Abstract
Objective
We wished to ascertain if there is an association between symptoms of Attention-Deficit/Hyperactivity Disorder (ADHD) and home environment in children with ADHD and non-ADHD siblings, controlling for other environmental measures.
Method
96 children with ADHD combined type (ADHD-CT) and their siblings participated in the study. Parent and teacher Conners’ rating scales were completed and home environment was assessed using the Middle Childhood and Early Adolescent Home Observation for Measurement of the Environment (HOME). ADHD symptoms were assessed for correlation with HOME in children with ADHD-CT and non-ADHD siblings and multiple regression analysis was used to control for gender, socio-economic status, exposure to nicotine, exposure to alcohol in utero, birth weight, gestational age, pregnancy and perinatal risk factors. The presence of oppositional disorders was assessed for association with HOME score in those with ADHD-CT. The multiple regression analysis was repeated controlling for environmental factors and for oppositional disorders in those with ADHD-CT. Oppositional symptoms were assessed for correlation with HOME score in non-ADHD siblings.
Results
Teacher-rated hyperactive/impulsive scores correlated with HOME (r = −.27, p <.01) in children with ADHD-CT. This association remained significant when other environmental factors and oppositional disorders were controlled for. Environmental factors and gender contributed to 30% of the variance of ADHD symptoms in ADHD-CT. Parent-rated hyperactive/impulsive scores also correlated with HOME (r = −.28, p < .05) for non-ADHD siblings. An association between HOME and diagnosis of oppositional defiant disorder or CD was found for children with ADHD-CT and between HOME and oppositional symptoms in non-ADHD siblings.
Conclusions
The home environment has a small but significant association with hyperactive/impulsive symptoms in children with ADHD-CT and non-ADHD siblings. This association remained when other environmental factors were taken into account. Oppositional symptoms are associated with home environment in ADHD-CT and in non-ADHD siblings.
Keywords: Attention Deficit Hyperactivity Disorder, HOME, environmental factors, oppositional disorders
Introduction
Attention-Deficit/Hyperactivity Disorder (ADHD) is characterised by symptoms of inattention, hyperactivity and impulsivity before seven years of age, with symptoms at home and school. Twin, family and adoption studies support a genetic component to ADHD (Schachar & Wachsmuth, 1990; Frick et al., 1991; Levy et al., 1997; Sprich et al., 2000) with heritability estimated at 76% (Faraone et al., 2005). Molecular genetic studies have identified several genetic variants of minor effect, many in genes involved in monoaminergic neurotransmission (Brookes, et al., 2006; Banaschewski et al, 2010). However candidate gene studies explain only a small amount of the genetic component of ADHD; genome wide scan studies to date have not identified genes of major effect (Franke et al., 2009), and the focus of ADHD research is moving to gene-environment interactions and endophenotype studies (Banaschewski et al, 2010).
Early child psychiatry research focused on environmental factors. Childhood psychiatric disorders are associated with severe marital discord, paternal psychopathology, maternal psychiatric disorder, large family size, fostered children and low social status (Rutter, 1973). These adversity factors (excluding fostered children) are associated with ADHD (Biederman et al., 1995), and associated with ADHD after controlling for the effects of maternal smoking, gender and parental ADHD (Biederman et al., 2002). A longitudinal study has shown that family adversity predicts psychiatric disorders in children, and in particular early onset disorder, disorders in boys, and conduct disorders (Blanz et al., 1991). A more recent study has shown that children with ADHD combined type have more risk factors for family adversity than community controls, and that oppositional defiant symptoms and conduct disorder symptoms are related to family adversity factors (Counts et al., 2005).
Adoption studies support the theory that early childhood environment may influence the development of ADHD. Adopted children have higher rates of ADHD than non-adopted children (Keyes et al., 2008; Beverly et al., 2008)and those adopted later have higher rates of ADHD (Beverly et al., 2008). Early deprivation may have a contributory or causal role in ADHD, as those exposed to early deprivation had higher levels of inattention/overactivity than those with no history of deprivation (Kreppner et al., 2001). A meta-analysis of adoption studies found that early deprivation was associated with externalizing symptoms (Juffer & van Ijzendoorn, 2005). A significant effect of duration of deprivation on the level of inattention/overactivity has been shown, with effects not attenuating over time (Kreppner et al., 2001).
We hypothesize that the quality of the home environment might be associated with the risk of developing symptoms of ADHD in a similar way but to a less severe effect as the effect of early deprivation on symptoms of ADHD. In a multifactorial model, children with a biological or genetic predisposition to ADHD may be more vulnerable to developing ADHD in certain home environments. Our theory has parallels with the dynamic developmental theory of ADHD (Sagvolden et al., 2005), which hypothesizes that altered dopaminergic function in ADHD causes altered reinforcement of behaviour, giving rise to a failure to inhibit certain responses and to symptoms of ADHD in some environments; there is an interplay between the individual’s surroundings and the biological predisposition so that symptoms of ADHD develop in some environments and not in others. The aim of this study is to look for an association between the quality of the home environment and symptoms of ADHD and related oppositional disorders in children with ADHD and their siblings. We expect to find a dose dependent association between symptoms of ADHD and the quality of the home environment, with a more supportive home environment associated with less symptoms of ADHD. We also expect to find an association between the home environment and the presence of oppositional disorders which are often associated with ADHD.
The sample is a subset of the International Multicentre ADHD Genetics Study (IMAGE), a genetics study of 1400 families funded by the National Institute of Mental Health, (Brookes et al., 2006). The home environment was measured using the Home Observation for Measurement of the Environment (HOME) (Bradley & Caldwell, 1977). Other environmental factors of known or suspected association with ADHD were controlled for in the analysis.
Methods
Study Sample
Families participating in the IMAGE study in Ireland (N=156) were invited to participate in this study, after ethical approval by relevant ethics committees. Inclusion and exclusion criteria were as for IMAGE (Brookes et al., 2006); all participating families had a child with ADHD combined type (ADHD-CT) aged 6–17 years attending a child and adolescent child psychiatry service, a sibling in the same age range and one or both parents available to participate in the study. Families were excluded (following enquiry of parents) if siblings were half siblings, adopted or fostered. Families were excluded if the research diagnosis of ADHD-CT was not confirmed (N= 8), if the child with ADHD-CT or sibling had a diagnosis of epilepsy (N=1), autism spectrum disorder (N=5), IQ<70 (N=9), or history of a metabolic or neurological disorder. For this study, siblings with a diagnosis of ADHD were excluded. 133 families participated in IMAGE in Ireland; 104 of these completed the IMAGE assessment and had a research diagnosis of ADHD-CT confirmed and did not meet any of the exclusion criteria for IMAGE. This study commenced some time after the IMAGE study in Ireland, recruiting 96 families. The probands from this group did not differ significantly from the total Irish IMAGE group in terms of ADHD severity measured by parent and teacher Conners’ or socio-economic status. The ADHD proband group had a mean age of 10.94 +/− 3.06 years, range of 5–17 years and male: female ratio of 80:16 (83% male). The sibling group had a mean age of 10.38 +/− 3.00 years, range of 6–17 years and male: female ratio of 43:51 (45.7% male). There was no significant difference in the mean age of the ADHD group and the sibling group (mean diff = .56; standard error of difference =0.44; 95% CI = −.31 – 1.42; t = 1.26; p = .21). There was a significant difference in the gender ratio of the proband and the sibling group (chi = 29.40; df = 1; p <0.001).
Measures used
The research diagnosis of ADHD-CT was confirmed using data from the Parental Account of Children’s Symptoms semi-structured interview (PACS) (Chen & Taylor, 2006), Conners’ rating scale data (Conners, 2001) and the Strengths and Difficulties Questionnaire, (Goodman, 1997). Initially all children participating in the study (probands and siblings) were screened for ADHD using the parent and teacher Conners’ rating scales. Those with a T-score of 63 or greater on column “N” (DSM-IV Total) of the Conners’ scale were further assessed for symptoms of ADHD using the PACS semi-structured interview. The use of the cut- off point of 63 (90th percentile) on the Conners T score to identify those who were for a more detailed assessment of ADHD symptoms using the PACS semi structured interview reduced the likelihood of missing a case of ADHD, especially in the sibling group. This method of screening all child participants for ADHD was used in the IMAGE study and facilitated a comprehensive assessment for ADHD symptoms in all child participants. The Strengths and Difficulties Questionnaire (SDQ) was used to measure clinical impairment (a requirement for the diagnosis of ADHD) and screen for autistic spectrum disorder. Clinical impairment was measured using the impact supplement part of the parent and teacher SDQ. A child was considered clinically impaired if the parent or teacher responded that the child had difficulties in emotions, concentration, behaviour or being able to get on with other people, and that these difficulties were upsetting or distressing the child or interfering with the child’s home life or friendships or classroom learning or leisure activities or peer relationships or that these difficulties were putting a burden on the parent/teacher or the family/class as a whole.
The Hypescheme data capture system (Curran et al., 2000)was used to extract DSM-IV symptoms from the Conners’, PACS and SDQ data. Hence DSM-IV diagnoses of ADHD-CT, ADHD inattentive type, ADHD hyperactive type or no ADHD were generated, as well as oppositional defiant disorder (ODD) and conduct disorder (CD). Four subtests of the WISC (Wechsler, 1991) were performed (LB) for each child and the IQ calculated using Sattler’s method (Sattler, 1992). For this study, ADHD symptoms were measured using the parent and teacher Conners’ rating scale, with inattention measured using the T-score for column L and hyperactivity/impulsivity measured using the T-score for column M.
Probands and siblings with autistic spectrum disorder were excluded from the sample using the Social Communication Questionnaire (SCQ) (Berument et al., 1999; Rutter et al., 2003) and the prosocial scale of the SDQ as follows. Children with a score of 15 or higher on the parent completed SCQ, or a score of 4 or less on the parent or teacher prosocial scale of the SDQ or for whom the researcher had a clinical suspicion that the child may have an autism spectrum disorder were further assessed using the autism part of the PACS semistructured interview. This contains 12 questions which assess for symptoms in the three domains of autism (communication domain, reciprocal social interaction domain and restricted, repetitive, stereotyped patterns of behaviour domain). An algorithm applied to the autism part of the PACS was used to classify children into those with No Autism, Atypical Autism and Typical Autism. Children identified as having atypical autism or typical autism were then excluded from the IMAGE study and from this study on the HOME and ADHD.
The HOME assessments were performed by the first author with the child’s parent in the home, with the child present. The PACS semi-structured interview was also performed at this visit; Conners’ parent and teacher rating scales were posted to the parent(s) approximately 10 days before the planned visit, and were returned to the researcher either at the home visit or immediately prior to the visit. Each HOME assessment was performed using both interview and observation techniques, as described in the HOME manual (Caldwell & Bradley, 2001). The manual provides guidance on the performance of the HOME, with a comprehensive explanation of each item and some examples for scoring HOME assessments; no other specific training is required. Each item on the HOME is designated an item which is rated on observation (O) or by a structured interview question (I) or by either observation or structured interview (E). The first author performed the HOME with the parent with regard to the identified proband in the family. A small subset of the sample (7 sibling pairs non-concordant for ADHD) had HOME assessments of both the proband and sibling. It was found that proband and sibling percentage total HOME scores were highly correlated (r = .97, p <.001, two tailed) and hence no further sibling HOME assessments were performed. The percentage HOME score of the ADHD-CT proband was taken to represent the sibling HOME score for statistical analysis in this study.
The Middle Childhood (MC) HOME was performed for those under 10 years (Bradley et al., 1988) and contains 59 items; the Early Adolescent (EA) HOME was performed for those 10 years and over (Bradley et al., 2000) and contains 60 items which are scored positive or negative by the rater. Hence maximum HOME scores of 59 and 60 respectively are possible. Higher HOME scores indicate an enriched home environment. MC HOME items are grouped into eight subscales: I Responsivity; II Encouragement of Maturity; III Emotional Climate; IV Learning Materials & Opportunities; V Enrichment; VI Family Companianship; VII Family Integration and VIII Physical Environment. The EA HOME has seven subscales: I Physical Environment; II Learning Materials; III Modeling; IV Fostering Self-Sufficiency; V Regulatory Activities; VI Family Companianship; VII Acceptance. The HOME has been used extensively in research and in clinical settings (Casey & Bradley, 1982; Totsika & Sylva, 2004) and has been shown to be valid in another European population (Burston et al., 2005). Studies have shown that the HOME has an inter-observer agreement level of 80–90% (Totsika & Sylva, 2004).
The following environmental measures were taken for children with ADHD-CT and siblings by parental interview: exposure to nicotine in utero, exposure to alcohol in utero, birth weight, gestational age, the presence of pregnancy and perinatal risk factors. Exposure to nicotine was measured on a three point scale: no exposure (score= 0), exposure to less than 20 cigarettes daily (score= 1), exposure to 20 cigarettes daily or more (score= 2) at some time during pregnancy. Exposure to alcohol was also measured on a three point scale: no exposure (score= 0), exposure to less than 2 units per week (score= 1), exposure to 2 units per week or greater (score= 2). Perinatal risk factors (as per IMAGE protocol) were classified as ‘Definite Disorder’ if any of the following factors were definitely present: birth weight less than 1.5kg, gestational age less than 29 weeks, APGAR score less than 2 at 5 minutes, special care baby unit for more than 24 hours, severe infection, severe metabolic disturbance (hypoglycaemia, acidosis, hypocalcaemia), seizures/convulsions in the first month of life, and as ‘Possible Disorder’ if any of these factors were suspected. Pregnancy risk factors (as per IMAGE protocol) were classified as ‘Definite Disorder’ if any of the following risk factors listed were definitely present: antepartum hemorrhage, pre-eclamptic toxaemia, hypertension, diabetes, severe infection, smoking more than 20 cigarettes daily for at least 3 months of pregnancy, use of benzodiazepines, anticonvulsants or other drugs thought to be associated with ADHD in the offspring and as ‘Possible Disorder’ if any of these factors were suspected. Each parent’s socioeconomic status was recorded on a 5 point scale (professional, clerk, services/sales, labourer, other/homemaker) according to the IMAGE protocol, and probands and siblings donated blood or saliva samples for the IMAGE study, along with one or both parents.
Statistical Analysis
Mean HOME total and subscale scores were calculated. Each MC or EA HOME score was converted into a percentage so the total group of children with ADHD-CT could be studied together, following personal communication with Robert Bradley, an author of the HOME tool. For each child who had the Middle Childhood HOME performed, the child’s score was divided by 59 (the maximum possible MC HOME score) and multiplied by 100 to convert it to the percentage HOME score. Similarly for each child who had the Early Adolescent HOME performed, the child’s score was divided by 60 (the maximum possible EA HOME score) and multiplied by 100 to convert it to the percentage HOME score. The percentage total HOME scores were assessed for correlation with symptoms of hyperactivity/impulsivity and symptoms of inattention (parent and teacher Conners’ Rating scales) in those with ADHD-CT. Data were analysed using SPSS version 12. Non-parametric tests were used, as the Conners’ data were not normally distributed. HOME subscale scores were analysed if a significant correlation between total HOME and ADHD symptoms was found. The group with ADHD-CT was divided into those with and those without ODD and the mean percentage HOME score was compared in each group. The group with ADHD-CT was then divided into those with and without CD and the mean percentage HOME score was compared in each group. Finally multiple regression analysis was performed to assess the effect of gender, HOME score and measured environmental factors listed above on hyperactivity/impulsivity.
The analysis was repeated for their non-ADHD siblings, with siblings excluded if they had a PACS diagnosis of ADHD or a DSM-IV Conners’ T-score greater than 63. The percentage HOME score of the ADHD-CT proband was taken to represent the family HOME score and correlated with the sibling Conners’ hyperactivity/impulsivity and inattention. Where there was more than one non-ADHD sibling in a family, the mean Conners’ sibling score was calculated and used for correlation with the HOME score. The non-ADHD siblings’ oppositional symptoms scores (parent and teacher-rated Conners’ column A T-score) were also correlated with the HOME score. Finally the effects of HOME and measured environmental factors on siblings’ symptoms of ADHD were explored by performing a standard multiple regression analysis.
Results
Probands
35 children with ADHD-CT had a mean total MC HOME score of 43.1, SD = 8.0. 61 early adolescents with ADHD-CT had a mean total EA HOME score of 43.1, SD = 9.6. The mean percentage total HOME score for the total group was 72.8, SD = 14.3, N = 96. The mean HOME subscales scores and published mean subscale scores (Bradley et al., 1988; Bradley et al, 2000) can be seen in Table 1.
Table 1.
Middle Childhood and Early Adolescent HOME mean subscale scores in ADHD probands
| Middle Childhood HOME | N | Mean | Std. Deviation | Published Expected Mean* | Published Std. Deviation* |
|---|---|---|---|---|---|
| I. Responsivity | 35 | 7.83 | 1.52 | 8.4 | 2.3 |
| II. Encouragement of Maturity | 35 | 5.17 | 1.62 | 4.8 | 1.6 |
| III. Emotional Climate | 35 | 5.43 | 1.54 | 6.0 | 1.6 |
| IV. Learning Materials & Opportunities | 35 | 5.54 | 1.34 | 5.2 | 2.0 |
| V. Enrichment | 35 | 5.34 | 1.76 | 3.4 | 2.2 |
| VI. Family Companianship | 35 | 5.06 | 1.03 | 4.1 | 1.4 |
| VII. Family Integration | 35 | 3.03 | 0.92 | 2.4 | 1.2 |
| VIII. Physical Environment | 35 | 5.69 | 1.88 | 6.8 | 1.7 |
| Total MC HOME | 35 | 43.1 | 8.0 | 41.6 | 9.0 |
| Early Adolescent HOME | |||||
| I. Physical Environment | 61 | 5.15 | 1.93 | 6.60 | 1.04 |
| II. Learning Materials | 61 | 6.90 | 2.61 | 7.98 | 2.01 |
| III. Modeling | 61 | 6.21 | 2.05 | 8.00 | 1.68 |
| IV. Fostering Self-sufficiency | 61 | 4.67 | 1.30 | 4.16 | 1.31 |
| V. Regulatory Activities | 61 | 8.13 | 1.61 | 9.00 | 1.17 |
| VII. Family Companianship | 61 | 5.33 | 1.80 | 5.94 | 1.55 |
| VIII. Acceptance | 61 | 6.62 | 1.85 | 8.69 | 0.77 |
| Total EA HOME | 61 | 43.1 | 9.6 | 51.05 | 5.77 |
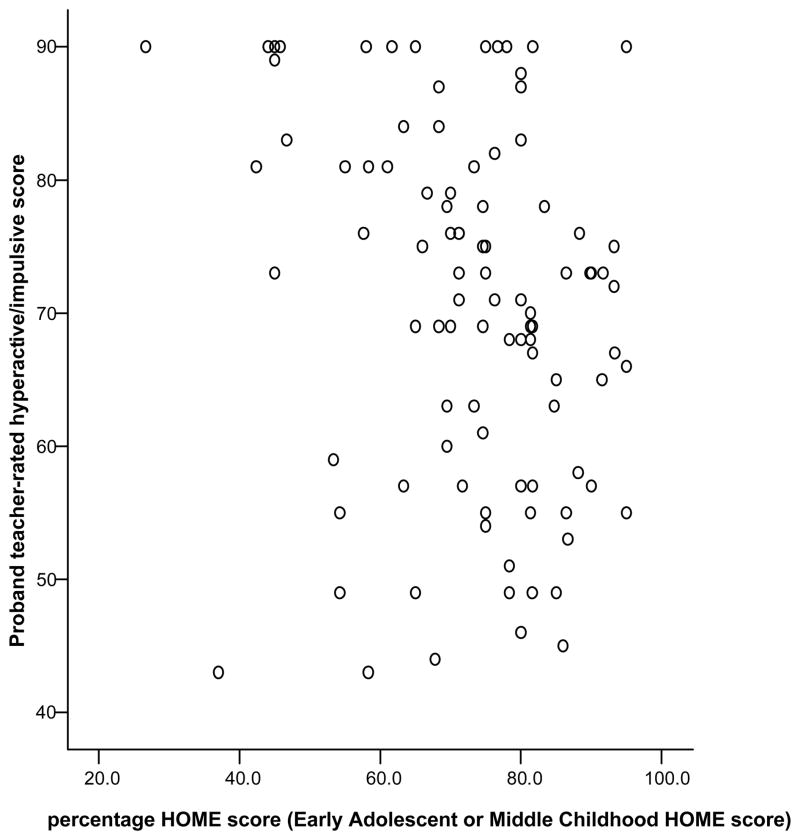
Higher total HOME scores were correlated with lower teacher-rated hyperactivity/impulsivity (r = −.27, p =.008, N=96) (see Figure 1). There was no significant relationship between HOME and parent-rated ADHD scores or teacher-rated inattentive scores.
Figure 1. Correlation of proband teacher-rated hyperactive/impulsive score and percentage HOME score.
r = −.27, p =0.008, N=96. Note: a higher percentage of HOME score (such as in the 80–100 range) indicates a more supportive home environment.
HOME subscales were correlated with teacher-rated hyperactivity/impulsivity in those with ADHD-CT to ascertain if one aspect of the home environment correlated with teacher-rated hyperactivity/impulsivity. The sample size for subscale correlation is smaller than for correlation with the Total HOME (N=96), due to different MC and EA subscales. Using the MC HOME, a negative correlation was found with physical environment (r = −.358, p = .035) and with learning materials and opportunities (r = −.39, p = .021). Using the EA HOME, a negative correlation was found with learning materials (r = −.35, p = .006). In summary, children with ADHD-CT aged less than 10 years who had a poorer physical home environment or less learning opportunities had greater teacher-rated hyperactivity/impulsivity than those with a better physical home environment or those with more learning opportunities. Early adolescents with ADHD-CT who had less access to learning materials had greater teacher-rated hyperactivity/impulsivity than those with more access to learning materials.
34 children had ADHD-CT without ODD and had a significantly higher mean percentage HOME score (76.93) than the 62 children with ADHD-CT and comorbid ODD (70.48) (mean diff = 6.46, p = .018, 95% CI = 1.14 – 11.77). 74 children had ADHD-CT without CD and had a significantly higher mean percentage HOME score (75.58) than the 22 children with ADHD-CT and comorbid CD (63.30) (mean diff = 12.28, p < .001, 95% CI = 5.83 – 18.72). The group with ODD and the group with CD had less supportive home environments than those without these comorbid disorders.
Multiple linear regression analysis was performed with HOME score and environmental factors entered simultaneously as independent variables and teacher-rated hyperactivity/impulsivity as the dependent variable (see Table 2). Gender was also entered as a predictor in the multiple regression analysis, as the ADHD group had a significantly higher ratio of males to females than the sibling group.
Table 2.
Regression analysis: Environmental factors and teacher-rated hyperactivity/impulsivity in ADHD combined type probands
| Environmental Risk Factors | Unstandardized Coefficients | Standardized Coefficients | |||
|---|---|---|---|---|---|
| B | Std. Error | Beta | tolerance | p | |
| (Constant) | 117.189 | 42.150 | |||
| HOME score | −.286 | .128 | −.313 | −2.233 | .030 |
| Birth weight | 1.041 | 3.375 | .042 | .309 | .759 |
| Gestational age | −1.083 | .956 | −.148 | −1.133 | .262 |
| Cigs exposure in utero | 6.396 | 2.489 | .362 | 2.570 | .013 |
| Alcohol exposure in utero | 2.642 | 2.021 | .150 | 1.307 | .196 |
| Pregnancy risk factors | −3.706 | 2.401 | −.224 | −1.543 | .128 |
| Perinatal risk factors | 3.239 | 2.361 | .166 | 1.372 | .176 |
| Father’s socioeconomic status | −2.366 | 1.747 | −.186 | −1.355 | .181 |
| Mother’s socioeconomic status | 1.564 | 1.349 | .145 | 1.159 | .251 |
| gender | 11.963 | 3.816 | .338 | 3.135 | .003 |
The proband group had a mean birth weight of 3.5 kg (SD = 0.6kg) and mean gestational age of 39.9 weeks (range 33 – 44 weeks). The majority were not exposed to nicotine in utero (53% scored 0 vs 29% scored 1 vs 18% scored 2); the majority were not exposed to alcohol in utero (52% scored 0 vs 31% scored 1 vs 17% scored 2). A large proportion did not have pregnancy risk factors as described above (39% scored 0 vs 29.5% scored 1 vs 31.5% scored 2); the majority did not have perinatal risk factors (71% scored 0 vs 16% % scored 1 vs 13% scored 2). All socio-economic groups were represented, with a large proportion of fathers from socioeconomic group 3 (48.4%) and from socio-economic group 4 (30.1%). Many mothers were from socio-economic group 5 (43.6%) and from socio-economic group 3 (35.1%).
The environmental factors and the effects of gender explained 30% of the variance in teacher-rated hyperactivity/impulsivity (adjusted R square = .307; F = 3.93 df = 10, p < .001). The regression line can be explained by the following equation: teacher-rated hyperactive/impulsive symptoms = 117.19 − 0.29(percentage HOME score)+1.04(birth weight) − 1.08(gestational age) + 6.40(cig exposure) + 2.64(alcohol exposure) − 3.71(pregnancy risk factors) + 3.24(perinatal risk factors) − 2.37(father’s socio) + 1.56(mother’s socio) + 11.96(gender), using unstandardized co-efficient figures from Table 2, with gender expressed as 1 = male and 2 = female The relative contribution of each variable to the regression line can be seen from the tolerance values in Table 2; variables which have a tolerance value of >2 or <-2 contribute more to the equation than other environmental factors. The largest contribution is from gender (tolerance = 3.1. p = .003), followed by exposure to nicotine in utero (tolerance = 2.57, p = .013) and by the percentage HOME score (tolerance = −2.23, p = .03). Thus the percentage of HOME score and exposure to nicotine in utero remained significant factors which were related to teacher-rated hyperactivity/impulsivity when other environmental factors and gender were adjusted for.
Post hoc multiple regression analysis was performed controlling for ODD and CD as well as environmental factors listed above. When ODD and CD were added to the model, a linear relationship remained, with oppositional, environmental and gender factors together describing 67% of the variance in teacher-rated hyperactivity/impulsivity (adjusted R squared = .67, F = 7.48, df = 12, p < .001). The relative contribution of the HOME became greater, (tolerance = −5.24, p < .001); the relative contribution of paternal socio-economic status became significant (tolerance = −2.5, p = .02) and the contribution of exposure to nicotine in utero was no longer significant.
Siblings
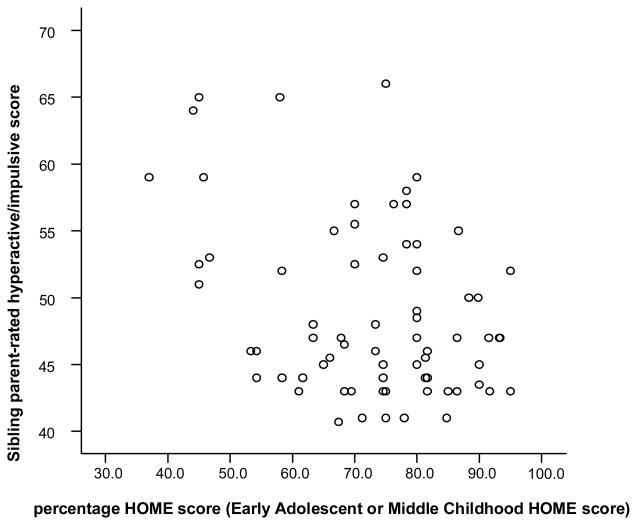
79 families had a non-ADHD sibling or siblings who contributed data to this analysis; 94 siblings were included in the analysis; 23 were excluded due to a diagnosis or suspected diagnosis of ADHD. Each family was represented only once: an average ADHD score for all non-ADHD siblings in the family was used. Parent-rated hyperactivity/impulsivity for siblings correlated with the HOME score (r = −.284, p = .011, N = 79) (see Figure 2). There was no correlation between HOME score and parent-rated inattentiveness or teacher-rated ADHD symptoms in siblings. HOME score correlated negatively with mean sibling oppositional scores rated by parents (r = −.220, p = .033), but not by teachers (r = −.191, p= 0.68).
Figure 2. Correlation of sibling parent-rated hyperactive/impulsive score and HOME score.
r = −.284, p = .011, N = 79
Multiple regression analysis was performed to assess if sibling parent-rated hyperactivity/impulsivity was predicted by measured environmental factors, including HOME score (see Table 3). The sibling group had a mean birth weight of 3.6 kg (SD = 0.5kg) and mean gestational age of 39.6 weeks (range 34 – 43 weeks). A large proportion did not have pregnancy risk factors (40.5% scored 0 vs 33% scored 1 vs 26.5% scored 2); the majority did not have perinatal risk factors (75% scored 0 vs 15% scored 1 vs 10% scored 2); the majority were not exposed to nicotine in utero (60.5% scored 0 vs 27.5% scored 1 vs 12% scored 2); the majority were not exposed to alcohol in utero (58% scored 0 vs 26.5% scored 1 vs 15.5% scored 2). The parental socio-economic groupings were the same as that of the probands.
Table 3.
Regression analysis: Environmental factors and parent-rated hyperactivity/impulsivity in non-ADHD siblings
| Unstandardized Coefficients | Standardized Coefficients | ||||
|---|---|---|---|---|---|
| B | Std. Error | Beta | t | p | |
| (Constant) | 60.321 | 30.126 | 2.002 | .052 | |
| HOME score | −.160 | .089 | −.288 | −1.799 | .080 |
| Birth weight | 1.999 | 2.648 | .118 | .755 | .455 |
| Gestational age | −.270 | .654 | −.063 | −.413 | .682 |
| Cigs exposure in utero | 1.934 | 1.865 | .185 | 1.037 | .306 |
| Alcohol exposure in utero | −.483 | 1.332 | −.054 | −.363 | .719 |
| Pregnancy risk factors | 1.498 | 1.434 | .179 | 1.045 | .303 |
| Perinatal risk factors | −1.348 | 1.672 | −.139 | −.806 | .425 |
| Father’s socioeconomic status | .739 | 1.108 | .118 | .666 | .509 |
| Mother’s socioeconomic status | .199 | .856 | .038 | .233 | .817 |
| gender | −.431 | 2.294 | −.032 | −.188 | .852 |
Regression equation: Parent-rated hyperactive/impulsive symptoms = 60.32 − .16(percentage HOME score) + 1.99(birth weight) − 0.27(gestational age) + 1.93(cig exposure) − .48(alcohol exposure)+ 1.50(preg risk factors) −1.35(perinatal risk factors) + 0.74(father’s socio) + .20(mother’s socio)−0.43(gender)
Note: gender was expressed as 1 = male and 2 = female
These environmental factors and gender in siblings predicted just 6.5% of the variance of parent-rated hyperactivity/impulsivity (R square = .256, adjusted R square = 0.065, N = 94), with residual factors more important than gender and the measured environmental factors. The model of gender and environmental factors did not predict parent-rated hyperactivity/impulsivity effectively (Regression: F = 1.339, df = 10, p = .245) and of the environmental factors measured, the closest to significance was the family HOME score (see Table 3).
Discussion
Mean HOME scores in this study (MC HOME mean = 43, SD = 8.0; EA HOME mean = 43, SD = 9.6) are similar to published means for USA samples (MC HOME mean = 42, Bradley et al., 1988; EA HOME mean = 51, Bradley et al., 2000) and a Scottish sample (MC HOME mean = 40; EA HOME mean = 38, Burston et al., 2005). A less supportive home environment significantly correlated with more teacher-rated hyperactivity/impulsivity and a greater risk of oppositional disorders in those with ADHD-CT, and was associated with more parent-rated hyperactivity/impulsivity and oppositionality in non-ADHD siblings. The association between HOME score and hyperactivity/impulsivity is in keeping with results of previous studies which have shown that family adversity is associated with ADHD (Biederman et al., 1995; Biederman et al., 2002; Counts et al., 2005). A previous longitudinal study of inattentive/hyperactive symptoms in children of alcoholic parents has shown that early home environment predicts later inattention/hyperactivity (Jester et al., 2005). In our study we separated symptoms of ADHD into symptoms of inattention and symptoms of hyperactivity/impulsivity, and found that home environment is associated with hyperactive/impulsive symptoms and not with symptoms of inattention. It is possible that our sample size was too small to identify an association with inattention, despite all probands having combined type ADHD, and hence symptoms of inattention and of hyperactivity/impulsivity. Alternatively the home environment (in particular physical environment and learning opportunities) may influence hyperactive-impulsive symptoms but not inattentive symptoms.
Our study also differs from previous studies on psychosocial adversity (Biederman et al., 1995; Jester et al., 2005) as we controlled for other environmental factors which could influence symptoms of ADHD. We showed that the association between home environment and hyperactivity/impulsivity in those with ADHD-CT was not due gender or to measured confounding environmental factors, nor due to comorbid oppositional disorders, using multiple regression analysis. In fact the contribution of HOME score to teacher-rated hyperactivity/impulsivity in those with ADHD-CT was greater when oppositional disorders were controlled for.
It is interesting that for children with ADHD-CT, teacher-rated hyperactivity/impulsivity correlated with HOME score while in non-ADHD siblings, parent-rated hyperactivity/impulsivity correlated with HOME score. The correlation with teacher-rated hyperactivity/impulsivity in ADHD-CT is likely to be a real finding as both the HOME and teacher-rated symptoms were measured objectively. Parents rated children with ADHD-CT as having more hyperactivity/impulsivity (mean = 83.12 +/− 8.30, range = 59–90) than did their teachers; (mean = 70.30 +/− 13.51, range = 43–90). The tighter range of parent-rated Conners’ scores makes it more difficult for any correlation with the HOME to be seen in the group with ADHD-CT – a bigger sample may show a correlation between HOME score and hyperactivity/impulsivity rated by parents as well as teachers.
In non-ADHD siblings there was a greater range in parent-rated hyperactivity/impulsivity (range = 41–66, mean = 48.56+/−6.28) than teacher-rated (range = 42–62, mean score = 49.03+/−5.69), and the HOME correlated with parent and not with teacher-rated Conners’ scores. Perhaps non-ADHD children can control any tendency towards hyperactivity/impulsivity in the classroom but allow their “true selves” to be seen at home, where their parents can identify some children with higher hyperactivity/impulsivity. If the study were repeated in a larger sample of children with ADHD-CT and their siblings, there may be a greater range of hyperactivity/impulsivity in those with ADHD-CT at home and a greater range of hyperactivity/impulsivity in those without ADHD in the classroom setting, and a correlation between hyperactivity/impulsivity and HOME may be seen in those with ADHD-CT and in their non-ADHD siblings at home and at school.
The association between home environment and hyperactive/impulsive symptoms is in keeping with our hypothesis that the quality of the home environment is associated with the risk of developing symptoms of ADHD, although we did not find an association with attentional symptoms. While these data do not prove a causal relationship, the study of children with ADHD and their siblings allows debate on the possibility of a causal relationship between home environment and hyperactive/impulsive symptoms. If ADHD symptoms in probands with ADHD-CT were causing a poor home environment, we would not expect an association between HOME score and hyperactive/impulsive symptoms in non-ADHD siblings. However if a less supportive home environment was partly causing ADHD in the child with ADHD-CT it could be expected that a sibling brought up in the same environment would have partial symptoms of ADHD. The affected proband may have a greater genetic predisposition to the disorder, and thus develop ADHD-CT while the sibling has just a few symptoms of the disorder.
The association between home environment and hyperactivity/impulsivity in probands may be gene-environment interaction effect. An interaction between polymorphisms of the DAT1 genotype and psychosocial adversity has been previously reported in ADHD (Laucht et al., 2007) and an interaction between severe early institutional deprivation and DAT1 genotype has been shown to influence symptoms of ADHD in children adopted from Romania (Stevens et al., 2009). It would be interesting to ascertain if there is a gene-environment interaction between the current home environment (HOME) and genes predisposing to ADHD, including DAT1.
Previous studies used Rutter’s psychosocial adversity index (Blanz et al., 1991; Biederman et al., 2002, Counts et al., 2005) with many measures of adversity at a societal level rather than family level. In contrast, the HOME places particular emphasis on how family life is adapted to the child’s developmental needs – features which a family could modify. If we can show a decrease in ADHD symptoms with an intervention to improve home environment then a causal relationship will be proven. Clinical services could then advise parents on how to provide a more supportive home environment.
Our multiple regression analysis suggests that 30% of the variance of hyperactivity/impulsivity in those with ADHD-CT is due to environmental and gender factors, with a mixture of shared and non-shared environmental factors. This is in keeping with the finding that ADHD is 76% heritable, with approximately 24% of the variance due to non-shared environmental effects (Faraone et al., 2005). The model of environmental factors was however a poor predictor of hyperactivity/impulsivity in siblings, and other factors may explain the variance of symptoms in non-ADHD siblings better than environmental factors.
The HOME subscale analysis highlighted the importance of a supportive learning environment and mirrors the finding that learning environment was consistently associated with early motor and social developmental (Bradley et al., 2001). Also in younger children with ADHD, a better physical environment and greater family integration factors were associated with less hyperactivity/impulsivity. Hence in families with more structure (where there was a child with ADHD-CT), symptoms of ADHD were less pronounced.
It is an advantage of this study that children with ADHD-CT and their non-ADHD siblings were studied as the association between hyperactivity/impulsivity and home environment could be analysed in those with and without the disorder. It is also a strength that recognised research tools and objective measures were used. It is a further strength that only one ethnic group was studied as mean HOME scores vary for different ethnic groups (Bradley et al., 1996; Bradley et al., 2001). There are also some limitations to this study. All data are correlational so we can only speculate on the direction of causation. The sample size was relatively small, although the sample size was sufficiently large to study nine independent environmental factors using multiple regression analysis. There was some inter-correlation of environmental variables; however all variables studied were previously listed as of known importance in ADHD. It is also a limitation that the groups were relatively small when the sample with ADHD was divided into those with and without oppositional defiant disorder and those with and without conduct disorder. However despite the size of the groups, a dose dependent relationship was found between HOME score and the presence of oppositional defiant disorder and conduct disorder, with a higher mean percentage HOME score (more supportive) in those with oppositional defiant disorder (70.48) than those with conduct disorder (63.30). These findings are in keeping with the study hypothesis and in keeping with the findings of previous studies on family adversity and oppositional disorders (Blanz et al., 1991, Counts et al., 2005). It is a further limitation that the HOME assessor was aware of the child’s clinical diagnosis of ADHD and of the research hypothesis (as she devised the study), although she was not aware if the research diagnosis of ADHD had been confirmed or not. It may have improved the study if the HOME was assessed separately for probands and for siblings, although it was noted that proband and sibling HOME scores were strongly correlated in the subset for whom this was performed. It should be noted that it is considered unlikely that children influence the quality of the home environment. Robert Bradley, co-author of the HOME, stated that “children whose characteristics are not extreme may have little impact on the type or quality of experience received. That is, they do not cause parents to reverse judgement regarding what the child needs” (Bradley, 1994). The high correlation of HOME scores in probands and siblings and the known information about home environment makes it unlikely that the diagnosis of ADHD in the proband could influence the results of the HOME and ADHD symptoms analysis for siblings.
In conclusion a more supportive home environment was associated with less hyperactivity/impulsivity in children with ADHD-CT and their non-ADHD siblings. A more supportive home environment was also associated with lower rates of ODD and CD in those with ADHD-CT, and with lower parent-rated oppositional scores in non-ADHD siblings. In those with ADHD-CT, environmental factors and gender accounted for 30% of the variance of hyperactivity/impulsivity, and the association between home environment and hyperactivity/impulsivity remained even when gender and other environmental factors were taken into account. However in siblings an environmental factors and gender model did not fit the data well, and the model of environmental factors and gender did not explain the variance of symptoms of ADHD. A gene-environment interaction between home environment and genes associated with ADHD could explain the difference between proband and sibling hyperactivity/impulsivity for siblings brought up in the same home environment.
Key Messages.
A supportive home environment is associated with less hyperactive/impulsive symptoms in children with ADHD and their siblings.
A supportive home environment is associated with less oppositional symptoms in children with ADHD and their siblings.
Acknowledgments
We would like to thank the families who participated in this study, especially the children. We would also like to thank child psychiatrists of the South Western area of the Eastern Regional, Midlands, North Eastern, South Eastern Area, and Mid Western Areas of the Health Services Executive and to thank the Hyperactive and Attention Deficit Disorder family support group (HADD). We would like to acknowledge the IMAGE Consortium, a multi-site, international effort supported by NIMH grant R01MH62873 to S. Faraone.
Contributor Information
Aisling Mulligan, Email: aisling.mulligan@ucd.ie.
Richard Anney, Email: anneyr@tcd.ie.
Louise Butler, Email: butlerlm@tcd.ie.
Myra O’Regan, Email: moregan@tcd.ie.
Thomas Richardson, Email: thomashughrichardson@googlemail.com.
Edyta Maria Tulewicz, Email: sikstus@yahoo.com.
Michael Fitzgerald, Email: fitzi@iol.ie.
Michael Gill, Email: mgill@tcd.ie.
References
- Banaschewski T, Becker K, Scherag S, Franke B, Coghill D. Molecular genetics of attention-deficit/hyperactivity disorder: an overview. European Child & Adolescent Psychiatry. 2010;19:237–57. doi: 10.1007/s00787-010-0090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: diagnostic validity. British Journal of Psychiatry. 1999;175:444–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- Beverly BL, McGuinness TM, Blanton DJ. Communication and academic challenges in early adolescence for children who have been adopted from the former Soviet Union. Language, Speech, and Hearing Services in Schools. 2008;39:303–13. doi: 10.1044/0161-1461(2008/029). [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV, Monuteaux MC. Differential effect of environmental adversity by gender: Rutter’s Index of Adversity in a Group of Boys and Girls with and without ADHD. American Journal of Psychiatry. 2002;159:1556–1562. doi: 10.1176/appi.ajp.159.9.1556. [DOI] [PubMed] [Google Scholar]
- Biederman J, Milberger S, Faraone SV, Kiely K, Guite J, Mick E, Ablon JS, Warburton R, Reed E, Davis SG. Impact of adversity on functioning and comorbidity in children with attention-deficit hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 1995;34:1495–503. doi: 10.1097/00004583-199511000-00017. [DOI] [PubMed] [Google Scholar]
- Blanz B, Schmidt MH, Esser G. Familial adversities and child psychiatric disorders. Journal of Child Psychology and Psychiatry. 1991;32:939–950. doi: 10.1111/j.1469-7610.1991.tb01921.x. [DOI] [PubMed] [Google Scholar]
- Bradley R, Caldwell B. Home observation for measurement of the environment: a validation study of screening efficiency. American Journal of Mental Deficiency. 1977;81:417–20. [PubMed] [Google Scholar]
- Bradley RH, Caldwell BM, Rock SL, Hamrick HM, Harris P. Home Observation for Measurement of the Environment: Development of a Home Inventory for use with families having children 6 to 10 years old. Contemporary Educational Psychology. 1988;13:58–71. [Google Scholar]
- Bradley RH. Home Inventory: Review and Reflections. In: Reese HW, editor. Advances in Child Development and Behavior. Vol. 25. Academic Press, Inc; San Diego, USA: 1994. pp. 256–259. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF, Whiteside-Mansell L. Life at Home: Same Time, Different Places -- An Examination of the HOME Inventory in different cultures. Early Development and Parenting. 1996;5:251–269. [Google Scholar]
- Bradley RH, Corwyn RF, Burchinal M, McAdoo HP, Coll CG. The home environments of children in the United States Part II: relations with behavioral development through age thirteen. Child Development. 2001;72:1868–1886. doi: 10.1111/1467-8624.t01-1-00383. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF, Caldwell BM, White-Mansell L, Wasserman GA, Mink IT. Measuring the home environment of children in early adolescence. Journal of Research on Adolescence. 2000;10:247–288. [Google Scholar]
- Bradley RH, Corwyn RF, McAdoo HP, Coll CG. The home environments of children in the United States Part I: Variations by age, ethnicity, and poverty status. Child Development. 2001;72:1844–1867. doi: 10.1111/1467-8624.t01-1-00382. [DOI] [PubMed] [Google Scholar]
- Brookes K, Xu X, Chen W, Zhou K, Neale B, Lowe N, Anney R, Franke B, Gill M, Ebstein R, Buitelaar J, Sham P, Cambell D, Knight J, Androu P, Altink M, Arnold R, Boer F, Buschgens C, Butler L, Christiansen H, Feldman L, Fleischman K, Fliers E, Howe-Forbes R, Goldfarb A, Heise A, Gabriels I, Korn-Lubetzki I, Marco R, Medad S, Minderaa R, Mulas F, Muller U, Mulligan A, Rabin K, Rommelse N, Sethna V, Sorohan J, Uebel H, Psychogiou L, Weeks A, Barrett R, Craig I, Banascheweski T, Sonuga-Barke E, Eisenberg J, Kuntsi J, Manor I, McGuffin P, Miranda A, Oades RD, Plomin R, Roeyers H, Rothenberger A, Sergeant J, Steinhausen HC, Taylor E, Thompson M, Faraone SV, Asherson P. The analysis of 51 genes in DSM-IV combined type attention deficit hyperactivity disorder: association signals in DRD4, DAT1 and 16 other genes. Molecular Psychiatry. 2006;11:934–953. doi: 10.1038/sj.mp.4001869. [DOI] [PubMed] [Google Scholar]
- Burston A, Puckering C, Kearney E. At HOME in Scotland: validation of the home observation for measurement for the environment inventory. Child: Care, Health & Development. 2005;31:533–538. doi: 10.1111/j.1365-2214.2005.00546.x. [DOI] [PubMed] [Google Scholar]
- Caldwell B, Bradley RH. Home inventory and Administration manual. University of Arkansas for Medical Sciences and University of Arkansas at Little Rock; Little Rock, AR, USA: 2001. [Google Scholar]
- Casey PH, Bradley RH. The impact of the home environment on children’s development: clinical relevance for the pediatrician. Journal of Developmental & Behavioral Pediatrics. 1982;3:146–152. doi: 10.1097/00004703-198209000-00005. [DOI] [PubMed] [Google Scholar]
- Chen W, Taylor EA. Parental account of children’s symptoms (PACS), ADHD phenotypes and its application to molecular genetic studies. In: Oades R, editor. Attention-deficit/hyperactivity disorder and the hyperkinetic syndrome: Current ideas and ways forward. Nova Science Publishing Inc; Hauppauge, NY, USA: 2006. pp. 3–20. [Google Scholar]
- Conners CK. Conners’ Rating Scales- Revised (CRS-R) Technical Manual. Multi-Health Systems Inc; Toronto, Canada: 2001. [Google Scholar]
- Counts CA, Nigg JT, Stawicki JA, Rappley MD, Von Eye A. Family adversity in DSM-IV ADHD combined and inattentive subtypes and associated disruptive behavior problems. Journal of the American Academy of Child & Adolescent Psychiatry. 2005;44:690–698. doi: 10.1097/01.chi.0000162582.87710.66. [DOI] [PubMed] [Google Scholar]
- Curran S, Newman S, Taylor E, Asherson P. Hypescheme: an operational criteria checklist and minimum data set for molecular genetic studies of attention deficit and hyperactivity disorders. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2000;96B:244–250. doi: 10.1002/1096-8628(20000612)96:3<244::aid-ajmg2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren M, Sklar P. Advancing the neuroscience of ADHD – molecular genetics of Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Franke B, Neale BM, Faraone SV. Genome-wide association studies in ADHD. Human Genetics. 2009;126:13–50. doi: 10.1007/s00439-009-0663-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick PJ, Lahey BB, Christ MAG, Loeber R, Green S. History of childhood behavior problems in biological relatives of boys with Attention-Deficit Hyperactivity Disorder and Conduct Disorder. Journal of Clinical Child Psychology. 1991;20:445–451. [Google Scholar]
- Goodman R. The Strengths and Difficulties Questionnaire: A Research Note. Journal of Child Psychology and Psychiatry. 1997;38:581–586. doi: 10.1111/j.1469-7610.1997.tb01545.x. [DOI] [PubMed] [Google Scholar]
- Jester JM, Nigg JT, Adams K, Fitzgerald HE, Puttler LI, Wong MM, Zucker RA. Inattention/hyperactivity and aggression from early childhood to adolescence: Heterogeneity of trajectories and differential influence of family environment characteristics. Development and Psychopathology. 2005;17:99–125. doi: 10.1017/50954579405050066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juffer F, van Ijzendoorn MH. Behaviour problems and mental health referrals of international adoptees, a meta-analysis. Journal of the American Medical Association. 2005;293:2501–2515. doi: 10.1001/jama.293.20.2501. [DOI] [PubMed] [Google Scholar]
- Keyes MA, Sharma A, Elkins IJ, Iacono WG, McGue M. The Mental Health of US adolescents adopted in infancy. Archives of Pediatrics & Adolescent Medicine. 2008;162:419–425. doi: 10.1001/archpedi.162.5.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreppner JM, O’Connor TG, Rutter M and the English and Romanian Adoptees Study Team . Can Inattention/Overactivity Be An institutional deprivation syndrome? Journal of Abnormal Child Psychology. 2001;29:513–528. doi: 10.1023/a:1012229209190. [DOI] [PubMed] [Google Scholar]
- Laucht M, Skowronek MH, Becker K, Schmidt MH, Esser G, Schulze TG, Rietschel M. Interacting effects of the dopamine transporter gene and psychosocial adversity on attention-deficit/hyperactivity disorder symptoms among 15-year-olds from a high-risk community sample. Archives of General Psychiatry. 2007;64:585–590. doi: 10.1001/archpsyc.64.5.585. [DOI] [PubMed] [Google Scholar]
- Levy F, Hay D, McStephen M, Wood C, Waldman I. Attention-deficit hyperactivity disorder: A category or a continuum? Genetic analysis of a large-scale twin study. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:734–744. doi: 10.1097/00004583-199706000-00009. [DOI] [PubMed] [Google Scholar]
- Rutter M. Why are London Children so Disturbed? Proceeding of the Royal Society of Medicine, 1973, 66 1221–1225. In: Taylor E, Green J, editors. Research and innovation on the road to modern child psychiatry, Classic papers by Professor Michael Rutter. The Royal College of Psychiatrists; UK: 2001. [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. The Social Communication Questionnaire Manual. Western Psychological Services; Los Angeles, USA: 2003. [Google Scholar]
- Sagvolden T, Johansen EB, Aase H, Russell VA. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behavioral and Brain Sciences. 2005;28:397–468. doi: 10.1017/S0140525X05000075. [DOI] [PubMed] [Google Scholar]
- Sattler JM. Assessment of children: WISC-III and WPPSI-R supplement. San Diego: CA: 1992. [Google Scholar]
- Schachar R, Wachsmuth R. Hyperactivity and parental psychopathology. Journal of Child Psychology and Psychiatry. 1990;31:381–392. doi: 10.1111/j.1469-7610.1990.tb01576.x. [DOI] [PubMed] [Google Scholar]
- Sprich S, Biederman J, Crawford MH, Mundy E, Faraone SV. Adoptive and biological families of children and adolescents with ADHD. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39:1432–1437. doi: 10.1097/00004583-200011000-00018. [DOI] [PubMed] [Google Scholar]
- Stevens SE, Kumsta R, Kreppner JM, Brookes KJ, Rutter M, Sonuga-Barke EJS. Dopamine transporter gene polymorphism moderates the effects of severe deprivation on ADHD symptoms: developmental continuities in gene–environment interplay. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2009;150B:753–761. doi: 10.1002/ajmg.b.31010. [DOI] [PubMed] [Google Scholar]
- Totsika V, Sylva K. The Home Observation for Measurement of the Environment Revisited. Child and Adolescent Mental Health. 2004;9:25–35. doi: 10.1046/j.1475-357X.2003.00073.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Examiner’s Manual: Wechsler Intelligence Scale for Children. 3. Psychological Corporation; New York, USA: 1991. [Google Scholar]