Abstract
Osteosarcoma is a devastating tumor of bone, primarily affecting adolescents. Osteosarcoma tumors are notoriously radioresistant. Radioresistant cancers, including osteosarcoma, typically exhibit a considerable potential for relapse and development of metastases following treatment. Relapse and metastatic potential can, in part, be due to a specific radioresistant subpopulation of cells with stem-like characteristics, cancer stem cells, which maintain the capacity to regenerate entire tumors. In the current study, we have investigated whether in vitro treatments with parthenolide, a naturally occurring small molecule that interferes with NF-κB signaling and has various other effects, will re-sensitize cancer stem cells and the entire cell population to radiotherapy in osteosarcoma. Our results indicate that parthenolide and ionizing radiation synergistically induce cell death in LM7 osteosarcoma cells. Importantly, the combination treatment results in a significant reduction in the viability of both the overall population of osteosarcoma cells and the cancer stem cell subpopulation. This effect is dependent on the ability of parthenolide to induce oxidative stress. Therefore, as a supplement to current multimodal therapy, parthenolide may sensitize osteosarcoma tumors to radiation and greatly reduce the prevalence of relapse and metastatic progression.
Keywords: osteosarcoma, radiation, cancer stem cells, parthenolide, NF-κB, ROS
Introduction
Osteosarcomas are highly aggressive primary bone tumors of osteoblastic origin which predominantly affect children and young adults. In addition to having a high potential for producing pulmonary metastases, osteosarcoma tumors typically demonstrate significant radioresistance (Fuchs and Pritchard, 2002). Consequently, conventional treatment is unable to prevent metastatic progression in 30–40% of patients (Lisle et al., 2008). In order to generate a more effective and comprehensive mode of treatment, it may be necessary to specifically target mechanisms utilized by osteosarcoma cells to confer radioresistance. The NF-κB signaling pathway has been implicated in a wide range of cellular processes, many of which are modulated by cancer cells to promote tumor growth (Hanahan and Weinberg, 2000; Greten and Karin, 2005; Li et al., 2005). Of particular importance, NF-κB mediates a cell’s response to stressful stimuli such as radiation-induced DNA damage (Basu et al., 1998). NF-κB is a fast-acting heterodimeric transcription factor which is sequestered in an inactive state in the cytoplasm, and translocates to the nucleus upon pathway stimulation. The cJun N-terminal kinase (JNK) signaling pathway plays an important role in radiation-induced apoptosis, especially in the absence of p53 signaling frequently observed in osteosarcoma tumors (Vogelstein et al., 2000). In cells with constitutively active NF-κB, JNK is inhibited, preventing cellular apoptotic response to DNA damage (Kuwabara et al., 2003; Dent et al., 2003). As a result, NF-κB activation is considered a key instigator of therapeutic resistance in osteosarcoma. Our previous data demonstrate NF-κB upregulation and NF-κB dependent radioresistance in osteosarcoma cell line SaOS2 in contrast to a control osteoblastic cell line, hFOB (Eliseev et al., 2005). Concurrently, LM7, a highly aggressive derivative of the SaOS2 line demonstrates similar elevation of NF-κB.
In addition to conferring radioresistance, NF-κB activation in osteosarcoma may also be responsible for disruption of the osteoblastic differentiation pathway (Liu and Shuai, 2001). Traditionally, mesenchymal stem cells that have committed to osteogenesis become osteoprogenitor cells, and further differentiate into osteoblasts, which are responsible for bone formation. NF-κB plays a vital role in this process by orchestrating a subset of processes involved in the development of osseous tissue. In cells with constitutively active NF-κB, this intricate signaling cascade is interrupted, resulting in incomplete differentiation and the maintenance of cells with stem-like properties (Mohseny et al., 2009; Tang et al., 2008). A small subpopulation of pluripotent, apoptosis-resistant stem cells possesses the capacity to generate a complex and heterogeneous osteosarcoma tumor (Siclari and Qin, 2010; Clarcke et al., 2006; Ischenko et al., 2008; Koch et al., 2010). Because these cancer stem cells are generally considered responsible for initiation and growth of primary tumors, they also retain the ability to trigger relapse and escape the primary tumor site to create metastases. Recent studies have pinpointed a membrane protein, CD133, which is able to identify, and through FACS, enrich cancerous cell populations for cancer stem cells (Tirino et al., 2008; Singh et al., 2004; Ricci-Vitiani et al., 2007; Yin et al., 2007; Monzani et al., 2007; Gibbs et al, 2005). The ability to isolate cancer stem cells has enabled the development of specific CSC targeting methods, a task which is vital to prevent relapse and metastasis in osteosarcoma.
We have previously shown that compared to non-tumorigenic osteoblasts, osteosarcoma cells transfected to stably express the NF-κB constitutive inhibitor, mIκB, demonstrate decreased cell growth accompanied by increased apoptotic markers. Exposure to radiation enhances this effect, suggesting that inhibition of NF-κB increases sensitivity to radiation, leading to apoptosis. Parthenolide is a naturally occurring small molecule that interferes with NF-κB (Kishida et al., 2007), and would potentially provide a clinically applicable method of reducing NF-κB in osteosarcoma patients, enabling radiation therapy to play a more effective role in cancer treatment. We propose that parthenolide, as a supplement to current therapeutic regimens, will increase the sensitivity of cancerous cells to radiotherapy, providing a more complete eradication of malignant tissue. Although cancer stem cells demonstrate significant resistance to chemotherapy and radiation (Siclari and Qin, 2010; Bao et al., 2006; Liu et al., 2006; Hirschmann-Jax et al., 2004; Szotek et al., 2006) , parthenolide treatment appears to greatly reduce the number of cancer stem cells in culture. The combination of parthenolide treatment and irradiation was able to synergistically induce cell death in cultured osteosarcoma cells, including the cancer stem cell subpopulation. Because parthenolide is already implemented for the treatment of migraines (Murphy et al., 1988), it should be considered a superior candidate for inclusion in modern multimodal therapeutic regimens for osteosarcoma.
Materials and Methods
Materials
Chemicals were obtained from Sigma unless otherwise noted. Cell culture media, media supplements and antibiotics were obtained from Invitrogen. Primary antibodies for p65 and lamin were from Santa Cruz. PE conjugated antibodies for CD133, used in flow cytometry analysis and FACS, were obtained from eBioscience. Active caspase-3 labeling kit for flow cytometry was from BD Bioscience. Secondary antibodies, Precision Plus molecular weight markers, dry milk, and buffer ingredients were from Bio-Rad. Hoechst 33342 stain was from Invitrogen. FuGENE® HD transfection reagent was from Roche.
Cell Culture and Treatment
Human osteosarcoma SaOS2 cells and human fetal osteoblast hFOB 1.19 were obtained from ATCC, and LM7 cells were a kind gift of Dr. Eugene Kleinerman of MD Anderson Cancer Center. Osteosarcoma cells were cultured in DMEM, and hFOB cells were cultured in DMEM/F12 at 37°C. All media was supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin mixture. Seeding densities were kept constant and adjusted for different plating surface areas. All cells were cultured for 24 hours before receiving treatment. Parthenolide dissolved in DMSO, or DMSO alone was added to cell culture media for appropriate cells as described in figure legends. Cells receiving radiation treatment were irradiated with 10Gy of γ-radiation using a cesium source.
Dual luciferase promoter-reporter assay
Cells plated on 12-well plates were transfected with either the NF-κB-luc or AP1-luc firefly luciferase reporter at 0.5 µg per well. The renilla luciferase promoterless reporter, pRL-TK, produced by Promega, was co-transfected at 0.05 µg per well as a reference. FuGENE® HD reagent (Roche) was used for transfections. After 24 hours, cells were treated as indicated in figure legends, lysed, and firefly and renilla luciferase activities were measured using a Dual Luciferase Reporter Assay System (Promega) in an Optocomp 1 luminometer according to the manufacturer’s instructions. The firefly luciferase signal was normalized to the renilla luciferase signal and expressed as relative luminescence units (RLU).
Cell Fractionation and Western Blotting
Nuclear fractions were prepared using the NE-PER nuclear extraction kit (Pierce) according to the manufacturer’s protocol. Protein concentrations were measured using the Bradford Assay. Twenty-five micrograms of total protein per sample were mixed 1:1 with 2× Laemmli buffer, boiled, and subjected to electrophoresis through NuPage precast 4–12% gradient polyacrylamide gels (Invitrogen) followed by electroblotting onto polyvinylidene difluoride membranes (Bio-Rad). Blots were blocked with 5% dry milk emulsified in PBS containing 0.01% Tween-20 (PBST), probed with anti-p65 primary antibody resuspended in 2.5% dry milk in PBST at 2 µg/mL, incubated with horseradish peroxidase-conjugated secondary antibody resuspended in 2.5% dry milk in PBST at 0.2 µg/mL, resolved using SuperSignal WestPico chemiluminescent substrate from Thermo Scientific (Rockford, IL), and photographed. To verify equal loading, blots were stripped in Re-Blot Plus stripping buffer produced by Millipore (Billerica, MA), reprobed with anti-lamin antibody resuspended in 2.5% milk in PBST at 2 µg/mL. Band intensities were measured using densitometry and Adobe software.
Cell Viability Assay
Cells were plated on 96-well plates, grown for 24 hours, treated as stated in figure 2B, and cell viability was assessed using the LIVE/DEAD Viability/Cytotoxicity Kit for mammalian cells (Invitrogen) according to the manufacturer’s protocol. Fluorescence intensity was determined using a BioTek microplate reader.
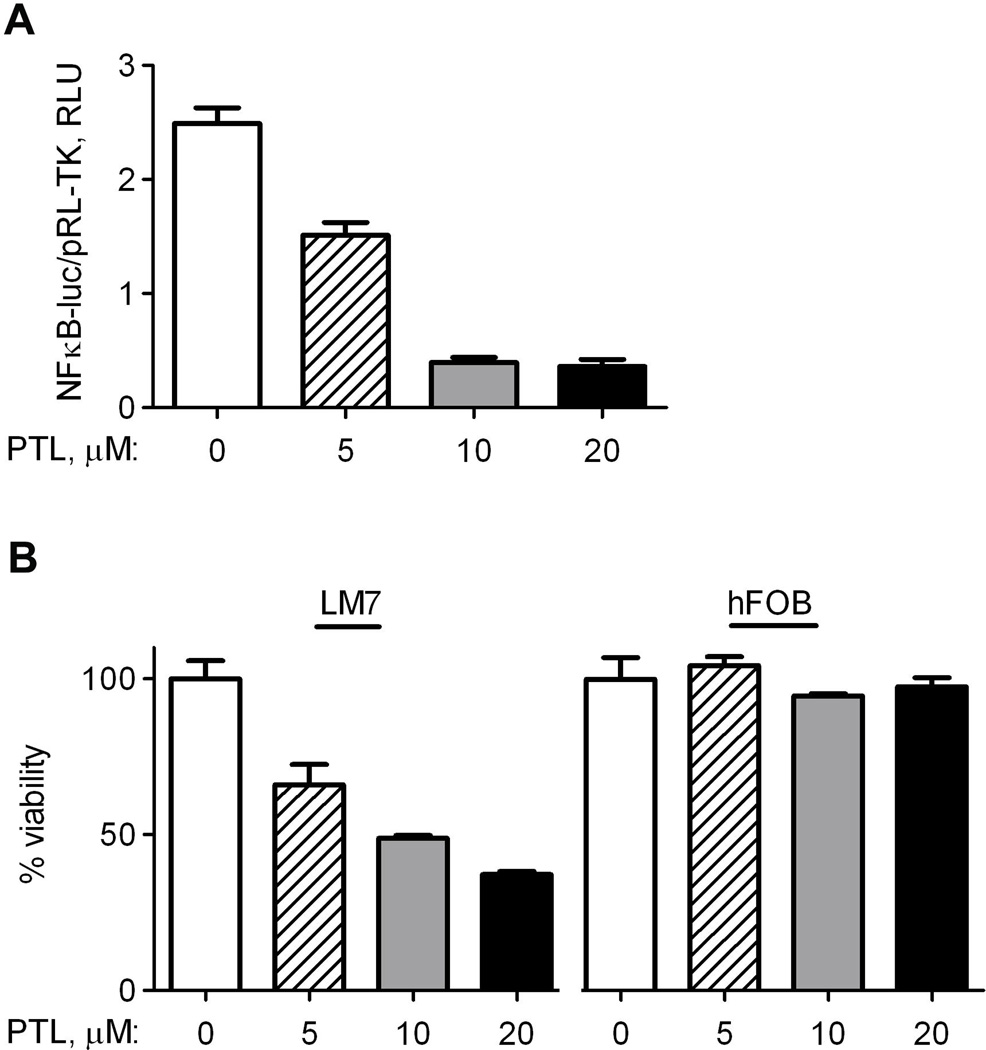
Fig. 2.
Parthenolide demonstrates dose-dependent effect on NF-κB activity and viability of LM7 osteosarcoma cells. DMSO as a vehicle control or parthenolide (PTL) was added to cell culture medium at indicated concentrations for 24 hrs. A) Transfected LM7 cells from Fig. 1A were treated for 24 hrs. NF-κB-induced firefly luciferase luminescence was compared to basally expressed renilla luciferase luminescence as a reference, and plotted as Relative Luminescence Units (RLU); B) LM7 and hFOB cells were treated for 24 hrs. Cell viability was assessed using the LIVE/DEAD Viability/Cytotoxicity Kit from Invitrogen. Data are Means ± SE (n=4).
Cell Growth Assay
Cells were plated onto 12-well plates at 2×105 cells per well, grown for 24 hours, treated as stated in figure 4B, trypsinized, pelleted, resuspended in 1mL PBS and counted using the Cellometer automated cell counter (Nexcelcom Bioscience).
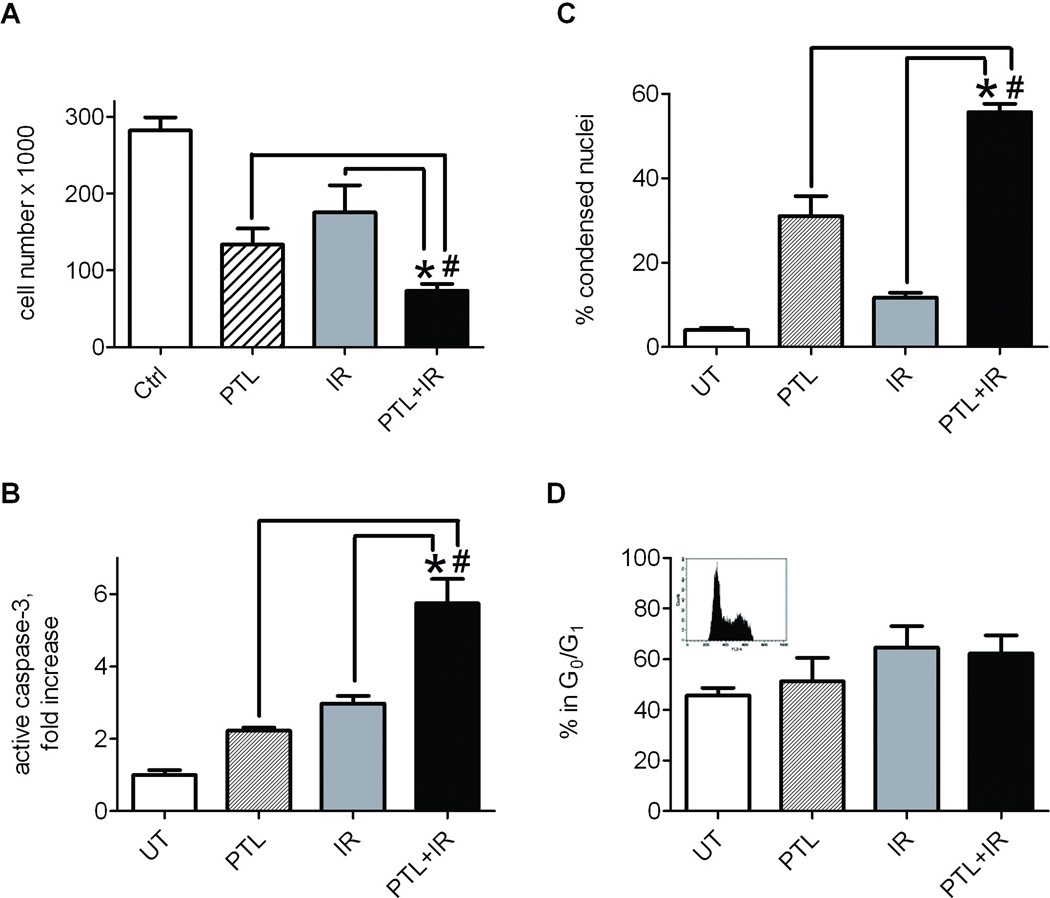
Fig. 4.
Combination treatment with parthenolide and radiation synergistically inhibits growth and induces apoptosis in LM7 osteosarcoma cells. LM7 cells were treated with either DMSO or parthenolide at 10 µM, DMSO and 10Gy ionizing radiation or both parthenolide and radiation. In the latter parthenolide was added 4 hrs before irradiation. At 24 hrs after irradiation cells were collected. A) LM7 cells were detached and counted in a CelloMeter automatic cell counter. B) After treatments LM7 cells were stained with Hoechst33342. The number of cells with condensed apoptotic nuclei was counted against the total number of cells. C) After treatments LM7 cells were fixed and incubated with PE-conjugated anti-active caspase-3 antibody and assayed using flow cytometry. PE positive cell counts of treated cells were compared to those of untreated cells, and plotted as average fold change. D) Cells were stained with propidium iodide and subjected to flow cytometry to determine cell cycle distribution. Box insert shows representative flow cytometry histogram. Data are Means ± SE (n=4). * indicates P<0.05 when compared to cells exposed only to radiation. # indicates P<0.05 when compared to cells treated with parthenolide alone.
Cell Cycle Analysis
Cell cycle phase distribution was analyzed with flow cytometry. Cells plated on 6-well plates were grown for 24 hours, synchronized in a serum-free medium for 24 hours, treated as described in the Results section and harvested. Collected cells were treated with RNase A at 1 µg/µL and stained with propidium iodide (50 ng/µL). Samples were analyzed by flow cytometry on FACSCantoII (Becton-Dickinson) and cell cycle phase distribution was determined.
Nuclear Condensation Assay
Cells plated on 24-well plates were grown for 24 hours, treated as stated in figure 4B, and stained with Hoechst3334 at 1 µM in cell culture media. Fluorescent nuclear signal was visualized using the Zeiss AxioVert inverted microscope under UV illumination and cells with condensed apoptotic nuclei were counted against the total number of cells.
Active Caspase-3, CD133 and DCFDA Labeling, Flow Cytometry and Fluorescence Activated Cell Sorting
To determine activation of the intrinsic apoptotic pathway, cells plated on 6-well plates were grown for 24 hours, treated as stated in Fig 4C, trypsinized, fixed and labeled with PE-conjugated anti-active caspase-3 antibody using labeling kit from BD Bioscience according to the manufacturer’s instructions. The resulting number of fluorescing cells was quantified using Flow Cytometry on a FACSCantoII and quantities determined for samples that received treatment were compared to untreated samples. To determine the percentage of viable cells expressing the CD133 antigen, cells were labeled with 1 µg/mL PE-conjugated anti-CD133 antibody in binding buffer (PBS+0.1% fetal bovine serum). To isolate the CD133+ subpopulation in LM7 cells for a sarcosphere formation assay, untreated LM7 cells were trypsinized, labeled with antibody and then separated into CD133− and CD133+ subpopulations using fluorescence activated cell sorting on a FACSAriaII. To assess cellular ROS content, cells were loaded with DCFDA at 1 µM for 30 min at 37°C. Some cells were double labeled with DCFDA and PE-conjugated anti-CD133 antibody and DCFDA signal in CD133+ and CD133− subpopulations was measured on a FACSCantoII.
Sphere Formation and Clonogenicity Assay
To determine the ability of CD133+ cells to form sarcospheres in culture, sorted LM7 cells were plated on semi-solid medium (agar) in 6 well plates at a density of 1000/well, grown for seven days, stained with Crystal Violet, and photographed. To assess cell capacity to form colonies in various treatment conditions, cells were treated, then trypsinized, resuspended in cell culture medium and plated at a density of 1000 cells/well in 6-well plates. After 10 days, cells were stained with Crystal Violet and counted. Colony numbers from treated cells were compared to those from untreated cells.
Oxygen consumption
Rates of antimycin A-sensitive oxygen consumption in cell suspensions were measured with a Clark-type oxygen electrode (Microelectrodes Inc) in a custom-made air-tight temperature-controlled chamber at 37°C. Three hundred µl of medium containing 6×105 re-suspended cells was added in the absence or presence of antimycin A at 1 µM; and the chamber was sealed. After signal stabilization, oxygen consumption was recorded for 15 min and then a reducing agent, dithionite, was added to calibrate for 0% oxygen content. The signal trace was digitized on a computer connected to the electrode via a data acquisition device (DATAQ Instruments) using version 2.54 WinDaq software (DATAQ Instruments).
Statistical analysis
Experiments were repeated 3 to 5 times. Data were analyzed using Prism 5.01 (GraphPad Software). Mean values and standard errors were calculated, and the statistical significance was established using either Student's t-test or one-way analysis of variance (ANOVA), as appropriate. Data with P<0.05 were considered statistically significant.
Results
NF-κB pathway is stimulated in osteosarcoma cells
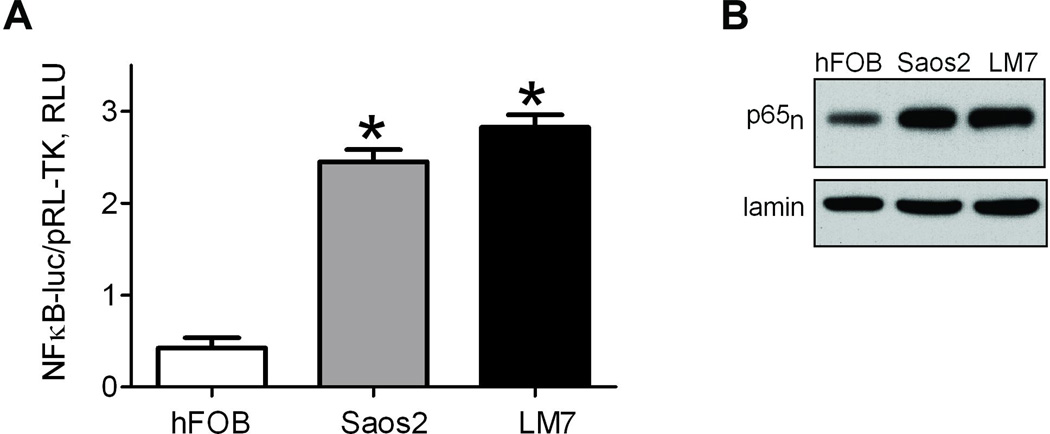
We have previously shown that NF-κB plays important role in osteosarcoma radioresistance (Eliseev et al., 2005). In our previous study we used SaOS2 cell line. LM7 cell line is a tumorigenic and metastatic derivative of the SaOS2 cell line developed without any genetic manipulations (Duan et al., 2004) and representing a reliable and clinically relevant osteosarcoma model. We therefore focused on this cell line and compared it to the maternal and previously studied SaOS2 as well as to a non-malignant hFOB cell line. We first determined the degree of basal NF-κB transcriptional activity in osteosarcoma-derived cell lines, SaOS2 and LM7, and in a human fetal osteoblast progenitor cell line, hFOB, using an NF-κB promoter luciferase reporter assay. Fig 1A shows that both osteosarcoma lines demonstrated at least a four-fold increase in basal NF-κB activity compared to the non-malignant control. To confirm this observation, nuclear fractions were isolated from these same cell lines and probed for the NF-κB subunit p65 via Western Blot (Fig 1B). The presence of p65 in the nucleus indicates an active NF-κB signaling cascade, as the inactive form is sequestered in the cytoplasm. Both osteosarcoma cell lines showed significantly increased nuclear p65 compared to the control, validating the observation of elevated NF-κB signaling in osteosarcoma vs control cells.
Fig. 1.
Activation of NF-κB in osteosarcoma cells. A) Intrinsic NF-κB activity measured in hFOB and osteosarcoma cells, Saos2 and LM7. Cells were transfected with NF-κB-luc reporter and promoterless pRL-TK to demonstrate NF-κB transcriptional activity. NF-κB induced firefly luciferase luminescence was compared to basally expressed renilla luciferase luminescence as a reference, and plotted as Relative Luminescence Units (RLU). Data are Means ± SE (n=4). *indicates P<0.05 when compared to control level in hFOB; B) Activation of NF-κB as determined by nuclear localization of NF-κB subunit, p65. Nuclear fractions from hFOB, Saos2, and LM7 were collected and the level of p65 protein was determined by Western Blot, with lamin as a loading control. Blot is a representative of 3.
Parthenolide suppresses NF-κB and exhibits a dose-dependent and selective effect on osteosarcoma cell viability
To determine the effect of parthenolide on NF-κB signaling in osteosarcoma, LM7 cells were incubated in media supplemented with parthenolide at a working concentration of 0 µM, 5 µM, 10 µM, or 20 µM for 24 hours. As seen in Fig 2A, an NF-κB luciferase reporter assay indicated progressive inhibition of NF-κB signaling at increasing parthenolide concentrations. At parthenolide concentrations of 10 µM and 20 µM, inhibition of NF-κB activity plateaued with a 5-fold decrease. To assess the effect of parthenolide concentrations on viability of osteosarcoma cell lines vs. a non-cancerous osteoblast cell line in our hFOB-SaOS2-LM7 model, the Live-Dead assay was used to measure intracellular esterase activity and membrane permeability as described in Methods, and viable cells were quantified using a plate reader (Fig 2B). Concentrations up to 20 µM parthenolide had a negligible effect on non-malignant hFOB osteoblast viability. In contrast, LM7 osteosarcoma cells reacted adversely to parthenolide, showing a decrease in viability to below 50% of the control at a working concentration of 20 µM. These data indicate that parthenolide selectively and dose-dependently eliminates osteosarcoma cells but not non-malignant osteoblasts.
Parthenolide potentiates the effects of ionizing radiation in osteosarcoma cells
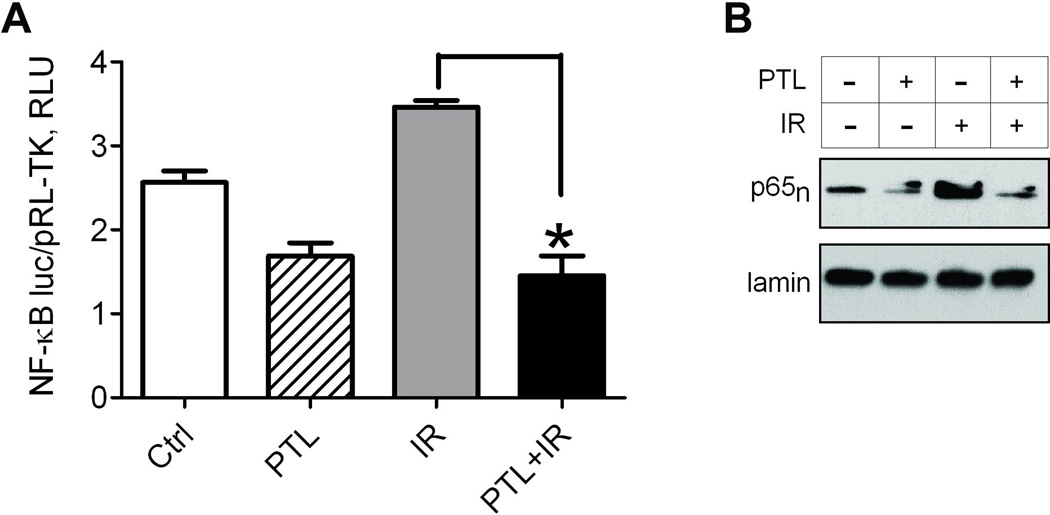
Because elevated NF-κB signaling in osteosarcoma may confer radioresistance (Eliseev et al., 2005), the suppression of NF-κB by parthenolide is hypothesized to increase the susceptibility of cells to ionizing radiation. In Fig. 3A, a reporter-promoter assay was used to demonstrate further activation of NF-κB in LM7 cells following exposure to radiation compared to untreated LM7 cells, indicating initiation of an NF-κB-mediated protective response to stress that may lead to evasion of apoptosis (Naugler and Karin, 2008; Van Antwerp et al, 1996). Pre-treatment with 10 µM parthenolide for 4 hours suppressed NF-κB signaling and maintained similarly low levels of NF-κB transactivational activity following exposure to 10Gy ionizing radiation. Concurrently, significantly reduced p65 was detected in nuclear fractions of LM7 cells pre-treated with parthenolide, including cells that received radiation treatment (Fig 3B). Cells exposed to radiation in the absence of parthenolide exhibited elevated nuclear p65 compared to control cells. The effects of parthenolide and radiation on cell numbers and induction of apoptosis were also assessed in our osteosarcoma cell model. Cells were plated, treated or not with parthenolide and radiation, detached, and counted (Fig 4A). Cells exposed to either parthenolide or radiation displayed a moderate decrease in cell numbers compared to untreated cells, and cells exposed to both parthenolide and radiation demonstrated a significant 3-fold decrease in cell count, suggesting that the cells may have undergone cell death via apoptosis. Induction of apoptosis was confirmed in two individual assays. Staining with Hoechst33342 revealed condensed, apoptotic nuclei which were counted vs. the total number of nuclei (Fig 4B). Cells incubated with parthenolide and exposed to radiation exhibited a significant percentage of apoptotic nuclei, almost 10 fold greater than untreated controls. Cells treated with parthenolide or gamma irradiation alone contained an average of 31% and 12% apoptotic nuclei, respectively, while cells receiving a combination of treatments demonstrated an apoptotic subpopulation reaching 56%. Active caspase-3 is an indicator that the apoptosis pathway has been activated in cells. Figure 4C illustrates an almost 6 fold increase in active caspase-3 in cells treated with parthenolide and radiation compared to untreated cells while cells treated with parthenolide or radiation alone showed a 2–3 fold increase. In addition we have studied whether the parthenolide- and radiation-induced cell demise was in part due to the effect on cell cycle. We performed cell cycle analysis using propidium iodide staining and flow cytometry and found no change in the number of cells in G0/G1 phase in the parthenolide pre-treated irradiated group when compared to radiation alone group (Fig. 4D). Together, these data indicate that parthenolide potentiates radiation-induced apoptosis without noticeable effect on cell cycle.
Fig. 3.
Parthenolide treatment suppresses radiation induced NF-κB signaling in LM7 cells. LM7 cells were treated with either DMSO or parthenolide at 10 µM, DMSO and 10Gy ionizing radiation or both parthenolide and radiation. In the latter parthenolide was added 4 hrs before irradiation. At 24 hours after irradiation cells were collected. A) NF-κB transcriptional activity was assessed with firefly luciferase luminescence vs. control renilla luciferase luminescence, and plotted as Relative Luminescence Units (RLU). Data are Means ± SE (n=4). *indicates P<0.05 when compared to level in cells exposed only to radiation; B) Activation of NF-κB as determined by nuclear localization of NF-κB subunit, p65. Nuclear fractions were collected and the level of p65 protein was determined by Western Blot, with Lamin as a loading control. Blot is a representative of 3.
Osteosarcoma stem-like cells are synergistically suppressed by parthenolide and ionizing radiation
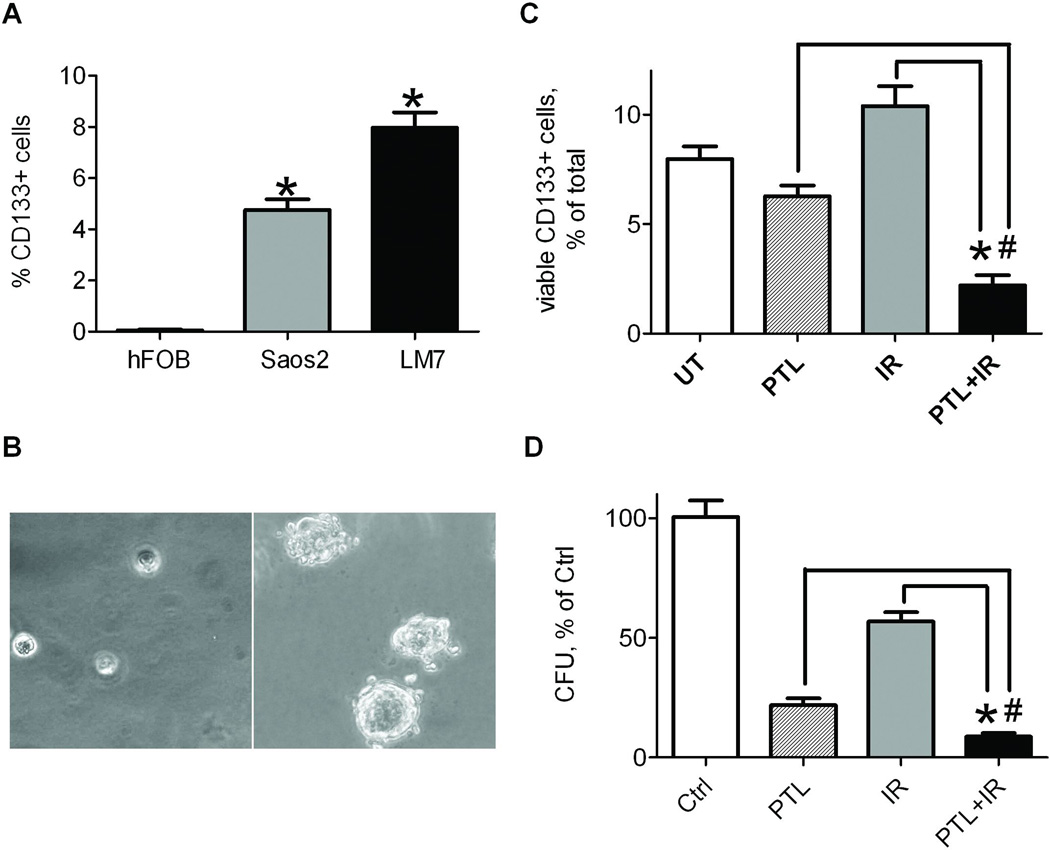
Considering that cancer stem-like cells may be main culprits of tumor relapse after irradiation, we have focused on the effect of parthenolide and radiation on this subpopulation of cells. We first determined the percentage of stem-like cells in two osteosarcoma cell lines and a control cell line of osteoblastic lineage by probing cells for the CD133 surface marker specific for osteosarcoma stem-like cells (Tirino et al., 2008) and subjecting them to flow cytometry. Both osteosarcoma derived cell lines contained a subpopulation of CD133+ cells that were not seen in the non-malignant osteoblastic cell line, hFOB. Consistent with previous studies by Tirino et al.(2008), our data show that SaOS2 cells contain a 5% subpopulation of cells that express CD133, while the more aggressive and metastatic LM7 line features an average of 8% CD133 positive cells. The sorted LM7 cells were plated in semi-solid medium and cultured for 7 days to observe sphere forming potential (Fig 5B). CD133+ cells succeeded at forming sarcospheres, while CD133− cells did not, confirming stem-like characteristics of CD133+ cells. To assess the ability of parthenolide to suppress the population of stem-like cells in osteosarcoma, LM7 cells were treated with parthenolide or radiation, or a combination, and compared to untreated cells. Following treatment(s), surviving cells were probed for CD133 and the number of positive cells was determined using flow cytometry (Fig 5C). We focused on surviving cell population because this population is most relevant to tumor recurrence following treatments. Figure 5C shows that parthenolide alone slightly reduced the CD133+ subpopulation of surviving LM7 cells; radiation, on the other hand, increased the percentage of CD133+ stem-like cells in a surviving population from 8 to 11%, confirming radioresistance of this subpopulation; and a combination of parthenolide and ionizing radiation was very effective and reduced the CD133+ subpopulation to 2% of total surviving cells. To confirm that colony formation is dependent on the presence of CD133+ cells in a population, LM7 cells were subjected to treatment, plated at low density, and allowed to grow for 10 days. Cells were stained with crystal violet to observe colony formation, and colonies were counted (Fig 5D). Irradiation demonstrated the most minimal effect, reducing colony formation to 57% of control. The number of colonies formed following parthenolide pre-treatment was significantly reduced, to 22% with parthenolide alone and 9% when parthenolide and irradiation were used in combination. These data indicate that parthenolide, especially when combined with ionizing radiation, effectively eliminates a pool of CD133+ stem-like cells in osteosarcoma.
Fig. 5.
CD133+ stem-like cell subpopulation in osteosarcoma cell lines is suppressed synergistically with combination treatment of parthenolide and radiation. A) hFOB, Saos2, and LM7 cells were probed with PE-conjugated anti-CD133 antibody and assayed using flow cytometry. *indicates P<0.05 when compared to hFOB cells. B) LM7 cells were probed with PE-conjugated anti-CD133 antibody and subjected to FACS. Isolated CD133+ and CD133− populations were plated on semi-solid media and cultured for 7 days to determine sphere formation potential. Sphere colonies were present in the cultured CD133+ cells but not in the CD133− cells. C) LM7 cells were treated with either DMSO or parthenolide at 10 µM, DMSO and 10Gy ionizing radiation or both parthenolide and radiation. In the latter parthenolide was added 4 hrs before irradiation. At 24 hrs after irradiation surviving cells were collected, probed with PE-conjugated anti-CD133 antibody and assayed using flow cytometry. The number of PE positive cells in each treatment condition was compared to the number in the untreated controls and plotted as % of total. D) LM7 cells were treated as described above in (C). 24 hours after treatments, cells were detached, plated at low density (1000 cells/well in 6-well plates), grown for 10 days, and stained with Crystal Violet. The number of colonies were counted for each treatment condition and compared to untreated controls. Data was plotted as Colony-Forming Units (CFU), % of control. Data in (A, C and D) are Means ± SE (n=4). * in (C, D) indicates P<0.05 when compared to cells exposed to radiation alone. # in (C, D) indicates P<0.05 when compared to cells treated with parthenolide alone.
The effect of parthenolide on osteosarcoma stem-like cells is dependent on ROS
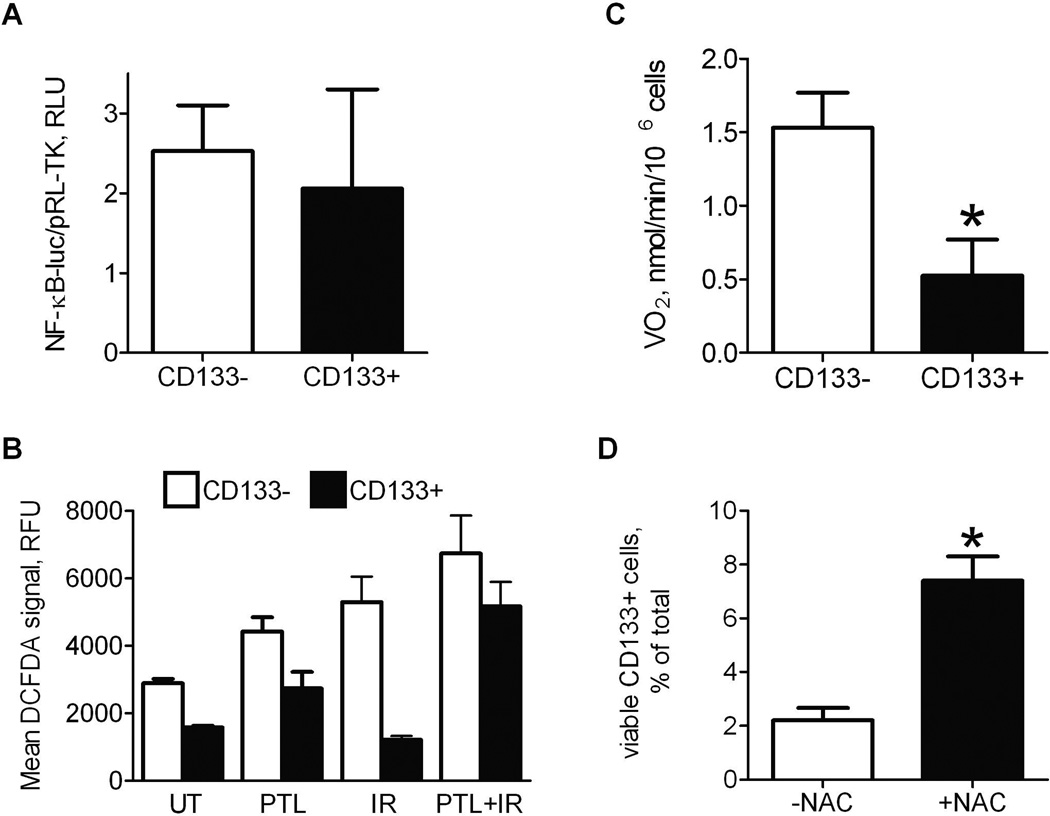
To elucidate the mechanism of radiosensitization of CD133+ stem-like osteosarcoma cells by parthenolide, we first assessed whether NF-κB activity differ in CD133+ and CD133− LM7 subpopulations. If NF-κB activity is significantly higher in CD133+ cells, it may explain decreased sensitivity of this subpopulation to ionizing radiation. Correspondingly, JNK pathway activity, which is inhibited by NF-κB as shown in our previous work (Eliseev et al., 2005), may be decreased contributing to radioresistance of the CD133+ subpopulation. We, therefore, assessed both NF-κB and JNK pathway activities in CD133+ and CD133− subpopulations by transfecting LM7 cells with NF-κB or AP1 luciferase reporters, sorting cells for CD133 and measuring NF-κB-luc and AP1-luc reporter activities in CD133+ and CD133− subpopulations. Figure 6A shows that NF-κB activity is similar in both subpopulations. JNK pathway activity is also not significantly different in these subpopulations (Data not shown). Therefore, decreased sensitivity of CD133+ stem-like osteosarcoma cells is unlikely due to an increased NF-κB or correspondingly decreased JNK pathway activity.
Fig. 6.
Radiosensitization of CD133+ stem-like cells by parthenolide is dependent on its pro-oxidant effect. A) LM7 cells were transfected with NF-κB-luc reporter and RL-TK, probed with PE-conjugated anti-CD133 antibody and subjected to FACS. Isolated CD133+ and CD133− subpopulations were lyzed and assayed for NF-κB activity; B) Cells were treated as described in Figure 5, stained with DCFDA and with PE-conjugated CD133 antibody, and simultaneously assayed for DCFDA and PE signals using flow cytometry; C) Cells were treated as described above and sorted for CD133 using FACS. Cell respiration, VO2, was measured in isolated CD133+ and CD133− subpopulations using Clark-type oxygen electrode; D) Number of surviving CD133+ cells after treatment with parthenolide and radiation in the absence or presence of N-acetyl cysteine (NAC) was determined using flow cytometry. Data are Means ± SE (n=4). * indicates P<0.05 when compared to CD133− cells (A, C) or control (D).
Another well described effect of parthenolide, other than NF-κB inhibition, is its ability to induce oxidative stress and ROS production (Wen et al., 2002; Sun et al., 2010). We, therefore, studied whether the observed radioresistance in osteosarcoma stem-like cells and radiosensitization by parthenolide are due to the involvement of ROS. First, we measured ROS in CD133+ and CD133− subpopulations using DCFDA and flow cytometry. Figure 6B shows that the basal level of ROS in CD133+ cells is significantly lower than that in CD133− cells. Importantly, our data are in concert with previous reports showing decreased ROS levels in CSCs (Diehn et al., 2009). Radiation induces ROS in CD133− cells but not in CD133+ cells. Parthenolide, however, causes ROS burst in both subpopulations and also potentiates the pro-oxidant effect of ionizing radiation. Thus, these data demonstrate that parthenolide has a strong pro-oxidant effect especially when combined with ionizing radiation in CD133+ stem-like osteosarcoma cells that originally have significantly lower ROS levels when compared to CD133− cells.
Cells that have stem-like characteristics have been shown to have decreased oxidative metabolism due to suppressed mitochondrial respiration and, therefore, lower ROS content because mitochondria are the major source of ROS in cells (Noble et al., 2003; Chen et al., 2010). To confirm this, we measured oxygen consumption, which was antimycin A-sensitive and, thus, mitochondrial, in CD133+ and CD133− cells. Figure 6C shows that oxygen consumption is indeed significantly lower in CD133+ cells. This could explain lower ROS levels in this subpopulation of osteosarcoma cells. Finally, we wanted to find out whether the radiosensitizing effect of parthenolide in CD133+ osteosarcoma cells was due to its ROS-inducing effect. We, therefore, treated LM7 cells with parthenolide and radiation in the presence of ROS scavenger, N-acetylcysteine (NAC), and found that NAC effectively rescued CD133+ cells and abolished the radiosensitizing effect of parthenolide. These data indicate that the radiosensitizing effect of parthenolide on CD133+ cells is ROS-dependent.
Discussion
Osteosarcomas are notoriously chemo- and radioresistant, resulting in an incomplete eradication of malignant cells during treatment and a relatively low five-year post-treatment survival rate of 50–60% (Goorin and Schwartzentruber, 2003). Comprehensive elimination of cancerous cells is necessary to prevent relapse and is contingent upon identification of the mechanisms utilized by cancer cells to evade treatment. However, an extensive network of cellular signals influences a cell’s commitment to the cancer phenotype, obscuring the detection of mechanisms that may be fundamental to osteosarcoma. Several genes and pathways with aberrant activity profiles have been consistently identified in osteosarcoma cells including but not limited to ABCG2, COX-2, and NF-κB. ABCG2 and COX-2 are recognized as important markers for osteosarcoma, though little is known regarding their role in osteosarcoma maintenance. ABCG2 is involved in cellular efflux of xenobiotic compounds, and elevated expression is responsible for resistance to chemotherapy in a variety of cancers (Lou and Dean, 2007). Elevated expression of COX-2, a mediator of inflammatory response, is associated with tumor development, invasion, and metastasis (Zhao et al., 2011). Both ABCG2 and COX-2 may play integral roles in osteosarcoma maintenance, though neither has been associated with radioresistance. Because radiotherapy can be a highly effective means of controlling the propagation of cancer cells, pathways that contribute to radioresistance are considered a primary concern for this study. The NF-κB signaling pathway has been implicated as a primary contributor to radioresistance in osteosarcomas (Eliseev et al., 2005) and has been shown to play a key role in the maintenance of cancer stem cells (Tang et al., 2008). Consequently, it has been suggested that resistance to radiation is a key property held by cancer stem cells (Ischenko et al., 2008; Bao et al., 2006). While cancer stem cells are an elusive and important target for preventing metastasis and relapse, NF-κB mediated radioresistance is a broad feature of cancerous cells and should be considered a preliminary focus.
For a cell to develop cancer-like properties, it must achieve nearly unlimited replicative potential. Malignant cells must retain a self-sufficiency in growth-promoting factors, insensitivity to growth-inhibitory signals, ability to evade apoptosis, and sustained capability for angiogenesis (Hanahan and Weinberg, 2000). NF-κB activation plays a role in all of these cellular phenomena (Greten and Karin, 2005; Li et al., 2005). Our data demonstrate a clear upregulation of NF-κB transcriptional activity in osteosarcoma cells as compared to control osteoblasts, supporting the currently accepted role of NF-κB involvement in carcinogenesis. While a connection to cancer cell maintenance is proposed for NF-κB, its upregulated activity can only be identified as a potential participant in malignancy.
Parthenolide is a naturally occurring sesquiterpene lactone that interferes with NF-κB signaling (Kwok et al., 2001; Hehner et al., 1999), obstructing signaling pathways required for the maintenance and progression of malignant cells. In our data, viability of the LM7 osteosarcoma-derived cell line was reduced to less than 50% when treated with a 10 µM concentration of parthenolide, while the viability of a non-cancerous cell line of osteoblastic origin was unaffected. This finding suggests that parthenolide could effectively reduce tumor size in vivo without compromising healthy tissues. Parthenolide has been shown to effectively induce apoptosis in a variety of cancer cell lines (Zhang et al, 2004; Wen et al., 2002), and our data demonstrate a similar effect in LM7 cells. Cells treated with 10 µM parthenolide exhibited a significant increase in the percentage of condensed apoptotic nuclei, as well as a two-fold increase in active caspase-3, suggesting induction of apoptosis. A variety of cellular signals may mediate the pro-apoptotic action of parthenolide. The activity of histone deacetylase (HDAC) enzymes are a common means for cells to regulate the expression of apoptotic genes, and elevated activity in osteosarcoma has been shown to reduce the sensitivity of tumor cells to apoptotic stimuli (Koshkina et al., 2011). For this reason, histone deacetylase inhibitors are frequently included in combination cancer therapy (Carraway et al., 2007). However, HDAC inhibitors primarily induce apoptosis through the increased expression of Fas receptors, which participate in the extrinsic apoptotic pathway. The principle interest of this study is induction of the intrinsic apoptotic program in response to ionizing radiation. The pathways by which parthenolide induces apoptosis are important to consider in future analyses, though the mechanism by which cells can be sensitized to radiation is the main focus of this study.
The reduction of NF-κB activity implicates parthenolide as a useful tool to sensitize cancerous cells to ionizing radiation (Eliseev et al., 2005; Chendil et al., 2004; Egan et al., 2004). In non-pathogenic cell lines with minimal basal levels of NF-κB activity, exposure to ionizing radiation has been shown to stimulate the JNK pathway, leading to initiation of the intrinsic apoptotic program (Kuwabara et al., 2003; Dent et al., 2003). Elevated NF-κB signaling in osteosarcoma enables cells to evade radiation-induced apoptosis by inhibiting JNK pathway signaling (Eliseev et al., 2005). In addition, our data indicate that irradiation further induces NF-κB activity, subsequently escalating the resistance to radiation. In our studies, parthenolide was able to effectively reduce NF-κB activity in LM7 cells and maintain decreased NF-κB signaling following irradiation. Corresponding with reduced NF-κB, LM7 cells subjected to ionizing radiation after receiving parthenolide treatment displayed a synergistic increase in the percentage of condensed apoptotic nuclei and a 5-fold increase in active caspase-3. Congruently, the number of live cells in culture was greatly reduced following combined parthenolide treatment and radiotherapy compared to control cells and cells treated with either parthenolide or radiation alone. The evidence suggests that parthenolide may be an effective tumor reducing agent in vivo and a useful therapeutic supplement to radiotherapy for patients with osteosarcoma.
While general eradication of malignant cells is an essential component of cancer therapy, a more specific targeting of cancer stem cells may be crucial to prevent relapse. Solid tumors are suggested to be composed of a heterogeneous population of cells which perform distinct functions in tumor growth and maintenance (Ailles and Weissman, 2007; Al-Hajj and Clarke, 2004). A subtle subpopulation of tumor cells is represented by cancer stem cells, which are responsible for initiating, repairing, and sustaining tissue (Ailles and Weissman, 2007; Al-Hajj and Clarke, 2004). Cells with stem-like characteristics carry the potential to self-renew and differentiate, enabling them to regenerate an entire tumor following treatment. The CD133 antigen is a pentaspan membrane glycoprotein that has been able to pinpoint cancer initiating subpopulations in the brain, colon, liver, skin, and recently bone (Tirino et al., 2008; Singh et al., 2004; Ricci-Vitiani et al., 2007; Yin et al., 2007; Monzani et al., 2007; Gibbs et al., 2005; Fargeas et al., 2003). Cells that have been isolated for the presence of CD133 are capable of forming sarcospheres, an identifying characteristic of cancer stem cells (Ischenko et al., 2008; Tang et al., 2007). For our studies, osteosarcoma cells and osteoblastic cells were probed for CD133 and subjected to FACS. As expected, a CD133 positive subpopulation was absent in the non-cancerous osteoblastic cell line hFOB, whereas osteosarcoma derived cell lines SaOS2 and LM7 contain a CD133 positive subpopulation. Cultured SaOS2 cells accommodate an average of 5% CD133 positive cells, while the more aggressive and metastatic LM7 cell line contains an 8% subpopulation presenting the antigen. This finding suggests that population density of cancer stem cells may be linked to aggressiveness of the cancer, although this has yet to be verified. CD133 expressing cells isolated from cultured LM7 cells were able to form sarcospheres in semi-solid medium, while cells negative for CD133 were unable to form spheres, confirming stemness of the CD133 positive subpopulation.
Because parthenolide treatment has been shown to have anti- NF-κB activity, and NF-κB plays a key role in cancer stem cell maintenance (Mohseny et al., 2009; Tang et al., 2008), we hypothesized that parthenolide may reduce the population of cancer stem cells. Our results showed that treatment with parthenolide was able to diminish the population of CD133+ cells in the LM7 line, and the effect was significantly enhanced with exposure to 10Gy radiation. The CD133+ subpopulation of dual-treated cultures was reduced to less than 3% of total LM7 population, while irradiation alone increased the CD133+ subpopulation from 8 to 11% of total viable cells consistent with previous findings in tumor types other than osteosarcoma (Bao et al., 2006). Colony formation assays illustrated that parthenolide pre-treatment in conjunction with irradiation was able to significantly reduce colony formation of LM7 cells. The observed increased radioresistance of the CD133+ subpopulation could be due to higher NF-κB activity and correspondingly lower JNK activity in this subpopulation; however our results indicate no difference in activities of the above pathways between CD133+ and CD133− cells. We, however, found that CD133+ osteosarcoma cells have significantly lower ROS levels and decreased oxidative metabolism. These data are consistent with previous works showing decreased oxidative metabolism in stem-like normal and cancer cells (Noble et al., 2003; Diehn et al., 2009). In addition to NF-κB inhibition effect, parthenolide has a strong pro-oxidant effect (Wen et al., 2002; Sun et al., 2010). Our data indicate that parthenolide was able to induce ROS not only in CD133− cells but also in CD133+ cells that originally have significantly lower ROS levels and are protected from ROS burst during irradiation. Although further studies are needed to fully elucidate the involvement of ROS in the effect of parthenolide in the CD133+ stem-like subpopulation of osteosarcoma cells, our data clearly demonstrate that the radiosensitizing effect of parthenolide is dependent on ROS.
Taken together, our data provide evidence for radiosensitizing effect of parthenolide in osteosarcoma cells and in a subset of osteosarcoma stem-like cells and suggest a starting point for further studies on the mechanism of osteosarcoma radioresistance and of parthenolide action. Our results implicate parthenolide as an effective supplement to the current treatment of osteosarcoma. With no known side effects, parthenolide may be considered a critical candidate for inclusion in osteosarcoma treatment regimens.
Acknowledgements
The authors thank Dr. Eugenie Kleinerman of MD Anderson Cancer Center for providing LM7 cells. This study was supported by grant NCRR/NIH KL2 RR 024136 to Dr. Roman Eliseev, the Karen D’Amico Foundation, and the Department of Orthopaedics of the University of Rochester.
References
- Ailles LE, Weissman IL. Cancer stem cells in solid tumors. Curr Opin Biotechnol. 2007;18(5):460–466. doi: 10.1016/j.copbio.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Al-Hajj M, Clarke MF. Self-Renewal and solid tumor stem cells. Oncogene. 2004;23(43):7274–7282. doi: 10.1038/sj.onc.1207947. [DOI] [PubMed] [Google Scholar]
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- Basu S, Rosenzweig KR, Youmell M, Price BD. The DNA-dependent protein kinase participates in the activation of NF kappa B following DNA damage. Biochem Biophys Res Commun. 1998;247(1):79–83. doi: 10.1006/bbrc.1998.8741. [DOI] [PubMed] [Google Scholar]
- Chen CT, Hsu SH, Wei YH. Upregulation of mitochondrial function and antioxidant defense in the differentiation of stem cells. Biochim Biophys Acta. 2010;1800:257–263. doi: 10.1016/j.bbagen.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Chendil D, Ranga RS, Meigooni D, Sathishkumar S, Ahmed MM. Curcumin confers radiosensitizing effect in prostate cancer cell line PC-3. Oncogene. 2004;23:1599–1607. doi: 10.1038/sj.onc.1207284. [DOI] [PubMed] [Google Scholar]
- Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells-perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- Dent P, Yacoub A, Fisher PB, Hagan MP, Grant S. MAPK pathways in radiation responses. Oncogene. 2003;22:5885–5896. doi: 10.1038/sj.onc.1206701. [DOI] [PubMed] [Google Scholar]
- Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, Joshua B, Kaplan MJ, Wapnir I, Dirbas F, Somlo G, Garberoglio C, Paz B, Shen J, Lau SK, Quake SR, Brown JM, Weissman IL, Clarke MF. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Jia SF, Zhou Z, Langley RR, Bolontrade MF, Kleinerman ES. Association of αvβ3 integrin expression with the metastatic potential and migratory and chemotactic ability of human osteosarcoma cells. Clin Exp Metastasis. 2004;21(8):747–753. doi: 10.1007/s10585-005-0599-6. [DOI] [PubMed] [Google Scholar]
- Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblst differentiation. Cell. 1997;89:747. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- Egan LJ, Eckmann L, Greten FR, Chae S, Li ZW, Myhre GM, Robine S, Karin M, Kagnoff MF. IkappaB-kinase beta-dependent NF-kappaB activation provides radioprotection to the intestinal epithelium. Proc Nat Acad Sci USA. 2004;101:2452–2457. doi: 10.1073/pnas.0306734101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliseev RA, Zuscik MJ, Schwarz EM, O’Keefe RJ, Drissi H, Rosier RN. Increased Radiation-Induced Apoptosis of SaOS2 Cells via Inhibition of NFκB: A role for c-Jun N-Terminal Kinase. Journal Cell Biochem. 2005;96:1262–1273. doi: 10.1002/jcb.20607. [DOI] [PubMed] [Google Scholar]
- Fargeas CA, Corbeil D, Huttner WB. AC133 antigen, CD133, prominin-1, prominin-2, etc.: prominin family gene products in need of a rational nomenclature. Stem Cells. 2003;21:506–508. doi: 10.1634/stemcells.21-4-506. [DOI] [PubMed] [Google Scholar]
- Fuchs B, Pritchard DJ. Etiology of Osteosarcoma. Clin Orthop Rel Res. 2002;397:40–52. doi: 10.1097/00003086-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Gibbs CP, Kukekov VG, Reith JD, Tchigrinova O, Suslov ON, Scott EW, Ghivizzani SC, Ignatova TN, Steindler DA. Stem-like cells in bone sarcomas: implications for tumorigenesis. Neoplasia. 2005;7:967–976. doi: 10.1593/neo.05394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goorin AM, Schwartzentruber DJ. Presurgical chemotherapy compared with immediate surgery and adjuvant chemotherapy for nonmetastatic osteosarcoma. J Clin Oncol. 2003;21:1574–1580. doi: 10.1200/JCO.2003.08.165. [DOI] [PubMed] [Google Scholar]
- Greten FR, Karin M. NF-κB: Linking inflammation and immunity to cancer development and progression. Nature Rev. Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The Hallmarks of Cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hehner SP, Hofmann TG, Droge W, Schmitz ML. The anti-inflammatory sesquiterpene lactone parthenolide inhibits NF-kappa B by targeting the I kappa B kinase complex. J Immunol. 1999;163:5617–5623. [PubMed] [Google Scholar]
- Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, Goodell MA, Brenner MK. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci USA. 2004;101 doi: 10.1073/pnas.0400067101. 14228-14223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ischenko I, Seeliger H, Schaffer M, Jauch KW, Bruns CJ. Cancer stem cells: how can we target them? Curr Med Chem. 2008;15:3171–3184. doi: 10.2174/092986708786848541. [DOI] [PubMed] [Google Scholar]
- Kishida Y, Yoshikawa H, Myoui A. Parthenolide, a Natural Inhibitor of Nuclear Factor-κB, Inhibits Lung Colonization of Murine Osteosarcoma Cells. Clin Cancer Res. 2007;13:59–67. doi: 10.1158/1078-0432.CCR-06-1559. [DOI] [PubMed] [Google Scholar]
- Koch U, Krause M, Baumann M. Cancer stem cells at the crossroads of current cancer therapy failures-Radiation oncology perspective. Semin Cancer Biol. 2010;20(2):116–124. doi: 10.1016/j.semcancer.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Kuwabara M, Takahashi K, Inanami O. Induction of apoptosis through the activation of SAPK/JNK followed by the expression of death receptor Fas in X-irradiated cells. J Rad Res. 2003;44:203–209. doi: 10.1269/jrr.44.203. [DOI] [PubMed] [Google Scholar]
- Kwok BH, Koh B, Ndubuisi MI, Elofsson M, Crews CM. The anti-inflammatory natural product parthenolide from the medicinal herb Feverfew directly binds to and inhibits IkappaB kinase. Chem Biol. 2001;8:759–766. doi: 10.1016/s1074-5521(01)00049-7. [DOI] [PubMed] [Google Scholar]
- Li Q, Withoff S, Verma I. Inflammation-associated cancer: NF-κB is the lynchpin. Trends Immunol. 2005;26:318–325. doi: 10.1016/j.it.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Lisle JW, Choi JY, Horton JA, Allen MJ, Damron TA. Metastatic Osteosarcoma Gene Expression Differs In Vitro and In Vivo. Clin Orthop Relat Res. 2008;466:2071–2080. doi: 10.1007/s11999-008-0309-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Shuai K. Induction of apoptosis by protein inhibitor of activated Stat1 through c-Jun NH2-terminal kinase activation. J Biol Chem. 2001;276:36624–36631. doi: 10.1074/jbc.M101085200. [DOI] [PubMed] [Google Scholar]
- Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Lu L, Irvin D, Black KL, Yu JS. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohseny AB, Szuhai K, Romeo S, Buddingh EP, Briaire-de Bruijn, de Jong D, van Pel M, Cleton-Jansen AM, Hogendoorn PC. Osteosarcoma originates from mesenchymal stem cells in consequence of aneuploidization and genomic loss of Cdkn2. J Pathol. 2009;219:294–305. doi: 10.1002/path.2603. [DOI] [PubMed] [Google Scholar]
- Monzani E, Facchetti F, Galmozzi E, Corsini E, Benetti A, Cavazzin C, Gritti A, Piccinini A, Porro D, Santinami M, Invernici G, Parati E, et al. Melanoma contains CD133 and ABCG2 positive cells with enhanced tumorigenic potential. Eur J Cancer. 2007;43:935–946. doi: 10.1016/j.ejca.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Murphy JJ, Heptinstall S, Mitchell JR. Randomised double-blind placebo-controlled trial of feverfew in migraine prevention. Lancet. 1988;2:189–192. doi: 10.1016/s0140-6736(88)92289-1. [DOI] [PubMed] [Google Scholar]
- Naugler WE, Karin M. NF-κB and cancer – identifying targets and mechanisms. Current Opinion in Genetics and Development. 2008;18:19–26. doi: 10.1016/j.gde.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble M, Smith J, Power J, Mayer-Proschel M. Redox state as a central modulator of precursor cell function. Ann NY Acad Sci. 2003;991:251–271. doi: 10.1111/j.1749-6632.2003.tb07481.x. [DOI] [PubMed] [Google Scholar]
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer initiating cells. Nature. 2007;445(7123):111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- Siclari VA, Qin L. Targeting the osteosarcoma cancer stem cell. Journal of Orthopaedic Surgery and Research. 2010;5:78. doi: 10.1186/1749-799X-5-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman Rm, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Sun Y, St. Clair DK, Xu Y, Crooks PA, St. Clair WH. A NADPH oxidase–dependent redox signaling pathway mediates the selective radiosensitization effect of parthenolide in prostate cancer cells. Cancer Res. 2010;70:2880–2890. doi: 10.1158/0008-5472.CAN-09-4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J, You K, Lee SY, Song CH, Kim DG. Oxidative Stress-mediated Apoptosis. THE ANTICANCER EFFECT OF THE SESQUITERPENE LACTONE PARTHENOLIDE. J Biol Chem. 2002;277:38954–38964. doi: 10.1074/jbc.M203842200. [DOI] [PubMed] [Google Scholar]
- Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connoll D, Foster R, Dombrowski D, Preffer F, Maclaughlin DT, Donahoe PK. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc Natl Acad Sci USA. 2006;103:11154–11159. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C, Ang BT, Pervaiz S. Cancer stem cell: target for anti-cancer therapy. FASEB J. 2007;21:3777–3785. doi: 10.1096/fj.07-8560rev. [DOI] [PubMed] [Google Scholar]
- Tang N, Song WX, Luo J, Haydon RC, He TC. Osteosarcoma development and stem cell differentiation. Clin Orthop Rel Res. 2008;466:2114–2130. doi: 10.1007/s11999-008-0335-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirino V, Desiderio V, d’Aquino R, De Francesco F, Pirozzi G, Galderisi U, Cavaliere C, De Rosa A, Papaccio G. Deterction and Characterization of CD133+ Cancer Stem Cells in Human Solid Tumours. PLoS ONE. 2008;3(10):e346. doi: 10.1371/journal.pone.0003469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274(5288):787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- Wen J, You KR, Lee SY, Song CH, Kim DG. Oxidative stress-mediated apoptosis. The anticancer effect of the sesquiterpene lactone parthenolide. J Biol Chem. 2002;277:38954–38964. doi: 10.1074/jbc.M203842200. [DOI] [PubMed] [Google Scholar]
- Yin S, Li J, Hu C, Chen X, Yao M, Yan M, Jiang G, Ge C, Xie H, Wan D, Yang S, Zheng S, Gu J. CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int J Cancer. 2007;120:1444–1450. doi: 10.1002/ijc.22476. [DOI] [PubMed] [Google Scholar]
- Zhang S, Ong CN, Shen HM. Critical roles of intracellular thiols and calcium in parthenolide-induced apoptosis in human colorectal cancer cells. Cancer Lett. 2004;208:143–153. doi: 10.1016/j.canlet.2003.11.028. [DOI] [PubMed] [Google Scholar]