Abstract
Background
Human and animal studies suggest that testosterone may have antidepressant effects. In this study, we sought to investigate the molecular mechanisms underlying the antidepressant effects of testosterone within the hippocampus, an area that is fundamental in the etiology of depression.
Methods
The effects of testosterone replacements in gonadectomized adult male rats were investigated using the sucrose preference and forced swim tests. We explored possible effects of testosterone on hippocampal neurogenesis and gene expression of stress-related molecules. Through the use of viral vectors we pursued the antidepressant molecular mechanism(s) of testosterone in mediating anhedonia, and manipulated extracellular signal-regulated kinase 2 (ERK2) expression in the dentate gyrus in gonadectomized rats with testosterone replacements.
Results
Testosterone had antidepressant effects, likely mediated by aromatization to estrogen metabolites, in the sucrose preference and FSTs despite having no effects on hippocampal cell proliferation or survival. We found a testosterone-dependent regulation of hippocampal ERK2 expression. Functionally, reducing ERK2 activity within the dentate gyrus induced anhedonia in gonadectomized rats receiving testosterone supplementation, while the overexpression of ERK2 rescued this behavior in gonadectomized rats.
Conclusions
These results implicate a role for ERK2 signaling within the dentate gyrus area of the hippocampus as a key mediator of the antidepressant effects of testosterone.
Keywords: testosterone, depression, ERK2, neurogenesis, hippocampus, 17β-estradiol
Introduction
Affective disorders are twice as likely to occur in women as in men (1–4) implicating a critical role for gonadal hormones in their etiology. In particular, testosterone has mood-enhancing properties and antidepressant effects in men (5). In fact, increased incidence of hypogonadism occurs in men with major depressive disorder (MDD) (6, 7)= and testosterone replacement effectively improves mood (7–9). In rodents, testosterone has antidepressant effects in aged male mice (10), and protective effects against the development of depression-like behaviors in rats (11). These studies suggest a modulatory role for testosterone in the regulation of depressive disorders; however the molecular mechanism(s) and brain site(s) of its actions are not well characterized.
Alterations of neurotrophic factors including brain-derived neurotrophic factor (BDNF) in limbic regions, such as the hippocampus, are associated with the treatment and/or onset of depression (12). Since increased BDNF expression and enhancement of neurogenesis in the dentate gyrus (DG) occur following treatment with antidepressants (13–18), it is possible that the antidepressant effects of testosterone are mediated through an increase in hippocampal BDNF expression and neurogenesis.
In addition to neurogenic changes, antidepressant treatments are associated with neuroendocrine regulation of the hypothalamic-pituitary-adrenal (HPA) axis. Indeed, major depression is associated with dysregulation of the HPA axis, possibly reflecting decreased glucocorticoid receptor (GR) activity (19). In fact, GR function imbalance might be a contributing factor in depression. Moreover, antidepressant treatments increase GR expression in the hippocampus (20), suggesting a key role for GR in the development and treatment of depression (for review see (21)).
Testosterone may interact with directly androgen receptors or through aromatization to estrogen metabolites in the brain to stimulate the mitogen activated protein kinase (MAPK) pathway (22), a fundamental signaling pathway and critical regulator of emotional responses. Chronic stress is associated with decreased protein expression of extracellular signal-regulated kinase 2 (ERK2) in the hippocampus. Both stress-induced depressive-like behaviors and ERK2 expression were reversed by chronic treatment with fluoxetine (23). Furthermore, chronic administration of lithium or valproate, mood stabilizers used in the treatment of manic depression, stimulates the MAPK pathway in the rat hippocampus (24). Interestingly, depressive-like symptoms were negatively correlated with ERK2 activation in the rat hippocampus (25). These studies implicate ERK activity within the hippocampus as a potential molecular target in the treatment of depression.
In this work, we investigated the role of testosterone and its metabolites in mediating depressive-like behaviors and explored possible underlying molecular mechanisms. In gonadectomized rats, we examined effects of testosterone on hippocampal cell proliferation and survival, and changes in gene expression of stress related molecules including BDNF, GR, and ERK2. Using viral vectors, we manipulated ERK2 activity in the DG and provided evidence for the role of testosterone-dependent ERK2 activity in mediating depressive-like behavior.
Materials and Methods
Experimental Design
Experiment 1: The effects of testosterone and its' metabolites on depressive-like behaviors
Rats were sham-operated or gonadectomized and received placebo, low, or high dose testosterone pellet implants. Twenty-one days later, all rats underwent the sucrose preference test (SPT) followed one week later by the forced swim test (FST). Rats were sacrificed 7 days later under basal, non-stressful conditions, hippocampi collected and the effects of gonadectomy and testosterone replacements on the expression of stress related molecules (BDNF, GR, ERK2) examined. A separate group of rats were gonadectomized and received placebo, β-estradiol 3-benzoate, or 5α- dihydrotestosterone (DHT) supplementation. Their behavior was analyzed two weeks later in the FST.
Experiment 2: The effects of testosterone on neurogenesis
Rats were gonadectomized and received placebo (n=12) or high dose (n=12) testosterone pellets. To examine cell proliferation, rats were injected with bromo-2'-deoxyuridine (BrdU) 20 days after gonadectomy and sacrificed 24 h later. To examine cell survival, rats were injected with BrdU on three occasions, once every 24 hrs, beginning on the day of gonadectomy and sacrificed 21 days later. Saline or imipramine contained in mini osmotic pumps was administered to control rats to examine the effects on cell proliferation (protocol adapted from (26)).
Experiment 3: The role of ERK2 in the antidepressant effects of testosterone
Sham-operated and gonadectomized rats receiving placebo or low dose testosterone pellet supplementation were injected with HSV viral vectors containing GFP, dominant negative ERK2-GFP (dnERK2), or the ERK2 overexpressing GFP form (wtERK2) into the DG. All rats underwent the SPT for 7 days beginning 24 h after viral injections. Rats were sacrificed on the 8th day and brains were collected for injection placement verification.
General Methods
Animals
Adult male Sprague-Dawley rats, weighing 250–270g, were purchased from Charles River (Wilmington, MA, USA), pair-housed in 43×21.5×25.5cm Plexiglas cages, and kept on a 12h: 12h light: dark cycle (lights on at 0700 hours). Food and water was available ad libitum except during testing. All behavioral experiments, except the SPT, were conducted during the first 4 h of the light phase of the light/dark cycle and were in accordance with the NIH Guide for Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Florida State University.
Surgery
Rats were anesthetized with a ketamine (70 mg/kg)/xylazine (10 mg/kg) mixture (i.p.). Bupivicaine (0.25% solution; 0.4mL/kg) was applied topically as analgesic and the non-steroidal anti-inflammatory drug meloxicam (1.0 mg/mL) was injected subcutaneously.
Gonadectomy/sham surgeries
A 1–2 cm ventral midline incision was made in the scrotum to expose the tunica. The tunica was pierced and both testes were extracted to expose the underlying blood vessels, which were ligated with silk suture. The testes were excised and all vessels and ducts were placed back into the tunica prior to suturing.
Hormone supplementation
Following gonadectomy/sham surgery, 60-day slow release testosterone (low dose, 25mg/pellet; high dose, 100mg/pellet), β-estradiol 3-benzoate (0.1mg/pellet), 5α-dihydrotestosterone (DHT; 12.5mg/pellet), or placebo pellets (Innovative Research of America, Sarasota Fl) were inserted subcutaneously into gonadectomized males 10 cm from a small 2 cm incision below the shoulder blades.
Osmotic minipumps
Rats were anesthetized as indicated earlier, and Alzet Osmotic Minipumps (Alza, Mountain View, CA) for 30-day administration (Model 2ML4), containing imipramine HCl dissolved in saline (Sigma-Aldrich, St. Louis, MO; n=6; 20mg/kg/day) or saline (n=6) were implanted subcutaneously in the dorsal rear flank region.
Viral vector infusions and transgene detection
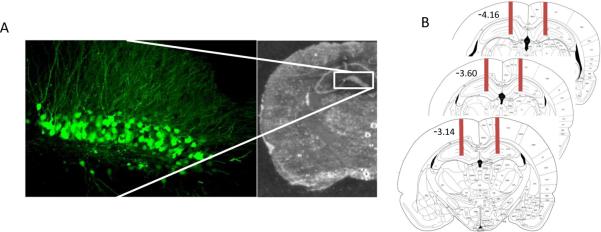
Herpes simplex viral (HSV) vectors containing green fluorescent protein (GFP), dnERK2-GFP, or wt-ERK2-GFP, were a generous gift from Dr. Eric Nestler (Mount Sinai School of Medicine, NY). These vectors have been previously described (27, 28), and validated in vivo and in vitro (29–31). The average titer of the recombinant virus stocks was 4.0 × 107 infectious units/mL. For stereotaxic delivery of the viral constructs, rats were bilaterally microinjected (1.0 μl per side over 10 min) into the dorsal region of the DG (anteroposterior, −4.3 mm; lateral, ±3 mm; dorsoventral, −4.7 mm from bregma (32)). All rats had correct placements between −3.14 and −4.16 from bregma (see figure 7).
Figure 7.
HSV vector injection placement. (A) Photomicrograph showing representative cannula placement of the lesion created during HSV injection into the dentate gyrus and confocal image illustrating the subsequent location of GFP expression three days after HSV-GFP injection. (B) Representative images adapted from The Rat Brain in Stereotaxic Coorndinates (32) illustrating the total area (−3.14 to −4.16 from bregma) affected by stereotaxic injection.
Maximal transgene expression for these vectors has been reported to occur on days 3–4 after injection (33, 34). To examine the time course of viral expression, we injected a separate group of rats with HSV-GFP (n=6) and sacrificed them 3 days (n=2), 5 days (n=2), or 8 days (n=2) later using transcardial perfusion with phosphate buffered saline (PBS, pH 7.4), followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4). Brains were extracted, postfixed at 4°C in paraformaldehyde overnight, sectioned at 30 μm on a vibratome (Leica VT1000S), and GFP expression was observed under a confocal microscope (Leica, TCS SP2).
To determine the efficacy with which the HSV-dnERK2 vector compromised ERK2 activity, rats were injected with HSV-GFP (n=4) or HSV- dnERK2-GFP (n=4) and sacrificed 3 days later. We examined the effects of the dnERK2, on the phosphorylation status of an ERK2 downstream target gene-mitogen and stress activated protein kinase (MSK) in the DG. Eight additional rats were injected with HSV-GFP (n=4) or HSV-wt-ERK2 (n=4) and sacrificed 4 days later to analyze ERK2 overexpression.
Behavioral Tests
Sucrose preference test
The SPT consisted of a two-bottle choice paradigm as described previously (35). Briefly, rats were given access, in experiment 1, to 0.25% sucrose for the first 48 h, 0.5% sucrose for the next 48 h, and 1% sucrose for the last 48 hrs of the test, and, in experiment 3, to 1% sucrose for 7 consecutive days. Sucrose preference was tested without prior exposure to sucrose.
Locomotor Activity
Rats were placed in a large (1m × 1m) open field (MED Associates Inc., St. Albans, Vermont) under dim lighting and were allowed to freely explore the arena for 10 minutes. Rats' behavior was recorded by a digital camcorder placed directly above the arena. A 5 × 5 grid was drawn on the bottom of the arena and the numbers of grid crosses were determined.
Forced swim test
The FST is a two day procedure. On day 1, rats were placed for 15 min in large inescapable Plexiglas cylinders (30 × 45 cm) filled with 25°C water to a depth of 30 cm in a dimly lit room. On day 2, the rats were again forced to swim for 5 min under the same conditions. The cylinders were emptied and cleaned between rats. Rats' behavior was videotaped, and the latency to the first immobility, and the total time spent immobile were analyzed. Immobility was defined as minimal movements required only to remain afloat (36).
Testosterone levels
Blood samples were centrifuged at 4°C for 10 min at 5,000 g and plasma was stored at −80°C until assayed. Total plasma testosterone concentrations were quantified by solid-phase 125I RIA, using “Coat- a-count” kits from Siemens Healthcare Diagnostics (Los Angeles, CA) with a sensitivity of 4 ng/dL.
Hippocampal cell proliferation and survival
For cell proliferation studies, rats received a single injection of BrdU (200mg/kg, i.p.) 20 days following surgeries, and were sacrificed 24 h later. For cell survival studies, rats received BrdU injections (200 mg/kg i.p.) for 3 consecutive days immediately following surgery, and were sacrificed 21 days after the first BrdU injection. Animals were anesthetized with sodium pentobarbital (100mg/kg) and were transcardially perfused; brains were extracted, postfixed as indicated earlier, cryoprotected in increasing sucrose solutions (24 h in 10%, 24 h in 20%, and 48 h in 30%) at 4°C, and frozen at −80°C until further processing.
Immunohistochemistry for BrdU
Thirty μm thick sections were obtained from throughout the hippocampus, mounted on poly-L-lysine-coated slides and stored at −80°C until use. Tissue to be labeled was blocked with normal goat serum (NGS; Vector labs) in 0.1M PBS pH 7.4 for I h at room temperature and then incubated overnight at 4°C in 1:100 rat anti-BrdU antibody (Abcam, ab6326). Slides were incubated at room temperature for 2 h in 1:1000 anti-rat IgG biotinylated antibody (Sigma, B7139), followed by 1 h incubation with biotin-peroxidase complex (Vector labs), and 3,3'-diaminobenzadine (DAB) solution (Vector labs), air-dried and cover slipped.
Quantification of BrdU labeled cells
Stereological procedures were conducted by a blind investigator and were based on computerized stereology (StereoInvestigator, MicroBrightField, Inc., Colchester, VT) using a Leica fluorescence microscope (Ctr6000) at 400× magnification. Sampling of every 6th section began approximately −2.56 mm from bregma where the DG first becomes clearly visible and ended at approximately −4.16 mm from bregma where the hippocampus has dorsal and ventral components in coronal section (32). Cell density of the dorsal hippocampus was calculated for each subject as the total number of BrdU positive cells within the granule cell layer and the hilus of the DG divided by the volume (mm3) of the DG estimated for each rat using Cavalieri's method (37) (Table 1).
Table 1.
Volume (mean ± SEM) of the dentate gyri. There were no significant differences among groups. Gnx = gonadectomized, IMI = imipramine.
| Experiment | Treatment | Dentate Volume (mm3) |
|---|---|---|
| Cell Proliferation | Gnx +Placebo | 9.033 ± 0.377 |
| Gnx + Testosterone | 9.145 ± 0.322 | |
| Sham + Saline | 8.273 ± 0.237 | |
| Sham + IMI | 8.502 ± 0.261 | |
| Cell Survival | Gnx + Placebo | 8.098 ± 0.173 |
| Gnx + Testosterone | 8.916 ± 0.351 |
Semi-Quantitative Real-Time PCR
Total RNA was extracted from both hippocampi and cDNA synthesis was carried out as previously described (35). Nicotinamide adenine dinucleotide dehydrogenase (NADH) was used as the reference gene for normalization of all target genes. The forward and reverse primer sequences were 5'-CCATAAGGACGCGGACTTGTAC-3' and 5'-AGACATGTTTGCGGCATCCAGG-3' for BDNF, 5'-CCACTGCAGGAGTCTCACAA-3' and 5'-CCAGCAGTGACACCAAGGTA-3' for GR, 5'-GACAAGGGCTCAGAGGACTG-3' and 5'-ACGGCTCAAAGGAGTCAAGA-3' for ERK2, and 5'-CTATTAATCCCCGCCTGACC-3' and 5'-GGAGCTCGATTTGTTTCTGC-3' for NADH, respectively. The normalized data is expressed as percent of control, with control sham animals set at 100%.
Western Blot
Total proteins were extracted from both hippocampi, or from the DG at the site of the injection to confirm HSV viral construct efficacy. Protein samples were processed as described previously (35). Immunoblots were incubated overnight (4°C) with ERK1/2 (Cell Signaling Technology; 1:1000) and GAPDH (Cell Signaling Technology; 1:1000), phospho-ERK1/2 (Cell Signaling Technology, 1:1000) and ERK1/2 (1:1000), or phospho-MSK (Cell Signaling Technology; 1:1000) and MSK (Santa Cruz Biotechnology; 1:1000), antibodies, washed and incubated 1 h with goat anti-rabbit IR Dye 680LT (Li-COR Biosciences; 1:10000) or donkey anti-goat IR Dye 800CW (Li-COR; 1:10000) fluorescent secondary antibodies, and visualized using an Odyssey infrared imaging system (Li-COR Biosciences). Quantification was done using NIH ImageJ (http://rsbweb.nih.gov/ij). Normalized data are expressed as percent of control, with control sham animals set at 100%.
Statistical analysis
Results were analyzed using one-way analysis of variance (ANOVA) followed by post-hoc Fisher tests where appropriate. Repeated ANOVA was used for sucrose preference results in experiment 3. P values < 0.05 were considered statistically significant.
Results
Determination of serum testosterone levels
Gonadectomized rats receiving low dose testosterone supplementation had serum testosterone levels comparable to sham gonadectomized animals. Gonadectomy and placebo supplementation resulted in nearly undetectable levels (Figure 1; p<0.05), whereas high dose supplementation produced extraphysiological serum testosterone levels significantly different from all other treatment groups (p<0.0001).
Figure 1.
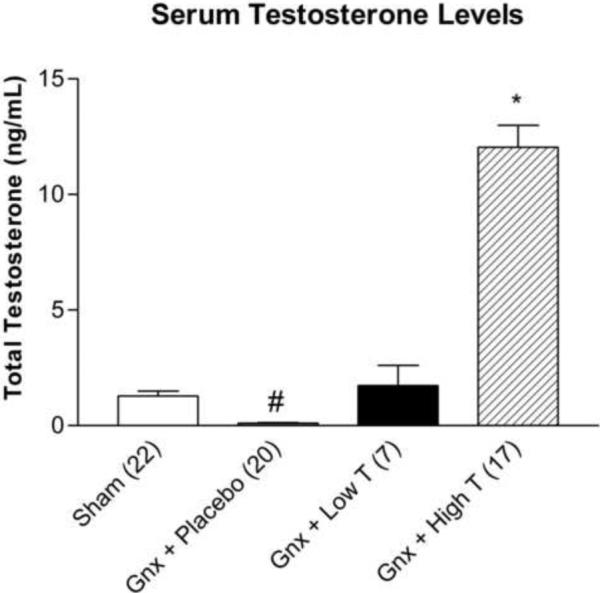
Serum testosterone levels (mean ± SEM) from sham gonadectomized (n= 22), and gonadectomized animals receiving placebo (n=20), low dose (n=7), or high dose testosterone supplementation (n= 17). *p<0.0001 compared to sham, gonadectomized + placebo, low, and high dose testosterone, #p<0.05 compared to sham, gonadectomized + low, and high dose testosterone treatment groups; Gnx = gonadectomized.
The effects of testosterone on depressive-like behaviors
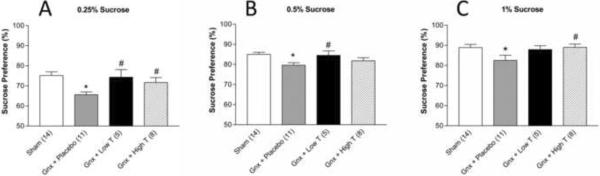
Three weeks after gonadectomy, locomotor activity was unaffected in sham and gonadectomized males receiving placebo (21.429 ± 5.995, sham; 17.500 ± 4.752, gonadectomized + placebo; F(1,11)=0.251, p>0.05). In the SPT, at 0.25% (Figure 2A; F(3,34)=4.846, p<0.05), 0.5% (Figure 2B; (F(3,32)=3.497, p<0.05) and 1% sucrose concentrations (Figure 2C; F(3,32)=4.8, p<0.05), gonadectomized rats receiving placebo exhibited decreased sucrose preference compared to sham and gonadectomized animals receiving low or high dose testosterone supplementation. No significant differences in water intake between treatment groups were detected (51.560 ± 3.229 g, sham; 47.892 ± 3.970 g, gonadectomized + placebo; 49.193 ± 0.707 g, gonadectomized + low dose testosterone; 53.908 ± 4.628 g, gonadectomized + high dose testosterone; F(3,36)=0.587, p>0.05).
Figure 2.
Sucrose preference. Gonadectomized + placebo rats exhibit decreased sucrose preference at (A) 0.25% (B) 0.5% and (C) 1% sucrose concentrations, compared with sham gonadectomized and gonadectomized animals receiving low and high dose testosterone supplementation (n= 5–14 per group). *p<0.05 compared to sham; #p<0.05 compared to placebo; Gnx = gonadectomized.
In the FST, latency to immobility (Figure 3A; F(3,34)=7.809, p<0.05) and total time spent immobile (Figure 3B; F(3,34)=13.006, p<0.0001) were significantly affected by testosterone levels. Gonadectomy with placebo supplementation resulted in a significant increase in total time spent immobile and a decrease in latency to immobility, compared to sham, high dose testosterone or low dose testosterone treated rats (p<0.05). Gonadectomized rats with DHT replacement had increased immobility and decreased latency, similar to gonadectomized animals with placebo (Figure 3 C–D), while estradiol benzoate replacement decreased immobility and increased latency to immobility (Figure 3 C–D; F(2,15)=3.704; p<0.05), suggesting that the antidepressant effects of testosterone are mediated by estrogenic effects following aromatization.
Figure 3.
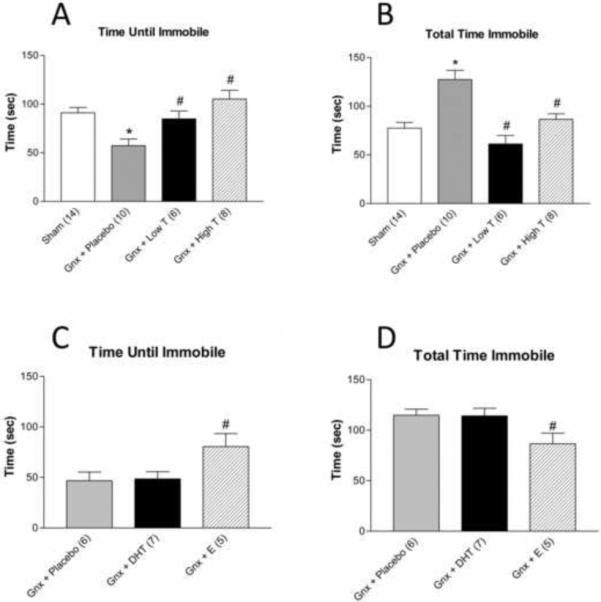
Porsolt FST. Gonadectomized rats receiving placebo pellets (A) demonstrate a shorter time until immobility and (B) spend a longer total time immobile compared to sham and gonadectomized animals receiving low and high dose testosterone supplementation. Gonadectomized rats receiving estradiol benzoate pellets (C) demonstrate an increased time until immobility and (D) spend a shorter total time immobile compared to gonadectomized rats receiving placebo or dihydrotestosterone supplements (n=5–14 per group). *p<0.05 compared to sham; #p<0.05 compared to placebo; Gnx = gonadectomized, T = testosterone, DHT = 5α-dihydrotestosterone, E = β-estradiol 3-benzoate.
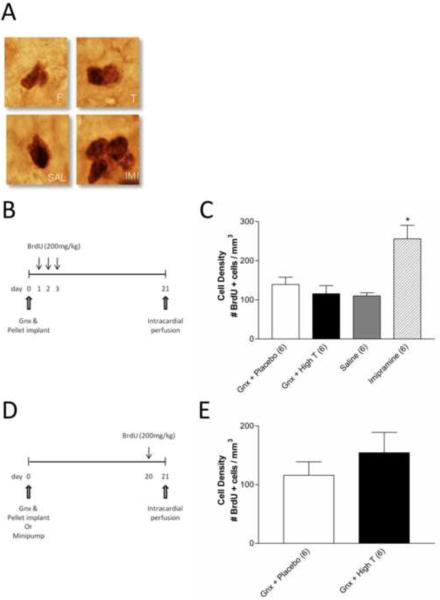
Effects of testosterone on hippocampal cell proliferation and survival
Chronic imipramine treatment resulted in a significant increase in the density of BrdU positive cells in the DG compared to saline treated animals (Figure 4C, p<0.05). Testosterone supplementation did not affect proliferation (Figure 4C, p>0.05) or cell survival (Figure 4E, p>0.05) as the densities of BrdU positive cells were similar in gonadectomized testosterone- or placebo-treated rats. The volume (mm3) of the DG was unchanged with all treatments (Table 1).
Figure 4.
Testosterone does not affect hippocampal cell proliferation or survival. (A) Representative photomicrographs (magnification ×400) of newly proliferated BrdU positive cells located in the subgranular zone between the granule cell layer and the hilus in the DG of adult male rats from one of four treatment groups; Gnx + Placebo (top left), Gnx + Testosterone (top right), Saline minipump (bottom left), and Imipramine minipump (bottom right). (B) Schematic representation of the timeline used for the cell proliferation studies. (C) The density of BrdU positive cells in the DG 24h after a single 200 mg/kg BrdU injection. Gonadectomized rats receiving high dose testosterone supplementation did not differ from those receiving placebo, however chronic imipramine treatment significantly increased cell proliferation compared to all other groups (n=6 per group). (C) Schematic representation of the timeline used for cell survival. (D) The density of cells surviving 21 days after the first of three BrdU injections did not differ in gonadectomized animals receiving placebo (n=6) or testosterone pellet supplementation (n=6). *p<0.05; Gnx = gonadectomized, P = Placebo, T = Testosterone, SAL = Saline, IMI = Imipramine
Effects of testosterone on hippocampal mRNA expression
Neither hippocampal BDNF (Figure 5A) nor GR (Figure 5B) mRNA expression was affected by testosterone. Gonadectomized animals receiving placebo pellet supplementation had significantly decreased ERK2 mRNA levels compared to sham-operated and gonadectomized rats receiving testosterone supplementation (F(3,26)=2.477; p <0.05).
Figure 5.
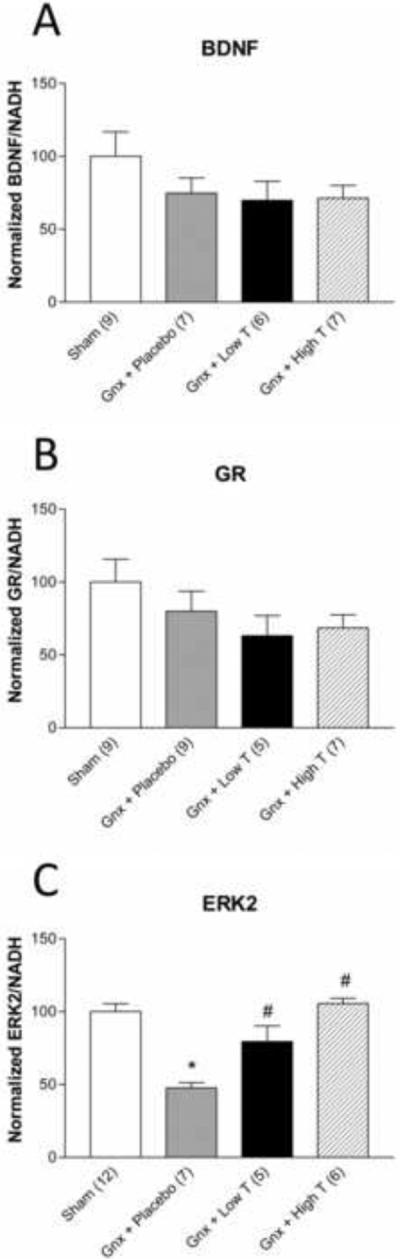
The effects of testosterone on the mRNA expression of brain derived neurotrophic factor (BDNF), glucocorticoid receptor (GR), and extracellular signal regulated kinase (ERK2) in the hippocampus of adult male sham gonadectomized (n=9–12), or gonadectomized animals receiving placebo (n=7–9), low (n=5–6), or high dose supplementation (n=6–7). Testosterone levels do not significantly affect mRNA expression of (A) BDNF or (B) GR, but (C) positively regulate ERK2 expression in the hippocampus. *p<0.05 compared to sham; #p<0.05 compared to placebo; Gnx = gonadectomized.
Effects of testosterone on hippocampal ERK protein expression
Total and phosphorylated hippocampal ERK1 protein (Figure 6A,B) were unaffected by serum testosterone levels. Gonadectomized, placebo-treated rats had significantly decreased hippocampal ERK2 expression compared to both sham-operated and gonadectomized rats receiving high dose testosterone supplementation (Figure 6C, F(3,18)=3.572, p<0.05). No significant effects of testosterone levels on hippocampal ERK2 phosphorylation were observed (Figure 6D).
Figure 6.
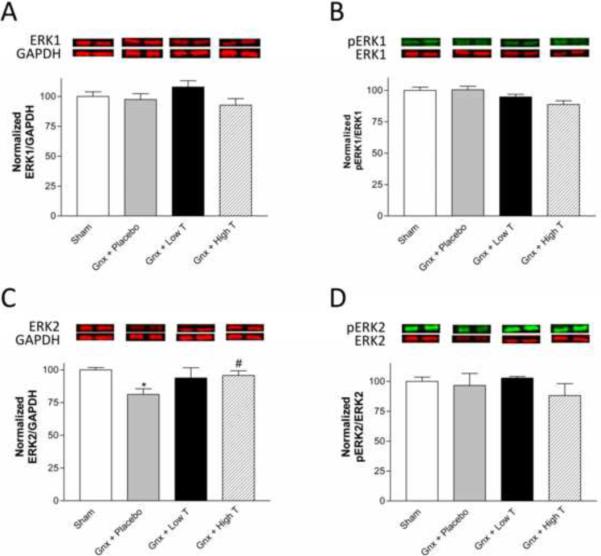
The effects of testosterone on hippocampal ERK total protein and phosphorylation status. Testosterone levels do not significantly affect ERK1 protein expression (A) pERK1/ERK1 protein expression (B), or pERK2/ERK2 protein expression (D), but positively regulate ERK2 expression (C) in the hippocampus. *p<0.05 compared to sham; #p<0.05 compared to placebo. Gnx = gonadectomized.
The antidepressant effects of testosterone are mediated by ERK2
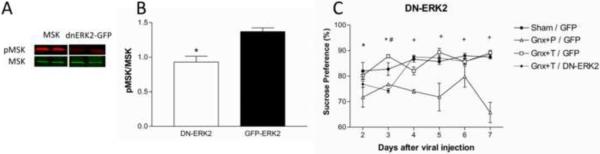
In order to investigate whether the antidepressant effects of testosterone were mediated by ERK2, we used HSV vectors expressing GFP alone, GFP and wtERK2, or GFP and dnERK2 to manipulate endogenous ERK2 expression and signaling the DG (Figure 7).
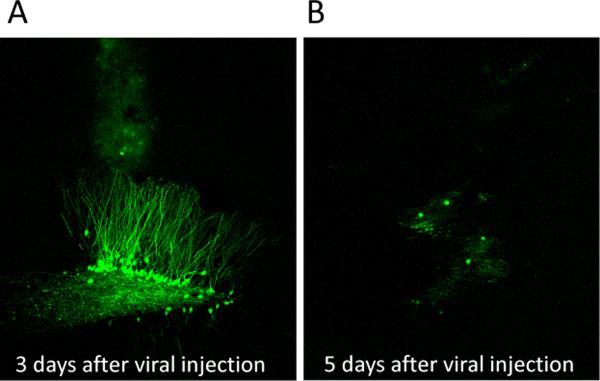
The HSV encoded proteins are highly expressed 3 days after injection (Figure 8A) and greatly diminished by day 5 (Figure 8B). Compared to GFP, overexpression of dnERK2-GFP induced a significant decrease in the phosphorylation of a major ERK2 substrate, MSK, in the DG 3 days after injection (Figure 9A–B, F(1,6)=18.854, p<0.05). We measured sucrose preference for 7 days beginning 1 d after injection of HSV encoding GFP alone or dnERK2-GFP (Figure 9C), and observed significant group (F(3,100)=24.479, p<0.0001), time (F(5,100)=6.312, p<0.0001), and interaction effects (F(15,100)=4.660, p<0.0001). As expected, gonadectomized rats receiving placebo exhibited decreased sucrose preference compared to sham and gonadectomized rats receiving testosterone supplementation on every examined day (p<0.05). On day 3, when viral vector expression was high (p<0.0001; Figure 8A) and ERK2 activity low (Figure 9A–B), gonadectomized rats receiving low dose testosterone supplementation but infected with the dnERK2-GFP virus exhibited significant signs of anhedonia that were comparable to gonadectomized placebo-treated rats infected with the control GFP virus. However, they differed significantly from sham-operated GFP-infected rats and from gonadectomized rats infected with the control GFP virus and treated with low testosterone. By day 5 after the injection, when viral protein expression was low (Figure 6B), the sucrose preference behavior in these rats increased to the same extent as sham-operated rats.
Figure 8.
Confocal images of herpes simplex virus (HSV) expressing GFP in the dentate gyrus. (A) Extensive GFP expression can be visualized in the dentate gyrus three days after viral vector injection however, (B) 5 days after viral injection GFP expression is greatly diminished.
Figure 9.
Downregulation of the ERK2 pathway induces anhedonia. (A) Western blot images and (B) quantification showing decreased pMSK/MSK in the dentate gyrus three days after viral injection of the HSV dominant negative ERK2 vector compared with HSV-GFP indicating compromised activity of the endogenous ERK2 by the dominant negative. (C) Adult male rats exposed to dnERK2-GFP exhibit anhedonia as measured by decreased sucrose preference similar to gonadectomized animals receiving placebo during the time period when the viral construct is highly expressed. HSV-dnERK2-GFP animals increase their sucrose preference to the level of sham and gonadectomized animals receiving testosterone supplementation. Gonadectomized animals receiving placebo continue to exhibit anhedonia for up to 7 days. *p<0.05 placebo HSV-GFP vs sham HSV-GFP and gonadectomized + testosterone + HSV-GFP; #p<0.05 gonadectomized + testosterone + HSV-dnERK2-GFP; +p<0.05 placebo HSV-GFP vs sham HSV-GFP and gonadectomized + testosterone + HSV-GFP and gonadectomized + testosterone + HSV-dnERK2-GFP. Gnx = gonadectomized, DN ERK2 = dominant negative ERK2, T = testosterone, P = placebo.
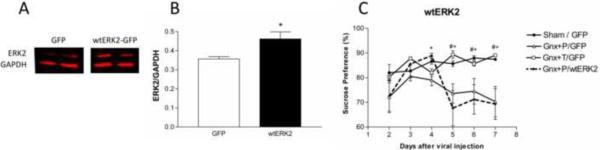
Overexpression of ERK2 using the wtERK2-GFP construct resulted in increased ERK2 levels compared to GFP controls within the DG 4 d after injections (Figure 10 A–B; F(1,6)=7.341, p<0.05). We measured sucrose preference behaviors in these animals for 7 d, beginning 1 d after viral injection (Figure 10C), and observed significant time (F(5,155)=3.461, p<0.05) and interaction effects (F(15,155)=2.457, p<0.05). Gonadectomized animals receiving placebo displayed decreased sucrose preference compared to sham and gonadectomized animals receiving testosterone supplementation on every day examined (p < 0.05) except day 2. Gonadectomized rats receiving placebo and infected with the wtERK2-GFP virus construct exhibited a trend for increased sucrose preference compared to gonadectomized placebo-treated rats infected with the GFP control virus on day 3, and a significant increase on day 4 (p<0.0001); this effect was no longer apparent by day 5. On days 5–7, when viral expression is greatly diminished, both groups of gonadectomized placebo-treated rats infected with either the GFP, or the wtERK2-GFP virus, were significantly different from sham-operated and gonadectomized rats receiving testosterone supplementation and infected with the control GFP virus (p<0.05).
Figure 10.
Overexpression of ERK2 in gonadectomized animals rescues anhedonia. (A) Western blot images and (B) quantification showing increased ERK2 expression 4 days after viral injection. (C) Adult male gonadectomized rats receiving placebo pellets exposed to wtERK2 exhibit increased sucrose preference compared with gonadectomized rats receiving placebo and GFP when ERK2 is overexpressed. HSV wt-ERK2-GFP animals exhibit anhedonia comparable to gonadectomized animals receiving placebo when the viral construct is no longer expressing. *p<0.05 gonadectomized + placebo + HSV-wtERK2-GFP Vs gonadectomized + placebo + HSV-GFP; #p<0.05; #p<0.05 gonadectomized + placebo + HSV-wtERK2-GFP Vs sham + HSV-GFP, +p<0.05 gonadectomized + placebo + HSV-GFP Vs sham + HSV-GFP. Gnx = gonadectomized, DN ERK2 = dominant negative ERK2, T = testosterone, P = placebo.
Discussion
Our findings showed that gonadectomized rats developed depressive-like behaviors which could be alleviated with either physiological (low dose) or extraphysiological (high dose) testosterone supplementation, and that the antidepressant effects of testosterone are likely mediated by aromatization to estrogen. Moreover, gonadectomy and testosterone replacements had no effect on hippocampal cell proliferation, cell survival, or BDNF or GR mRNA expression. The present findings provide evidence for testosterone-dependent regulation of ERK2 within the hippocampus and demonstrate the necessity of ERK2 function in mediating the antidepressant effects of testosterone. Indeed, ERK2 activation may be a common downstream signaling pathway used by various treatments to relieve symptoms of depression.
Gonadal hormones such as testosterone have profound organizational effects during development, as well as reversible activational effects in adulthood. The organizational effects of testosterone result in an irreversible masculinization of the brain that, undoubtedly, impacts adult emotional behavior with significant contribution to the incidence of sex differences in depression. In the present study, elimination of the peripheral source of testosterone by gonadectomy of adult male rats permitted the investigation of the activational effects of testosterone on depressive-like behaviors.
While prolonged loss of testosterone in adult males may result in decreased locomotor activity after a long testing period occurring 1–3 hours into the dark cycle (38), or hypophagia 27 days following gonadectomy (39), we did not observe significant differences in our treatment groups at the time of testing. In the present study, locomotion occurred during the light cycle and sucrose testing between 14–21 days post-surgery, thus potentially confounding effects are unlikely.
Neurogenesis in the DG of the hippocampus is facilitated by chronic antidepressant treatment (13), therefore representing a potential mechanism mediating the antidepressant effects of testosterone. In agreement with a previous study (40), (but see (26)), we report no effects of testosterone on cell proliferation or survival despite a significant increase in our positive control imipramine. The importance of enhanced neurogenesis in mediating the therapeutic effects of antidepressant treatment has been recently challenged (41, 42) and the manifestation of behavioral improvement following antidepressant administration has been shown to occur even in the absence of neurogenesis (43–45). These studies, and ours, suggest that mood-enhancing effects of pharmacological antidepressants may not require hippocampal neurogenesis.
Although the molecular mechanisms remain unclear, the pathophysiology of MDD includes excessive stimulation of the HPA axis, resulting in elevated glucocorticoid levels and reduced feedback inhibition of the axis (46–49). Through the activation of glucocorticoid receptors, excess glucocorticoids may contribute to stress-induced structural changes in the hippocampus (50), a phenomenon often associated with reduced neurotrophic factor activity. The neurotrophic theory of depression involves a reduction of hippocampal BDNF expression, which can be reversed by antidepressant treatment (51–53). Accordingly, BDNF may offset stress-induced structural changes in the hippocampus. Here, no testosterone-dependent changes in hippocampal GR and BDNF mRNAs, or neurogenesis were observed, despite clear antidepressant effects. These findings are in line with recent reports indicating that antidepressants de-suppress translation of neurotrophic factors' mRNAs (54). It is therefore likely that testosterone can still up-regulate GR or BDNF protein levels.
Activated by testosterone and its metabolites (58), the MAPK/ERK pathway is a major convergence point for signaling pathways related to cell growth, differentiation, and neuronal plasticity (55–57). Recently proposed theories implicate signaling pathways related to synaptic plasticity to be critical to the molecular mechanisms of antidepressants, with particular focus on the ERK pathway regarding emotional responses (13, 23, 24, 59). Stress-mediated disruption of the ERK signaling pathway could cause depressive-like behaviors; an effect reversed by fluoxetine treatment (23). Here, we report testosterone-dependent regulation of ERK2 mRNA and protein expression within the hippocampus. Compromising ERK2 activity in gonadectomized male rats, specifically within the DG, eliminates the antidepressant effects of testosterone supplementation. Conversely, ERK2 overexpression (without testosterone supplementation) in gonadectomized rats rescues anhedonia. These findings highlight an important role of ERK2 signaling in the antidepressant effects of testosterone. The substantial number of ERK2 substrates warrants future studies to delineate the downstream target(s) of ERK2 implicated in the antidepressant mechanism of testosterone.
The antidepressant effects of testosterone may be mediated through androgen receptors distributed throughout the hippocampal formation, or may depend on local conversion to metabolites (63). We investigated the effects of two major testosterone metabolites on the behavior of gonadectomized rats in the FST and report antidepressant effects of estradiol benzoate, but not DHT supplementation. Conversion to 17β-estradiol by P450-aromatase enzyme has been shown to occur in the hippocampus (64), rich in estrogen receptors (65), where they exhibit neuroprotective effects regarding aging, neurodegeneration, neurogenesis, excitotoxicity, and ischemia (66, 67). It is therefore conceivable that the antidepressant effects of testosterone can be mediated by aromatization to 17β-estradiol. It should be noted that prominent sex differences exist in the influence of estrogens, particularly on neurogenesis with females showing a greater response than males (68). Indeed, chronic 17β-estradiol administration increases cell proliferation in female but not male rats (69). The effects of gonadal hormones and their metabolites on neurogenesis and emotional state are complex and depend on several factors, warranting future investigations to determine the contributions of testosterone and its metabolites in the etiology of depression.
We have demonstrated antidepressant effects of testosterone in adult male rats, independent of neurogenesis, but rather directly involving ERK2-mediated signaling within the DG. The observed antidepressant effects are likely mediated via estrogen metabolites given that estradiol benzoate, but not DHT supplementation, reversed behavioral despair in gonadectomized male rats. Antidepressant effects of testosterone supplementation are abolished when ERK2 activity is compromised within the DG, and ERK2 overexpression can rescue anhedonic behaviors in placebo-treated gonadectomized rats. These findings implicate a critical role for hippocampal ERK2 signaling in mediating the antidepressant effects of testosterone and warrant future studies delving into the regulation of the MAPK pathway as a potential specific molecular target of future antidepressant drugs.
Acknowledgements
This work was supported by funds from the National Institute of Mental Health (R01MH087583), the FSU College of Medicine and an FSU Developing Scholar Award to Mohamed Kabbaj. FSU College of Medicine Core lab facility is acknowledged for assistance with the confocal laser microscope. We thank Dr. Eric Nestler, from The Mount Sinai Medical Center, New York, for the HSV viral vector constructs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Earls F. Sex differences in psychiatric disorders: origins and developmental influences. Psychiatric developments. 1987;5:1–23. [PubMed] [Google Scholar]
- 2.Angst J, Gamma A, Gastpar M, Lepine JP, Mendlewicz J, Tylee A. Gender differences in depression. Epidemiological findings from the European DEPRES I and II studies. European archives of psychiatry and clinical neuroscience. 2002;252:201–209. doi: 10.1007/s00406-002-0381-6. [DOI] [PubMed] [Google Scholar]
- 3.Bebbington P, Dunn G, Jenkins R, Lewis G, Brugha T, Farrell M, et al. The influence of age and sex on the prevalence of depressive conditions: report from the National Survey of Psychiatric Morbidity. International review of psychiatry, (Abingdon, England) 2003;15:74–83. doi: 10.1080/0954026021000045976. [DOI] [PubMed] [Google Scholar]
- 4.Kessler RC. Epidemiology of women and depression. Journal of affective disorders. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- 5.Kanayama G, Amiaz R, Seidman S, Pope HG., Jr. Testosterone supplementation for depressed men: current research and suggested treatment guidelines. Experimental and clinical psychopharmacology. 2007;15:529–538. doi: 10.1037/1064-1297.15.6.529. [DOI] [PubMed] [Google Scholar]
- 6.McIntyre RS, Mancini D, Eisfeld BS, Soczynska JK, Grupp L, Konarski JZ, et al. Calculated bioavailable testosterone levels and depression in middle-aged men. Psychoneuroendocrinology. 2006;31:1029–1035. doi: 10.1016/j.psyneuen.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham GR, Cordero E, Thornby JI. Testosterone replacement with transdermal therapeutic systems. Physiological serum testosterone and elevated dihydrotestosterone levels. Jama. 1989;261:2525–2530. [PubMed] [Google Scholar]
- 8.McNicholas TA, Dean JD, Mulder H, Carnegie C, Jones NA. A novel testosterone gel formulation normalizes androgen levels in hypogonadal men, with improvements in body composition and sexual function. BJU international. 2003;91:69–74. doi: 10.1046/j.1464-410x.2003.04016.x. [DOI] [PubMed] [Google Scholar]
- 9.Vermeulen A, Kaufman JM. Diagnosis of hypogonadism in the aging male. Aging Male. 2002;5:170–176. [PubMed] [Google Scholar]
- 10.Frye CA, Walf AA. Depression-like behavior of aged male and female mice is ameliorated with administration of testosterone or its metabolites. Physiology & behavior. 2009;97:266–269. doi: 10.1016/j.physbeh.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solomon MB, Karom MC, Norvelle A, Markham CA, Erwin WD, Huhman KL. Gonadal hormones modulate the display of conditioned defeat in male Syrian hamsters. Hormones and behavior. 2009;56:423–428. doi: 10.1016/j.yhbeh.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perera TD, Coplan JD, Lisanby SH, Lipira CM, Arif M, Carpio C, et al. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J Neurosci. 2007;27:4894–4901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madsen TM, Treschow A, Bengzon J, Bolwig TG, Lindvall O, Tingstrom A. Increased neurogenesis in a model of electroconvulsive therapy. Biological psychiatry. 2000;47:1043–1049. doi: 10.1016/s0006-3223(00)00228-6. [DOI] [PubMed] [Google Scholar]
- 16.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science, (New York, NY. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 18.Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- 19.Holsboer F. Stress, hypercortisolism and corticosteroid receptors in depression: implications for therapy. Journal of affective disorders. 2001;62:77–91. doi: 10.1016/s0165-0327(00)00352-9. [DOI] [PubMed] [Google Scholar]
- 20.Lopez JF, Vazquez DM, Chalmers DT, Watson SJ. Regulation of 5-HT receptors and the hypothalamic-pituitary-adrenal axis. Implications for the neurobiology of suicide. Annals of the New York Academy of Sciences. 1997;836:106–134. doi: 10.1111/j.1749-6632.1997.tb52357.x. [DOI] [PubMed] [Google Scholar]
- 21.Anacker C, Zunszain PA, Carvalho LA, Pariante CM. The glucocorticoid receptor: pivot of depression and of antidepressant treatment? Psychoneuroendocrinology. 2011;36:415–425. doi: 10.1016/j.psyneuen.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, et al. Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. The EMBO journal. 2000;19:5406–5417. doi: 10.1093/emboj/19.20.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qi X, Lin W, Li J, Li H, Wang W, Wang D, et al. Fluoxetine increases the activity of the ERK-CREB signal system and alleviates the depressive-like behavior in rats exposed to chronic forced swim stress. Neurobiology of disease. 2008;31:278–285. doi: 10.1016/j.nbd.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Einat H, Yuan P, Gould TD, Li J, Du J, Zhang L, et al. The role of the extracellular signal-regulated kinase signaling pathway in mood modulation. J Neurosci. 2003;23:7311–7316. doi: 10.1523/JNEUROSCI.23-19-07311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qi X, Lin W, Li J, Pan Y, Wang W. The depressive-like behaviors are correlated with decreased phosphorylation of mitogen-activated protein kinases in rat brain following chronic forced swim stress. Behavioural Brain Research. 2006;175:233–240. doi: 10.1016/j.bbr.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 26.Spritzer MD, Galea LA. Testosterone and dihydrotestosterone, but not estradiol, enhance survival of new hippocampal neurons in adult male rats. Developmental neurobiology. 2007;67:1321–1333. doi: 10.1002/dneu.20457. [DOI] [PubMed] [Google Scholar]
- 27.Robinson MJ, Harkins PC, Zhang J, Baer R, Haycock JW, Cobb MH, et al. Mutation of position 52 in ERK2 creates a nonproductive binding mode for adenosine 5'-triphosphate. Biochemistry. 1996;35:5641–5646. doi: 10.1021/bi952723e. [DOI] [PubMed] [Google Scholar]
- 28.Neve RL, Howe JR, Hong S, Kalb RG. Introduction of the glutamate receptor subunit 1 into motor neurons in vitro and in vivo using a recombinant herpes simplex virus. Neuroscience. 1997;79:435–447. doi: 10.1016/s0306-4522(96)00645-8. [DOI] [PubMed] [Google Scholar]
- 29.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 30.Russo SJ, Bolanos CA, Theobald DE, DeCarolis NA, Renthal W, Kumar A, et al. IRS2-Akt pathway in midbrain dopamine neurons regulates behavioral and cellular responses to opiates. Nature neuroscience. 2007;10:93–99. doi: 10.1038/nn1812. [DOI] [PubMed] [Google Scholar]
- 31.Iniguez SD, Vialou V, Warren BL, Cao JL, Alcantara LF, Davis LC, et al. Extracellular signal-regulated kinase-2 within the ventral tegmental area regulates responses to stress. J Neurosci. 2010;30:7652–7663. doi: 10.1523/JNEUROSCI.0951-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paxinos GWC. The rat brain in stereotaxic coordinates. 3 ed. Academic Press; San Diego: 1998. [Google Scholar]
- 33.Carlezon WA, Jr., Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, et al. Regulation of cocaine reward by CREB. Science, (New York, NY. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- 34.Barrot M, Olivier JD, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, et al. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11435–11440. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hollis F, Duclot F, Gunjan A, Kabbaj M. Individual differences in the effect of social defeat on anhedonia and histone acetylation in the rat hippocampus. Hormones and behavior. 59:331–337. doi: 10.1016/j.yhbeh.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behavioural pharmacology. 1997;8:523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, et al. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. Apmis. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- 38.Adler A, Vescovo P, Robinson JK, Kritzer MF. Gonadectomy in adult life increases tyrosine hydroxylase immunoreactivity in the prefrontal cortex and decreases open field activity in male rats. Neuroscience. 1999;89:939–954. doi: 10.1016/s0306-4522(98)00341-8. [DOI] [PubMed] [Google Scholar]
- 39.Gentry RT, Wade GN. Androgenic control of food intake and body weight in male rats. J Comp Physiol Psychol. 1976;90:18–25. doi: 10.1037/h0077264. [DOI] [PubMed] [Google Scholar]
- 40.Buwalda B, van der Borght K, Koolhaas JM, McEwen BS. Testosterone decrease does not play a major role in the suppression of hippocampal cell proliferation following social defeat stress in rats. Physiology & behavior. 2010;101:719–725. doi: 10.1016/j.physbeh.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 41.Vollmayr B, Simonis C, Weber S, Gass P, Henn F. Reduced cell proliferation in the dentate gyrus is not correlated with the development of learned helplessness. Biological psychiatry. 2003;54:1035–1040. doi: 10.1016/s0006-3223(03)00527-4. [DOI] [PubMed] [Google Scholar]
- 42.Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, et al. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Molecular psychiatry. 2006;11:514–522. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- 43.Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA, et al. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Molecular psychiatry. 2009;14:764–773. 739. doi: 10.1038/mp.2008.119. [DOI] [PubMed] [Google Scholar]
- 44.Holick KA, Lee DC, Hen R, Dulawa SC. Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology. 2008;33:406–417. doi: 10.1038/sj.npp.1301399. [DOI] [PubMed] [Google Scholar]
- 45.Huang GJ, Bannerman D, Flint J. Chronic fluoxetine treatment alters behavior, but not adult hippocampal neurogenesis, in BALB/cJ mice. Molecular psychiatry. 2008;13:119–121. doi: 10.1038/sj.mp.4002104. [DOI] [PubMed] [Google Scholar]
- 46.Gillespie CF, Nemeroff CB. Hypercortisolemia and depression. Psychosomatic medicine. 2005;67(Suppl 1):S26–28. doi: 10.1097/01.psy.0000163456.22154.d2. [DOI] [PubMed] [Google Scholar]
- 47.Sapolsky RM. Stress hormones: good and bad. Neurobiology of disease. 2000;7:540–542. doi: 10.1006/nbdi.2000.0350. [DOI] [PubMed] [Google Scholar]
- 48.Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biological psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- 49.Barden N. Implication of the hypothalamic-pituitary-adrenal axis in the physiopathology of depression. J Psychiatry Neurosci. 2004;29:185–193. [PMC free article] [PubMed] [Google Scholar]
- 50.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiological reviews. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 51.Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular medicine. 2004;5:11–25. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- 52.Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Archives of general psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 53.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 54.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen Z, Gibson TB, Robinson F, Silvestro L, Pearson G, Xu B, et al. MAP kinases. Chemical reviews. 2001;101:2449–2476. doi: 10.1021/cr000241p. [DOI] [PubMed] [Google Scholar]
- 56.Schafe GE, Atkins CM, Swank MW, Bauer EP, Sweatt JD, LeDoux JE. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of pavlovian fear conditioning. J Neurosci. 2000;20:8177–8187. doi: 10.1523/JNEUROSCI.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. Journal of neurochemistry. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- 58.Cheng J, Watkins SC, Walker WH. Testosterone activates mitogen-activated protein kinase via Src kinase and the epidermal growth factor receptor in sertoli cells. Endocrinology. 2007;148:2066–2074. doi: 10.1210/en.2006-1465. [DOI] [PubMed] [Google Scholar]
- 59.Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nature medicine. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- 60.Tsai MJ, O'Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annual review of biochemistry. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 61.Razandi M, Pedram A, Park ST, Levin ER. Proximal events in signaling by plasma membrane estrogen receptors. The Journal of biological chemistry. 2003;278:2701–2712. doi: 10.1074/jbc.M205692200. [DOI] [PubMed] [Google Scholar]
- 62.Song RX, McPherson RA, Adam L, Bao Y, Shupnik M, Kumar R, et al. Linkage of rapid estrogen action to MAPK activation by ERalpha-Shc association and Shc pathway activation. Molecular endocrinology, (Baltimore, Md. 2002;16:116–127. doi: 10.1210/mend.16.1.0748. [DOI] [PubMed] [Google Scholar]
- 63.Tabori NE, Stewart LS, Znamensky V, Romeo RD, Alves SE, McEwen BS, et al. Ultrastructural evidence that androgen receptors are located at extranuclear sites in the rat hippocampal formation. Neuroscience. 2005;130:151–163. doi: 10.1016/j.neuroscience.2004.08.048. [DOI] [PubMed] [Google Scholar]
- 64.Konkle AT, McCarthy MM. Developmental time course of estradiol, testosterone, and dihydrotestosterone levels in discrete regions of male and female rat brain. Endocrinology. 2011;152:223–235. doi: 10.1210/en.2010-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weiland NG, Orikasa C, Hayashi S, McEwen BS. Distribution and hormone regulation of estrogen receptor immunoreactive cells in the hippocampus of male and female rats. The Journal of comparative neurology. 1997;388:603–612. doi: 10.1002/(sici)1096-9861(19971201)388:4<603::aid-cne8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 66.McEwen B. Estrogen actions throughout the brain. Recent progress in hormone research. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- 67.Barha CK, Lieblich SE, Galea LA. Different forms of oestrogen rapidly upregulate cell proliferation in the dentate gyrus of adult female rats. Journal of neuroendocrinology. 2009;21:155–166. doi: 10.1111/j.1365-2826.2008.01809.x. [DOI] [PubMed] [Google Scholar]
- 68.Galea LA. Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res Rev. 2008;57:332–341. doi: 10.1016/j.brainresrev.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 69.Barker JM, Galea LA. Repeated estradiol administration alters different aspects of neurogenesis and cell death in the hippocampus of female, but not male, rats. Neuroscience. 2008;152:888–902. doi: 10.1016/j.neuroscience.2007.10.071. [DOI] [PubMed] [Google Scholar]