Rudolph MG. A hinge in the distal end of the PACSIN 2 F-BAR domain may contribute to membrane-curvature sensing. Journal of molecular biology. 2010;400:129–136. doi: 10.1016/j.jmb.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Pompe T, Renner L, Werner C. Nanoscale features of fibronectin fibrillogenesis depend on protein-substrate interaction and cytoskeleton structure. Biophysical Journal. 2005;88:527–534. doi: 10.1529/biophysj.104.048074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prange MT, Margulies SS. Regional, directional, and age-dependent properties of the brain undergoing large deformation. J Biomech Eng. 2002;124:244–252. doi: 10.1115/1.1449907. [DOI] [PubMed] [Google Scholar]
- Puchner EM, Alexandrovich A, Kho AL, Hensen U, Schafer LV, Brandmeier B, Grater F, Grubmuller H, Gaub HE, Gautel M. Mechanoenzymatics of titin kinase. Proc Natl Acad Sci U S A. 2008;105:13385–13390. doi: 10.1073/pnas.0805034105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. The Journal of comparative neurology. 1972;145:61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- Raper J, Mason C. Cellular strategies of axonal pathfinding. Cold Spring Harb Perspect Biol. 2010;2:a001933. doi: 10.1101/cshperspect.a001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recknor JB, Sakaguchi DS, Mallapragada SK. Directed growth and selective differentiation of neural progenitor cells on micropatterned polymer substrates. Biomaterials. 2006;27:4098–4108. doi: 10.1016/j.biomaterials.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Roca-Cusachs P, Alcaraz J, Sunyer R, Samitier J, Farre R, Navajas D. Micropatterning of single endothelial cell shape reveals a tight coupling between nuclear volume in G1 and proliferation. Biophys J. 2008;94:4984–4995. doi: 10.1529/biophysj.107.116863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussigne M, Bianco IH, Wilson SW, Blader P. Nodal signalling imposes left-right asymmetry upon neurogenesis in the habenular nuclei. Development. 2009;136:1549–1557. doi: 10.1242/dev.034793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez A, Buguin A, Silberzan P, Ladoux B. Is the mechanical activity of epithelial cells controlled by deformations or forces? Biophys J. 2005;89:L52–54. doi: 10.1529/biophysj.105.071217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaita K, Nair PM, Petit RS, Neve RM, Das D, Gray JW, Groves JT. Restriction of receptor movement alters cellular response: physical force sensing by EphA2. Science. 2010;327:1380–1385. doi: 10.1126/science.1181729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandquist JC, Swenson KI, Demali KA, Burridge K, Means AR. Rho kinase differentially regulates phosphorylation of nonmuscle myosin II isoforms A and B during cell rounding and migration. The Journal of biological chemistry. 2006;281:35873–35883. doi: 10.1074/jbc.M605343200. [DOI] [PubMed] [Google Scholar]
- Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosshauer B, Dreesmann L, Schaller HE, Sinis N. Synthetic nerve guide implants in humans: a comprehensive survey. Neurosurgery. 2006;59:740–747. doi: 10.1227/01.NEU.0000235197.36789.42. discussion 747–748. [DOI] [PubMed] [Google Scholar]
- Siechen S, Yang S, Chiba A, Saif T. Mechanical tension contributes to clustering of neurotransmitter vesicles at presynaptic terminals. Proc Natl Acad Sci U S A. 2009;106:12611–12616. doi: 10.1073/pnas.0901867106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater SJ, Kelly MB, Taddeo FJ, Ho C, Rubin E, Stubbs CD. The modulation of protein kinase C activity by membrane lipid bilayer structure. The Journal of biological chemistry. 1994;269:4866–4871. [PubMed] [Google Scholar]
- Straley KS, Foo CW, Heilshorn SC. Biomaterial design strategies for the treatment of spinal cord injuries. Journal of neurotrauma. 2010;27:1–19. doi: 10.1089/neu.2009.0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukharev S, Corey DP. Mechanosensitive channels: multiplicity of families and gating paradigms. Sci STKE. 2004;2004:re4. doi: 10.1126/stke.2192004re4. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Price LS, Koffer A. Rho controls cortical F-actin disassembly in addition to, but independently of, secretion in mast cells. The Journal of biological chemistry. 1999;274:38140–38146. doi: 10.1074/jbc.274.53.38140. [DOI] [PubMed] [Google Scholar]
- Svitkina TM, Surguchova IG, Verkhovsky AB, Gelfand VI, Moeremans M, De Mey J. Direct visualization of bipolar myosin filaments in stress fibers of cultured fibroblasts. Cell Motil Cytoskeleton. 1989;12:150–156. doi: 10.1002/cm.970120304. [DOI] [PubMed] [Google Scholar]
- Thery M, Racine V, Pepin A, Piel M, Chen Y, Sibarita JB, Bornens M. The extracellular matrix guides the orientation of the cell division axis. Nature cell biology. 2005;7:947–953. doi: 10.1038/ncb1307. [DOI] [PubMed] [Google Scholar]
- Thibault KL, Margulies SS. Age-dependent material properties of the porcine cerebrum: effect on pediatric inertial head injury criteria. J Biomech. 1998;31:1119–1126. doi: 10.1016/s0021-9290(98)00122-5. [DOI] [PubMed] [Google Scholar]
- Tsuruma A, Tanaka M, Yamamoto S, Shimomura M. Control of neural stem cell differentiation on honeycomb films. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2008;313:536–540. [Google Scholar]
- Tyska MJ, Dupuis DE, Guilford WH, Patlak JB, Waller GS, Trybus KM, Warshaw DM, Lowey S. Two heads of myosin are better than one for generating force and motion. Proc Natl Acad Sci U S A. 1999;96:4402–4407. doi: 10.1073/pnas.96.8.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nature reviews Molecular cell biology. 2009;10:75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- Weiss PA. In vitro experiments on the factors determining the course of the outgrowing nerve fiber. JExpZool. 1934;68:393–448. [Google Scholar]
- Wen S, Li H, Liu J. Dynamic signaling for neural stem cell fate determination. Cell Adh Migr. 2009;3:107–117. doi: 10.4161/cam.3.1.7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wess TJ. Collagen fibril form and function. Adv Protein Chem. 2005;70:341–374. doi: 10.1016/S0065-3233(05)70010-3. [DOI] [PubMed] [Google Scholar]
- Willits RK, Skornia SL. Effect of collagen gel stiffness on neurite extension. J Biomater Sci Polym Ed. 2004;15:1521–1531. doi: 10.1163/1568562042459698. [DOI] [PubMed] [Google Scholar]
- Wu ZZ, Kisaalita WS, Wang L, Zachman AL, Zhao Y, Hasneen K, Machacek D, Stice SL. Effects of topography on the functional development of human neural progenitor cells. Biotechnol Bioeng. 2010;106:649–659. doi: 10.1002/bit.22715. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Lee AC, Suter DM, Lee GU. Topography and nanomechanics of live neuronal growth cones analyzed by atomic force microscopy. Biophysical Journal. 2009;96:5060–5072. doi: 10.1016/j.bpj.2009.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Wirtz D, Kuo SC. Mechanics of living cells measured by laser tracking microrheology. Biophys J. 2000;78:1736–1747. doi: 10.1016/S0006-3495(00)76725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim EK, Pang SW, Leong KW. Synthetic nanostructures inducing differentiation of human mesenchymal stem cells into neuronal lineage. Experimental cell research. 2007;313:1820–1829. doi: 10.1016/j.yexcr.2007.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Bellamkonda RV. Dorsal root ganglia neurite extension is inhibited by mechanical and chondroitin sulfate-rich interfaces. Journal of neuroscience research. 2001;66:303–310. doi: 10.1002/jnr.1225. [DOI] [PubMed] [Google Scholar]
- Yue X, Dreyfus C, Kong TA, Zhou R. A subset of signal transduction pathways is required for hippocampal growth cone collapse induced by ephrin-A5. Dev Neurobiol. 2008;68:1269–1286. doi: 10.1002/dneu.20657. [DOI] [PMC free article] [PubMed] [Google Scholar]