Abstract
The use of molecular assays to improve diagnosis have been routinely applied for the last 25 years. Assays that guide therapy have a similar history, however their evolution has lacked the focus on analytic integrity required for the molecularly targeted therapy of today. New molecularly targeted agents require assays of greater precision/quantitation to predict the likelihood of response; identifying patients whose tumors will respond, while at the same time excluding and protecting those patients whose tumors will not respond or treatment will cause unacceptable toxicity. The handling of tissue has followed a “fit-for-purpose” approach focused on appropriateness for diagnostic needs, which is less rigorous than the demands of new molecular assays that interrogate DNA, RNA and proteins in quantitative multiplex assays. There is a new appreciation of the importance and fragility of tissue specimens as the source of analytes to direct therapy. By applying a total test paradigm and defining and measuring sources of variability in specimens, a set of specifications can be developed that can be deployed into the clinical care environment to ensure that a specimen is appropriate for analysis and will return a true result.
Introduction
The examination of tissue for the determination of diagnosis and guidance of optimal therapy has been practiced since the advent of surgery. The late 19th century saw the advent of microscopic examination of tissue, and substantial advancement in the classification of disease [1]. The basic form of histo-morphological examination of excised tissue stained with contrast agents of hematoxylin and eosin has been in widespread use for over a century, and remains the cornerstone of diagnostic anatomic pathology. Beyond defining a tumor, this approach is able to provide substantial prognostic information that clinicians routinely rely on to guide therapy. The most common examples being the status of surgical margins, spread of disease and differentiation state of the tumor (grade) which can all be combined into the stage of a tumor that predicts outcome. This approach of histopathology is dependent on a fund of knowledge. With the advent of molecular biology it is now possible to extend beyond the histo-morphology of a tumor and probe the tumor for specific molecular alterations that portend behavior or are targets of therapy. Some of these alterations are observable at the level of histo-morphology; however most are more accurately measured at the DNA, RNA or protein level. Many of these analytes can also be measured in body fluids; however this approach introduces additional challenges. Ultimately the goal is to link the use of biomarkers to functionally guide therapy beyond prognosis and to predict response to therapy. The capacity to use biomarkers to monitor response at the molecular level offers new tools to fine-tune therapy, preventing toxicity, and identifying treatment failure at an earlier time point than disease progression as measured by tumor mass.
As histopathology pre-dated molecular pathology, the handling and processing of specimens has been optimized for histopathology, with little/no reference to molecular biology [2]. Biospecimens protocols have evolved to follow the needs of the assays performed. From the alternative perspective, the handling of biospecimens has been well recognized as contributing to assay variability and issues in assay validation [3, 4]. To address these issues, some tissues are amenable to repeated sampling, without concern of substantial tissue heterogeneity or sampling issues, such that every sample can be assumed identical, and in these situations, “molecular friendly” means of preservation, optimization for the analyte of interest, rather than histo-morphologic examination, can be applied. Many of the commonly employed means of preservation of tissue in a clinical setting are optimized in such a manner that biomolecules are damaged or destroyed. A class of fixatives and preservatives that attempts to overcome these issues, generically termed “molecularly friendly” have been developed, but are not feasible as replacement agents. The most common example of this is flow cytometry. Unfortunately this approach cannot be applied to the vast majority of tumors.
The development, validation and application of integral biomarkers is facing a number of challenges [5,6]. One challenge is the demands of new assays on the biospecimen. It is impractical to replace entirely the current methods of biospecimen collection, processing and handling. The solution to improved integral biomarker assays is the combination of a) evolution of biospecimen protocols b) appreciation of the limitations of the current collection of biospecimens, and efforts to harmonize new biomarker assays to perform within this context and c) and integrated approach to the development and validation of integral biomarker assays. The difference between how a biospecimen is handled in a clinical setting and in a research setting must be reduced (see Poste et al [5]). The discovery pipeline of new biomarkers is strong, however validation continues to be the rate limiting step. Bringing the “laboratorian” actively into the process of assay validation, early in the process should help address this bottleneck.
Total Test
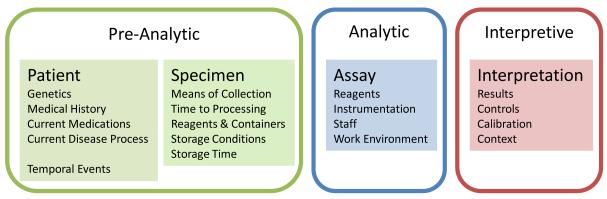
The application of the Total Test Paradigm has substantially improved the means by which tests are developed and validated [7,8]. A Test is divided into three elements: pre-analytic, analytic, and interpretive (post-analytic) phases (Figure 1) [8]. The pre-analytic phase will be discussed in depth, but can be summarized as everything before the assay. The analytic phase is the assay- the actions of performing the test, and its reagents. The interpretive (post-analytic) phase implies interpreting the test results by means of a standardized method, within the context of the disease. This paradigm is equally applicable to all diagnostic procedures in clinical and anatomic pathology, from histo-morphologic examination of tissue to measuring the specific gravity of urine. The goal is to qualify a specimen for a defined assay, and obtain a result within the context of the patient and the underlying disease question.
Figure 1.
The elements of the total test paradigm overlaid on the testing process for the patient to results.
Pre-Analytic Phase
Within the laboratory, the pre-analytic phase is everything pertaining to the specimen, before the performance of the assay. This over simplification is an attempt to capture the concept that the biospecimen represents the patient, and the physiology of the patient is equally important to the collecting and handling of the specimen once it is obtained from the patient. The pre-analytic phase may be divided into two elements – those pertaining to the patient for which variables cannot be controlled and elements from the collection of the specimen, until analytic phase, for which specification and control are feasible [7]. Elements of the pre-analytic phase include the patients’ past medical history, genetic and environmental elements, and medications. The FDA has become much more aware and concerned about patient-derived factors [9], and test developers will need to investigate these issues more fully. The act of collection (method and setting), the containers, time in transit, temperature of the specimen, intervening preparations/separations and stabilizations, and storage until the assay is conducted are the additional common elements of the pre-analytic phase. Unfortunately there is no exhaustive, or prioritized list of pre-analytic factors, as they are variable based on biospecimen and analyte. From the perspective of assay development, it is essential to test the pre-analytic variables that are anticipated to be encountered in a clinical setting, encompassing both the patient-derived factors and the specimen handling factors. The challenge is determining when an assay is sufficiently robust that additional testing of pre-analytic factors is not required.
Another challenge for the test developer is the poor appreciation and standardization in current clinical practice of the pre-analytic variables from collection of the specimen until the analytic phase [2,10]. Most commonly the issue is the belief by a test developer that a specification has a singular industry-standard practice, but in fact is best characterized as a practice for which there is substantial undocumented variability. Examples are as simple as “invert tube to mix”, where details of the number of times and the speed at which inversion is to be done are not described. This variability may not only be present between laboratories, but within laboratories or instruments in laboratories. In some instances the specification may in fact lack appropriate detail, and summarize a set of actions of conditions, introducing unrecognized variables. This can be perceived as the difference between the how the protocol is written in the guidelines, and how it is performed on a daily basis in the laboratory.
An example commonly encountered is “tissue was fixed and paraffin embedded per standard protocol”. Even within a single facility, it is routine to have multiple protocols for tissue impregnation (the formal term for embedding). Chung et al. demonstrated the substantial variation in RNA quality based on differences in fixation time or tissue impregnation conditions, both at the total RNA level as well as with specific mRNAs [10]. The authors were able to definitively demonstrate that fixation was a critical element of tissue processing, and that under-fixation was as detrimental as over-fixation (with a final recommendation of 24 hours), and that longer tissue processing times resulted in improved biomolecule preservation, presumably as the result of better extraction of water. The authors concurrently explored differences in buffers used in the manufacture of neutral buffered formalin. In this study, the authors uncovered multiple variables that have direct impact on new assays on formalin fixed, paraffin embedded tissue, that had been confounding previous studies, but had been unappreciated.
Differences in pre-analytic handling factors with serum are substantial. That lack of specification in blood collection, handling and preparation has the potential to alter results has been well reviewed [11,12,13]. Examples of commonly encountered maneuvers with the capacity to introduce relevant differences include a) instructions for inversion of the specimen at time of collection b) centrifugation conditions ( g-force, temperature) and c) storage conditions. Although the majority of the specifications are empirically derived, the validation of the specifications is frequently narrow and may be based on analysis of a single analyte, despite the fact that the preservation method is designed for multiple analytes. This issue is a function of the cost of comprehensive validation as well the rapid evolution of testing protocols, where adoption of an existing specification is commonly employed to expedited introduction. Although specification of handling is highly standardized, practice is poorly monitored, and only in the instance of assay failure is retrospective (site-specific) analysis carried out - often documenting deviation from the protocol specifications.
Substantial Challenges
Collection of biospecimens is not a new effort - analysis of tissue and fluids for diagnostic purposes is well over a century old. Specimen handing and preparation has evolved over this time period with the increasing demands of the laboratory. Matching an assay to a biospecimen should start with the examination of the current clinical guidelines and determination of their adequacy of the purpose. The introduction of new biospecimen handling protocols is expensive and comes with substantial barriers to validation and introduction into wide spread use. The goal should be to minimize new protocols, and when necessary, align them as closely as feasible with current practice. In the instance of tissue, the adoption of formalin was based on the need for an aseptic, non-flammable means of storing tissue for microscopic examination. The introduction of buffers to formalin was to improve histomorphology [2]. The advent of immunohistochemistry and the desire to obtain RNA from formalin fixed, paraffin embedded tissue has resulted in substantial efforts to understand and improve tissue processing with new recommendations on neutral buffered formalin, the length of fixation and tissue processing protocols., however efforts to replace neutral buffered formalin have not had substantial success [8, 10]
The forensics of pre-analytic failure provide the basis of ongoing advances in specimen handling. The lack of stability of RNA in formalin fixed paraffin embedded tissue [14, 15] has resulted into new guidelines in tissue storage [16]. New blood collection tubes for proteomics were developed by BD to address the failings of ETDA containing tubes for serum proteomics [11, 17] These new blood collection tubes introduced protease inhibitors that facilitated mass-spectrometry analysis of the serum proteome, while the removal of EDTA reduced artifacts introduced into the specimen from the cellular components of whole blood. The challenge is providing salient guidance to investigators and laboratorians as to what variables require development of a risk stratification approach to ensure biospecimens collected for assay have sufficient quality and quantity to allow accurate testing.
Selection of assays for replication rarely takes into account the quality of the specification of the test (pre-analytic, analytic or interpretative) and is driven by the quality of purported utility. The challenge for the investigator replicating a previously described biomarker is the detail in the specification of the specimens, the assay itself, and the interpretation.Historically, information has come to light when assays fail, and investigators have the integrity, resources and opportunity to determine why the assay failed. Unfortunately, investigators often lack resources and opportunity, and either drop the potential biomarker, or publish that the results could not be replicated. Determination of the root cause of a failure to replicate/validate an assay is often beyond the skills and resources of those performing the replication/validation. Too often the assumption is that the assay is “ready out of the box”, when in fact the assay remains in evolution through the validation phase [3].Often samples used in the initial description of a biomarker are “specimens of convenience” – pre-existing specimens not collected for the study- and specification of biospecimen related factors is more a historical recounting of what the specimens were rather than a description of appropriateness of the biospecimens. Application of the “fit-for-purpose” paradigm is a useful means of addressing the frequently encountered variables that limit the utility of a biospecimen, however it may not be readily apparent that some variables have never been tested. As noted previously, Chung et al demonstrated the substantial difference in RNA quality based on tissue impregnation time, a variable that had never been investigated previously [10].
Multiple parallel efforts are underway to assist investigators in this regard. The NCI OBBR has developed a best practices document for biospecimen processing, which are referenced to empiric data and provide a starting place for any specimen collection effort [18 ] These guidelines are in their second generation, and function as a tool for researchers to understand the current recommended clinical specimen collection specifications. These guidelines attempt to be comprehensive, for all biospecimens, providing a framework for collection, management and use of biospecimens in research. Applied correctly, this document also provides a checklist to examine deviations from best practices.
Working with the NCI Division of Cancer Treatment and Diagnosis (DCTD), a consortium of assay experts have developed template-based questionnaires to assist investigators in defining and addressing those biospecimen questions that may come up in the process of a pre-IDE meeting with the FDA[19]. These questions can be generalized into first-order questions, which an investigator should be able to address without consultation with test developers (Table 1) and second-order questions, which are issues routinely tested and evaluated in the process of assay hardening (Table 2). As with many aspects of medicine, there is substantial specialization, and the capacity to develop a new, clinically validated assay suitable for introduction into patient care is no exception. The development of clinical-lab-ready assays requires the collaboration of the investigator who initially discovers the biomarker, as well as advanced laboratorians who will specify the assay, and interrogate sources of variability, defining an assay, prior to its clinical validation, demonstrating clinical utility [7]. In the following sections, we have attempted to summarize the strengths and weaknesses of some bioanalytes commonly tested. The current data are insufficient for the list to be exhaustive, or to even prioritize these issues effectively.
Table 1.
Examples of first-order biospecimen issues for new biomarkers.
| Intended use of the assay? |
| Clinical Utility – How useful is the diagnostic within its intended clinical use? |
| What additional information is required for an accurate test result? |
| Analyte - What biomolecule is being analyzed? |
| Specimen Type - What are the type(s) of tissue/fluid for analysis? |
| Does a clinical specification exist for this biospecimen? |
| Is this specification adequate for this test? |
| Have specimens been serially tested to demonstrate the specimens is stable in storage? |
| Can the assay be replicated in another laboratory with the original specimens? |
| Can the assay be replicated in the original laboratory with submitted specimens? |
Table 2.
Examples of second-order biospecimen issues for new biomarkers.
| Minimum specimen size? |
| μl of fluid, μg of tissue, number of cells |
| Are non-standard specimen handling requirements utilized? |
| frozen tissue, alternative fixatives, non-routine blood collection tubes |
| Have common variables of biospecimen handing been tested as a source of assay variability? |
| fluids - temperature, time, separation technologies |
| tissue - fixative, fixation times, processing times, specimen storage |
| Have other analytes been demonstrated to interfere with the assay? |
| Is there a document that describes what an adequate vs inadequate biospecimen for this assay is? |
Fluids as a Biospecimen
Fluids, and their constituent non- proteinaceous components, are the best characterized analytes in reference to pre-analytic variables, generally falling within the specialty of Clinical Pathology. Serum/plasma is the most common fluid analyzed [20], followed by urine [21 and 18]. This diverse universe of analytes is the starting point for understanding analytes in tissue.
DNA
DNA is clearly the most robust analyte with reference to pre-analytic variables. Quality issues typically center on the length of DNA fragments obtained from the specimen, and whether the length of the DNA impacts assay performance [14]. For in situ based assays, little qualitative data exist. aCGH has gained substantial ground as a means of examining copy number variations. DNA fragment length has been documented to impact “call rates” on aCGH platforms, resulting in modification of cut-offs for the presence of a genomic alteration. The net effect is that aCGH with shorter DNA fragements provides less information and is less capable of defining small regions of loss or gain [22]. At this time, there is little data on pre-analytic factors and epigenetic modifications, however the development of the single-nucleotide sequencers will have the potential to interrogate the genome at a level not obtainable previously.
Sequence-derived biomarkers are a rapidly developing field. Numerous techniques have been widely applied in a screening modality for the last two decades, but all relied on confirmation by Sanger sequencing as a confirmatory approach. With the development of “next-generation” sequencing technologies, it is impractical to apply Sanger sequencing as a confirmatory step, as this approach becomes a bottle-neck. False-positive mutations are frequently attributed to specimen fixation and processing issues, especially with reference to tissue [23]. As noted above, the central theme of tissue is purity of target. These false-positive mutations are believed to occur because of alterations to the DNA by means of chemical crosslinking of the fixatives. In this instance the connection between pre-analytic and analytic phases of the assay plays a substantial role in defining the baseline false discovery rate of the assay. It is anticipated that each instrument/assay would have its own false positive rate, specific to the instrumentation and protocol applied, and that this information can only be defined empirically [ 24] . This is a subject of substantial concern in the development of sequence-based biomarkers, and will have direct impact on assay sensitivity, specificity and false-positive rates. This challenge has brought greater interest in the development of clinically feasible “molecular friendly” non-crosslinking fixatives for tissue, however this approach faces other challenges.
RNA
In contrast to DNA, RNA is the most labile analyte commonly analyzed. The nature of cellular processes has evolved such that mRNAs are encoded with multiple elements that impact the stability of RNA, as a mechanism of regulation of the production of proteins [2]. RNA is a very inviting target for measurement as a biomarker; molecular biology makes its measurement relatively easy by means of PCR and hybridization techniques, and the linkage of is highly regulated expression to patho-physiology. A key element of assay design is probe placement and in the case of PCR, amplicon size.
Assays for the presence or absence of specific mRNAs can be constructed to avoid the majority of pre-analytic challenges that are commonly encountered [10]. More importantly, concurrent measurement of “housekeeping” RNAs, provides the essential quality control check for both the analyte and the assay. In situ arrays have improved in recent years, such as the introduction of chromagenic in situ assays for Her2 amplifcation (SPOT-Light® HER2 CISH from Life Technologies), while traditional northern blot analysis or RT-PCR assays can be specified to provide binary (present or absent) information.
As the desire for information from RNA increases, the demands on the specimen increase concurrently. Quantitative RT-PCR is a powerful tool, but is very dependent on the quality of starting RNA. This quality is measured in both integrity and quantity [2]. Concurrent with quantitative RNA assays are multiplex assays. Fundamentally, both present the same complexities – a lack of a defined “denominator of quality” of starting RNA. The general approach has been to increase the number of “control genes”, most commonly housekeeping genes in an effort to better define the quality of the RNA being subject to analysis. In the case of OncoType Dx, 5 of 21 genes are used to ensure adequacy of the assay results, and provide a form of denominator by which to measure the target RNAs[ 25]. In contrast, microRNAs appear to suffer fewer of the challenges encountered with mRNA. The size of the microRNAs appears to be the salient feature [26].
Protein
Protein is the analyte we have the most experience with as a clinical diagnostic in tissue. Immunohistochemistry as a technique is over 70 years old [27], and has seen widespread clinical application for over 20 years with formalin fixed paraffin embedded tissue. This substantial experience has both confirmed its general utility as well as unmasked the weaknesses in the assay. With the advent of heat mediated antigen retrieval, immunohistochemistry has widened the breath of potential targets and sensitivity of the technique in general [28].
The overwhelming pre-analytic variable in immuno histochemistry in the clinical setting remains fixation time [10] . Although other variables are documented to have substantial impact on assay performance, fixation time remains poorly controlled in general practice [2]. It is demonstrated that antigen retrieval can offset differences in fixation time, increasing antigen “availabilty” in tissues that have been fixed for longer times. Application of this approach, while improving assay sensitivity, has a negative effect on assay calibration and specificity [29].
Only recently addressed in a systematic fashion, and of substantial concern is the veracity of clinical samples stored for prolonged periods of time [2]. It has been well documented that RNA degrades in FFPE samples over periods measured in 5 and 10 year units [15]. At present no means of preventing degradation has been demonstrated to entirely halt the process [16]. The measurement of modified proteins, most commonly phospho-proteins as indicators of cellular signaling offers many opportunities. Phospho-proteins are best envisioned as delicate as mRNA, and are susceptible to both hypoxic/ischemic affects as well as damage by specimen handling and fixation [10].
Although nucleic acids can be assayed in body fluids, present from the degradation of cellular components, they are a less common analyte than secreted proteins. As the methods for assaying the non-protein components of fluids are well developed, only slight modification has been required to ensure robust pre-analytic performance of proteins found in the serum/plasma. Tubes with EDTA are most commonly deployed, however specialized tubes are being used with increasing frequency. It should be noted that temperature of storage/transportation and means of centrifugation have been demonstrated as important pre-analytic variables.
Conclusions
Biospecimen handling is an evolutionary process that continues to evolve to address the demands of the clinical environment. The development of robust biomarkers based on nucleic acids and proteins requires a more rigorous evaluation of factors that impact analyte stability and variability. The NCI Office of Biorepositories and Biospecimens (OBBR) has recently updated their Best Practices [18] which provides current, data-driven guidelines, while acknowledging the limitations of current practice. Unfortunately there is no comprehensive set of recommendations on biospecimens and methods of evaluating the pre-analytic phase of a test. The factors determining the optimal processing of patient samples are biospecimen, analyte and assay specific. Our current understandings of the factors that determine the quality of a biospecimen are limited, and guidelines require constant evaluation and revision. To date, the only unified set of guideline are those put forth for breast cancer clinical trials by Leland-Jones, et al. [14] These guidelines, already four years old, and becoming out of date, only provide a framework of the issues concerning other malignancies. Ultimately, success in translational research will require the standardization of specimen handling along the lines of what is currently seen in clinical chemistry, which will then lead to improved biomarker test performance. One element of these efforts is to move away from the ad hoc nature of specimen collection for research and to introduce and adhere to specifications for collection and analyte stabilization that are found in the clinical diagnostic setting. A second aspect of this approach is the adoption of a “blood-tube paradigm” for the collection of biospecimens beyond blood. The immediate segregation of biospecimens into analyte-optimized biospecimen collection protocols will enhance the diversity and quality of end assays. Lastly, elevation of the collection and handling of biospecimens in clinical research beyond an “add-on task” and providing appropriate budget and staffing support is critical for new biomarker development. Biospecimen collection will continue to advance, providing more robust assays, but this progress will be driven by a fit-for-purpose model. Standardization and performance standards will be required to obtain the uniform quality of biospecimens demanded by the emerging biomarkers.
References
- 1.Gal AA. In search of the origins of modern surgical pathology. Adv Anat Pathol. 2001 Jan;8(1):1–13. doi: 10.1097/00125480-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Hewitt SM, Lewis FA, Cao Y, Conrad RC, Cronin M, Danenberg KD, et al. Tissue handling and specimen preparation in surgical pathology: issues concerning the recovery of nucleic acids from formalin-fixed, paraffin-embedded tissue. Arch Pathol Lab Med. 2008 Dec;132(12):1929–35. doi: 10.5858/132.12.1929. [DOI] [PubMed] [Google Scholar]
- 3.Chau CH, Rixe O, McLeod H, Figg WD. Validation of analytic methods for biomarkers used in drug development. Clin Cancer Res. 2008;14:5967–76. doi: 10.1158/1078-0432.CCR-07-4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Owzar K, Barry WT, Jung SH, Sohn I, George SL. Statistical challenges in preprocessing in microarray experiments in cancer. Clin Cancer Res. 2005;14:5959–66. doi: 10.1158/1078-0432.CCR-07-4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poste G, Carbone DP, Parkinson DR, Verweij J, Hewitt S, Jessup JM. Leveling the playing field: bringing development of biomarkers and molecular diagnostics up to the standards for drug development. Clin Cancer Res. 2012;18 doi: 10.1158/1078-0432.CCR-11-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schilsky RL, Doroshow JH, LeBlanc M, Conley BA. Development and use of integral assays in clinical trials. Clin Cancer Res. 2012;18 doi: 10.1158/1078-0432.CCR-11-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hewitt SM, Takikita M, Braunschweig T, Chung J-Y. Promises and challenges of predict tissue biomarkers. Biomarkers Med. 2007;1(2):313–318. doi: 10.2217/17520363.1.2.313. [DOI] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute (CLSI) Quality Assurance for Design Control and Implementation of Immunohistochemical Assays: Approved Guidelines – Second Edition. Clinical and Laboratory Standards Institute; 940 West Valley Road, Suite 1400, Wayne Pennsylvania 19087 USA: 2011. CLSI document I/LA28-A2 (ISBN 1-56238-745-6) [Google Scholar]
- 9.Meshinchi S, Hunger SP, Aplenc R, Adamson PC, Jessup JM. Lessons learned from the Investigational Device Exemption (IDE) review of Children’s Oncology Group Trial AAML1031. Clin Cancer Res. 2012;18 doi: 10.1158/1078-0432.CCR-11-2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung JY, Braunschweig T, Williams R, Guerrero N, Hoffmann KM, Kwon M, et al. Factors in tissue handling and processing that impact RNA obtained from formalin-fixed, paraffin-embedded tissue. J Histochem Cytochem. 2008 Nov;56(11):1033–42. doi: 10.1369/jhc.2008.951863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rai AJ, Vitzthum F. Effects of preanalytical variables on peptide and protein measurements in human serum and plasma: implications for clinical proteomics. Expert Rev Proteomics. 2006 Aug;3(4):409–26. doi: 10.1586/14789450.3.4.409. [DOI] [PubMed] [Google Scholar]
- 12.Barelli S, Crettaz D, Thadikkaran L, Rubin O, Tissot JD. Plasma/serum proteomics: pre-analytical issues. Expert Rev Proteomics. 2007 Jun;4(3):363–70. doi: 10.1586/14789450.4.3.363. [DOI] [PubMed] [Google Scholar]
- 13.Pieragostino D, Petrucci F, Del Boccio P, Mantini D, Lugaresi A, Tiberio S, et al. Pre-analytical factors in clinical proteomics investigations: impact of ex vivo protein modifications for multiple sclerosis biomarker discovery. J Proteomics. 2010 Jan 3;73(3):579–92. doi: 10.1016/j.jprot.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Leyland-Jones BR, Ambrosone CB, Bartlett J, Ellis MJ, Enos RA, Raji A, et al. Recommendations for collection and handling of specimens from group breast cancer clinical trials. J Clin Oncol. 2008 Dec 1;26(34):5638–44. doi: 10.1200/JCO.2007.15.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cronin M, Pho M, Dutta D, Stephans JC, Shak S, Kiefer MC, et al. Measurement of gene expression in archival paraffin-embedded tissues: development and performance of a 92-gene reverse transcriptase-polymerase chain reaction assay. Am J Pathol. 2004 Jan;164(1):35–42. doi: 10.1016/S0002-9440(10)63093-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie R, Chung JY, Ylaya K, Williams RL, Guerrero N, Nakatsuka N, et al. Factors influencing the degradation of archival formalin-fixed paraffin-embedded tissue sections. J Histochem Cytochem. 2011 Apr;59(4):356–65. doi: 10.1369/0022155411398488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yi J, Liu Z, Craft D, O’Mullan P, Ju G, Gelfand CA. Intrinsic peptidase activity causes a sequential multi-step reaction (SMSR) in digestion of human plasma peptides. J Proteome Res. 2008 Dec;7(12):5112–8. doi: 10.1021/pr800396c. [DOI] [PubMed] [Google Scholar]
- 18. http://biospecimens.cancer.gov/bestpractices/
- 19.Dancey JE, Dobbin KK, Groshen S, Jessup JM, Hruszkewycz AH, Koehler M, et al. Guidelines for the development and incorporation of biomarker studies in early clinical trials of novel agents. Clin Cancer Res. 2010 Mar 15;16(6):1745–55. doi: 10.1158/1078-0432.CCR-09-2167. [DOI] [PubMed] [Google Scholar]
- 20.Barker PE, Wagner PD, Stein SE, Bunk DM, Srivastava S, Omenn GS. Standards for plasma and serum proteomics in early cancer detection: a needs assessment report from the national institute of standards and technology--National Cancer Institute Standards, Methods, Assays, Reagents and Technologies Workshop, August 18-19, 2005. Clin Chem. 2006 Sep;52(9):1669–74. doi: 10.1373/clinchem.2006.067249. Epub 2006 Jul 13. [DOI] [PubMed] [Google Scholar]
- 21.Court M, Selevsek N, Matondo M, Allory Y, Garin J, Masselon CD, Domon B. Toward a standardized urine proteome analysis methodology. Proteomics. 2011 Mar;11(6):1160–71. doi: 10.1002/pmic.201000566. [DOI] [PubMed] [Google Scholar]
- 22.Tuefferd M, De Bondt A, Van Den Wyngaert I, Talloen W, Verbeke T, et al. Genome-wide copy number alterations detection in fresh frozen and matched FFPE samples using SNP 6.0 arrays. Genes Chromosomes Cancer. 2008 Nov;47(11):957–64. doi: 10.1002/gcc.20599. [DOI] [PubMed] [Google Scholar]
- 23.Koboldt DC, Ding L, Mardis ER, Wilson RK. Challenges of sequencing human genomes. Brief Bioinform. 2010 Sep;11(5):484–98. doi: 10.1093/bib/bbq016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nothnagel M, Herrmann A, Wolf A, Schreiber S, Platzer M, Siebert R, et al. Technology-specific error signatures in the 1000 Genomes Project data. Hum Genet. 2011 Oct;130(4):505–16. doi: 10.1007/s00439-011-0971-3. [DOI] [PubMed] [Google Scholar]
- 25.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004 Dec 30;351(27):2817–26. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 26.Klopfleisch R, Weiss AT, Gruber AD. Excavation of a buried treasure--DNA, mRNA, miRNA and protein analysis in formalin fixed, paraffin embedded tissues. Histol Histopathol. 2011 Jun;26(6):797–810. doi: 10.14670/HH-26.797. [DOI] [PubMed] [Google Scholar]
- 27.Coons AH, Creech HJ, Jone RN, et al. The demonstration of pneumococcal antigen in tissues by use of fluorescent antibodies. J Immunol. 1942;45:159–170. [Google Scholar]
- 28.Shi SR, Key ME, Kalra KL. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 1991 Jun;39(6):741–8. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein NS, Hewitt SM, Taylor CR, Yaziji H, Hicks DG, Members of Ad-Hoc Committee On Immunohistochemistry Standardization Recommendations for improved standardization of immunohistochemistry. Appl Immunohistochem Mol Morphol. 2007 Jun;15(2):124–33. doi: 10.1097/PAI.0b013e31804c7283. [DOI] [PubMed] [Google Scholar]