Abstract
Non-viral transfection is a promising technique which could be used to increase the therapeutic potential of stem cells. The purpose of this study was to explore practical culture parameters of relevance in potential human mesenchymal stem cell (hMSC) clinical and tissue engineering applications, including type of polycationic transfection reagent, N/P ratio and dose of polycation/pDNA polyplexes, cell passage number, cell density, and cell proliferation. The non-viral transfection efficiency was significantly influenced by N/P ratio, polyplex dose, cell density, and cell passage number. hMSC culture conditions that inhibited cell division also decreased transfection efficiency, suggesting that strategies to promote hMSC proliferation may be useful to enhance transfection efficiency in future tissue engineering studies. Non-viral transfection treatments influenced hMSC phenotype, including the expression level of the hMSC marker CD105, and the ability of hMSCs to differentiate down the osteogenic and adipogenic lineages. The parameters found here to promote hMSC transfection efficiency, minimize toxicity, and influence hMSC phenotype may be instructive in future non-viral transfection studies and tissue engineering applications.
Introduction
Non-viral transfection has become an important approach in tissue engineering applications, as it allows for forced overexpression of specific proteins that influence cell behavior. Protein overexpression is particularly important in controlling stem cell processes and products. For example, stem cells have been transfected to influence cell survival (Jo, et al., 2007, Song, et al., 2005), differentiation (Dragoo, et al., 2003), telomerase activity (Poh, et al., 2005), therapeutic protein production (Wang, et al., 2009), and new tissue formation (Huang, et al., 2005, Madry, et al., 2002, Shea, et al., 1999). Therefore, the non-viral transfection of stem cells could enable new approaches in tissue engineering to meet key therapeutic needs.
Human mesenchymal stem cells (hMSCs) may be useful targets for transfection, as they differentiate into multiple clinically relevant cell types including bone, fat, and cartilage (Pittenger, et al., 1999) and their lineage-specific differentiation of cells can be strongly influenced by cellular gene expression (Friedenstein, et al., 1970, Pittenger, Mackay, Beck, Jaiswal, Douglas, Mosca, Moorman, Simonetti, Craig and Marshak, 1999). For example, forced overexpression of the cytokines bone morphogenetic protein-2 (Hosseinkhani, et al., 2006) or insulin-like growth factor-1 (Koch, et al., 2005) via DNA delivery has been shown to induce osteogenic differentiation of hMSCs. In addition, Garcia and coworkers have shown that forced overexpression of the regulatory gene Cbfa1/Runx2 promotes osteogenic differentiation of MSCs (Byers and Garcia, 2004). Forced overexpression of the transcription factor Sox9 has also been used to promote chondrogenesis by MSCs (Tsuchiya, et al., 2003). However, in each of these applications the potential utility of non-viral transfection approaches is critically dependent on the MSC transfection efficiency, as well as practical issues such as cost and scalability.
A variety of approaches can be used to modulate the non-viral transfection efficiency of hMSCs. For example, 25kDa branched Polyethyleneimine (PEI), Lipofectamine 2000, poly-l-lysine-palmitic acid, poly-l-lysine, cationized dextran, Fugene, and Effectene have been explored as polycationic transfection reagents in hMSC culture (Baksh, et al., 2007, Clements, et al., 2006, Clements, et al., 2007a, Farrell, et al., 2007, Jo, Nagaya, Miyahara, Kataoka, Harada-Shiba, Kangawa and Tabata, 2007). A common theme in these approaches is the use of polycationic vectors to condense plasmid DNA (pDNA) into “polyplexes”, neutralize DNA’s charge, and promote endosomal escape into the cytoplasm. In these various studies, the transfection efficiency of hMSCs - defined as the number of cells expressing a fluorescent reporter divided by the total number of cells - is typically less than 20% unless there are multiple rounds of transfection or other additives, like chloroquine (Clements, et al., 2007b), are used to increase the transfection efficiency. In addition, it is likely that the efficiency of DNA delivery to cells is highly context-dependent, and cell culture parameters such as cell density, cell proliferation rate, and media components may strongly influence non-viral DNA uptake and expression (Audouy, et al., 2000).
The choice of non-viral transfection parameters for tissue engineering applications must be both efficient and practical to be clinically relevant. Effective non-viral transfection in hMSC-based biotechnology applications, such as large scale cell production for drug screening or cell therapy, will likely require reagents and culture conditions that are low cost and scalable. Currently, only PEI- and calcium phosphate-mediated transfection are considered scalable and cost effective for liter-scale cultures (Durocher, et al., 2002). For example, PEI has been used to transfect mammalian cells in 20 liter WAVE bioreactors (Haldankar, et al., 2006), 50L orbital shaker bioreactors (Stettler, et al., 2007), and 100L stirred tank bioreactors (Tuvesson, et al., 2008). Also, PEI costs 4000–5000 times less per liter than several commercially available transfection reagents (Morrow, 2008). PEI has been used in clinical trials, which suggests that it could be used for clinical applications as well as other biotechnology applications (Anwer, et al., 2010). Together, these previous studies suggest that PEI is one of the few practical non-viral vectors currently available for large scale transfections for tissue engineering applications.
Optimized cell culture conditions will be critical for the success of any hMSC-based approach in which hMSC proliferation, transfection efficiency, and multipotency must be tightly regulated. Therefore, this study was designed to explore standard cell culture parameters that may influence hMSC transfection, with a particular emphasis on PEI as a transfection reagent for the practical reasons outlined in the previous paragraph. The particular focus is on parameters that can be easily adapted for tissue engineering applications, including the cell density, the cell passage number, the type of non-viral vector (60kDa branched PEI, Superfect, and JetPEI), the N/P ratio (the ratio of primary amines on PEI to phosphate groups on pDNA), and dose of PEI:pDNA polyplexes. Results indicate that each parameter studied influences hMSC transfection and viability. We also observed differences in the multipotency of hMSCs after transfection in preliminary experiments using immunostaining for a common hMSC cell surface marker, CD105, and by observing their differentiation down the osteogenic and adipogenic lineages in vitro.
Materials and Methods
Cell Culture
hMSCs were purchased from Cambrex (East Rutherford, New Jersey) and grown in Basal Media [Dulbecco’s Modified Eagle Media (Mediatech Inc, Herdon, VA) supplemented with 1% Penicillin/Streptomycin (Gibco, Carlsbad, California) and 10% Mesenchymal Stem Cell Qualified Fetal Bovine Serum (Invitrogen, Carlsbad, California)]. 24 hrs. prior to transfection hMSCs were passaged using standard cell culture techniques and 5000 cells/cm2 (unless otherwise noted) were plated in 1ml of media in wells of a 24 well plate (BD Biosciences, San Jose, CA). Four cell culture wells per condition were prepared, treated, and analyzed. The values on graphs represent means and standard deviations. Student’s T-tests were used to assess differences between individual data points, and comparisons with p<0.05 were considered statistically significant.
Transfection Methods
PEI stock solutions (Sigma. St. Louis, MO) were formulated by diluting PEI in ultrapure H2O (18MΩ resistance) to a final concentration of 10μM unless otherwise indicated. The pH of the solution was adjusted to 7.4 by adding 2N HCl (Fisher Scientific, Fairlawn, NJ). The solution was filtered through a 0.22μM pore size PTFE Membrane (Millipore, Bedford, MA). Superfect (Qiagen), and JetPEI (Polyplus Transfection, New York, New York) were used per manufacturer’s recommendations with a dose of 1μg/well plasmid DNA (pDNA). The pDNA used throughout this study was pEGFP-N1 (Clontech, Mountain View, CA) which was acquired from the Waisman Clinical Biomanufacturing Facility (Madison, WI). PEI and pDNA were added to separate containers of 150mM sterile NaCl and allowed to equilibrate for 5 minutes. The N/P ratios were calculated using the technique outlined by Boussif and colleagues (1ul of 10μM 60kDa PEI stock solution= 10nmol primary amine/1ug of pDNA=3nmol phosphate) (Boussif, et al., 1995). The polyplex solutions were then pipetted gently 5 times and centrifuged for 1 second. The pDNA and transfection reagents were incubated for 25 minutes to allow for complexation to occur. During this incubation, hMSCs were washed with phosphate-buffered saline (PBS) and fresh serum-containing media was added. After the incubation period, the pDNA:transfection agent polyplexes were added to the cell culture media.
hMSC Transfection Efficiency and Cytotoxicity
Transfected cells were observed at predetermined times on an Olympus IX51 epifluorescent microscope (Olympus, Tokyo, Japan). Digital micrographs were captured using a Hamamatzu C4742-05 CCD Camera (Hirakuchi, Hamakita-City, Japan) and processed using Simple PCI software (Hamatzu, Hamakita-City, Japan). The transfection efficiency of a treatment was quantified using a method that involved counting the number of GFP+ cells on the cell culture substrate and dividing by the total number of cells measured using the CellTiter-Blue Assay (Promega, Madison, WI) on a Synergy HT plate reader (Biotek, Santa Barbara, CA). The CellTiter-Blue Assay works on the principle that metabolically active cells convert a non-fluorescent dye, resazurin, into a fluorescent end product resorufin which can be measured using a fluorimeter. Transfected cell viability was also evaluated using the CellTiter-Blue Assay (Promega, Madison, WI).
Characterizing the Influence of hMSC Division on Transfection
Experiments to characterize the influence of hMSC division on transfection involved inhibiting division using either a soluble inhibitor (mitomycin C, Sigma, Saint Louis, MO) or gamma radiation. First, a preliminary experiment was performed to determine the appropriate dose of mitomycin C needed to inhibit division, but not substantially decrease hMSC viability. The mitomycin C dose was optimized by plating 3000 hMSCs and then treating them with 0–100μg/ml mitomycin C for 0–6 hrs. After treatment with mitomycin C or treatment with 6000 rad gamma radiation (Cs-137 source), transfection procedures were conducted as previously described.
Immunostaining
The CD105 expression of hMSCs was evaluated using a mouse monoclonal CD105 antibody conjugated to Phycoerythrin (Abcam, Cambridge, MA) using standard immunocytochemistry techniques. Vectorshield DAPI (Vector Labs, Burlingame, CA) was used to stain DNA using manufacturer’s recommendations. CD105 Antibody-Phycoerythrin fluorescence intensity was quantified by measuring fluorescence emission of >50 cells per culture condition with ImageJ software (Freeware, NIH, Bethesda, MD).
Differentiation Protocols
Osteogenic Differentiation: hMSCs were grown to different densities (30% confluency was considered low density, 70% confluency was considered high density) in hMSC growth media. After the desired density was reached, the hMSCs were washed with osteogenic induction media [Basal Media + 0.1uM Dexamethasone, 10mM β-Glycerol Phosphate, and 50uM Ascorbic Acid 2-Phosphate (Cambrex, East Rutherford, NJ) with 2mM L-Glutamine (Hyclone, Logan, Utah), and 10% MCGS Serum (Cambrex, East Rutherford, NJ)]. The osteogenic induction medium was replaced every three days. Alkaline Phosphatase Staining was performed using manufacturer’s instructions (Genehunter Corporation, Nashville, TN) 7 days post osteogenic induction and Alizarin Red Staining (Acros, Geel, Belgium) was performed using standard cytochemistry techniques.
Adipogenic Differentiation: hMSCs were grown to different densities (30% confluency was considered low density, 100% confluency was considered high density) in hMSC basal media which was then replaced with Adipogenic Induction Media [basal media + 1μM Dexamethasone (MP Biomedicals, Solon, OH), 10μg/ml recombinant human insulin (MP Biomedicals, Solon, OH), and 0.5mM 3-isobutyl-1-methyl xanthine (Sigma-Aldrich, St. Louis, MO)] for 48 hrs. After the 48 hr. induction, the hMSCs were washed with PBS and replaced with basal media. hMSCs were stained with Oil Red O 14 days post induction using standard cytochemistry techniques.
Results
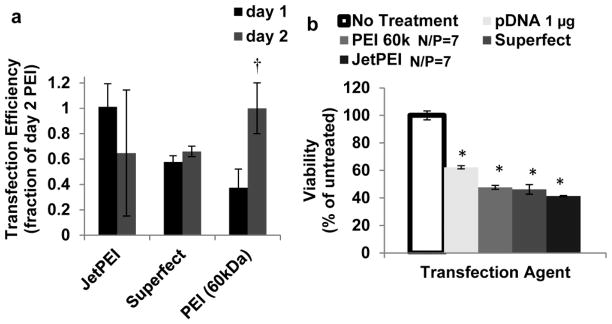
Non-viral transfection efficiency and viability of hMSCs were similar for each transfection reagent tested (Fig. 1). Naked pDNA alone was unable to transfect hMSCs and induce Green Fluorescent Protein expression (GFP+) (data not shown). For comparison, each transfection efficiency was normalized to N/P=7 60kDa branched PEI’s efficiency after 48 hr. (2.9±0.6% of total cells GFP+). Superfect, JetPEI, and 60kDa branched PEI produced similar transfection efficiencies after 24 hr. (1.01±0.18, 0.58±0.05, and 0.37±0.15, of 48 hr. N/P=7 60kDa branched PEI respectively) and 48 hr. (0.65±0.49, 0.66±0.042, and 1.00±0.20, of 48 hr. N/P=7 60kDa branched PEI respectively) (Fig. 1a). It is significant to note that the image-based method used to measure transfection efficiency was similar and slightly more conservative than the transfection efficiency measurement acquired using flow cytometry (Fig. S1). Each transfection procedure decreased hMSC viability over 48 hrs. compared to untreated hMSCs (Fig. 1b).
Fig. 1.
Effect of transfection reagents on transfection efficiency and hMSC viability. a) Transfection efficiency of passage 6 hMSCs with pDNA complexed with JetPEI, Superfect, or 60k branched PEI 1 and 2 days post transfection normalized to PEI’s transfection efficiency after 2 days. † Denotes significant difference between day 1 and day 2 transfection efficiencies. * Denotes significant increase compared to 60kDa branched PEI (t test, p<0.05). b) Normalized viability of passage 6 hMSCs transfected with pDNA alone or complexed with 60kDa branched PEI, Superfect, or JetPEI 48 hours post transfection. * Denotes significant difference with untreated hMSCs (t test, p<0.05)
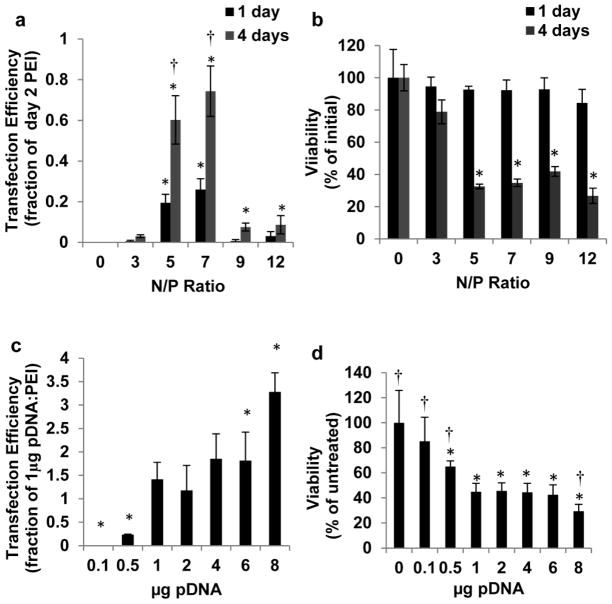
60kDa branched PEI was explored in greater detail as a transfection reagent since it is well-characterized, commercially available, low cost, and chemically modifiable. Increasing the N/P ratio from 0–7 in PEI/pDNA solutions resulted in higher hMSC transfection efficiencies. 4 days post treatment the highest transfection efficiencies were produced with N/P ratios of 5 (0.60±0.12 of 48hr. N/P=7 60kDa branched PEI) and 7 (0.74±0.12 of 48hr. N/P=7 60kDa branched PEI). Increasing the N/P ratio greater than 7 led to decreasing transfection efficiencies (Fig. 2a). Increasing the N/P ratio from 0–12 had no significant effect on hMSC viability 24 hrs. post treatment. However, N/P ratios greater than 5 significantly decreased cell viability 48 hrs. post treatment (Fig. 2b).
Fig. 2.
Characterization of PEI:pDNA polyplex-mediated hMSC transfection. a) Normalized transfection efficiency of passage 6 hMSCs transfected with 60kDa branched PEI:1 μg pDNA polyplexes formed at different N/P ratios. * Denotes significant difference compared to a N/P ratio of 0. † denotes significant difference in transfection efficiency between day 1 and 4 post transfection (t test, p<0.05). b) Viability of passage 6 hMSCs transfected with 60kDa branched PEI:1 μg pDNA polyplexes formed at N/P ratios from 0–12 one and four days post transfection. * Denotes significant difference compared to a N/P ratio of 0 (t test, p<0.05). c) Normalized transfection efficiency of passage 6 hMSCs transfected with 0–8 μg 60kDa branched PEI:pDNA polyplexes formed at a N/P ratio of 7. * Denotes significant difference compared to 1μg pDNA (t test, p<0.05). d) Normalized viability of passage 6 hMSCs transfected with 60kDa branched PEI:pDNA polyplexes with 0–8μg pDNA. † Denotes significant difference with 1μg pDNA. * Denotes significant difference with 0μg pDNA (t test, p<0.05)
There was a clear trade-off between transfection efficiency and cell viability as the PEI/pDNA polyplex concentration was varied. Increasing polyplex concentrations produced higher hMSC transfection efficiencies, and hMSCs treated with 8μg/ml pDNA polyplexes (N/P=7) produced the greatest transfection efficiency (3.28±0.41 of 48 hr. N/P=7 60kDa branched PEI) (Fig. 2c). However, 8μg/ml pDNA polyplexes (N/P=7) also reduced hMSC viability to 29.4±5.5% when compared to untreated cells. 1, 2, and 4 μg/ml pDNA polyplexes produced statistically equivalent transfection efficiencies and viability (Fig. 2c–d). Although doses of 0.1 and 0.5 μg/ml PEI/pDNA polyplexes were less toxic to hMSCs, their transfection efficiencies were significantly lower than higher doses (Fig. 2c–d).
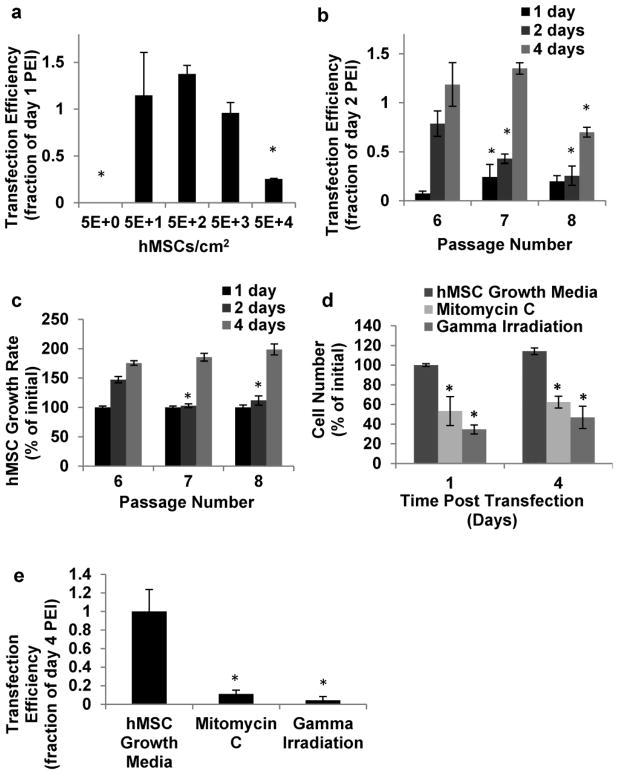
The seeding density of hMSCs in culture and the hMSC passage number significantly influenced transfection via PEI/pDNA polyplexes. Cell seeding densities of 50–5000 hMSCs/cm2 had the highest transfection efficiencies 24 hrs. post treatment (Fig. 3a). Passage 7 (P7) hMSCs had the highest transfection efficiency 24 hrs. after treatment, while P6 hMSCs had the highest transfection efficiency 48 hrs. post treatment, and both P6 and P7 hMSCs had higher transfection efficiencies than P8 hMSCs 96 hr. post treatment (Fig. 3b). To determine whether passage-dependent differences in transfection efficiency may be due to differences in cell proliferation rate, we also measured the growth rate of each hMSC passage number. Passage 7 and 8 hMSCs had a slower growth rate 48 hrs. post seeding when compared to passage 6 hMSCs(p<0.05). However, the numbers of passage 6, 7, and 8 hMSCs in culture were not significantly different 96 hrs. post seeding (Fig. 3c).
Fig. 3.
Characterization of the cell culture parameters that affect transfection efficiency when hMSCs were treated with 1μg pDNA complexed with 60kDa PEI at an N/P ratio of 7. a) Normalized transfection efficiency of passage 6 hMSCs seeded at a density of 5-5*104 hMSCs/cm2. * Denotes significant difference from 5*102 hMSCs/cm2 (t test, p<0.05). b) Normalized transfection efficiency of passage 6–8 hMSCs. * Denotes significant difference from passage 6 hMSC’s (t test, p<0.05). c) Normalized growth rate of transfected passage 6–8 hMSCs. * Denotes significant difference from passage 6 hMSCs (t test, p<0.05). d) Normalized cell number of hMSCs that were not treated, treated with mitomycin C, or treated with gamma radiation. * Denotes significant difference from untreated hMSCs (t test, p<0.05). e) Normalized transfection efficiency of hMSCs that were untreated, treated with mitomycin C, or treated with gamma radiation. * Denotes significant difference from untreated hMSCs (t test, p<0.05)
To further characterize the potential influence of hMSC proliferation rate on transfection efficiency, we observed transfection in PEI/pDNA-containing hMSC cultures in which proliferation was inhibited by either a pharmacological inhibitor (mitomycin C) or gamma irradiation. Mitomycin C inhibited hMSC proliferation after 2, 4, and 6 hr. of exposure when its concentration was ≥11.1 μg/ml. Greater concentrations of mitomycin C were cytotoxic, as indicated by a decrease in cell viability within 2 hr. of mitomycin C treatment (Fig. S2), and we therefore used 11.1 μg/ml mitomycin C in transfection studies. Notably, this dose was similar to the 10μg/ml mitomycin C used in a previous study to inhibit hMSC proliferation (McBeath, et al., 2004). In a separate set of experiments we used 6000 rad gamma irradiation as an alternative mechanism to inhibit hMSC proliferation (Fig. 3d–e), and this dose was identical to the 6000 rad dose used in a previous study to inhibit fibroblast division (Iuchi, et al., 2006). Mitomycin C and gamma irradiation each slowed the rate of hMSC proliferation 24 hr. and 96 hr. post treatment, but did not produce a decrease in cell number due to cell death (Fig. 3d). Importantly, hMSCs treated with mitomycin C and gamma irradiation had transfection efficiencies significantly lower than hMSCs cultured in growth media 96 hr. post treatment (Fig. 3e).
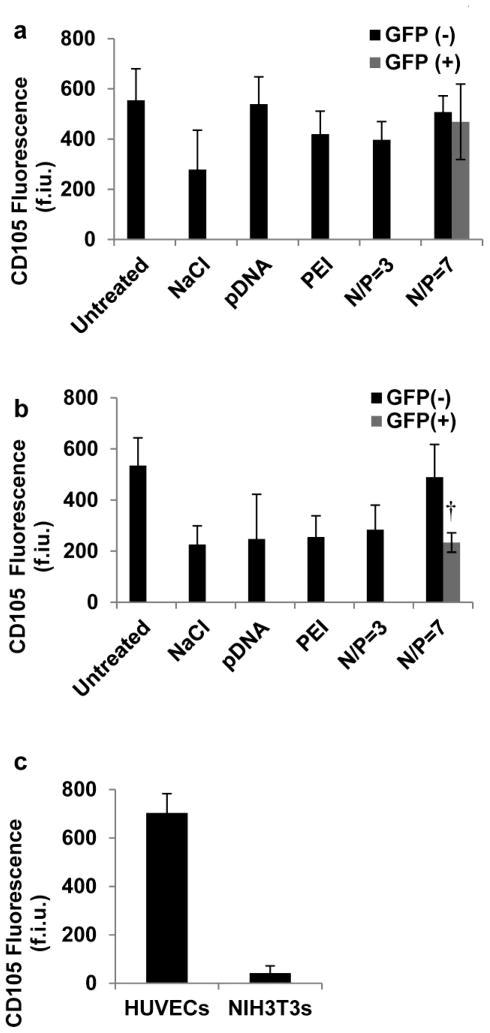
Expression of CD105, a cell surface marker associated with multipotent hMSCs, was significantly decreased at particular timepoints after transfection treatment. CD105 expression was initially unaffected by transfection treatment, as measured by immunostaining 24 hrs. post treatment (Fig. 4a). 48 hrs. post treatment CD105 expression was significantly reduced when treated with either 150mM NaCl, 1μg/ml pDNA, N/P=7 PEI without plasmid DNA, or PEI/pDNA N/P= 3 compared to untreated hMSCs. In addition, the CD105 expression of transfected EGFP+ hMSCs (treated with an N/P=7) was significantly less than that of EGFP− hMSCs in the same cultures (Fig. 4b). As expected, HUVECs had strong CD105 fluorescence (703±80 f.i.u.) and served as a positive control, while NIH3T3 cells had weak CD105 staining (42±30 f.i.u.) and served as a negative control (Fig. 4c) (Fonsatti, et al., 2003).
Fig. 4.
CD105 expression of hMSCs. Quantification of cells that did not express GFP (GFP−) and cells that did express (GFP+) CD105-Phycoerythrin fluorescence intensity in different culture conditions on day a) 1 and b) 2. c) Quantification of HUVEC and NIH3T3 CD105-Phycoerythrin fluorescence intensity. †denotes significant difference in CD105 fluorescence intensity compared to GFP− hMSCs (t test, p<0.05)
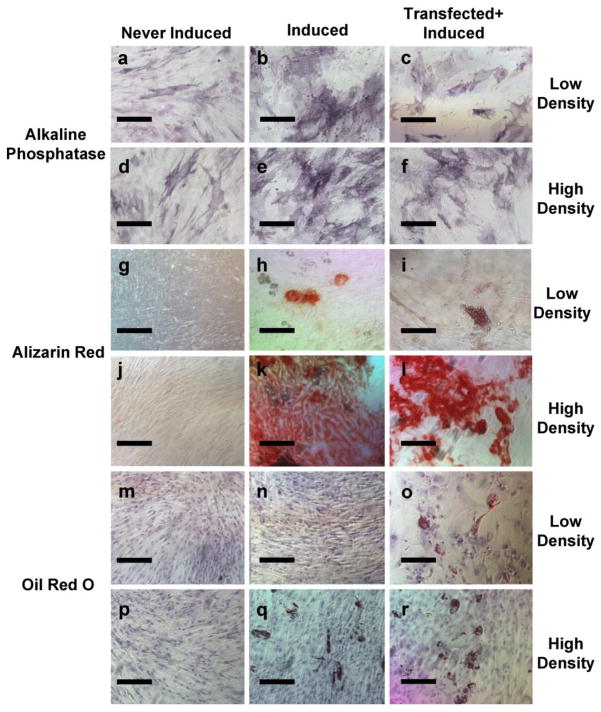
Transfection treatment and cell density may alter hMSC differentiation potential down the osteogenic and adipogenic lineages. hMSCs that were not cultured in osteogenic induction medium did not show positive Alizarin Red staining or enhanced Alkaline Phosphatase staining, as expected. hMSCs cultured in osteogenic induction medium showed enhanced alkaline phosphatase staining, and hMSCs treated with 1μg pDNA complexed with 60kDa PEI at an N/P ratio=7 resulted in less alkaline phosphatase staining than untreated hMSCs, whether they were induced to differentiate at low or high cell densities (Fig. 5a–f). Treatment with 1μg pDNA complexed with 60kDa PEI at an N/P ratio=7 did not change the amount of Alizarin Red staining, regardless of the hMSC density (Fig. 5g–l).
Fig. 5.
Preliminary insights into differentiation potential of hMSCs after transfection. a–f) Alkaline Phosphatase-stained hMSCs that had been either never induced to differentiate, induced to differentiate, or transfected and induced to differentiate at low or high cell densities. g–l) Alizarin Red-stained hMSCs that had been either never induced to differentiate, induced to differentiate, or transfected and induced to differentiate at low or high cell densities. m–r) Oil Red O-stained hMSCs that had been either never induced to differentiate, induced to differentiate, or transfected and induced to differentiate at low or high cell densities. Scalebar denotes 20μm
hMSCs that were never cultured in adipogenic induction media had no Oil Red O staining, as expected. hMSCs treated with 1μg pDNA complexed with 60kDa PEI at an N/P ratio=7 showed positive Oil Red O staining, whether they were induced to differentiate at low or high cell densities. Untreated hMSCs did not stain positively for Oil Red O when they were induced to differentiate at low seeding densities, but did stain positively for Oil Red O when they were induced to differentiate at high hMSC seeding density (Fig. 5m–r). Taken together, these results suggest that treatment of hMSCs with 1μg pDNA complexed with 60kDa PEI at an N/P ratio=7 influences their differentiation down the osteogenic and adipogenic lineages.
Discussion
Stem cell-based applications have design requirements that instruct the choice of an appropriate transfection technique. For example, tissue engineering (Palsson and Bhatia, 2004) and drug screening (Eglen, et al., 2008) approaches can each require billions of cells, and often include multiple distinct cell types. Therefore, the cell source for many tissue engineering applications should be capable of proliferating in culture and differentiating into multiple somatic cell types. In view of this requirement, hMSCs were chosen as the cell source in this study since they can be readily expanded in culture and can differentiate into multiple mesenchymal cell types. Toward that end, we examined the influence of a series of parameters on hMSC transfection efficiency and viability, including cell culture parameters (cell seeding density, cell passage number, and cell division) and polyplex properties (polyplex N/P ratio, and polyplex dose). Taken together, our results indicate that each of these parameters significantly influence hMSC transfection efficiency and viability, and our initial analysis of hMSC markers and differentiation potential suggests that transfection treatment may also influence hMSC phenotype.
hMSC seeding density, proliferation rate, and passage number had a significant impact on hMSC transfection efficiency and viability. The decrease in transfection efficiency at low hMSC seeding densities (Fig. 3a) could be attributed to the high dose of PEI:pDNA per number of cells, leading to a high proportional decrease in hMSC viability. The decrease in transfection efficiency at high cell seeding densities could be attributed in part to a decrease in hMSC division rate due to contact inhibition, which has been observed in previous studies (Lucarelli, et al., 2003). Importantly, hMSC proliferation also influenced transfection efficiency when probed more directly, as transfection efficiency was significantly reduced when hMSC proliferation was inhibited with the pharmacological inhibitor mitomycin C or gamma radiation (Fig. 3d+e). It has been hypothesized that pDNA only diffuses into the nucleus when the nuclear membrane breaks down during mitosis (Brunner, et al., 2000, Kunath, et al., 2003, Tseng, et al., 1999), and our current study provides supporting evidence that cell division may be necessary for hMSC transfection. Thus, we can hypothesize that in future large scale transfections for tissue engineering applications, culture conditions that promote sustained high rates of hMSC division may provide optimal conditions for non-viral transfection. Notably, hMSC’s have been shown to divide for over 10 passages using standard cell culture techniques (Bruder, et al., 1997), and work has begun on the robotic passaging of hMSCs for large scale biotechnology applications (Thomas, et al., 2007, Thomas, et al., 2008). Automation of cell culture and real time data processing of these cultures may ultimately enable optimization of hMSC division rates and, in turn, optimization of non-viral transfection efficiency.
The relatively high hMSC passage numbers used in this study were chosen based on the need for large numbers of hMSCs in tissue engineering, and on previous studies that have demonstrated multipotency of hMSCs at higher passage number. Bruder et al. serially passaged hMSC’s to quantify growth rates and osteogenic potential and found no decrease in growth rate between P6 and P8. P6–P8 hMSC’s were also able to upregulate their alkaline phosphatase expression greater than 10 fold when stimulated with osteogenic media (Bruder, Jaiswal and Haynesworth, 1997). Therefore, in this manuscript hMSC division rates, transfection efficiency, and differentiation potential were characterized at P6–P8. P7 and P8 hMSCs had a slightly lower cell number than P6 hMSCs after 48 hrs. in culture, but by 96 hrs. there were no significant differences in hMSC number when comparing the three passages (Fig. 3c). These results are consistent with those in previous studies, in which P1, P4, and P7 hMSCs had similar growth rates for up to 10 days in culture (Bruder, Jaiswal and Haynesworth, 1997). Although 4 days post seeding the passage number had no significant effect on hMSC proliferation, there was still a significant decrease in transfection efficiency between P6 and P8 hMSCs (Fig. 3b). hMSC transfection efficiency was similarly shown to decrease in with increased passage numbers in a previous study, in which a polyamidoamine dendrimer was used as a non-viral vector (Santos, et al., 2009). Further research will be necessary to characterize the mechanism dictating the decrease in transfection efficiency with increased hMSC passage numbers.
It is noteworthy that the PEI-mediated hMSC transfection efficiencies measured in this study are low (0–10%), but consistent with the MSC transfection efficiencies observed in this study (Fig. 1) and previous studies using other non-viral vectors (Baksh, Yao and Tuan, 2007, Clements, Incani, Kucharski, Lavasanifar, Ritchie and Uludag, 2007a, Farrell, Pepin, Kucharski, Lin, Xu and Uludag, 2007). Therefore, biotechnology applications that require higher transfection efficiencies may benefit from alternative techniques, such as electroporation or viral transduction. 70% hMSC transfection efficiencies have been reported using electroporation-based methods (Baksh, Yao and Tuan, 2007), and viral vectors have induced greater than 80% hMSC transfection efficiencies (Love, et al., 2007). However, high transfection efficiencies may not be necessary in all applications. For example, Park et al. encapsulated hMSCs with PEI/pDNA polyplexes encoding bone morphogenetic protein-7 (BMP-7) in a chitosan-alginate hydrogel and demonstrated that the BMP-7 produced by a subset of hMSCs could induce mineralized tissue formation in the hydrogel matrix (Park, et al., 2007). Other similar cell therapy approaches have used non-viral vectors to induce MSCs to produce glial cell line-derived neurotrophic factor (Bolliet, et al., 2008) and endostatin (Sun, et al., 2009) at levels that are likely to have physiologic effects. These previous findings indicate that some tissue engineering applications may indeed benefit from non-viral gene delivery, and that the parameters we explore in the current manuscript may be useful to enhance non-viral transfection efficiency in these applications.
Also consistent with previous reports, hMSC viability was significantly decreased by the various polycationic transfection reagents (Clements, Incani, Kucharski, Lavasanifar, Ritchie and Uludag, 2007b, Gwak and Kim, 2008, Saraf, et al., 2008) (Fig. 1). The mechanism of polycation toxicity is not well understood (Wiethoff and Middaugh, 2003), but cell signals associated with necrosis have been observed from liver (Tousignant, et al., 2000) and muscle cells (Brazeau, et al., 1998) after PEI-mediated transfection. As the different transfection reagents explored had similar transfection efficiencies, we decided to characterize PEI-mediated transfection in further detail for multiple reasons. First, PEI has been extensively studied and has a known chemical structure that is amenable to covalent modification. In addition, despite the widespread availability of many different polycationic transfection agents, only PEI has been considered economical and available for the liter-scale cultures needed for many biotechnology applications (Geisse, 2009). We particularly chose 60kDa branched PEI as a representative PEI, as branched PEIs of 10kDa-1.6MDa have been used widely (Fischer, et al., 1999, Godbey, et al., 1999), and lower molecular weight PEIs within this range have been predominantly used in recent studies (Choosakoonkriang, et al., 2003). Taken together, these findings suggest that PEI may be a particularly suitable vector for stem cell-based applications, and this reagent was therefore explored in detail in this study.
Experiments in which the formulation and dose of PEI:pDNA polyplexes were varied demonstrated a trade-off between transfection efficiency and cell viability, and revealed conditions that may be optimal for hMSC transfection (Fig. 2). In general, the hMSC transfection efficiency was decreased when PEI exceeded a high enough concentration to achieve measurable transfection (Fig. 2b), and this phenomenon has been observed with other cell lines in previous studies (Boussif, Lezoualc’h, Zanta, Mergny, Scherman, Demeneix and Behr, 1995, Horbinski, et al., 2001, Shin, et al., 2005). The mechanism behind this optimum range of polyplex N/P ratios can be attributed to previous observations that at low N/P ratios the PEI:pDNA polyplexes do not have a net positive charge on their surfaces and do not have small enough hydrodynamic radii to be endocytosed. Conversely, when the N/P ratio is too high PEI exerts toxic effects on the cells (Lungwitz, et al., 2005).
Increasing the dose of PEI/pDNA produced increasingly higher transfection efficiencies (Fig. 2c). However, increasing the dose also lead to increased hMSC toxicity (Fig. 2d). One reason for this increase in toxicity could be unbound PEI in PEI:pDNA formulations, as described in previous studies (Clamme, et al., 2003, Finsinger, et al., 2000), and the unbound PEI produces increased cytotoxic effects (Boussif, Lezoualc’h, Zanta, Mergny, Scherman, Demeneix and Behr, 1995, Godbey, et al., 2001). Taken together these findings indicate that the PEI/pDNA ratio and dose must be high enough to achieve measureable transfection, but low enough to avoid toxic effects or interference with tissue formation by hMSCs.
Previous studies have defined hMSCs as SH2+ and CD105+ mononuclear cells derived from bone marrow, although it has been more recently shown that SH2 antibodies bind to an epitope on CD105 (Barry, et al., 1999). Therefore, for the purposes of our study, normal hMSC phenotype was defined by expression of CD105. Transfection treatment decreased CD105 expression (Fig. 4), suggesting potential effects on hMSC phenotype. In addition, transfection treatment led to apparent differences in osteogenic and adipogenic induction of hMSCs. Specifically, transfection treatment led to a decrease in alkaline phosphatase staining after osteogenic induction at both low and high seeding densities, and led to an increase in Oil Red O staining of hMSCs after adipogenic induction at a low seeding density (Fig. 5). Taken together, these results offer preliminary evidence that transfection treatment influences hMSC differentiation potential, perhaps by influencing expression of canonical hMSC markers (e.g. CD105). Notably, previous studies with other cell lines have also shown that treatment with non-viral vectors can induce changes in gene expression (Akhtar and Benter, 2007, Omidi, et al., 2005, Omidi, et al., 2003), and some of the genes explored in these previous studies are known regulators of hMSC differentiation. However, further studies will be needed to mechanistically delineate the effects of transfection treatment on hMSC phenotype.
Conclusion
Non-viral transfection efficiency and viability of hMSCs are dependent on both the cell culture environment and the formulation of polycation/pDNA polyplexes. Cell culture conditions that were previously demonstrated to promote hMSC multipotency also resulted in the greatest transfection efficiencies and cell division rate (i.e. P6–7 hMSCs, low seeding density, basal media). Alternatively, hMSC culture conditions that inhibited cell division also decreased transfection efficiency (i.e. culture conditions containing mitomycin C, hMSCs treated with gamma radiation, high hMSC cell seeding density). Preliminary experiments demonstrated that transfection treatment influenced the expression of the hMSC marker CD105 as well as the ability of hMSCs to differentiate down the osteogenic and adipogenic lineages. The relationships between cell culture parameters and non-viral transfection efficiency explored here could be used to instruct future approaches for efficient non-viral transfection of hMSCs for tissue engineering applications, and future studies will be needed to further delineate the mechanism governing effects of transfection treatment on hMSC phenotype.
Supplementary Material
To provide a comparison of this technique to another commonly used measurement of transfection efficiency we also performed an experiment in which hMSCs were treated with 1μg of PEI:pDNA with a N/P ratio of 7, and characterized for expression of EGFP three days post transfection using flow cytometry. The hMSC transfection efficiency was 5.2±2.4% as measured by flow cytometry(Fig. S1 a+b), and was similar and slightly more conservative (2.9±0.6%) when measured via image analysis (Fig. 1a)
Flow Cytometry Method
hMSCs were seeded at a concentration of 5000 hMSCs/cm2 in a 6-well culture plate. 24 hours later the hMSCs were transfected with 1μg pDNA that was complexed with 60kDa branched PEI at an N/P ratio of 7. Three days post-transfection the media was removed and the hMSCs were washed twice with 1ml of phosphate buffered saline, treated for 5 minutes with trypsin, and washed a final time with hMSC growth media. The hMSC concentration was then adjusted to 2*106 hMSCs/ml. The hMSCs were placed on ice and analyzed using a FACSVantage SE cell sorter (BD Biosciences San Jose, CA). hMSC viability was assessed with forward-area and side scatter. GFP+ and GFP− cells were then sorted from this double-gated technique in fetal bovine serum.
hMSC proliferation after treatment with 0–100μg/ml mitomycin C for 2–6 hours
Acknowledgments
The authors are grateful for support from the AO Research Foundation (GENEDEL exploratory research grant to W.L.M.) and the National Science Foundation (Graduate Research Fellowship to W.J.K, and CAREER award #0745563 to W.L.M.)
References
- Akhtar S, Benter I. Toxicogenomics of non-viral drug delivery systems for RNAi: potential impact on siRNA-mediated gene silencing activity and specificity. Adv Drug Deliv Rev. 2007;59:164–182. doi: 10.1016/j.addr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Anwer K, Barnes MN, Fewell J, Lewis DH, Alvarez RD. Phase-I clinical trial of IL-12 plasmid/lipopolymer complexes for the treatment of recurrent ovarian cancer. Gene Ther. 2010;17:360–369. doi: 10.1038/gt.2009.159. [DOI] [PubMed] [Google Scholar]
- Audouy S, Molema G, de Leij L, Hoekstra D. Serum as a modulator of lipoplex-mediated gene transfection: dependence of amphiphile, cell type and complex stability. J Gene Med. 2000;2:465–476. doi: 10.1002/1521-2254(200011/12)2:6<465::AID-JGM141>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25:1384–1392. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- Barry FP, Boynton RE, Haynesworth S, Murphy JM, Zaia J. The monoclonal antibody SH-2, raised against human mesenchymal stem cells, recognizes an epitope on endoglin (CD105) Biochem Biophys Res Commun. 1999;265:134–139. doi: 10.1006/bbrc.1999.1620. [DOI] [PubMed] [Google Scholar]
- Bolliet C, Bohn MC, Spector M. Non-viral delivery of the gene for glial cell line-derived neurotrophic factor to mesenchymal stem cells in vitro via a collagen scaffold. Tissue Eng Part C Methods. 2008;14:207–219. doi: 10.1089/ten.tec.2008.0168. [DOI] [PubMed] [Google Scholar]
- Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci U S A. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazeau GA, Attia S, Poxon S, Hughes JA. In vitro myotoxicity of selected cationic macromolecules used in non-viral gene delivery. Pharm Res. 1998;15:680–684. doi: 10.1023/a:1011954516233. [DOI] [PubMed] [Google Scholar]
- Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64:278–294. doi: 10.1002/(sici)1097-4644(199702)64:2<278::aid-jcb11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Brunner S, Sauer T, Carotta S, Cotten M, Saltik M, Wagner E. Cell cycle dependence of gene transfer by lipoplex, polyplex and recombinant adenovirus. Gene Ther. 2000;7:401–407. doi: 10.1038/sj.gt.3301102. [DOI] [PubMed] [Google Scholar]
- Byers BA, Garcia AJ. Exogenous Runx2 expression enhances in vitro osteoblastic differentiation and mineralization in primary bone marrow stromal cells. Tissue Eng. 2004;10:1623–1632. doi: 10.1089/ten.2004.10.1623. [DOI] [PubMed] [Google Scholar]
- Choosakoonkriang S, Lobo BA, Koe GS, Koe JG, Middaugh CR. Biophysical characterization of PEI/DNA complexes. J Pharm Sci. 2003;92:1710–1722. doi: 10.1002/jps.10437. [DOI] [PubMed] [Google Scholar]
- Clamme JP, Azoulay J, Mely Y. Monitoring of the formation and dissociation of polyethylenimine/DNA complexes by two photon fluorescence correlation spectroscopy. Biophys J. 2003;84:1960–1968. doi: 10.1016/S0006-3495(03)75004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements BA, Bai J, Kucharski C, Farrell LL, Lavasanifar A, Ritchie B, Ghahary A, Uludag H. RGD conjugation to polyethyleneimine does not improve DNA delivery to bone marrow stromal cells. Biomacromolecules. 2006;7:1481–1488. doi: 10.1021/bm060073w. [DOI] [PubMed] [Google Scholar]
- Clements BA, Incani V, Kucharski C, Lavasanifar A, Ritchie B, Uludag H. A comparative evaluation of poly-L-lysine-palmitic acid and Lipofectamine 2000 for plasmid delivery to bone marrow stromal cells. Biomaterials. 2007a;28:4693–4704. doi: 10.1016/j.biomaterials.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Clements BA, Incani V, Kucharski C, Lavasanifar A, Ritchie B, Uludag H. A comparative evaluation of poly-L-lysine-palmitic acid and Lipofectamine (TM) 2000 for plasmid delivery to bone marrow stromal cells. Biomaterials. 2007b;28:4693–4704. doi: 10.1016/j.biomaterials.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Dragoo JL, Choi JY, Lieberman JR, Huang J, Zuk PA, Zhang J, Hedrick MH, Benhaim P. Bone induction by BMP-2 transduced stem cells derived from human fat. J Orthop Res. 2003;21:622–629. doi: 10.1016/S0736-0266(02)00238-3. [DOI] [PubMed] [Google Scholar]
- Durocher Y, Perret S, Kamen A. High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells. Nucleic Acids Res. 2002:30. doi: 10.1093/nar/30.2.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eglen RM, Gilchrist A, Reisine T. An overview of drug screening using primary and embryonic stem cells. Comb Chem High Throughput Screen. 2008;11:566–572. doi: 10.2174/138620708785204108. [DOI] [PubMed] [Google Scholar]
- Farrell LL, Pepin J, Kucharski C, Lin X, Xu Z, Uludag H. A comparison of the effectiveness of cationic polymers poly-L-lysine (PLL) and polyethylenimine (PEI) for non-viral delivery of plasmid DNA to bone marrow stromal cells (BMSC) Eur J Pharm Biopharm. 2007;65:388–397. doi: 10.1016/j.ejpb.2006.11.026. [DOI] [PubMed] [Google Scholar]
- Finsinger D, Remy JS, Erbacher P, Koch C, Plank C. Protective copolymers for nonviral gene vectors: synthesis, vector characterization and application in gene delivery. Gene Ther. 2000;7:1183–1192. doi: 10.1038/sj.gt.3301227. [DOI] [PubMed] [Google Scholar]
- Fischer D, Bieber T, Li YX, Elsasser HP, Kissel T. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: Effect of molecular weight on transfection efficiency and cytotoxicity. Pharm Res. 1999;16:1273–1279. doi: 10.1023/a:1014861900478. [DOI] [PubMed] [Google Scholar]
- Fonsatti E, Altomonte M, Nicotra MR, Natali PG, Maio M. Endoglin (CD105): a powerful therapeutic target on tumor-associated angiogenetic blood vessels. Oncogene. 2003;22:6557–6563. doi: 10.1038/sj.onc.1206813. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- Geisse S. Reflections on more than 10 years of TGE approaches. Protein Expr Purif. 2009;64:99–107. doi: 10.1016/j.pep.2008.10.017. [DOI] [PubMed] [Google Scholar]
- Godbey WT, Wu KK, Mikos AG. Poly(ethylenimine) and its role in gene delivery. J Control Release. 1999;60:149–160. doi: 10.1016/s0168-3659(99)00090-5. [DOI] [PubMed] [Google Scholar]
- Godbey WT, Wu KK, Mikos AG. Poly(ethylenimine)-mediated gene delivery affects endothelial cell function and viability. Biomaterials. 2001;22:471–480. doi: 10.1016/s0142-9612(00)00203-9. [DOI] [PubMed] [Google Scholar]
- Gwak SJ, Kim BS. Poly(lactic-co-glycolic acid) nanosphere as a vehicle for gene delivery to human cord blood-derived mesenchymal stem cells: comparison with polyethylenimine. Biotechnol Lett. 2008;30:1177–1182. doi: 10.1007/s10529-008-9676-7. [DOI] [PubMed] [Google Scholar]
- Haldankar R, Li DQ, Saremi Z, Baikalov C, Deshpande R. Serum-free suspension large-scale transient transfection of CHO cells in WAVE bioreactors. Mol Biotechnol. 2006;34:191–199. doi: 10.1385/mb:34:2:191. [DOI] [PubMed] [Google Scholar]
- Horbinski C, Stachowiak MK, Higgins D, Finnegan SG. Polyethyleneimine-mediated transfection of cultured postmitotic neurons from rat sympathetic ganglia and adult human retina. BMC Neurosci. 2001;2:2. doi: 10.1186/1471-2202-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinkhani H, Azzam T, Kobayashi H, Hiraoka Y, Shimokawa H, Domb AJ, Tabata Y. Combination of 3D tissue engineered scaffold and non-viral gene carrier enhance in vitro DNA expression of mesenchymal stem cells. Biomaterials. 2006;27:4269–4278. doi: 10.1016/j.biomaterials.2006.02.033. [DOI] [PubMed] [Google Scholar]
- Huang YC, Simmons C, Kaigler D, Rice KG, Mooney DJ. Bone regeneration in a rat cranial defect with delivery of PEI-condensed plasmid DNA encoding for bone morphogenetic protein-4 (BMP-4) Gene Ther. 2005;12:418–426. doi: 10.1038/sj.gt.3302439. [DOI] [PubMed] [Google Scholar]
- Iuchi S, Marsch-Moreno M, Velez-DelValle C, Easley K, Kuri-Harcuch W, Green H. An immortalized drug-resistant cell line established from 12–13-day mouse embryos for the propagation of human embryonic stem cells. Differentiation. 2006;74:160–166. doi: 10.1111/j.1432-0436.2006.00067.x. [DOI] [PubMed] [Google Scholar]
- Jo J, Nagaya N, Miyahara Y, Kataoka M, Harada-Shiba M, Kangawa K, Tabata Y. Transplantation of genetically engineered mesenchymal stem cells improves cardiac function in rats with myocardial infarction: benefit of a novel nonviral vector, cationized dextran. Tissue Eng. 2007;13:313–322. doi: 10.1089/ten.2006.0133. [DOI] [PubMed] [Google Scholar]
- Koch H, Jadlowiec JA, Campbell PG. Insulin-like growth factor-I induces early osteoblast gene expression in human mesenchymal stem cells. Stem Cells Dev. 2005;14:621–631. doi: 10.1089/scd.2005.14.621. [DOI] [PubMed] [Google Scholar]
- Kunath K, von Harpe A, Fischer D, Peterson H, Bickel U, Voigt K, Kissel T. Low-molecular-weight polyethylenimine as a non-viral vector for DNA delivery: comparison of physicochemical properties, transfection efficiency and in vivo distribution with high-molecular-weight polyethylenimine. J Control Release. 2003;89:113–125. doi: 10.1016/s0168-3659(03)00076-2. [DOI] [PubMed] [Google Scholar]
- Love Z, Wang F, Dennis J, Awadallah A, Salem N, Lin Y, Weisenberger A, Majewski S, Gerson S, Lee Z. Imaging of mesenchymal stem cell transplant by bioluminescence and PET. J Nucl Med. 2007;48:2011–2020. doi: 10.2967/jnumed.107.043166. [DOI] [PubMed] [Google Scholar]
- Lucarelli E, Beccheroni A, Donati D, Sangiorgi L, Cenacchi A, Del Vento AM, Meotti C, Bertoja AZ, Giardino R, Fornasari PM, Mercuri M, Picci P. Platelet-derived growth factors enhance proliferation of human stromal stem cells. Biomaterials. 2003;24:3095–3100. doi: 10.1016/s0142-9612(03)00114-5. [DOI] [PubMed] [Google Scholar]
- Lungwitz U, Breunig M, Blunk T, Gopferich A. Polyethylenimine-based non-viral gene delivery systems. Eur J Pharm Biopharm. 2005;60:247–266. doi: 10.1016/j.ejpb.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Madry H, Padera R, Seidel J, Langer R, Freed LE, Trippel SB, Vunjak-Novakovic G. Gene transfer of a human insulin-like growth factor I cDNA enhances tissue engineering of cartilage. Hum Gene Ther. 2002;13:1621–1630. doi: 10.1089/10430340260201716. [DOI] [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- Morrow KJ. Optimizing transient gene expression. Genet Eng News. 2008;28:54–59. [Google Scholar]
- Omidi Y, Barar J, Akhtar S. Toxicogenomics of cationic lipid-based vectors for gene therapy: impact of microarray technology. Curr Drug Deliv. 2005;2:429–441. doi: 10.2174/156720105774370249. [DOI] [PubMed] [Google Scholar]
- Omidi Y, Hollins AJ, Benboubetra M, Drayton R, Benter IF, Akhtar S. Toxicogenomics of non-viral vectors for gene therapy: a microarray study of lipofectin- and oligofectamine-induced gene expression changes in human epithelial cells. J Drug Target. 2003;11:311–323. doi: 10.1080/10611860310001636908. [DOI] [PubMed] [Google Scholar]
- Palsson B, Bhatia S. Tissue engineering. Pearson Prentice Hall; Upper Saddle River, N.J: 2004. [Google Scholar]
- Park DJ, Choi JH, Leong KW, Kwon JW, Eun HS. Tissue-engineered bone formation with gene transfer and mesenchymal stem cells in a minimally invasive technique. Laryngoscope. 2007;117:1267–1271. doi: 10.1097/MLG.0b013e31805f680e. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Poh M, Boyer M, Solan A, Dahl SL, Pedrotty D, Banik SS, McKee JA, Klinger RY, Counter CM, Niklason LE. Blood vessels engineered from human cells. Lancet. 2005;365:2122–2124. doi: 10.1016/S0140-6736(05)66735-9. [DOI] [PubMed] [Google Scholar]
- Santos JL, Oramas E, Pego AP, Granja PL, Tomas H. Osteogenic differentiation of mesenchymal stem cells using PAMAM dendrimers as gene delivery vectors. J Control Release. 2009;134:141–148. doi: 10.1016/j.jconrel.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Saraf A, Hacker MC, Sitharaman B, Grande-Allen KJ, Barry MA, Mikos AG. Synthesis and conformational evaluation of a novel gene delivery vector for human mesenchymal stem cells. Biomacromolecules. 2008;9:818–827. doi: 10.1021/bm701146f. [DOI] [PubMed] [Google Scholar]
- Shea LD, Smiley E, Bonadio J, Mooney DJ. DNA delivery from polymer matrices for tissue engineering. Nat Biotechnol. 1999;17:551–554. doi: 10.1038/9853. [DOI] [PubMed] [Google Scholar]
- Shin JY, Suh D, Kim JM, Choi HG, Kim JA, Ko JJ, Lee YB, Kim JS, Oh YK. Low molecular weight polyethylenimine for efficient transfection of human hematopoietic and umbilical cord blood-derived CD34+ cells. Biochim Biophys Acta. 2005;1725:377–384. doi: 10.1016/j.bbagen.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Song H, Kwon K, Lim S, Kang SM, Ko YG, Xu Z, Chung JH, Kim BS, Lee H, Joung B, Park S, Choi D, Jang Y, Chung NS, Yoo KJ, Hwang KC. Transfection of mesenchymal stem cells with the FGF-2 gene improves their survival under hypoxic conditions. Mol Cells. 2005;19:402–407. [PubMed] [Google Scholar]
- Stettler M, Zhang XW, Hacker DL, De Jesus M, Wurm FM. Novel orbital shake bioreactors for transient production of CHO derived IgGs. Biotechnol Prog. 2007;23:1340–1346. doi: 10.1021/bp070219i. [DOI] [PubMed] [Google Scholar]
- Sun XD, Jeng L, Bolliet C, Olsen BR, Spector M. Non-viral endostatin plasmid transfection of mesenchymal stem cells via collagen scaffolds. Biomaterials. 2009;30:1222–1231. doi: 10.1016/j.biomaterials.2008.10.020. [DOI] [PubMed] [Google Scholar]
- Thomas RJ, Chandra A, Liu Y, Hourd PC, Conway PP, Williams DJ. Manufacture of a human mesenchymal stem cell population using an automated cell culture platform. Cytotechnology. 2007;55:31–39. doi: 10.1007/s10616-007-9091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RJ, Hourd PC, Williams DJ. Application of process quality engineering techniques to improve the understanding of the in vitro processing of stem cells for therapeutic use. J Biotechnol. 2008;136:148–155. doi: 10.1016/j.jbiotec.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Tousignant JD, Gates AL, Ingram LA, Johnson CL, Nietupski JB, Cheng SH, Eastman SJ, Scheule RK. Comprehensive analysis of the acute toxicities induced by systemic administration of cationic lipid: Plasmid DNA complexes in mice. Hum Gene Ther. 2000;11:2493–2513. doi: 10.1089/10430340050207984. [DOI] [PubMed] [Google Scholar]
- Tseng WC, Haselton FR, Giorgio TD. Mitosis enhances transgene expression of plasmid delivered by cationic liposomes. BBA Gene structure and expression. 1999;1445:53–64. doi: 10.1016/s0167-4781(99)00039-1. [DOI] [PubMed] [Google Scholar]
- Tsuchiya H, Kitoh H, Sugiura F, Ishiguro N. Chondrogenesis enhanced by overexpression of sox9 gene in mouse bone marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2003;301:338–343. doi: 10.1016/s0006-291x(02)03026-7. [DOI] [PubMed] [Google Scholar]
- Tuvesson O, Uhe C, Rozkov A, Lullau E. Development of a generic transient transfection process at 100 L scale. Cytotechnology. 2008;56:123–136. doi: 10.1007/s10616-008-9135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang M, Abarbanell AM, Weil BR, Herrmann JL, Tan J, Novotny NM, Coffey AC, Meldrum DR. MEK mediates the novel cross talk between TNFR2 and TGF-EGFR in enhancing vascular endothelial growth factor (VEGF) secretion from human mesenchymal stem cells. Surgery (St Louis) 2009;146:198–205. doi: 10.1016/j.surg.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Wiethoff CM, Middaugh CR. Barriers to nonviral gene delivery. J Pharm Sci. 2003;92:203–217. doi: 10.1002/jps.10286. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
To provide a comparison of this technique to another commonly used measurement of transfection efficiency we also performed an experiment in which hMSCs were treated with 1μg of PEI:pDNA with a N/P ratio of 7, and characterized for expression of EGFP three days post transfection using flow cytometry. The hMSC transfection efficiency was 5.2±2.4% as measured by flow cytometry(Fig. S1 a+b), and was similar and slightly more conservative (2.9±0.6%) when measured via image analysis (Fig. 1a)
Flow Cytometry Method
hMSCs were seeded at a concentration of 5000 hMSCs/cm2 in a 6-well culture plate. 24 hours later the hMSCs were transfected with 1μg pDNA that was complexed with 60kDa branched PEI at an N/P ratio of 7. Three days post-transfection the media was removed and the hMSCs were washed twice with 1ml of phosphate buffered saline, treated for 5 minutes with trypsin, and washed a final time with hMSC growth media. The hMSC concentration was then adjusted to 2*106 hMSCs/ml. The hMSCs were placed on ice and analyzed using a FACSVantage SE cell sorter (BD Biosciences San Jose, CA). hMSC viability was assessed with forward-area and side scatter. GFP+ and GFP− cells were then sorted from this double-gated technique in fetal bovine serum.
hMSC proliferation after treatment with 0–100μg/ml mitomycin C for 2–6 hours