Abstract
Background
Minocycline exhibits anti-inflammatory properties independent of its antibiotic activity, ameliorating inflammatory responses in monocytes and macrophages. However, the mechanisms of minocycline anti-inflammatory effects are only partially understood.
Methods
Human circulating monocytes were cultured in the presence of lipopolysaccharide (LPS), 50 ng/ml, and minocycline (10–40 µM). Gene expression was determined by RT-PCR, cytokine and prostaglandin E2 (PGE2) release by ELISA, protein expression, phosphorylation and nuclear translocation by Western blotting.
Results
Minocycline significantly reduced the inflammatory response in LPS-challenged monocytes, decreasing LPS-induced transcription of pro-inflammatory tumor-necrosis factor alpha (TNF-α), interleukin-1 beta, interleukin-6 (IL-6) and cyclooxygenase-2 (COX-2), and the LPS-stimulated TNF-α, IL-6 and PGE2 release. Minocycline inhibited LPS-induced activation of the lectin-like oxidized low density lipoprotein receptor-1 (LOX-1), NF-κB, LPS-induced TNF-α factor (LITAF) and the Nur77 nuclear receptor. Mechanisms involved in the anti-inflammatory effects of minocycline include a reduction of LPS-stimulated p38 mitogen-activated protein kinase (p38 MAPK) activation and stimulation of the phosphoinositide 3-kinase (PI3K)/Akt pathway.
Conclusions
We provide novel evidence demonstrating that the anti-inflammatory effects of minocycline in human monocytes include, in addition to decreased NF-κB activation, abrogation of the LPS-stimulated LOX-1, LITAF, Nur77 pathways, p38 MAPK inhibition and PI3K/Akt activation. Our results reveal that minocycline inhibits points of convergence of distinct and interacting signaling pathways mediating multiple inflammatory signals which may influence monocyte activation, traffic and recruitment into the brain.
General significance
Our results in primary human monocytes contribute to explain the profound anti-inflammatory and protective effects of minocycline in cardiovascular and neurological diseases and may have direct translational relevance.
Keywords: Minocycline, Lipopolysaccharide, Nuclear receptor, LITAF, Inflammation, Human monocytes
1. Introduction
It is well-established that systemic immune responses to infection are associated with vulnerability and progression of neurodegenerative diseases [1]. Minocycline is a tetracycline antibiotic with multiple properties in addition to its antibiotic activity, including free radical scavenging and amelioration of apoptosis, inflammation and protein misfolding [2]. These properties may be responsible for minocycline therapeutic effects in pre-clinical models of neurodegenerative disease, including direct neuroprotection and reduction of microglia inflammatory responses [2–5].
Minocycline blocks, in vivo, bacterial endotoxin (lipopolysaccharide, LPS)-induced adhesion of leukocytes to cerebrovascular endothelial cells, and tumor necrosis factor-α (TNF-α) production in the brain [6]. Anti-inflammatory effects of minocycline have also been reported in vitro in monocytes [7] and in macrophages [8,9]. These results indicate that the neuroprotective effects of minocycline include reduction of monocyte activation, trafficing and recruitment into the brain. However, the precise mechanisms of minocycline anti-inflammatory effects in monocytes are incompletely understood.
To further establish the mechanisms of minocycline anti-inflammatory actions, we studied the effect of minocycline on LPS-stimulated human circulating monocytes in culture. First, we determined the anti-inflammatory effects of minocycline in our preparation. Then, we addressed a number of mechanisms associated with LPS-induced inflammation: a) lectin-like oxidized low density lipoprotein receptor-1 (LOX-1), a major inducer of vascular inflammation and leukocyte recruitment [10]; b) nuclear factor κB (NF-κB), a master regulator of inflammatory responses [11]; c) Nur77, a member of the NR4A subfamily of nuclear receptors, regulating LPS induction of inflammatory factors and involved in atherosclerosis and macrophage activation [12,13]; d) lipopolysaccharide-induced TNF-α factor (LITAF) transcription factor [14,15], an important determinant of sensitivity and resistance to LPS [16]; e) the participation of p38 mitogen-activated protein kinase (p38 MAPK) [17] and the role of the interacting phosphoinositide 3-kinase/Akt (PI3K/Akt) pathway [18,19].
2. Materials and methods
2.1. Reagents
Cell culture media and supplements were obtained from Invitrogen (Carlsbad, CA). Lipopolysaccharide (LPS, Escherichia coli serotype 055:B5) and minocycline were purchased from Sigma-Aldrich (St. Louis, MO). Primers for real-time PCR were synthesized by BioServe (Beltsville, MD) and are listed in Table 1; SYBR Green PCR Master Mix for qPCR was purchased from Applied Biosystems (Foster City, CA); the remaining reagents for RNA isolation and reverse transcription were from Invitrogen. Primary antibodies used for Western blot analysis were: rabbit polyclonal anti-NFκB-p65 antibody (1:2000, Millipore, Billerica, MA); mouse polyclonal anti-COX-2 (1:1000, Cayman Chemical, Ann Arbor, MI); rabbit anti-LOX-1 (1:200) and rabbit anti-Nur77 (1:1500) were from Santa Cruz Biotechnology (Santa Cruz, CA); rabbit anti-phospho-p38 MAPK (1:1000), mouse anti-phospho-Akt (Ser473) (1:1000), rabbit anti-LITAF (1:1000), rabbit anti-β-actin (1:1000), and rabbit anti-histone H4 (1:1000), all from Cell Signaling Technology (Danvers, MA). Secondary antibodies for Western blot analysis were: donkey anti-rabbit IgG (1:5000, Amersham BioSciences, Piscataway, NJ); goat anti-mouse IgG (1:10,000, Jackson ImmunoResearch, West Grove, PA). SuperSignal West Dura Substrate for chemiluminescent detection was purchased from Thermo Fisher Scientific (Pittsburg, PA). All other chemicals were obtained from Sigma-Aldrich unless otherwise stated.
Table 1.
List of PCR primers used in the study
| Gene | Accession # | Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|---|
| 18S rRNA | X03205 | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG |
| COX-2 | NM_000963 | GATTGCCCGACTCCCTTGG | AACTGATGCGTGAAGTGCTG |
| IκB-α | NM_020529 | CGGACTGCCCTTCACCTC | ACATCAGCCCCACACTTCAA |
| IL-1β | NM_000576 | GTCATTCGCTCCCACATTCT | CTACTTCTTGCCCCCTTTGA |
| IL-6 | NM_000600 | CCAGTACCCCCAGGAGAAGAT | GAGGATGTACCGAATTTGTTTGTC |
| LITAF | NM_004862 | CTCTTCCCGTCCCTGATGA | TGTTAATAGCCCCCTCCTTGTA |
| LOX-1 | NM_002543 | CTTTGATGCCCCACTTATTTAG | GGTTTGCCTTCTTCTGACAT |
| Nur77 | NM_002135 | ACGGCTACACAGGAGAGTTTGA | GGTGGCTGAGGACGAGGAT |
| TNF-α | NM_000594 | CCAGCTGGAGAAGGGTGAC | AGGCGTTTGGGAAGGTTG |
rRNA, ribosomal RNA; COX-2, cyclooxygenase-2; IκB-α, inhibitor of kappa B alpha; IL, interleukin; LITAF, Lipopolysaccharide-induced tumor necrosis factor-alpha factor; LOX-1, Lectin-like Oxidized Low-density Lipoprotein Receptor 1; Nur77, nuclear receptor subfamily 4 group A member 1; TNF-α, tumor necrosis factor alpha.
2.2. Cell culture
Human monocytes from human peripheral blood mononuclear cells were isolated by density gradient centrifugation of heparinized blood, collected by leukaphoresis of normal volunteers at the Department of Transfusion Medicine, National Institutes of Health (NIH). Purified populations of non-activated monocytes were prepared by counterflow centrifugal elutriation on a Beckman elutriation system [20]. Monocyte preparations were enriched to > 90% as determined by flow cytometry. Monocytes were allowed to attach to cell culture plates for 2 hours at 37 °C in humidified 5% CO2-95% air atmosphere, and non-adherent cells were removed.
Human monocytes were seeded at 2 × l06 cells/ml in serum-free Dulbecco’s modified Eagle’s medium (DMEM), GlutaMAX™-I with 50 µg/ml gentamicin and cultured in 100 mm culture dishes or 6-well plates, at 37°C in humidified 5% CO2-95% air atmosphere. The next day after platting the cells, monocytes were preincubated with vehicle or minocycline for two hours. Minocycline was prepared as 4 mM stock in deionized water. After preincubation with vehicle or minocycline, monocytes were incubated in the presence of LPS (50 ng/ml). LPS was added for 4 hours previous to PCR analysis and cytokine release determinations in cell culture supernatants.
For each determination, monocytes from at least three donors were studied individually and data are presented as means ± SEM. All experiments were approved by the NIH's Office of Human Subjects Research (OHSR).
2.3. RNA isolation and real-time PCR
Total RNA was isolated individually from lysed human monocytes using 1 ml TRIzol reagent (Invitrogen), followed by purification using an RNeasy Mini kit (Qiagen, Valencia, CA) according to the manufacturer’s specifications. Synthesis of complementary DNA (cDNA) was performed by using 0.5 µg of total RNA and Super-Script III first-Strand Synthesis kit (Invitrogen). Quantitative real-time polymerase chain reaction (PCR) was performed on DNA Engine Opticon™ (MJ Research, Waltham, MA) with SYBR Green PCR Master Mix. PCR was performed in a 20 µl reaction mixture containing 10 µl SYBR Green PCR Master Mix, 2 µl cDNA and 0.3 µM of each primer for a specific target. The amplification conditions consisted of one denaturation/activation cycle at 95°C for 10 min, followed by 40–45 cycles at 95°C for 15 sec and 60°C for 60 sec followed by final extension step for 10 min at 72°C. Serial dilutions of cDNA from the same source as samples were used to obtain a calibration curve. The individual targets for each sample were quantified by determining the cycle threshold (Ct) and by using calibration curves. The relative amount of the target was normalized with the housekeeping gene 18S rRNA.
2.4. Western blot analysis
Human monocytes were preincubated for 2 h with minocycline or vehicle followed by addition of 50 ng/ml LPS or saline for indicated time. To determine NF-κB-p65, LITAF and Nur77 nuclear translocation, nuclear protein extracts were prepared using a Nuclear Extraction kit (Pierce, Rockford, IL), according to the manufacturer's instructions. To prepare whole-cell protein extracts, the cells were lysed in Tris-Glycine SDS lysis buffer (Invitrogen) and the lysate was boiled for 10 min. Protein concentration was determined with a BCA Assay kit (Thermo Fisher Scientific). The extracted nuclear or whole-cell proteins were separated by electrophoresis on 10% SDS-PAGE gels and transferred onto PVDF membranes. The membranes were blocked for 1 h in casein-based blocking buffer (Sigma-Aldrich) and incubated overnight at 4°C with the primary antibody followed by washing and exposure to the secondary antibody for 1 h at room temperature. β-actin and histone H4 were used as loading controls for whole-cell and nuclear samples, respectively. The membranes were exposed to SuperSignal West Dura Substrate for chemiluminescent detection.
2.5. Measurement of prostaglandin E2 (PGE2) production
Human monocytes were preincubated for 2 h with minocycline or vehicle followed by addition of 50 ng/ml LPS or saline. The cell supernatants were collected 24 h after the addition of LPS, and PGE2 concentration was measured by PGE2 EIA kit (Cayman Chemical) according to the manufacturer's instructions.
2.6. Determination of cytokine release
Human monocytes were preincubated for 2 h with minocycline or vehicle followed by addition of 50 ng/ml LPS or saline. The cell supernatants were collected 4 h after the addition of LPS, and the IL-6 and TNF-α concentrations were measured by human ELISA kit (Invitrogen) according to the manufacturer's instructions.
2.7. Statistical analysis
Statistical significance was determined using GraphPad Prism 5 Software (GraphPad Software, San Diego, CA). Multiple group comparisons were performed by one-way ANOVA followed by Newman-Keuls posttest. Differences were considered statistically significant at P < 0.05. Values are expressed as the mean ± SEM.
3. Results
3.1. Minocycline inhibits LPS-induced inflammatory response
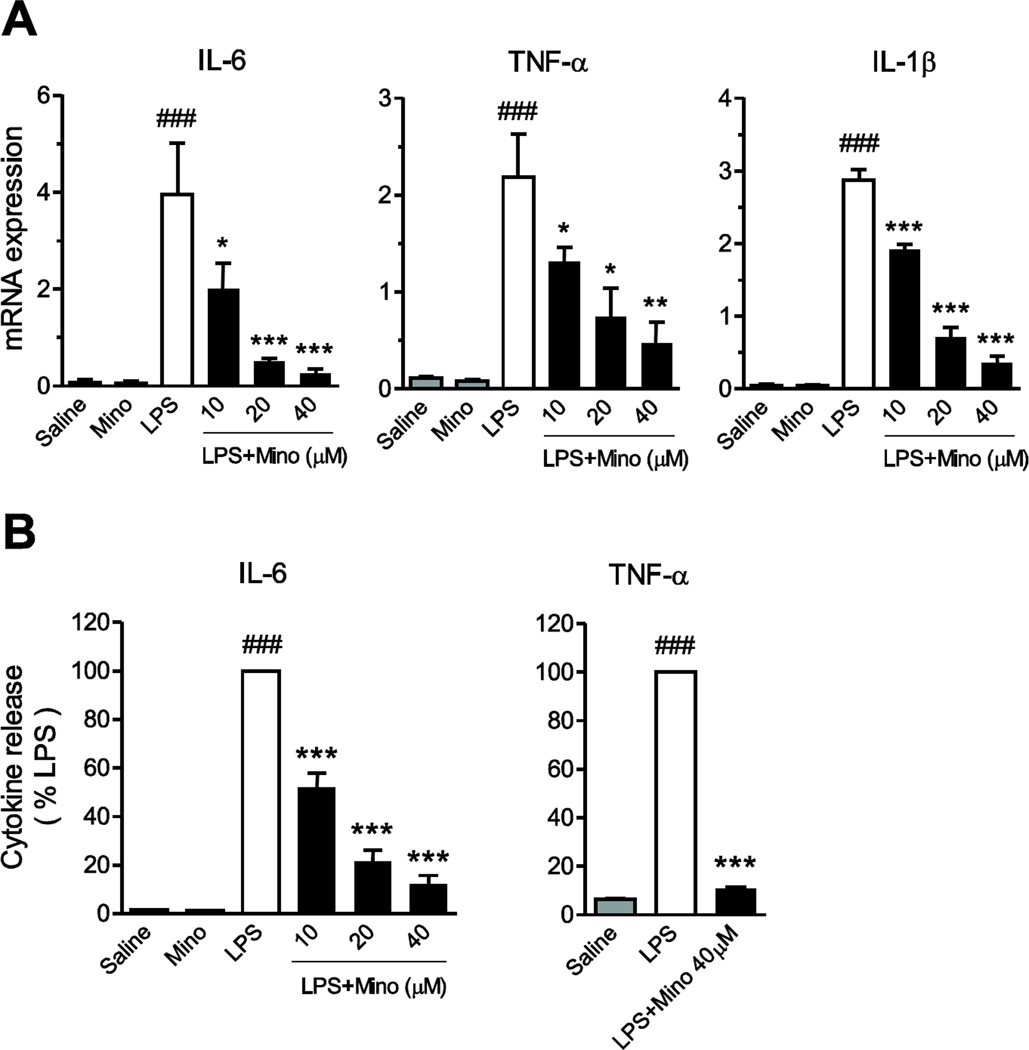
Incubation of primary human monocytes in the presence of LPS upregulated TNF-α, IL-1β and IL-6 mRNA expression, and TNF-α and IL-6 release (Fig. 1). Minocycline concentration-dependently reduced the LPS effects (Fig. 1).
Fig. 1.
Minocycline suppresses LPS-induced cytokine gene expression and release. (A) Primary human monocytes were pretreated with 0, 10, 20, 40 µM minocycline (Mino) for 2 h and then treated with LPS (50 ng/ml) for another 4 h. IL-6, TNF-α and IL-1β mRNA levels (A), and IL-6 and TNF-α release (B) were determined by RT-PCR and specific ELISA as described in Materials and Methods, respectively. Data are presented as means ± SEM of at least three different monocyte preparations assayed individually. Individual groups were compared by one-way ANOVA followed by Newman-Keuls posttest. *P<0.05, **P<0.01, ***P<0.001 compared with LPS; ###P<0.001 compared with Saline.
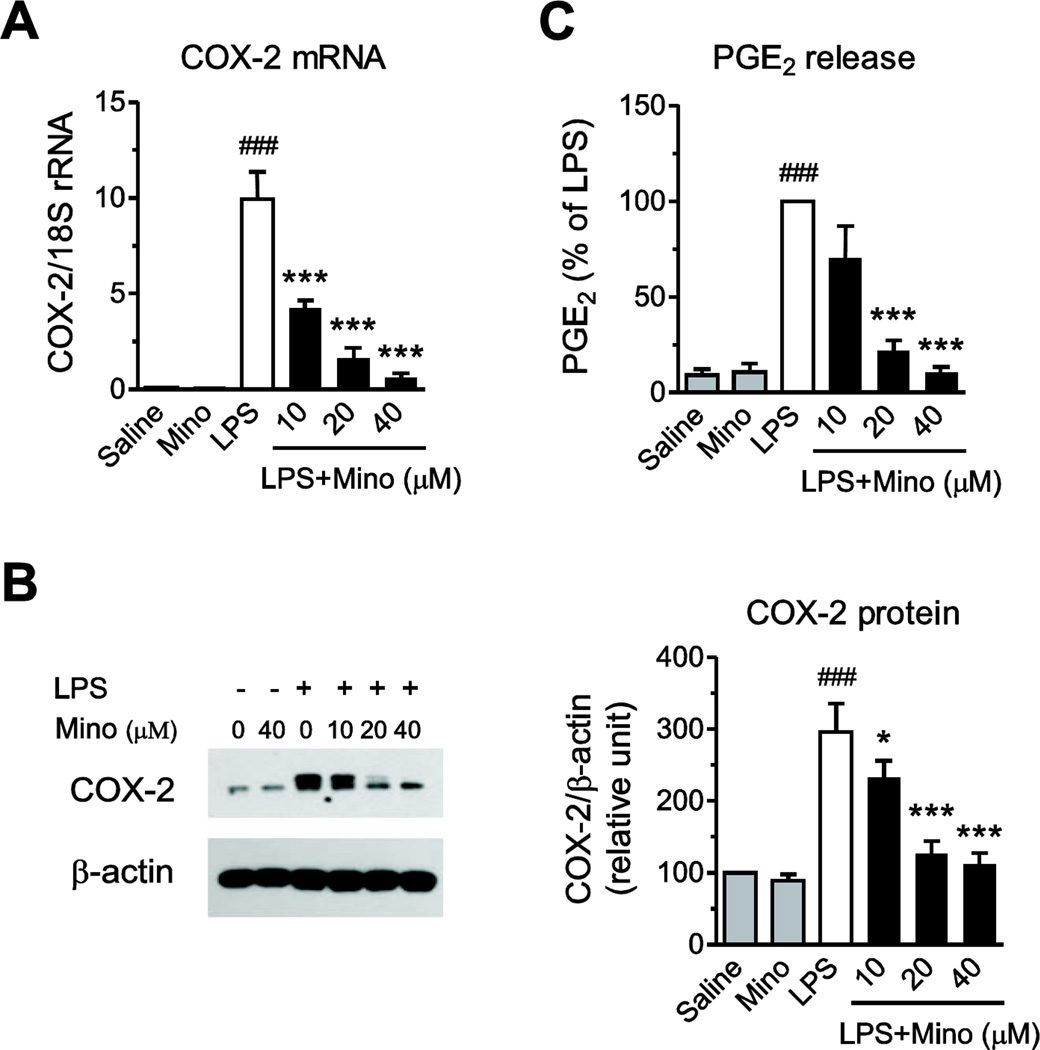
LPS produced a major induction in COX-2 mRNA expression, a three-fold increase in COX-2 protein expression, and a ten-fold stimulation of PGE2 release in primary human monocytes (Fig. 2). Minocycline concentration-dependently decreased the LPS effects (Fig. 2).
Fig. 2.
Minocycline decreases LPS-induced COX-2 mRNA and protein expression, and PGE2 release. Human monocytes were pre-incubated for 2 h with vehicle or minocycline (Mino) and subsequently exposed to 50 ng/ml LPS. COX-2 mRNA expression (A) was quantified by RT-PCR after 4 h of incubation with LPS; COX-2 protein expression (B) and cumulative PGE2 release (C) in monocytes exposed to LPS for 24 h was determined by Western blot and EIA as described in Materials and Methods, respectively. Representative Western blot (B) is shown. Data are presented as means ± SEM from three independent experiments. Statistical significance was determined with one-way ANOVA followed by Newman-Keuls posttest. *P<0.05, ***P<0.001 compared with LPS; ###P<0.001 compared with Saline.
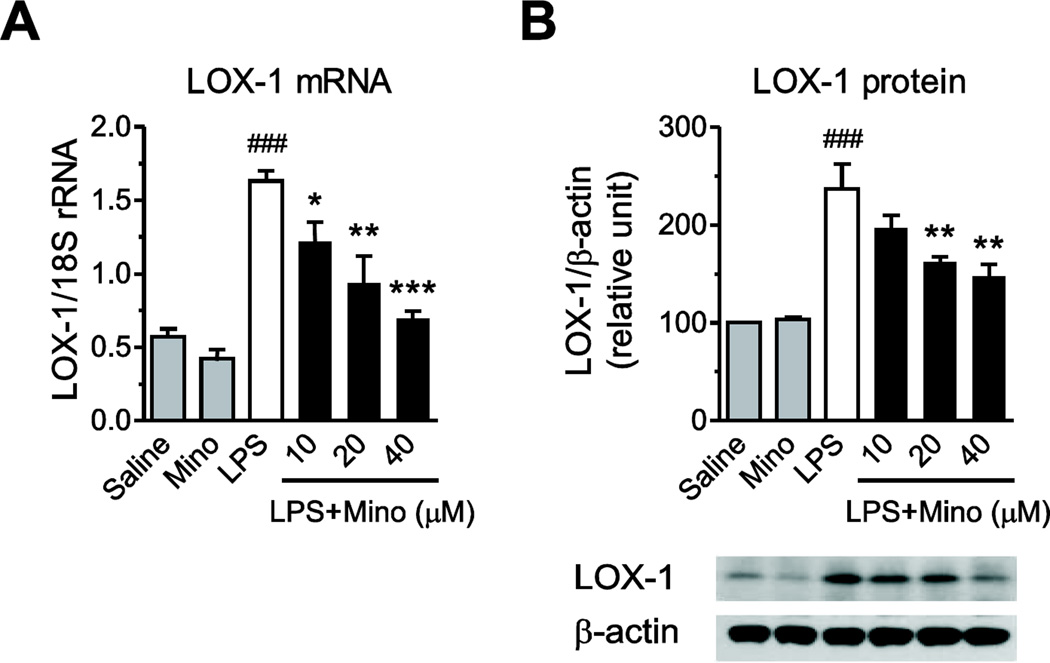
LPS increased LOX-1 gene expression by three-fold and doubled LOX-1 protein expression in primary human monocytes (Fig. 3). Minocycline concentration-dependently reduced these effects of LPS (Fig. 3).
Fig. 3.
Minocycline reduces LPS-induced LOX-1 mRNA and protein expression. Human monocytes were pre-incubated for 2 h with vehicle or minocycline (Mino) and subsequently exposed to 50 ng/ml LPS. LOX-1 mRNA expression (A) was quantified by RT-PCR after 4 h of incubation with LPS; LOX-1 protein expression (B) in monocytes exposed to LPS for 24 h was determined by Western blot as described in Materials and Methods. Representative Western blot (B) is shown. Data are presented as means ± SEM from three independent experiments. Statistical significance was determined with one-way ANOVA followed by Newman-Keuls posttest. *P<0.05, **P<0.01, ***P<0.001 compared with LPS; ###P<0.001 compared with Saline.
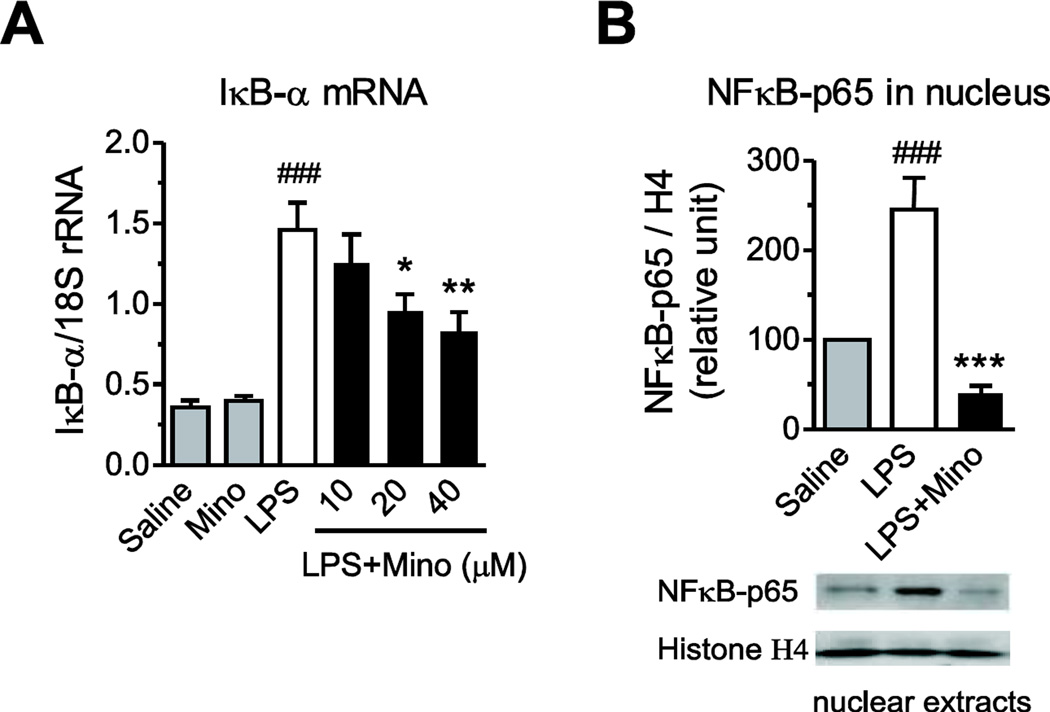
3.2. Minocycline prevents LPS-induced activation of NF-κB, Nur77 and LITAF
LPS produced a strong NF-κB activation in primary human monocytes, as evidenced by a three-fold increase in IκB-α gene expression and NF-κB-p65 nuclear translocation (Fig. 4). Minocycline concentration-dependently decreased the LPS-induced IκB-α mRNA expression and completely abrogated the LPS-stimulated NF-κB-p65 nuclear translocation (Fig. 4).
Fig. 4.
Minocycline decreases LPS-induced IκB-α mRNA expression and NF-κB nuclear translocation. Human monocytes were pre-incubated for 2 h with vehicle or minocycline (Mino) and subsequently exposed to LPS. IκB-α mRNA expression (A) was quantified by RT-PCR after 4 h of incubation with LPS; nuclear proteins were extracted from monocytes exposed to LPS for 2 h and used to measure NF-κB p65 protein expression (B) by Western blot to determine NF-κB nuclear translocation. Representative Western blot (B) is shown. Data are presented as means ± SEM from three independent experiments. Statistical significance was determined with one-way ANOVA followed by Newman-Keuls posttest. *P<0.05, **P<0.01, ***P<0.001 compared with LPS; ###P<0.001 compared with Saline.
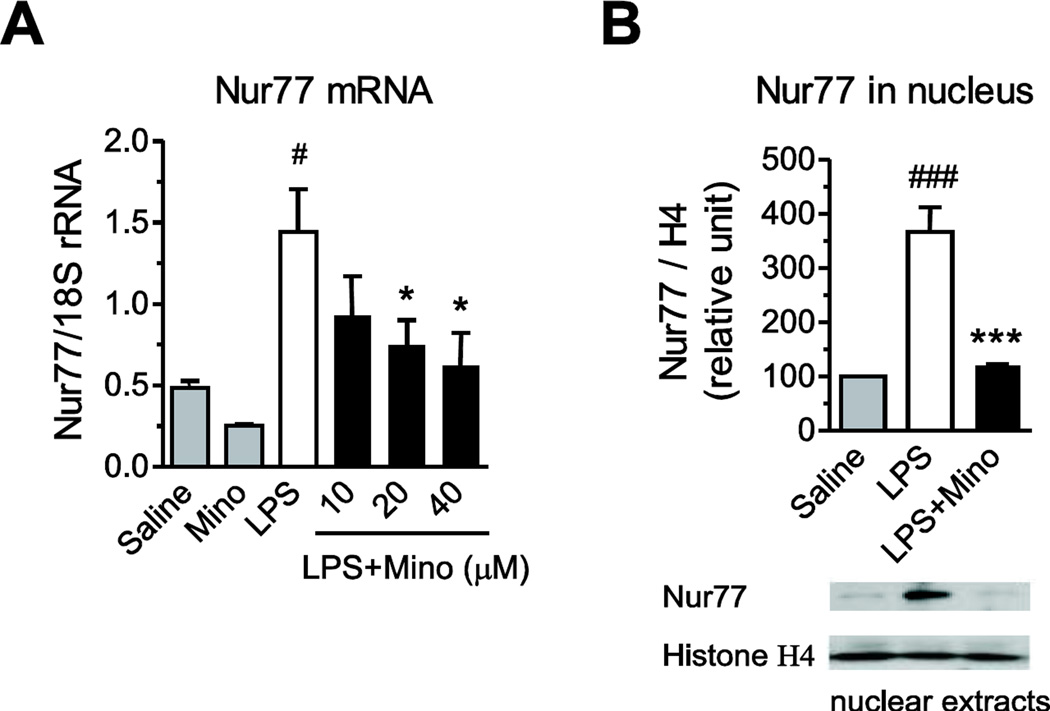
Incubation of primary human monocytes with LPS produced three-fold increases in Nur77 mRNA expression and nuclear translocation (Fig. 5). Minocycline concentration-dependently reduced the LPS-induced Nur77 gene expression and prevented Nur77 nuclear translocation (Fig. 5).
Fig. 5.
Minocycline decreases LPS-induced Nur77 mRNA expression and nuclear translocation. Human monocytes were pre-incubated for 2 h with vehicle or minocycline (Mino) and subsequently exposed to LPS. Orphan nuclear receptor Nur77 mRNA expression (A) was quantified by RT-PCR after 4 h of incubation with LPS; nuclear proteins were extracted from monocytes exposed to LPS for 2 h and used to measure Nur77 protein expression (B) by Western blot to determine Nur77 nuclear translocation. Representative Western blot (B) is shown. Data are presented as means ± SEM from three independent experiments. Statistical significance was determined with one-way ANOVA followed by Newman-Keuls posttest. *P<0.05, ***P<0.001 compared with LPS; #P<0.05, ###P<0.001 compared with Saline.
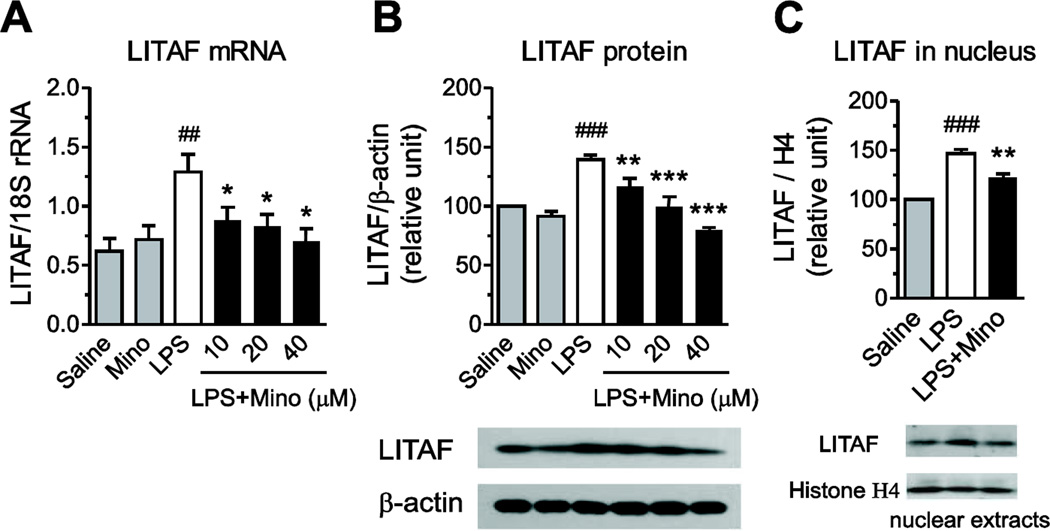
LPS significantly enhanced LITAF mRNA and protein expression and its nuclear translocation in primary human monocytes (Fig. 6). Minocycline concentration-dependently reduced the LPS-induced LITAF mRNA and protein expression, and significantly decreased the enhanced LITAF nuclear translocation elicited by LPS (Fig. 6).
Fig. 6.
Minocycline decreases LPS-induced LITAF receptor mRNA and protein expression, and nuclear translocation. Human monocytes were pre-incubated for 2 h with vehicle or minocycline (Mino) and subsequently exposed to 50 ng/ml LPS. LITAF mRNA expression (A) was quantified by RT-PCR after 4 h of incubation with LPS; LITAF protein expression (B) in monocytes exposed to LPS for 24 h was determined by Western blot. Nuclear proteins were extracted from monocytes after 2 hr-incubation with LPS and used to measure LITAF protein expression (C) by immunoblotting to determine LITAF nuclear translocation. Data are presented as means ± SEM from three independent experiments. Statistical significance was determined with one-way ANOVA followed by Newman-Keuls posttest. *P<0.05, **P<0.01, ***P<0.001 compared with LPS; ##P<0.01, ###P<0.001 compared with Saline.
3.3. Mechanisms involved in the minocycline anti-inflammatory effects
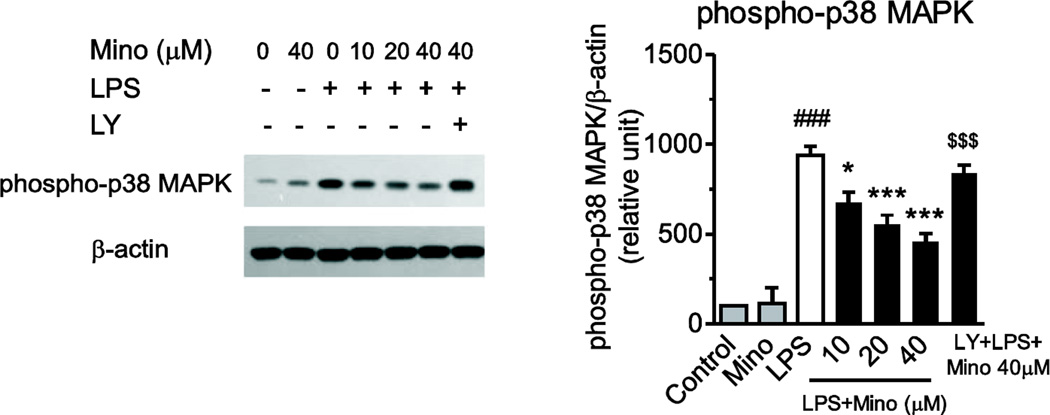
Incubation of primary human monocytes with LPS increased p38 MAPK phosphorylation by eight-fold (Fig. 7). Minocycline concentration-dependently and significantly reduced this effect (Fig. 7).
Fig. 7.
Minocycline inhibits LPS-induced p38 MAPK phosphorylation. Minocycline (Mino) was added 2 h before LPS treatment and, after 30 min, p38 MAPK phosphorylation was measured by immunoblotting. LPS increased phosphorylation of p38 MAPK, and pretreatment with minocycline inhibited this expression in a concentration-dependent manner. The PI3K/Akt pathway inhibitor LY294002 (LY, 10 µM) blocked the minocycline effect. Representative immunoblot is shown. Data are presented as means ± SEM from three independent experiments. Statistical significance was determined with one-way ANOVA followed by Newman-Keuls posttest. *P<0.05, ***P<0.001 compared with LPS; ###P<0.001 compared with Control; $$$P<0.001 compared with LPS+40 µM Mino.
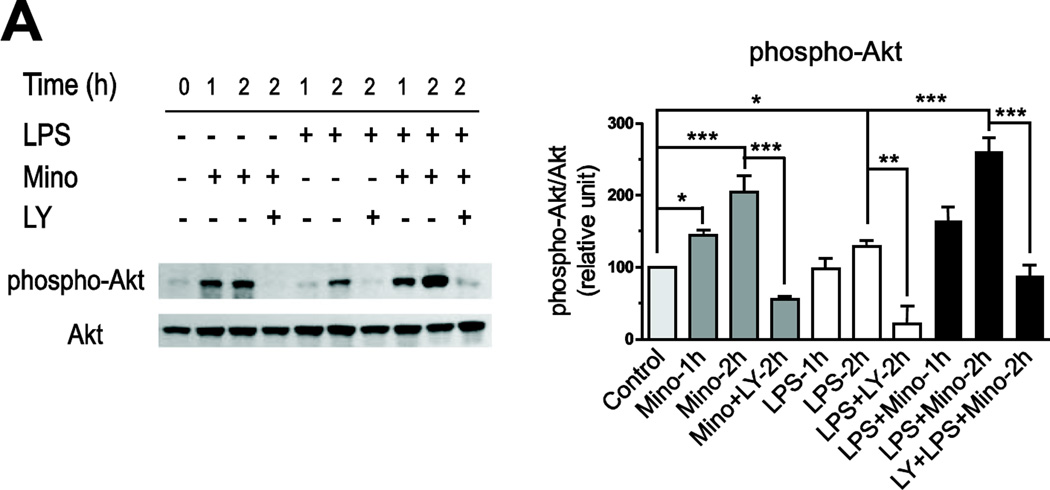
Incubation of primary human monocytes with LPS produced a small but significant increase in Akt phosphorylation (Fig. 8A). Minocycline, when administered alone, time-dependently enhanced Akt phosphorylation and remarkably increased Akt phosphorylation when co-incubated with LPS (Fig. 8A).
Fig. 8.
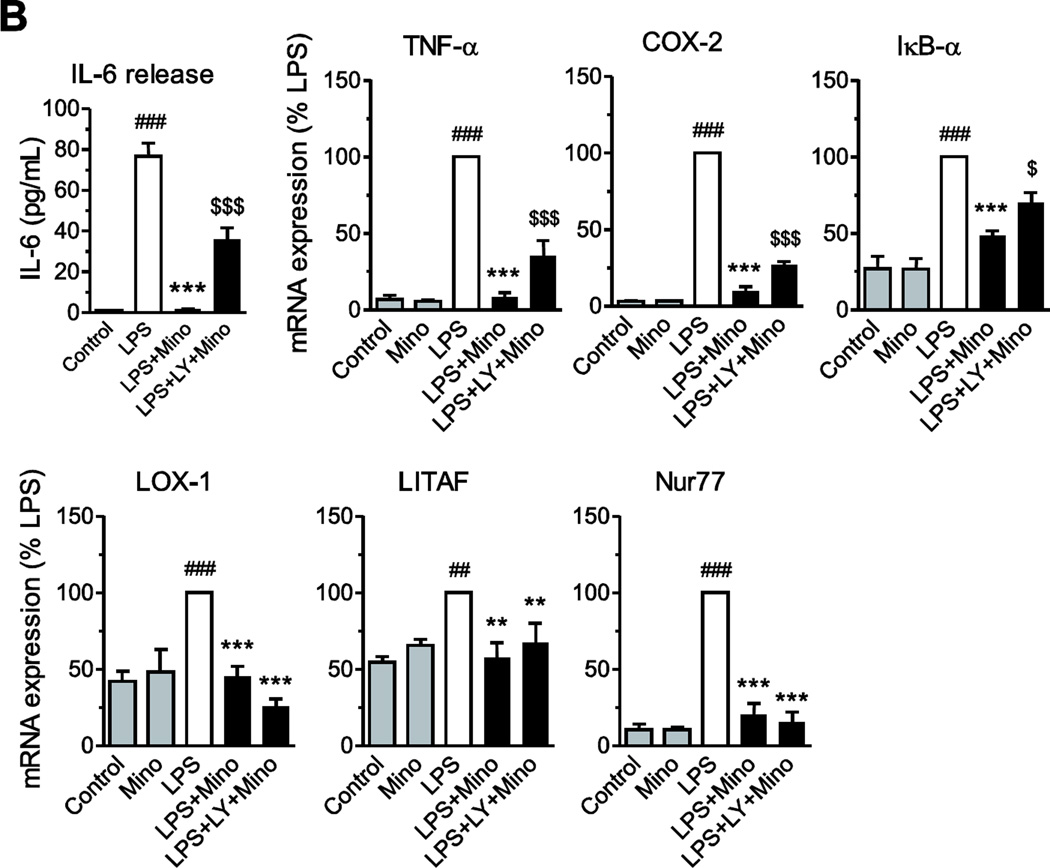
The anti-inflammatory effects of minocycline are partly mediated through Akt activation. (A) Minocycline (Mino) enhances Akt activity. Human monocytes were treated with minocycline (40 µM) alone, LPS (50 ng/ml) alone, or in combination (with minocycline being added 1 hr before LPS). The phosphorylation of Akt was determined at different time points as described in Materials and Methods. Minocycline alone or in combination with LPS increased phosphorylation of Akt in a time-dependent manner, and pretreatment with specific PI3K/Akt pathway inhibitor LY294002 (LY, 10 µM) prevented this effect. *P<0.05, **P<0.01, ***P<0.001. (B) Monocytes were pretreated for 2 h with LY294002 (LY, 10 µM) before minocycline (40 µM) followed by LPS (50 ng/ml) incubation. After 4 h of LPS treatment, IL-6 release was quantified in culture medium by specific ELISA, and the mRNA expression of TNF-α, COX-2, IκB-α, LOX-1, LITAF and Nur77 (C) was quantified by RT-PCR as described in Material and Methods. Data are presented as means ± SEM from three independent experiments. Statistical significance was determined with one-way ANOVA followed by Newman-Keuls posttest. **P<0.01, ***P<0.001 compared with LPS; ##P<0.01, ###P<0.001 compared with Control; $P<0.05, $$$P<0.001 compared with LPS+Mino.
The PI3K/Akt pathway inhibitor LY294002 significantly decreased Akt phosphorylation in the presence of LPS (Fig. 8A) and also abrogated the effect of minocycline on Akt phosphorylation (Fig. 8A). In addition, LY294002 eliminated the inhibitory effect of minocycline on LPS-induced p38 MAPK phosphorylation (Fig. 7).
In multiple instances, the anti-inflammatory effects of minocycline were partially abrogated by concurrent incubation in the presence of LY294002 (Fig. 8B). LY294002 significantly diminished minocycline effects on the LPS-induced IL-6 release, and TNF-α, COX-2, and IκB-α mRNA expression (Fig. 8B). Conversely, LY294002 did not affect minocycline effects on the LPS-induced LOX-1, LITAF and Nur77 mRNA expression (Fig. 8B).
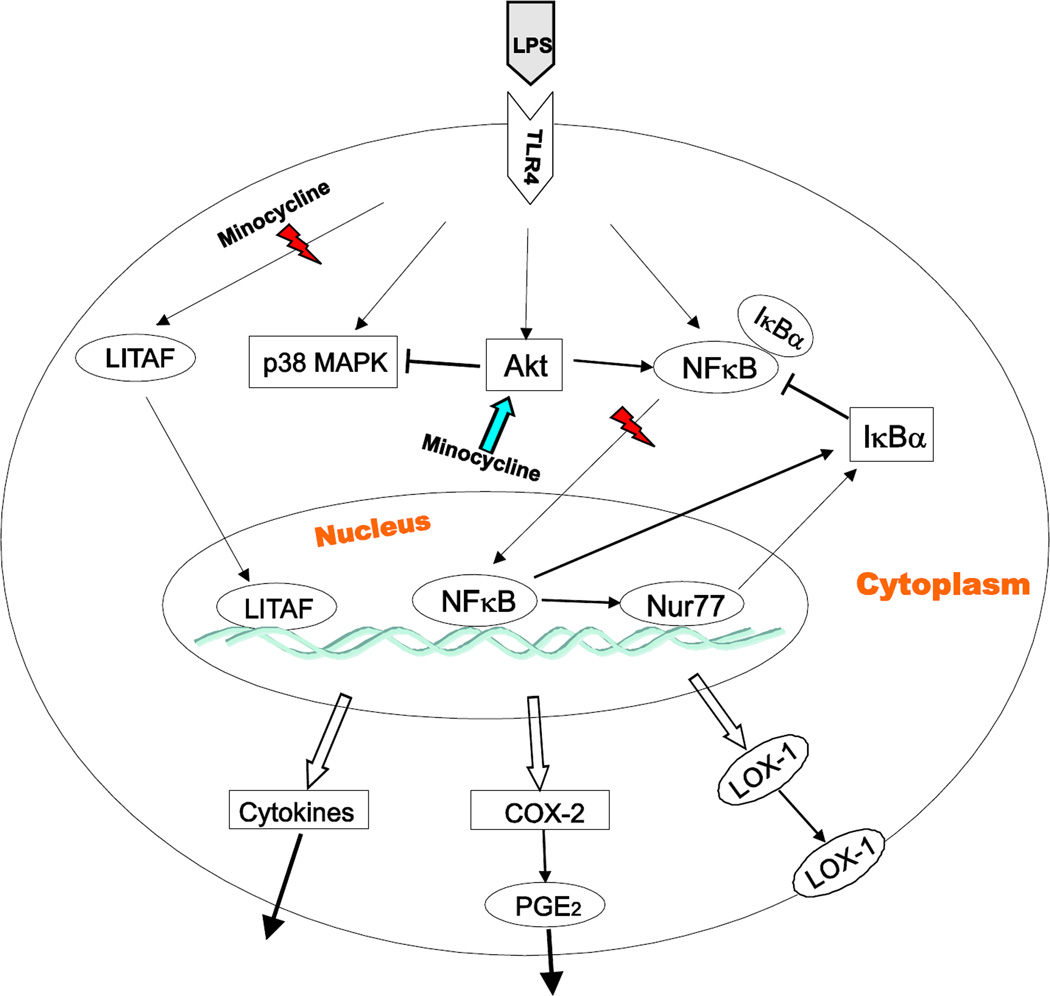
Figure 9 represents some of the target mechanisms involved in minocycline amelioration of LPS-induced inflammation as discussed above.
Fig. 9.
Scheme for the proposed pathway involved in minocycline anti-inflammation in primary human monocytes. The pro-inflammatory effects of LPS in primary human monocytes are the consequence of binding to its TLR-4 receptor, which in turn enhance LITAF production, p38 MAPK and Akt activation, and the nuclear translocation of the transcription factors NF-κB, Nur77 and LITAF. This results in the release of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6), COX-2 expression and release of its product PGE2 and transmembrane receptor LOX-1 production. Minocycline abrogates the pro-inflammatory effects of LPS by a number of mechanisms including inhibition of p38 MAPK activation, LITAF transcription, and LITAF, NF-κB and Nur77 nuclear translocation, and activation of PI3K/Akt pathway. Akt-dependent inactivation of p38 MAPK may be partially responsible for minocycline anti-inflammation effect in human monocytes.
4. Discussion
The principal findings of our study are: a) minocycline ameliorates the innate immune response in primary human monocytes challenged with a substantial but subseptic amount of LPS, including decreased induction of pro-inflammatory cytokines, PGE2 formation and release, as well as reduced activation of LOX-1, LITAF and Nur77; b) minocycline effects are mediated, at least in part, by inhibition of NF-κB and p38 MAPK activation, the later being dependent on the stimulation of the PI3K/Akt pathway.
We selected a subseptic concentration of LPS, capable of mounting a substantial inflammatory response while being in the same order of magnitude as the concentrations frequently encountered in chronic infections, periodontal disease and metabolic disorders [21,22]. Our study, in agreement with previous reports, demonstrates that in monocytes this concentration of LPS leads to a profound pro-inflammatory response associated with activation of traditional pro-inflammatory NF-κB and p38 MAPK pathways [11,17,23].
In our study, minocycline substantially reduced LPS-induced NF-κB activation, demonstrating that this pathway is an important target for its anti-inflammatory effects. We also found that minocycline ameliorated LPS-induced LOX-1 activation. LPS induces LOX-1 through a mechanism involving NF-κB activation [24–26], and we demonstrated that inhibition of LPS-induced LOX-1 expression by minocycline parallels minocycline inhibition of NF-κB activation. LOX-1 is a multifunctional receptor rapidly induced in macrophages by oxidized low-density lipoproteins and inflammatory cytokines such as TNF-α and IL-1β [10,27]. LOX-1 induction stimulates further formation of inflammatory cytokines and adhesion molecules [10] contributing to vascular inflammation and leukocyte recruitment and migration, as well as to the pathogenesis of cardiovascular disease [26,28]. It has been reported that LOX-1 inhibition decreases the levels of the LPS receptor TLR4 [29]. Thus, our results suggest that minocycline may interfere with the positive feedback loop between TLR4 and LOX-1 stimulation.
An additional novel finding in this study was the discovery that minocycline prevented the LPS-induced activation of the NR4A nuclear receptor Nur77. Nur77 is another NF-κB target gene induced in macrophages by inflammatory stimuli including LPS and increasing activation of inflammatory genes [12]. This being the case, we may hypothesize that decreased Nur77 activation is an additional mechanism involved in the anti-inflammatory effect of minocycline. However, NR4A nuclear receptors regulate inflammation in a manner highly dependent on cell type and context. It was reported that the NR4A nuclear receptors may also participate in inhibitory loops reducing inflammatory responses [13,30]. Thus, the reduction of LPS-induced Nur77 activation may also be interpreted as a reduction of an inhibitory feed back loop, consequence of the reduced inflammatory response produced by minocycline.
Our studies also reveal another novel mechanism for anti-inflammatory effect of minocycline, the inhibition of LPS-induced LITAF activation. Initially characterized as an LPS-dependent major TNF-α inducer, it was later reported that LITAF activation induces production not only of TNF-α but also of other inflammatory cytokines such as IL-1β and IL-6 [16], that LITAF is an important factor mediating macrophage activation during acute and chronic inflammation [15,31], and that its degree of activation determines sensitivity and resistance to LPS [16].
Minocycline substantially reduced p38 MAPK phosphorylation in response to LPS. Thus, our results support the hypothesis that p38 MAPK is an important target for the anti-inflammatory effects of minocycline in circulating monocytes [3,32]. Inhibition of p38 MAPK by minocycline in monocytes parallels the p38 MAPK inhibition by minocycline in microglia, reported to be partially responsible for its neuroprotective effects [4]. LPS-induced phosphorylation of p38 MAPK has been proposed as a context-dependent upstream stimulator of NF-κB activation [33]. However, p38 MAPK and NF-κB may be regulated independently of each other. For example, LOX-1 induction may be upregulated independently of NF-κB through p38 MAPK stimulation [34]. Similarly, LPS-induction of TNF-α and other inflammatory cytokines is the consequence of stimulation of at least two transcription factors, NF-κB and LITAF, regulated by different mechanisms. Stimulation of inflammatory cytokines following LITAF phosphorylation and nuclear translocation is mediated through p38 MAPK phosphorylation, as part of a pathway linking TLR4-MyD88-LITAF and independent of NF-κB [14,15].
A most important finding of our study is the demonstration that exposure to minocycline alone significantly enhances Akt phosphorylation, indicative of activation of the PI3K/Akt pathway. It is known that PI3K/Akt plays an important role in the negative regulation of LPS-induced acute inflammatory responses and limiting LPS induction of inflammatory cytokines involving NF-κB nuclear translocation [18,19,35,36]. This indicates that PI3K/Akt plays a major role in the balance and control of the inflammatory response to LPS.
We confirmed the role of the PI3K/Akt pathway with the demonstration that incubation with the PI3K/Akt inhibitor LY294002 partially reverses the ameliorating effect of minocycline on LPS-induced IL-6 release, TNF-α and COX-2 mRNA expression, and NF-κB activation. Moreover, inhibition of PI3K/Akt pathway completely abolished the effect of minocycline on LPS-induced p38 MAPK phosphorylation. This indicates that the effect of minocycline on p38 MAPK activity is exclusively dependent on PI3K/Akt signaling. These results demonstrate that minocycline activation of the PI3K/Akt pathway plays a significant role in its anti-inflammatory effects. Conversely, PI3K/Akt inhibition does not significantly affect the minocycline effects on LPS-induced LOX-1, Nur77 or LITAF activation, suggesting additional mechanisms involved in the anti-inflammatory effect of minocycline.
Our findings may have important translational value. The anti-inflammatory effects of minocycline were apparent even at a low concentration of 10 µM used in this in vitro study, in the same range of the peak plasma concentrations of 6 mg/l (equals 13 µM) achieved after a single oral administration of 100 to 200 mg of minocycline in humans [37]. A number of clinical trials have reported beneficial effects of minocycline for the treatment of stroke, spinal cord injury and multiple sclerosis [38–40]. Conversely, minocycline treatment was not effective for the treatment of amyotrophic lateral sclerosis (ALS) [41], Parkinson’s disease [42] or Huntington’s disease [43]. More studies are necessary to test the beneficial effects of minocycline in selective neurodegenerative diseases.
In conclusion, we report that the inhibition of LPS-induced inflammatory response by minocycline in primary human monocytes includes not only the well-established NF-κB and p38 MAPK pathways, but additional mechanisms including inhibition of LOX-1, Nur77 and LITAF pathways. PI3K/Akt activation plays a partial but important role in minocycline anti-inflammatory effects by reducing NF-κB and p38 MAPK-associated or independent mechanisms limiting inflammatory challenge and preserving homeostasis. These observations expand the proposed mechanisms of minocycline therapeutic effect and contribute to clarify the minocycline beneficial effects in acute inflammatory diseases, including amelioration of vascular inflammation and leukocyte recruitment, resistance to LPS-induced toxicity and reduced development of chronic inflammatory conditions. We demonstrate these multiple effects in primary human monocytes, further supporting the therapeutic potential of minocycline in cardiovascular and brain disorders.
Highlights.
We study minocycline effects on LPS inflammation in primary human monocytes.
We examine LPS-induction of cytokines, NF-κB, p38 MAPK, Akt, LOX-1, LITAF and Nur77.
Minocycline significantly inhibits all LPS effects.
Minocycline effects are partly the consequence of Akt activation.
The results clarify novel mechanisms of minocycline anti-inflammatory effects.
Acknowledgements
This study was supported by the Division of Intramural Research Programs, National Institute of Mental Health, National Institutes of Health, Department of Health and Human Services, USA.
Abbreviations
- Akt
protein kinase B
- COX-2
cyclooxygenase-2
- IκB-α
inhibitor of kappa B alpha
- IL-1β
interleukin-1 beta
- IL-6
interleukin-6
- LITAF
lipopolysaccharide -induced tumor-necrosis factor alpha factor
- LOX-1
lectin-like oxidized low density lipoprotein receptor-1
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinases
- NF-κB
nuclear factor kappa B
- PGE2
prostaglandin E2
- PI3K
phosphoinositide 3-kinase
- TLR4
toll-like receptor 4
- TNF-α
tumor-necrosis factor alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perry VH, Newman TA, Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nat. Rev. Neurosci. 2003;4:103–112. doi: 10.1038/nrn1032. [DOI] [PubMed] [Google Scholar]
- 2.Noble W, Garwood CJ, Hanger DP. Minocycline as a potential therapeutic agent in neurodegenerative disorders characterised by protein misfolding. Prion. 2009;3:78–83. doi: 10.4161/pri.3.2.8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stirling DP, Koochesfahani KM, Steeves JD, Tetzlaff W. Minocycline as a neuroprotective agent. Neuroscientist. 2005;11:308–322. doi: 10.1177/1073858405275175. [DOI] [PubMed] [Google Scholar]
- 4.Kim HS, Suh YH. Minocycline and neurodegenerative diseases. Behav. Brain. Res. 2009;196:168–179. doi: 10.1016/j.bbr.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 5.Defaux A, Zurich MG, Honegger P, Monnet-Tschudi F. Minocycline promotes remyelination in aggregating rat brain cell cultures after interferon-γ plus lipopolysaccharide-induced demyelination. Neuroscience. 2011;187:84–92. doi: 10.1016/j.neuroscience.2011.04.053. [DOI] [PubMed] [Google Scholar]
- 6.Zhou H, Lapointe BM, Clark SR, Zbytnuik L, Kubes P. A requirement for microglial TLR4 in leukocyte recruitment into brain in response to lipopolysaccharide. J. Immunol. 2006;177:8103–8110. doi: 10.4049/jimmunol.177.11.8103. [DOI] [PubMed] [Google Scholar]
- 7.Campbell JH, Burdo TH, Autissier P, Bombardier JP, Westmoreland SV, Soulas C, González RG, Ratai EM, Williams KC. Minocycline inhibition of monocyte activation correlates with neuronal protection in SIV neuroAIDS. PLoS One. 2011;6:e18688. doi: 10.1371/journal.pone.0018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dutta K, Mishra MK, Nazmi A, Kumawat KL, Basu A. Minocycline differentially modulates macrophage mediated peripheral immune response following Japanese encephalitis virus infection. Immunobiology. 2010;215:884–893. doi: 10.1016/j.imbio.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Dunston CR, Griffiths HR, Lambert PA, Staddon S, Vernallis AB. Proteomic analysis of the anti-inflammatory action of minocycline. Proteomics. 2011;11:42–51. doi: 10.1002/pmic.201000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogura S, Kakino A, Sato Y, Fujita Y, Iwamoto S, Otsui K, Yoshimoto R, Sawamura T. Lox-1: the multifunctional receptor underlying cardiovascular dysfunction. Circ. J. 2009;73:1993–1999. doi: 10.1253/circj.cj-09-0587. [DOI] [PubMed] [Google Scholar]
- 11.Baker RG, Hayden MS, Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pei L, Castrillo A, Tontonoz P. Regulation of macrophage inflammatory gene expression by the orphan nuclear receptor Nur77. Mol. Endocrinol. 2006;20:786–794. doi: 10.1210/me.2005-0331. [DOI] [PubMed] [Google Scholar]
- 13.Bonta PI, van Tiel CM, Vos M, Pols TW, van Thienen JV, Ferreira V, Arkenbout EK, Seppen J, Spek CA, van der Poll T, Pannekoek H, de Vries CJ. Nuclear receptors Nur77, Nurr1, and NOR-1 expressed in atherosclerotic lesion macrophages reduce lipid loading and inflammatory responses. Arterioscler. Thromb. Vasc. Biol. 2006;26:2288–2294. doi: 10.1161/01.ATV.0000238346.84458.5d. [DOI] [PubMed] [Google Scholar]
- 14.Tang X, Marciano DL, Leeman SE, Amar S. LPS induces the interaction of a transcription factor, LPS-induced TNF-alpha factor, and STAT6(B) with effects on multiple cytokines. Proc. Natl. Acad. Sci. 2005;102:5132–5137. doi: 10.1073/pnas.0501159102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang X, Metzger D, Leeman S, Amar S. LPS-induced TNF-alpha factor (LITAF)-deficient mice express reduced LPS-induced cytokine: Evidence for LITAF-dependent LPS signaling pathways. Proc. Natl. Acad. Sci. 2006;103:13777–13782. doi: 10.1073/pnas.0605988103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srinivasan S, Leeman SE, Amar S. Beneficial dysregulation of the time course of inflammatory mediators in lipopolysaccharide-induced tumor necrosis factor alpha factor-deficient mice. Clin. Vaccine. Immunol. 2010;17:699–704. doi: 10.1128/CVI.00510-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma FY, Ikezumi Y, Nikolic-Paterson DJ. Macrophage signaling pathways: a novel target in renal disease. Semin. Nephrol. 2010;30:334–344. doi: 10.1016/j.semnephrol.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Guha M, Mackman N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J. Biol. Chem. 2002;277:32124–32132. doi: 10.1074/jbc.M203298200. [DOI] [PubMed] [Google Scholar]
- 19.Luyendyk JP, Schabbauer GA, Tencati M, Holscher T, Pawlinski R, Mackman N. Genetic analysis of the role of the PI3K-Akt pathway in lipopolysaccharide-induced cytokine and tissue factor gene expression in monocytes/macrophages. J. Immunol. 2008;180:4218–4226. doi: 10.4049/jimmunol.180.6.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egidy G, Friedman J, Viswanathan M, Wahl LM, Saavedra JM. CGP-42112 partially activates human monocytes and reduces their stimulation by lipopolysaccharides. Am. J. Physiol. 1997;273:C826–C833. doi: 10.1152/ajpcell.1997.273.3.C826. [DOI] [PubMed] [Google Scholar]
- 21.Li HL, Suzuki J, Bayna E, Zhang FM, Dalle Molle E, Clark A, Engler RL, Lew WY. Lipopolysaccharide induces apoptosis in adult rat ventricular myocytes via cardiac AT(1) receptors. Am. J. Physiol. Heart. Circ. Physiol. 2002;283:H461–H467. doi: 10.1152/ajpheart.00701.2001. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz EA, Zhang WY, Karnik SK, Borwege S, Anand VR, Laine PS, Su Y, Reaven PD. Nutrient modification of the innate immune response: a novel mechanism by which saturated fatty acids greatly amplify monocyte inflammation. Arterioscler. Thromb. Vasc. Biol. 2010;30:802–808. doi: 10.1161/ATVBAHA.109.201681. [DOI] [PubMed] [Google Scholar]
- 23.Larrayoz IM, Pang T, Benicky J, Pavel J, Sánchez-Lemus E, Saavedra JM. Candesartan reduces the innate immune response to lipopolysaccharide in human monocytes. J. Hypertens. 2009;27:2365–2376. doi: 10.1097/HJH.0b013e3283314bc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagase M, Abe J, Takahashi K, Ando J, Hirose S, Fujita T. Genomic organization and regulation of expression of the lectin-like oxidized low-density lipoprotein receptor (LOX-1) gene. J. Biol. Chem. 1998;273:33702–33707. doi: 10.1074/jbc.273.50.33702. [DOI] [PubMed] [Google Scholar]
- 25.Shibata Y, Kume N, Arai H, Hayashida K, Inui-Hayashida A, Minami M, Mukai E, Toyohara M, Harauma A, Murayama T, Kita T, Hara S, Kamei K, Yokode M. Mulberry leaf aqueous fractions inhibit TNF-alpha-induced nuclear factor kappaB (NF-kappaB) activation and lectin-like oxidized LDL receptor-1 (LOX-1) expression in vascular endothelial cells. Atherosclerosis. 2007;193:20–27. doi: 10.1016/j.atherosclerosis.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Yoshimoto R, Fujita Y, Kakino A, Iwamoto S, Takaya T, Sawamura T. The Discovery of LOX-1, its Ligands and Clinical Significance. Cardiovasc. Drugs. Ther. 2011 Aug 2; doi: 10.1007/s10557-011-6324-6. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hofnagel O, Luechtenborg B, Stolle K, Lorkowski S, Eschert H, Plenz G, Robenek H. Proinflammatory cytokines regulate LOX-1 expression in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2004;24:1789–1795. doi: 10.1161/01.ATV.0000140061.89096.2b. [DOI] [PubMed] [Google Scholar]
- 28.Navarra T, Del Turco S, Berti S, Basta G. The lectin-like oxidized low-density lipoprotein receptor-1 and its soluble form: cardiovascular implications. J. Atheroscler. Thromb. 2010;17:317–331. doi: 10.5551/jat.3228. [DOI] [PubMed] [Google Scholar]
- 29.Nowicki M, Müller K, Serke H, Kosacka J, Vilser C, Ricken A, Spanel-Borowski K. Oxidized low-density lipoprotein (oxLDL)-induced cell death in dorsal root ganglion cell cultures depends not on the lectin-like oxLDL receptor-1 but on the toll-like receptor-4. J. Neurosci. Res. 2010;88:403–412. doi: 10.1002/jnr.22205. [DOI] [PubMed] [Google Scholar]
- 30.You B, Jiang YY, Chen S, Yan G, Sun J. The orphan nuclear receptor Nur77 suppresses endothelial cell activation through induction of IkappaBalpha expression. Circ. Res. 2009;104:742–749. doi: 10.1161/CIRCRESAHA.108.192286. [DOI] [PubMed] [Google Scholar]
- 31.Bushell KN, Leeman SE, Gillespie E, Gower AC, Reed KL, Stucchi AF, Becker JM, Amar S. LITAF Mediation of Increased TNF-α Secretion from Inflamed Colonic Lamina Propria Macrophages. PLoS One. 2011;6:e25849. doi: 10.1371/journal.pone.0025849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piao CS, Kim JB, Han PL, Lee JK. Administration of the p38 MAPK inhibitor SB203580 affords brain protection with a wide therapeutic window against focal ischemic insult. J. Neurosci. Res. 2003;73:537–544. doi: 10.1002/jnr.10671. [DOI] [PubMed] [Google Scholar]
- 33.Muthusamy V, Piva TJ. The UV response of the skin: a review of the MAPK, NFkappaB and TNFalpha signal transduction pathways. Arch. Dermatol. Res. 2010;302:5–17. doi: 10.1007/s00403-009-0994-y. [DOI] [PubMed] [Google Scholar]
- 34.Ishiyama J, Taguchi R, Yamamoto A, Murakami K. Palmitic acid enhances lectin-like oxidized LDL receptor (LOX-1) expression and promotes uptake of oxidized LDL in macrophage cells. Atherosclerosis. 2010;209:118–124. doi: 10.1016/j.atherosclerosis.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Schabbauer G, Tencati M, Pedersen B, Pawlinski R, Mackman N. PI3K-Akt pathway suppresses coagulation and inflammation in endotoxemic mice. Arterioscler. Thromb. Vasc. Biol. 2004;24:1963–1969. doi: 10.1161/01.ATV.0000143096.15099.ce. [DOI] [PubMed] [Google Scholar]
- 36.Bommhardt U, Chang KC, Swanson PE, Wagner TH, Tinsley KW, Karl IE, Hotchkiss RS. Akt decreases lymphocyte apoptosis and improves survival in sepsis. J. Immunol. 2004;172:7583–7591. doi: 10.4049/jimmunol.172.12.7583. [DOI] [PubMed] [Google Scholar]
- 37.Saivin S, Houin G. Clinical pharmacokinetics of doxycycline and minocycline. Clin. Pharmacokinet. 1988;15:355–366. doi: 10.2165/00003088-198815060-00001. [DOI] [PubMed] [Google Scholar]
- 38.Lampl Y, Boaz M, Gilad R, Lorberboym M, Dabby R, Rapoport A, Anca-Hershkowitz M, Sadeh M. Minocycline treatment in acute stroke: an open-label, evaluator-blinded study. Neurology. 2007;69:1404–1410. doi: 10.1212/01.wnl.0000277487.04281.db. [DOI] [PubMed] [Google Scholar]
- 39.Casha S, Zygun D, McGowan D, Yong VW, Hurlbert JR. Neuroprotection with minocycline after spinal cord injury: results of a double blind, randomized, controlled pilot study. Neurosurgery. 2009;65:410–411. [Google Scholar]
- 40.Metz LM, Li D, Traboulsee A, Myles ML, Duguette P, Godin J, Constantin M, Yong VW. GA/minocycline study investigators, Glatiramer acetate in combination with minocycline in patients with relapsing-remitting multiple sclerosis: results of a Canadian, multicenter, double-blind, placebo-controlled trial. Mult. Scler. 2009;15:1183–1194. doi: 10.1177/1352458509106779. [DOI] [PubMed] [Google Scholar]
- 41.Gordon PH, Moore DH, Miller RG, Florence JM, Verheijde JL, Doorish C, Hilton JF, Spitalny GM, MacArthur RB, Mitsumoto H, Neville HE, Boylan K, Mozaffar T, Belsh JM, Ravits J, Bedlack RS, Graves MC, McCluskey LF, Barohn RJ, Tandan R Western ALS Study Group. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomized trial. Lancet Neurol. 2007;6:1045–1053. doi: 10.1016/S1474-4422(07)70270-3. [DOI] [PubMed] [Google Scholar]
- 42.NINDS NET-PD Investigators. A pilot clinical trial of creatine and minocycline in early Parkinson disease: 18-month results. Clin. Neuropharmacol. 2008;31:141–150. doi: 10.1097/WNF.0b013e3181342f32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas M, Ashizawa T, Jankovic J. Minocycline in Huntington’s disease: a pilot study. Mov. Disord. 2004;19:692–695. doi: 10.1002/mds.20018. [DOI] [PubMed] [Google Scholar]