Abstract
We have shown that the rat can quantitatively predict the verapamil-cyclopsorine A (CsA) drug-drug interaction (DDI) at the human blood-brain barrier (BBB). In addition, the potency (EC50) of CsA to inhibit rat BBB P-gp can be predicted from in vitro studies in MDRI-transfected cells. To assess if these excellent agreements extend to other substrates, we determined the magnitude of P-gp-based DDI at the rat BBB between loperamide (Lop) or its metabolite, N-desmethyl Lop (dLop), and escalating CsA blood concentrations. The percent increase in the brain:blood Lop concentration ratio was described by the Hill equation, Emax=2000%, EC50=7.1 μM and γ=3.7. The potency (EC50) of CsA to inhibit P-gp at the rat BBB was independent of the substrate used (verapamil, Lop, or dLop). Like the verapamil-CsA DDI, the potency (EC50) of CsA to inhibit rat BBB P-gp could be predicted from studies in MDRI-transfected cells. When 11C-Lop was co-administered with a 10 mg/kg i.v. infusion of CsA 1yielding ~5.6 uM CsA blood concentration) to healthy volunteers, the brain distribution of 11C-radioactivity was increased by 110% 1. When corrected for diffusible Lop metabolite(s), this translates into an increase in 11C-Lop brain distribution of 457%. Based on our rat data, we estimated a remarkably similar value at 5.6 μM blood CsA concentration, 588% increase in Lop brain distribution. These data support our conclusion that the rat is a promising model to predict P-gp based DDI at the human BBB.
Keywords: P-glycoprotein, blood-brain-barrier, Cyclosporine A, Loperamide, N-desmethyl Loperamide, drug-drug interaction, in vitro to in vivo correlation
INTRODUCTION
P-glycoprotein (P-gp), an ATP-dependent efflux transporter, is recognized as an important component of the blood-brain barrier (BBB) 2. Localized at the luminal membrane of brain endothelial cells 3, P-gp protects the brain by restricting the entry of harmful compounds and drugs 2. The importance of P-gp at the BBB has been demonstrated in numerous small animal studies, mostly in mice and rats. In rodents, when the genes encoding P-gp (mdr1a/b) are ablated or P-gp is chemically inhibited, the brain distribution of numerous chemically diverse drugs is increased from 800 to 3600% 3; 4; 5; 6; 7. While these rodent studies raise concerns for clinically significant drug-drug interactions (DDI) at the human BBB, these concerns are only relevant if the rodent is truly predictive of DDI at the human BBB. Until recently, it was not possible to test this hypothesis due to our inability to quantitatively and non-invasively measure drug distribution across the human BBB. Using noninvasive, quantitative, positron-emission tomography (PET) to assess P-gp-based DDI at the human BBB, we reported excellent quantitative correlation between the rat and the human for the verapamil-cyclosporine A (CsA) DDI 8; 9. At identical CsA blood concentration (~3 μM), the increase in brain:plasma concentration ratio of labeled verapamil in the human and the rat was 79 and 75% respectively. In addition, the potency of CsA to inhibit rat BBB P-gp was virtually identical to its potency to inhibit human P-gp in MDRI-transfected cells. Despite these excellent correlations, the study examined only one P-gp substrate-inhibitor pair. Therefore, studies in the rat and the human, with other P-gp substrates and inhibitors, are needed to determine if DDI at the rat BBB are predictive of those at the human BBB. This is particularly important for P-gp because it demonstrates allostery with multiple binding sites and therefore has the potential for substrate-dependent DDI 10; 11; 12; 13; 14; 15.
To assess if the above excellent agreements extend to other substrates, we determined the magnitude of P-gp-based DDI at the rat BBB between loperamide (Lop) or its metabolite, N-desmethyl Lop (dLop), and escalating CsA blood concentrations. In addition, we determined if the potency of CsA to inhibit P-gp at the rat BBB correlates with that obtained in MDR1-transfected cells. Using a similar study design as that of our 11C-verapamil-CsA PET study, Passchier et al., showed that 11C-Lop CNS distribution increased 110% when co-administered with a 10 mg/kg i.v. infusion of CsA 1. Therefore, this study provides us an opportunity to determine whether the rat is predictive of this DDI at the human BBB.
METHODS
Materials
Loperamide (Lop) was purchased from Sigma-Aldrich (St. Louis, MO). N-desmethyl Loperamide (dLop) was a kind gift from GlaxoSmithKline. Cyclosporine A (Sandimune, 50 mg/mL) was purchased from Abbott Laboratories (Chicago, IL). Lop was formulated as 5 mg/mL in 650 mg Cremophore® EL and 32.9% ethanol by volume. All other reagents were of the highest grade available from commercial sources.
Animals
Male Sprague Dawley rats (8-10 wks, ~300g) were purchased from Taconic Farms (Hudson, NY) and housed in a temperature- and humidity-controlled room with a 12-h light/dark cycle with free access to food and water. The experimental protocol was approved by the Institutional Animal Care and Use Committee of the University of Washington. All experimental procedures were conducted according to the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC, 1996).
Experimental protocol
Under isoflurane anesthesia (5% induction, 1~1.5% maintenance at 1.0 L/min), each animal was cannulated in both the left or right femoral artery and vein. Anesthesia was maintained throughout the experiment. The anesthesia plane and the condition of the animal were evaluated by a routine tail/toe pinching test, respiration rate and the palpebral reflex test. CsA (or saline) (0, 1.3, 2.3, 3.5, 5.0, 7.0, or 10.0 mg/kg in 0.1 mL) and Lop (3 mg/kg in 0.1 mL) were administered as an i.v. bolus, followed by a constant rate i.v. infusion of CsA plus Lop (0, 1.6, 3.0, 4.5, 6.5, 9.0, or 13.0 mg/kg of CsA plus 0.3 mg Lop/kg/h at the rate of 0.5 mL/h) via the femoral vein to achieve pseudo-steady-state blood CsA concentrations of 0, 2.7 (3.3), 4.2 (5.0), 6.0 (7.2), 8.3 (10), 12.0 (14.4), 15.0 (18.0) μM (μg/mL) respectively. CsA blood samples (~0.5 mL) were collected in heparinized tubes via the femoral artery at 0 (pre-CsA), 30, 45, and 60 min. Hematocrit in each blood sample (~50 μL) was determined immediately following each blood draw. Immediately following the 60-min. blood draw, the animal was sacrificed by decapitation and the brain harvested. This time point was chosen based on reported Lop pharmacokinetic studies in rats16; 17 along with our pilot study in rats. Collectively, the literature and our pilot study results indicate that this duration was adequate for Lop and its metabolite, dLop, to reach pseudo-equilibrium between the brain and plasma, and exhibit linear pharmacokinetics at the Lop, dLop plasma concentrations studied16; 17; 18. Blood CsA concentrations were determined within 24 h by LC/MS (Department of Laboratory Medicine at the University of Washington Medical Center), while the brain and plasma samples were stored at -20°C until analysis for Lop/dLop concentration by LC/MS.
Plasma and brain tissue analysis
Brain samples (0.4- 0.7 g) were homogenized with PBS (100 μL PBS per 100 mg brain). The plasma and brain homogenate (100 μL) samples were precipitated with 1:1 acetonitrile containing 500 ng/mL of saquinavir (internal standard), followed by centrifugation at 20,800 G. The supernatant was then injected (5-35 μl) onto an Agilent XDB-C18 reverse-phase column (2.1 × 50 mm, 5 μm, with an Agilent XDB-C18 guard column, 2.1 × 12.5 mm, 5 μm; Agilent Technologies, Santa Clara, CA) eluted at 0.25 mL/min with a gradient mobile phase consisting of mobile phase A, 0.1% acetic acid in water, and mobile phase B, 1:1 of methanol: acetonitrile. The gradient was adjusted linearly as follows: 65% A/35% B for the first 1 min, 5% A/95% B from 1 to 4 min, maintained at 5% A/95% B from 4 to 6.4 min, 65% A/35% B from 6.4 min to 6.5 min, and maintained at this composition from 6.5 to 7.5 min. The mass spectrometer was operated in atmospheric pressure-ionization-electro-spray (API-ES) mode (spray chamber: gas temp 350°C, Vcap (+) 3500 V). The standard curve contained 0.31 to 625 ng/mL Lop and dLop in plasma. Quality control samples of 1.2, 20 and 313 ng/mL Lop and dLop were prepared in both plasma and brain homogenate. The limit of detection for Lop and dLop in tissue and plasma samples was 0.6 ng/g or 0.6 ng/mL respectively.
Data Analysis
The brain:plasma ratio of Lop and dLop was adjusted for vascular contamination. We previously found that the brain vascular volume in the rat is 26.3 ± 10 μL/g 8. Using this value, we estimated the vascular content of Lop/dLop in each brain sample. Next, the brain Lop/dLop concentration for each animal was corrected for the corresponding contamination from the vascular Lop/dLop content. Using nonlinear regression (WinNonlin®; Pharsight Corporation), the Hill equation was fitted (using uniform weighting) to the percent increase in the brain:plasma ratio of Lop or dLop as a function of blood CsA concentration. Unless otherwise stated, data are presented as mean ± S.D. Analysis of variance, followed by Student's t-test was used to determine the statistical significance of the difference (p < 0.05) between experimental groups.
RESULTS
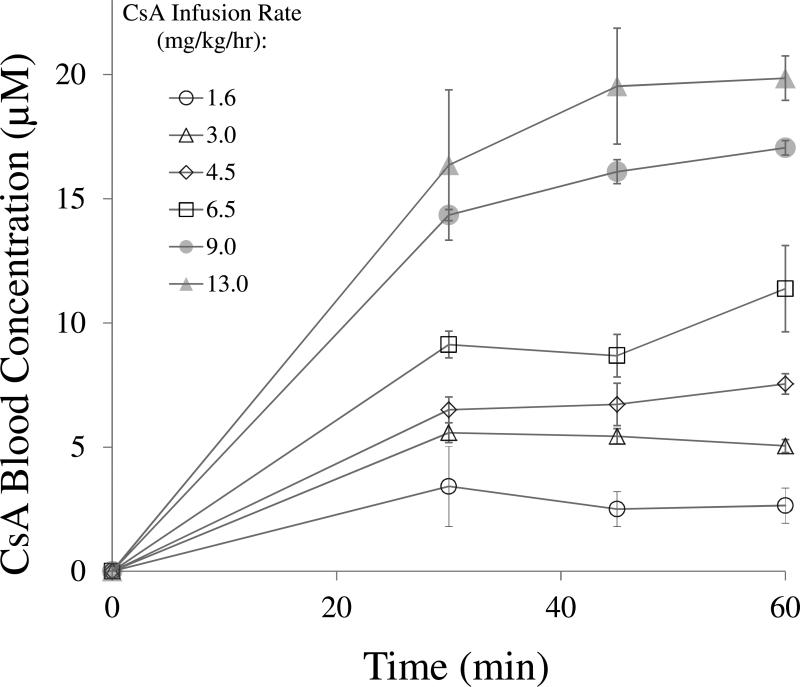
Except at the higher CsA infusion rates, the targeted pseudo-steady-state blood CsA concentrations were achieved before the experimental end point (60 min) (Fig. 1). The blood-CsA concentrations achieved at the higher targeted CsA groups (11.4 ± 1.7, 17.1 ± 0.3 and 19.9 ± 0.9 μM) differed from the expected values (8.3, 12.0 and 15.0 μM, respectively) most likely due to nonlinearity in the pharmacokinetics of CsA.
Fig 1.
Except at the higher CsA infusion rates, the expected pseudo-steady-state blood CsA concentrations (mean±SD of at least n=3) were achieved by 30 min after an i.v. loading dose of CsA followed by an i.v. infusion of CsA.
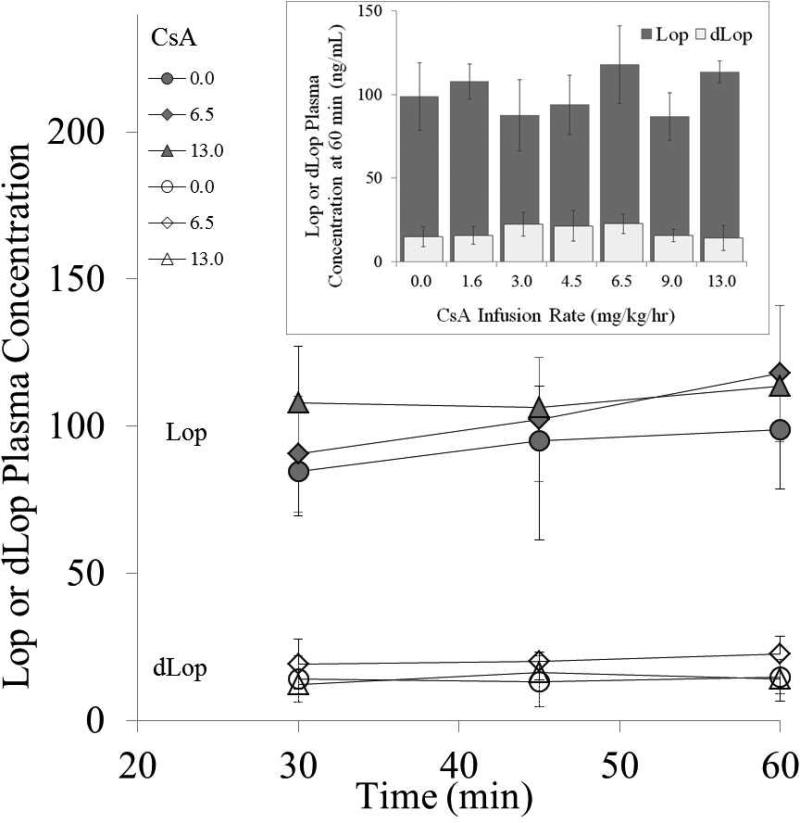
Targeted pseudo-steady-state plasma Lop and dLop concentrations were also achieved and maintained for the duration of the experiment (Fig. 2). The presence of CsA did not significantly affect the pseudo-steady-state plasma Lop or dLop concentrations (Student's t-test) at the experimental end point (60 min) (Fig. 2 insert).
Fig 2.
Target Lop (shaded circle, diamond and triangle) and dLop (open circle, diamond and triangle) pseudo-steady-state plasma concentrations (mean±SD of at least n=3) were achieved by 30 minutes and maintained thereafter. For clarity, only three CsA-infusion-rate groups are shown. Inset: Lop (shaded bar) and dLop (open bar) steady-state plasma concentrations (mean±SD at 60 min.) were not significantly affected by the presence of CsA (Student's t-test).
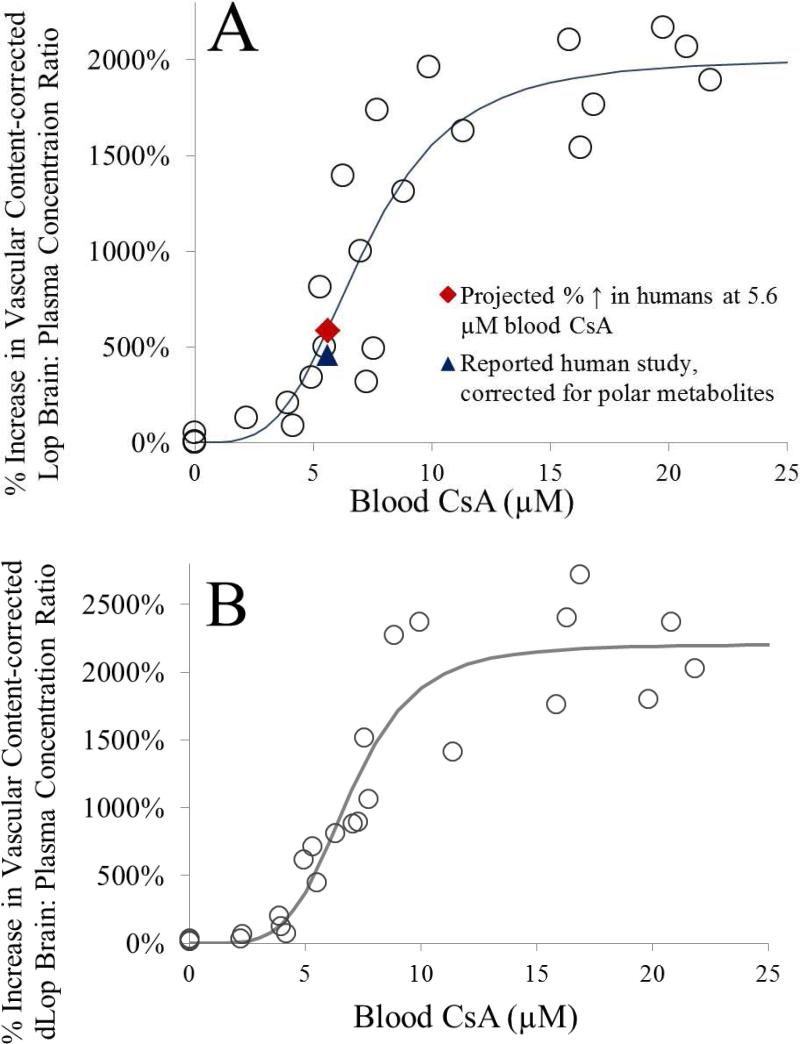
In the absence of CsA, the brain:plasma concentrations for Lop and dLop were 0.10 ± 0.02 (n=5) and 0.14 ± 0.04 (n=5), respectively. When corrected for vascular content, the brain:plasma concentrations for both Lop and dLop changed modestly to 0.07 ± 0.02 (n=5) and 0.11 ± 0.04 (n=5), respectively. With increasing CsA blood concentration, the percent corrected Lop and dLop brain:plasma concentration ratio increased in a sigmoidal fashion (Fig 3a and 3b). Fitting the Hill equation to the data yielded the following estimates (% CV of the estimate) for Lop and dLop respectively: Emax = 2000% (8.5%), EC50 = 7.1 μM (8.6%), γ = 3.7 (28.9%) and Emax = 2200% (1.3%), EC50 = 6.9 μM (0.4%), γ = 4.8 (1.2%) (Fig. 3).
Fig 3.
With increasing CsA-blood concentrations, the percent increase in the vascular-content corrected brain:plasma Lop (A) or dLop (B) concentration ratio, expressed relative to that in the control group (absence of CsA), increased as the blood-CsA concentration increased. (A) Fitting the Hill equation to the Lop data yielded the following estimates (% CV of the estimate): Emax = 2000% (8.5%), EC50 = 7.1 μM (8.6%), and γ = 3.7 (28.9%). For the reported PET study by Passchier et al. (estimated blood CsA concentration of 5.6 μM), the rat model predicted a 588% increase in the Lop brain:plasma concentration ratio (filled diamond) versus the observed 110% increase in total brain: plasma radioactivity. However, on correcting the latter for the labeled diffusible metabolites, the predicted increase in Lop brain:plasma concentration ratio (457%; filled triangle) was comparable with that predicted by the rat model. (B) Fitting the Hill equation to the dLop data yielded the following estimates (% CV of the estimate): Emax = 2200% (1.3%), EC50 = 6.9 μM (0.4%), and γ = 4.8 (1.2%). The consistency in CsA EC50 values between Lop, dLop and verapamil (EC50 7.9 μM) indicates that CsA inhibits P-gp in a substrate-independent manner.
DISCUSSION
Similar to the verapamil-CsA study8, vascular content correction had little effect on the magnitude of brain distribution of Lop or dLop in the absence of CsA. In addition, the maximum increase in the brain distribution of Lop (2000%) or dLop (2200%) produced by CsA was comparable to that of the CsA-verapamil interaction (2630%)8.
In order to compare our rat data to the human PET data of Passchier et al. 1, the blood CsA concentrations achieved in the human study should be available. Unfortunately they are not. Therefore, we estimated these concentrations based on our human 11C-verapamil-CsA DDI study. Since the i.v. infusion rate of CsA in the Passchier study was double that in our study, based on linear pharmacokinetics, we predicted that the average blood CsA concentration achieved in their study was 5.6 μM. At 5.6 μM blood CsA concentration, our model predicted a 588% increase in the brain distribution of Lop (Fig. 3A, shaded diamond). This estimated percent increase is much higher than the 110% observed in the CsA-Lop PET human study 1. However, this discrepancy between our rat study and the reported human study is likely due to significant metabolism of Lop in humans. In our rat study, we measured the unchanged Lop concentrations in the brain and plasma while in the human PET study; imaging is unable to distinguish between labeled Lop and its labeled metabolites. Thus, we estimated the brain concentration of the unchanged Lop in the PET study based on the following data. First, Passchier et al reported that 76% of the radioactivity in the plasma was due to 11C-Lop and 24% was due to its metabolites (presumably polar metabolites) 1. Second, the brain: plasma concentration ratio of the Lop polar metabolites in the mouse is ~0.54 16; 19; 20; 21. Assuming the same is true in humans and that in the absence of P-gp inhibition, the human brain: plasma ratio of 11C-Lop is similar to that in mice, one can correct the observed 110% increase for the presence of polar labeled metabolites in the human brain which would also be imaged in the PET study. When such a correction is made, the corrected but estimated increase in the 11C-Lop brain: plasma ratio at 5.6 μM CsA blood concentrations is 457%, a number similar to the value predicted by our rat data (588%, Figure 3A). Several assumptions were made in arriving at this metabolite-corrected estimate. A rigorous 11C-Lop PET study needs to be conducted in humans to test some of these assumptions. If our predictions are correct, then CNS toxicity of Lop may occur when P-gp at the human BBB is significantly inhibited. In a recent review, Vandenbossche and coworkers examined 595 post-market Lop case reports and 10 clinical Lop drug interaction studies 22 but found no evidence of clinically significant drug interactions with Lop. However, these studies may not have achieved unbound plasma concentration of the P-gp inhibitor/perpetrator that significantly inhibits P-gp at the human BBB.
CsA was equipotent in inhibiting the in vivo efflux of verapamil, Lop or dLop across the rat BBB (EC50 [mean±SD of the estimate] 7.2 ± 0.5, 7.1 ± 0.6 and 6.9 ± 0.4 μM and γ 3.7 ± 0.8, 3.7 ± 1.1, and 4.8 ± 0.6 respectively) 8. Although the CsA EC50 and γ appear to be independent of the substrate used, we have recently reported substrate dependence in a number of model P-gp inhibitors such as quinine and itraconazole 23 in an in vitro P-gp drug interaction study. In that study, depending on the probe substrate used (verapamil-bodipy or prazosin-bodipy), the the potency (EC50) of quindine, itraconazole and verapamil to inhibit P-gp differed by 20, 10 or 2-fold, respectively 23. Given these data from our laboratory and the reported variable sensitivity of dLop to P-gp modulators in vivo 24, additional animal studies using Lop as the substrate and quinidine or itraconazole as the inhibitor seems to be the next logical step to further investigate the substrate dependency of P-gp inhibition in vivo. A value of γ (Hill coefficient) >1 is suggestive of possible P-gp allosterism. This is especially plausible for P-gp given its large drug binding pocket and multiple drug binding sites10; 23; 24. However, other factors may play a role in in vivo studies in giving rise to a γ of > 1.
The comparable CsA Emax between the Lop-CsA, dLop-CsA, and verapamil-CsA studies suggest that the role of P-gp and other processes (e.g. passive diffusion) in the brain distribution of these three drug substrates is similar. However, if the drug substrate has a higher affinity for P-gp (i.e. lower brain distribution in the absence of P-gp activity) and/or if P-gp plays a larger role in preventing the brain distribution of that drug (vs. other processes such as diffusion), than the impact of inhibiting P-gp by CsA may be much greater on its brain distribution than that for Lop, dLop or verapamil. Based on a survey of the literature, P-gp appears to play a larger role in excluding nelfinavir from the brain. Nelfinavir has one of the largest increases in the brain:blood concentration ratio in mdr1a/b(-/-) vs. wild-type mice (3400% increase, versus 900% for verapamil) 5; 7. Thus nelfinavir seems a logical P-gp substrate for a future study on the maximum magnitude of change in the brain distribution of a P-gp drug in the presence of an inhibitor. Studying the CsA-nelfinavir interaction at the BBB will also provide another opportunity to determine if such an interaction is substrate dependent (i.e. CsA EC50).
For this study, our in vivo unbound CsA EC50 for Lop (calculated to be 0.50 ± 0.04 μM) was remarkably consistent with the in vitro unbound EC50 (0.78 ± 0.04 μM) reported by others25 (unbound EC50 value was computed using the reported CsA fraction unbound of 7% in the rat (Bernareggi and Rowland, 1991)). This is consistent with the finding in our prior in vitro-in vivo correlation study using verapamil-CsA as the P-gp substrate pair (CsA in vitro EC50 of 0.6 ± 0.3 μM vs. in vivo unbound EC50 of 0.47 ± 0.004 μM at the rat BBB26). The present data provide further support to the notion that inhibition of P-gp at the rat BBB is consistent with that observed with human P-gp expressed in LLCPK1-MDR1 cell line.
In summary, the above CsA-Lop interaction provides support to our conclusion based on our previous CsA-verapamil data that the rat is a promising model to predict P-gp based drug interactions at the human BBB. In addition, the potency (EC50) of CsA to inhibit P-gp appears to be substrate independent when Lop, dLop or verapamil are used as the substrates. Furthermore, there is a remarkable in vitro (LLCPK1-MDR1 cells) to in vivo correlation of the potency of CsA to inhibit P-gp. However, given the dearth of human data available, additional human studies with other substrate-inhibitor pairs are needed to further validate whether the rat, together with in vitro studies in LLCPK1 cells, can be used to predict the magnitude of P-gp drug interactions at the human BBB.
ACKNOWLEDGEMENT
This work was supported by NCRR Grant TL1 RR 025016, the ITHS TL1 Multidisciplinary Predoctoral Clinical Research Training Program (PHS2271) and NIH grants GM032165, and RCNS068404.
Abbreviations
- P-gp
P-glycoprotein
- BBB
blood-brain-barrier
- CsA
Cyclosporine A
- Lop
Loperamide
- dLop
N-desmethyl Loperamide
- PET
positron emission tomography
- DDI
drug-drug interaction
Footnotes
Recommended section: Absorption, Distribution, Metabolism, and Excretion
Supporting information available: this information is available free of charge via the Internet at http://pubs.acs.org/.
REFERENCES
- 1.Passchier J, Comley R, Salinas C, Rabiner E, Gunn R, Cunningham V, Wilson A, Houle S, Gee A, Laruelle M. Blood brain barrier permeability of [11C]loperamide in humans under normal and impaired P-glycoprotein function. J NUCL MED MEETING ABSTRACTS. 2008;49:211P. [Google Scholar]
- 2.Eyal S, Hsiao P, Unadkat JD. Drug interactions at the blood-brain barrier: fact or fantasy? Pharmacol Ther. 2009;123:80–104. doi: 10.1016/j.pharmthera.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schinkel AH, Wagenaar E, Mol CA, van Deemter L. P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest. 1996;97:2517–24. doi: 10.1172/JCI118699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hendrikse NH, Schinkel AH, de Vries EG, Fluks E, Van der Graaf WT, Willemsen AT, Vaalburg W, Franssen EJ. Complete in vivo reversal of P-glycoprotein pump function in the blood-brain barrier visualized with positron emission tomography. Br J Pharmacol. 1998;124:1413–8. doi: 10.1038/sj.bjp.0701979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim RB, Fromm MF, Wandel C, Leake B, Wood AJ, Roden DM, Wilkinson GR. The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J Clin Invest. 1998;101:289–94. doi: 10.1172/JCI1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choo EF, Kurnik D, Muszkat M, Ohkubo T, Shay SD, Higginbotham JN, Glaeser H, Kim RB, Wood AJ, Wilkinson GR. Differential in vivo sensitivity to inhibition of P-glycoprotein located in lymphocytes, testes, and the blood-brain barrier. J Pharmacol Exp Ther. 2006;317:1012–8. doi: 10.1124/jpet.105.099648. [DOI] [PubMed] [Google Scholar]
- 7.Choo EF, Leake B, Wandel C, Imamura H, Wood AJ, Wilkinson GR, Kim RB. Pharmacological inhibition of P-glycoprotein transport enhances the distribution of HIV-1 protease inhibitors into brain and testes. Drug Metab Dispos. 2000;28:655–60. [PubMed] [Google Scholar]
- 8.Hsiao P, Sasongko L, Link JM, Mankoff DA, Muzi M, Collier AC, Unadkat JD. Verapamil P-glycoprotein transport across the rat blood-brain barrier: cyclosporine, a concentration inhibition analysis, and comparison with human data. J Pharmacol Exp Ther. 2006;317:704–10. doi: 10.1124/jpet.105.097931. [DOI] [PubMed] [Google Scholar]
- 9.Sasongko L, Link JM, Muzi M, Mankoff DA, Yang X, Collier AC, Shoner SC, Unadkat JD. Imaging P-glycoprotein transport activity at the human blood-brain barrier with positron emission tomography. Clin Pharmacol Ther. 2005;77:503–14. doi: 10.1016/j.clpt.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL, Chang G. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718–22. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin C, Berridge G, Higgins CF, Mistry P, Charlton P, Callaghan R. Communication between multiple drug binding sites on P-glycoprotein. Mol Pharmacol. 2000;58:624–32. doi: 10.1124/mol.58.3.624. [DOI] [PubMed] [Google Scholar]
- 12.Martin C, Berridge G, Mistry P, Higgins C, Charlton P, Callaghan R. The molecular interaction of the high affinity reversal agent XR9576 with P-glycoprotein. Br J Pharmacol. 1999;128:403–11. doi: 10.1038/sj.bjp.0702807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin C, Berridge G, Mistry P, Higgins C, Charlton P, Callaghan R. Drug binding sites on P-glycoprotein are altered by ATP binding prior to nucleotide hydrolysis. Biochemistry. 2000;39:11901–6. doi: 10.1021/bi000559b. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro AB, Fox K, Lam P, Ling V. Stimulation of P-glycoprotein-mediated drug transport by prazosin and progesterone. Evidence for a third drug-binding site. Eur J Biochem. 1999;259:841–50. doi: 10.1046/j.1432-1327.1999.00098.x. [DOI] [PubMed] [Google Scholar]
- 15.Taub ME, Podila L, Ely D, Almeida I. Functional assessment of multiple P-glycoprotein (P-gp) probe substrates: influence of cell line and modulator concentration on P-gp activity. Drug Metab Dispos. 2005;33:1679–87. doi: 10.1124/dmd.105.005421. [DOI] [PubMed] [Google Scholar]
- 16.Miyazaki H, Nambu K, Matsunaga Y, Hashimoto M. Disposition and metabolism of [14C]loperamide in rats. Eur J Drug Metab Pharmacokinet. 1979;4:199–206. doi: 10.1007/BF03189427. [DOI] [PubMed] [Google Scholar]
- 17.Heykants J, Michiels M, Knaeps A, Brugmans J. Loperamide (R 18 553), a novel type of antidiarrheal agent. Part 5: the pharmacokinetics of loperamide in rats and man. Arzneimittelforschung. 1974;24:1649–53. [PubMed] [Google Scholar]
- 18.Kamali F, Adriaens L, Huang ML, Woestenborghs R, Emanuel M, Rawlins MD. Dose proportionality study of loperamide following oral administration of loperamide oxide. Eur J Clin Pharmacol. 1992;42:693–4. doi: 10.1007/BF00265940. [DOI] [PubMed] [Google Scholar]
- 19.Zoghbi SS, Liow JS, Yasuno F, Hong J, Tuan E, Lazarova N, Gladding RL, Pike VW, Innis RB. 11C-loperamide and its N-desmethyl radiometabolite are avid substrates for brain permeability-glycoprotein efflux. J Nucl Med. 2008;49:649–56. doi: 10.2967/jnumed.107.047308. [DOI] [PubMed] [Google Scholar]
- 20.Lazarova N, Zoghbi SS, Hong J, Seneca N, Tuan E, Gladding RL, Liow JS, Taku A, Innis RB, Pike VW. Synthesis and evaluation of [N-methyl-11C]N-desmethyl-loperamide as a new and improved PET radiotracer for imaging P-gp function. J Med Chem. 2008;51:6034–43. doi: 10.1021/jm800510m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshida K, Nambu K, Arakawa S, Miyazaki H, Hashimoto M. Metabolites of loperamide in rats. Biomed Mass Spectrom. 1979;6:253–9. doi: 10.1002/bms.1200060606. [DOI] [PubMed] [Google Scholar]
- 22.Vandenbossche J, Huisman M, Xu Y, Sanderson-Bongiovanni D, Soons P. Loperamide and P-glycoprotein inhibition: assessment of the clinical relevance. J Pharm Pharmacol. 2010;62:401–12. doi: 10.1211/jpp.62.04.0001. [DOI] [PubMed] [Google Scholar]
- 23.Zolnerciks JK, Booth-Genthe CL, Gupta A, Harris J, Unadkat JD. Substrate- and species-dependent inhibition of p-glycoprotein-mediated transport: Implications for predicting in vivo drug interactions. J Pharm Sci. 2011 doi: 10.1002/jps.22566. [DOI] [PubMed] [Google Scholar]
- 24.Moerman L, Wyffels L, Slaets D, Raedt R, Boon P, De Vos F. Antiepileptic drugs modulate P-glycoproteins in the brain: A mice study with (11)C-desmethylloperamide. Epilepsy Res. 2011;94:18–25. doi: 10.1016/j.eplepsyres.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 25.Corkill G, Turner D, Pufong B, Gill H, Fessey R, Dilworth C. In vitro evaluation of P-glycoprotein inhibition using loperamide as a probe substrate. 10th European ISSX Meeting. 2008 [Google Scholar]
- 26.Hsiao P, Bui T, Ho RJ, Unadkat JD. In vitro-to-in vivo prediction of P-glycoprotein-based drug interactions at the human and rodent blood-brain barrier. Drug Metab Dispos. 2008;36:481–4. doi: 10.1124/dmd.107.018176. [DOI] [PubMed] [Google Scholar]