Abstract
Dravet syndrome (DS) is a childhood disorder associated with loss-of-function mutations in SCN1A and is characterized by frequent seizures and severe cognitive impairment. Animal studies have revealed new insights into the mechanisms by which mutations in this gene, encoding the type I voltage-gated sodium channel (Nav1.1), may lead to seizure activity and cognitive dysfunction. In this review, we further consider the function of fast-spiking GABAergic neurons, one cell type particularly affected by these mutations, in the context of the temporal coordination of neural activity subserving cognitive functions. We hypothesize that disruptions in GABAergic firing may directly contribute to the poor cognitive outcomes in children with DS, and discuss the therapeutic implications of this possibility.
Keywords: Scn1a, Nav1.1, Dravet syndrome, interneuron, oscillations, epileptic encephalopathy, EEG, epilepsy, sodium channel, cognitive impairment
Introduction
Dravet syndrome (DS), also termed Severe Myoclonic Epilepsy in Infancy, was first described in 1978 by Charlotte Dravet [1] and later recognized in the 1989 revised classification of epilepsies by the International League Against Epilepsy [2]. The incidence has been estimated at between 1:20,000 and 1:40,000, and it occurs more often in males than females with a ratio of 2:1 [3,4]. DS is a childhood epilepsy disorder associated with devastating effects on cognitive development. Children with DS often have severe intellectual disability and will be dependent on caregiver support throughout their adult life. In this review, we describe the clinical and cognitive features of this syndrome and the current treatments available. We also present the underlying mechanisms believed to be involved in both seizure susceptibility and cognitive impairment. While the presence of frequent and severe seizures during development is likely to impact cognitive function, we hypothesize that the underlying mechanisms responsible for seizures in DS may also directly affect cognitive processes and long-term outcome.
Clinical and Electroencephalographic Features
Children with DS suffer from seizures that are resistant to therapy and may be frequent and severe. Beginning around 5 months of age, affected children typically present with generalized or unilateral prolonged febrile seizures [5]. However, a variety of other seizure types later appear, including myoclonic, atonic, generalized tonic-clonic, absence, complex partial, and, less often, tonic. Frequent episodes of status epilepticus and ongoing susceptibility to hyperthermia-induced seizures are key clinical features. Reflex or stimulus-provoked seizures, defined as seizures which are reliably evoked by a stimulus, are also very common in DS. More than 50% have photosensitive seizures [6]. Dalla Bernardina et al. [7] observed that strong photosensitivity is associated with a poorer prognosis in children with DS. Although reflex seizures in DS are most often triggered by visual stimuli, stress or emotional state (i.e. when chastised or when entering an unfamiliar environment) has also been observed to trigger seizures [8]. Seizures tend to evolve throughout development. Between age 1 and 4 years, episodes of status epilepticus with fever, as well as episodes of nonconvulsive status epilepticus, are frequent. After 5 years of age, convulsive status epilepticus tends to be less frequent, and nocturnal generalized tonic-clonic seizures predominate. Adults also show persistence of seizures and fever susceptibility; nocturnal generalized tonic-clonic is the most common seizure type, and typically far fewer absence and myoclonic seizures are seen [9,10].
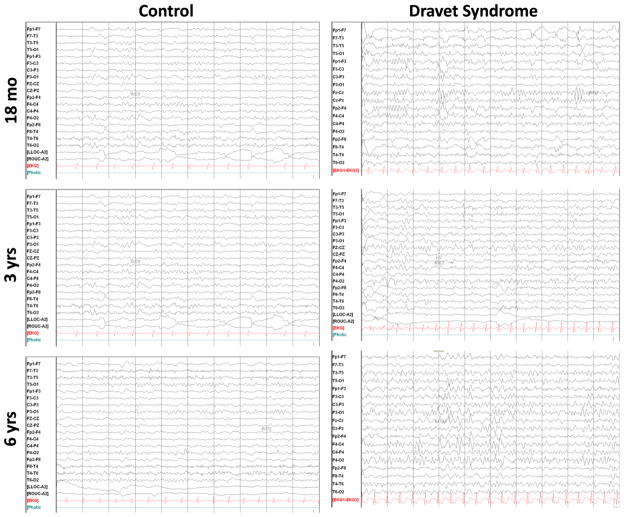
Electroencephalogram findings are typically normal in the first year of life, but parallel to the onset of afebrile seizures, the EEG shows characteristic changes with the appearance of paroxysmal epileptiform abnormalities. Generalized spike-wave and polyspike waves constitute the most frequent abnormality with multifocal spikes and slowing of the background reported in about 50% of children (Figure 1). Conversely, 40–50% of children may show a normal background. Sleep architecture remains preserved in many children, but abnormal patterns including activation of epileptiform activity and the spectrum of electrical status epilepticus have been described. As seizures tend to become nocturnal in older children and adults with DS, it is not surprising that epileptiform abnormalities may appear only during sleep over time [6]. Alterations in brain rhythms, or oscillations, have also been found on analysis of the EEG. Power spectral analysis of the scalp EEG in children between 1 and 9 years of age reveals an age-dependent alteration in oscillatory activity characterized by an overall slowing of the EEG (Figures 1 & 2). Calculated as a percentage of total power in the EEG from 0 to 25 Hz, we also observed a decrease in the alpha band (8–12 Hz) and increase in the theta band (4–7 Hz) compared to controls in children between ages 3 and 9 years [11]. In adult patients (18 years of age or older), an absence of occipital background alpha band activity has been found to correlate with poor mental status [12].
Figure 1.
Examples of EEGs from age-matched healthy control and DS children. The EEGs are from six children. The children classified as controls had EEGs obtained because of possible seizures. All of these children had normal developmental assessments and neurological examinations. No discernible changes were noted between the child with DS and the control at 18 months of age. However, a progressive change is observed over the first 6 years. In both the 3 and 6 year-old children, the EEG had slower frequencies in the child with DS than the healthy controls, and this difference is most noticeable at 6 years of age.
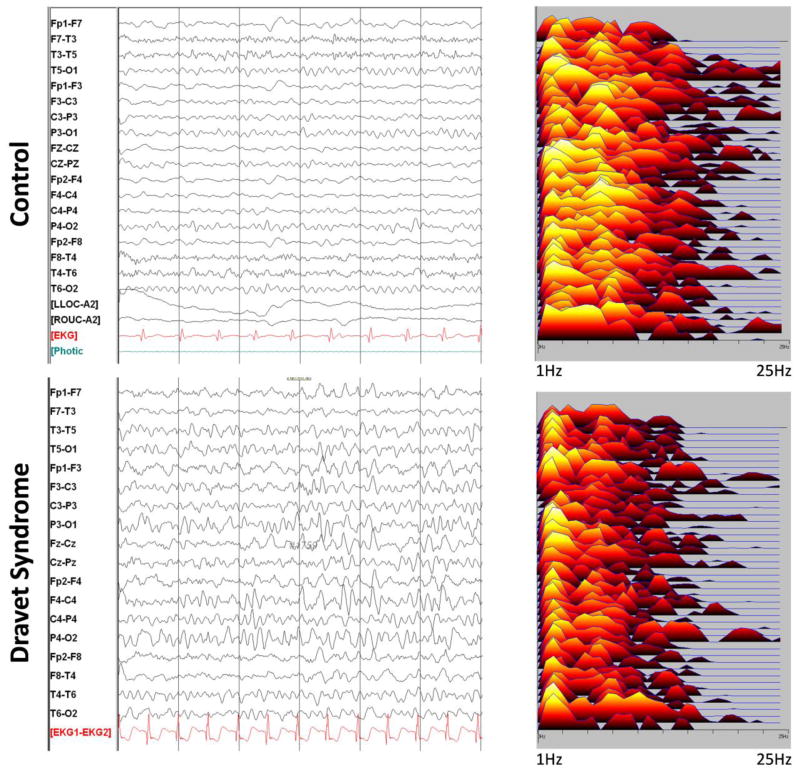
Figure 2.
EEG and power spectral analysis of a child with DS and a healthy control at 6 years of age. In the power spectral analysis on the right each line represents an electrode pair corresponding to the EEG on the left. Note that the spectral analysis of the child with DS was dominated by frequencies in the delta range whereas in the control, alpha range frequencies were prominent. Older children with DS typically have slower EEGs than age-matched controls.
Cognitive and Behavioral Impairment
While early-life seizures are perhaps the most striking feature of DS, the most debilitating consequences of the disease are often the associated cognitive and behavioral impairments. In most patients, normal development slows or regresses in the first few years of life and may never substantially improve. During the first year, prior to increases in frequency of febrile and afebrile seizures, infants appear to develop normally. However, during the second year, a progressive decline is observed in multiple domains of cognitive function. Psychomotor, visuospatial and language development are impaired, and social development is severely affected with patients displaying recalcitrance, mood instability, affective indifference, or autistic behaviors [9,13]. Intellectual disability is reported in the majority of cases [14,15].
The pattern and time-course of these impairments in cognitive development have been further revealed in longitudinal studies. Wolff et al. [16] documented a steep decline in the developmental quotient (DQ) during the first 4 to 6 years to around 20 to 40 percent of normal (N=14). This pattern appeared to occur in all but two children who declined only moderately (DQ 60–80%). Visuomotor performance was poor (DQ 15–35%) in all children after age 4, and language was also significantly impaired but more heterogeneous. It is important to note that since the DQ is a relative measure, this may represent an early plateauing of development rather than overt developmental regression. A similar pattern of cognitive and developmental impairment was found by Ragona et al. [17] using the Griffith’s General Quotient. They observed a dramatic decrease in the developmental profile in the first 4 years to 20 to 60 percent of normal (N=8). The authors also reported behavioral problems associated with intellectual disability in 21 of 37 patients, including hyperactivity, attention deficit and opposition. Together, these studies demonstrated a consistent pattern of cognitive and behavioral impairment characterized by slowed developmental progress during the first 4 to 6 years of life.
Subsequent studies have evaluated functional outcomes in adulthood and reveal that early cognitive impairments are persistent and chronically debilitating. By age 25 as many as 71% have IQ scores below 50 [18]. Akiyama et al. [12] further revealed deficits in language skills in adulthood that are striking. Out of 31 patients, five had simple conversation skills and some reading skills, nine had primitive conversation, nine could speak only a few words, and seven had no language skills at all. Interestingly, among all patients, including those with better outcomes, intellectual ability was not significantly correlated with seizure phenotype. Consistent with these observations, Genton et al. [15] reported major language impairment in 87% of patients (N=24). As a result of these impairments, adults with Dravet syndrome are often not able to live independently and require caregiver support throughout their lives.
Treatment
Effective treatment of seizures in patients with DS has been elusive. In a retrospective, single-center study of 53 patients evaluated between 1990 and 2004, seizures were well-controlled with anti-epileptic drugs (AEDs) in only 2 patients (3.8%). These two patients responded well to valproic acid associated with clobazam [19]. Topiramate has had some success as an add-on therapy. Among three separate studies, a greater than 50% reduction in seizure frequency was observed in 56% to 78% of patients [20–22]. In addition to topiramate, there are reports of limited success with a number of other AEDs, as well as with the ketogenic diet [23]. In 2005, Sankar, Wheless and Dravet proposed a treatment regimen beginning with valproic acid monotherapy with or without the ketogenic diet. When seizure control is not satisfactory, they recommended the addition of topiramate or clobazam and stiripentol, and ethosuximide, levetiracetam or zonisamide should also be considered if seizures persist [24].
Unfortunately, recommendations for the treatment of patients with DS are largely unsupported by placebo-controlled, randomized, clinical trials (RCTs). Despite reports of limited efficacy with clobazam, valproic acid, topiramate and the ketogenic diet, only stiripentol has been tested in this population in a well-designed RCT. In France, stiripentol was compared to placebo as an add-on therapy to clobazam and valproate with a 2 month follow-up period. This study found a greater than 50% reduction in seizure frequency (tonic or tonic-clonic) in the second month of follow-up in 15 out of 21 patients (71%) receiving stiripentol compared to only 1 out of 20 patients (5%) receiving placebo [25]. A second RCT was conducted in Italy in a smaller group with an identical study design, but the results of this second study have not yet been published [26]. Although the results of these trials were significant, the period of follow-up was short.
Despite the severity of cognitive impairments in DS and the impact they have on the quality of life of both children and caregivers, the causes of these cognitive impairments are uncertain. It is possible that frequent seizures contribute to cognitive impairment. Indeed, developmental seizures affect cognition in rodents [27–29], and interictal spikes may contribute to transient disruptions in cognitive function [30,31]. However, while reducing seizure frequency is important, considering cognitive and seizure outcomes independently may also be beneficial. In DS, there is so far little evidence that seizure severity correlates with cognitive outcome or that current anti-epileptic treatments prevent cognitive impairment. In fact, in a 2010 study by Akiyama et al. [12], only the presence or absence of the occipital alpha rhythm was correlated with mental status in adulthood, whereas frequency of status epilepticus and generalized spike-waves on the EEG were not. This suggests that, in addition to seizures, other factors may contribute to cognitive impairment. In the following sections, we propose that the alterations in neuronal synchrony that are responsible for seizures are also involved in altered brain oscillations and information processing contributing to impaired cognitive function.
In summary, patients with DS suffer from marked impairments in cognitive function early in development that tend to be permanent. Their language, intelligence and social skills are low, and they are often dependent on caregiver support as adults. There is a great need for new treatments that will improve cognitive outcomes. However, the causes of cognitive impairment have not yet been elucidated. As we will discuss, evidence from genetic studies and animal models has provided important insight into the underlying cellular and neural network mechanisms that may help explain the clinical phenotype. Understanding these mechanisms in the context of normal brain physiology will greatly benefit future efforts to improve patient care.
SCN1A Mutations cause DS
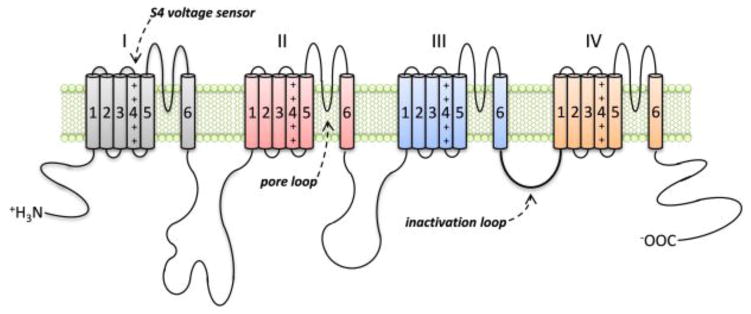
A remarkable feature of DS is its strong association with de novo mutations in a particular gene. Approximately 80% of patients with DS carry mutations in SCN1A [32–37], encoding the type I voltage-gated sodium channel (Nav1.1) alpha subunit (Figure 3). Nav1.1 belongs to a larger family of nine sodium channel proteins that also associate with, and are modulated by, smaller beta subunits [38]. These voltage-gated sodium channels are important for the excitability of cells such as neurons and myocytes, and in the brain they are critical for the initiation and propagation of action potentials in neurons. The Nav1.1 protein comprises four repeated domains (DI-DIV) of six transmembrane segments each (S1–S6), which are associated with different functions in the channel. The S4 segments act as the voltage sensors and activate opening of the channel in response to changes in membrane potential, and a loop on the cytosolic side of the membrane connecting DIII and DIV is involved with channel inactivation through interactions with the S5–S6 loops in DIII and DIV. The four pore loops between segments S5 and S6, which form the lining of the channel pore, also confer selectivity of the channel for the sodium ion [39–41]. Therefore, depending on the location and properties of the amino-acid affected, mutations in SCN1A will affect the function of Nav1.1 differently.
Figure 3.
The type I voltage-gated sodium channel (Nav1.1) alpha subunit. The alpha-subunit is composed of four repeated domains (I–IV) of six transmembrane segments each (S1–S6). The four pore loops between segments S5 and S6, which form the lining of the channel pore, confer selectivity of the channel for the sodium ion. The S4 segments act as the voltage sensors and activate opening of the channel in response to changes in membrane potential, and a loop on the cytosolic side of the membrane connecting domains III and IV is involved with channel inactivation. Both activation and inactivation of the channel are triggered by depolarization of the cell membrane and are characterized by fast kinetics. Opening of the channel produces an inward sodium conductance and rapid depolarization of the cell membrane, which are critical for initiating action potentials in neurons. Mutations in children with DS are often found in the key functional regions of the channel, particularly the S4 voltage sensors and S5–S6 pore loop regions.
The varying severity of these mutations appears to correlate with the spectrum of severity of SCN1A-associated epileptic disorders. For example, some patients (10–15%) diagnosed with Generalized Epilepsy with Febrile Seizures Plus (GEFS+) have SCN1A mutations. They also present with early-life seizures, particularly hyperthermia-induced seizures. Seizures in GEFS+ are usually less frequent and less severe than in DS, and they often stop after puberty. In contrast to DS, patients with GEFS+ rarely have significant cognitive and behavioral impairments [42–44]. In view of the heterogeneity in the clinical phenotype of patients with SCN1A mutations diagnosed with GEFS+ or DS, several authors have proposed a “Febrile Seizures↔GEFS+↔DS” continuum, whereby more severe SCN1A mutations tend to lead to more severe types of epilepsy [45–47]. Consistent with this view, Zuberi et al. [48] examined the genotype-phenotype relationship in 273 people with SCN1A mutations. They found that the Grantham Score (GS) associated with these mutations, a measure for the dissimilarity of amino acids, was higher in patients with DS. The greatest proportion of truncating mutations was also found in DS cases, whereas almost all GEFS+ mutations were missense. Interestingly, missense mutations occurred with a higher-than-expected frequency in the S4 voltage sensors and S5-S6 pore loop regions, a finding reflective of the functional importance of these regions. Moreover, within these regions, the magnitude of change in amino acid polarity occurring as a result of the mutation was significantly greater in patients with DS compared to patients with GEFS+. This finding in particular supports the idea that mutations resulting in greater alterations in the properties of the channel are associated with more severe phenotypes. However, it is also important to note that other factors, such as genetic background, may contribute to the severity of the phenotype. In addition, the relationship between SCN1A mutations and phenotype severity is not always clear. For instance, in the context of a family with an inherited SCN1A mutation (approximately 5% of DS cases) both the DS and GEFS+ phenotypes can be observed [49].
The expression profile of SCN1A may also relate to the developmental onset of the epilepsy phenotype. There are four main voltage-gated sodium channels expressed in the central nervous system: Nav1.1, encoded by the gene SCN1A, along with Nav1.2, Nav1.3 and Nav1.6, encoded by the genes SCN2A, SCN3A and SCN8A, respectively. The expression patterns of these channels change during development. Nav1.1 and Nav1.6 expression increases during development [50–52], with Nav1.1 expression reaching adult levels over the first month in rodents [50,51]. Interestingly, Nav1.2 and Nav1.3 expression appears to decline during postnatal development [51,52]. However, between brain regions Nav1.2 expression changes may vary [53]. Overall, these reports suggest there is a relative switch in the expression of these voltage-gated sodium channels in the brain that occurs gradually during development, over the first four weeks of life in rodents [51–53]. It is also during this period that mice with complete and partial Scn1a deletion show reduced survival and increased susceptibility to spontaneous or hyperthermia-induced seizures [54,55]. This evidence supports the hypothesis that the relative role of Nav1.1 in the brain increases during early development. Although the exact functional implications of these expression changes have not yet been determined, it is possible that a similar process occurs in humans and is associated with the timing of susceptibility to febrile seizures and slowed cognitive development. Indeed, in humans Nav1.1 expression in the hippocampus and cortex also increases during early development, peaking around 7 to 9 months of age [56], and this is precisely a period that often coincides with the onset of the epilepsy phenotype in children with DS.
To summarize, there is a strong association of SCN1A mutations with DS, and the evidence suggests a relationship between the location and type of SCN1A mutation and the severity of the epilepsy phenotype. Furthermore, the developmental expression of Nav1.1 in the brain is consistent with the early developmental onset of clinical symptoms. Considering that the voltage-gated sodium channels are critical for action potential generation and propagation in neurons, it is not surprising that mutations in SCN1A are detrimental to the normal functioning of the nervous system. However, since loss of function in one of these channels would be expected to impair neuronal firing, it is perhaps not intuitive that mutations in SCN1A lead to neuronal hyperexcitability and seizures. In the following sections, we discuss evidence from animal studies that have helped elucidate the mechanisms by which SCN1A mutations may cause both seizures and cognitive impairment.
Mechanism for Hyperexcitability and Seizures
The mechanism by which SCN1A loss-of-function mutations cause increased susceptibility to seizures was first elucidated by Yu et al. [54] using a Scn1a knock-out (KO) mouse model. As in DS, they observed that mice that were heterozygous for the Scn1a deletion were susceptible to febrile (hyperthermia-induced) seizures and had spontaneous seizures [55]. To investigate the mechanism by which Scn1a haploinsufficiency leads to seizures, they evaluated sodium currents in dissociated hippocampal interneurons and pyramidal cells. They found that sodium currents in inhibitory, GABAergic interneurons, but not excitatory pyramidal cells, were substantially reduced. Consistent with this finding, they further demonstrated that action potential firing frequency was impaired only in interneurons. Moreover, the impairment in firing was greater when a larger current was used for stimulation, suggesting that the ability of interneurons to provide inhibitory input to postsynaptic cells may be impaired to the greatest extent during times of increased demand. These results suggest that increased neuronal excitability in disorders linked to SCN1A loss-of-function mutations may be caused by a principal impairment in the ability of interneurons to provide appropriate inhibition in neuronal networks, thus leading to an imbalance between excitation and inhibition in the brain [45,54,55]. This mechanism has been supported by the development of several other animal models created with human SCN1A mutations, and their results are in accordance with the impairments seen by Yu et al. [50,51,54,57].
The hypothesis that SCN1A-associated epileptic disorders are caused by impaired function of inhibitory interneurons is supported by the expression pattern of Nav1.1. Although Nav1.1 is expressed throughout the central nervous system, including in pyramidal neurons [58], Nav1.1 has been reported, in particular, in the neocortex and hippocampus to colocalize with cells expressing parvalbumin (PV), a marker for fast-spiking GABAergic interneurons. In the cerebellum, PV+ purkinje cells also express Nav1.1, a finding consistent with the observation that many patients show signs of ataxia [59]. Immunohistochemical evidence also suggests that Nav1.1 is clustered in the proximal portion of the axon initial segment of interneurons [60,61], precisely a region that is critical for the initiation and generation of action potentials. The expression of Nav1.1 in PV+ GABAergic neurons in the central nervous system is, therefore, consistent with the electrophysiology data indicating that this cell type is susceptible to SCN1A deficits.
Based on the evidence from mouse models, it is clear that loss-of-function mutations of SCN1A cause functional impairments in GABAergic neurons and leads to a loss of appropriate inhibition in neuronal networks. The finding that cortical pyramidal neurons were relatively spared in these studies further supports the concept of a net imbalance between excitation and inhibition in the brain leading to an increase in susceptibility to seizures. However, a number of questions remain to be addressed. Patients with DS also suffer from severe cognitive and behavioral impairments that begin during development and rarely improve [16,17]. Yet, the mechanisms responsible for their cognitive outcome are uncertain. Is cognitive impairment caused by the effect of seizures during development, interneuron dysfunction, or both? Furthermore, in considering appropriate treatment strategies, will re-establishing the balance between excitation and inhibition be sufficient, or will it also be necessary to restore function to the cells that are affected by SCN1A mutations?
Role of Interneuron Function in Neural Processing and Cognition
In considering the impact of SCN1A mutations on cognition in the context of DS, it is necessary to consider the functional role of Nav1.1 in the normal and developing brain. The evidence from animal models discussed above supports the view that SCN1A mutations in DS cause a functional impairment in the firing of forebrain GABAergic interneurons. More specifically, evidence from several studies demonstrates that PV+ interneurons express Nav1.1 and may be particularly affected by SCN1A mutations [50,56,61]. Therefore, although this sodium channel may also have important roles in other cells, in the following sections we will focus on its role in the PV+ GABAergic cell type. As we explain below, these cells play critical roles in the spatiotemporal patterning of neural network activity during cognitive processes. For this reason, even a partial physiological impairment in their firing may yield substantial disturbances in cognition and behavior. Thus, this underlying interneuron dysfunction may contribute to the severe cognitive impairments in individuals with DS in parallel with the effects of seizures.
Interneurons were classically described as short-axon neurons with connections between ‘input’ and ‘output’ principal cells and were recognized for their role in modulating excitability via GABA-mediated inhibition. However, it is has been generally accepted that this is an oversimplified description. Interneurons are more diverse than initially thought, and they serve more complex roles than just the regulation of excitability. At the simplest level, they can be divided into those that project onto the dendritic versus the perisomatic domain of their target cells. Dendritic inhibition is thought to control the excitatory synaptic inputs of pyramidal cells, whereas perisomatic inhibition controls their output [62]. Perisomatic-targeting interneurons form synapses onto the axon initial segments, proximal dendrites or soma of pyramidal cells and can typically be identified by their expression of PV [62–65]. By innervating the region on or near the soma, these PV+ interneurons are ideally positioned to control action potential initiation. They are also synaptically and electrically coupled to each other, and each cell innervates large populations of pyramidal cells – as many as 1500 in the hippocampus [66–69]. These properties, together with their fast-spiking activity, confer PV+ perisomatic-targeting interneurons the ability to synchronize large populations of neurons at the millisecond resolution. Therefore, they are critical for regulating the synchrony and temporal patterning of neural networks. A good example of this is their role in the well-characterized brain oscillations in the gamma and theta frequency bands.
Gamma oscillations are a particular brain rhythm in the 30 to 100 Hz frequency range and have been associated with several cognitive functions, including auditory, visual, olfactory, and somatosensory processing, as well as memory and attention [70–73]. Abnormal gamma oscillations have also been reported in diseases involving dysfunctional cognitive processing, including schizophrenia, Alzheimer’s disease, and ADHD [74,75]. Functionally, gamma oscillations have been shown to increase information flow in interneuron-pyramidal cell connections [76], and different gamma frequencies may support information flow for different input sources in neural circuits [77]. Several studies have demonstrated that PV+ interneurons play an important role in generating cortical gamma oscillations [78–81]. This is well-illustrated by the observation that suppressing PV+ interneuron signaling reduces gamma power in vivo, and stimulating this cell population is sufficient to induce gamma [76,82]. Consistent with these findings, reduced PV+ interneuron signaling causes impaired spatial working memory and recognition of novel spatial arrangements [82]. Thus, gamma oscillations are one type of brain rhythm that are important for cognitive processes and require the proper function of PV+ fast-spiking interneurons, the primary cell type believed to be affected in DS.
Theta oscillations (5–12 Hz in rodents; 4–7 Hz in humans) also play a critical role in cognitive function and provide a temporal framework for neural network activity. Theta oscillations have received much attention for their prominent role in the hippocampal system as they are the most striking EEG feature of this brain region during exploration and rapid eye movement (REM) sleep. In rodents, theta oscillations are strongly tied to spatial cognition and have been associated with working memory, sensorimotor integration, spatial recognition memory, and learning and recall [83–87]. In humans, theta oscillations also correlate with spatial working memory, difficulty of virtual maze navigation, sensorimotor integration, learning and recall in a virtual water maze, and image identity recognition [88–91]. PV+ perisomatic-targeting interneurons are also critical for theta generation. In the hippocampus, fast-spiking interneuron discharge is modulated by theta rhythm [92,93], and the stimulation of single PV+ interneurons in a hippocampal slice is able to synchronize pyramidal cells at theta frequencies [94]. In support of their important role in hippocampal theta activity, selectively blocking synaptic transmission of PV+ interneurons in the CA1 region of the hippocampus directly impairs spatial working memory, consistent with the effect of reduced theta oscillations [95]. Therefore, together with gamma oscillations, theta oscillations represent a second type of neural network activity that is critical for cognitive function and relies on the precise firing of PV+ interneurons.
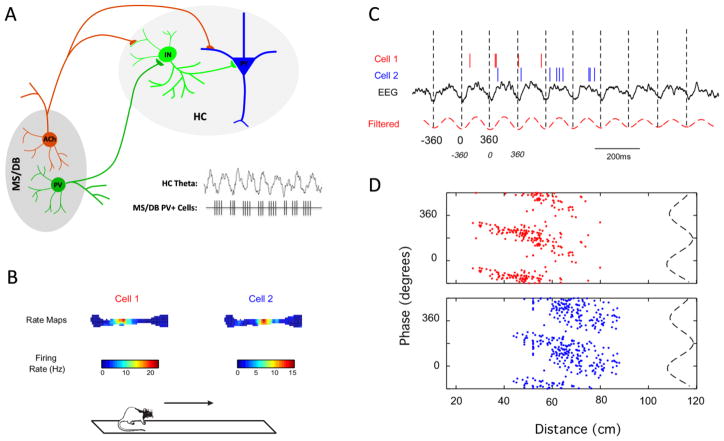
The function of PV+ GABAergic neurons in distal network structures is also required for the proper generation of hippocampal and cortical oscillations. For example, the medial septum and diagonal band of Broca (MSDB) is an important pacemaker for theta oscillations in the hippocampus and cortex [96]. The MSDB contains two well-characterized populations of neurons – cholinergic cells expressing acetylcholine and GABAergic cells expressing PV [97,98] (Figure 4A). The cholinergic population has received much attention, in part due to its relation to Alzheimer’s disease, and is indeed important for modulating theta oscillations in the hippocampus. However, the generation of theta oscillations also requires the coordinated action of PV+ GABAergic neurons in the MSDB [96,99–102]. These MSDB PV+ neurons project onto interneurons in the hippocampus, subsequently affecting large populations of pyramidal cells. Moreover, a population of these cells fires in a rhythmic bursting pattern, temporally leading the hippocampal theta, suggesting that they serve as a pacemaker through the patterning of local hippocampal interneurons [96,100,103–105]. Consistent with the important role of these cells in patterning theta oscillations in the hippocampus, selective damage to GABAergic neurons in the MSDB impairs performance on a hippocampal-dependent task of spatial working memory [106]. Although the effect of SCN1A deficits on the PV+ GABAergic cell population in the MSDB has not yet been investigated, one might predict that the firing of these cells would also be affected by loss of function of Nav1.1, and consequently, the ability of the MSDB to coordinate neural network activity in distal brain structures would be affected.
Figure 4.
The septo-hippocampal network and phase precession. A) The medial septum (MS) and diagonal band of Broca (DB), the hippocampal formation (HC) and the neural connections between these two brain regions through the fimbria fornix comprise the septo-hippocampal network. Cholinergic (Ach) and PV+ GABAergic neurons (PV) projecting from the MS/DB to the hippocampus are important for patterning and modulating theta oscillations. The population of PV+ projection neurons in the MS/DB fires in a bursting pattern, preceding the peak of each hippocampal theta cycle, and exerts a pacemaking role on hippocampal theta oscillations through the patterning of local hippocampal interneurons (IN). The significance of such temporal coordination of hippocampal network activity is well-illustrated by phase precession (B–D). B) Firing rate maps of two place cells recorded in the rodent hippocampus during performance on a linear track. C) While the rat crosses the linear track, place cells discharge at specific phases of the ongoing hippocampal EEG, shifting increasingly earlier in phase with each theta cycle. D) This phenomenon, termed phase precession, can be visualized by the phase-distance plots for each cell, with each theta cycle divided in 360º from trough to trough. Phase precession is believed to be important for spatial cognition by encoding the distance and event sequence as the animal traverses the corresponding place fields. Therefore, mutations in SCN1A that affect the firing of PV+ neurons in the septo-hippocampal network are likely to impair this important type of coordinated network activity.
The precise relationship of theta oscillations to local single-cell activity in the hippocampus perhaps best exemplifies the importance of oscillatory network activity in shaping pyramidal cell output during cognitive processing. One phenomenon, termed phase precession, describes a temporal relationship between network theta oscillations and CA1 place cell firing that is associated with spatial cognition. Place cells in the CA1 region of the hippocampus display a systematic temporal relationship with the local field potential oscillations, firing preferentially in phase with the peak of the theta cycle. Importantly, as an animal crosses through a place field, the firing of the corresponding place cell precesses, or shifts increasingly earlier with each theta cycle [107] (Figure 4B-D). The exact mechanisms controlling phase precession are still debated, but perisomatic-targeting interneurons are believed to play an important role [108–111]. PV+ interneuron input to CA1 pyramidal cells is indeed required to reproduce phase precession in an ex vivo slice model [112]. Functionally, phase precession is postulated to be important for spatial cognition by encoding the distance travelled through the place field, as well as the sequence of events through multiple place fields [113–116]. In support of this view, reduced phase precession in animals with preserved place fields is associated with severe spatial memory impairment [117,118]. Altered phase precession has also been found in a rat model of temporal lobe epilepsy associated with marked cognitive impairment in the water maze [119]. Thus, temporal coordination of network oscillations and single-cell activity is critical for normal cognitive processes. This is well-illustrated by phase precession in the hippocampus, and it is one neural network phenomenon susceptible to interneuron dysfunction in disease.
To summarize, PV+ GABAergic neurons serve a complex role in normal brain functions beyond balancing excitation with inhibition. They are critical for the synchronization and spatiotemporal patterning of neural activity, including for brain oscillations, such as gamma and theta. The precise temporal coordination of these neural network patterns is essential for cognitive processes, and therefore this is one mechanism by which impairments in PV+ interneurons can disrupt cognitive function. Although the effects of SCN1A mutations on brain oscillations and information processing have not yet been directly tested, we believe that the deficit in PV+ interneuron firing caused by SCN1A mutations may impair the normal function of these brain processes. Therefore, this may be one mechanism that is responsible for cognitive impairment in DS.
Developmental Role of Interneurons
The cellular and physiological changes in the brain involved in the development of normal cognitive functions may also be of particular relevance to understanding the role of SCN1A mutations in DS. For example, the developmental increase in Scn1a expression in rodents parallels both the neurophysiological development of fast-spiking interneurons and the development of spatial cognition. During the second and third postnatal weeks, the action potential width decreases and firing frequency increases in PV+ fast-spiking interneurons, conferring their conversion from a slow to a fast signaling cell [120,121]. The observation that interneurons of Scn1a knockout mice fail to develop a narrow action potential width characteristic of mature cells suggests that Scn1a expression is important for this conversion [54]. This is also the period during which place cells develop, mean theta frequency increases [122,123], and performance on spatial tasks begins to improve [124,125]. These changes also occur in a similar time frame as the increase in susceptibility to hyperthermia-induced seizures in Scn1a+/− mice during the third postnatal week [55]. Together, the timing of the above developmental changes makes this period especially susceptible to functional deficits affecting neural network activity. Although direct causal relationships have not been determined, it is likely that SCN1A deficits in DS impact multiple developmental processes in the neural substrates affecting cognition. As such, the underlying neurobiology of SCN1A mutations in this disease may concurrently lend to the severely impaired development of normal cognitive function in parallel with a seizure disorder, wherein the impact of seizures on neural development subsequently worsens cognition.
Conclusions
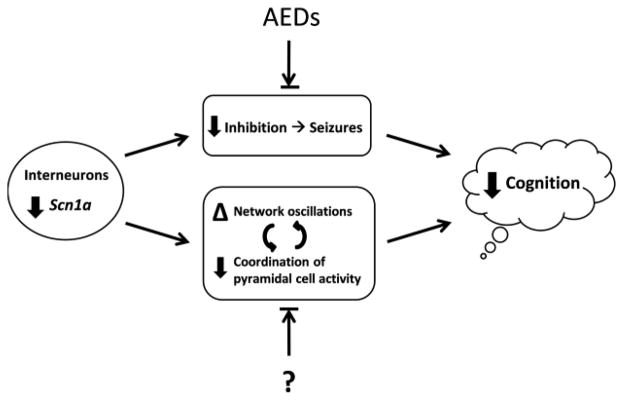
DS is a childhood disorder associated with loss-of-function mutations in SCN1A that is characterized by frequent, refractory seizures and permanent cognitive impairment. An overall slowing of the EEG is observed after the first year, including alterations in oscillatory activity. Animal studies have revealed that SCN1A mutations causing loss of function of Nav1.1 impair the firing of GABAergic neurons compared to pyramidal cells. Among this cell type, the fast-spiking, PV+ population is affected. These cells are critical for maintaining precise temporal dynamics in cortical and subcortical networks, including the patterning and generation of rhythmic oscillations, such as theta and gamma in the hippocampus and cortex. Their role in coordinating activity in neural networks is essential for higher cognitive functions, including attention, learning, and memory. Therefore, the effect of SCN1A mutations on the function of these cells in the context of normal network activities is likely to contribute to impairments in cognitive abilities and poor mental outcomes in DS. Moreover, this may represent a mechanism independent from the effects of seizures (Figure 5). Future efforts to develop therapies that will improve cognitive outcome may benefit by targeting these mechanisms.
Figure 5.
A working model for possible contributions to cognitive impairment in DS. Loss-of-function mutations in SCN1A are found in children with DS. Animal studies have revealed that loss of function in Nav1.1, encoded by SCN1A, causes impaired firing of GABAergic interneurons relative to pyramidal cells. This is believed to cause an imbalance between excitation and inhibition, leading to seizures. The frequent and severe seizures observed in DS affect cognitive function. In addition, the functional deficit in GABAergic neurons likely alters the normal function of neural network activity known to be critical for cognitive functions. Examples may include alterations in theta and gamma oscillations, and loss of coordination of pyramidal cell ensembles involved in information processing. We hypothesize that these effects directly contribute to cognitive impairment independently of the contribution from seizures. Current antiepileptic drugs (AEDs) rebalance the relative amounts of excitation and inhibition in the brain to reduce the severity and frequency of seizures. However, it is unknown (“?”) whether treatments can ameliorate the alterations in network activity that are important for cognitive function. Future therapeutic strategies may benefit from consideration of these additional mechanisms that affect cognitive outcome.
Highlights.
Dravet syndrome is a childhood disorder associated with mutations in SCN1A.
It is characterized by frequent seizures and severe cognitive impairment.
We propose that dysfunction in GABAergic neurons leads to altered brain oscillations.
Both seizures and altered oscillations may contribute to cognitive impairment.
Acknowledgments
Supported by grants from NINDS to GLH, PPLS, RCS (NS074450 and NS073083), and ACB (F31NS077537-01), and by the Emmory R. Shapses Research Fund. RCS is supported by Great Ormond Street Hospital Children’s Charity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dravet C. Les epilepsies graves de l’enfant. La Vie medicale. 1978;8:543–8. [Google Scholar]
- 2.Proposal for Revised Classification of Epilepsies and Epileptic Syndromes. Commission on Classification and Terminology of the ILAE. Epilepsia. 1989;30(4):389–99. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- 3.Hurst DL. Epidemiology of severe myoclonic epilepsy of infancy. Epilepsia. 1990;31(4):397–400. doi: 10.1111/j.1528-1157.1990.tb05494.x. [DOI] [PubMed] [Google Scholar]
- 4.Yakoub M, Dulac O, Jambaque I, Chiron C, Plouin P. Early diagnosis of severe myoclonic epilepsy in infancy. Brain & development. 1992;14(5):299. doi: 10.1016/s0387-7604(12)80147-1. [DOI] [PubMed] [Google Scholar]
- 5.Dravet C. The core Dravet syndrome phenotype. Epilepsia. 2011 Apr;52( Suppl 2):3–9. doi: 10.1111/j.1528-1167.2011.02994.x. [DOI] [PubMed] [Google Scholar]
- 6.Bureau M, Dalla Bernardina B. Electroencephalographic characteristics of Dravet syndrome. Epilepsia. 2011 Apr;52( Suppl 2):13–23. doi: 10.1111/j.1528-1167.2011.02996.x. [DOI] [PubMed] [Google Scholar]
- 7.Bernardina BD, Capovilla G, Chiamenti C, Trevisan E, Colamaria V, Fontana E. Cryptogenic myoclonic epilepsies of infancy and early childhood: nosological and prognostic approach. In: Wolf P, Dam M, Janz D, editors. Advances in Epileptology. New York: Raven Press; 1987. pp. 175–9. [Google Scholar]
- 8.Morse RP. Dravet syndrome: inroads into understanding epileptic encephalopathies. The Journal of Pediatrics. 2011 Mar;158(3):354–9. doi: 10.1016/j.jpeds.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 9.Dravet C, Bureau M, Oguni H, Fukuyama Y, Cokar O. Advances in Neurology. John Libbey Eurotext; 2005. Severe myoclonic epilepsy in infancy (Dravet syndrome) pp. 71–102. [PubMed] [Google Scholar]
- 10.Jansen FE, Sadleir LG, Harkin LA, Vadlamudi L, McMahon JM, Mulley JC, et al. Severe myoclonic epilepsy of infancy (Dravet syndrome): recognition and diagnosis in adults. Neurology. 2006 Dec 26;67(12):2224–6. doi: 10.1212/01.wnl.0000249312.73155.7d. [DOI] [PubMed] [Google Scholar]
- 11.Holmes GL, Bender AC, Wu EX, Scott RC, Lenck-Santini PP, Morse RP. Maturation of EEG oscillations in children with sodium channel mutations. Brain & Development. 2011 Sep 20; doi: 10.1016/j.braindev.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akiyama M, Kobayashi K, Yoshinaga H, Ohtsuka Y. A long-term follow-up study of Dravet syndrome up to adulthood. Epilepsia. 2010 Jun;51(6):1043–52. doi: 10.1111/j.1528-1167.2009.02466.x. [DOI] [PubMed] [Google Scholar]
- 13.Chieffo D, Battaglia D, Lettori D, Del Re M, Brogna C, Dravet C, et al. Neuropsychological development in children with Dravet syndrome. Epilepsy research. 2011 Apr 5; doi: 10.1016/j.eplepsyres.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Ragona F. Cognitive development in children with Dravet syndrome. Epilepsia. 2011 Apr;52(Suppl 2):39–43. doi: 10.1111/j.1528-1167.2011.03000.x. 2010. [DOI] [PubMed] [Google Scholar]
- 15.Genton P, Velizarova R, Dravet C. Dravet syndrome: the long-term outcome. Epilepsia. 2011 Apr;52( Suppl 2):44–9. doi: 10.1111/j.1528-1167.2011.03001.x. [DOI] [PubMed] [Google Scholar]
- 16.Wolff M, Cassé-Perrot C, Dravet C. Severe myoclonic epilepsy of infants (Dravet syndrome): natural history and neuropsychological findings. Epilepsia. 2006 Jan;47( Suppl 2):45–8. doi: 10.1111/j.1528-1167.2006.00688.x. [DOI] [PubMed] [Google Scholar]
- 17.Ragona F, Brazzo D, De Giorgi I, Morbi M, Freri E, Teutonico F, et al. Dravet syndrome: early clinical manifestations and cognitive outcome in 37 Italian patients. Brain & Development. 2010 Jan;32(1):71–7. doi: 10.1016/j.braindev.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Ohtsuka Y, Maniwa S, Ogino T, Yamatogi Y, Ohtahara S. Severe Myoclonic Epilepsy in Infancy: A Long Term Follow Up Study. The Japanese Journal of Psychiatry and Neurology. 1991;45(2):416–8. doi: 10.1111/j.1440-1819.1991.tb02506.x. [DOI] [PubMed] [Google Scholar]
- 19.Caraballo RH, Fejerman N. Dravet syndrome: a study of 53 patients. Epilepsy research. 2006 Aug;70( Suppl 1):S231–8. doi: 10.1016/j.eplepsyres.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 20.Nieto-Barrera M, Lillo MM, Rodriguez-Collado C, Candau R, Correa A. Epilepsia mioclonica severa de la infancia. Estudio epidemiologico analitico. Revista de neurologia. 2000;30(7):620–4. [PubMed] [Google Scholar]
- 21.Kröll-Seger J, Portilla P, Dulac O, Chiron C. Topiramate in the treatment of highly refractory patients with Dravet syndrome. Neuropediatrics. 2006 Dec;37(6):325–9. doi: 10.1055/s-2007-964867. [DOI] [PubMed] [Google Scholar]
- 22.Coppola G, Capovilla G, Montagnini A, Romeo A, Spanò M, Tortorella G, et al. Topiramate as add-on drug in severe myoclonic epilepsy in infancy: an Italian multicenter open trial. Epilepsy research. 2002 Mar;49(1):45–8. doi: 10.1016/s0920-1211(02)00010-4. [DOI] [PubMed] [Google Scholar]
- 23.Caraballo RH, Cersósimo RO, Sakr D, Cresta A, Escobal N, Fejerman N. Ketogenic diet in patients with Dravet syndrome. Epilepsia. 2005 Sep;46(9):1539–44. doi: 10.1111/j.1528-1167.2005.05705.x. [DOI] [PubMed] [Google Scholar]
- 24.Sankar R, Wheless JW, Dravet C, Guerrini R, Medina M, Bureau M, et al. Treatment of myoclonic epilepsies in infancy and early childhood. Advances in Neurology. 2005;95:289–98. [PubMed] [Google Scholar]
- 25.Chiron C, Marchand MC, Tran A, Rey E, d’ Athis P, Vincent J, et al. Stiripentol in severe myoclonic epilepsy in infancy: a randomized placebo-controlled syndrome-dedicated trial. STICLO study group. Lancet. 2000 Nov 11;356(9242):1638–42. doi: 10.1016/s0140-6736(00)03157-3. [DOI] [PubMed] [Google Scholar]
- 26.Kassaï B, Chiron C, Augier S, Cucherat M, Rey E, Gueyffier F, et al. Severe myoclonic epilepsy in infancy: a systematic review and a meta-analysis of individual patient data. Epilepsia. 2008 Feb;49(2):343–8. doi: 10.1111/j.1528-1167.2007.01423.x. [DOI] [PubMed] [Google Scholar]
- 27.Huang L, Cilio MR, Silveira DC, McCabe BK, Sogawa Y, Stafstrom CE, et al. Long-term effects of neonatal seizures: a behavioral, electrophysiological, and histological study. Developmental Brain Research. 1999 Dec 10;118(1–2):99–107. doi: 10.1016/s0165-3806(99)00135-2. [DOI] [PubMed] [Google Scholar]
- 28.Holmes GL, Gairsa JL, Chevassus-Au-Louis N, Ben-Ari Y. Consequences of neonatal seizures in the rat: morphological and behavioral effects. Annals of Neurology. 1998 Dec;44(6):845–57. doi: 10.1002/ana.410440602. [DOI] [PubMed] [Google Scholar]
- 29.Karnam HB, Zhou J-L, Huang L-T, Zhao Q, Shatskikh T, Holmes GL. Early life seizures cause long-standing impairment of the hippocampal map. Experimental Neurology. 2009 Jun;217(2):378–87. doi: 10.1016/j.expneurol.2009.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleen JK, Scott RC, Holmes GL, Lenck-Santini PP. Hippocampal interictal spikes disrupt cognition in rats. Annals of Neurology. 2010 Feb;67(2):250–7. doi: 10.1002/ana.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holmes GL, Lenck-Santini P-P. Role of interictal epileptiform abnormalities in cognitive impairment. Epilepsy & Behavior. 2006 May;8(3):504–15. doi: 10.1016/j.yebeh.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 32.Harkin La, McMahon JM, Iona X, Dibbens L, Pelekanos JT, Zuberi SM, et al. The spectrum of SCN1A-related infantile epileptic encephalopathies. Brain. 2007 Mar;130(Pt 3):843–52. doi: 10.1093/brain/awm002. [DOI] [PubMed] [Google Scholar]
- 33.Sugawara T, Mazaki-Miyazaki E, Fukushima K, Shimomura J, Fujiwara T, Hamano S, et al. Frequent mutations of SCN1A in severe myoclonic epilepsy in infancy. Neurology. 2002 Apr;58(7):1122–4. doi: 10.1212/wnl.58.7.1122. [DOI] [PubMed] [Google Scholar]
- 34.Ohmori I, Ouchida M, Ohtsuka Y, Oka E, Shimizu K. Significant correlation of the SCN1A mutations and severe myoclonic epilepsy in infancy. Biochemical and biophysical research communications. 2002 Jul;295(1):17–23. doi: 10.1016/s0006-291x(02)00617-4. [DOI] [PubMed] [Google Scholar]
- 35.Meisler MH, Kearney JA. Sodium channel mutations in epilepsy and other neurological disorders. Journal of Clinical Investigation. 2005;115(8):2010. doi: 10.1172/JCI25466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meisler MHMH, O’Brien JE, Sharkey LMLM. Sodium channel gene family: epilepsy mutations, gene interactions and modifier effects. Journal of Physiology. 2010 Jun;588(11):1841–8. doi: 10.1113/jphysiol.2010.188482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Claes L, Del-Favero J, Ceulemans B, Lagae L, Van Broeckhoven C, De Jonghe P. De novo mutations in the sodium-channel gene SCN1A cause severe myoclonic epilepsy of infancy. American Journal of Human Genetics. 2001 Jun;68(6):1327–32. doi: 10.1086/320609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Isom LL, De Jongh KS, Catterall WA. Auxiliary subunits of voltage-gated ion channels. Neuron. 1994 Jun;12(6):1183–94. doi: 10.1016/0896-6273(94)90436-7. [DOI] [PubMed] [Google Scholar]
- 39.Beneski DA, Catterall WA. Covalent labeling of protein components of the sodium channel with a photoactivable derivative of scorpion toxin. PNAS. 1980 Jan;77(1):639–43. doi: 10.1073/pnas.77.1.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hartshornes RP, Catterall WA. The sodium channel from rat brain. The Journal of Biological Chemistry. 1984;259(3) [PubMed] [Google Scholar]
- 41.Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000 Apr;26(1):13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- 42.Gambardella A, Marini C. Clinical spectrum of SCN1A mutations. Epilepsia. 2009 May;50( Suppl 5):20–3. doi: 10.1111/j.1528-1167.2009.02115.x. [DOI] [PubMed] [Google Scholar]
- 43.Stafstrom CE. Severe epilepsy syndromes of early childhood: the link between genetics and pathophysiology with a focus on SCN1A mutations. Journal of Child Neurology. 2009;24(8 suppl):15S. doi: 10.1177/0883073809338152. [DOI] [PubMed] [Google Scholar]
- 44.Escayg A, MacDonald B, Meisler M, Baulac S, Huberfeld G, An-Gourfinkel I, et al. Mutations of SCN1A, encoding a neuronal sodium channel, in two families with GEFS+2. Nature Genetics. 2000 May;24:343–5. doi: 10.1038/74159. [DOI] [PubMed] [Google Scholar]
- 45.Catterall WA, Kalume F, Oakley JC. Nav1. 1 channels and epilepsy. Journal of Physiology. 2010 Jun;588(11):1849–59. doi: 10.1113/jphysiol.2010.187484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Escayg A, Goldin AL. Sodium channel SCN1A and epilepsy: Mutations and mechanisms. Epilepsia. 2010 May;51(9):1650–8. doi: 10.1111/j.1528-1167.2010.02640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ragsdale DS. How do mutant Nav1.1 sodium channels cause epilepsy? Brain Research Reviews. 2008 Jun;58(1):149–59. doi: 10.1016/j.brainresrev.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 48.Zuberi SM, Brunklaus A, Birch R, Reavey E, Duncan J, Forbes GH. Genotype-phenotype associations in SCN1A-related epilepsies. Neurology. 2011 Feb;76(7):594–600. doi: 10.1212/WNL.0b013e31820c309b. [DOI] [PubMed] [Google Scholar]
- 49.Scheffer IE, Zhang Y-H, Jansen FE, Dibbens L. Dravet syndrome or genetic (generalized) epilepsy with febrile seizures plus? Brain & Development. 2009 May;31(5):394–400. doi: 10.1016/j.braindev.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 50.Ogiwara I, Miyamoto H, Morita N, Atapour N, Mazaki E, Inoue I, et al. Na(v)1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. Journal of Neuroscience. 2007 May 30;27(22):5903–14. doi: 10.1523/JNEUROSCI.5270-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mashimo T, Ohmori I, Ouchida M, Ohno Y, Tsurumi T, Miki T, et al. A missense mutation of the gene encoding voltage-dependent sodium channel (Nav1.1) confers susceptibility to febrile seizures in rats. Journal of Neuroscience. 2010 Apr 21;30(16):5744–53. doi: 10.1523/JNEUROSCI.3360-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liao Y, Deprez L, Maljevic S, Pitsch J, Claes L, Hristova D, et al. Molecular correlates of age-dependent seizures in an inherited neonatal-infantile epilepsy. Brain. 2010 May;133:1403–14. doi: 10.1093/brain/awq057. [DOI] [PubMed] [Google Scholar]
- 53.Beckh S, Noda M, Lübbert H, Numa S. Differential regulation of three sodium channel messenger RNAs in the rat central nervous system during development. The EMBO journal. 1989 Dec;8(12):3611–6. doi: 10.1002/j.1460-2075.1989.tb08534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu FH, Mantegazza M, Westenbroek RE, Robbins CA, Kalume F, Burton KA, et al. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nature Neuroscience. 2006 Sep;9(9):1142–9. doi: 10.1038/nn1754. [DOI] [PubMed] [Google Scholar]
- 55.Oakley JC, Kalume F, Yu FH, Scheuer T, Catterall WA. Temperature- and age-dependent seizures in a mouse model of severe myoclonic epilepsy in infancy. PNAS. 2009 Mar;106(10):3994–9. doi: 10.1073/pnas.0813330106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang W, Takashima S, Segawa Y, Itoh M, Shi X, Hwang S-K, et al. The developmental changes of Na(v)1.1 and Na(v)1.2 expression in the human hippocampus and temporal lobe. Brain Research. 2011 May;1389:61–70. doi: 10.1016/j.brainres.2011.02.083. [DOI] [PubMed] [Google Scholar]
- 57.Martin MS, Dutt K, Papale La, Dubé CM, Dutton SB, de Haan G, et al. Altered function of the SCN1A voltage-gated sodium channel leads to gamma-aminobutyric acid-ergic (GABAergic) interneuron abnormalities. The Journal of Biological Chemistry. 2010 Mar 26;285(13):9823–34. doi: 10.1074/jbc.M109.078568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang B, Dutt K, Papale L, Rusconi R, Shankar A, Hunter J, et al. A BAC transgenic mouse model reveals neuron subtype-specific effects of a Generalized Epilepsy with Febrile Seizures Plus (GEFS+) mutation. Neurobiology of Disease. 2009 Jul;35(1):91–102. doi: 10.1016/j.nbd.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kalume F, Yu FH, Westenbroek RE, Scheuer T, Catterall WA. Reduced sodium current in Purkinje neurons from Nav1.1 mutant mice: implications for ataxia in severe myoclonic epilepsy in infancy. Journal of Neuroscience. 2007 Oct;27(41):11065–74. doi: 10.1523/JNEUROSCI.2162-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duflocq A, Le Bras B, Bullier E, Couraud F, Davenne M. Nav1.1 is predominantly expressed in nodes of Ranvier and axon initial segments. Molecular and Cellular Neurosciences. 2008 Oct;39(2):180–92. doi: 10.1016/j.mcn.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 61.Lorincz A, Nusser Z. Cell-type-dependent molecular composition of the axon initial segment. Journal of Neuroscience. 2008 Dec 31;28(53):14329–40. doi: 10.1523/JNEUROSCI.4833-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Freund T. Interneuron Diversity series: Rhythm and mood in perisomatic inhibition. Trends in Neurosciences. 2003 Sep;26(9):489–95. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- 63.Maccaferri G. Interneuron Diversity series: Hippocampal interneuron classifications–making things as simple as possible, not simpler. Trends in Neurosciences. 2003 Oct;26(10):564–71. doi: 10.1016/j.tins.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 64.Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nature Reviews. Neuroscience. 2004 Oct;5(10):793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 65.Kawaguchi Y, Kondo S. Parvalbumin, somatostatin and cholecystokinin as chemical markers for specific GABAergic interneuron types in the rat frontal cortex. Journal of neurocytology. 2003;31(3–5):277–87. doi: 10.1023/a:1024126110356. [DOI] [PubMed] [Google Scholar]
- 66.Galarreta M, Hestrin S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature. 1999 Nov;402(6757):72–5. doi: 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- 67.Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999 Nov;402(6757):75–9. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- 68.Galarreta M, Hestrin S. Electrical and chemical synapses among parvalbumin fast-spiking GABAergic interneurons in adult mouse neocortex. PNAS. 2002 Sep;99(19):12438–43. doi: 10.1073/pnas.192159599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sik A, Penttonen M, Ylinen A, Buzsáki G. Hippocampal CA1 interneurons: an in vivo intracellular labeling study. Journal of Neuroscience. 1995;15(10):6651. doi: 10.1523/JNEUROSCI.15-10-06651.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Herrmann CS, Fründ I, Lenz D. Human gamma-band activity: a review on cognitive and behavioral correlates and network models. Neuroscience and Biobehavioral Reviews. 2010 Jun;34(7):981–92. doi: 10.1016/j.neubiorev.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 71.Fries P, Nikolić D, Singer W. The gamma cycle. Trends in Neurosciences. 2007 Jul;30(7):309–16. doi: 10.1016/j.tins.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 72.Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annual Review of Neuroscience. 2009 Jan;32:209–24. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- 73.Sauvé K. Gamma-band synchronous oscillations: recent evidence regarding their functional significance. Consciousness and Cognition. 1999 Jun;8(2):213–24. doi: 10.1006/ccog.1999.0383. [DOI] [PubMed] [Google Scholar]
- 74.Herrmann CS, Demiralp T. Human EEG gamma oscillations in neuropsychiatric disorders. Clinical Neurophysiology. 2005 Dec;116(12):2719–33. doi: 10.1016/j.clinph.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 75.Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nature reviews. Neuroscience. 2010 Mar;11(2):100–13. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 76.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009 Jun;459(7247):698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, Jensen O, et al. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature. 2009 Nov;462(7271):353–7. doi: 10.1038/nature08573. [DOI] [PubMed] [Google Scholar]
- 78.Bragin A, Jando G, Nadasdy Z, Hetke J, Wise K, Buzsaki G. Gamma (40–100 Hz) oscillation in the hippocampus of the behaving rat. Journal of Neuroscience. 1995;15(1):47. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bartos M, Vida I, Frotscher M, Meyer A, Monyer H, Geiger JRP, et al. Fast synaptic inhibition promotes synchronized gamma oscillations in hippocampal interneuron networks. PNAS. 2002 Oct;99(20):13222–7. doi: 10.1073/pnas.192233099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mann EO, Suckling JM, Hajos N, Greenfield Sa, Paulsen O. Perisomatic feedback inhibition underlies cholinergically induced fast network oscillations in the rat hippocampus in vitro. Neuron. 2005 Jan;45(1):105–17. doi: 10.1016/j.neuron.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 81.Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nature reviews. Neuroscience. 2007 Jan;8(1):45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- 82.Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, Lebeau FEN, Bannerman DM, et al. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007 Mar 15;53(4):591–604. doi: 10.1016/j.neuron.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 83.Winson J. Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science. 1978;201(4351):160. doi: 10.1126/science.663646. [DOI] [PubMed] [Google Scholar]
- 84.Givens B. Stimulus-evoked resetting of the dentate theta rhythm: relation to working memory. Neuroreport. 1996 Dec;8(1):159–63. doi: 10.1097/00001756-199612200-00032. [DOI] [PubMed] [Google Scholar]
- 85.Bland BH, Oddie SD. Theta band oscillation and synchrony in the hippocampal formation and associated structures: the case for its role in sensorimotor integration. Behavioural Brain Research. 2001 Dec;127(1–2):119–36. doi: 10.1016/s0166-4328(01)00358-8. [DOI] [PubMed] [Google Scholar]
- 86.Seager Ma, Johnson LD, Chabot ES, Asaka Y, Berry SD. Oscillatory brain states and learning: Impact of hippocampal theta-contingent training. PNAS. 2002 Feb;99(3):1616–20. doi: 10.1073/pnas.032662099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Benchenane K, Peyrache A, Khamassi M, Tierney PL, Gioanni Y, Battaglia FP, et al. Coherent theta oscillations and reorganization of spike timing in the hippocampal- prefrontal network upon learning. Neuron. 2010 Jun 24;66(6):921–36. doi: 10.1016/j.neuron.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 88.Caplan JB, Madsen JR, Schulze-Bonhage A, Aschenbrenner-Scheibe R, Newman EL, Kahana MJ. Human theta oscillations related to sensorimotor integration and spatial learning. Journal of Neuroscience. 2003 Jun;23(11):4726–36. doi: 10.1523/JNEUROSCI.23-11-04726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kahana MJ, Sekuler R, Caplan JB, Kirschen M, Madsen JR. Human theta oscillations exhibit task dependence during virtual maze navigation. Nature. 1999;399(6738):781–4. doi: 10.1038/21645. [DOI] [PubMed] [Google Scholar]
- 90.Rutishauser U, Ross IB, Mamelak AN, Schuman EM. Human memory strength is predicted by theta-frequency phase-locking of single neurons. Nature. 2010 Apr;464(7290):903–7. doi: 10.1038/nature08860. [DOI] [PubMed] [Google Scholar]
- 91.Caplan JB, Madsen JR, Raghavachari S, Kahana MJ. Distinct patterns of brain oscillations underlie two basic parameters of human maze learning. Journal of Neurophysiology. 2001;86(1):368. doi: 10.1152/jn.2001.86.1.368. [DOI] [PubMed] [Google Scholar]
- 92.Ranck JB. Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats. I. Behavioral correlates and firing repertoires. Experimental Neurology. 1973;41(2):461–531. doi: 10.1016/0014-4886(73)90290-2. [DOI] [PubMed] [Google Scholar]
- 93.Klausberger T, Magill PJ, Márton LF, Roberts JDB, Cobden PM, Buzsáki G, et al. Brain-state-and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421(6925):844–8. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- 94.Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyl P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378(2):75–8. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- 95.Murray AJ, Sauer J-F, Riedel G, McClure C, Ansel L, Cheyne L, et al. Parvalbumin-positive CA1 interneurons are required for spatial working but not for reference memory. Nature Neuroscience. 2011 Mar;14(3):297–9. doi: 10.1038/nn.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Buzsaki G. Theta Oscillations in the Hippocampus. Neuron. 2002 Jan;33(3):325–40. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- 97.Dutar P, Bassant MH, Senut MC, Lamour Y. The septohippocampal pathway: structure and function of a central cholinergic system. Physiological Reviews. 1995 Apr;75(2):393–427. doi: 10.1152/physrev.1995.75.2.393. [DOI] [PubMed] [Google Scholar]
- 98.Morris NP, Harris SJ, Henderson Z. Parvalbumin-immunoreactive, fast-spiking neurons in the medial septum/diagonal band complex of the rat: intracellular recordings in vitro. Neuroscience. 1999 Jan;92(2):589–600. doi: 10.1016/s0306-4522(99)00026-3. [DOI] [PubMed] [Google Scholar]
- 99.Stewart M, Fox SE. Two populations of rhythmically bursting neurons in rat medial septum are revealed by atropine. Journal of Neurophysiology. 1989 May;61(5):982–93. doi: 10.1152/jn.1989.61.5.982. [DOI] [PubMed] [Google Scholar]
- 100.Brazhnik ES, Fox SE. Action potentials and relations to the theta rhythm of medial septal neurons in vivo. Experimental Brain Research. 1999 Aug;127(3):244–58. doi: 10.1007/s002210050794. [DOI] [PubMed] [Google Scholar]
- 101.Givens BS, Olton DS. Cholinergic and GABAergic modulation of medial septal area: effect on working memory. Behavioral Neuroscience. 1990 Dec;104(6):849–55. doi: 10.1037//0735-7044.104.6.849. [DOI] [PubMed] [Google Scholar]
- 102.Bland BH, Trepel C, Oddie SD, Kirk IJ. Intraseptal microinfusion of muscimol: effects on hippocampal formation theta field activity and phasic theta-ON cell discharges. Experimental Neurology. 1996 Apr;138(2):286–97. doi: 10.1006/exnr.1996.0067. [DOI] [PubMed] [Google Scholar]
- 103.Varga V, Hangya B, Kránitz K, Ludányi A, Zemankovics R, Katona I, et al. The presence of pacemaker HCN channels identifies theta rhythmic GABAergic neurons in the medial septum. Journal of Physiology. 2008 Aug 15;586(16):3893–915. doi: 10.1113/jphysiol.2008.155242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hangya B, Borhegyi Z, Szilágyi N, Freund TF, Varga V. GABAergic neurons of the medial septum lead the hippocampal network during theta activity. Journal of Neuroscience. 2009 Jun;29(25):8094–102. doi: 10.1523/JNEUROSCI.5665-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tóth K, Freund TF, Miles R. Disinhibition of rat hippocampal pyramidal cells by GABAergic afferents from the septum. Journal of Physiology. 1997 Apr 15;500( Pt 21997):463–74. doi: 10.1113/jphysiol.1997.sp022033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pang KCHKCH, Jiao X, Sinha S, Beck KDKD, Servatius RJRJ. Damage of GABAergic neurons in the medial septum impairs spatial working memory and extinction of active avoidance: Effects on proactive interference. Hippocampus. 2011 Aug;21(8):835–46. doi: 10.1002/hipo.20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.O’Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3(3):317–30. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- 108.Ego-Stengel V, Wilson MA. Spatial selectivity and theta phase precession in CA1 interneurons. Hippocampus. 2007;17(2):161–74. doi: 10.1002/hipo.20253. [DOI] [PubMed] [Google Scholar]
- 109.Maurer AP, Cowen SL, Burke SN, Barnes Ca, McNaughton BL. Phase precession in hippocampal interneurons showing strong functional coupling to individual pyramidal cells. Journal of Neuroscience. 2006 Dec;26(52):13485–92. doi: 10.1523/JNEUROSCI.2882-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Geisler C, Diba K, Pastalkova E, Mizuseki K, Royer S, Buzsáki G. Temporal delays among place cells determine the frequency of population theta oscillations in the hippocampus. PNAS. 2010 Apr;107(17):7957–62. doi: 10.1073/pnas.0912478107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mann EO, Paulsen O. Role of GABAergic inhibition in hippocampal network oscillations. Trends in Neurosciences. 2007 Jul;30(7):343–9. doi: 10.1016/j.tins.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 112.Losonczy A, Zemelman BV, Vaziri A, Magee JC. Network mechanisms of theta related neuronal activity in hippocampal CA1 pyramidal neurons. Nature Neuroscience. 2010 Jul;13(8):967–72. doi: 10.1038/nn.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Skaggs WE, McNaughton BL, Wilson Ma, Barnes Ca. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus. 1996 Jan;6(2):149–72. doi: 10.1002/(SICI)1098-1063(1996)6:2<149::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 114.Huxter J, Burgess N, O’Keefe J. Independent rate and temporal coding in hippocampal pyramidal cells. Nature. 2003;425(6960):828–32. doi: 10.1038/nature02058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lengyel M, Szatmáry Z, Erdi P. Dynamically detuned oscillations account for the coupled rate and temporal code of place cell firing. Hippocampus. 2003 Jan;13(6):700–14. doi: 10.1002/hipo.10116. [DOI] [PubMed] [Google Scholar]
- 116.Dragoi G, Buzsáki G. Temporal encoding of place sequences by hippocampal cell assemblies. Neuron. 2006 Apr;50(1):145–57. doi: 10.1016/j.neuron.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 117.Robbe D, Montgomery SM, Thome A, Rueda-Orozco PE, McNaughton BL, Buzsaki G. Cannabinoids reveal importance of spike timing coordination in hippocampal function. Nature Neuroscience. 2006 Dec;9(12):1526–33. doi: 10.1038/nn1801. [DOI] [PubMed] [Google Scholar]
- 118.Robbe D, Buzsáki G. Alteration of theta timescale dynamics of hippocampal place cells by a cannabinoid is associated with memory impairment. Journal of Neuroscience. 2009 Oct;29(40):12597–605. doi: 10.1523/JNEUROSCI.2407-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lenck-Santini P-P, Holmes GL. Altered phase precession and compression of temporal sequences by place cells in epileptic rats. Journal of Neuroscience. 2008 May;28(19):5053–62. doi: 10.1523/JNEUROSCI.5024-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Goldberg EM, Jeong H-Y, Kruglikov I, Tremblay R, Lazarenko RM, Rudy B. Rapid Developmental Maturation of Neocortical FS Cell Intrinsic Excitability. Cerebral Cortex. 2011 Mar;21(3):666–82. doi: 10.1093/cercor/bhq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Doischer D, Hosp JA, Yanagawa Y, Obata K, Jonas P, Vida I, et al. Postnatal differentiation of basket cells from slow to fast signaling devices. Journal of Neuroscience. 2008 Nov;28(48):12956–68. doi: 10.1523/JNEUROSCI.2890-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Scott RC, Richard GR, Holmes GL, Lenck-Santini P-P. Maturational dynamics of hippocampal place cells in immature rats. Hippocampus. 2011 Apr;21(4):347–53. doi: 10.1002/hipo.20789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wills TJ, Cacucci F, Burgess N, O’Keefe J. Development of the Hippocampal Cognitive Map in Preweanling Rats. Science. 2010 Jun 17;328(5985):1573–6. doi: 10.1126/science.1188224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cimadevilla JM, Fenton AA, Bures J. Transient sex differences in the between-sessions but not in the within-session memory underlying an active place avoidance task in weanling rats. Behavioral Neuroscience. 2001;115(3):695–703. doi: 10.1037//0735-7044.115.3.695. [DOI] [PubMed] [Google Scholar]
- 125.Lucas MM, Lenck-Santini P-P, Holmes GL, Scott RC. Impaired cognition in rats with cortical dysplasia: additional impact of early-life seizures. Brain. 2011 Jun;134:1684–93. doi: 10.1093/brain/awr087. [DOI] [PMC free article] [PubMed] [Google Scholar]