Abstract
Objective
To evaluate children’s cardiovascular disease (CVD) risk factors as predictors of parents’ subsequent CVD, type 2 diabetes (T2DM), high blood pressure (HBP).
Study design
26-year prospective follow-up of 852 5-19 year-old black and white schoolchildren (mean age 12, LRC 1973-8), and parents (mean age 40), 519 families, Princeton Schools, Cincinnati, Ohio. Schoolchildren reassessed in the Princeton Follow-up study (PFS 1999-2003) at mean age 39; CVD, T2DM, HBP history of their 1038 parents by mean age 66. Assess relationships of childhood risk factors with parental CVD, T2DM, and HBP. Child-probands identified by triglyceride (TG), blood pressure (BP), LDL cholesterol (LDLC), BMI, and glucose above and HDL cholesterol (HDLC) below established cutoffs.
Results
Pediatric HBP (p= .006) and low HDLC (p=.018) predicted parental CVD ≤ age 50. Pediatric HBP (p = .02) and high TG (p= .03) predicted parental CVD ≤ age 60. Pediatric high TG (p=.009) and high LDLC (p=.04) predicted parental CVD by age 66. Pediatric high BMI (p=.0006) predicted parental T2DM. Pediatric high BMI (p = .003) and black race (p = .004) predicted parental HBP.
Conclusions
Pediatric risk factors identify families with parents at increased risk for CVD, T2DM, and HBP, emphasizing the utility of the child as proband.
Keywords: cardiovascular disease; type 2 diabetes mellitus, hypertension; familial aggregation of risk factors
Approaches to prevent and treat cardiovascular disease (CVD) have been guided in part by aggregation of CVD in families, first noted by Osler a century ago1. Factors aggregating in case-families vs. comparison-families were identified as potential coronary heart disease (CHD) risk factors, including reports of increased total cholesterol in siblings of CHD patients. 2-4 Later studies reported elevated LDL-cholesterol in offspring of patients with premature myocardial infarction.5-8 Community-based studies of unselected families found significant familial associations for cholesterol, lipoprotein cholesterols,9, 10 and blood pressure. 9 These studies led to recommendations for screening pediatric patients11, 12 for high risk levels of CVD risk factors to permit early intervention, especially LDL cholesterol13 and hypertension. 14 Because most children get annual well-child visits, screening children for lipids, blood pressure, obesity, and glucose 12 could identify both children and their parents at increased risk to CVD, type 2 diabetes mellitus (T2DM), and high blood pressure (HBP) following the same reasoning that supports the NCEP11 algorithm.
Relying on significant child-parent correlations for risk factors, children with high risk factors are more likely than children free of risk factors to have parents with high levels of the same risk factors. 15,16 Polonsky et al reported that children with elevated LDLC or high TG/HDLC ratios were more likely to have parents with the same disorder as themselves, concluding that lipid disorders in parents can be predicted by LDLC and TG/HDLC in their children15. Gidding et al measured lipids of parents of hypercholesterolemic children, concluding “when children with hypercholesterolemia are identified, parents should also have lipids assessed.”16 However, Chen et al studied parents and offspring in 477 families, and concluded “…the predictability of parent’s dyslipidemia from their children’s disorder was modest,” and that “…sensitivity and positive predictive values are not high enough to be useful as a selective screening tool.”17 These reports15, 16, 17 focused on parental risk factors, not health outcomes. Within this framework, our specific aim in the current study was to evaluate the use of risk factor screening results of 5 to 19 year old schoolchildren to predict families at high risk for parental CVD, T2DM, HBP outcomes 26 years later, and identify parents for early intervention by reason of their children’s elevated risk factors.
METHODS
All data were collected following protocols approved by the Children’s Hospital Institutional Review Board, with signed informed consent.18,19
We used longitudinal data from the NHLBI Princeton Follow-up Study (PFS, 1999-2003), a 22-30 year follow-up of black and white former schoolchildren and their parents first studied in the NHLBI Lipid Research Clinics (LRC, 1973-1978).18, 19
The Princeton LRC5 and PFS20 have both been described previously. Briefly, the Princeton LRC was a multistage survey of lipids and other CVD risk factors in US and Canadian communities. The Princeton LRC studied students in grades 1 through 12 and a 50% subset of their parents, selected by family. The student population in LRC was 72% white and 28% black, with a mean age of 12.3 ± 3.4 years. Eighty-four percent of eligible students participated at the initial LRC study visit and 91% of eligible students participated at subsequent visits; participation rates did not differ significantly between races. At Visit 1, total cholesterol and TG were measured and family relationships of students and household adults were identified. At Visit 2, complete fasting lipid profiles, blood pressure, glucose, and body mass index (kg/cm2) were measured on random and hyperlipidemic subsets of the Visit 1 participants. At Visit 3, the first degree relatives of random participants at Visit 2 plus all Visit 2 subjects with total cholesterol and/or TG in the 99th percentile had complete fasting lipid profiles, glucose, and body mass index (kg/cm2) measured. Visit 1 ran from September 1973 through June 1975. About 6 weeks after Visit 1, Visit 2 was started, which ran throughout the school year and into the summer of 1975. Visit 3 started in January 1976 and ran for two years.
The PFS was conducted in adults, 22 to 30 years after their initial pediatric (age 5 to 19) LRC sampling to assess changes in CVD risk factor correlations from the period of shared households to separate households and to assess the relation of pediatric risk factors to subsequent health events. PFS eligibility was restricted to former students that participated at LRC Visit 2 and had a sibling or parent at Visit 2 plus all former students and parents participating at Visit 3. The subjects’ own and their parents’ CVD, T2DM and HBP status were obtained by questionnaire. There was no contact with the former schoolchildren during intervals in these studies.
After an overnight fast, in 852 children and in 422 parents the following biosample measurements (TG, HDLC, LDLC, SBP, DBP, BMI, glucose) were made in children and parents at the LRC assessment and at the subsequent PFS study 26 years later.
Diagnosis of CVD, Diabetes, and High Blood Pressure
At PFS, CVD was defined as myocardial infarction, coronary artery bypass graft, angioplasty, ischemic stroke, and carotid or peripheral artery bypass surgery. Information about parental CVD, T2DM, and HBP was obtained by interview with both the parents and with former students. The parents’ CVD, T2DM and HBP positive status was determined by family using the parents’ report if the parent participated in PFS and by the offsprings’ reports if not.
Diagnosis of diabetes was based on World Organization of Health criteria, fasting glucose ≥ 7 mmol/l (126 mg/dl) and/or self-report of diabetes with treatment by a physician.21 In PFS we did not have a measurement of C-peptides or diabetes autoantibody levels, the gold standard methods of distinguishing type 1 from type 2 diabetes. 21 Ten children with type I diabetes mellitus at LRC were removed from the analysis cohort assessed in the current study.
High blood pressure at the PFS visit was defined as a systolic and/or diastolic blood pressure ≥140/90 mmHg or taking blood pressure medication prescribed by a physician.
At the PFS, information regarding medication use was obtained by interview from both former schoolchildren and their parents, including the question “are you currently taking medicine to lower cholesterol, or medicine to lower blood pressure?”
Pediatric Risk Factor Cutoffs
Pediatric high LDLC was defined as ≥110 mg/dl.22 Pediatric metabolic syndrome risk factor cutoffs23 were used as follows: high TG (≥110 mg/dl), low HDLC (≤50 mg/dl in girls, ≤40 in boys), high BP (≥90th age-height specific percentile), high glucose (≥100 mg/dl), and high BMI (≥85th CDC 2000 age-sex specific percentile).
Statistical Methods
Analyses (first univariate, then multivariate) were focused on the question, do childhood risk factors for CVD, T2DM, and HBP predict parental CVD, T2DM, and HBP outcomes.
First, summary data describing the parent and student cohorts at the LRC and PFS were calculated. For LDLC and LDLC/HDLC summary data, offspring and parents taking cholesterol-lowering were excluded; and for SBP and DBP summary data, offspring and parents taking blood pressure lowering medications were excluded.
Second, univariate associations of pediatric high TG, LDLC, BP, glucose, BMI, and low HDLC with parental outcomes (CVD, T2DM, HBP) were calculated providing associated relative risk and confidence intervals. If a family had more than one child with risk factors determined at the LRC, the worst risk factor value for each factor was used in analyses. A family was counted as a case-family for each outcome if either the mother or father had the outcome. The relative risk of the outcome associated with each pediatric risk factor was calculated as the risk ratio in families with ≥ 1 child with an abnormal value for the risk factor to families with no abnormal child probands.
Using pediatric (age 5 to 19) risk factor status as screening tests for parental outcomes (CVD, T2DM, HBP), sensitivity, specificity, positive predictive value, and negative predictive value were calculated, and the significance of the associations between children’s risk factors and parental events were assessed by X2 analyses.
Third, Spearman correlations were calculated between children’s CVD risk factors and their parents’ risk factors in 95 families where data were complete for both parents and ≥1 child at both LRC and PFS. The mean value for father’s and mother’s measures (mid-parent), and the mean value for their offspring (mid-child) were used. Because the ages of children at PFS (median age =39) were similar to parents at LRC (median age =39), correlations between former children at PFS and their parents at LRC were also assessed. In calculations of parent-offspring LDLC and LDLC/HDLC correlations at PFS, participants who reported taking a cholesterol lowering drug were first included and then excluded from analyses, to allow an assessment of how cholesterol-lowering drug use affected parent—offspring LDLC measures. In calculations of parent-offspring SBP and DBP correlations at PFS, participants who reported taking a blood pressure lowering drug were first included and then excluded from analyses.
Risk factors at the LRC in parents who had developed CVD, T2DM, or high blood pressure by PFS (mean age 66) were compared with those in parents who had not developed CVD, T2DM, or high blood pressure by PFS.
Because the parents’ CVD, T2DM and HBP status were available from both the parents’ reports and from offspring’s reports on their parents in 95 families, the concordance of the parents’ and offspring’s reports was calculated, and McNemar’s test was used to check for discordance (over- or under-reporting by offspring). Parental CVD, T2DM and HBP were counted as positive either reported by parents themselves or by their children.
Fourth, in families with health-outcome data available for both parents, stepwise logistic regression analysis was used to identify significant, independent pediatric predictors for parental CVD, T2DM and HBP at the PFS. Explanatory variables included race, offspring’s risk factors at the LRC, all categorized: TG, LDLC, blood pressure, BMI and glucose [high vs not high], and HDLC [low vs not low]. The dependent variables were parental CVD ≤ age 50 vs all other, CVD ≤ age 60 vs all other, all CVD endpoints vs no CVD, parental T2DM vs no T2DM, and parental HBP vs no HBP. Preset cutoffs were used to maximize clinical utility for the pediatrician and family physician. Separately, we re-ran the stepwise logistic regressions using the childhood risk factor as continuous variables (taking mean values in each family) to predict parental outcome. In these models, more explanatory variables were added including children’s age, sex, maturation stages at LRC, and sibship size. In the absence of Tanner staging, maturation stages during childhood at the LRC for boys were age-driven,25 non-pubertal (age <12), mixed non pubertal and pubertal (ages 12-15), and pubertal (age ≥ age 15). Girls were categorized as pre-menarchal and post menarchal.
Fifth, parents’ CVD-free years were counted as the youngest age of CVD in the father or mother in families where parental CVD occurred, and in families without parental CVD, the age (mean of father and mother) at PFS interview was counted as censored CVD-free years used in survival analysis. Kaplan-Meier survival curves were plotted with strata by number of children’s risk factors in TG and LDLC (0 -- both TG and LDLC not high; 1 -- TG high or LDLC high; 2 -- both TG and LDLC high). Parents’ expected CVD-free years in these strata were estimated using SAS LIFEREG procedure, adjusted for race.
RESULTS
In the LRC-PFS, there were 852 former children from 519 families with pediatric risk factor values measured in the LRC (mean age 12.3, ages 5-19) and follow-up at PFS, with report of health status for both mother and father (Table I). In addition, in the LRC (mean age 39.5) and PFS (mean age 66.4), there were 422 parents with sampling of risk factors for CVD, T2DM, and HBP, belonging to 319 families. Summary data for the participating former children and their parents are presented in Table I for both the LRC and PFS.
Table 1.
Childhood (LRC) and young adult (PFS) cardiovascular risk factors in 852 children from 519 families1
| Mean ±SD at LRC | Mean ±SD at PFS | Spearman correlations (values at LRC and PFS) | |
|---|---|---|---|
| Race | W 631 (74%), B 221 (26%) | ||
| Gender | M 396 (46%), F 456 (54%) | ||
| Age (yr) | 12.3 ±3.4 | 38.6 ±3.7 | |
| TG (mg/dl) | 77 ±37 | 136 ±131 | r =0.35, p<.0001 |
| HDLC (mg/dl) | 55 ±12 | 45 ±15 | r =0.47, p<.0001 |
| LDLC (mg/dl) a | 105 ±28 | 121 ±35 | r =0.51, p<.0001 |
| LDL/HDL a | 2.02 ±0.74 | 2.95 ±1.33 | r =0.48, p<.0001 |
| SBP (mmHg) b | 103 ±12 | 119 ±15 | r=0.22, p<.0001 |
| DBP (mmHg) b | 62 ±12 | 78 ±11 | r=0.19, p<.0001 |
| BMI (kg/m2) | 20.0 ±4.3 | 28.6 ±6.9 | r =0.39, p<.0001 |
| Glucose (mg/dl) | 85 ±8 | 90 ±23 | r =0.17, p<.0001 |
| Young adult (LRC) and older adult (PFS) cardiovascular risk factors in 422 parents who had measures at LRC and at PFS | |||
| Mean ±SD at LRC | Mean ±SD at PFS | Spearman correlations | |
| Race | W 335 (79%), B 87 (21%) | ||
| Gender | M 165 (39%), F 257 (61%) | ||
| Age (yr) | 39.5 ±6.5 | 66.4 ±6.5 | |
| TG (mg/dl) | 125 ±76 | 153 ±94 | r =0.43, p<.0001 |
| HDLC (mg/dl) | 53 ±14 | 46 ±15 | r =0.63, p<.0001 |
| LDLC (mg/dl) a | 125 ±32 | 128 ±34 | r =0.44, p<.0001 |
| LDL/HDL a | 2.48 ±1.03 | 2.94 ±1.19 | r =0.57, p<.0001 |
| SBP (mmHg) b | 113 ±11 | 134 ±18 | r =0.20, p=.045 |
| DBP (mmHg) b | 75 ±9 | 78 ±11 | r =0.11, p=.28 |
| BMI (kg/m2) | 26.3 ±4.9 | 30.0 ±6.2 | r =0.65, p<.0001 |
| Glucose (mg/dl) | 90 ±14 | 105±37 | r =0.37, p<.0001 |
(had information of CVD status on father and mother)
excluded cholesterol lowering medication users (31 [4%] offspring, 148 [35%] parents)
excluded BP lowering medication users (63 [7%] offspring, 222 [53%] parents)
Comparing children’s reports of their parents’ CVD, T2DM, and HBP status with reports by the parents themselves in 95 families where both were obtained at PFS revealed that the data were highly concordant: 87% concordant for CVD, 91% for T2DM, and 86% for HBP. There were no significant over- or under- reporting in children’s reports, McNemar’s p>0.17, data not shown.
Of the 519 families, there were 243 (47%) families with parental CVD events by the time of the PFS. Age of first CVD event was recorded in 228 families, the 10th percentile of age being 43 years, the 25th 50 years, the 50th 58 years, the 75th 63 years, and the 90th 69 years. Of 513 families with data on parental T2DM, 190 families (37%) had parental T2DM. Of 499 families with data on parental BP, 347 (70%) had parental HBP at PFS.
By univariate analyses, the risk of CVD at PFS was significantly greater (p<.05) in families with pediatric high TG or high LDLC (Table IV; available at www.jpeds.com), risk of parental T2DM was higher (p<.05) in families with pediatric high BMI (Table V; available at www.jpeds.com), and risk of parental HBP was higher (p<.05) with high pediatric BMI, LDLC, or BP (Table VI; available at www.jpeds.com).
Table IV.
In 519 Families, Percentage of Families with Parental CVD Events by Offsprings’ Pediatric Risk Factor Status
| Pediatric Risk Factor | # Families | Using pediatric risk factor as screening test for parental CVD
|
||||||
|---|---|---|---|---|---|---|---|---|
| # families with CVD | Relative Risk 95% CI | Sensitivity | specificity | Positive predicted value | Negative predicted value | p | ||
|
BMI (≥85th CDC 2000 age-gender specific percentile as high)
| ||||||||
| High | 151 (30%) | 77 (51%) | 1.15 | 33% | 73% | 51% | 55% | Χ2=1.79, p=.18 |
|
| ||||||||
| Not high | 355 (70%) | 158 (45%) | 0.94-1.39 | |||||
|
| ||||||||
|
TG (≥110 mg/dl as high)
| ||||||||
| High | 103 (20%) | 59 (57%) | 1.30 | 24% | 84% | 57% | 56% | Χ2=5.65, p=.018 |
|
| ||||||||
| Not high | 416 (80%) | 184 (44%) | 1.06-1.58 | |||||
|
| ||||||||
|
HDLC (≤50 F, ≤40 M as low)
| ||||||||
| Low | 173 (34%) | 85 (49%) | 1.09 | 36% | 68% | 49% | 55% | Χ2=0.81, p=.37 |
|
| ||||||||
| Not Low | 336 (66%) | 151 (45%) | 0.90-1.33 | |||||
|
| ||||||||
|
LDLC (≥110 mg/dl as high)
| ||||||||
| High | 234 (46%) | 122 (52%) | 1.26 | 52% | 59% | 52% | 59% | Χ2=5.98, p=.015 |
|
| ||||||||
| Not high | 276 (54%) | 114 (41%) | 1.05-1.52 | |||||
|
| ||||||||
|
BP (≥90th age-height specific percentile as high)
| ||||||||
| High | 62 (14%) | 35 (56%) | 1.26 | 17% | 89% | 56% | 55% | Χ2=2.86, p=.091 |
|
| ||||||||
| Not high | 394 (86%) | 177 (45%) | 0.98-1.60 | |||||
|
| ||||||||
|
Glucose (≥100 mg/dl as high)
| ||||||||
| High | 29 (6%) | 12 (41%) | 0.88 | 5% | 94% | 41% | 53% | Χ2=0.32, p=.57 |
|
| ||||||||
| Not High | 479 (94%) | 224 (47%) | 0.57-1.38 | |||||
Table V.
In 519 Families, Percentage of Families with Parental T2DM by Offsprings’ Pediatric Risk Factor Status
| Pediatric Risk Factor | # Families | Using pediatric risk factor as screening test for parental T2DM
|
||||||
|---|---|---|---|---|---|---|---|---|
| # families with T2DM | Relative Risk 95% CI | Sensitivity | specificity | Positive predicted value | Negative predicted value | p | ||
|
BMI (≥85th CDC 2000 age-gender specific percentile as high)
| ||||||||
| High | 148 (30%) | 72 (49%) | 1.53 | 39% | 76% | 49% | 68% | Χ2=12.69, p=.0004 |
|
| ||||||||
| Not High | 352 (70%) | 112 (32%) | 1.23-1.92 | |||||
|
| ||||||||
|
TG (≥110 mg/dl as high)
| ||||||||
| High | 102 (20%) | 43 (42%) | 1.18 | 23% | 82% | 42% | 64% | Χ2=1.43, p=.23 |
|
| ||||||||
| Not high | 411 (80%) | 147 (36%) | 0.91-1.53 | |||||
|
| ||||||||
|
HDLC (≤50 F, ≤40 M as low)
| ||||||||
| Low | 173 (34%) | 73 (42%) | 1.24 | 39% | 69% | 42% | 66% | Χ2=3.17, p=.075 |
|
| ||||||||
| Not Low | 331 (66%) | 113 (34%) | 0.98-1.56 | |||||
|
| ||||||||
|
LDLC (≥110 mg/dl as high)
| ||||||||
| High | 230 (46%) | 88 (38%) | 1.06 | 47% | 55% | 38% | 64% | Χ2=0.24, p=.62 |
|
| ||||||||
| Not high | 274 (54%) | 99 (36%) | 0.84-1.33 | |||||
|
| ||||||||
|
BP (≥90th age-height specific percentile as high)
| ||||||||
| High | 62 (14%) | 28 (45%) | 1.30 | 17% | 88% | 45% | 65% | Χ2=2.49, p=.11 |
|
| ||||||||
| Not high | 388 (86%) | 135 (35%) | 0.96-1.76 | |||||
|
| ||||||||
|
Glucose (≥100 mg/dl as high)
| ||||||||
| High | 29 (6%) | 11 (38%) | 1.03 | 6% | 94% | 38% | 63% | Χ2=0.015, p=.90 |
|
| ||||||||
| Not High | 473 (94%) | 174 (37%) | 0.64-1.67 | |||||
Table VI.
In 519 Families, Percentage of Families with Parental HBP by Offsprings’ Pediatric Risk Factor Status
| Pediatric Risk Factor | # Families | Using pediatric risk factor as screening test for parental HBP
|
||||||
|---|---|---|---|---|---|---|---|---|
| # families with HBP | Relative Risk 95% CI | Sensitivity | specificity | Positive predicted value | Negative predicted value | p | ||
|
BMI (≥85th CDC 2000 age-gender specific percentile as high)
| ||||||||
| High | 147 (30%) | 118 (80%) | 1.23 | 35% | 80% | 80% | 35% | Χ2=10.93, p=.0009 |
|
| ||||||||
| Not High | 340 (70%) | 222 (65%) | 1.10-1.37 | |||||
|
| ||||||||
|
TG (≥110 mg/dl as high)
| ||||||||
| High | 100 (20%) | 71 (71%) | 1.02 | 20% | 81% | 71% | 31% | Χ2=0.094, p=.76 |
|
| ||||||||
| Not high | 399 (80%) | 277 (69%) | 0.89-1.18 | |||||
|
| ||||||||
|
HDLC (≤50 F, ≤40 M as low)
| ||||||||
| Low | 170 (35%) | 127 (75%) | 1.11 | 50% | 61% | 75% | 35% | Χ2=5.48, p=.019 |
|
| ||||||||
| Not Low | 319 (65%) | 214 (67%) | 0.99-1.25 | |||||
|
| ||||||||
|
LDLC (≥110 mg/dl as high)
| ||||||||
| High | 228 (47%) | 171 (75%) | 1.15 | 50% | 61% | 75% | 35% | Χ2=5.48, p=.019 |
|
| ||||||||
| Not high | 262 (53%) | 171 (65%) | 1.02-1.29 | |||||
|
| ||||||||
|
BP (≥90th age-height specific percentile as high)
| ||||||||
| High | 60 (14%) | 49 (82%) | 1.22 | 16% | 92% | 82% | 33% | Χ2=5.14, p=.023 |
|
| ||||||||
| Not high | 377 (86%) | 253 (67%) | 1.06-1.40 | |||||
|
| ||||||||
|
Glucose (≥100 mg/dl as high)
| ||||||||
| High | 28 (6%) | 18 (64%) | 0.92 | 5% | 93% | 64% | 30% | Χ2=0.42, p=.52 |
|
| ||||||||
| Not High | 461 (94%) | 323 (70%) | 0.69-1.22 | |||||
By multivariate analysis, the risk of parental premature CVD before age 50 yrs was greater in families with high pediatric BP and low HDLC (p<.05) and the risk of parental CVD ≤ age 60 yrs was higher in families with high pediatric BP and high TG (p<.05) (Table II). The risk of parental CVD at any age (median age 58 at CVD event) was higher in families with high pediatric TG and LDLC (Table II).
Table 2.
Logistic Regression Models for 452 Families with Parental CVD, T2DM, HBP at PFS
| Parental outcome | Pediatric Predictors | OR, [95% CI], p |
|---|---|---|
| CVD ≤age 50 (62 Yes, 390 no) 452 observations used AUC=0.634 | BP (high vs not high) | 2.49, [1.29-4.80], p=.006 |
| HDLC (low vs not low) | 1.93, [1.12-3.34], p=.018 | |
| CVD ≤age 60 (128 Yes, 324 no) 452 observations used AUC=0.582 | BP (high vs not high) | 1.95, [1.11-3.42], p=.021 |
| TG (high vs not high) | 1.75, [1.07-2.87], p=.026 | |
| CVD any age (211 Yes, 241 no) 452 observations used AUC=0.591 | TG (high vs not high) | 1.90, [1.18-3.07], p=.009 |
| LDLC (high vs not high) | 1.50, [1.03-2.19], p=.036 | |
| T2DM (162 Yes, 285 no) 447 observations used AUC=0.577 | BMI (high vs not high) | 2.10, [1.38-3.20], p=.0006 |
| Hypertension (299 Yes, 134 no) 433 observations used AUC=0.618 | BMI (high vs not high) | 2.16, [1.31-3.56], p=.003 |
| Race (Black vs White) | 2.16, [1.28-3.65], p=.004 |
Stepwise selection from explanatory variables race and mean value of offspring’s pediatric risk factors at LRC: BMI, BP, TG, HDLC, LDLC, and glucose
Pediatric high BMI was a significant, independent predictor of parental T2DM (Table II).
High pediatric BMI and black race were significant, independent predictors of parental HBP (Table II).
The shortest parental CVD-free time was observed in families where children had both high TG and LDLC, with intermediate CVD-free time where children had either high TG or LDLC, and the longest CVD-free time where children had neither high TG nor high LDLC (Figure). For each abnormal factor added (high pediatric TG or high pediatric LDLC), the expected parental CVD-free years were decreased to 97%, p=.04, adjusted for race.
Figure.
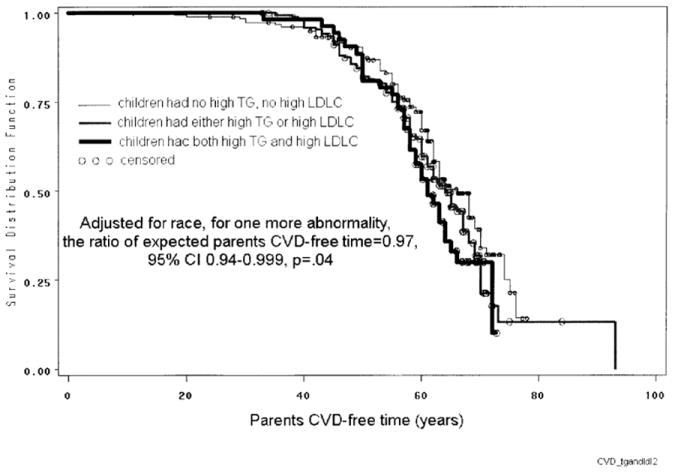
Kaplan-Meier survival curves. Parental CVD-free time related to both high TG and LDLC in children.
Children’s puberty status was not a significant explanatory variable for parental outcomes (Table III). Using the continuous measures of children’s at LRC, childhood TG was a significant predictor for parental CVD (≤ age 50 yrs, ≤ age 60, and at any age [by age 66]; Table III). Children’s LDLC and age at LRC were significant predictors of CVD by age 66 (Table III). Children’s BMI at LRC was a significant predictor for parental T2DM (Table III). Children’s DBP, sex, race, sib ship size were significant predictors for parental HBP (Table III). Whether childhood explanatory variables for parental CVD or T2DM were used as categorical (Table II) or continuous, the significant predictors were the same, TG and LDLC for CVD, BMI for T2DM (Tables II and III)
Table 3.
Logistic Regression Models for 452 Families with Parental CVD, T2DM, HBP at PFS.
| Parental outcome | Pediatric Predictors | OR, [95% CI], p |
|---|---|---|
| CVD ≤age 50 (62 Yes, 390 no) 452 observations used AUC=0.628 | TG at LRC, unit=10mg/dl | 1.11, [1.04-1.18], p=.002 |
| CVD ≤age 60 (128 Yes, 324 no) 452 observations used AUC=0.614 | TG at LRC, unit=10mg/dl | 1.11, [1.05-1.18], p=.0004 |
| CVD any age (211 Yes, 241 no) 452 observations used AUC=0.634 | TG at LRC, unit=10mg/dl | 1.07, [1.001-1.13], p=.048 |
| LDLC at LRC, unit=10mg/dl | 1.10, [1.03-1.19], p=.008 | |
| Age at LRC, unit=year | 1.11, [1.03-1.19], p=.004 | |
| T2DM (162 Yes, 285 no) 447 observations used AUC=0.591 | BMI at LRC, unit=5kg/m2 | 1.38, [1.08-1.77], p=.01 |
| Hypertension (299 Yes, 134 no) 433 observations used AUC=0.684 | DBP at LRC, unit=10 mmHg | 1.41, [1.17-1.71], p=.0004 |
| Sex (Male=1, Female=2) | 1.88, [1.16-3.03], p=.01 | |
| Race (White=1, Black=2) | 2.38, [1.37-4.13], p=.002 | |
| Number of siblings | 1.70, [1.29-2.26], p=.0002 |
Stepwise selection from explanatory variables: race and mean value of offspring’s pediatric measures at LRC: age, sex, BMI, SBP, DBP, TG, HDLC, LDLC, glucose, maturation stage, and number of siblings
In 95 families where data were available for ≥ 1 child and both parents at the LRC and at the PFS, there were significant correlations between midvalues of parents and midvalues of offspring’s risk factors (Table VII; available at www.jpeds.com). At the LRC with mean age of children 12 and parents 39 years, there were significant child-parent correlations (all p<.05) for TG (r=.40), HDLC (r=0.56), LDLC (r=.40), BMI (r=.33), and glucose (r=.47) (Table VII). At the PFS (offspring’s mean age 39 and parents’ mean age 66), there were significant (all p<.05) correlations between adult offspring and parents for TG (r=.32), HDLC (r=0.31), LDLC (r=.29), SBP (r=0.31), BMI (r=.44), and glucose (r=.40) (Table VII).
Table VII.
Correlations between midvalues of parents and midvalues of offspring’s risk factors (TG, HDLC, LDLC, LDLC/HDLC, SBP, DBP, BMI, Glucose) in 95 families with measures on father, mother, and ≥ 1 offspring
| Schoolchildren mean ±SD | Parents mean ±SD | Correlations between children and their parents | ||
|---|---|---|---|---|
| At LRC | ||||
|
| ||||
| Age (yr) | 12.2 ±3.1 | 39.3 ±5.5 | ||
|
| ||||
| TG (mg/dl) | 82 ±36 | 126 ±48 | r=0.40, p<.0001 | |
|
| ||||
| HDLC (mg/dl) | 55 ±10 | 53 ±11 | r=0.56, p<.0001 | |
|
| ||||
| LDLC (mg/dl) | 101 ±24 | 135 ±26 | r=0.40, p<.0001 | |
|
| ||||
| LDL/HDL | 1.91 ±0.60 | 2.76 ±0.75 | r=0.48, p<.0001 | |
|
| ||||
| SBP (mmHg) | 106 ±12 | 121 ±15 | r= -0.05, p=.76 | |
|
| ||||
| DBP (mmHg) | 62 ±13 | 80 ±11 | r= -0.06, p=.71 | |
|
| ||||
| BMI (kg/m2) | 20.3 ±4.1 | 26.2 ±3.3 | r=0.33, p=.0024 | |
|
| ||||
| Glucose (mg/dl) | 85 ±8 | 90 ±9 | r=0.47, p<.0001 | |
|
| ||||
| At PFS | Correlations between children at PFS (median age 39) and parents at LRC (median age 39) | |||
|
| ||||
| Age (yr) | 38.8 ±3.4 | 66.2 ±5.6 | ||
|
| ||||
| TG (mg/dl) | 136 ±81 | 147 ±55 | r=0.32, p=.0016 | r=0.38, p=.0002 |
|
| ||||
| HDLC (mg/dl) | 46 ±14 | 45 ±10 | r=0.31, p=.0020 | r=0.37, p=.0004 |
|
| ||||
| LDLC (mg/dl) | 118 ±28 | 118 ±27 | r=0.29, p=.0044 | r=0.25, p=.018 |
| 113 ±29a | 131 ±27 a | r=0.42, p=.0053 a | ||
|
| ||||
| LDL/HDL | 2.89 ±1.03 | 2.87 ±0.85 | r=0.17, p=.095 | r=0.28, p=.0071 |
| 2.71 ±1.00 a | 3.13 ±0.95 a | r=0.22, p=.16 a | ||
|
| ||||
| SBP (mmHg) | 119 ±12 | 136 ±13 | r= 0.31, p=.0019 | r= -0.0068, p=.96 |
| 112 ±9 b | 129 ±16 b | r=0.29, p=.12 b | ||
|
| ||||
| DBP (mmHg) | 78 ±9 | 77 ±8 | r= 0.22, p=.029 | r= -0.054, p=.69 |
| 74 ±7 b | 74 ±11 b | r=0.38, p=.038 b | ||
|
| ||||
| BMI (kg/m2) | 27.2 ±4.8 | 29.4 ±4.3 | r=0.44, p<.0001 | r=0.42, p<.0001 |
|
| ||||
| Glucose (mg/dl) | 88 ±18 | 103±28 | r=0.40, p<.0001 | r=0.25, p=.020 |
excluded parents (n=68) and schoolchildren (n=2) taking lower-cholesterol medication at PFS
excluded parents (n=92) and schoolchildren (n=12) taking lower-BP medication at PFS
At PFS, 68 of the 190 parents (36%) in the 95 families (where measures were available from both parents and ≥ 1 child) were taking cholesterol-lowering drugs as were 2 of 210 offspring (1%). In PFS, after dropping families where subjects were taking cholesterol-lowering medications, the adult offspring-parent LDLC correlation was 0.42 (Table VII).
At PFS, 92 of the 190 parents (48%) and 12 of 210 (6%) offspring were taking blood pressure lowering drugs. After dropping families where subjects were taking blood pressure-lowering medications, the adult offspring-parent SBP correlation was 0.29, and adult offspring- parent DBP correlation was 0.38 (Table VII). The correlation coefficients between adult offspring and parents did not differ when correlation coefficients were calculated with and without exclusion of subjects taking cholesterol lowering or blood pressure lowering medications, (p>0.05) (Table VII).
Offspring at PFS were about the same age (39) as their parents had been at LRC (Table VII). Between offspring at PFS and their parents at LRC, there were significant offspring: parent correlations for TG (r=.38), HDLC (r=0.37), LDLC (r=.25), BMI (r=.42), and glucose (r=.25) (Table VII).
In 422 parents who had lipid measures at the LRC and outcome information on CVD, T2DM, and HBP at PFS, comparing the group who developed CVD by PFS by age 66 with the group who did not, the CVD group had significantly higher TG, LDL, DBP, BMI and glucose, and lower HDL at LRC at age 39 (Table VIII; available at www.jpeds.com). The parents with T2DM by PFS had higher TG, BMI and glucose, and lower HDL at LRC than the group that did not develop T2DM (Table VIII). The parents with HBP by PFS had higher TG, LDL, SBP, DBP, BMI and glucose at LRC than the group that had no HBP (Table VIII).
Table VIII.
Comparisons in risk factor measures at LRC of parents who, by age 66 in PFS had CVD, T2DM, or HBP vs parents free of CVD, T2DM, or HBP at PFS
| Had CVD at PFS Mean ±SD at LRC | No CVD at PFS Mean ±SD at LRC | Comparison | |
|---|---|---|---|
| Race | W 75 (74%), B 27 (26%) | W 257 (81%), B 59 (19%) | Χ2 =2.87, p=.090 |
| Gender | M 60 (59%), F 42 (41%) | M 103 (33%), F 213 (67%) | Χ2 =22.30, p<.0001 |
| Age (yr) | 42.5 ±7.2 | 38.5 ±5.9 | p<.0001 |
| TG (mg/dl) | 161 ±90 | 115 ±68 | p<.0001, pa <.0001 |
| HDLC (mg/dl) | 49 ±14 | 55 ±13 | p<.0001, pa =.0002 |
| LDLC (mg/dl) | 144 ±37 | 131 ±35 | p=.0049, pa =.0141 |
| LDL/HDL | 3.23 ±1.29 | 2.59 ±1.05 | p<.0001, pa <.0001 |
| SBP (mmHg) | 123 ±17 | 119 ±14 | p=.66, pa =.35 |
| DBP (mmHg) | 83 ±11 | 78 ±12 | p =.0068, pa =.040 |
| BMI (kg/m2) | 27.4 ±5.2 | 25.9 ±4.8 | p =.013, pa =.0131 |
| Glucose (mg/dl) | 94 ±24 | 88±9 | p =.0002, pa =.0025 |
| Had T2DM at PFS Mean ±SD at LRC | No T2DM at PFS Mean ±SD at LRC | Comparison | |
| Race | W 76 (74%), B 27 (26%) | W 237 (82%), B 53 (18%) | Χ2 =2.95, p=.086 |
| Gender | M 44 (43%), F 59 (57%) | M 109 (38%), F 181 (62%) | Χ2 =0.84, p=.36 |
| Age (yr) | 39.2 ±7.2 | 39.4 ±6.2 | p=.56 |
| TG (mg/dl) | 163 ±97 | 114 ±64 | p<.0001 |
| HDLC (mg/dl) | 49 ±14 | 55 ±14 | p<.0001 |
| LDLC (mg/dl) | 140 ±38 | 133 ±35 | p=.088 |
| LDL/HDL | 3.13 ±1.27 | 2.61 ±1.06 | p=.0002 |
| SBP (mmHg) | 123 ±18 | 119 ±14 | p=.25 |
| DBP (mmHg) | 81 ±13 | 79 ±12 | p =.18 |
| BMI (kg/m2) | 29.9 ±5.7 | 25.1 ±4.1 | p <.0001 |
| Glucose (mg/dl) | 96 ±25 | 88±8 | p <.0001 |
| Had HBP at PFS Mean ±SD at LRC | No HBP at PFS Mean ±SD at LRC | Comparison | |
| Race | W 187 (72%), B 71 (28%) | W 135 (89%), B 16 (11%) | Χ2 =16.3, p<.0001 |
| Gender | M 98 (38%), F 160(62%) | M 63 (42%), F 88 (58%) | Χ2 =0.56, p=.46 |
| Age (yr) | 39.7 ±6.5 | 38.9 ±6.4 | p=.16 |
| TG (mg/dl) | 130 ±80 | 114 ±64 | p=.025 |
| HDLC (mg/dl) | 54 ±14 | 53 ±13 | p=.84 |
| LDLC (mg/dl) | 137 ±37 | 129 ±34 | p=.0076 |
| LDL/HDL | 2.78 ±1.14 | 2.64 ±1.13 | p=.099 |
| SBP (mmHg) | 125 ±15 | 113 ±10 | p<.0001 |
| DBP (mmHg) | 83 ±12 | 75 ±8 | p <.0001 |
| BMI (kg/m2) | 27.2 ±5.4 | 24.6 ±3.3 | p <.0001 |
| Glucose (mg/dl) | 91 ±17 | 88±9 | p =.0069 |
For numerical measures, p: comparison by Wilcoxon test; p a LS means adjusted for age
DISCUSSION
Multiple studies have provided evidence supporting screening of children for high risk levels of CVD risk factors to permit early intervention26-35. Whether childhood risk factors cause adult CVD directly, or do so by tracking into adulthood 36, 37 is not well understood. Berenson 38 has advocated universal screening of children for CVD risk. Conventionally, however, it is the parental history of CVD serves as an indication for screening for lipid abnormalities in children. 39, 40,41 The Expert Panel on Blood Cholesterol in Children and Adolescents recommended targeted screening only for children with a family history positive for premature CVD or parental hypercholesterolemia (≥240 mg/dl) 11, 42 and the American Academy of Pediatrics endorsed using these guidelines. 12 The effectiveness of the Expert Panel-Academy of Pediatrics guidelines 12 depends on several factors: 1) the parents’ own pattern of health care utilization, 2) their knowledge of their lipid levels, 3) their awareness of the importance of informing their children’s physician or clinic about their family history; 4) the provider’s knowledge of the family history, and 5) the “Balkanization” of the family’s health care providers. Moreover, the 40 year old ostensibly healthy parent is unlikely to have systematic 43 or practically successful 44 screening for CVD risk factors, and the mother may predominantly have only gynecological care. Identification of CVD risk factors in the child can directly facilitate primary prevention 45 in the child through young adulthood, and also focus diagnostic attention on the potentially high risk parent.
Our finding that pediatric risk factors predict parents’ CVD, T2DM, and HBP outcomes emphasizes the utility of the child as proband. The associations between childhood risk factors and parental CVD events reflect the familial aggregation of triglycerides, HDL and LDL cholesterol, blood pressure, and obesity, as well as the metabolic syndrome.46, 47 In a study of 94 families, Reis et al 48 reported that parents of children with hypertension, obesity, or hypertriglyceridemia had 15 times, 6 times, or 5 times increased odds of having the same risk factors as their children. Reis et al 48 concluded that Identification of cardiovascular disease risk factors in children predicts elevated cardiovascular disease risk in their parents. They48 noted that “…because children access primary care more frequently than adults, children can potentially serve as the index case to identify families at increased risk for cardiovascular disease.”
In the current report, the Princeton LRC and Follow-up Study together indicate that pediatric risk factors measured at mean age 12 identify families at risk for future parental CVD, T2DM, and hypertension by age 66, in large degree due to underlying offspring-parent risk factor correlations during and even after the period of the shared household. Thus, in 5-19 year old children, pediatric TG and LDLC (high or total distribution) predicted parental CVD by mean age 66. CVD-free time was longest in parents whose children had neither high TG nor high LDLC, and was shorter if children had either high TG or high LDLC, or both. Moreover, pediatric BMI (high or total distribution) was associated with parental T2DM by age 66, and high pediatric DBP, black race were associated with parental HBP by age 66. Screening for lipids, BMI, and blood pressure in 5-19 year old children thus identifies families where parents at high risk for CVD, T2DM, and HBP.
At mean age 39, those parents who, by age 66 had CVD, T2DM, and hypertension, had, at their LRC screening, significantly higher abnormal risk factor levels, so that, triggered by their children’s risk factors, in young adulthood they could have been recognized as individuals at increased risk for later CVD, T2DM, and hypertension. Thus, identification of parents stimulated by documentation of risk factors in their children, would identify a relatively young cohort at age 39, where primary prevention could be initiated to prevent later CVD, T2DM, and hypertension.
The power of child-parent correlations for risk factors for CVD, T2DM, and hypertension was illuminated by the finding between children at PFS (median age 39) and their parents at LRC (also median age 39) of significant mid-parent mid-offspring correlations for TG, HDLC, LDLC, BMI, and glucose.
Our findings are congruent with those of Schrott et al 49 who compared CHD and stroke mortality in family members identified by schoolchildren with high total cholesterol (≥ 95th percentile), mid-range total cholesterol (5th < TC < 95th percentile) and low total cholesterol (< 5th percentile) subsets of the Muscatine Iowa Study. Our report differs from that of Schrott et al, 49 focusing specifically on the parents, not the larger family, and on future development of CVD, not contemporaneous CVD status. Our findings are also consistent with earlier observations that coronary artery disease aggregates in families.50
Screening during childhood for risk factors for CVD, T2DM, and hypertension is valuable in prediction of parental disease, as well as an approach to primary prevention of CVD, T2DM, and hypertension in children as they become young adults. Pediatric risk factors for atherosclerosis are associated with young adult atherosclerotic lesions, 26,32 carotid intimal-medical thickening, 27-30 and cardiovascular disease (CVD) events. 31 Increased carotid intimal medial thickness (CIMT) in young adults is associated with high total cholesterol and hypertension in childhood. 33 CVD risk factor status in adolescence predicts increased CIMT in adulthood, independent of adult risk factors. 34 Children with the metabolic syndrome 35 are at 2 to 3 times the risk of having high CIMT and T2DM as adults compared with those free of the metabolic syndrome at youth. We have previously reported 31 that pediatric triglycerides were consistently and independently associated with CVD in the 4th-5th decade of life.
Pediatric risk factors identify families at risk for future parental CVD, T2DM, and HBP, in large degree due to underlying offspring-parent risk factor correlations. There is value in screening children for risk factors for CVD, T2DM, and HBP, with the child as the index case identifying families at increased risk for CVD, T2DM, and HBP. Moreover, increased risk factors for CVD, T2DM, and HBP in parents suggest increased risk factors in children, compared with their peers. The fact that risk for CVD, T2DM, and HBP runs in families, and neither pediatricians nor internists pay sufficient attention to this, is a public health issue of importance for both children and their parents.
Acknowledgments
Supported by National Institutes of Health (HL62394 to J.M.) and the Lipoprotein Research Fund of the Jewish Hospital of Cincinnati (to C.G. and P.W.).
LIST OF ABBREVIATIONS
- LRC
Lipid Research Clinics
- PFS
Princeton School Follow-up Study
- CVD
cardiovascular disease
- CHD
coronary heart disease
- T2DM
type 2 diabetes mellitus
- HBP
high blood pressure
- DBP
diastolic blood pressure
- SBP
systolic blood pressure
- TG
triglyceride
- HDLC
high density lipoprotein cholesterol
- LDLC
low density lipoprotein cholesterol
- BMI
body mass index
- NCEP
National Cholesterol Education Program
- CIMT
carotid intimal-medical thickening
- NHLBI
National Heart, Lung, and Blood Institute
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Osler W. Lumberigon Lectures. Lancet. 1910;1910:2839. [Google Scholar]
- 2.Boas EP, Parets AD, Adlesberg D. Hereditary disturbance of cholesterol metabolism; a factor in the genesis of atherosclerosis. Am Heart J. 1948;35:611–22. doi: 10.1016/0002-8703(48)90646-2. [DOI] [PubMed] [Google Scholar]
- 3.Schaefer LE, Drachman SR, Steinberg AG, Adlersberg D. Genetic studies on hypercholesteremia: frequency in a hospital population and in families of hypercholesteremic index patients. Am Heart J. 1953;46:99–116. doi: 10.1016/0002-8703(53)90243-9. [DOI] [PubMed] [Google Scholar]
- 4.Adlersberg D, Parets AD, Boas EP. Genetics of atherosclerosis; studies of families with xanthoma and unselected patients with coronary artery disease under the age of 50 years. J Am Med Assoc. 1949;141:246–54. doi: 10.1001/jama.1949.02910040008002. [DOI] [PubMed] [Google Scholar]
- 5.Glueck CJ, Fallat RW, Tsang R, Buncher CR. Hyperlipemia in progeny of parents with myocardial infarction before age 50. Am J Dis Child. 1974;127:70–5. doi: 10.1001/archpedi.1974.02110200072010. [DOI] [PubMed] [Google Scholar]
- 6.Hennekens CH, Jesse MJ, Klein BE, Gourley JE, Blumenthal S. Cholesterol among children of men with myocardial infarction. Pediatrics. 1976;58:211–7. [PubMed] [Google Scholar]
- 7.Chase HP, O’Quin RJ, O’Brien D. Screening for hyperlipidemia in childhood. JAMA. 1974;230:1535–7. [PubMed] [Google Scholar]
- 8.Tamir I, Bojanower Y, Levtow O, Heldenberg D, Dickerman Z, Werbin B. Serum lipids and lipoproteins in children from families with early coronary heart disease. Arch Dis Child. 1972;47:808–10. doi: 10.1136/adc.47.255.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson BC, Epstein FH, Kjelsberg MO. Distributions and Familial Studies of Blood Pressure and Serum Cholesterol Levels in a Total Community--Tecumseh, Michigan. J Chronic Dis. 1965;18:147–60. doi: 10.1016/0021-9681(65)90098-6. [DOI] [PubMed] [Google Scholar]
- 10.Morrison JA, Kelly K, Horvitz R, Khoury P, Laskarzewski PM, Mellies MJ, et al. Parent-offspring and sibling lipid and lipoprotein associations during and after sharing of household environments: the Princeton school district family study. Metabolism. 1982;31:158–66. doi: 10.1016/0026-0495(82)90129-9. [DOI] [PubMed] [Google Scholar]
- 11.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 12.Daniels SR, Greer FR. Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122:198–208. doi: 10.1542/peds.2008-1349. [DOI] [PubMed] [Google Scholar]
- 13.The Lipid Research Clinics Coronary Primary Prevention Trial results. II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA. 1984;251:365–74. [PubMed] [Google Scholar]
- 14.Five-year findings of the hypertension detection and follow-up program. II. Mortality by race-sex and age Hypertension Detection and Follow-up Program Cooperative Group. JAMA. 1979;242:2572–7. doi: 10.1001/jama.1979.03300230028022. [DOI] [PubMed] [Google Scholar]
- 15.Polonsky SM, Simbartl LA, Sprecher DL. Triglyceride and high-density lipoprotein cholesterol: predicting disorders in parents from their children. Pediatrics. 1994;94:824–31. [PubMed] [Google Scholar]
- 16.Gidding SS, Whiteside P, Weaver S, Bookstein L, Rosenbaum D, Christoffel K. The child as proband High prevalence of unrecognized and untreated hyperlipidemia in parents of hyperlipidemic children. Clin Pediatr (Phila) 1989;28:462–5. doi: 10.1177/000992288902801006. [DOI] [PubMed] [Google Scholar]
- 17.Chen W, Srinivasan SR, Bao W, Wattigney WA, Berenson GS. The relationship of conjoint traits of dyslipidemias between young offspring and their parents in a community-based sample. Prev Med. 1997;26:717–23. doi: 10.1006/pmed.1997.0197. [DOI] [PubMed] [Google Scholar]
- 18.Morrison JA, Friedman LA, Wang P, Glueck CJ. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J Pediatr. 2008;152:201–6. doi: 10.1016/j.jpeds.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Obesity and cardiovascular disease risk factors in black and white girls: the NHLBI Growth and Health Study. Am J Public Health. 1992;82:1613–20. doi: 10.2105/ajph.82.12.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrison JA, Glueck CJ, Horn PS, Wang P. Childhood predictors of adult type 2 diabetes at 9- and 26-year follow-ups. Arch Pediatr Adolesc Med. 2010;164:53–60. doi: 10.1001/archpediatrics.2009.228. [DOI] [PubMed] [Google Scholar]
- 21.Dabelea D, Bell RA, D’Agostino RB, Jr, Imperatore G, Johansen JM, Linder B, et al. Incidence of diabetes in youth in the United States. Jama. 2007;297:2716–24. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- 22.Magnussen CG, Raitakari OT, Thomson R, Juonala M, Patel DA, Viikari JS, et al. Utility of currently recommended pediatric dyslipidemia classifications in predicting dyslipidemia in adulthood: evidence from the Childhood Determinants of Adult Health (CDAH) study, Cardiovascular Risk in Young Finns Study, and Bogalusa Heart Study. Circulation. 2008;117:32–42. doi: 10.1161/CIRCULATIONAHA.107.718981. [DOI] [PubMed] [Google Scholar]
- 23.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988-1994. Arch Pediatr Adolesc Med. 2003;157:821–7. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 24.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Curr Opin Cardiol. 2006;21:1–6. doi: 10.1097/01.hco.0000200416.65370.a0. [DOI] [PubMed] [Google Scholar]
- 25.Biro FM, Lucky AW, Huster GA, Morrison JA. Pubertal staging in boys. J Pediatr. 1995;127:100–2. doi: 10.1016/s0022-3476(95)70265-2. [DOI] [PubMed] [Google Scholar]
- 26.McMahan CA, Gidding SS, Malcom GT, Tracy RE, Strong JP, McGill HC., Jr Pathobiological determinants of atherosclerosis in youth risk scores are associated with early and advanced atherosclerosis. Pediatrics. 2006;118:1447–55. doi: 10.1542/peds.2006-0970. [DOI] [PubMed] [Google Scholar]
- 27.Juonala M, Magnussen CG, Venn A, Dwyer T, Burns TL, Davis PH, et al. Influence of age on associations between childhood risk factors and carotid intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study, the Childhood Determinants of Adult Health Study, the Bogalusa Heart Study, and the Muscatine Study for the International Childhood Cardiovascular Cohort (i3C) Consortium. Circulation. 2010;122:2514–20. doi: 10.1161/CIRCULATIONAHA.110.966465. [DOI] [PubMed] [Google Scholar]
- 28.Juonala M, Viikari JS, Kahonen M, Taittonen L, Laitinen T, Hutri-Kahonen N, et al. Life-time risk factors and progression of carotid atherosclerosis in young adults: the Cardiovascular Risk in Young Finns study. Eur Heart J. 2010;31:1745–51. doi: 10.1093/eurheartj/ehq141. [DOI] [PubMed] [Google Scholar]
- 29.Magnussen CG, Venn A, Thomson R, Juonala M, Srinivasan SR, Viikari JS, et al. The association of pediatric low- and high-density lipoprotein cholesterol dyslipidemia classifications and change in dyslipidemia status with carotid intima-media thickness in adulthood evidence from the cardiovascular risk in Young Finns study, the Bogalusa Heart study, and the CDAH (Childhood Determinants of Adult Health) study. J Am Coll Cardiol. 2009;53:860–9. doi: 10.1016/j.jacc.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freedman DS, Dietz WH, Tang R, Mensah GA, Bond MG, Urbina EM, et al. The relation of obesity throughout life to carotid intima-media thickness in adulthood: the Bogalusa Heart Study. Int J Obes Relat Metab Disord. 2004;28:159–66. doi: 10.1038/sj.ijo.0802515. [DOI] [PubMed] [Google Scholar]
- 31.Morrison JA, Glueck CJ, Horn PS, Yeramaneni S, Wang P. Pediatric triglycerides predict cardiovascular disease events in the fourth to fifth decade of life. Metabolism. 2009;58:1277–84. doi: 10.1016/j.metabol.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berenson GS, Srinivasan SR, Bao W, Newman WP, 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338:1650–6. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 33.Davis PH, Dawson JD, Riley WA, Lauer RM. Carotid intimal-medial thickness is related to cardiovascular risk factors measured from childhood through middle age: The Muscatine Study. Circulation. 2001;104:2815–9. doi: 10.1161/hc4601.099486. [DOI] [PubMed] [Google Scholar]
- 34.Raitakari OT, Juonala M, Kahonen M, Taittonen L, Laitinen T, Maki-Torkko N, et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003;290:2277–83. doi: 10.1001/jama.290.17.2277. [DOI] [PubMed] [Google Scholar]
- 35.Magnussen CG, Koskinen J, Chen W, Thomson R, Schmidt MD, Srinivasan SR, et al. Pediatric metabolic syndrome predicts adulthood metabolic syndrome, subclinical atherosclerosis, and type 2 diabetes mellitus but is no better than body mass index alone: the Bogalusa Heart Study and the Cardiovascular Risk in Young Finns Study. Circulation. 2010;122:1604–11. doi: 10.1161/CIRCULATIONAHA.110.940809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daniels SR. Can lipid and lipoprotein concentrations in childhood predict adult atherosclerosis? J Am Coll Cardiol. 2009;53:870–1. doi: 10.1016/j.jacc.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 37.Nadeau KJ, Maahs DM, Daniels SR, Eckel RH. Childhood obesity and cardiovascular disease: links and prevention strategies. Nat Rev Cardiol. 2011;8:513–25. doi: 10.1038/nrcardio.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berenson GS, Pickoff AS. Preventive cardiology and its potential influence on the early natural history of adult heart diseases: the Bogalusa Heart Study and the Heart Smart Program. Am J Med Sci. 1995;310(Suppl 1):S133–8. doi: 10.1097/00000441-199512000-00024. [DOI] [PubMed] [Google Scholar]
- 39.Schwandt P, Haas GM, Liepold E. Lifestyle and cardiovascular risk factors in 2001 child-parent pairs: the PEP Family Heart Study. Atherosclerosis. 2010;213:642–8. doi: 10.1016/j.atherosclerosis.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 40.Dennison BA, Kikuchi DA, Srinivasan SR, Webber LS, Berenson GS. Parental history of cardiovascular disease as an indication for screening for lipoprotein abnormalities in children. J Pediatr. 1989;115:186–94. doi: 10.1016/s0022-3476(89)80063-0. [DOI] [PubMed] [Google Scholar]
- 41.Screening for lipid disorders in children: US Preventive Services Task Force recommendation statement. Pediatrics. 2007;120:e215–9. doi: 10.1542/peds.2006-1812. [DOI] [PubMed] [Google Scholar]
- 42.National Cholesterol Education Program (NCEP): highlights of the report of the Expert Panel on Blood Cholesterol Levels in Children and Adolescents. Pediatrics. 1992;89:495–501. [PubMed] [Google Scholar]
- 43.Greenland P, Smith SC, Jr, Grundy SM. Improving coronary heart disease risk assessment in asymptomatic people: role of traditional risk factors and noninvasive cardiovascular tests. Circulation. 2001;104:1863–7. doi: 10.1161/hc4201.097189. [DOI] [PubMed] [Google Scholar]
- 44.Boulware LE, Marinopoulos S, Phillips KA, Hwang CW, Maynor K, Merenstein D, et al. Systematic review: the value of the periodic health evaluation. Ann Intern Med. 2007;146:289–300. doi: 10.7326/0003-4819-146-4-200702200-00008. [DOI] [PubMed] [Google Scholar]
- 45.Nguyen QM, Srinivasan SR, Xu JH, Chen W, Kieltyka L, Berenson GS. Utility of childhood glucose homeostasis variables in predicting adult diabetes and related cardiometabolic risk factors: the Bogalusa Heart Study. Diabetes Care. 2010;33:670–5. doi: 10.2337/dc09-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morrison JA, Ford ES, Steinberger J. The pediatric metabolic syndrome. Minerva Med. 2008;99:269–87. [PubMed] [Google Scholar]
- 47.Morrison JA, Friedman LA, Gray-McGuire C. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: the Princeton Lipid Research Clinics Follow-up Study. Pediatrics. 2007;120:340–5. doi: 10.1542/peds.2006-1699. [DOI] [PubMed] [Google Scholar]
- 48.Reis EC, Kip KE, Marroquin OC, Kiesau M, Hipps L, Jr, Peters RE, et al. Screening children to identify families at increased risk for cardiovascular disease. Pediatrics. 2006;118:e1789–97. doi: 10.1542/peds.2006-0680. [DOI] [PubMed] [Google Scholar]
- 49.Schrott HG, Clarke WR, Wiebe DA, Connor WE, Lauer RM. Increased coronary mortality in relatives of hypercholesterolemic school children: the Muscatine study. Circulation. 1979;59:320–6. doi: 10.1161/01.cir.59.2.320. [DOI] [PubMed] [Google Scholar]
- 50.Mahoney LT, Burns TL, Stanford W, Thompson BH, Witt JD, Rost CA, et al. Coronary risk factors measured in childhood and young adult life are associated with coronary artery calcification in young adults: the Muscatine Study. J Am Coll Cardiol. 1996;27:277–84. doi: 10.1016/0735-1097(95)00461-0. [DOI] [PubMed] [Google Scholar]


