Abstract
Hematopoietic stem cells (HSCs) reside in complex bone marrow (BM) microenvironments where niche-induced signals regulate hematopoiesis. Focal adhesion kinase (Fak) is a non-receptor protein tyrosine kinase, which plays an essential role in many cell types, where its activation controls adhesion, motility and survival. Fak expression is relatively increased in HSCs compared to progenitors and mature blood cells. Therefore we explored its role in HSC homeostasis. We have used the Mx1-Cre inducible conditional knockout mouse model to investigate the effects of Fak deletion in bone marrow compartments. Results. The total number as well as the fraction of cycling Lin-Sca-1+c-kit+ (LSK) cells is increased in Fak−/− mice, compared to controls, while hematopoietic progenitors and mature blood cells are unaffected. BM cells from Fak−/− mice exhibit enhanced, long-term (i.e. 20 week duration) engraftment in competitive transplantation assays. Intrinsic Fak function was assessed in serial transplantation assays, which showed that HSCs (Lin-Sca-1+c-kit +CD34-Flk-2-cells) sorted from Fak−/− mice have similar self-renewal and engraftment ability on a per cell basis as wild type HSCs. When Fak deletion is induced following engraftment of Fakfl/flMx1-Cre+ BM cells into wild type recipient mice, the number of LSKs is unchanged. In conclusion, Fak inactivation does not intrinsically regulate HSC behavior and is not essential for steady-state hematopoiesis. However, widespread Fak inactivation in the hematopoietic system induces an increased and activated HSC pool size, potentially as a result of altered reciprocal interactions between HSCs and their microenvironment.
Keywords: Focal adhesion kinase, Hematopoietic stem cell, Homeostasis, Engraftment
Hematopoiesis is a physiological process where a small number of hematopoietic stem cells (HSCs) residing in the bone marrow (BM) proliferate and differentiate to generate the full complement of blood and immune cells 1, 2. Through a step-wise, highly regulated procedure, HSCs self-renew and differentiate into increasingly less-rare progenitors and ultimately more-mature blood cells 3-5. When HSCs divide, they are faced with several fate decisions including self-renewal (symmetric and asymmetric cell division), differentiation, apoptosis or migration. These cell fate decisions are thought to occur in distinct bone marrow microenvironments, e.g. niches 6, 7. HSC niches, defined by soluble regulators, cell-cell and cell-extracellular matrix interactions, are presumed to exist within distinct anatomical regions, e.g. metaphysis and/or diaphysis of the BM. However the metaphyseal and diaphyseal regions, as well as the potential niches within these regions, are significantly different. The metaphyses are made up of cancellous (trabecular) bones. The diaphysis is primarily made up of a tube of cortical (compact) bone that defines the femoral shaft 8. HSCs may lodge in distinct regions of the metaphyses, where (like in the diaphyses) each region may have multiple niches associated with it, e.g. osteoblastic, vascular/endothelial niche, CXCL12-expressing reticular cell niche 7, 9-11. Importantly, mature hematopoietic cells including monocytes and osteoclasts also contribute to the niche environment 12, 13. Distinct niches likely exist for HSCs and may selectively or collectively promote different aspects of lineage differentiation and self-renewal. Several genetic mouse models demonstrated that alterations in hematopoietic microenvironments are critical for inducing and/or sustaining hematopoietic disease 14, 15. Therefore there is a growing interest to better understand the molecular pathways governing HSC fate decisions intrinsically and/or extrinsically. In this regard, the non-receptor protein tyrosine kinase Focal Adhesion Kinase (FAK), has been proposed to play an important role in regulating bone marrow microenvironmental signals for HSCs 6, because of its key role as an integrator of intracellular signaling downstream of integrins (e.g. VLA-4 and VLA-5) and growth factors (e.g. CXCL12 and c-kit ligand) 16. FAK is ubiquitously expressed in many cell types, where its activation controls multiple processes of cell behavior, including cell adhesion, motility and survival 16-18. Because Fak deletion is embryonic lethal, FAK function was first extensively studied in mouse embryonic fibroblasts. Following integrin and/or growth factor receptor activation, FAK is catalytically activated and undergoes autophosphorylation at Tyr397, which serves as a binding site for Src-homology 2 (SH2) domain containing Src family kinases. The FAK-Src kinase complex leads to further phosphorylation at additional FAK sites and to the recruitment and activation of multiple downstream signaling proteins. In fibroblasts, FAK plays an important anti-apoptotic role in the nucleus under conditions of stress 19. Recent studies employing conditional Fak gene inactivation approaches have revealed a role for Fak in many cell types (e.g. neuronal, endothelial) including hematopoietic progenitors of erythroid, lymphoid, myeloid and megaloblastic lineage20-24. The mechanisms by which FAK functions and is activated in these different cell types are not fully defined but several studies indicate that they differ in some aspects from activation pathways in fibroblasts 22, 25-27. For example, inhibition of FAK expression or activity can cause apoptosis in some cell types but not others implying that different FAK signaling pathways might exist depending on cell type 25. In preliminary studies, we found that Fak is preferentially expressed in HSCs compared to more differentiated committed progenitor cells. We therefore speculated that Fak might be of particular importance in regulating HSC homeostasis. To this end, an inducible conditional knockout mouse strain (Fakfl/fl Mx1-Cre+) was generated in which the Fak gene could be stably deleted in the bone marrow compartment upon administration of poly(I)-poly(C) (pIpC). Contrary to our prediction, Fak was dispensable for steady-state HSC homeostasis. Although Fak deletion in the bone marrow led to enhanced, long-term (i.e. 20 week duration) engraftment of bone marrow cells in a competitive repopulation assay, this effect was not due to an increase in intrinsic HSC self renewal and/or transplantation ability on a per cell basis but rather the result of an increased HSC pool, which was generated when Fak was inactivated in both the hematopoietic and non-hematopoietic compartment. These findings suggest that FAK signaling may influence reciprocal interactions between HSCs and their niche.
Materials and Methods
Mice
Mx1-Cre and ROSA26-EGFP reporter mice were generously provided by Dr. Stuart Orkin. Fakfl/fl mice 20 were backcrossed for 5 generations onto the C57BL/6 (CD45.2+) background. Congenic mice (CD45.1+, B6.SJL-Ptprca/BoAiTac) were purchased from Taconic (Albany, NY USA). Eight- to twelve-week-old mice received 5 doses of pIpC (25 μg/g of body weight every other day, Sigma, St. Louis, MO USA) injection intraperitoneally. Genotypes and deletion efficiency were determined by PCR or quantitative PCR. All the experiments were performed with the mice at 2 weeks post last pIpC treatment unless otherwise described. Animals were handled and housed in accordance with the guidelines of the Children’s Hospital Boston Animal Care and Use Committee.
Flow Cytometry and Antibodies
Murine single-cell suspensions were prepared from BM, spleen or peripheral blood (PB) after erythrocyte depletion. Cells were stained with antibodies, as indicated: HSC analysis, Biotin-conjugated lineage markers (CD3e, CD4, CD8, B220, CD19, Gr-1, Mac-1, Ter119), Sca-1-APC, c-kit-PE-Cy7, CD34-FITC, Flk-2-PE and Streptavidin-APC-Cy7; PαS cell analysis: CD45-PE, Ter119-PE, Sca-1-FITC, PDGFRα-APC; apoptosis analysis, Annexin-V-PE and 7-AAD; chimerism analysis, CD45.1-PE, CD45.2-APC, B220-APC-Cy7, CD3e-PE-Cy7 and Gr-1-FITC. All antibodies were purchased from BD Biosciences (San Jose, CA USA) and eBiosciences (San Diego, CA USA). Analysis was performed on FACSCanto and sorting was performed on FACSAria. All data were analyzed by FlowJo software.
Colony-Forming Unit in Culture (CFU-C) assay
Bone marrow cells (2×104), spleen cells (1×105), or PB cells (1×105) were cultured in 1mL methycellulose medium with recombinant cytokines and erythropoietin (Stemcell Technologies, Vancouver, CA). Colonies were scored on Day 7.
Cell Cycle Analysis
Hoechst and Ki-67 staining was used to examine cell cycle status. Briefly, bone marrow cells were first stained for LSK surface markers using above described antibodies, fixed and permeabilized with the BD Cytofix/Cytoperm Fixation/permeabilization Solution kit (BD Biosciences, San Jose, CA USA) according to the manufacturer’s recommendations. The cells were next incubated with Ki-67-FITC (BD Biosciences, San Jose, CA USA) on ice for 30 minutes, followed by staining with 10μM Hoechst 33342 (Invitrogen, Carlsbad, CA USA), and analyzed by flow cytometry.
Transplantation
All the recipient mice were lethally irradiated (2 doses of 5.2 Gy, 3 hours apart) on the day of transplantation. For competitive repopulation assay, test donor BM cells (CD45.2+) were mixed with competitor BM cells (CD45.1+/CD45.2+) at a 1:1 ratio and injected into recipients intravenously. For serial transplantation assay, 125 sorted donor long-term HSCs (LT-HSCs, Lin−Sca-1+c-kit+ CD34−Flk-2−, CD45.2+) were competitively transplanted with 2×105 competitor BM cells (CD45.1+/CD45.2+) into primary recipients (CD45.1+). At 16 weeks post-transplantation the donor-derived LT-HSCs were sorted from primary recipients and 125 purified LT-HSCs were injected intravenously into secondary recipients along with 2×105 competitor cells. For reciprocal transplantation, 3×106 wild type BM cells (CD45.1+) were transplanted into either Fakfl/fl or Fakfl/flMx1-Cre+ Mice. Two to four recipients/genotype/experiment were transplanted, and the experiment was carried out in duplicates.
Competitive Homing Assay
LSK cells were sorted from BM samples. 2×105 Fak-deleted or control LSKs were labelled with either CFSE-green or CMTMR-red (Invitrogen, Carlsbad, CA USA), respectively. The labelled cells were mixed at a 1:1 ratio and injected into non-irradiated animals intravenously. Four hours later, mononuclear BM cells were harvested from femurs and analyzed by flow cytometry for homed cells.
Statistical Analysis
Statistical analysis was carried out using two-tailed Student’s t test assuming unequal variance.
Results
In preliminary experiments, we first determined the expression pattern of Fak in normal hematopoietic stem and progenitor cells. Phenotypically defined long-term HSCs (LT-HSCs, Lin−Sca-1+c-kit+CD34−Flk-2−), short-term HSCs (ST-HSCs, Lin−Sca-1+c-kit+CD34+Flk-2−), multipotent progenitors (MPPs, Lin−Sca-1+c-kit+CD34+Flk-2+) and hematopoietic progenitor cells (HPCs, Lin− c-kit+) were sorted from wild type mouse BM samples and Fak expression was examined by quantitative RT-PCR. The expression level of Fak mRNA was highest in LT-HSCs, and decreased 3-fold when LT-HSCs transitioned to ST-HSCs. Fak expression remained relatively low in subsequent hematopoietic progenitor cell populations (Fig. S1).
Fak Is Efficiently Deleted in the Hematopoietic and Non-hematopoietic Bone Marrow Compartment Following pIpC Injection
Fak knockout mice are embryonic lethal, which precludes the analysis of Fak in bone marrow hematopoiesis in adults in vivo. To overcome this experimental restriction, we have generated inducible conditional Fak knockout mice by crossing Fakfl/fl mice with Mx1-Cre transgenic mice, in which Cre expression is driven by an interferon-inducible promoter. Administration of poly(I)-poly(C) (pIpC) and induction of Mx1-Cre leads to efficient excision of floxed alleles in both BM hematopoietic and non-hematopoietic cells. At 2 weeks after 5 doses of pIpC treatment, more than 95% of floxed Fak gene sequence was deleted in Fak−/−Mx1-Cre+ mice BM cells and FAK protein became undetectable (Fig. 1A and 1B). The deletion of Fak gene in BM microenvironment cells was also highly efficient (Fig. 1C). Moreover by monitoring for EGFP expression in Fak−/− Mx1-Cre+EGFP+ mice we found that Fak gene was efficiently excised in BM cell populations enriched for hematopoietic stem and progenitor cells (Fig. 1D). We further confirmed that Fak deletion was not associated with potential compensation by FAK related protein, Pyk-2 28 (Fig. S2). Fak inactivation was also demonstrated in the non-hematopoietic compartment and similar to the hematopoietic compartment, was sustained for over 12 weeks (Fig. 1C). Among the non-hematopoietic cells, we determined that Fak was inactivated in cells expressing PDFRα and Sca-1 (PαS cells) (Fig. S3). PαS cells are enriched for mesenchymal stem cells that support hematopoiesis in vivo 29.
Figure 1. Efficient and stable deletion of floxed Fak allele in Fakfl/flMx1-Cre+ BM compartments after 5 doses of pIpC injection.
(A) Fak deletion was examined by PCR genotyping and western blotting of BM cells from pIpC treated mice. The genotyping PCR product consisted of floxed Fak (400bp band) and Cre-mediated excised fragment (326bp band). Western blot with ß-actin was included for loading controls. (B) Excision efficiency of floxed Fak gene sequence was monitored by qPCR. Genomic DNA was isolated from BM cells of pIpC injected WT (Fakfl/fl) or KO (Fak−/−Mx1-Cre+) mice. The DNA level of undeleted floxed Fak allele was normalized with internal control 18s ribosomal RNA. (C) Fak deletion in non-hematopoietic BM cells. Left, flow cytometric characterization of BM CD45−Ter119− cells from a WT mouse. Right, PCR genotyping was performed with genomic DNA from CD45−Ter119− and whole BM cells from a WT mouse (Lane 1 and 2), a KO mouse (Lane 3 and 4), or a KO mouse – 12 weeks post pIpC (Lane 5 and 6). (D) Left, flow cytometric analysis of EGFP expression in LSKs and Lin−c-Kit+ cells in BM of control (Fakfl/flEGFP+) and knockout (Fak−/−Mx1-Cre+EGFP+) mice. Right, PCR genotyping was performed with the genomic DNA isolated from LSKs, Lin−c-Kit+ and unsorted BM cells of a knockout mouse. All mice were 2 weeks post last pIpC injection unless otherwise indicated.
Fak Deletion in the BM Leads to an Increase in LSK Cells, But Not in Lineage Committed Progenitor Cells
The frequency and absolute numbers of LSK, CD34+ and CD34− LSK were equivalent in Fakfl/fl and Fakwt/-Mx1-Cre+ mice (data not shown) and thus Fakfl/fl mice were used as WT controls. The total cellularity in BM was comparable between Fak knockout (KO, Fak−/− Mx1-Cre+) and wild type (WT, Fakfl/fl) mice at two weeks post pIpC injections (Fig. 2A). The frequencies and numbers of HSC-enriched LSK cells (Lin−Sca-1+c-kit+) and CD34+LSKs (enriched for ST-HSCs) increased 2-fold in Fak KO mice BM when compared with WT controls, while the CD34−LSK population (enriched for LT-HSCs) was unchanged (Fig. 2C and 2D). We also measured the frequency and absolute number of hematopoietic progenitor cells of each lineage in BM of WT and KO animals at 2 weeks post-pIpC. Both the percentage and number of CMP (common myeloid progenitors, Lin− Sca-1+c-kit+CD34+FcγRlow), GMP (granulocyte-monocyte progenitors, Lin−Sca-1+c-kit− CD34+FcγRhigh), MEP (megakaryocyte-erythrocyte progenitors, Lin−Sca-1+c-kit−CD34− FcγR−) and CLP (common lymphoid progenitors, Lin−IL7-R+Sca-1lowc-kitlow) were not statistically different. Similarly the numbers of circulating mature cells in PB were comparable in WT and KO animals (Fig. S4). We also questioned if Fak deletion in the BM would cause HSC mobilization. To address this question, we compared the number of CFU-C, frequency and absolute number of LSK cells in spleen (Fig. 2 E-H) and PB (Fig. 2 I-K). We found no evidence for increased circulating LSK in PB of Fak deleted mice. However, we did detect a two-fold increase in LSK cells in the spleen of Fak deleted mice, which we hypothesize reflects murine hematopoiesis in spleen 30.
Figure 2. Fak deletion leads to increased LSK cells in BM.
(A) BM cellularity was similar in Fak knockout (Fak−/−Mx1-Cre+) and control (Fakfl/fl) mice. BM cells were from two femurs and two tibias. (B) Representative flow cytometry profile of LSKs, CD34+LSKs and CD34−LSKs in BM of a control mouse. (C and D) Comparison of frequency and absolute number of LSKs, CD34+LSKs and CD34−LSKs in BM in control or knockout animals. n=7/genotype. (E-H) Comparison of total cells, number of CFU, frequency of LSKs and absolute number of LSKs in spleen of Fak knockout (Fak−/−Mx1-Cre+) and wild type (Fakfl/fl) mice. n≥7/genotype. (I-K) Comparison of number of CFU, frequency of LSKs and absolute number of LSKs in PB of Fak knockout (Fak−/−Mx1-Cre+) and wild type (Fakfl/fl) mice. n=5/genotype. All animals were 2 weeks after last pIpC treatment. *P<0.05. Data are mean±SD.
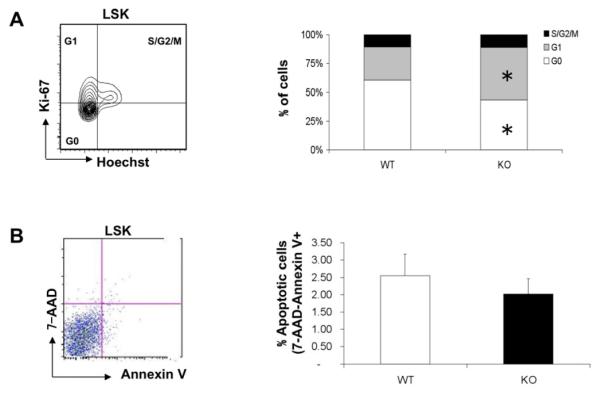
Fak Deletion in the BM Increases the Cycling Status of LSK Cells
The increased BM HSC pool could be due to several reasons including cell cycle progression in HSCs. Hence we determined the cell cycle status of Fak-deleted HSC population by Hoechst and Ki-67 staining. A 1.5-fold decrease of HoechstlowKi-67− (G0) cells and 1.5-fold increase of HoechsthighKi-67+ (G1) cells in Fak−/− LSKs were observed compared to controls (Fig. 3A), suggesting the presence of fewer quiescent LSK cells in Fak knockout mice BM. In addition, 7-AAD and Annexin V staining demonstrated that the relative frequency of apoptotic Fak−/− LSK cells was similar to WT control cells (Fig. 3B).
Figure 3. Fak−/−Mx1-Cre+ mice have reduced quiescent LSK cells.
(A) Left, representative cell cycle analysis (Hoechst and Ki-67 staining) on BM LSK cells of a wild type mouse. Right, % of WT or KO LSK cells in G0, G1 and S/G2/M phase. n=4/genotype. (B) Left, representative flow cytometry analysis of 7-AAD and Annexin V staining of WT BM LSK cells. Right, percentage of apoptotic cells in LSKs derived from freshly isolated BM cells. n≥3/genotype. All the animals were examined 2 weeks after last pIpC injection. *P<0.05. Data are mean±SD.
Fak-deleted BM Cells Exhibit Enhanced Engraftment of Long-term Duration
We next sought to determine if the increase in LSK cells of Fak deleted BM correlated with an increase in phenotypically and functionally defined HSCs. To this end, in vivo competitive repopulation assays were first performed using fixed number of whole bone marrow cells. At 2 weeks post-pIpC injection, 5×105 Fak-deleted or WT donor BM cells (CD45.2+) were mixed with 5×105 competitor BM cells (CD45.1+/CD45.2+), and then injected into lethally irradiated recipient mice (CD45.1+). The contribution of donor-derived cells (% of CD45.2+ cells) was analyzed using PB samples at different time-points post-transplantation (Fig. 4A). About 60% KO donor-derived (CD45.2+) cells were detected at 4, 6, 12 and 20 weeks post transplantation, in comparison to about 25% WT donor-derived cells in PB (Fig. 4B). Moreover, PB chimerism in side scatterhigh granulocytes, which is considered a good indicator of transplanted HSC number and/or function 31, was 3-fold higher in PB of KO recipients (Fig. 4C). Lineage reconstitution in PB was similar between WT and KO donor-derived cells (Fig. 4D) suggesting that Fak deletion did not affect peripheral blood mature B, T and myeloid cell survival. The enhanced engraftment of long-term duration revealed by the competitive repopulation assay suggested that Fak inactivation could have led to an increase in HSC number and/or function. HSC function was therefore tested in serial transplantation assays, whereby 125 freshly sorted donor LT-HSCs (Lin−Sca-1+c-kit+CD34−Flk-2−) from Fak-deleted and WT control mice were competitively transplanted to primary and secondary recipients. The mice transplanted with Fak deleted HSCs exhibited similar PB chimerism at different post-transplantation time-points when compared to control mice (Fig. S5), indicating that Fak-deletion did not affect the self-renewal and/or transplantation ability of LT-HSCs on a per cell basis. In separate competitive homing assay experiments, we found that whole BM (intra and extra-vascular compartment) homing ability of Fak KO LSK cells compared to WT LSK cells was not statistically different (P=0.57) (Fig. S6). These results suggest that the enhanced repopulation ability of Fak deleted BM cells is due to an increased HSC pool with long-term engraftment potential.
Figure 4. Fak-deleted BM Cells Exhibit Enhanced Engraftment of Long-term Duration.
(A) Schematic diagram of competitive repopulation assay. (B) The chimerism of recipients was determined with PB cells at different time points. n≥5/genotype, *P<0.05. CD45.2+ cells were sorted from both BM and PB samples of recipients at 20 weeks after transplantation. Genomic DNA was extracted followed by PCR genotyping. Lane1 and 2, CD45.2+ cells from a KO recipient’s PB and BM; lane 3 and 4, CD45.2+ cells from a WT recipient’s PB and BM. (C) The chimerism was also measured in whole BM cells and PB granulocytes (B220−Gr-1+side scatterhigh) of recipients at 20 weeks post transplantation. n≥5/genotype, *P<0.05. (D) Lineage reconstitution was comparable in WT and KO donor-derived (CD45.2+) cells at 20 weeks post transplantation. Data are mean±SD.
Fak Does Not Intrinsically Regulate Steady-State Hematopoiesis
While earlier studies utilizing the Mx1-Cre approach noted efficient and sustained gene deletion in HSCs, it is currently appreciated that Mx1-Cre mediated deletion following pIpC injection also occurs in niche cells 32, 33, which we also observed in BM of Fak KO mice (Fig. 1C). Two complimentary strategies were used to explore the relative contribution of Fak inactivation in HSC and niche cells to the observed hematopoietic phenotype of Fak−/−Mx1-Cre+ mice. In the first approach, Fak inactivated HSCs are maintained in a WT bone marrow environment. Here 2×105 either floxed Fak (Fakfl/flMx1-Cre+) or WT (Fakfl/fl) BM cells along with the same number of competitor BM cells were transplanted into lethally irradiated recipient mice. Five weeks after transplantation, the mice were treated with pIpC to induce Fak deletion and monitored up to 20 weeks (Fig. 5A). No difference was detected in BM chimerism of LSK cells at 20 weeks post-pIpC, or in PB and PB granulocyte chimerism at 4, 8, 12 and 20 weeks post-pIpC (Fig. 5B). This finding suggests that Fak is not an intrinsic factor in steady-state HSC homeostasis.
Figure 5. Fak Does Not Intrinsically Regulate Steady-State Hematopoiesis.
(A) The chimeric mice were generated by transplanting 2×105 either floxed Fak (Fakfl/flMx1-Cre+) or WT (Fakfl/fl) BM cells along with same number of competitor BM cells into lethally irradiated recipient mice. Five weeks post transplantation, the mice were treated with pIpC to induce Fak deletion and PB chimerism was analyzed at the indicated time points. (B) The chimerisms of PB (Left), PB granulocytes (Upper Right) and LSKs in BM (Lower Right) were not statistically different between WT and KO recipients. P-values are 0.13, 0.30 and 0.92, respectively. CD45.2+ cells were sorted from PB samples of a WT (lane 1) and KO (lane 2) recipient at 20 weeks after transplantation. Genomic DNA was isolated followed by PCR genotyping. n=7/genotype. Data are mean±SEM.
To assess the relative contribution of Fak inactivation in niche cells a reciprocal transplantation assay was performed wherein WT HSCs are maintained in a Fak deleted BM microenvironment. BM cells from CD45.1+ congenic mice were transplanted into lethally irradiated either Fakfl/fl or Fakfl/flMx1-Cre+mice (CD45.2+). The animals were allowed to recover and reconstitute hematopoiesis for 5 weeks, and then treated with pIpC to delete Fak in the bone marrow microenvironment cells. PB and BM samples were examined at 2 weeks after the last pIpC injection (Fig. 6A). The numbers of LSK cells in the BM of Fak KO and WT recipients were not statistically different (Fig. 6B). Moreover, PB chimerism and PB granulocytes exhibited similar levels in KO animals compared to WT controls (Fig. 6C). Hence the ablation of Fak in the BM microenvironment alone did not recapitulate the hematopoietic phenotype, i.e. increase in LSK cells, of Fak−/−Mx1-Cre+ mice, thus arguing against an essential role for extrinsic Fak regulation of HSC homeostasis.
Figure 6. Fak deletion in BM microenvironment niche cells alone does not affect HSC homeostasis.
(A) Schematic diagram of reciprocal transplantation. (B) The chimerisms of PB (Left, P=0.63) and PB granulocytes (Right, P=0.55) were not statistically different between WT and KO recipients. (C) Both frequency and absolute number of LSKs in CD45.1+ donor-derived BM cells were similar between WT and KO recipients at 2 weeks post pIpC. Both p-values are 0.5. n≥3/genotype. Data are mean± SD.
Discussion
HSCs are enriched in the BM cell population that does not express cell-surface markers present on lineage committed hematopoietic cells, but does express the stem cell antigen (Sca-1) and c-kit surface antigens: LSK (Lin−Sca-1+c-kit+) cells. While assaying more surface antigens improves the purity of HSCs within the LSK population 34, in vivo functional assays are considered by many investigators to be the gold-standard for identifying HSCs 35. In our Fak−/−Mx1-Cre+ mouse model, the Fak gene was deleted in both HSCs and the BM microenvironment including mesenchymal stem/stromal cells. A 2-fold increase was detected in number and frequency of LSK cells in BM cells of Fak KO mice compared to WT controls, which correlated with a 2-3 fold enhanced engraftment of long-term duration in a competitive repopulation assay. This phenotype of Fak-deleted BM cells could be due to increased HSC number and/or enhanced HSC function. Unlike the role of Fak in hematopoietic progenitor cells 36, in vitro cell proliferation and transwell migration assays showed that Fak−/− HSCs did not differ from WT HSCs (data not shown). Also, since Fak-deleted HSC exhibit similar self-renewal and transplantation ability as WT HSCs in vivo, we concluded that Fak deletion in the BM affects HSC number rather than function. Curiously however, flow cytometric analyses showed that the number of CD34−LSK cells, which are enriched for more quiescent, i.e. designated as LT-HSCs, was not affected by Fak deletion. In contrast, the number of CD34+LSK cells, enriched for more activated, i.e. designated as ST-HSCs, was increased 2-3 fold suggesting that this cell population was responsible for the enhanced, long-term engraftment of Fak-deleted BM cells. These data could be explained by recent studies, which have challenged the classical hierarchy diagram depicting progenitors arising in an orderly fashion from a prototypical LT-HSC. Importantly, HSC are believed to reversibly switch in vivo from quiescence to self-renewal and back to quiescence 37. Moreover, HSCs with long-term repopulation potential are found within both the quiescent and activated, cycling fractions of LSK cells38. Thus, HSCs in vivo represent groups of cells with varying developmental and functional potential driven by cell autonomous pathways (intrinsic determinants) and instructive cues from the niches (extrinsic determinants) in which HSCs reside. To explore to what extent Fak intrinsically and/or extrinsically regulates HSC pool size, two different types of transplantation assays were performed so that Fak was deleted either only in the hematopoietic compartment or only in the BM microenvironment. In both instances, the increase in number of LSK cells, as observed in Fak KO mice was not statistically significant (Fig. 5B and 6C). These results suggested that the expanded HSC pool observed in Fak−/− Mx1-Cre+ mice, wherein the Fak inactivation occurs in both the hematopoietic and non-hematopoietic environment may be due to altered interactions between HSCs and their microenvironment. Further analyses showed that Fak−/− LSK cells isolated from Fak KO mice contained a lower fraction of quiescent cells, i.e. in G0 cell cycle stage, and consistent with the increased number of CD34+LSK cells. However, HSCs of Fak−/−Mx1-Cre+ mice was not eventually exhausted after secondary transplantation. It is possible, however, that the two transplantations represented an insufficient stress signal to cause HSC exhaustion since infrequently a minimum of three transplantations can be necessary to demonstrate HSC exhaustion 39, 40. Indeed, a principal cell biologic function of FAK is to promote cell survival in cells under conditions of stress 19, 24. In summary, we establish that under steady-state conditions Fak is not an essential intrinsic factor of HSC homeostasis. However, Fak inactivation in both hematopoietic and non-hematopoietic cells in BM leads to an increase in HSC pool size with significantly fewer cells in G0 phase than WT HSCs. Since Fak signaling regulates integrin and CXCL12-induced adhesion 16, 36, it is speculated that Fak plays a role in HSC quiescence maintained by interactions between HSC and the microenvironment 33, 41.
Acknowledgments
Support and Financial Disclosure Declaration
This work was supported by NIH grants P01HL095489-01A1 and T32HL066987 to L.E.S. The authors indicate no financial conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100:157–168. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- 3.Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 4.Yang L, Bryder D, Adolfsson J, et al. Identification of Lin(−)Sca1(+)kit(+)CD34(+)Flt3− short-term hematopoietic stem cells capable of rapidly reconstituting and rescuing myeloablated transplant recipients. Blood. 2005;105:2717–2723. doi: 10.1182/blood-2004-06-2159. [DOI] [PubMed] [Google Scholar]
- 5.Adolfsson J, Mansson R, Buza-Vidas N, et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 7.Yin T, Li L. The stem cell niches in bone. J Clin Invest. 2006;116:1195–1201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greene DA, Naughton GA. Adaptive skeletal responses to mechanical loading during adolescence. Sports Med. 2006;36:723–732. doi: 10.2165/00007256-200636090-00001. [DOI] [PubMed] [Google Scholar]
- 9.Kiel MJ, Yilmaz OH, Iwashita T, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 10.Sugiyama T, Kohara H, Noda M, et al. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 12.Chow A, Lucas D, Hidalgo A, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011;208:261–271. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lymperi S, Ersek A, Ferraro F, et al. Inhibition of osteoclast function reduces hematopoietic stem cell numbers in vivo. Blood. 2011;117:1540–1549. doi: 10.1182/blood-2010-05-282855. [DOI] [PubMed] [Google Scholar]
- 14.Raaijmakers MH, Mukherjee S, Guo S, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464:852–857. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walkley CR, Olsen GH, Dworkin S, et al. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell. 2007;129:1097–1110. doi: 10.1016/j.cell.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 17.Sieg DJ, Hauck CR, Ilic D, et al. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000;2:249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- 18.Glodek AM, Honczarenko M, Le Y, et al. Sustained activation of cell adhesion is a differentially regulated process in B lymphopoiesis. J Exp Med. 2003;197:461–473. doi: 10.1084/jem.20021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim ST, Chen XL, Lim Y, et al. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol Cell. 2008;29:9–22. doi: 10.1016/j.molcel.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beggs HE, Schahin-Reed D, Zang K, et al. FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron. 2003;40:501–514. doi: 10.1016/s0896-6273(03)00666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen TL, Park AY, Alcaraz A, et al. Conditional knockout of focal adhesion kinase in endothelial cells reveals its role in angiogenesis and vascular development in late embryogenesis. J Cell Biol. 2005;169:941–952. doi: 10.1083/jcb.200411155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hitchcock IS, Fox NE, Prevost N, et al. Roles of focal adhesion kinase (FAK) in megakaryopoiesis and platelet function: studies using a megakaryocyte lineage specific FAK knockout. Blood. 2008;111:596–604. doi: 10.1182/blood-2007-05-089680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Y, Zhu BM, Harley B, et al. SOCS3 protein developmentally regulates the chemokine receptor CXCR4-FAK signaling pathway during B lymphopoiesis. Immunity. 2007;27:811–823. doi: 10.1016/j.immuni.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Vemula S, Ramdas B, Hanneman P, et al. Essential role for focal adhesion kinase in regulating stress hematopoiesis. Blood. 2010;116:4103–4115. doi: 10.1182/blood-2010-01-262790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18:516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Le Y, Honczarenko M, Glodek AM, et al. CXC chemokine ligand 12-induced focal adhesion kinase activation and segregation into membrane domains is modulated by regulator of G protein signaling 1 in pro-B cells. J Immunol. 2005;174:2582–2590. doi: 10.4049/jimmunol.174.5.2582. [DOI] [PubMed] [Google Scholar]
- 27.Palazzo AF, Eng CH, Schlaepfer DD, et al. Localized stabilization of microtubules by integrin- and FAK-facilitated Rho signaling. Science (New York, N.Y. 2004;303:836–839. doi: 10.1126/science.1091325. [DOI] [PubMed] [Google Scholar]
- 28.Schaller MD. Cellular functions of FAK kinases: insight into molecular mechanisms and novel functions. J Cell Sci. 2010;123:1007–1013. doi: 10.1242/jcs.045112. [DOI] [PubMed] [Google Scholar]
- 29.Morikawa S, Mabuchi Y, Kubota Y, et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med. 2009;206:2483–2496. doi: 10.1084/jem.20091046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yohei Morita AI, Satoshi Okamura, Sachie Suzuki, Hiroitsu Nakuchi, Hideo Ema. Functional charcterization of hematopoietci stem cells in the spleen. Experimental Hematology. 2010;39:351–359. doi: 10.1016/j.exphem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Bhattacharya D, Bryder D, Rossi DJ, et al. Rapid lymphocyte reconstitution of unconditioned immunodeficient mice with non-self-renewing multipotent hematopoietic progenitors. Cell Cycle. 2006;5:1135–1139. doi: 10.4161/cc.5.11.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larsson J, Ohishi M, Garrison B, et al. Nf2/merlin regulates hematopoietic stem cell behavior by altering microenvironmental architecture. Cell stem cell. 2008;3:221–227. doi: 10.1016/j.stem.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walkley CR, Shea JM, Sims NA, et al. Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell. 2007;129:1081–1095. doi: 10.1016/j.cell.2007.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiel MJ, Yilmaz OH, Iwashita T, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 35.Purton LE, Scadden DT. Limiting factors in murine hematopoietic stem cell assays. Cell stem cell. 2007;1:263–270. doi: 10.1016/j.stem.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 36.Glodek AM, Le Y, Dykxhoorn DM, et al. Focal adhesion kinase is required for CXCL12-induced chemotactic and pro-adhesive responses in hematopoietic precursor cells. Leukemia. 2007;21:1723–1732. doi: 10.1038/sj.leu.2404769. [DOI] [PubMed] [Google Scholar]
- 37.Wilson A, Laurenti E, Oser G, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 38.Takizawa H, Regoes RR, Boddupalli CS, et al. Dynamic variation in cycling of hematopoietic stem cells in steady state and inflammation. J Exp Med. 2011;208:273–284. doi: 10.1084/jem.20101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamminga LM, Bystrykh LV, de Boer A, et al. The Polycomb group gene Ezh2 prevents hematopoietic stem cell exhaustion. Blood. 2006;107:2170–2179. doi: 10.1182/blood-2005-09-3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ito K, Hirao A, Arai F, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 41.Arai F, Suda T. Maintenance of quiescent hematopoietic stem cells in the osteoblastic niche. Ann N Y Acad Sci. 2007;1106:41–53. doi: 10.1196/annals.1392.005. [DOI] [PubMed] [Google Scholar]