Figure 1.
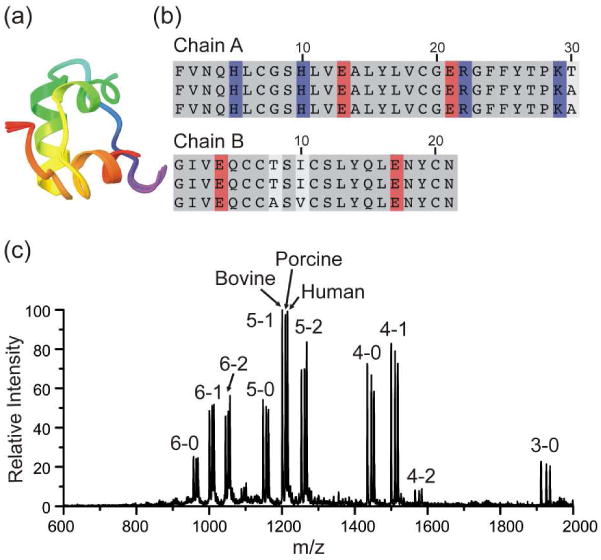
(a) Backbone structural alignment for the three variants of insulin. (b) Sequence alignment for human, porcine and bovine insulin from top to bottom, respectively. Sequence variation (white), basic residues (blue) and acidic residues (red) are highlighted. (c) ESI-MS spectrum of insulin from bovine, porcine, human and 18C6 in water. Peaks are labeled by (charge state) – (number of 18C6 adducts). The results are similar for all three proteins.
