Abstract
Sleep benefits memory across a range of tasks for young adults. However, remarkably little is known of the role of sleep on memory for healthy older adults. We used two tasks, one assaying motor skill learning and the other assaying non-motor/declarative learning, to examine off-line changes in performance in young (20–34 yrs), middle-aged (35–50 yrs), and older (51–70 yrs) adults without disordered sleep. During an initial session, conducted either in the morning or evening, participants learned a motor sequence and a list of word pairs. Memory tests were given twice, 12 and 24 hours after training, allowing us to analyze off-line consolidation after a break that included sleep or normal wake. Sleep dependent performance changes were reduced in older adults on the motor sequence learning task. In contrast, sleep dependent performance changes were similar for all three age groups on the word pair learning task. Age-related changes in sleep or networks activated during encoding or during sleep may contribute to age-related declines in motor sequence consolidation. Interestingly, these changes do not affect declarative memory.
Keywords: Sleep, memory, declarative, procedural, aging, consolidation
1. Introduction
Memories benefit from sleep (e.g., Peigneux et al. 2001; Stickgold & Walker 2005). While this effect has been extensively documented across a wide range of task domains, this performance enhancement is diminished in older adults: Off-line changes in performance on a motor sequence learning task did not differ for intervals of wake and sleep for adults 45–80 yrs of age (Spencer et al. 2007). Likewise, Siengsukon and Boyd (2008; Siengsukon & Boyd 2009) found that healthy older adults show no sleep-related changes in motor sequence learning regardless of the participants’ level of awareness.
Whether older adults show sleep dependent gains in other task domains remains unclear. Aly and Moscovitch (2010) recently compared episodic recall of personal events and experimental narratives following an interval of overnight sleep and daytime waking. While older adults (69–80 yrs) exhibited greater forgetting over both intervals relative to young adults, the protection of memory by sleep, relative to wake, did not differ across the age groups. However, Backhaus and colleagues (2007) suggested that sleep-dependent consolidation (SDC) of declarative memories is reduced in middle-aged adults (48–55 yrs). In an associated word-pair learning task, young adults had greater recall following early-night (SWS-rich) sleep than late-night (REM-rich) sleep. Middle-aged adults showed no significant change in performance following either sleep epoch. Interestingly, sleep was beneficial for middle-aged adults that garnered the same amount of SWS as the young adults. Thus, changes in SDC with aging may be related to changes in sleep physiology.
In the present study, we directly compared off-line performance changes in young, middle-aged, and older adults. We adopted this design to examine two issues. First, to date, studies of age-related changes in SDC have been contradictory: For example, using tasks associated with declarative learning, one study reported spared SDC in older adults (Aly & Moscovitch 2010), whereas another reported that some middle-aged adults exhibited reduced SDC (Backhaus et al. 2007). A limitation with this work is that these studies used binary contrasts, comparing younger adults to either middle-aged or older adults. By testing young, middle-aged, and older adults on the same set of tasks, we will better characterize age-related changes associated with sleep-dependent consolidation.
Second, we sought to directly compare SDC for two tasks across these age groups. Research on SDC in healthy young adults has favored a distinction in the processes underlying SDC for motor and sensorimotor tasks, tasks that are historically classified as procedural learning tasks, relative to processes underly SDC for non-motor, declarative learning tasks (e.g., Diekelmann et al. 2009; Payne 2010; Plihal & Born 1997; Stickgold et al. 2001; Wilhelm et al. 2008). The two tasks adopted here, a motor sequence learning task and a word pair learning task, were chosen to represent this distinction from the SDC literature. Notably, these tasks are both expected to engage the hippocampus at encoding (Keele et al. 2003; Schendan et al. 2003), a feature hypothesized to characterize tasks which are benefitted by sleep in young adults (Cai et al. 2009; Rauchs et al. 2011; Spencer et al. 2006). The motor sequence learning task, and the serial reaction time variant in particular, was specifically selected to allow us to replicate the previously observed decline in SDC in healthy older adults (Spencer et al. 2007) given that not all forms of motor sequence learning are subject to such benefits even in young adults (Nemeth et al. 2010; Robertson et al. 2004; Spencer et al. 2006). The word-pair learning task was selected given that it has been well established to benefit from sleep in young adults (e.g., Ellenbogen et al. 2006; Plihal & Born 1997; Tucker & Fishbein 2008).
One proposed distinction between SDC for motor learning and SDC for classic declarative learning tasks is their unique reliance in regards to physiological measures of sleep. SDC for word-pair learning and other episodic memory tasks has been associated with slow wave sleep (SWS; Plihal & Born 1997; Tucker et al. 2006) which dramatically drops from young to older adulthood (Danker-Hopfe et al. 2005; Ohayon et al. 2004). SDC for motor sequence learning is associated with nREM2 sleep (Walker et al. 2002) which is largely protected through the middle-age period (Danker-Hopfe et al. 2005; Ohayon et al. 2004). Based on these distinctions, we predicted a difference in the influence of age on SDC for these two tasks.
2. Methods
2.1 Participants
Eighty-seven participants, 20–70 yrs of age, were tested. Participants were divided into three age groups: Young adults (20–34 yrs; n=24), middle-aged adults (35–50 yrs; n=32), and older adults (51–70 yrs; n=31). Gender was approximately evenly distributed across all age groups (Young: 10 female/14 male; Middle-aged: 14 female/18 male; Older: 13 female/18 male). Data from an additional 6 participants (Young = 2; Middle-aged = 1; Older = 3) were excluded for self-reported mid-day naps greater than 0.5 hrs in duration. Based on a questionnaire given at the time of recruitment, none of the participants reported sleep disorders, sleep-affecting medications, uncorrected vision problems, or a history of neurological disorders or impairment. The groups did not differ reliably in terms of education (F(1,85)=2.6; p=.11), although the mean for the Young group (14.6 years) was lower than for two older groups (Middle: 17.2; Older: 16.5) due to the fact that many in the Young group were still in college. The sample size even within each age group exceeds that used in previous studies of SDC that have used a between-subject design (Ellenbogen et al. 2006; Plihal & Born 1997; Spencer et al. 2007), and thus, should provide sufficient power to detect group differences. All procedures were approved by the institutional review board at University of California, Berkeley and University of Massachusetts, Amherst.
2.2 Sequence-learning task
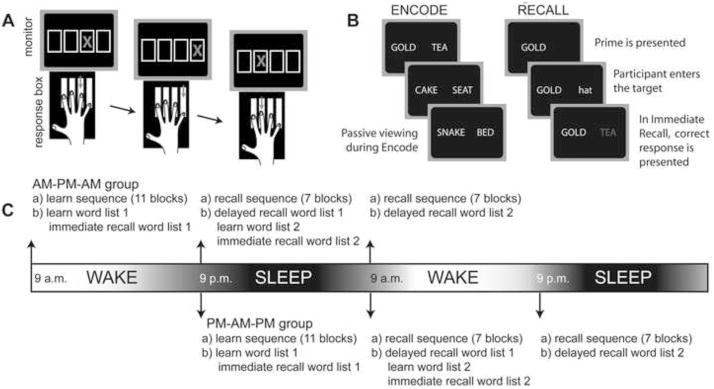
The sequence-learning task was a modified serial reaction time task (Nissen & Bullemer 1987) identical to that used in our previous work demonstrating an age-related decline in SDC (Spencer et al. 2007; Spencer et al. 2006). In this task, participants were instructed to press one of four response keys based on the spatial position of a visual stimulus presented on a computer monitor (Figure 1A). A horizontal row of four boxes was displayed in the center of the screen at all times. A trial was cued with the presentation of an ‘X’ in one of the four boxes. Participants were instructed to press the key corresponding to the spatial location of the stimulus. Responses were made with the four fingers (thumb excluded) of the non-dominant hand. Immediately following a response, the cue disappeared and, following a 100-ms inter-trial interval, the next cue appeared. Blocks were composed of 120 trials. At the end of each block, feedback indicating the mean reaction time (RT) and number of errors for that block was presented.
Figure 1.
Task and procedures. A) In the motor sequence-learning task, participants pressed a key in response to the position of each stimulus. B) During the encode phase of the word-pair learning task, participants passively viewed the word pairs. During the immediate and delayed recall phases, participants were given a cue and were to respond with the corresponding target word. Feedback was provided in the immediate recall phase. C) Timeline for the two subgroups within each age group (the order of the sequence and word pair tasks was randomized).
On “sequence blocks”, the cues were presented in a repeating 10-item sequence. The grammar of the sequence was the same for all participants, but the mapping between sequence element and stimulus/response location was randomly assigned across participants. Thus, for one participant, the sequence was 4-3-1-4-2-1-3-4-1-2 whereas for another it would be 1-2-4-1-3-4-2-1-4-3. To probe sequence learning, the cues were selected at random on some blocks (see Procedure). In these “random blocks”, we imposed three constraints that were also present in the sequence blocks: The frequency of each cue location was matched to that in sequence blocks, the cue could not appear in the same location on successive trials, and 3-element trills (e.g., 1-3-1 or 2-4-2) were excluded.
Participants were told that the cues would follow a sequence on most blocks (explicit; Spencer et al. 2006). The instructions emphasized that speed was the primary measure of interest and that by learning the sequence, they would be able to respond faster while maintaining good, but not necessarily perfect, accuracy.
2.3 Word-pair learning task
Following Ellenbogen et al. (2009), we used a word-pair learning task in which the word-pairs were semantically unrelated. For each participant, 128 words were randomly selected from a list of 168 single-syllable, high-frequency, concrete nouns (see Donohue & Spencer 2011). Words were randomly paired to create two lists of 32 word pairs (e.g., CAT-COACH, DESK-ICE). One list was used for the wake condition and the other for the sleep condition (see below). There were three phases to the task: encode, immediate recall and delayed recall (Figure 1B). During the encode phase, word pairs from a single list were presented for 5 s on a computer monitor in front of the participant (inter-stimulus interval between pairs was 100 ms). Participants were instructed to study each pair of words for subsequent recall. To facilitate learning, participants were instructed to use a mnemonic strategy. Specifically, they were told: “To help you remember the pairs, it is helpful to think of associations between the pairs. For instance, if the words were FRAME-SHOE you might try to picture in your mind a framed painting of a shoe.”
The immediate recall phase followed the completion of the encode phase. The first word from twenty-eight of the word pairs (eliminating the first and last two pairs for primacy and recency effects as in: Plihal & Born 1997) was displayed on the left side of the screen and participants were instructed to say the corresponding word for that pair. Participants were asked to guess if they did not know the answer however a response of “I don’t know” was also accepted. The experimenter entered the participant’s response into the computer. If the response was incorrect, the correct response was displayed on the computer monitor for 750 ms. The list was repeated until performance reached 62% or when the list had been repeated a maximum of five times. The order of items was randomized for each presentation of the list.
The delayed recall phase was administered after a 12-hr break. This test was identical to the immediate recall phase with two exceptions. First, the list of words was presented only once. Second, feedback was not presented following an incorrect response. The order of items was again randomized for each presentation of the list.
2.4 Procedure
Participants in each age group were divided into two subgroups (see Table 1). The AM-PM-AM group started Session 1 in the morning (between 7–10 a.m.) and learning was assessed that evening (Session 2: 12 hrs after Session 1) and on the following morning (Session 3: 24 hrs after Session 1; Figure 1C). The PM-AM-PM group started Session 1 in the evening (between 7–10 p.m.) and Sessions 2 and 3 took place the next day (12 and 24 hrs after Session 1, respectively).
Table 1.
Demographic and neuropsychological means for each group. Numbers in parentheses are standard deviations. WAIS III Scores are scaled sub-scores.
| Session order | Mean age | Dominant hand | WAIS III Scores | WMS | |||
|---|---|---|---|---|---|---|---|
| WM Index | Arithmetic | Digit Span | Letter-# Sequence | Spatial Span | |||
|
Young adults
| |||||||
| APA | 26.1 (4.9) | 12 R, 1L | 107.6 (17.8) | 11.9 (3.7) | 11.0 (3.4) | 11.4 (2.5) | 11.1 (3.0) |
| PAP | 25.7 (4.0) | 11 R | 116.9 (16.8) | 12.4 (2.3) | 11.4 (4.3) | 13.9 (2.2) | 12.6 (2.9) |
|
| |||||||
|
Middle-aged adults
| |||||||
| APA | 44.3 (6.8) | 13 R, 1 L | 100.7 (14.6) | 10.0 (3.2) | 9.3 (2.9) | 11.0 (3.7) | 11.5 (3.4) |
| PAP | 43.7 (5.0) | 17 R, 1 L | 117.3 (11.2) | 12.3 (2.3) | 12.4 (3.7) | 14.0 (2.9) | 11.8 (2.1) |
|
| |||||||
|
Older-age adults
| |||||||
| APA | 63.5 (6.2) | 14 R, 1 L | 107.7 (11.5) | 11.0 (3.6) | 11.6 (3.3) | 11.6 (1.6) | 11.6 (2.8) |
| PAP | 62.8 (5.5) | 15 R, 1 A | 115.3 (7.0) | 11.9 (1.3) | 13.6 (2.9) | 13.0 (1.2) | 10.3 (2.5) |
Dominant hand R = right; L = left; A = ambidextrous
During training (Session 1), participants completed 11 blocks of the sequence-learning task (120 responses/block) with the stimuli following the 10-element sequence on blocks 1–7, 9, and 11. The stimuli were selected at random on blocks 8 and 10. Participants also completed the encode and immediate recall phases of the word-pair task using the first of two generated word-pair lists. In Session 2, participants completed 7 blocks of the sequence-learning task (with the stimuli selected at random on block 6, only) and the delayed recall phase for the word-pair list learned in Session 1. Following a short delay (approximately 20 min in which sleep assessments and other forms were completed), participants completed the encode and immediate recall phases of the word-pair task with using the second word list. In Session 3, participants again performed 7 blocks of the sequence-learning task (again only block 6 being random). For the word-pair task, participants completed the delayed recall phase with the word pairs that had been learned in Session 2. The order of the tasks was counterbalanced across participants, but within subjects, task order was maintained across the three sessions.
At the end of Session 3, participants were given a survey to assess their explicit knowledge of the sequence and identify strategies used for the word-pair task (i.e., did they imagine an integration of the objects as instructed). They also completed a neuropsychological battery composed of the arithmetic, digit span, and letter-number sequencing subtests from the Wechsler Adult Intelligence Scale (WAIS-III), the spatial-span subtest of the Wechsler Memory Scale (WMS-III), and the Mini-Mental Status Exam (MMSE). In a limited number of participants (n = 8), this battery was not performed due to time constraints; for these participants, only the MMSE was administered.
To assess subjective sleep quality and quantity, an abbreviated Wake-time Diary (Smith et al. 2003) was given following the morning session of the second day (Session 2 for the PM-AM-PM group and Session 3 for the AM-PM-AM group). To assess daytime activities including napping and caffeine intake, an abbreviated Bedtime Diary (Smith et al. 2003) was given in the evening session. Diaries have been shown to have high levels of agreement with polysomnographic measures of sleep duration and overall quality (e.g., Rogers et al. 1993). Participants also completed the Pittsburgh Sleep Quality Index (PSQI) which provides a subjective estimate of sleep over the past 30 days (Buysse et al. 1989).
2.5 Analysis
The median reaction time (RT) was computed for each block on the sequence learning task, following the standard convention (e.g., Gomez-Beldarrain et al. 1998; Nissen & Bullemer 1987; Shin & Ivry 2003; Willingham et al. 1989). Given our interest in off-line effects, we compared performance between the end of session x and the beginning of session x+1. Thus, our dependent variable is the change in reaction time for sequence blocks across sessions. For the first inter-session interval, this difference is the average of the median RTs for blocks 9 and 11 from session 1 minus the average of the median RTs for blocks 1 and 2 from session 2. For the second inter-session interval, this difference reflects the average of the median RTs for blocks 5 and 7 in session 2 minus the average of the median RTs for blocks 1 and 2 from session 3. To account for differences in RT across age groups, the off-line changes in performance were normalized to the participant’s median RT (for further discussion, see: Spencer et al. 2007; Spencer et al. 2006; Walker et al. 2002). Thus, we computed a Difference Value (DV):
where seqRT is the median RT for the specified sequence blocks from a particular phase.
The primary statistical analyses were based on analysis of variance (ANOVA) with the between-subjects factors Age Group (Young, Middle, Old) and Session Order (PM-AM-PM, AM-PM-AM) and the within-subject factor of Interval Type (Sleep, Wake).
3. Results
3.1 Sleep assessments
We estimated the amount of sleep in the overnight interval from the subjective reports. For the Young, Middle-aged, and Older participants, the mean values were 6.9 hrs (sd = 1.0 hrs), 6.8 hrs (sd = 1.4 hrs), and 6.6 hrs (sd = 1.3 hrs), respectively. While the means suggest a slight decline in sleep duration, the main effect of Age Group was not significant (F(2,81)=.33, p=.72). Similarly, the effect of Session Order was not significant (F(1,81)=.01, p=.93) nor was the Age Group by Session Order interaction (F(2,81)=.78, p=.46). As assessed by sleep diaries, the average reported number of nighttime awakenings was .41 (sd=.62). This measure did not differ across the age groups (F(2,81)=1.6, p=.21). Other measures of sleep quality (subjective sleep quality from PSQI and sleep diaries) also did not differ across groups (all F’s <1).
3.2 Neuropsychological evaluations
All participants scored in the normal range on the MMSE, measures of working memory (WM Index, Digit-Span, Letter-Number Sequencing, Spatial Span), and measures of attention/concentration (Arithmetic, Digit-Span, Letter-Number Sequencing). Individuals who did not perform the full neuropsychological battery all scored 29 or higher on the MMSE. Importantly, there were no significant differences between age groups on any of the neuropsychological measures (all F<1; Table 1).
3.3 Sequence-learning task
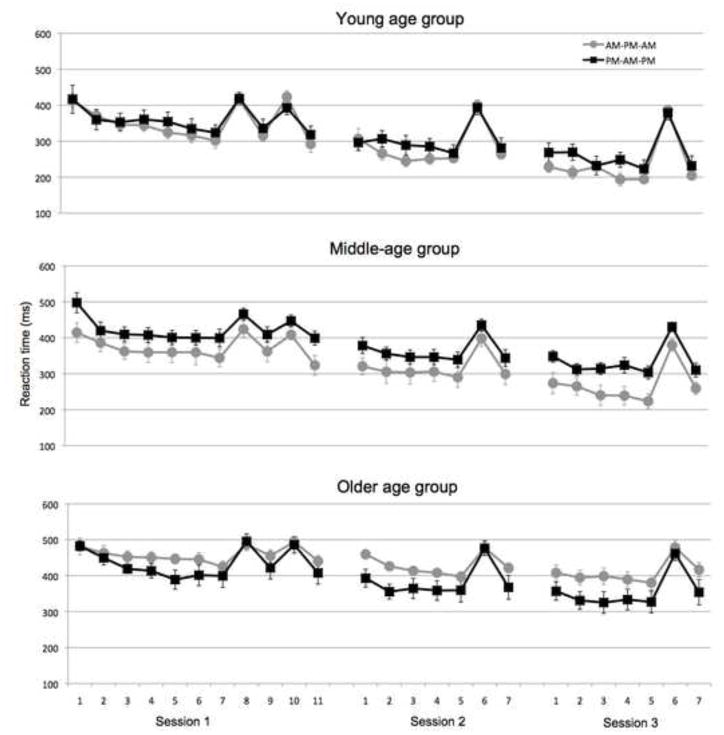
As is typical for a serial reaction time task, errors were low (mean error rate = 5.8%) and did not differ by Age Group (F(2,81)=.19, p=.83). Therefore, subsequent analyses are restricted to the RT-based measures. Learning curves for all groups are presented in Figure 2.
Figure 2.
Median reaction times across blocks for the sequence-learning task. Error bars represent standard error.
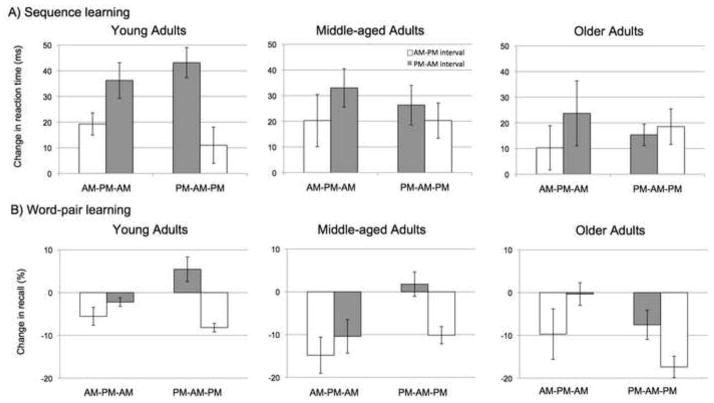
We first compared the change in RT across sessions separated by intervals with sleep compared to the change over intervals spent fully awake. The main effects of Age (F(2,81) = 1.6, p=.21) and Session Order (F(1,81)=.77, p=.38) were not significant. The main effect of Interval Type neared significance (F(1,81)=3.7, p=.056). Importantly, this latter effect differed across the age groups as indicated by a significant Age and Interval Type interaction (F(2,81)=3.1, p=.048; Figure 3).
Figure 3.
Intersession performance changes for A) motor-sequence learning and B) word-pair learning. Error bars represent standard error.
Given that RTs differed across age groups (main effect of Age: F(2,81)=12.2, p<.001; Figure 2), we also compared a normalized measure of off-line changes in RT (see Methods) as used previously (Spencer et al., 2007; Walker et al., 2002). Based on the DV, significant main effects were observed for the factors Age Group (F(2,81)=4.1, p=.02) and Interval Type (F(1,81)=9.60, p=.003). The main effect of Session Order (F(1,81)=.11, p=.75) and interactions of Session Order with Interval Type (F(1,81)=.57, p=.45) and Age (F(2,81)=2.2, p=.11) were not significant. Importantly, however, the interaction of Age Group and Interval Type was again significant (F(2,81)=4.0, p=.02). To explore this interaction, post-hoc comparisons were performed on the DV scores for each of the three groups. For this analysis, a two-tailed paired t-test was used, comparing the size of the DV after sleep (PM to AM) with the size of the DV after a wake interval (AM to PM). The Young age group showed a clear benefit of sleep (t(46)=−3.3, p=.002). However, performance changes over the sleep and wake intervals did not differ for the Middle-aged (t(62)=−1.1, p=.27) and Older (t(60)=− 49, p=.63) groups (Figure 4).
Figure 4.
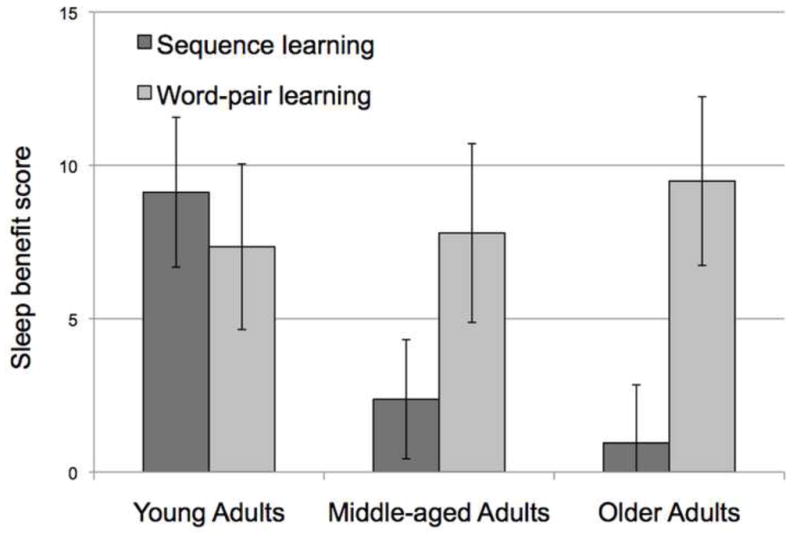
Sleep benefit scores for each task (see text). Error bars represent standard error.
We considered whether diminished sleep-dependent memory consolidation in the Middle-Aged and Older groups might be the result of attenuated sequence learning. That is, if participants fail to learn the sequence initially, there may be less information to consolidate, even given our normalization procedure. To examine this issue, we computed a learning score for session 1. Motor sequence learning is measured as the slowing exhibited on the random probe blocks (blocks 8 and 10) relative to the surrounding sequence blocks (blocks 7, 9, and 11). All three groups exhibited robust sequence learning, with means of 72, 60, and 54 ms for the Young, Middle-aged, and Older groups, respectively. The main effect of Age was not reliable (F(2,81)=2.3, p=.11). The main effect of Session Order was marginally significant (F(1,81)=3.5, p=.07), with slightly larger learning scores when session 1 was in the morning. The interaction of Session Order and Age was not significant (F(1,81)=1.0, p=.36). To account for the differences in RT across age groups, we also examined normalized learning scores. The learning score was normalized by dividing by the participants’ mean sequence reaction time in session 1 (mean of blocks 7, 9, and 11). A similar pattern was observed based on this normalized learning score (main effect of Age: F(2,81)=2.34, p=.103; Order: F(1,81)=2.2, p=.14). However, given this near-significant effect of age, we also examined whether the sleep benefit is a function of initial learning by examining the correlation between the learning score and the sleep benefit. To do this, we computed a Sleep Benefit Score, defined as the intersession performance change over sleep minus the intersession performance change over wake:
The correlation between the Sleep Benefit Score and the normalized learning score was not significant (r=.14, p=.20).
We examined whether the age-related decline in SDC might not be specific to sequence learning per se, but rather reflect a more general difference in performance. To address this, changes in performance on the random blocks across sessions were examined. Neither main effects of Interval Type (F(1,168)=2.3, p=.14) nor Age Group (F(2,168)=.65, p=.52) were observed. In addition, the interaction of Age and Interval Type did not approach significance (F(2,168)=.91, p=.41). In sum, the diminished SDC in our two older groups does not appear to reflect a general problem in sequence learning, nor a general absence of change across 12-hour breaks. Rather, the effect of age was limited to SDC for sequence learning.
Finally, given that age can be considered a continuous variable, we used multiple regression with age and total sleep time as independent predictor variables. The dependent variable was the Sleep Benefit Score defined above. The adjusted R2 was low (.03) and the model neared significance (F(2,84) = 2.4, p=.09). Total sleep time was not a significant predictor in this model (b=.007, t(85)=.68, p=.49). However the negative effect of age on the Sleep Benefit Score was marginally significant (b=−.002, t(85)=−1.9, p=.063). This result is consistent with the conclusion that SDC was diminished with increasing age as reported previously (Spencer et al. 2007).
3.4 Word-pair learning
For the word-pair learning task, performance in the delayed recall phase was generally diminished relative to the corresponding immediate recall phase. This pattern indicates that there is some forgetting over the 12-hour breaks, a process that would work at odds with any benefits from off-line consolidation. Nonetheless, we can compare the changes over sleep and wake to determine if forgetting is reduced following sleep.
Despite our imposition of a learning criterion during the immediate recall phase for the word-pair learning task (62%), there was a main effect of Age on immediate recall accuracy (F(2,81)=4.6, p=.009). The Young group performed better (74%) than the Older group (65%) (F(1,51)=7.8, p=.007), with the Middle-aged group’s rate falling between these two values (71%). This difference reflects the fact that the number of participants failing to reach criterion by the end of the fifth training list increased with age (1 Young, 3 Middle-aged, and 6 Older adults). Not surprisingly, after excluding these individuals, the main effect of Age on initial learning scores was no longer significant (F(2,75)=2.2, p=.14). Comparing the change in recall over intervals with sleep or wake for individuals reaching criterion, the effect of Interval Type was significant (F(1,151)=14.3, p<.001; Figure 3B), but the Age Group × Interval Type interaction was not (F(2,151)=.06, p=.94). Averaged over all groups, participants recalled 2.5% fewer words during delayed recall following sleep (compared to the last cycle of immediate recall). The comparable value following wake was 10.7%. Thus, sleep attenuated the degree of forgetting over a 12-hour delay. The main effect of Session Order was not significant (F(1,151)=.21, p=.65) nor did this factor interact with Age Group (F(2,151)=.49, p=.61) or Interval Type (F(1,151)=1.8, p=.18).
Within-group paired t-tests comparing changes in recall over wake and sleep intervals, again using only those meeting the learning criterion, were significant for Young (t(22)=3.1, p=.005), Middle-aged (t(28)=−2.7, p=.01) and Older adults (t(24)=−2.9, p=.007). Thus, all three age groups showed a benefit from sleep on the word-pair learning task. For the Young participants, the mean percentage of correctly recalled pairs was actually greater at the delayed phase compared to immediate recall. For the Middle-aged and Older participants, the rate of forgetting was larger, but sleep reduced the rate of forgetting, and to a similar degree as that observed in the Young partici pants (Figure 3B).
We again regressed age and total sleep time against a Sleep Benefit Score computed for each participant on the word-pair learning task. This score was calculated as the difference of the intersession changes in recall accuracy for the sleep interval relative to the wake interval:
where IR is immediate recall and DR is delayed recall. However, the model was not significant (F(2,74) = .28, p=.76). Thus, age does not account for a significant portion of the variance on this task, consistent with the conclusion that the benefit of sleep was similar across age groups for word-pair learning.
Finally, we considered whether eliminating those who failed to reach our initial learning criterion created a bias against finding an age-related effect on consolidation for word-pair learning. This question seemed especially important given that the motor sequence-learning task did not have a performance criterion. When all participants were included in the analysis, there was a trend for forgetting to be more pronounced with age, (main effect of Age Group: F(2,162)=2.65, p=.07), but the Age Group by Interval Type interaction (F(2,162)=.11, p=.89) remained non-significant.
3.5 Task interactions
The preceding analyses suggest that age has a differential effect on sleep-dependent changes in memory for sequence learning and word-pair learning. A second way to examine this issue is by looking at correlations between the two tasks: Similar mechanisms should produce a positive correlation between measures of sleep-dependent changes for the two tasks. At odds with this hypothesis, there was no correlation between the Sleep Benefit Scores for the two tasks (r=−.07, ns). Interestingly, the correlation was negative, although not significant (r=−.29, p=.17), when the analysis was restricted to the Young adults, the group that benefited from sleep on both tasks. The Sleep Benefit Scores are also useful for illustrating the relative sleep benefit for the two tasks across the age groups (Figure 4).
One concern with our design is that participants were tested on two different tasks in each session. This may have introduced interference between consolidation processes for sequence learning and word-pair learning (Brown & Robertson 2007). However, Task Order (sequence task first v. word-pair learning task first) did not affect the Sleep Benefit Score for either task (F(1,162)=.65, p=.42) or interact with Age Group (F(2,162)=2.1, p=.12).
While the preceding analyses suggest that Task Order did not influence performance, we note that the magnitude of sleep-related changes on sequence learning was lower in the Younger group in this study (8.8% improvement) compared to our previous report (18% improvement; Spencer et al. 2006). There is an age difference in that the current study age range was 21–35 year, whereas in our earlier study, it was 18–24. The attenuation of off-line learning may reflect this age difference or it may reflect the inclusion of multiple tasks in the test session.
4. Discussion
These results present an intriguing dissociation between age-related variations in two tasks that have been widely employed in the literature on SDC (Diekelmann et al. 2009; Payne 2010). Consistent with our previous report (Spencer et al. 2007) and work of others (Siengsukon & Boyd 2008; Siengsukon & Boyd 2009), older adults failed to show sleep-related changes in performance on a sequence learning task, our proxy of motor learning. Notably, middle-aged adults also showed a reduced performance benefit over sleep relative to wake. In contrast, SDC was observed in all age groups on the word-pair task. Sleep benefit scores for the word-pair and motor sequence learning tasks were not correlated in the present study, providing further support for the hypothesis that distinct processes underlie sleep-dependent consolidation for these tasks.
At first blush, it would be tempting to argue that this dissociation reflects a disproportionate affect of age on SDC for procedural relative to declarative learning. However, we (Spencer et al. 2006) and others (Keele et al. 2003) have argued that a simple procedural/declarative distinction cannot be applied to sequence learning, perhaps akin to a similar dissociation emerging in category learning literature (Ashby & Crossley 2010). In fact, earlier work (Keele et al. 2003; Schendan et al. 2003) suggests that the explicit variant of motor sequence learning used here, engages the hippocampus at encoding just as would be expected of word pair learning. Thus, while we cannot rule out the possibility that the aging-related reductions in hippocampal volume and functional engagement (Buckner 2004; Hedden & Gabrieli 2004; Spreng et al. 2010) underlie the present dissociation, we consider this unlikely. However, it is also possible that older adults do not utilize hippocampal-based learning processes at encoding in the same way young adults do. In a recent neuroimaging study, motor sequence learning was associated with increased striatal but decreased medial temporal lobe activation in young adults. However, for older adults, learning was paralleled by increases in both striatal and medial temporal lobe activation (Rieckmann et al. 2010). In the present study, there was no difference in initial learning at a behavioral level. Nonetheless, underlying processes engaged during encoding could contribute to the distinction in SDC for these two tasks irrespective of how they may be classified in terms of memory systems. Further examination including neuroimaging is warranted.
Alternatively, the function sleep serves may vary for these tasks. Memory consolidation is often marked by performance enhancements following sleep (Stickgold 2005), a pattern observed for the young adults here on the motor sequence learning task. We did not observe a similar overall level of post-sleep improvement on the word-pair learning task in either group. The sleep benefit for word-pair learning was manifest as an attenuation of forgetting. In contrast, Tucker et al (2006) reported that recall was enhanced by 45% following a nap compared to 28% following an equivalent wake interval. Likewise, Backhaus et al (2007) found greater recall following sleep relative to immediate recall prior to the break for both young and middle-age adults. However, given that feedback was provided throughout immediate recall in those studies, it is difficult to distinguish between changes associated with feedback during the immediate recall phase and sleep-dependent enhancement. In other words, even in the final presentation of the list during immediate recall, the correct response was given for each incorrect response. As such, performance changes between immediate recall and delayed recall not only reflect time but also the benefit of this additional exposure to the correct pairing. While feedback was also provided in the present study, the benefit of feedback and the 12-hr delay was likely different than previous studies (Backhaus et al. 2007; Plihal & Born 1999; Tucker et al. 2006) given that our study used semantically unassociated pairs. With this in mind, it is possible then that sleep only passively protects word-pair learning in the form used here (i.e., unrelated pairs) while sleep may actively process and enhance motor sequence learning (as well as, perhaps, semantically associated word pairs). If such is the case, our results may reflect a decline in active sleep-dependent processing (e.g., neural replay, shift to cortical storage) with age whereas the function of sleep in passively protecting memories from interference (decreased stimulation and encoding of new memories) is unchanged. Such an explanation could account for the discrepancy between the decline in sleep-dependent consolidation in middle-aged adults reported by Backhaus (2007) and not observed here. In that study, the active enhancement of existing associations by sleep was lost in the middle-aged group. In the present study and, likewise, the report of Aly & Moscovitch (2010), the protection of new associations by sleep was not changed by increasing age.
Finally, one might consider changes in sleep physiology that may account for the present distinction. Sleep-dependent changes for word-pair learning tasks have been associated with SWS (Plihal & Born 1997; Tucker et al. 2006) while SDC for the motor sequence learning task has been associated with nREM2 (Walker et al. 2002). Drawing upon normative data on sleep physiology, the effects of aging do not appear to be uniform across the sleep cycle, nor are they simply related to the total time in sleep stages: SWS declines rapidly across the adult lifespan while the time spent in nREM2 remains relatively constant (Danker-Hopfe et al. 2005; Ohayon et al. 2004). Thus, the current results go against a simple mapping between behavior and sleep stages given that the spared SDC in our older groups was observed for the task linked to a sleep stage that shows an aging effect. Future work measuring related physiological changes is warranted to examine whether measures of the quality of these sleep stages may, instead, be critical.
It is interesting to note that the dissociation we observe here with age parallels that reported in children. Compared to young adults, 6–8 year old children showed a similar magnitude of sleep-dependent consolidation on a word-pair task but reduced sleep-dependent changes on a sequence-learning task (Wilhelm et al. 2008). It may be that sleep-related changes for motor learning exhibit an inverted U-shaped function with a peak in early adulthood. This is supported by results in the Middle-aged group: it seems that only in young adults is motor sequence learning enhanced so strongly over sleep for healthy populations (Fischer et al. 2007; Siengsukon & Boyd 2008; Siengsukon & Boyd 2009; Wilhelm et al. 2008). While the basis for this function is unknown, it is clear that lifespan changes in SDC require further consideration.
Acknowledgments
R. M. C. Spencer was supported by NIH K99/R00AG029710. RBI was supported by NIH P01 NS040813. The authors would like to thank Amanda Schultz and Nola Klemfuss for their help in recruitment and data collection.
Footnotes
Disclosure Statement
The authors have no actual or potential conflicts of interest including any financial, personal or other relationships with other people or organizations within three years of beginning the work submitted that could inappropriately influence (bias) their work. All procedures were approved by the institutional review board (IRB) at University of California, Berkeley and University of Massachusetts, Amherst.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aly M, Moscovitch M. The effects of sleep on episodic memory in older and younger adults. Memory. 2010;18:327–34. doi: 10.1080/09658211003601548. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Crossley MJ. The neurobiology of categroization. In: Mareschal D, Quinn PC, Lea SE, editors. The Making of Human Concepts. New York: Oxford University Press; 2010. pp. 75–98. [Google Scholar]
- Backhaus J, Born J, Hoeckesfeld R, Fokuhl S, Hohagen F, Junghanns K. Midlife decline in declarative memory consolidation is correlated with a decline in slow wave sleep. Learn Mem. 2007;14:336–41. doi: 10.1101/lm.470507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM, Robertson EM. Off-line processing: reciprocal interactions between declarative and procedural memories. J Neurosci. 2007;27:10468–75. doi: 10.1523/JNEUROSCI.2799-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cai DJ, Shuman T, Gorman MR, Sage JR, Anagnostaras SG. Sleep selectively enhances hippocampus-dependent memory in mice. Behav Neurosci. 2009;123:713–9. doi: 10.1037/a0016415. [DOI] [PubMed] [Google Scholar]
- Danker-Hopfe H, Schafer M, Dorn H, Anderer P, Saletu B, Gruber G, Zeitlhofer J, Kunz D, Barbanoj MJ, Himanen SL, Kemp B, Penzel T, Roschke J, Dorffner G. Percentile reference charts for selected sleep parameters for 20- to 80-year-old healthy subjects from the SIESTA database. Somnologie. 2005;9:3–14. [Google Scholar]
- Diekelmann S, Wilhelm I, Born J. The whats and whens of sleep-dependent memory consolidation. Sleep Med Rev. 2009;13:309–21. doi: 10.1016/j.smrv.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Donohue K, Spencer RMC. Continuous re-exposure to environmental sound cues during sleep does not improve memory for semantically unrelated word pairs. Journal of Cognitive Education and Psychology. 2011;10:1–10. doi: 10.1891/1945-8959.10.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbogen JM, Hulbert JC, Jiang Y, Stickgold R. The sleeping brain’s influence on verbal memory: boosting resistance to interference. PLoS One. 2009;4:e4117. doi: 10.1371/journal.pone.0004117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbogen JM, Hulbert JC, Stickgold R, Dinges DF, Thompson-Schill SL. Interfering with theories of sleep and memory: sleep, declarative memory, and associative interference. Current Biology. 2006;16:1290–4. doi: 10.1016/j.cub.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Fischer S, Wilhelm I, Born J. Developmental differences in sleep’s role for implicit off-line learning: comparing children with adults. J Cogn Neurosci. 2007;19:214–27. doi: 10.1162/jocn.2007.19.2.214. [DOI] [PubMed] [Google Scholar]
- Gomez-Beldarrain M, Garcia-Monco JC, Rubio B, Pascual-Leone A. Effect of focal cerebellar lesions on procedural learning in the serial reaction time task. Experimental Brain Research. 1998;120:25–30. doi: 10.1007/s002210050374. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Keele SW, Ivry RB, Mayr U, Hazeltine E, Heuer H. The cognitive and neural architecture of sequence representation. Psychological Review. 2003;110:316–339. doi: 10.1037/0033-295x.110.2.316. [DOI] [PubMed] [Google Scholar]
- Nemeth D, Janacsek K, Londe Z, Ullman MT, Howard DV, Howard JH., Jr Sleep has no critical role in implicit motor sequence learning in young and old adults. Exp Brain Res. 2010;201:351–8. doi: 10.1007/s00221-009-2024-x. [DOI] [PubMed] [Google Scholar]
- Nissen MJ, Bullemer P. Attentional requirements of learning: evidence from performance measures. Cognitive Psychology. 1987;19:1–32. [Google Scholar]
- Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- Payne JD. Memory consolidation, the diurnal rhythm of cortisol, and the nature of dreams: A new hypothesis. International review of neurobiology. 2010;92:101–136. doi: 10.1016/S0074-7742(10)92006-0. [DOI] [PubMed] [Google Scholar]
- Peigneux P, Laureys S, Delbeuck X, Maquet P. Sleeping brain, learning brain. The role of sleep for memory systems. Neuroreport. 2001;12:A111–24. doi: 10.1097/00001756-200112210-00001. [DOI] [PubMed] [Google Scholar]
- Plihal W, Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. Journal of Cognitive Neuroscience. 1997;9:534–547. doi: 10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- Plihal W, Born J. Memory consolidation in human sleep depends on inhibition of glucocorticoid release. NeuroReport. 1999;10:2741–2747. doi: 10.1097/00001756-199909090-00009. [DOI] [PubMed] [Google Scholar]
- Rauchs G, Feyers D, Landeau B, Bastin C, Luxen A, Maquet P, Collette F. Sleep contributes to the strengthening of some memories over others, depending on hippocampal activity at learning. J Neurosci. 2011;31:2563–8. doi: 10.1523/JNEUROSCI.3972-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieckmann A, Fischer H, Backman L. Activation in striatum and medial temporal lobe during sequence learning in younger and older adults: relations to performance. Neuroimage. 2010;50:1303–12. doi: 10.1016/j.neuroimage.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Robertson EM, Pascual-Leone A, Press DZ. Awareness modifies the skill-learning benefits of sleep. Current Biology. 2004;14:208–212. doi: 10.1016/j.cub.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Rogers AE, Caruso CC, Aldrich MS. Reliability of sleep diaries for assessment of sleep/wake patterns. Nursing Research. 1993;42:368–72. [PubMed] [Google Scholar]
- Schendan HE, Searl MM, Melrose RJ, Stern CE. An fMRI study of the role of the medial temporal lobe in implicit and explicit sequence learning. Neuron. 2003;37:1013–1025. doi: 10.1016/s0896-6273(03)00123-5. [DOI] [PubMed] [Google Scholar]
- Shin JC, Ivry R. Spatial and temporal sequence learning in patients with Parkinson’s disease or cerebellar lesions. Journal of Cognitive Neuroscience. 2003;15:1232–1243. doi: 10.1162/089892903322598175. [DOI] [PubMed] [Google Scholar]
- Siengsukon CF, Boyd LA. Sleep enhances implicit motor skill learning in individuals poststroke. Topics in Stroke Rehabilitation. 2008;15:1–12. doi: 10.1310/tsr1501-1. [DOI] [PubMed] [Google Scholar]
- Siengsukon CF, Boyd LA. Sleep to learn after stroke: implicit and explicit off-line motor learning. Neuroscience Letters. 2009;451:1–5. doi: 10.1016/j.neulet.2008.12.040. [DOI] [PubMed] [Google Scholar]
- Smith LJ, Nowakowski S, Soeffing JP, Orff HF, Perlis ML. The measurement of sleep. In: Perlis ML, Lichstein KL, editors. Treating sleep disorders: Principles and practice of behavioral sleep medicine. New York, NY: John Wiley & Sons; 2003. pp. 29–73. [Google Scholar]
- Spencer RM, Gouw AM, Ivry RB. Age-related decline of sleep-dependent consolidation. Learn Mem. 2007;14:480–484. doi: 10.1101/lm.569407. [DOI] [PubMed] [Google Scholar]
- Spencer RMC, Sunm M, Ivry RB. Sleep-dependent consolidation of contextual learning. Current Biology. 2006;16:1001–5. doi: 10.1016/j.cub.2006.03.094. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Wojtowicz M, Grady CL. Reliable differences in brain activity between young and old adults: a quantitative meta-analysis across multiple cognitive domains. Neurosci Biobehav Rev. 2010;34:1178–94. doi: 10.1016/j.neubiorev.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–8. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- Stickgold R, Hobson JA, Fosse R, Fosse M. Sleep, learning, and dreams: Off-line memory reprocessing. Science. 2001;294:1052–1057. doi: 10.1126/science.1063530. [DOI] [PubMed] [Google Scholar]
- Stickgold R, Walker MP. Memory consolidation and reconsolidation: what is the role of sleep? Trends in Neurosciences. 2005;28:408–415. doi: 10.1016/j.tins.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Tucker MA, Fishbein W. Enhancement of declarative memory performance following a daytime nap is contingent on strength of initial task acquisition. Sleep. 2008;31:197–203. doi: 10.1093/sleep/31.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker MA, Hirota Y, Wamsley EJ, Lau H, Chaklader A, Fishbein W. A daytime nap containing solely non-REM sleep enhances declarative but not procedural memory. Neurobiology of Learning and Memory. 2006;86:241–247. doi: 10.1016/j.nlm.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: Sleep-dependent motor skill learning. Neuron. 2002;35:205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- Wilhelm I, Diekelmann S, Born J. Sleep in children improves memory performance on declarative but not procedural tasks. Learn Mem. 2008;15:373–7. doi: 10.1101/lm.803708. [DOI] [PubMed] [Google Scholar]
- Willingham DB, Nissen MJ, Bullemer P. On the development of proceural knowledge. Memory and Cognition. 1989;15:1047–1060. doi: 10.1037//0278-7393.15.6.1047. [DOI] [PubMed] [Google Scholar]