Abstract
Objective
Re-operative cardiac surgery is complicated in part because of extensive adhesions encountered during the second operation. The purpose of this study was to examine the effects of alcohol with and without resveratrol (red wine vs. vodka), on post-operative pericardial adhesion formation in a porcine model of hypercholesterolemia and chronic myocardial ischemia.
Methods
Male Yorkshire swine were fed a high-cholesterol diet to simulate conditions of coronary artery disease followed by surgical placement of an ameroid constrictor to induce chronic ischemia. Post-operatively, control pigs continued their high-cholesterol diet alone, while the two experimental groups had diets supplemented with either red wine or vodka. Seven weeks after ameroid placement, all animals underwent re-operative sternotomy.
Results
Compared to controls, pericardial adhesion grade was markedly reduced in the vodka group while there was no difference in the wine group. Intramyocardial fibrosis was significantly reduced in the vodka group compared to controls. There was no difference in expression of proteins involved in focal adhesion formation between any groups (FAK, Int α5, Int β1, Paxillin, Vinculin, PYK2, PKCε, p-PKCε). The wine group exhibited elevated CRP levels vs. control and vodka group.
Conclusions
Post-operative vodka consumption markedly reduced the formation of pericardial adhesions and intramyocardial fibrosis while red wine had no effect. Analysis of protein expression did not reveal any obvious explanation for this phenomenon, suggesting a post-translational effect of alcohol on fibrous tissue deposition. The difference in adhesion formation in the vodka vs. wine groups may be due to increased inflammation in the wine group.
Introduction
Adhesion formation occurs in the mediastinum after cardiac surgical procedures and makes re-operative surgery particularly challenging, especially if the operation is performed within a few weeks of the initial surgery. These internal fibrinous attachments are thought to develop due to mesothelial trauma during an operation which can result from surgical handling, instrumentation, exposure to air, temperature changes, surgical glove powder, and foreign bodies such as suture materials and implanted devices.[1] Reduction in post-operative adhesion formation in cardiac surgery is a heavily researched topic by both the academic and biomedical industry sectors. A significant reduction or elimination of adhesions could drastically decrease the difficulty of re-operative cardiac surgery by reducing operative time, reducing intra- and post-operative bleeding, improving patient outcomes and even reducing operative costs. Small studies in animals have shown promising results in reducing adhesion formation with the use of bioabsorbable polymer films and barriers [2], topical application of growth factors [3], and the use of fibrilar collagen membranes [4] during the initial operation. However, large clinical trials are either lacking or have failed to produce a reduction of post-operative adhesions in any clinically relevant fashion.
A substance that could be given perioperatively in a safe and cost-effective manner to significantly reduce the degree of adhesions seen at re-operation would have enormous impact potential in cardiac surgery. A recent study examining the nanoscale architecture of cell to extracellular matrix (ECM) adhesions using three-dimensional super-resolution fluorescence microscopy delineated the protein interactions involved in adhesion formation.[5] The interruption of these molecular interactions or inhibition of the deposition and cross-linking of collagen could potentially reduce adhesion formation post-operatively. We hypothesized that the anti-inflammatory properties of resveratrol might modulate post-operative adhesion formation and designed an experiment in order to compare the effects of resveratrol-containing red wine to alcohol alone.
Methods
Animal Model
Starting at 4 weeks of age, 28 male Yorkshire miniswine (Parson’s Research, Amherst, MA) were fed a 500 g/day of a high-cholesterol diet throughout the 11-week experiment to simulate conditions of coronary artery disease (CAD). The hypercholesterolemic diet consisted of 4% cholesterol, 17.2% coconut oil, 2.3% corn oil, 1.5% sodium cholate, and 75% regular chow (Sinclair Research, Columbia, MO). All animals had unlimited access to drinking water. After 4 weeks of diet modification, all animals underwent ameroid constrictor placement (Research Instruments SW, Escondito, CA) to induce chronic cardiac ischemia. For all surgical procedures, anesthesia was induced with intramuscular injection of telazol (5 mg/kg), animals were endotracheally intubated and mechanically ventilated at 12–20 breaths/min. Anesthesia was maintained with a gas mixture of 1.5–2.0 L/min of O2 and 0.75%–3.0% isofluorane. Surgical approach for ameroid constrictor placement was via a mini-left thoracotomy, and a titanium ameroid constrictor (1.75–2.25 mm internal diameter) was placed around the proximal left circumflex coronary artery (LCx) just distal to its take-off from the left main coronary artery. The pericardium was re-approximated with 3 interrupted 4-0 nurolon sutures. Post-operative pain was controlled with Buprenex intramuscular injection (0.03mg/kg) at the end of the first operation and placement of a transdermal fentanyl patch (4 mcg/kg) continued for 72h post-operatively. All animals received peri-operative aspirin at 325mg/day for prophylaxis against thrombo-embolic events starting 1 day before the first operation and continuing for a total of 5 days.
Post-operatively the pigs were split into three groups according to diet supplementation. The high-cholesterol control group (HCC, n=9) continued the high-cholesterol diet for the remaining 7 weeks of the experiment. High-cholesterol wine pigs were supplemented with 375 mL of red wine (2009 Pinot Noir, Black Mountain Vineyard, Napa and Sonoma, CA) daily (12.5% EtOH/V, HCW, n=9). High-cholesterol vodka pigs were supplemented with 112 mL of vodka (Rubinoff Vodka, Somerville, MA) daily (40% EtOH/V, HCV, n=9).
Seven weeks after initial operation, at 15 weeks of age, all animals underwent a final non-survival operation via a median sternotomy. Pericardial adhesions were graded at the time of re-operation. Cardiac harvest included the collection of tissue from both the normal left ventricle (NV), perfused by the left anterior descending artery (LAD), as well as LV tissue from the area at risk (ARR), in the distribution of the LCx. Myocardial samples were either immediately frozen in liquid nitrogen at the time of harvest, or placed in a 10% formalin solution for paraffin embedment and tissue staining.
All experiments were approved by the Rhode Island Hospital Institutional Animal Care and Use Committees. Animals were cared for in accordance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals.” [6]
Pericardial Adhesion Grading
At the time of re-operation, after median sternotomy and exposure of the pericardium, epicardial to pericardial adhesions were evaluated using a grading system modified from a previously published porcine model.[3] Difficulty in separating the pericardium from epicardium was graded 0 – 3 as follows: 0 – adhesions did not exist; 1 – adhesions were filmy, light, with foamy dissection plane; 2 – adhesions were intermediate, requiring some sharp dissection, but mostly lysed by digital manipulation; 3 – adhesions were dense, easily bleeding, with marked obliteration of tissue plane, requiring exclusive sharp dissection. The overall adhesion rating for each pig was averaged into a mean for each group for comparison between groups.
Measurement of Intramyocardial Fibrosis
Paraffin sections of myocardial tissue from both the AAR and NV were prepared for trichrome staining. An area of myocardium free of vessels and representative of the entire sample was captured for each slide. Researchers were blinded to samples during staining, processing, and image analysis. Images were digitally captured at 10× magnification (Nikon Eclipse E800, Nikon Corporation, Japan) using SPOT Camera (Diagnostic Instruments, Sterling Heights, MI) and analyzed using Image J software (NIH, Bethesda, MD) to calculate the percent of fibrosis (blue) of total myocardium (red). Numbers are reported in percent of myocardium replaced by collagen (fibrosis).
Transmural Collagen Area Fraction
Paraffin sections of transmural myocardial tissue from both the AAR and NV were prepared for picosirius red staining. Images were digitally captured at 20× magnification (Aperio ScanScope, Vista, CA) and analyzed using Image J software to calculate the total transmural area (TA) in each sample, followed by measurement of the collagen area (CA) of the slide which stained red. Numbers are reported in collagen area fraction (CAF) where CAF = CA/TA.
Protein Expression
Sixty micrograms of total protein from the RIPA (Radio-Immunoprecipitation Assay) Buffer (Boston BioProducts, Ashland, MA) soluble fraction of myocardial lysates made from the NV tissue was fractionated by SDS-PAGE using the NuPage Novex Bis-Tris Mini Gel system (Invitrogen, Carlsbad, CA) and transferred to PVDF membranes (Millipore, Bedford, MA). Membranes were incubated with antibodies against Focal Adhesion Kinase (FAK), Integrin Alpha-5 (Int α5), Integrin Beta-1 (Int β1), Paxillin, Vinculin, Protein Tyrosine Kinase (PYK2, Cell Signaling, Danvers, MA), Protein Kinase C ε (PKCε) and phosphorylated Protein Kinase C ε (p-PKCε, Santa Cruz Biotechnology, Santa Cruz, CA) at dilutions recommended by the manufacturer, followed by the appropriate HRP-linked secondary antibodies (1:2000, Jackson Immunoresearch, West Grove, PA). Immune complexes were visualized via electrochemiluminescense (ECL) and photographed using GeneSnap software (Syngene, Cambridge, England). Densiometry of ECL signal was performed using Image J software (NIH, Bethesda, MD). To ensure and correct for equal protein loading, membranes were probed with GAPDH (Cell Signaling, Danvers, MA), a constitutively expressed housekeeping protein. Raw data collected as arbitrary light units from ECL fluorescence and Image J densiometry were averaged, and expressed in fold change (FC) as compared to HCC mean.
Blood Draws and Serum Studies
Blood was drawn under general anesthesia for serum analysis during the initial operation of ameroid placement and at the beginning of the re-operative sternotomy before the chest was opened. Three weeks after initial operation, animals were sedated with an intramuscular injection of Telazol (5 mg/kg) one hour after consumption of chow/supplement mixture, followed by percutaneous jugular venous blood sampling using anatomical landmarks. Serum was analyzed by the chemistry laboratory at the Rhode Island Hospital, Providence, RI.
Statistical Analysis
All results were reported as mean ± standard error of the mean (SEM). Weight gain, adhesion grade, intramyocardial fibrosis, transmural collagen area fraction, molecular studies, and CRP (C-reactive protein) at the time of reoperation were analyzed by comparing means of each group using one-way ANOVA followed by a post-hoc Bonferroni test using GraphPad Prism 5.0 Software (GraphPad Software Inc., San Diego, CA). Two-way ANOVA and Bonferroni post tests were used to compare changes in CRP in each group from the first to second operation. Student’s T-Test used for comparison between only two groups (Blood alcohol levels between HCW and HCV).
Results
Experimental Model
There were no wound infections, dehiscence or clinically different outcomes in wound healing in any of the animals. One animal from the HCW group died 9 days after ameroid placement. Necropsy did not reveal any obvious reason for death (which was likely due to ventricular arrhythmia) and the animal was excluded from analysis. Two animals from the HCC group died. One death occurred after jugular venous blood draw, presumably from a tension pneumothorax, and the other died during the initial ameroid placement secondary to technical intraoperative complications. Both were excluded from analysis, and replaced with new control animals.
Weight Gain
Weight gain from the first to second operations was not significantly different between the HCW and HCV groups (12.4 ± 0.591 kg vs. 11.7 ± 0.281 kg, p = 0.249). However, there was significantly less weight gained in both the HCW and HCV groups compared to the controls (16.5 ± 1.505 kg, p = 0.041 and p = 0.017 for HCW and HCV vs. HCC, respectively. ANOVA p = 0.0063).
Pericardial Adhesions
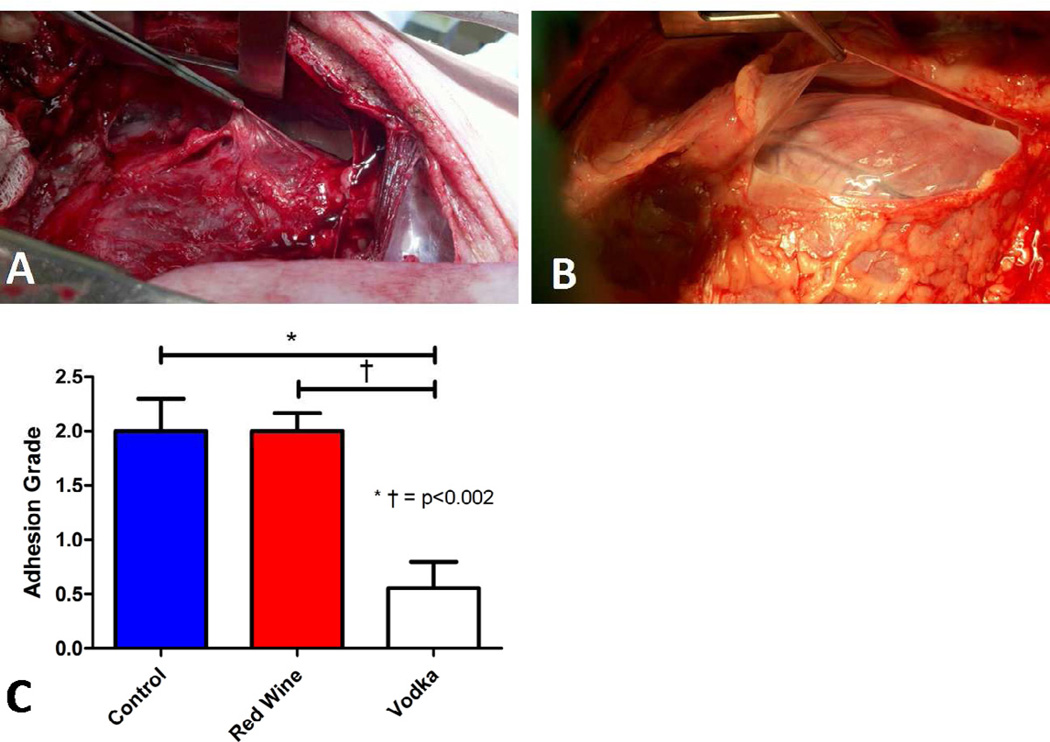
Mean adhesion grade at the time of second operation (median sternotomy) was markedly reduced in pigs supplemented with vodka. Using the grading system described in the methods, swine in the HCV group (Vodka) had significantly reduced adhesion scores as compared to either HCC (Control) or HCW (Red Wine) groups (ANOVA p = 0.0003, HCC vs. HCW p = 1, HCC vs. HCV p = 0.002, HCV vs. HCW p = 0.0002, Figure 1).
Figure 1.
Pericardial adhesions seen 7 weeks after ameroid placement during re-operative sternotomy in Control and Red Wine groups, A. Lack of pericardial adhesions observed in the Vodka group, B. Pericardial adhesion grade significantly reduced in Vodka group as compared to Control or Red Wine groups, C.
Intramyocardial Fibrosis
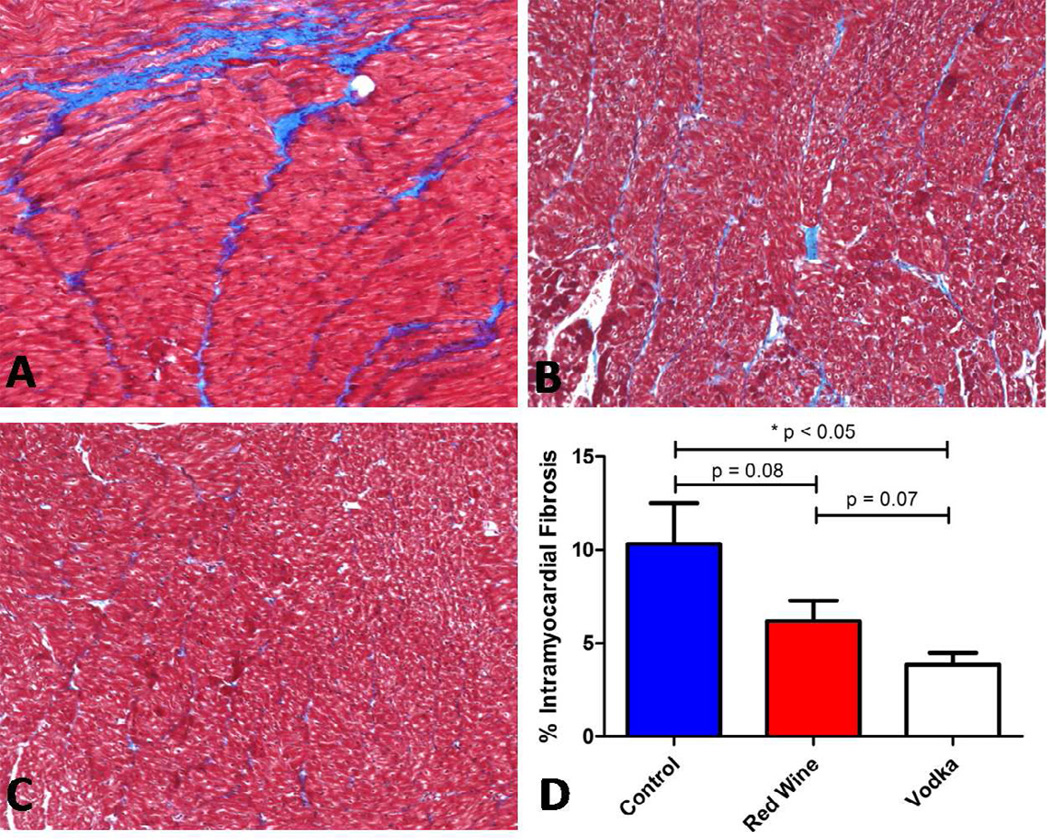
Trichrome staining of both the NV and AAR from each group showed grossly decreased intramyocardial collagen deposition in the pigs supplemented with vodka. Statistical analysis using imaging software revealed significantly reduced intramyocardial fibrosis in the HCV group (3.87 ± 0.621 % ) compared to controls (10.32 ± 2.19 %, p = 0.002, Figure 2). Though not reaching statistical significance, there were marked differences in fibrosis in the HCW (6.19 ± 1.10 %) group compared to controls (p = 0.081, HCW < HCC) as well as compared to the HCV group (p = 0.067, HCW > HCV. ANOVA p = 004).
Figure 2.
Trichrome staining showing intramyocardial collagen deposition (blue stain) replacing normal myocardium (red) of controls, A. Comparatively less collagen deposition is seen in Red Wine group, B, and minimal collagen deposition is seen in Vodka group, C. Analysis of the percent of intramyocardial fibrosis was significantly reduced in the Vodka group compared to controls, D.
Transmural Collagen Area Fraction
Picosirius red staining of transmural myocardial sections showed significantly less collagen deposition in pigs supplemented with vodka compared to both the control group as well as compared to the pigs supplemented with wine. Collagen area fraction (CAF) in HCC was 0.265 ± 0.021 as compared to HCW with 0.189 ± 0.025 and HCV with 0.058 ± 0.008 CAF. One-way ANOVA analysis for all groups determined p < 0.0001 and Bonferroni post-test determined all groups to be significantly different from each other (HCC vs. HCW p = 0.027, HCC vs. HCV p < 0.0001, HCW vs. HCV p < 0.0001).
Protein Expression
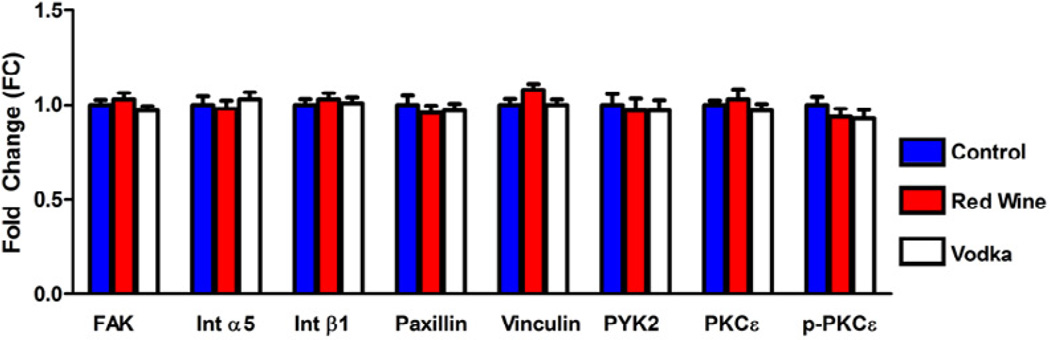
Western blot analysis of proteins involved in cell-ECM focal adhesion formation revealed no statistically significant differences in the expression of FAK, Int α5, Int β1, Paxillin, Vinculin, PYK2, PKCε, or p-PKCε in the NV (Figure 3).
Figure 3.
Expression of proteins involved in cell-ECM focal adhesion formation. There were no significant differences between groups. Levels represent fold change (FC) in mean as compared to controls.
Blood Alcohol Levels
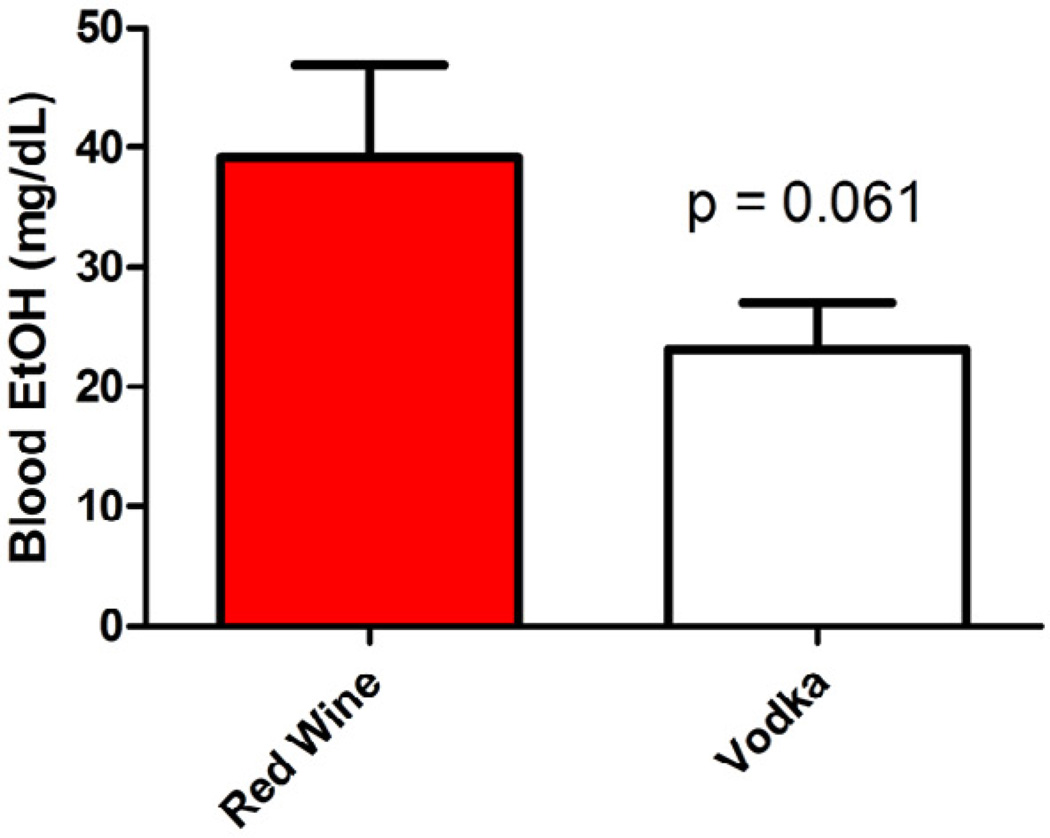
The mean serum ethanol level was lower in pigs receiving vodka compared to red wine (39.17 ± 7.71 mg/dL in HCW vs. 23.11 ± 3.86 mg/dL in HCV, p = 0.06, Figure 4). Blood was drawn from controls as well to verify that serum ethanol level was zero. A serum ethanol level of 40 mg/dL correlates with a blood alcohol content (BAC) of 0.04 on a standard breathalyzer.
Figure 4.
Blood alcohol levels higher in the Red Wine group compared to the Vodka group.
Markers of Inflammation
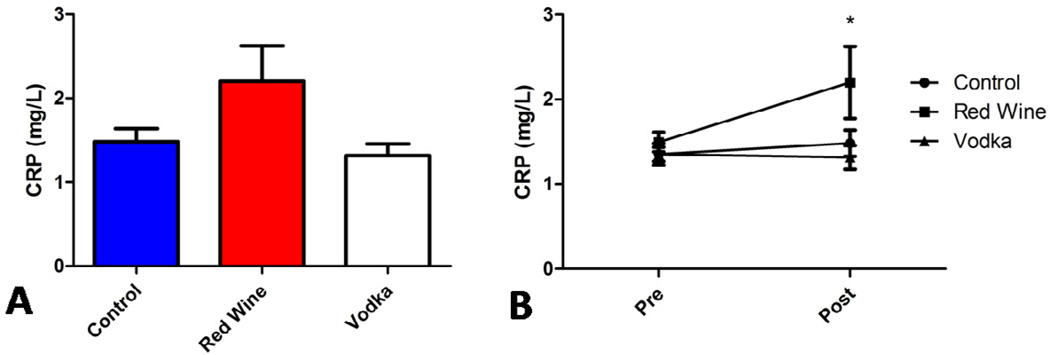
Serum C-reactive protein (CRP) levels were elevated in the HCW group (2.20 ± 0.425 mg/L) at the time of re-operative sternotomy compared to both HCV and HCC groups (1.32 ± 0.142 mg/L and 1.48 ± 0.154 mg/L respectively. ANOVA p = 0.0597). The change in serum CRP level from the first operation (Pre) to re-operative sternotomy (Post) was significantly different between groups (ANOVA p = 0.0263) with the largest difference between the HCW and HCV groups (p < 0.05, Figure 5). A trend toward similar changes in LDL (low-density lipoprotein) cholesterol was observed between groups (351, 552, 410 mg/dL in HCC, HCW, and HCV, respectively).
Figure 5.
CRP levels elevated in Red Wine group at re-operation, A. Changes in CRP significantly higher (*p < 0.05) in Red Wine group compared to Vodka group or Controls, B.
Discussion
In this study we tested the hypothesis that resveratrol-containing red wine would reduce pericardial adhesion formation as compared to alcohol alone. Surprisingly, we found that resveratrol-free vodka was significantly more effective at reducing adhesions over the seven week post-operative time period. This finding was supported by a statistically significant reduction in adhesion grade on a formalized scale. Interestingly, we did not find any difference in the expression of proteins known to be involved in focal adhesion formation between any groups (FAK, Int α5, Int β1, Paxillin, Vinculin, PYK2, PKCε, p-PKCε). One possible explanation for this negative finding in protein expression is that the mechanism of adhesion reduction is a post-translational modification effect on collagen deposition or cross-linking. Though there is overwhelming evidence that chronic alcohol abuse contributes to hepatic fibrosis, the effects of ethanol on cardiac and vascular tissues are not as well understood. Biomaterial research in the prevention of calcification and collagen deposition have shown ethanol pretreatment of porcine heart valves to be highly efficacious in preventing cuspal calcification and results in increased resistance to collagenase digestion. [7] Furthermore, in rabbit studies, oral administration of perillyl alcohol led to a 22% reduction in intimal thickness of arterialized vein grafts. [8] In our study the differences in both intramyocardial fibrosis and transmural collagen deposition (controls > red wine > vodka) between groups supports previous findings of the anti-fibrotic properties of ethanol in cardiac tissues and correlate with a reduction in adhesions. Another possibility is that there were, in fact, differences in the expression of proteins involved in focal adhesion formation, but that these differences are not apparent in the RIPA soluble lysate fractions which were analyzed in this experiment. As many of these cell-ECM adhesion proteins are at least in part trans-membrane, it is possible that differences would be appreciated in analysis of the insoluble lysate fraction.
In our groups experience with several hundred ameroid constrictor placements via mini left thoracotomy followed by median sternotomy several weeks later, extensive, dense, hemorrhagic adhesions are universally encountered at the time of reoperation. The dense adhesions seen in this experiment enveloping all surfaces of the epicardium, not just the area involved in the mini-thoracotomy in both the control and wine groups support the assumption that the thoracotomy is a sufficient operation to create dense, easily bleeding pericardial adhesions.
All animals in the study were given a high-cholesterol diet in order to replicate, as closely as possible the conditions present in human cardiac patients, the majority of who have a degree of hypercholesterolemia and diabetes or glucose intolerance. Studies of surgically induced chronic ischemia in otherwise healthy animals have proven to be inadequate simulators of human patients which have ischemic disease in the setting of multiple comorbidities.[9] This lack of translatability of large animal studies to clinical trials, though multifactorial, is due in large part to endothelial dysfunction which lacks in animal studies, yet is nearly omnipresent in human cardiac patients. Previous studies in swine have demonstrated that diet-induced hypercholesterolemia does, in fact, significantly alter endothelial function.[10, 11]
Moderate alcohol consumption in humans has been shown to increase plasma levels of high-density lipoproteins (HDL), or “good cholesterol.” Various lipoprotein functions are altered by alcohol intake, including the binding of HDL to cells, and the modulation of growth factors and the cytokine response.[12] The effects of alcohol on inflammatory factors have not been as well delineated, as the effects of alcohol on C-reactive protein (CRP) has yielded conflicting results ranging from different response curves depending on sex and body mass index [13] to having both pro- [14] and anti-inflammatory effects.[15, 16] According to the American Heart Association, CRP levels between 1.0 and 3.0 mg/L represent average risk for a cardiovascular event. [17] A recent study in human alcohol consumption that stratified not only for increasing amounts of alcohol use but also for beverage type found wine consumption to be related to an increase in HDL while beer and spirits were related to an increase in triglyceride levels. [18] In this study, both CRP and LDL cholesterol levels were higher at re-operation in the animals that received red wine, and the change in CRP from the first operation to the second was significantly higher in this group, while no difference existed between controls and the group receiving vodka. This elevated inflammatory state may explain why adhesions were seen in the wine group vs. vodka.
Understanding that hypercholesterolemia increases markers of inflammation and oxidative stress [9], and that alcohol consumption modifies lipoprotein function [12], as well as acting directly as an anti-inflammatory agent [16], it seems reasonable to deduce mechanisms by which vodka consumption decreases the inflammatory response: either by modulation of lipoprotein function in the setting of hypercholesterolemia, or by directly decreasing lipoprotein concentration thus decreasing the inflammatory response. Though this would be supported by the reduced adhesion formation seen in the vodka group, one would also expect a reduction in adhesion formation seen in the pigs receiving an equivalent amount of alcohol from wine; this was not observed.
Resveratrol, which is found in the skin of red grapes, is thought to have several cardioprotective qualities due to its antioxidant, pro-angiogenic, and anti-apoptotic properties. [19] Why were adhesions not seen in the wine group that was consuming the same volume of ethanol as the pigs supplemented with vodka? Vodka is a simple mixture of ethanol and water, in contrast to red wine, which is a complex mixture of many compounds. Just as the resveratrol in red wine has been shown to have anti-inflammatory properties, it is possible that another compound in red wine inhibits the effects of ethanol on the post-translational modification of collagen deposition and cross-linking, if that is in fact the mechanism of adhesion reduction facilitated by vodka.
It is worth mentioning that despite that the pigs were receiving identical volumes of alcohol in the red wine and vodka groups, the difference in serum ethanol levels between the groups was nearly a two-fold change in mean concentration (Figure 4). It is possible that there exists a “therapeutic window” for ethanol, as far as the inhibition of adhesion formation is concerned, and that the higher serum ethanol level in the wine group was outside this window. This explanation would be supported by observational studies in humans showing a U-shaped curve of CRP levels associated with alcohol consumption in which both non-drinkers and heavy drinkers had higher CRP concentrations than moderate drinkers.[16] In fact, this U-shaped curve was observed in our study as well with higher CRP levels in the non-drinkers (Controls) and the group with the highest blood alcohol level (Red Wine) compared to pigs with a lower blood alcohol level (Vodka). However, peak plasma concentrations for ethanol may vary as a function of rate of consumption or the concentration of alcohol in the substance being consumed. Thus it is possible that the peak plasma concentration of ethanol occurred at different times depending on whether the animal received red wine or vodka.
The use of a systemic agent for adhesion reduction is not a novel idea. The benefits of localized treatment are obvious as systemic treatments carry the burden of undesired non site-specific effects, such as with systemic administration of corticosteroids. However, as safe systemic agents are discovered and become available, current research is expanding into this realm. For example, recent studies in post operative adhesion formation in rabbits have found systemic treatment with the monoclonal antibody, sunitinib, to significantly inhibit the formation of adhesions. [20] The agents used in our study, red wine and vodka, have both been shown in the medical literature to be cardioprotective at moderate doses, supporting the safety of this agent for systemic administration. [18, 21, 22] Finally, the blood levels of ethanol in this study (approximately 20 – 40 mg/dL) correlate with “moderate consumption” as the legal level of intoxication for ethanol in most U.S. states is 80 mg/dL. Thus, these levels are quite physiologically possible in humans.
Limitations
The most obvious limitation of this study is that of translatability of this swine model of hypercholesterolemia and chronic ischemia to a reduction in post-operative pericardial adhesions in humans. As mentioned previously, this model of ischemia with concomitant risk-factor modification is designed to better replicate the human disease state that it intends to model. It is also possible that one type of vodka or red wine may produce results different than were observed in the present study. As there is yet no definitive mechanism for this observation of significant adhesion reduction, it would be premature to move into clinical trials. Occasionally during reoperative sternotomy in human cardiac patients, the mediastinum has very minimal pericardial adhesions. A feasible next-step clinical study would be to identify differences in gene and protein expression these patients in order the develop targets for novel therapeutic agents.
As with most large animal studies, due to small numbers in the experiments, it is possible that despite statistically significant differences these findings may not be replicated in a larger study or clinical trial. Finally, a definitive mechanism will need to be established before this therapy will be acceptable after heart surgery.
The lack of a definite mechanism presents an obstacle for translation to clinical trials from this stage in animal studies, and a definitive or putative mechanism will likely need to be realized before this happens. However, we do feel that this preliminary, large-animal study is pertinent to current clinical practice as these compounds are currently widely used by the general population including the cardiac surgery patient, and also pertinent to ongoing research in both systemic and local treatments for post-operative adhesion reduction.
Acknowledgments
Sources of Funding: Funding provided by grants from the National Heart, Lung, and Blood Institute (R01HL46716, R01HL69024, and R01HL85647, Dr.Sellke), NIH Training grant 5T32-HL094300 (Dr.Chu), and NIH Training grant 5T32-HL076134 (Dr.Lassaletta).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No disclosures or conflicts of interest to report
References
- 1.Holmdahl L, Risberg B, Beck DE, Burns JW, Chegini N, diZerega GS, et al. Adhesions: pathogenesis and prevention-panel discussion and summary. Eur J Surg Suppl. 1997;(577):56–62. [PubMed] [Google Scholar]
- 2.Kaushal S, Patel SK, Goh SK, Sood A, Walker BL, Backer CL. A novel combination of bioresorbable polymeric film and expanded polytetrafluoroethylene provides a protective barrier and reduces adhesions. J Thorac Cardiovasc Surg. 2011 Mar;141(3):789–795. doi: 10.1016/j.jtcvs.2010.11.043. [DOI] [PubMed] [Google Scholar]
- 3.Lopes JB, Dallan LA, Moreira LF, Campana Filho SP, Gutierrez PS, Lisboa LA, et al. Synergism between keratinocyte growth factor and carboxymethyl chitosan reduces pericardial adhesions. Ann Thorac Surg. 2010 Aug;90(2):566–572. doi: 10.1016/j.athoracsur.2010.03.086. [DOI] [PubMed] [Google Scholar]
- 4.Bel A, Kachatryan L, Bruneval P, Peyrard S, Gagnieu C, Fabiani JN, et al. A new absorbable collagen membrane to reduce adhesions in cardiac surgery. Interact Cardiovasc Thorac Surg. 2010 Feb;10(2):213–216. doi: 10.1510/icvts.2009.215251. [DOI] [PubMed] [Google Scholar]
- 5.Kanchanawong P, Shtengel G, Pasapera AM, Ramko EB, Davidson MW, Hess HF, et al. Nanoscale architecture of integrin-based cell adhesions. Nature. 2010 Nov 25;468(7323):580–584. doi: 10.1038/nature09621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark JD, Gebhart GF, Gonder JC, Keeling ME, Kohn DF. Special Report: The 1996 Guide for the Care and Use of Laboratory Animals. ILAR J. 1997;38(1):41–48. doi: 10.1093/ilar.38.1.41. [DOI] [PubMed] [Google Scholar]
- 7.Vyavahare NR, Hirsch D, Lerner E, Baskin JZ, Zand R, Schoen FJ, et al. Prevention of calcification of glutaraldehyde-crosslinked porcine aortic cusps by ethanol preincubation: mechanistic studies of protein structure and water-biomaterial relationships. J Biomed Mater Res. 1998 Jun 15;40(4):577–585. doi: 10.1002/(sici)1097-4636(19980615)40:4<577::aid-jbm9>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 8.Schachner T. Pharmacologic inhibition of vein graft neointimal hyperplasia. The Journal of thoracic and cardiovascular surgery. 2006 May;131(5):1065–1072. doi: 10.1016/j.jtcvs.2005.11.043. [DOI] [PubMed] [Google Scholar]
- 9.Boodhwani M, Sellke FW. Therapeutic angiogenesis in diabetes and hypercholesterolemia: influence of oxidative stress. Antioxid Redox Signal. 2009 Aug;11(8):1945–1959. doi: 10.1089/ars.2009.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lassaletta AD, Chu LM, Sellke FW. Therapeutic neovascularization for coronary disease: current state and future prospects. Basic Res Cardiol. 2011 Jun 29; doi: 10.1007/s00395-011-0200-1. [DOI] [PubMed] [Google Scholar]
- 11.Boodhwani M, Nakai Y, Mieno S, Voisine P, Bianchi C, Araujo EG, et al. Hypercholesterolemia impairs the myocardial angiogenic response in a swine model of chronic ischemia: role of endostatin and oxidative stress. The Annals of thoracic surgery. 2006 Feb;81(2):634–641. doi: 10.1016/j.athoracsur.2005.07.090. [DOI] [PubMed] [Google Scholar]
- 12.Brinton EA. Effects of ethanol intake on lipoproteins and atherosclerosis. Curr Opin Lipidol. 2010 Aug;21(4):346–351. doi: 10.1097/MOL.0b013e32833c1f41. [DOI] [PubMed] [Google Scholar]
- 13.Oliveira A, Rodriguez-Artalejo F, Lopes C. Alcohol intake and systemic markers of inflammation--shape of the association according to sex and body mass index. Alcohol Alcohol. 2010 Mar–Apr;45(2):119–125. doi: 10.1093/alcalc/agp092. [DOI] [PubMed] [Google Scholar]
- 14.Aziz-Seible RS, Lee SM, Kharbanda KK, McVicker BL, Casey CA. Ethanol feeding potentiates the pro-inflammatory response of kupffer cells to cellular fibronectin. Alcohol Clin Exp Res. 2011 Apr;35(4):717–725. doi: 10.1111/j.1530-0277.2010.01389.x. [DOI] [PubMed] [Google Scholar]
- 15.Paulson QX, Hong J, Holcomb VB, Nunez NP. Effects of body weight and alcohol consumption on insulin sensitivity. Nutr J. 2010;9:14. doi: 10.1186/1475-2891-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imhof A, Froehlich M, Brenner H, Boeing H, Pepys MB, Koenig W. Effect of alcohol consumption on systemic markers of inflammation. Lancet. 2001 Mar 10;357(9258):763–767. doi: 10.1016/S0140-6736(00)04170-2. [DOI] [PubMed] [Google Scholar]
- 17.Inflammation, Heart Disease and Stroke: The Role of C-Reactive Protein. 2011 [cited; Available from: http://www.americanheart.org/presenter.jhtml?identifier=4648.
- 18.Foerster M, Marques-Vidal P, Gmel G, Daeppen JB, Cornuz J, Hayoz D, et al. Alcohol drinking and cardiovascular risk in a population with high mean alcohol consumption. Am J Cardiol. 2009 Feb 1;103(3):361–368. doi: 10.1016/j.amjcard.2008.09.089. [DOI] [PubMed] [Google Scholar]
- 19.Wu JM, Hsieh TC. Resveratrol: a cardioprotective substance. Ann N Y Acad Sci. 2011 Jan;1215:16–21. doi: 10.1111/j.1749-6632.2010.05854.x. [DOI] [PubMed] [Google Scholar]
- 20.Meisel JA, Fallon EM, Le HD, Nehra D, de Meijer VE, Rodig SJ, et al. Sunitinib inhibits postoperative adhesions in a rabbit model. Surgery. 2011 Jul;150(1):32–38. doi: 10.1016/j.surg.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Hansel B, Thomas F, Pannier B, Bean K, Kontush A, Chapman MJ, et al. Relationship between alcohol intake, health and social status and cardiovascular risk factors in the Urban Paris-Ile-de-France Cohort: is the cardioprotective action of alcohol a myth? Eur J Clin Nutr. 2010 Jun;64(6):561–568. doi: 10.1038/ejcn.2010.61. [DOI] [PubMed] [Google Scholar]
- 22.Miyamae M, Kaneda K, Domae N, Figueredo VM. Cardioprotection by regular ethanol consumption: potential mechanisms and clinical application. Curr Drug Abuse Rev. 2010 Mar;3(1):39–48. doi: 10.2174/1874473711003010039. [DOI] [PubMed] [Google Scholar]