Abstract
Objectives
Therapeutic hypothermia is widely-employed for neuroprotection after cardiac arrest(CA). However, concern regarding elevated drug concentrations during hypothermia and increased adverse drug reaction risk complicates concurrent pharmacotherapy. Many commonly used medications in critically ill patients rely on the cytochrome P450(CYP) 3A isoform for their elimination. Therefore, our study objectives were to determine the effect of mild hypothermia on the in vivo pharmacokinetics of fentanyl and midazolam, two clinically-relevant CYP3A substrates, after CA and to investigate the mechanisms of these alterations.
Design
Prospective, randomized, controlled study
Setting
University research laboratory
Subjects
Thirty two adult male Sprague-Dawley rats
Interventions
An asphyxial CA rat model was used and mild hypothermia(33 °C) was induced 1h post injury by surface cooling and continued for 10 hours to mimic the prolonged clinical application of hypothermia accompanied by intensive care interventions. Fentanyl and midazolam were independently administered by intravenous infusion and plasma and brain concentrations were analyzed using ultra-performance liquid chromatography tandem mass spectrometry. Cyp3a2 protein expression was measured and a Michaelis-Menten enzyme kinetic analysis was performed at 37°C and 33°C using control rat microsomes.
Measurements and Main Results
Mild hypothermia decreased the systemic clearance of both fentanyl (61.5±11.5 to 48.9±8.95 mL/min/kg;p < 0.05) and midazolam (89.2±12.5 to 73.6±12.1 mL/min/kg;p < 0.05) after CA. The elevated systemic concentrations did not lead to parallel increased brain exposures of either drug. Mechanistically, no differences in Cyp3a2 expression was observed, but the in vitro metabolism of both drugs was decreased at 33 °C versus 37 °C through reductions in enzyme metabolic capacity rather than substrate affinity.
Conclusions
Mild hypothermia reduces the systemic clearances of fentanyl and midazolam in rats after CA through alterations in Cyp3a metabolic capacity rather than enzyme affinity as observed with other CYPs. Contrasting effects on blood and brain levels further complicates drug dosing. Consideration of the impact of hypothermia on medications whose clearance is dependent on CYP3A metabolism is warranted.
Keywords: hypothermia, cardiac arrest, drug metabolism, pharmacokinetics, midazolam, fentanyl
Introduction
Mild hypothermia, also known as targeted temperature management, is a recommended clinical intervention for neuroprotection. Mild hypothermia is generally defined as the controlled reduction of body temperature to 32-34°C for 12-48 hours and has been shown to decrease mortality and improve neurological outcomes after out-of-hospital cardiac arrest (CA)(1-3). Hypothermia alters the pharmacokinetics of several medications commonly used in CA patients. Clinical studies have reported toxic morphine concentrations in neonates(4), reduced systemic clearances of phenytoin(5) and midazolam(6) in adult traumatic brain injury patients, and temperature-dependent reductions in vecuronium(7) and midazolam(8) clearance in healthy volunteers. Little mechanistic data exist, but our lab and others have suggested an acute decrease in hepatic cytochrome p450(CYP)-mediated drug metabolism as the cause(9, 10). In preclinical studies, moderate cooling (30°C) substantially decreased the systemic clearance of a probe for CYP2E1 activity immediately after CA by reducing enzyme binding affinity(11). It is unknown whether this finding extends equally to other CYPs, the milder temperatures now applied clinically, or medications that are also CYP substrates.
CYP3A is the most important drug metabolizing enzyme family as it metabolizes approximately 50% of medications(12, 13). Variations in activity are a critical determinant of drug clearance and are involved in numerous clinically relevant drug-drug interactions observed in critically ill patients(14). Fentanyl and midazolam, as well as other common ICU medications, rely upon CYP3A for drug elimination. Given the complicated medication regimens that CA patients receive and the increased risk of adverse drug reactions in the intensive care unit(15), it is imperative to understand the effects of any new therapies on this enzyme system. Furthermore, current studies have focused on circulating drug levels in the blood. Whereas, the effects of hypothermia on brain tissue levels and subsequent brain pharmacodynamics, have not been explored. Given the fact that many sedatives and analgesics are metabolized by CYP3A system and have sites of action within the central nervous system, this represents an important knowledge gap for hypothermia optimization.
Based on these previous studies, we sought to investigate the impact of mild hypothermia on the pharmacokinetics of CYP3A-dependent drug metabolism after CA. We independently evaluated the disposition of fentanyl and midazolam in an animal model of CA. The specific study objectives were (1) to evaluate the effects of mild therapeutic hypothermia on the systemic clearance and brain penetration of fentanyl and midazolam during an extended period of mild hypothermia in a rat model of CA and (2) to identify the mechanisms underlying alterations in the systemic clearance by evaluating the effects of hypothermia on hepatic Cyp3a protein expression and in vitro enzyme kinetics.
Materials and Methods
Materials and chemicals
Fentanyl and midazolam for animal dosing were purchased from Hospira (Lake Forest, IL) and Bedford Labs, (Bedford, OH), respectively. Analytical standards of these medications, their primary metabolites (norfentanyl and 4-hydroxymidazolam), and deuterated internal standards were purchased from Cerilliant (Round Rock, TX). Organic solvents were purchased from ThermoFisher Scientific (Pittsburgh, PA). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
CA and hypothermia protocol
All studies were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh. Thirty-two healthy adult male Sprague-Dawley rats (300-350g) were purchased from Hilltop Laboratories (Scottsdale, PA) and acclimated for one week prior to study initiation. The animals were allowed free access to food and water and were housed on a 12-h light/dark cycle.
CA was induced as previously described(16, 17) with modifications for a prolonged time period of temperature management, and steady-state pharmacokinetic studies. Rats were anesthetized with 4% isoflurane via nose cone, endotracheally intubated, and mechanically ventilated to maintain a pCO2 of 35-45mmHg. Neuromuscular blockade was achieved with vecuronium 2 mg/kg and isoflurane was reduced to 1%. The left femoral artery, left femoral vein, and left jugular vein were cannulated for mean arterial pressures/blood gas sampling, pharmacokinetic sampling, and drug administration, respectively. A rectal probe was inserted for continuous body temperature measurement and electrocardiogram leads were placed. After preparative surgery, the CA arrest protocol was initiated. Inhaled anesthetics were washed out with 100% oxygen for 3 min followed by 2 min of room air to avoid the confounding cerebroprotective effects of inhaled anesthetics, thereby mimicking clinical CA. Vecuronium was administered and mechanical ventilation was disconnected for 8 minutes to induce an asphyxial CA. Approximately 5-6 min of asystole was confirmed by the electrocardiogram trace. Resuscitation was started by the administration of intravenous epinephrine (0.005 mg/kg) and sodium bicarbonate (1mEq/kg), reconnection of the ventilator (100% oxygen, 60 breaths per min), and providing chest compressions (200 per min) until restoration of spontaneous circulation (ROSC). Intravenous fluids (normal saline at 3mL/kg/h) and 0.5% isoflurane were re-initiated 30 min after ROSC.
Experimental manipulation of temperature was initiated 60 min after ROSC. Animals were randomized to mild hypothermia (33±0.5°C) or controlled normothermia (37±0.5°C) with target temperatures achieved via surface cooling (adjusted with ice bags, a fan, heating light, and heating pad) over 30 min followed by a 15 min stabilization period. The delay in hypothermia induction and target temperatures were selected to mimic clinical situations. This temperature was maintained through the 10h study duration.
Arterial blood gases, glucose, hematocrit, and electrolytes were measured prior to arrest; 10 min, 30 min, and 60 min after ROSC; at the end of the cooling and stabilization periods, and hourly during the pharmacokinetic study. Acidosis was corrected via infusion of sodium bicarbonate to maintain a pH=7.4. Blood pressure and electrocardiogram were also continuously recorded.
Drug administration and pharmacokinetic sampling
Rats were randomized to 1 of 4 groups in a 2×2 matrix: normothermia or hypothermia and either midazolam or fentanyl. After the 15 min stabilization period, midazolam (1.5mg/kg/h) or fentanyl (50μg/kg/h) was dosed by continuous intravenous infusion to clinically relevant, steady-state concentrations over 8 or 10 h, respectively. Plasma samples (0.3mL) were collected at baseline, 0.25,0.5,1 2 4,6,7, and 8 hours for midazolam, and at baseline, 1,2,3,4,6,8,9, and 10 h for fentanyl and were immediately frozen at -20°C. Sample volume was replaced with an equal volume of normal saline.
Brain tissue was also collected in order to measure drug distribution into this compartment during hypothermia. To minimize potential confounding effects of circulatory blood, transcardial perfusion with normal saline was conducted. Rats were killed by isoflurane overdose and blood washout was achieved by clamping the descending aorta, perforating the right atrium, and injecting 25mL of ice-cold normal saline into the left cardiac ventricle over 1 min. This rapid perfusion was identical in all treatment groups. Once perfused, brains were removed and frozen in liquid nitrogen and stored at -80°C. Liver tissue from each animal was also collected for Cyp3a expression analysis.
Drug analysis by UPLC-MS/MS
Fentanyl and midazolam total concentrations were measured in plasma and brain tissue by ultra-performance liquid chromatography tandem triple quadrupole mass spectometry (UPLC-MS/MS) using modifications of established methods(8, 18, 19). Fentanyl and Midazolam extractions were performed via 3mL Oasis MCX or 1mL Oasis HLB cartridges (Waters, Milford, MA), respectively, following the manufacturer's protocol. For fentanyl, plasma samples were acidified by adding 1 mL of 2% o-phosphoric acid containing internal standard (15ng/mL fentanyl-d5) to 100μL of plasma, prior to column loading. For midazolam, 1mL of water containing the internal standard, midazolam-d4, was added to 40μL of plasma. Brain tissue was processed using the same protocol following tissue homogenization using a Tissue Tearor (Biospec products, Bartlesville, OK) and centrifugation at 10,000 rpm (∼7000 g) for 30 min at 4°C. After the cartridge eluent was collected and evaporated to dryness under nitrogen, samples were reconstituted in 100μL 90% 0.15% formic acid/10% acetonitrile for fentanyl and 200μL 70% water/30% acetonitrile for midazolam. Chromatographic separation was achieved using a BEH-C18 1.7μm, 2.1×100mm column (Thermo, Pittsburgh, PA). The fentanyl mobile phase consisted of 0.15% formic acid (solution A) and acetonitrile (solution B) to provide a 0.2mL/min gradient elution as follows: initially 90%A/10%B for 1 min, then linearly increasing to 100%B over 3 min and holding for 1 min before returning to initial conditions over 0.5 min and holding for 2 min to achieve a total run time of 7.5 min. Midazolam mobile phase consisted of water containing 5% acetonitrile and 0.005% acetic acid (solution A) and acetonitrile containing 0.005% acetic acid (solution B) to provide a 0.4mL/min gradient elution as follows: initially 70%A/30%B for 4.5 min, then linearly increasing to 30%A/70%B over 0.5 min and holding for 0.5 min, before finally returning to initial conditions over 0.5 min and holding for 1 min to achieve a total run time of 7 min. Mass spectrometric detection of fentanyl (ion transition, 388→188), norfentanyl (233→84), midazolam (ion transition, 326→291) and 4-hyroxymidazolam (342→325,234) was performed using a TSQ Quantum Ultra MS/MS with an electrospray ionization source (Thermo, Waltham, MA) under positive mode with a collision gas pressure of 1.5 mTorr. Fentanyl specific conditions include a spray voltage of 3500V; collision energy of 23V; vaporizer and capillary temperature of 200°C and 350°C respectively; and sheath and auxiliary gas pressures of 60PSI and 35PSI, respectively. Midazolam specific conditions include a spray voltage of 4000V; collision energy of 27V; vaporizer and capillary temperature of 200°C and 255°C respectively; and sheath and auxiliary gas pressures of 60PSI and 55PSI, respectively. The XCalibur 2.0 software was used for data acquisition and analysis. Calibration curves for fentanyl and norfentanyl were linear from 0.125-200ng/mL (R2=0.9995) and 1-200ng/mL (R2=0.9990) with lower limits of quantification of 0.125ng/mL and 1ng/mL, respectively. Inter- and intra-assay variation was <15%. Calibration curves for midazolam and 4-hydroxymidazolam were linear from 5-1000ng/mL (R2=0.9996) and 0.5-75ng/mL (R2=0.9932) with lower limits of quantification of 5ng/mL.
Pharmacokinetic analysis
Pharmacokinetic parameters were estimated by noncompartmental methods. The plasma steady-state level was determined by averaging the last 3 plasma concentrations and by verifying that the coefficient of variance in each animal was less than 15%. Systemic clearance was calculated by dividing an animal's dosing rate by the steady state concentration. Brain tissue concentrations (ng/gm wet weight) were divided by the steady-state concentration to determine the brain to plasma ratio in each animal.
Microsomal preparation and Western blotting
Liver tissue microsomes were prepared by standard methods (20). Total protein was determined by bicinchoninic acid colorimetric assay (Thermo Pierce, Rockford, IL). Western blotting for Cyp3a2 protein expression was performed on liver harvested at the end of the study (∼10-12 h post-CA). Liver microsomes containing 2μg of protein were denatured with Laemmli sample buffer and heated for 10 min at 75°C. Samples were resolved on 4-15% Mini-Protein TGX polyacrylamide gels (Bio-Rad, Hercules, CA), transferred to Millipore IPFL membranes, and blocked with LI-COR Odyssey blocking reagent. Membranes were then probed with 1:4,000 primary mouse monoclonal anti-β-actin (Sigma-Aldrich, St. Louis, MO) and 1:6,000 primary rabbit anti-CYP3A2 (Millipore, Billerica, MA) followed by LI-COR 1:60,000 secondary goat anti-mouse IRDye® 680LT and 1:25,000 secondary goat anti-rabbit IRDye®800CW. All membrane incubations occurred in the dark for 1 h at room temperature while rocking. β-actin-normalized Cyp3a2 protein expression was quantified by densitometry using an Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, Nebraska). Assay linearity was confirmed in-membrane with controls for intermembrane comparisons.
Enzyme kinetic analysis
Microsomes from untreated rats were incubated under conditions of 200μg protein, 1mM reduced nicotinamide adenine dinucleotide phosphate and either fentanyl or midazolam in increasing concentrations from 1–150μM or 1–80μM, respectively. Duplicate samples were incubated for 12 min (fentanyl) or 2 min (midazolam) at either 37°C or 33°C in a circulating water bath. The reactions were stopped by a 10-fold dilution into ice-cold 2% o-phosphoric acid (fentanyl) or incubation buffer (midazolam) containing the deuterated metabolite internal standard. Samples were analyzed for norfentanyl or 4-hydroxymidazolam as described previously.
The effect of incubation temperature on metabolite formation rates was determined through Michaelis-Menten kinetic analysis using Graphpad Prism 5.04 (Graphpad Software, La Jolla, CA). Maximum velocity(Vmax) and the Michaelis-Menten constant(Km) were estimated by nonlinear regression of the raw data. The following equation was fit to the data: ν = Vmax · [S]/(Km + [S]) where ν is the velocity of the reaction and [S] is the substrate concentration. As some substrates can inhibit enzymatic activity at high concentrations, a model incorporating noncompetitive substrate inhibition was used where indicated: ν = Vmax · [S]/(Km + [S]·(1+[S]/Ki)), where Ki is the dissociation constant for binding. To determine if the more complex substrate inhibition model fit the data better, an extra sum of squares F-test was used. The data were also linearized via an Eadie-Hofstee plot for visual inspection.
Statistical analysis
For comparison of physiologic variables over time under normothermia and hypothermia conditions a repeated measures two-way analysis of variance with Bonferroni correction was used. Initial animal weight, resuscitation time, and total bicarbonate administered were compared between groups using unpaired Student's t-tests. To determine the effect of hypothermia on systemic clearances and brain to plasma ratios, unpaired Student's t-tests were used. A p-value less than 0.05 was considered statistically significant. All analyses were conducted using Graphpad Prism 5.04 (Graphpad Software, La Jolla, CA).
Results
Temperature management and physiologic variables
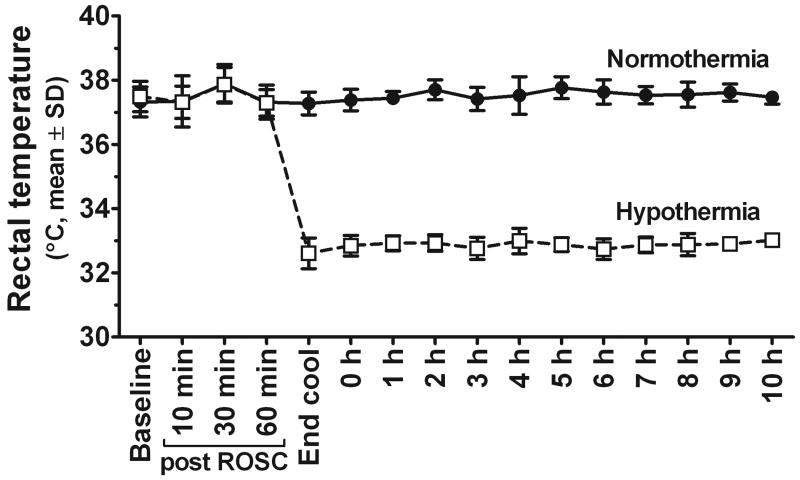
Surface cooling was effective in achieving and maintaining hypothermia and normothermia temperatures after CA (Figure 1). Groups were well-matched on weight, resuscitation time, and total bicarbonate replacement (Table 1). The mean PaO2 over the 8-10 h study period was higher in the hypothermia group after the CA (p < 0.05) as expected(11, 17). Hourly mean arterial pressure, pH, PaCO2, oxygen saturation, blood bicarbonate, lactate, glucose, hematocrit, lactate, and base deficit were not different between groups.
Figure 1.
Animals were well-controlled at target temperatures after CA by surface cooling.
Table 1. Physiological variables in rats following CA.
| Hypothermia | Normothermia | |
|---|---|---|
| Wt (g) | 403 ± 20 | 403 ± 16 |
| Resuscitation time (s) | 41 ± 11 | 36 ± 2 |
| Total HCO3- given (mEq) | 2.5 ± 1.1 | 2.3 ± 1.3 |
| Temp (°C) | 32.9 ± 0.3 | 37.5 ± 0.4* |
| MAP (mmHg) | 103 ± 15 | 101 ± 16 |
| pH | 7.38 ± 0.04 | 7.38 ± 0.07 |
| PaCO2 (mmHg) | 36.0 ± 5.1 | 36.3 ± 8.8 |
| PaO2 (mmHg) | 473 ± 33 | 439 ± 40* |
| HCO3- (mEq/L) | 21.2 ± 1.8 | 21.1 ± 2.2 |
| O2 saturation (%) | 99.6 ± 0.4 | 99.6 ± 0.4 |
| Lactate | 0.7 ± 0.3 | 0.8 ± 0.3 |
| Base deficit | -3.0 ± 1.6 | -2.9 ± 2.0 |
| Hct | 31 ± 4 | 30 ± 6 |
| Glucose | 63 ± 22 | 64 ± 23 |
Statistically significant hypothermia effect (p < 0.05). All values are mean ± SD of hourly measurements during the 8-10 h pharmacokinetic study.
Effect of hypothermia on the pharmacokinetics of fentanyl and midazolam following CA
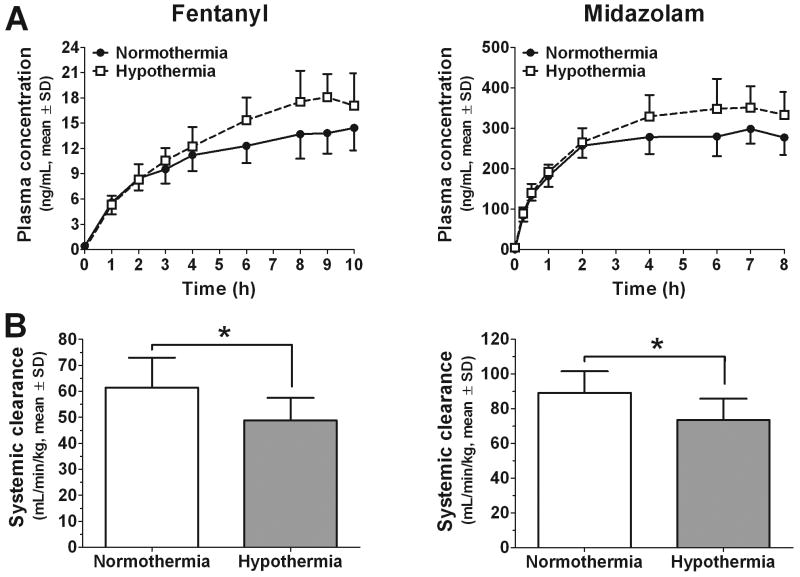
Concentration-time profiles (Figure 2) and specifically, the last three plasma concentrations demonstrate that steady-state was achieved. Hypothermia increased plasma concentrations of both fentanyl (n=10 hypothermia; n=9 normothermia) and midazolam (n=7 hypothermia; n=6 normothermia). This resulted in a significant decrease in the systemic clearances of fentanyl (20.5% decrease; 61.5±11.5 to 48.9±8.95mL/min/kg; p < 0.05) and midazolam (17.5% decrease; 89.2±12.5 to 73.6±12.1mL/min/kg; p<0.05) after CA.
Figure 2.
Panel A. Fentanyl and midazolam plasma time-concentration profiles were elevated under hypothermic conditions. Panel B. Hypothermia results in a modest decrease in the systemic clearances of both fentanyl (61.5±11.5 to 48.9±8.95 mL/min/kg) and midazolam (89.2±12.5 to 73.6±12.1 mL/min/kg) after CA. *Statistically significant effect (p < 0.05).
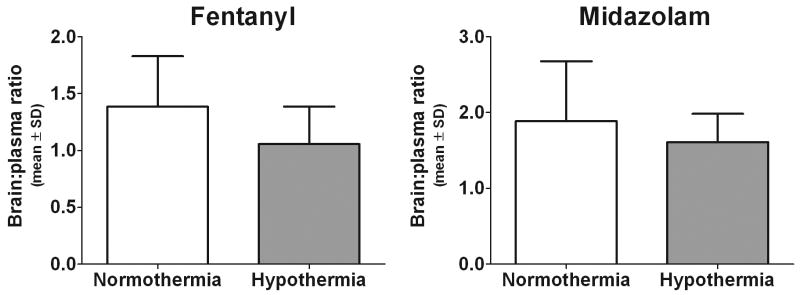
In contrast to the increased plasma concentrations, a nonsignificant trend towards decreased brain penetration of fentanyl during hypothermia was observed as evidenced by a lower brain to plasma concentration ratio (Figure 3; 1.39±0.444 to 1.06±0.330; p=0.08). Resultant brain concentrations were equivalent despite hypothermia treatment (18.8±4.85ng/gm wet weight in normothermia versus 18.4±5.37ng/gm wet weight in hypothermia animals; p=0.858). Hypothermia had less of an impact on midazolam brain penetration as the ratios were 1.89±0.792 under normothermia versus 1.61±0.375 during hypothermia (p=0.53). Brain tissue concentrations were 528±205 versus 550±108ng/gm wet weight (p=0.81) under normothermic and hypothermic conditions, respectively.
Figure 3.
Fentanyl and midazolam brain to plasma concentration ratios. A nonsignificant trend towards decreased brain penetration during hypothermia was observed for fentanyl (1.39±0.444 to 1.06±0.330; p=0.08), but was less evident for midazolam (1.89±0.792 to 1.61±0.375; p=0.53).
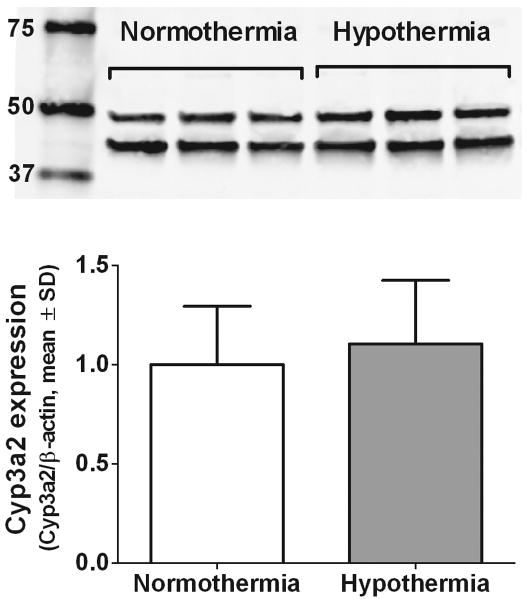
Effect of hypothermia on Cyp3a2 protein expression following CA
Rats killed at the end of the 8-10 h study showed no differences in liver microsomal β-actin normalized Cyp3a2 protein expression (Figure 4; normothermia, n=15, 1.00±0.293 vs. hypothermia, n=17, 1.10±0.320; p=0.346). Subgroup analyses of rats receiving either midazolam or fentanyl produced similar results. Cyp3a2 and β-actin expression was well-visualized via infrared imaging and all samples fell within the linear range of detection.
Figure 4.
Western blotting of Cyp3a2 (∼50 kDa) and β-actin (42 kDa) expression following CA and 8-10 h of either normothermia or hypothermia (representative 3 of each depicted). No differences in β-actin-normalized Cyp3a2 expression were observed by densitometry (n=15 normothermia, n=17 hypothermia; p = 0.346).
Effect of temperature on Cyp3a2 enzyme kinetics
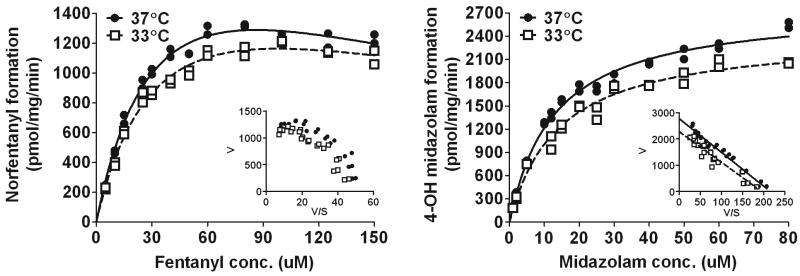
The best fit nonlinear regression curves and parameters derived from the Michaelis-Menten enzyme kinetic analyses are shown in Figure 5 and Table 2, respectively. For midazolam, the standard Michaelis-Menten equation fit well with R2 values of ≥0.98 for both the 37 °C and 33 °C curves and no improvement by applying the more complex substrate inhibition Michaelis-Menten model. For fentanyl, however, the norfentanyl formation became non-hyperbolic at higher concentrations. This characteristic “hook” in the upper region of the fentanyl Eadie-Hofstee transformation is representative of substrate inhibition at high concentrations. Indeed the substrate inhibition Michaelis-Menten equation fit better (p < 0.0001) with final R2 values of ≥ 0.98.
Figure 5.
Michaelis-Menten enzyme kinetic analysis in rat liver microsomes incubated at 37 °C or 33 °C. Cooling decreases the rate of formation of both norfentanyl and 4-OH midazolam (p<0.0001). Insets are Eadie-Hofstee that show differences in Vmax (y-intercept) for midazolam and a “hook” in the upper region of the fentanyl graph that is representative of substrate inhibition at high concentrations. No differences in the Michaelis-Menten constant, Km, (-slope) was observed with reduced temperature for either drug. V=velocity; S=substrate concentration.
Table 2. Michaelis-Menten enzyme kinetic parameter estimates in rat liver microsomes incubated at 37 °C or 33 °C.
| Fentanyl | Midazolam | |||
|---|---|---|---|---|
| 37 °C | 33 °C | 37 °C | 33 °C | |
| Vmax (pmol/mg/min) | 2480 ± 236 | 2080 ± 203 | 2780 ± 60.0 | 2430 ± 74.8* |
| Km (μM) | 39.8 ± 6.04 | 38.7 ± 6.15 | 12.9 ± 0.862 | 14.1 ± 1.36 |
| Ki (μM) | 186 ± 42.0 | 248 ± 67.5 | ||
Vmax, maximum velocity; Km, Michaelis-Menten constant. Ki, dissociation constant for the enzyme-inhibitor complex.
Statistically significant differences, p<0.01.
All data are represented as mean±SE.
A comparison of the curves at each temperature showed that hypothermia decreased the Cyp3a2-dependent metabolite formation rates for both drugs (p<0.0001). Cooling to 33°C resulted in a significantly lower midazolam enzymatic maximal velocity as parameter estimates for Vmax decreased (2780±60.0 pmol/mg/min at 37°C to 2430±74.8pmol/mg/min at 33°C; p=0.0009). The Eadie-Hofstee plot shows this difference as a changed y-intercept. There was a trend in decreased Vmax at the lower temperature for fentanyl (2480±236 pmol/mg/min at 37°C vs 2080±203 pmol/mg/min at 33°C; p=0.205). No differences in enzyme affinity were observed with either drug during cooling as the Eadie Hofstee plots had parallel slopes and the Michaelis-Menten constant, Km, was unchanged per model parameter estimates (p=0.900 for fentanyl and p=0.438 for midazolam).
Discussion
We report that mild hypothermia, as applied in a highly clinically relevant experimental CA paradigm with a 10 hour study duration alters the steady-state pharmacokinetics of two commonly used ICU medications, fentanyl and midazolam. Further, we demonstrate that these alterations are not due to changes in hepatic Cyp3a2 protein expression, the primary route of oxidative metabolism of both drugs. Finally, we found that in vitro Cyp3a2-dependent formation rates were decreased by cooling and that this reduction was the result of changes in maximal velocity rather than enzyme affinity. In order to mimic the clinical application of hypothermia we incorporated specific design elements into our CA and hypothermia protocols. In previous work, short duration (3 h), low temperature (30°C) hypothermia, and intravenous bolus of probes of CYP pathways were used in proof-of-concept studies to show that cooling could alter drug metabolism post CA(11, 17). In this study, we delayed the initiation of hypothermia for 1 h post ROSC, targeted a temperature of 33°C to match the now standard goal range of 32-34°C, and extended the duration of hypothermia to 8-10 h. Similarly, we selected the commonly used drugs, fentanyl and midazolam, at a dosing regimen that produced blood concentrations similar to those achieved in patients. The pharmacokinetic design employed continuous infusions to steady-state, to best estimate systemic clearance and to allow for brain to plasma tissue distribution ratios. Overall, this work suggests that Cyp3a-dependent metabolism of clinically-relevant medications is decreased by mild hypothermia after CA.
Mild hypothermia applied after CA resulted in a modest decrease in the systemic clearances of both midazolam and fentanyl in rats and is supported by published data. Fritz et al. cooled uninjured pigs to 31.6±0.2°C and showed that fentanyl concentrations increased 25%(21). Similarly, the application of 1 h of 32°C hypothermia to rats after an experimental traumatic brain injury significantly increased fentanyl concentrations approximately 20%(22). In both studies, short term fentanyl infusions were used and assessments were made while the drug was still accumulating, suggesting that differences in the systemic clearances and steady state concentrations may be far greater. For midazolam, clinical data exist, but are inconsistent. Fukuoka et al.(6) reported a “biphasic” concentration change in 8 brain-injured patients receiving 32–34°C hypothermia. Despite stable dosing, serum concentrations continued increasing linearly during the 3-4 day hypothermic period (never reaching a steady-state level) and achieved five-fold higher concentrations than in the 7 normothermic comparators. During rewarming, the levels fell drastically once body temperatures surpassed 35°C. In their pharmacokinetic analysis, these investigators reported a remarkable >100-fold difference in systemic clearance during hypothermia. Other data, however, suggest a less substantial effect of hypothermia on midazolam pharmacokinetics. In a randomized crossover study of healthy volunteers, we were able to lower core body temperature to 35.4°C and observed a significantly decreased formation of the major metabolite of midazolam (8). Population pharmacokinetic modelling predicted that midazolam clearance decreased 11.1% for each degree Celsius reduction in temperature from 36.5°C.
The current findings of a ∼20% decrease in the systemic clearance for both fentanyl and midazolam when prolonged mild hypothermia is applied after CA is a smaller magnitude reduction than previously observed. Findings with the CYP2E1 probe, chlorzoxazone, after the same asphyxial CA in rats and 3 h of 30°C hypothermia showed a >50% decrease in systemic clearance(11). Whether the differences in results are primarily attributable to the depth of cooling, which CYP isoform is involved, or drugs characteristics remains to be elucidated. However, the drug hepatic extraction ratios are likely to be an important factor. Contrary to midazolam in humans and chlorzoxazone humans/rats, both fentanyl and midazolam are high hepatic extraction drugs in rats with ratios of hepatic clearance to blood flow of ∼1 and 0.86 respectively (23, 24). As predicted by the well-stirred model of hepatic clearance(25-27), their hepatic clearance may therefore be more dependent on hepatic blood flow rather than significantly affected by changes in hepatic metabolic capacity in this species. Hepatic blood flow was not measured after CA in this experiment but has been shown to be decreased in hypothermic pigs(21), rats(28), and healthy volunteers(29). Overall, differences in the sensitivity of hepatic blood flow and enzyme metabolic capacity to hypothermia combined with the relative dependency of a given drug on each of these factors for its hepatic clearance may explain these observations.
Mechanistically, we found no differences in Cyp3a2 protein expression by western blotting, but discovered that cooling to 33°C decreases Cyp3a2-mediated fentanyl and midazolam metabolism in vitro through a reduction in enzyme capacity (Vmax). Collectively, this suggests that the decrease in systemic clearance of both drugs was due to a direct temperature-related decrease in enzyme activity in vivo rather than alterations in expression. To put the timing of this protein expression analysis into context, we previously reported that CA decreased Cyp3a RNA expression at 24 h, but not at 5 h post-injury in this model(17). The application of 3 h of moderate hypothermia (30°C) attenuated the down-regulation of this PXR-regulated gene through a proposed mechanism of blocking the increase in IL-6 after CA. The current work shows that 8-10 h of mild hypothermia does not alter Cyp3a2 protein expression 10-12 h after CA relative to controlled normothermia. This suggests that either 10-12 h is too early to observe the time-dependent down regulation of Cyp3a2 post CA or that mild hypothermia does not modulate CA-induced changes in Cyp3a2 protein expression. This work is also significant in that it shows that the mild hypothermic temperatures that are applied clinically noncompetitively lower the capacity of Cyp3a for drug metabolism. Previous in vitro Michaelis-Menten modeling of the effect of more aggressive cooling on Cyp2e1 activity suggested a different mode of inhibition. The reduction in chlorzoxazone metabolism at 30°C was attributed to a lower affinity of the enzyme for the drug rather than an enzyme capacity change as Km increased >2-fold and Vmax remained unaffected(11). In general, a temperature-dependency of Vmax is more consistent in the enzyme literature, but differences in Km have been also been observed(30).
Despite increased systemic exposures of both fentanyl and midazolam during mild hypothermia, the brain penetration was not significantly altered. Fentanyl brain to plasma concentration ratios trended towards a decrease by mild cooling. These contrasting effects of hypothermia on blood levels and brain penetration suggest a reduction in drug therapeutic index; concentrations are lower than expected in the central nervous system (the site of action) while systemic concentrations are potentially supratherapeutic. A lack of appreciation of this phenomenon may inadvertently lead to increased dosing to achieve efficacy at the expense of toxicity. Blood brain barrier (BBB) permeability is known to be determined by drug size, lipophilicity, and transporter protein interactions(31). Total drug concentration ratios can further be affected by plasma protein or brain tissue binding. Both drugs are lipophilic, small molecules that achieve high brain to plasma ratios (greater than 1) in control rats(32, 33). Fentanyl's brain distribution is also known to depend on both an uptake transporter, likely an organic anion transport protein, and to a minor extent, the efflux transporter p-glycoprotein at the BBB(34). It is unclear if rodent BBB integrity to small drug molecules early after CA is temperature sensitive. Some evidence for cooling effects on drug transporters has been published. Flux of the p-glycoprotein substrate, digoxin, is decreased ∼50% at 32°C (35) and transporter-mediated uptake of vecuronium shows a clear temperature dependency(36). Whether these in vitro results translate to alterations in brain concentrations due to changes in transporter activity remains an important area of study.
Limitations to our study should be considered. First, the rat asphyxial CA model used in this study, although well-established, produces a brain injury that is comprised of selective vulnerable neuronal death and thus may be mild relative to many clinically relevant CA insults. It may therefore underestimate some of the effect of CA and possible interactions between CA-mediated disturbances in metabolism and hypothermia. This is not a serious concern for the current work as this study was designed to evaluate the acute effect of hypothermia, not discern independent effects of CA. Second, our study was conducted in the rat model, which does not ideally mimic human P450 activity, particularly with respect to differences in extraction ratio of midazolam. Although species differences may affect the magnitude of the findings due to blood flow versus enzyme dependency, this study does clearly demonstrate the impact of mild hypothermia on fentanyl and midazolam pharmacokinetics. Finally, we focused on CYP metabolic activity and protein expression analysis to gain mechanistic insight into the alterations in systemic clearance. CA and hypothermia may also damage the liver directly and/or affect hepatic blood flow. Future work with include direct assessments liver histology and hepatic blood flow to better clarify the mechanisms of altered drug disposition.
Conclusions
We report, using sustained application of mild hypothermia in a CA model, that mild hypothermia alters the pharmacokinetics of two commonly used medications, fentanyl and midazolam. The systemic clearance of both drugs was decreased, but the resulting elevated systemic concentrations did not lead to an increased brain exposure. Mechanistically, we also demonstrated that the Cyp3a-dependent formation of the primary metabolites of both drugs was decreased at 33°C through a reduction in enzyme metabolic capacity. Our data indicate that the temperatures currently targeted in therapeutic hypothermia protocols that are in clinical use likely alter the disposition of medications whose clearance is dependent on CYP3A metabolism and/or liver blood flow. The observation that plasma concentrations and brain to blood ratio changes do not parallel one another further complicates pharmacotherapy. There is a strong need for well-designed pharmacokinetic studies to understand the magnitude and impact of such changes in CA patients. Given the prominent role CYP3A plays in the elimination of many medications, an appreciation of the impact of hypothermia is necessary for safe and effective drug therapy.
Acknowledgments
Grant support provided by the National Institutes of Health awards R01GM073031 (SMP), S10RR023461 (SMP), and P01NS30318 (PMK). Dr. Empey was supported by KL2RR024154 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would also like to acknowledge Dr. Dianxu Ren for the statistical review of this manuscript.
Footnotes
Presented, in part, at the SCCM 39th Critical Care Congress, Miami, FL, Jan 9-13, 2010.
The authors have not disclosed any potential conflicts of interest.
References
- 1.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 2.Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 3.Nolan JP, Morley PT, Vanden Hoek TL, et al. Therapeutic hypothermia after cardiac arrest: an advisory statement by the advanced life support task force of the International Liaison Committee on Resuscitation. Circulation. 2003;108(1):118–121. doi: 10.1161/01.CIR.0000079019.02601.90. [DOI] [PubMed] [Google Scholar]
- 4.Roka A, Melinda KT, Vasarhelyi B, et al. Elevated morphine concentrations in neonates treated with morphine and prolonged hypothermia for hypoxic ischemic encephalopathy. Pediatrics. 2008;121(4):e844–849. doi: 10.1542/peds.2007-1987. [DOI] [PubMed] [Google Scholar]
- 5.Iida Y, Nishi S, Asada A. Effect of mild therapeutic hypothermia on phenytoin pharmacokinetics. Ther Drug Monit. 2001;23(3):192–197. doi: 10.1097/00007691-200106000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Fukuoka N, Aibiki M, Tsukamoto T, et al. Biphasic concentration change during continuous midazolam administration in brain-injured patients undergoing therapeutic moderate hypothermia. Resuscitation. 2004;60(2):225–230. doi: 10.1016/j.resuscitation.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 7.Caldwell JE, Heier T, Wright PM, et al. Temperature-dependent pharmacokinetics and pharmacodynamics of vecuronium. Anesthesiology. 2000;92(1):84–93. doi: 10.1097/00000542-200001000-00018. [DOI] [PubMed] [Google Scholar]
- 8.Hostler D, Zhou J, Tortorici MA, et al. Mild Hypothermia Alters Midazolam Pharmacokinetics in Normal Healthy Volunteers. Drug Metab Dispos. 2010 doi: 10.1124/dmd.109.031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tortorici MA, Kochanek PM, Poloyac SM. Effects of hypothermia on drug disposition, metabolism, and response: A focus of hypothermia-mediated alterations on the cytochrome P450 enzyme system. Crit Care Med. 2007;35(9):2196–2204. doi: 10.1097/01.ccm.0000281517.97507.6e. [DOI] [PubMed] [Google Scholar]
- 10.Zhou J, Poloyac SM. The effect of therapeutic hypothermia on drug metabolism and response: cellular mechanisms to organ function. Expert Opin Drug Metab Toxicol. 2011 doi: 10.1517/17425255.2011.574127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tortorici MA, Kochanek PM, Bies RR, et al. Therapeutic hypothermia-induced pharmacokinetic alterations on CYP2E1 chlorzoxazone-mediated metabolism in a cardiac arrest rat model. Crit Care Med. 2006;34(3):785–791. doi: 10.1097/01.ccm.0000201899.52739.4f. [DOI] [PubMed] [Google Scholar]
- 12.Wilkinson GR. Drug metabolism and variability among patients in drug response. N Engl J Med. 2005;352(21):2211–2221. doi: 10.1056/NEJMra032424. [DOI] [PubMed] [Google Scholar]
- 13.Guengerich FP. Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol. 1999;39:1–17. doi: 10.1146/annurev.pharmtox.39.1.1. [DOI] [PubMed] [Google Scholar]
- 14.Spriet I, Meersseman W, de Hoon J, et al. Mini-series: II. clinical aspects. clinically relevant CYP450-mediated drug interactions in the ICU. Intensive Care Med. 2009;35(4):603–612. doi: 10.1007/s00134-008-1383-2. [DOI] [PubMed] [Google Scholar]
- 15.Cullen DJ, Sweitzer BJ, Bates DW, et al. Preventable adverse drug events in hospitalized patients: a comparative study of intensive care and general care units. Crit Care Med. 1997;25(8):1289–1297. doi: 10.1097/00003246-199708000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Fink EL, Alexander H, Marco CD, et al. Experimental model of pediatric asphyxial cardiopulmonary arrest in rats. Pediatr Crit Care Med. 2004;5(2):139–144. doi: 10.1097/01.pcc.0000112376.29903.8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tortorici MA, Mu Y, Kochanek PM, et al. Moderate hypothermia prevents cardiac arrest-mediated suppression of drug metabolism and induction of interleukin-6 in rats. Crit Care Med. 2009;37(1):263–269. doi: 10.1097/CCM.0b013e3181931ed3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Empey PE, Melick JA, Kochanek PM, et al. Effect of moderate induced hypothermia on fentanyl and midazolam clearance in a rat model of hypoxic cardiac arrest. Crit Care Med. 2009;37(12 (Supp)):A33. doi: 10.1097/CCM.0b013e31823779f9. Abstract 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Link B, Haschke M, Wenk M, et al. Determination of midazolam and its hydroxy metabolites in human plasma and oral fluid by liquid chromatography/electrospray ionization ion trap tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007;21(9):1531–1540. doi: 10.1002/rcm.2987. [DOI] [PubMed] [Google Scholar]
- 20.Rockich K, Blouin R. Effect of the acute-phase response on the pharmacokinetics of chlorzoxazone and cytochrome P-450 2E1 in vitro activity in rats. Drug Metab Dispos. 1999;27(9):1074–1077. [PubMed] [Google Scholar]
- 21.Fritz HG, Holzmayr M, Walter B, et al. The effect of mild hypothermia on plasma fentanyl concentration and biotransformation in juvenile pigs. Anesth Analg. 2005;100(4):996–1002. doi: 10.1213/01.ANE.0000146517.17910.54. [DOI] [PubMed] [Google Scholar]
- 22.Statler KD, Alexander HL, Vagni VA, et al. Moderate hypothermia may be detrimental after traumatic brain injury in fentanyl-anesthetized rats. Crit Care Med. 2003;31(4):1134–1139. doi: 10.1097/01.CCM.0000054864.43122.52. [DOI] [PubMed] [Google Scholar]
- 23.Bjorkman S, Redke F. Clearance of fentanyl, alfentanil, methohexitone, thiopentone and ketamine in relation to estimated hepatic blood flow in several animal species: application to prediction of clearance in man. J Pharm Pharmacol. 2000;52(9):1065–1074. doi: 10.1211/0022357001774985. [DOI] [PubMed] [Google Scholar]
- 24.Higashikawa F, Murakami T, Kaneda T, et al. In-vivo and in-vitro metabolic clearance of midazolam, a cytochrome P450 3A substrate, by the liver under normal and increased enzyme activity in rats. J Pharm Pharmacol. 1999;51(4):405–410. doi: 10.1211/0022357991772600. [DOI] [PubMed] [Google Scholar]
- 25.Pang KS, Rowland M. Hepatic clearance of drugs. III. Additional experimental evidence supporting the “well-stirred” model, using metabolite (MEGX) generated from lidocaine under varying hepatic blood flow rates and linear conditions in the perfused rat liver in situ preparation. J Pharmacokinet Biopharm. 1977;5(6):681–699. doi: 10.1007/BF01059690. [DOI] [PubMed] [Google Scholar]
- 26.Pang KS, Rowland M. Hepatic clearance of drugs. II. Experimental evidence for acceptance of the “well-stirred” model over the “parallel tube” model using lidocaine in the perfused rat liver in situ preparation. J Pharmacokinet Biopharm. 1977;5(6):655–680. doi: 10.1007/BF01059689. [DOI] [PubMed] [Google Scholar]
- 27.Pang KS, Rowland M. Hepatic clearance of drugs. I. Theoretical considerations of a “well-stirred” model and a “parallel tube” model. Influence of hepatic blood flow, plasma and blood cell binding, and the hepatocellular enzymatic activity on hepatic drug clearance. J Pharmacokinet Biopharm. 1977;5(6):625–653. doi: 10.1007/BF01059688. [DOI] [PubMed] [Google Scholar]
- 28.Nishida K, Okazaki M, Sakamoto R, et al. Change in pharmacokinetics of model compounds with different elimination processes in rats during hypothermia. Biol Pharm Bull. 2007;30(9):1763–1767. doi: 10.1248/bpb.30.1763. [DOI] [PubMed] [Google Scholar]
- 29.Leslie K, Sessler DI, Bjorksten AR, et al. Mild hypothermia alters propofol pharmacokinetics and increases the duration of action of atracurium. Anesth Analg. 1995;80(5):1007–1014. doi: 10.1097/00000539-199505000-00027. [DOI] [PubMed] [Google Scholar]
- 30.Scopes RK. The effect of temperature on enzymes used in diagnostics. Clin Chim Acta. 1995;237(1-2):17–23. doi: 10.1016/0009-8981(95)06060-q. [DOI] [PubMed] [Google Scholar]
- 31.Abbott NJ, Patabendige AAK, Dolman DEM, et al. Structure and function of the blood–brain barrier. Neurobiology of Disease. 2010;37(1):13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 32.Bjorkman S, Stanski DR, Verotta D, et al. Comparative tissue concentration profiles of fentanyl and alfentanil in humans predicted from tissue/blood partition data obtained in rats. Anesthesiology. 1990;72(5):865–873. doi: 10.1097/00000542-199005000-00017. [DOI] [PubMed] [Google Scholar]
- 33.Bjorkman S, Fyge A, Qi Z. Determination of the steady state tissue distribution of midazolam in the rat. J Pharm Sci. 1996;85(8):887–889. doi: 10.1021/js960113+. [DOI] [PubMed] [Google Scholar]
- 34.Elkiweri IA, Zhang YL, Christians U, et al. Competitive substrates for P-glycoprotein and organic anion protein transporters differentially reduce blood organ transport of fentanyl and loperamide: pharmacokinetics and pharmacodynamics in Sprague-Dawley rats. Anesth Analg. 2009;108(1):149–159. doi: 10.1213/ane.0b013e31818e0bd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin JS, Sakaeda T, Kakumoto M, et al. Effect of therapeutic moderate hypothermia on multi-drug resistance protein 1-mediated transepithelial transport of drugs. Neurol Med Chir (Tokyo) 2006;46:321–327. doi: 10.2176/nmc.46.321. discussion 327. [DOI] [PubMed] [Google Scholar]
- 36.Mol WE, Fokkema GN, Weert B, et al. Mechanisms for the hepatic uptake of organic cations. Studies with the muscle relaxant vecuronium in isolated rat hepatocytes. J Pharmacol Exp Ther. 1988;244(1):268–275. [PubMed] [Google Scholar]