Abstract
Significant correlations between obesity and incidence of various cancers have been reported. Obesity, considered a mild inflammatory process, is characterized by a high level of secretion of several cytokines from adipose tissue. These molecules have disparate effects, which could be relevant to cancer development. Among the inflammatory molecules, leptin, mainly produced by adipose tissue and overexpressed with its receptor (Ob-R) in cancer cells is the most studied adipokine. Mutations of leptin or Ob-R genes associated with obesity or cancer are rarely found. However, leptin is an anti-apoptotic molecule in many cell types, and its central roles in obesity-related cancers are based on its pro-angiogenic, pro-inflammatory and mitogenic actions. Notably, these leptin actions are commonly reinforced through entangled crosstalk with multiple oncogenes, cytokines and growth factors. Leptin-induced signals comprise several pathways commonly triggered by many cytokines (i.e, canonical: JAK2/STAT; MAPK/ERK1/2 and PI-3K/AKT1 and, non-canonical signaling pathways: PKC, JNK and p38 MAP kinase). Each of these leptin-induced signals is essential to its biological effects on food intake, energy balance, adiposity, immune and endocrine systems, as well as oncogenesis. This review is mainly focused on the current knowledge of the oncogenic role of leptin in breast cancer. Additionally, leptin pro-angiogenic molecular mechanisms and its potential role in breast cancer stem cells will be reviewed. Strict biunivocal binding-affinity and activation of leptin/Ob-R complex makes it a unique molecular target for prevention and treatment of breast cancer, particularly in obesity contexts.
Keywords: Leptin, Breast cancer, Tumor angiogenesis, Breast cancer stem cells, Leptin antagonist, Leptin signaling
1. Introduction
Obesity and overweight conditions are prevalent in the world. The World Health Organization (WHO) reported that more than 400 million people are obese, with a predicted increase to possibly reach 700 million by 2015 worldwide [1]. In the United States, the current epidemic of obesity in adults is 30%–35%, posing a major public health challenge [2–5]. Obesity or overweight conditions are associated with a significantly increased risk of development of various diseases, particularly cardiovascular disease [6], type 2 diabetes [7], hypertension [8], dyslipidemia [9], liver disease [10], as well as cancer [11–12]. Compelling evidence indicates over 13 different cancers including breast, cervical, colon or rectal, esophageal, gall bladder, kidney, liver, ovarian, pancreatic, stomach, uterine cancer, as well as multiple myeloma, non-Hodgkin lymphoma are associated with obesity [11–14].
How obesity is connected to cancer incidence is still an unexplainable or unanswered question. However, accumulated evidence shows that these two conditions have intertwined inflammatory patterns. In obesity, deregulated secretion of pro-inflammatory cytokines, chemokines and adipokines such as TNF-α, plasminogen activator inhibitor-1 (PAI-1), IL-1, IL-6, adiponectin and leptin from the expanding adipose tissue and inflammatory cells could make a clinically relevant contribution to the onset and progression of cancer [15–17]. However, the individual contributions of these factors to obesity-related cancers are often contradictory and not well understood in diverse scenarios. Among the above mentioned molecules, leptin has been the most studied adipokine since this protein was first cloned in 1994 [18].
The identification of spontaneous mutations in the leptin (ob or LEP) and Ob-R (db or LEPR) genes in mice opened up a new field in obesity research. Although allelic frequencies of ob and db polymorphisms show ethnic variation, systematic search for mutations showed low penetration and scarce number of affected individuals. The data suggests the lack of association between the genes under study and obesity. The lack of association could be due to the complex pathogenesis of obesity, which involves a number of genetic and environmental factors [19]. High leptin levels in obesity and overweight individuals or populations are clearly correlated with body fat and adipocyte size. Under these conditions leptin is unable to regulate appetite/size of fat deposits leading to a “leptin resistance status”, which could induce deregulated peripheral actions in many Ob-R expressing tissues. The molecular mechanisms underlying how obesity causes an increased risk of cancer are poorly understood, but compelling evidence shows that pandemic obesity and cancer incidence are connected [20]. A retrospective study on a large cohort of women (n=495,477) in the US reported by Calle et al., shows a significant correlation between increasing risk and higher body-mass-index values and death from breast cancer in obese/overweight women [21]. Other studies have also found that women with a higher percentage of adipose tissue/leptin levels have higher incidence of breast cancer [11, 22–23].
In light of the increasing reported role of leptin in several types of cancer [24–28], in this review we wish to focus on the role of leptin in breast cancer, highlighting the leptin-mediated signaling pathways and its potential as a drug target. Additionally, we will review and discuss recently identified molecular mechanisms of leptin in breast cancer, including its potential role in breast cancer stem cells (BCSC), tumor angiogenesis, as well as its crosstalk with other oncogenic signaling pathways.
2. Structure and function of leptin and leptin receptor
Leptin is a small 167-amino acid non-glycosilated protein with a molecular weight of 16 kDa, coded by the LEP gene, whose name is derived from the Greek word “leptos,” which means “thin.” The LEP gene is preserved in mammals providing a high sequence identity for leptin. Indeed, human leptin and mouse leptin share 84 percent sequence homology. The cDNA sequence encoding for leptin was identified on the mouse ob (obese) gene. A nonsense mutation in codon 105 (ob/ob) causes the lack of protein synthesis resulting in morbid obesity, hyperphagia, hypothermia, insulin resistance, and infertility [18]. The biological role of leptin in energy homeostasis was demonstrated by normalization of hyperphagy and obese phenotypes with recombinant leptin administration in rodents and humans [29–30]. In addition, leptin plays critical roles in the regulation of glucose homeostasis, reproduction, growth and the immune response [31–33]. It is currently believed that leptin binding to Ob-R induces the extracellular domains of Ob-R to form a homodimer which constitutes the functional unit responsible for leptin-mediated signals. Analysis of highly conserved regions in the leptin sequence and studies of a homology-based model of the leptin-Ob-R complex suggest that two helices of leptin (H1 and H3) contain the most important receptor binding sites [34]. The N-terminal region (94 amino acids) of leptin is essential for both the biological and the receptor binding activities [35].
The leptin receptor (Ob-R) belongs to a member of the class I cytokine receptor super-family, which includes the receptors of IL-1, IL-2, IL-6 and the growth hormone [36]. This super-family of receptors lacks autophosphorylation capabilities and needs auxiliary kinases for activation. Ob-R binding to leptin induces conformational changes that recruit JAKs (Janus kinases), which in turn phosphorylates Ob-R and activates STATs (signal transducers and activators of transcription). Six leptin receptor isoforms have been discovered so far, and are generated by mRNA alternative splicing [37]: the long isoform (OB-RL or OB-Rb) with full intracellular signaling capabilities and shorter isoforms with less biological activity (OB-RS) [36]. The large extracellular domain of Ob-R (816 amino acids) is common to all Ob-R forms, and the variable length cytoplasmatic tail (300 amino acid residues) distinguishes the several isoforms [38–39]. Soluble leptin receptor has also been reported in humans [37]. Leptin binding to Ob-R induces canonical (JAK2/STATs; MAPK/ERK 1/2, PI-3K/AKT) and non-canonical signaling pathways (PKC, JNK, p38 MAPK and AMPK) in diverse cell types. Among the leptin receptor isoforms, only Ob-Rb, which contains an intact intracellular domain, has the ability to activate the intracellular JAK-STAT pathway on ligand binding [40]. Tyr 1138 of Ob-Rb is required for leptin-mediated STAT3 signaling [41–43]. However, Ob-Ra, which contains a truncated intracellular domain, and unable to activate the STAT pathway [41] may still transduce signals by way of activation of JAK2, IRS-1 or extracellular factor-regulated kinases (ERKS) including MAPKs (mitogen-activated protein kinases) [42]. In addition, the SH2 domains of SOCS (suppressor of cytokine signaling) bind the phosphorylated tyrosine residues on the JAK2s regulating OB-Rb. SOCS-3 expression is leptin-induced and is a negative regulator of leptin signaling. The over-expression of SOCS-3 inhibits leptin-induced tyrosine phosphorylation of JAK2 and ERK activation by binding to phosphorylated Tyr 985 of OB-Rb [44]. Although, leptin-induced signaling occurs in normal peripheral tissues, high levels of leptin in obese women could lead to increased leptin signaling, which is a hallmark of the development of breast and other cancers.
3. Leptin signaling in normal breast development
Human placenta was earlier identified as an important source of leptin for fetal and neonatal growth [45]. Further, the role of mammary glands in providing leptin to the newborn was reported [46]. Leptin produced by human mammary epithelial cells was revealed by RT/PCR analysis and immunohistochemical staining of breast tissue, cultured mammary epithelial cells, and secretory epithelial cells present in human milk [46]. Analyses of micrographs of whole-mount preparations of mammary tissues for LepobLepob and LeprdbLeprdb leptin-deficient and leptin receptor-deficient mice, respectively, demonstrated a notable lack of mammary duct formation in both strains of genetically obese mice, compared with their respective lean counterparts, whose ductal branching was prominent [47]. These results indicate that both leptin and Ob-R are necessary for normal mammary gland development. In line with this finding, leptin expression was also found in epithelial cells from normal human breast tissues [48]. Adipose tissue is a component of normal breast tissue. Adipocytes account for more than 90% of human breast volume and secrete adipocytokines, including leptin. Leptin derived from breast adipose tissue can act as a paracrine positive stimulus for breast cancer growth and may also have pro-oncogenic actions on the stroma-vessel components. Many inflammatory compounds are secreted by the breast adipose tissue that can also induce the recruitment of macrophages [20]. Thereby, derived-adipose factors can increase tumor angiogenesis and growth. In fact, adipose tissue can act as an effector organ and influence both breast cancer risk and tumor behavior [12, 49–50].
4. Expression of leptin and Ob-R in human breast cancer and other cancer types
Data from reverse transcription-polymerase chain reaction, immunoblotting, and immunohistochemistry techniques show that leptin and Ob-R are weakly expressed in normal tissues or cells, but these molecules are overexpressed in various cancers of the lung, colon, uterus, ovary, and breast. In contrast, Ob-R was downregulated in bladder cancer [51]. However, no supporting or conflicting data on these earlier results on bladder cancer have been published to date. In the case of human breast cancer [52], ovarian cancer [53], prostate cancer [54] and skin melanoma [55], leptin was overexpressed in more than 80% cases of malignant tissues determined by immunohistochemistry techniques. In brain [56], breast [52], esophagus cancers [57], Ob-R was also overexpressed in more than 80% cases of malignant tissues according to immunohistochemistry results. There are two reports about Ob-R in thyroid papillary carcinoma, which showed different positive rates, 80% and 51%, respectively, probably due to different antibodies used in these studies [58–59]. To the best of our knowledge, there are no reports about either leptin or Ob-R expression in human pancreatic cancer. In recent groundbreaking studies conducted by Ishikawa et al, immunohistochemical analysis of normal breast tissues resulted in weaker staining levels of leptin in normal epithelial cells compared to adipocytes in adjacent adipose tissue. In sharp contrast, leptin production was enhanced in the majority of breast cancers. Their results also showed that compared to leptin, none of the normal mammary glands exhibited significant immunoreactivity to Ob-R, whereas Ob-R was expressed in most of the carcinoma cells [52].
Table I shows the relative levels of expression of leptin and Ob-R in different cancer types as evidenced via immunohistochemistry. Notice that the percentages of leptin or Ob-R proteins represent positive staining of tissues in which only cancer cells were evaluated for antigen expression, but the expression of leptin or Ob-R in stroma and endothelial cells was not determined (see Table I).
Table I.
Leptin and Ob-R expression in human cancer tissues by immunohistochemical staining
| Tissue | Subtype | Leptin | Ob-R | References |
|---|---|---|---|---|
| B-cell | Lymphoma | ND | 39.8% | [177] |
| Bladder | Carcinoma | ND | ↓↓ | [51] |
| Brain | Glioma | 55.2% | 80.5% | [56] |
| Breast | Carcinoma | 92% | 83% | [52] |
| Colon | Carcinoma | 73.5% | 72.5% | [255–256] |
| Endometrioid | Adenocarcinomas | 60% | 31% | [257] |
| Esophagus | Carcinoma | ND | 91% | [57] |
| Kidney | Carcinoma | ↑↑ | 38.6% | [258] |
| Liver | Carcinnoma | 60.3% | 53% | [259–260] |
| Lung | Adenocarcinoma | 69.0% | ND | [261] |
| Ovary | Carcinoma | 89.5% | 59.2% | [53] |
| Prostate | Cancer | 96.7% | 63.3% | [54] |
| Skin | Melanoma | 90.9% | 43.8% | [55] |
| Thyroid | Carcinoma | ND | 80% | [59] |
| Thyroid | Carcinoma | 37% | 51%, | [58] |
ND: Not determined; ↑↑, High serum leptin in cancer patients; ↓↓, low Ob-R expression
5. Leptin signaling and breast cancer stem cells
Stem cells (SC) are defined by their ability to undergo self-renewal, as well as multilineage differentiation. Classical models of carcinogenesis propose that any cell can be transformed by accumulation of oncogenic mutations in a random way, producing a tumor. According to this model, most of the cells in a fully developed tumor are equally malignant. In contrast, the SC theory of cancer proposes that tumors contain cancer cells that retain key SC properties [60]. These cancer stem cells (CSCs) initiate and drive carcinogenesis and differentiation contributing to tumor cellular heterogeneity through deregulation of the self-renewal process [61]. Thus, the population of CSC may be a risk factor for carcinogenesis [60].
Many human cancers including breast cancer possess an enhanced tumor-initiating capacity, can undergo self-renewal, partially recreate the cellular heterogeneity of the parental tumor, and seem to be generally more resistant than other cancer cells to conventional anticancer therapeutics. Over the last decade, studies have identified CSC in solid tumors including breast cancer that are responsible for tumor initiation, growth and metastasis [62–63]. Breast CSCs (BCSCs) are characterized by the expression of molecular markers (phenotype CD44+CD24−/ALDH+) [60]. Several factors could be linked to self-renewal of BCSC. Wnt, FGF (fibroblast growth factor), Notch, Hedgehog, and TGF-β/bone morphogenetic proteins (BMP) signaling cascades constitute the stem-cell signaling network, which plays a key role in the maintenance or homeostasis of pluripotent stem cells and CSC. Human embryonic stem cells (ESCs) are supported by FGF and TGF-β signals, whereas mouse ESCs by LIF (leukemia inhibitory factor) and canonical Wnt signals [64]. Associations between birth weight, maternal levels of Insulin-like growth factor 1(IGF-1), breast cancer risk in progeny and BCSC have also been reported [65]. Recently, several reports have revealed that leptin regulates or activates certain oncogenes, such as HER2 [66–67], Akt [68] as well as transcriptional factors, such as STAT3 [69], NF-κB [70] and Notch [71–72] which are critically associated with BCSC. In addition, leptin also activates the Notch signaling pathway [71, 73] that is important for the maintenance of SC or progenitors through the inhibition of differentiation [74–76] (See Fig 1). It is known that breast cancer cell lines contain BCSC. Although the percentage of CD44+/CD24− cells within cell lines does not correlate with tumorigenicity, these cells can form tumors and preferentially survive chemotherapy [77]. Interestingly, we have found leptin induces the expression of CD44 and ALDH1 in several cancer cell lines [78]. In a recent report, leptin deficiency in murine mammary tumor virus (MMTV)-Wnt-1 transgenic mice results in functional depletion of BCSCs leading to less tumor outgrowth [79]. More recently, Feldman et al also demonstrated that robust and selective expression of Ob-R is a characteristic feature of CSCs and of a broad array of embryonic and induced pluripotent stem cells [80]. CSCs exhibit sensitized responses to leptin, including the phosphorylation and activation of STAT3 and induction of Oct4 and Sox2, thereby establishing a self-reinforcing signaling module [80].
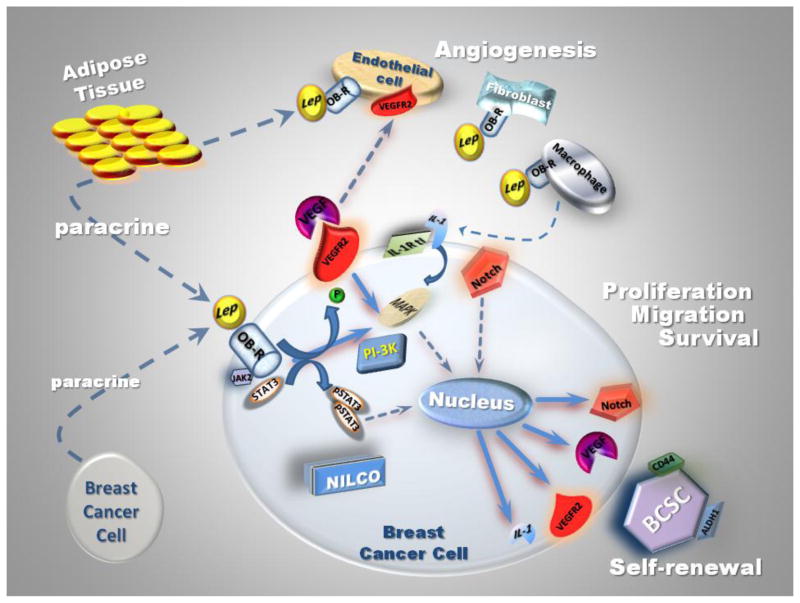
Fig. 1.
Triple-negative breast cancer (TNBC) is characterized by the lack of expression of ER, PgR, and HER-2. This difficult-to-treat form of breast cancer shows an undesirable tendency to overcome drug effectiveness. BCSCs are thought to be responsible for the development of drug-resistance and relapse of TNBC [77]. Basal-like breast cancer (BLBC) frequently expresses a CD44+/CD24− phenotype, which has been associated with a ‘stem-cell’ phenotype. These cells show resistance to conventional treatment and allow repopulation of the cancer. BCSC show some specific molecular alterations including activation of the Hedgehog and Notch pathway [81]. Leptin signaling reduces cisplatin effects on TNBC in vitro. Indeed, MDA-MB-231 (TNBC) cells treated with cisplatin (3.3 μg/ml) showed a significant reduction of cell proliferation/viability (≤ 30%) as determined by MTT assay. However, when these cells were co-incubated with cisplatin plus leptin an impressive recovery of cell proliferation (up to 150%) compared to basal conditions was observed [82]. The connection between leptin signaling and BCSC, as well as its crosstalk with many oncogenic pathways, including Notch, suggest that leptin signaling may be a viable drug target in the treatment of TNBC.
6. Leptin pro-angiogenic signature in breast cancer
Angiogenesis is critical for the growth of solid tumors [64]. The stimulation of this process in response to continuous pro-angiogenic factors by tumor cells results in a state of angiogenesis not normally present in tissues. Thus, anti-angiogenesis agents provide an important therapeutic option for prevention and treatment of breast cancer. Leptin, a regulator of energy homeostasis, is also a mitogenic and angiogenic cytokine that promotes anchorage, proliferation of microvessel and hematopoiesis, as well as increases the levels of several factors including cell cycle regulators [83–86]. Leptin induces the expression of several molecules (i.e., MMP-2, MMP-9, αvβ3 integrin, LIF, IL-1 and VEGF) in breast cancer cells that could further increase tumor angiogenesis. Leptin is as potent as VEGF in promoting tumor angiogenesis. In addition leptin phosphorylates VEGFR-2 independent of VEGF and upregulates Notch in endothelial and breast cancer cells. Moreover, leptin also acts on other stromal cells: fibroblasts and macrophages, which can secrete pro-angiogenic factors. Thus, leptin pro-angiogenic actions in breast cancer likely proceeds by mechanisms involving tumor endothelial (rapid response: leptin/Ob-R mediated pVEGFR2 and mid-term response: leptin-induced Notch), breast cancer (leptin induces VEGF expression, which induces tumor angiogenesis) and stromal cells (leptin induces the secretion of pro-angiogenic factors) (See Fig 1). However, leptin pro-angiogenic actions in breast cancer are mediated by poorly characterized mechanisms.
6.1. Metalloproteases and Integrins
The degradation of connective tissue/basement membrane proteins by MMPs is an important event for tumor angiogenesis [87]. Likewise, the interaction between integrin αvβ3 (vitronectin receptor) and the extracellular matrix is also crucial for angiogenesis and is involved in the VEGF-mediated activation of VEGFR2 [88–89] and metastasis of breast cancer cells [90]. We earlier reported that phenotypic changes of integrin and metalloproteinase secretion of the invasive human cytotrophoblast (whose invasive potential closely resembles cancer cells) were upregulated by leptin, IL-1, IL-6 and TGF-β [91]. Leptin induced some of the changes that cytotrophoblasts undergo to achieve a more invasive phenotype: upregulated α5 and α6 integrins and MMP-9 activity [91]. Moreover, leptin and IL-1β increased the expression of β3 integrin in Ishikawa cells (human epithelial endometrial cancer cell) [92]. In vivo, stimulation of circulating angiogenic cells with leptin increased their capacity to promote new vessel formation in the chorioallantoic membrane of chicken embryos and to improve neovascularization of ischemic murine hind limbs. These effects were linked to leptin/Ob-R-induced activation of JAK2 and phospholipase C (PLC) γ-mediated, which in turn induced Src kinase phosphorylation of αvβ5 integrins [93]. Furthermore, leptin induced the activation of αvβ5 and α4 integrins in human endothelial progenitor cells (EPCs) through STAT3 mechanisms. Leptin’s effects modulated the vascular remodeling and neointima formation in vitro and in vivo by increasing the number of EPCs adhering to vitronectin. In addition, leptin- enhanced the ability of EPCs to incorporate into a monolayer of human endothelial cells and the adherence of these cells to activated platelets. These findings suggest that leptin specifically modulates the adhesive properties and the homing potential of EPCs, thus enhancing their capacity to promote vascular regeneration in vivo [94]. Additionally, leptin increased the migration of human androgen-dependent (LnCaP) and independent (PC3 and DU145) prostate cancer cells and the expression of αvβ3 integrin. These leptin-induced effects were related to leptin activation of IRS-1, PI3K, Akt, and NF-κB signaling cascades [95]. In line with these findings, the expression of αvβ3 integrin was upregulated by leptin in human chondrosarcoma cells through the same signaling pathways [96].
6.2. Regulation of LIF and IL-1
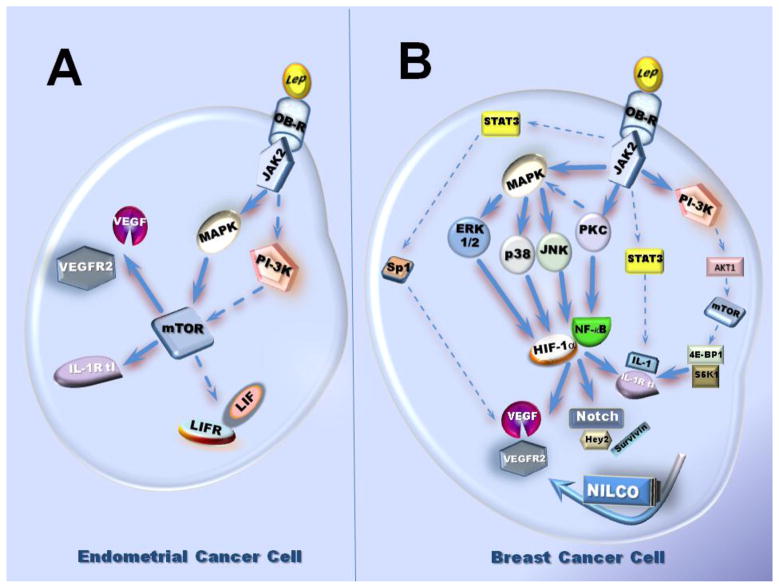
LIF (leukaemia inhibitory factor) and leptin have several similar features. Both of them are down-regulated by the suppressor of cytokine signaling-3 (SOCS-3). LIF signals through its receptor LIF-Rα (also called LIF-Rβ and gp130) by activating similar signaling pathways that those canonical pathways induced by leptin (JAK/STAT3, PI-3K/Akt and SHP2 [SH2 (Src homology 2) domain-containing tyrosine phosphatase 2]/MAPK) [97]. LIF is a key cytokine for maintaining self-renewal and pluripotency of embryonic stem cells. LIF is also an essential factor for embryo implantation and is involved in inflammation/angiogenesis inducing disparate biological responses in different cell types. Moreover, the angiogenic effects of LIF reported have been contradicting [86, 98–99]. LIF induces chemotaxis of mouse peritoneal macrophages in vitro and infiltration of macrophages in CNS lesions is delayed in LIF knock-out mice [100]. Leptin exerts differential effects on LIF regulation in diverse cell types. Leptin also induced opposing effects by increasing or repressing the secretion of LIF in monocytes and T cells, respectively. These results suggest leptin-induced LIF may modulate macrophage function [101]. In addition, leptin treatment to endometrial cancer cells (Ishikawa cells) increased the levels of phosphorylated STAT3, LIFR, and LIF. Moreover, treatment of Ishikawa cells with IL-1β also resulted in elevated levels of LIFR [102]. Furthermore, leptin induction of LIF/LIFR in human cancer endometrial cells (An3Ca, SK-UT2 and Ishikawa) involved JAK2, MAPK and mTOR (mammalian target of Rapamycin) activation [103].
IL-1 family belongs to pro-inflammatory/-angiogenesis cytokines, which is represented by two ligands: IL-1α, IL-1β, an antagonist: interleukin-1 receptor antagonist (IL-1Ra) and two receptors: IL-1R tI (type I receptor) and IL-1R tII (type II receptor) [104]. IL-1 plays a key role in the onset and development of the host reaction to invasion, being an important factor in the initiation of the inflammatory response and immune functions. IL-1 system is overexpressed in cancer cells and inflammatory cells invading tumor tissues. IL-1 is abundant at tumor sites, where it may affect carcinogenesis, tumor growth and invasiveness, and the patterns of tumor-host interactions and tumor angiogenesis [105]. Furthermore, IL-1 family, Notch and leptin crosstalk represents a major link among obesity, inflammation, angiogenesis and breast cancer progression [71, 106–108].
The IL-1 family and leptin are associated in several pathological situations, i.e., cachexia, cancer, reumathoid arthritis, and endogenous Cushing’s syndrome [106, 109–112], suggesting an interaction between them. In endometrial cancer cells, leptin induces the expression of IL-1 system [103]. Kinase inhibition studies showed that leptin increase of IL-1β levels in primary-EEC was mediated by JAK2/STAT3, PI-3K/AKT1 and mTOR signaling pathways (see Fig 2). In contrast, in benign immortalized HES and cancer-EEC, leptin-mediated increase in IL-1R tI involved the activation of JAK2/STAT3, MAPK/ERK1/2 and mTOR but PI-3K/AKT1 phosphorylation did not show a clear pattern. Notably, the highest levels of IL-1 R tI induced by leptin were seen in SK-UT2 cancer cells [103]. In MCF-7 xenograft model of breast cancer, MCF-7 cells produce a high level of IL-1α, which in turn induces leptin expression in stromal cells or in infiltrating immune cells recruited in the tumor microenvironnement [109]. We have recently reported that leptin increased the protein and mRNA levels of all components of the IL-1 system in breast cancer cells [113]. Leptin-induced canonical and non-canonical signaling pathways were all associated with IL-1 up-regulation. Furthermore, as it was shown for Notch, VEGF and VEGFR2, leptin upregulation of IL-1α promoter also involved the activation of SP1 and NF-κB transcription factors. Interestingly, blockade of IL-1R tI inhibited leptin-induced VEGF/VEGFR-2 expression. These data suggest that leptin pro-angiogenic signature is related to IL-1 [71]. Moreover, inhibition of IL-1 signaling by specific anti IL-1R tI antibodies also abrogated leptin-induced Notch, Hey2 and Survivin [71]. Then, a complex crosstalk between leptin and pro-angiogenic, inflammatory and mitogenic factors occurs in breast cancer (See Fig 2).
Fig. 2.
6.3. Leptin impact on VEGF/VEGFR-2 signaling
Signals triggered by VEGF (a major angiogenic factor) binding to its receptor, VEGFR-2 is required for normal and pathological proliferation, migration and differentiation of endothelial cells [114–115]. While VEGF and VEGFR-2 are not oncogenes, their expression is enhanced by several signals triggered in angiogenic tumors including breast cancer [114–115]. VEGFR-2, receptor type 2 (KDR or flk-1) is generally recognized to have a principal role in mediating VEGF-induced responses, and is considered as the earliest marker for endothelial cell development (Review see [89]). Moreover, VEGFR-2 directly regulates tumor angiogenesis [89, 115–116]. In addition to its angiogenic actions in endothelial cells the VEGF/VEGFR-2 paracrine-autocrine loop functions as an important survival mechanism in cancer cells [89].
Recently, leptin was reported to upregulate VEGF-A and VEGFR-2 in cancer cells [103, 117–118]. Leptin increased VEGFR-2 expression in endometrial [103] and breast cancer cells in vitro [69] and in vivo [117–118]. Interestingly, leptin upregulation of VEGF-A/VEGFR-2 was partially mediated by IL-1 system [113]. A comprehensive mechanism for leptin regulation of VEGF in breast cancer cells through activation of several canonical and non-canonical signaling pathways and NFκB and SP1 was recently reported [119] (see Fig. 2). Moreover, leptin induced rapid phosphorylation of VEGFR-2 (Y1175) in absence of VEGF in endothelial cells [120]. These leptin effects positively impacted on endothelial cell proliferation, survival and migration. Leptin-VEGFR-2 actions in endothelial cells involved a crosstalk between p38 kinase/Akt1 and COX-2 [120]. We have also found leptin induces pY951 VEGFR-2 in 4T1 mouse breast cancer cells. However, the biological significance of this finding needs further investigation.
Leptin is a known mitogenic, inflammatory and angiogenic factor for many tissues [84] and increases VEGF/VEGFR-2 expression in cancer cells [103, 117–118]. Therefore, leptin from corporal, mammary adipose or tumor tissues could affect cancer cells through autocrine and paracrine actions. Findings from our laboratory suggest that mouse (4T1, ER +) [117] and human breast cancer cells (MCF-7, ER+ and MDA-MB-231, ER-) express VEGFR-2 in vitro and in vivo [118]. Leptin increased the proliferation of 4T1 cells, but treatment with anti-VEGFR-2 antibody resulted in a further increase in leptin-induced VEGF concentrations in the medium. This suggests a feedback loop between VEGF expression and VEGFR-2 linked to cancer cell survival/proliferation that was later assessed by Aesoy et al in MCF-7 cells [121]. Remarkably, in mice hosting breast tumors either derived from 4T1 [117] or MCF-7 and MDA-MB231 cells [118], the inhibition of leptin signaling mediated an impressive reduction of tumor growth that paralleled a significant reduction of VEGF and VEGFR-2 levels [117–118]. However, the effects of inhibition of leptin signaling in these mouse models were more marked in MCF-7 ER+ cells [118]. We have also found that leptin activated NFκB, Sp1 and HIF-1α increasing the expression of Notch [71, 119] and VEGF mRNA and protein [119] in breast cancer cells under normoxic conditions. Furthermore, leptin-mediated activation of NFκB increased VEGFR-2 expression. Additionally, leptin increased promoter activities of VEGFR-2-Luc transfected-cells through several signaling pathways. Interestingly, the effects of leptin on VEGFR-2 were abrogated by a γ-secretase inhibitor. These data imply that a crosstalk between Notch and leptin is required for regulation of VEGFR-2 expression in breast cancer. Indeed, we have shown that the Notch, IL-1 and leptin crosstalk outcome (NILCO) is essential for leptin regulation of VEGF/VEGFR-2 in breast cancer [71]. Moreover, VEGFR-2 transcription and expression were heavily dependent upon VEGFR-2 gene methylation and histone acetylation that could be linked to leptin and Notch effects (Guo and Gonzalez-Perez, unpublished data). However, the molecular mechanisms of how leptin signaling regulates VEGFR-2 are largely unknown [122–124].
6.4. Leptin, macrophages, tumor stroma and breast cancer progression
It has long been recognized that once a critical mass of tumor cells is achieved, then the cancer cells initiate a colossal transformation of the microenvironment by secreting several factors. Many of these factors trigger angiogenesis needed for the tumor maintenance and growth. Accumulating data suggest that angiogenesis is controlled by opposing actions of an increasing number of pro- and anti-angiogenic factors. It is known that tumor stroma (noncancerous cells; that is, fibroblasts, immune and endothelial cells) could play a role in promoting tumor growth. Interestingly, data from SCID mice hosting human breast cancer cell xenografts suggested that stroma from MCF-7 breast cancer had higher levels of mouse VEGF than those from MDA-MB-231. These data were in contrast to the higher levels of human VEGF secreted by MDA-MB-231 in comparison with MCF-7 breast cancer cells. Thus, in addition to secrete VEGF the cancer cells (ie., MCF-7) can secrete factors may further promote angiogenesis via mouse tumor-stroma, consequently, contributing to tumor growth [118].
Recent evidence supports important roles for macrophages and adipocytes in breast cancer progression. Macrophage density (tumor associated macrophages, TAM) in breast cancer is specifically associated with poor tumor prognosis. These immune cells express diverse receptors that enable an active crosstalk with cancer and adipose cells [i.e., Ob-R, TLR4 (toll-like receptor 4, a lipopolysaccharide receptor also activated by fatty acids); CCR2 [(inflammatory chemokine (CCL2)/ chemoattractant protein -1 (MCP1) receptor] and ER] [125–128]. Macrophages respond to leptin stimulation by secreting pro-inflammatory and pro-angiogenic cytokines, i.e. IL-1, TNFα, IL-1, IL-6, IL-12 and nitric oxide, and thus may modulate macrophage phenotype: M1 (Th1 immunity-proinflammatory/cytotoxic) and M2 (suppress Th1/anti-inflammation/pro-angiogenesis) activation profiles [129]. Polarized M1 macrophages also colonize adipose tissue, particularly in obese individuals. These findings reinforce the idea that obesity is a state of chronic inflammation [130–131].
IL-1 secreted by macrophages, cancer and adipose cells all contribute to trigger signaling pathways that are shared by the TLR4 pathway [132]. Remarkably, in endometrial and breast cancer cells leptin-induced IL-1, which could further impact angiogenesis through intracellular and extracellular loops [102–103, 113]. Leptin and IL-1 synergic actions can activate NFκB that increase VEGF through an intracellular loop. It would appear that leptin-induced levels of IL-1 could enhance the synthesis of macrophage MCP-1 by breast cancer cells via an extracellular loop leading to the induction of angiogenesis upon the rise of recruitment and activation of infiltrating TAM. These actions would provide a link between pro-inflammatory and pro-angiogenic actions of leptin, IL-1, VEGF and macrophages in breast cancer progression.
Moreover, leptin can increase aromatase expression and ERα activation in macrophages, further contributing to macrophage recruitment [133]. After menopause, when the ovaries cease to produce estrogen, adipose tissue is the main source of estrogen. Moreover, breast adipose tissue may have enlarged or modified composition in postmenopausal-obese women, thus, locally providing higher amounts of estrogen, pro-inflammatory cytokines and adipokines such as leptin. Interestingly, it is known that leptin upregulates Ob-R receptor in tumor cells [134], however, we have found leptin downregulates Ob-R in TAM from murine breast cancer [Torroella-Kouri M, unpublished]. These data suggest that regulation of the leptin system may be different in macrophages and tumor cells. The biologic significance of these findings requests further investigation.
STAT3 constitutively activated in TAM is associated with the expression of several tumor-promoting genes [135]. Leptin signaling may also induce STAT3-mediated VEGF expression in macrophages [136]. TAM show activated oncogenic signaling pathways such as Notch and Wnt-β–catenin [137–138]. Probably, these signaling pathways are related to the TAM interplay with CSC. Indeed, glioma CSC induced M2-macrophages. In addition, macrophages induced tumorigenicity, self-renewal capacities and drug resistance in colon and lung CSC [139]. Furthermore, TAM regulates in a TGF-β dependent manner the epithelial-mesenchymal transition (EMT), which is associated with increased aggressiveness, invasive and metastatic potential of basal-like phenotype of tumor cells [140].
All the above data suggest that unexplored mechanisms are involved in an active crosstalk between macrophages-adipose and breast cancer cells. Investigations aimed to clarify these poorly understood relationships could lead to the discovery of novel ways to prevent and treat breast cancer.
7. Crosstalk between leptin signaling and oncogenic pathways in breast cancer
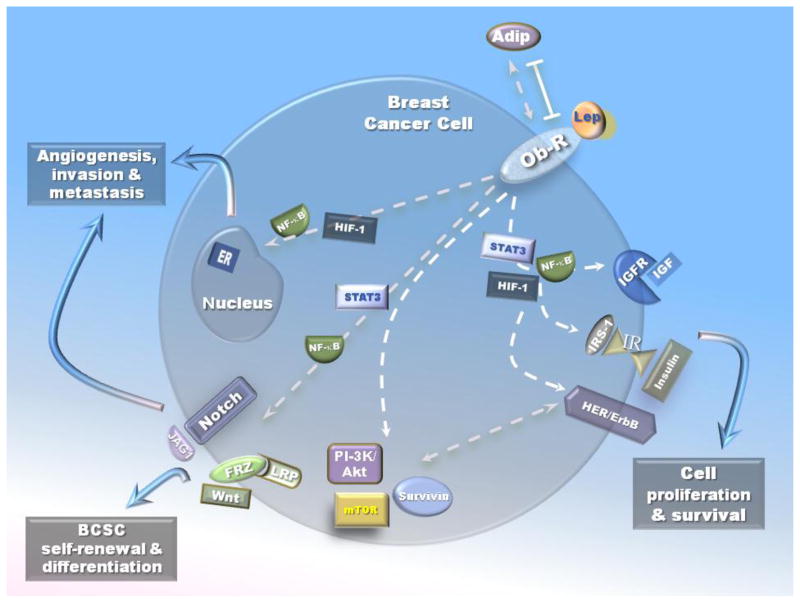
Leptin is a pleitropic adipocytokine that exerts mitogenic, pro-inflammatory and pro-angiogenic effects promoting anchorage, proliferation of breast cancer cells, microvessel and hematopoiesis and increases the levels of several factors including cell cycle regulators [117–118, 141]. As discussed before, leptin induces several canonical and non-canonical signaling pathways. These signals are essential for leptin to exert its biological effects in food intake, energy balance, and adiposity as well as in the immune and endocrine systems [142–143]. Many of the effects of leptin are attributable to effects in the central nervous system, particularly in the hypothalamus, a site of high Ob-Rb mRNA expression. Potentially important roles for membrane bound short-form receptors include the endocytosis and transport of leptin across the blood-brain barrier. Alternative splicing and proteolytic cleavage events also produce a circulating extracellular domain of Ob-R, which may affect the stability and/or availability of circulating leptin [32]. High levels of leptin - found in blood of obese individuals- fail to suppress feeding and decrease body weight/adiposity. This failure to prevent or mitigate obesity suggests a relative resistance to the catabolic effects of leptin action in obesity. Proposed mechanisms of leptin resistance include alterations in the transport of leptin across the blood-brain-barrier, alterations in cellular Ob-Rb signaling, perturbations in developmental programming, and others [144]. However, high levels of circulating leptin could over regulate signaling and expression of active Ob-R in peripheral tissues. These phenomena lead to the deregulation of leptin signaling, which contributes to breast cancer progression through its crosstalk with multiple oncogenic pathways (Fig 3).
Fig. 3.
7.1. Developmental signaling pathways
7.1.1 Wnt signaling pathway
Wnt signaling is a developmental signaling pathway that plays a critical role in regulating the normal development of the mammary gland. Deregulation of Wnt signaling is critical for breast cancer [145–146]. Fenton et al reported that genes regulating the Wnt/β-catenin-mediated pathway including Mdm2, Pik3r1, and Rb1 were upregulated by leptin inducing the survival and proliferation of colon epithelial cells possessing an Apc mutation but not normal cells [147]. These leptin effects were linked to IGF-I metabolism as in colon epithelial APC-mutated cells IGFBP-6 and IGF-1expression was upregulated, while IGFBP-2, IGFBP-3, IGFBP-4, IGFBP-5, and nephroblastoma overexpressed gene-protein (NOV) expression was downregulated by leptin treatment [147]. Interestingly, HES1and HES5, which are the downstream transducers of Notch signaling, could be activated by NOV, thus reducing cell differentiation [91]. Therefore, inhibition of leptin signaling may be an effective strategy for therapy and prevention of colonic adenoma and cancer, which shows activation of Wnt signaling [148]. More recent data suggest that leptin could also regulate the Wnt pathway contributing to breast cancer development. Indeed, leptin deficiency suppressed MMTV-Wnt-1 mammary tumor growth in obese mice, and abrogated tumor initiating cell survival [79]. We also found that leptin increased the levels of β-catenin and Notch in breast cancer cell cultures. The addition of Wnt-1 agonist to these cells also affected Notch levels. Likewise, the inhibition of leptin signaling decreased both Wnt and Notch expression in mammary tumors induced by DMBA in diet-induced-obesity mice (DIO) [149].
7.1.2. Notch signaling pathway
Notch is a family of mammalian transmembrane proteins that function as receptors for membrane bound ligands. There are four mammalian Notch genes, Notch1–Notch4, and five ligands, Jagged1, Jagged2 (homologs of Drosophila Serrate-like proteins), Delta-like 1 (DLL1), DLL3, and DLL4. Notch receptors have an extracellular domain made up of multiple epidermal growth factor domains (EGF), but the Notch intracellular domain is made up of many domain types [150]. The Notch proteins have been proven to affect diverse cell programs including angiogenesis, proliferation, differentiation, and apoptosis [72, 151]. Consequently, Notch influences organogenesis and morphogenesis [74]. The activation of a Notch receptor is triggered by ligands expressed on adjacent Jagged and Delta cells. In an earlier report, it was shown that leptin may regulate Jagged1 and Notch4 in human cord blood CD34+ cells and early differentiated human endothelial cells (HUVEC) possibly promoting cell differentiation [152]. We recently observed that leptin was able to upregulate Notch (ligands, receptors and targets) in HUVEC cells [153]. Moreover, leptin activated Notch signaling pathway (Notch1,3,4 NICD and CSL) in mouse (4T1, EMT6 and MMT) and human breast cancer cell lines (MCF-7 and MDA-MB-231) under normoxic conditions [71–72]. Leptin was demonstrated to increase the expression of both Notch receptors and ligands [71–72]. In these cells, leptin also up-regulated Notch-target genes, Hey2 and Survivin [89, 107]. Leptin-induced non-canonical signaling pathways (PKC, p38 and JNK) differentially impacted on CSL promoter activity and on the expression of IL-1 system [108]. Interestingly, leptin upregulatory effects on pro-angiogenic factors IL-1, VEGF/VEGFR-2, Notch, as well as OB-R were significantly abrogated by a γ-secretase inhibitor, DAPT as well as siRNA against CSL [71].
7.2. Growth factors
7.2.1. HER/ErbB
HER/ErbB (HER1/EGFR, HER3 and HER4) receptor tyrosine kinases bind several ligands (epidermal EGF; amphiregulin; heregulin or neu-mouse and TGF-α). HER2/neu (HER2 in humans and neu in mice) is an orphan receptor without a known ligand. The HER2/neu proto-oncogene (also known as c-erbB-2) encodes a 185 kDa transmembrane glycoprotein receptor known as HER2/neu or p185HER2 that has partial homology with EGFR and shares with that receptor intrinsic tyrosine kinase activity. It consists of three domains: a cysteine-rich extracellular domain, a transmembrane domain, and a short cytoplasmic domain. The HER2/neu gene has been implicated in cancer with special emphasis in breast cancer [154–155]. Amplification of HER2 occurs in 20%–25% of breast cancers and is also associated with an aggressive tumor phenotype and poor prognosis [156].
Importantly, HER2/neu could be transactivated by leptin/Ob-R signaling [92]. Furthermore, Ray et al [157] showed that leptin-induced proliferation of ER-positive (T47-D and MDA-MB-361) and ER-negative (MDA-MB-231 and SK-BR-3) breast cancer cell lines was HER2/neu dependant. Soma et al [158] further observed that leptin induced rapid-tyrosine phosphorylation of HER2 in SK-BR-3 cells that was partially reduced by an EGFR inhibitor, AG1478, or a JAK inhibitor, AG490. In these cells, leptin also induced pERK 1/2, which was mostly abrogated by a HER2 tyrosine kinase inhibitor, AG825. Therefore, leptin can transactivate HER2 through both EGFR and JAK 2 activation. In line with these findings, Ob-R and HER2 were also observed to physically interact and be coexpressed in several breast cancer cell lines; further suggesting leptin may contribute to enhanced HER2 activity [159]. Therefore, above emerging evidence suggests the existence of crosstalk between HER2/neu and Ob-R signaling pathways in breast cancer.
7.2.2. IGF signaling
Obesity correlated with high IGF-I [160] and leptin [161] levels in women without breast cancer. High expression levels of leptin [162] and IGF-I [163] were also positively associated with increased breast cancer risk. Both leptin and IGF-I are components of the metabolic system [164–165] and their levels are influenced by body mass index (BMI) [162, 166]. In addition, leptin can be regulated by other obesity-related stimuli such as insulin, estrogens, and hypoxic conditions [167]. In contrast, inconsistent data on leptin-induced regulation of IGF-I have been shown [168–169]. Interestingly, a bidirectional crosstalk between leptin and IGF-I signaling through EGFR signals were related to the invasion and migration of breast cancer cells in vitro (i.e., ER+, MCF-7 and TNBC, MDA-MB-231 and MDA-MB-468) [169]. This study further suggested that phosphorylation of Ob-R and IGF-IR subsequently activated downstream signaling molecules Akt, ERK, IRS-1, and IRS-2. EGF inhibitor erlotinib and dual tyrosine kinase inhibitor lapatinib inhibit leptin- and IGF-I-induced invasion and migration of breast cancer cells [169]. These data were later corroborated by using p- on Tyr1141, which is required for Ob-R to bind to STAT3 and subsequently activation by JAK2 [168]. However, these studies suggested leptin-IGF-I crosstalk is unidirectional, as leptin was not able to activate IGF-IR on Tyr 1131 or 1135/1136, which are critical for its mitogenic and transforming activity [168]. Conspicuously, the cell lines used in the study highly expressed HER2 (MCF-7, BT-474 and SKBR3), which may affect the crosstalk between leptin and IGF-I signaling. On the other hand, E2 is known to enhance both leptin/Ob-R and IGF-I/IGF-I receptor pathways. ERα actions on leptin/Ob-R pathway were suggested to be mediated by IGF-1 signaling pathway [170–171]. In summary, interactions between leptin/Ob-R and IGF-I/IGF-IR could contribute to the initiation and progression of breast cancer. Further research in this field is necessary to address these challenges.
7.3. Oncogenic kinases and Transcription factors
7.3. 1. PI-3K/Akt signaling pathway
PI-3K/Akt pathway plays a central role in a variety of cellular processes including cell growth, proliferation, motility, survival and angiogenesis in tumor cells including breast cancer [172–173]. The PI-3K/Akt pathway is also instrumental in EMT during carcinogenesis [174]. Moreover, many of the transforming events in breast cancer are a result of enhanced signaling of the PI3-K/Akt pathway [173, 175]. A number of studies have already established the central role of leptin-induced regulation of the PI-3K/Akt signaling pathway in several type of cancer [53, 56, 59, 176–182].
7.3.2. mTOR Signaling
The mammalian Target of Rapamycin (mTOR), a key protein kinase, controls signal transduction from various growth factors and upstream proteins to the level of mRNA translation and ribosome biogenesis [183–184]. mTOR has been intensely studied for over a decade as a central regulator of cell growth, proliferation, differentiation, autophagy, angiogenesis and survival [183, 185–186]. mTOR signaling has been reported to crosstalk with the leptin signaling pathway in several types of malignant cells [71, 103, 113, 187]. IGF-I induced phosporylation of Ob-R leucine could activate leptin expression via mTOR in adipocytes [93]. A recent review has provided an excellent overview of the detailed relationships between leptin and mTOR signaling [188].
mTOR is a ser/threo kinase that is often a downstream effector of PI-3K/Akt signaling pathway in breast and other types of cancer cells. Leptin-induced IL-1/IL-1Ra and VEGF in endometrial cancer cells involved PI-3K/Akt phosphorylation. Activated PI-3K/Akt was in turn strongly linked to mTOR-mediated activation of its downstream targets, S6K1 and 4BEP-1 [113]. However, MAPK pathway was identified as the preferential upstream regulator of mTOR in the induction of inflammatory/pro-angiogenic molecules in endometrial cancer cells [103]. mTOR can also phosphorylate Akt [189]. Aberrant activation of mTOR pathway was found in many types of cancer and thus may play a major role in breast cancer cell proliferation and resistance to anti-cancer drugs [190–192].
7.3.3. NF-κB signaling pathway
The family of NF-κB transcription factors is involved in the expression of key genes for innate and adaptive immunity, cell proliferation and survival, and lymphoid organ development. NF-κB, linked to tumor angiogenesis [119], is activated in a variety of cancers, including breast cancer [193–194]. NF-κB family, RelA (p65), RelB, c-Rel, p105/p50 and p100/p52 are evolutionarily conserved molecules that form hetero- or homodimers. The p65/p50 heterodimer, the most abundant form of NF-κB is regulated by the so-called canonical pathway [193, 195]. NF-κB is activated by many divergent stimuli, including pro-inflammatory cytokines such as interleukin-1β (IL-1β), epidermal growth factor (EGF), T- and B-cell mitogens, bacteria and lipopolysaccharides (LPS), viruses, viral proteins, double-stranded RNA, and physical and chemical stressors [194, 196]. Cellular stressors such as ionizing radiation and chemotherapeutic agents also activate NF-κB [197].
It is not surprising that NF-κB has been found to be activated by leptin in many tumors, such as glioma [198–199], chondrosarcoma [96], colon cancer [200–201], prostate cancer [95], as well as breast cancer [113, 119]. Leptin enhances migration, invasion and proliferation of malignant cells, possibly through activation of NF-κB [96, 199–200]. Our previously work strongly suggests that leptin regulates pro-angiogenic, pro-inflammatory and anti-apoptotic actions in breast cancer cells through activation of NF-κB [113, 117–119].
7.3.4. STAT3 signaling pathway
Signal transducer and activator of transcription (STAT3) has been placed at a central node in the development, progression, and maintenance of many human tumors [202–203]. STAT3 modulates the transcription of a variety of genes involved in the regulation of critical functions, including cell differentiation, proliferation, apoptosis, angiogenesis, metastasis, and immune responses. STAT3 protein is located in the cytoplasm in an inactive form and is activated via phosphorylation (pSTAT3) by the Janus tyrosine kinases (JAKs). The active form of STAT3 dimerizes and quickly translocates to the nucleus. For many cancers, elevated levels of activated STAT3 have been associated with poor prognosis, suggesting that STAT3 could be an attractive molecular target for the development of novel cancer therapeutics [204–205].
The STAT3 pathway is critical for mediating leptin actions on food intake, glucose metabolism, and weight gain, but it does not influence fertility [206]. A number of studies have also demonstrated that the STAT3 pathway is involved in leptin actions in cell migration [182, 207], proliferation [208–213], anti-apoptosis [179, 214–215] of malignant cells. STAT3 as a transcriptional factor regulates genes including cyclin D1[208, 210], cyclooxygenase (COX)-2 [180], VEGF [119, 216], leptin [216], human telomerase reverse transcriptase (hTERT) [217] and Survivin [218]. In addition, STAT3 could also directly or indirectly regulate IL-1, Notch [71, 113], as well as cancer stem cell markers, CD44 and ALDH1 (Guo and Gonzalez-Perez, unpublished).
7.3.5. HIF signaling pathway
Hypoxia-inducible factors (HIFs), key molecules overexpressed in response to oxygen deficiency, are master regulators of genes involved in tissue reoxygenation [219]. HIFs facilitate cancer progression by promoting tumor neoangiogenesis, cell motility, and invasion [220]. HIF-1 is a heterodimer consisting of a constitutively expressed HIF-1β subunit and an oxygen-regulated, unstable HIF-1α subunit. HIF-1 interactions with DNA are mediated through Hypoxia-Responsive Elements (HRE) [219]. Several studies have demonstrated that HIF-1 plays important roles in the development and progression of cancer or CSC through activation of various genes involved in angiogenesis, energy metabolism, vasomotor function, erythropoiesis, and cell survival [221–222].
Correlation between leptin and HIFs is bi-directional. HIFs can upregulate leptin signaling in several cell types. In trophoblast-derived BeWo cells exogenous HIF-1α markedly increased leptin-promoter luciferase-reporter activity [223]. Moreover, HIF-1α induced aberrant expression of leptin in ectopic endometriotic stromal cells, which may have a role in the etiology of endometriosis [224]. In endometrial cancer, the expression of HIF-1α was positively correlated with leptin (P < 0.0001, r = 0.573) and Ob-R (P = 0.020, r = 0.299) [225]. Furthermore, in human colorectal cancer HIF-1α and leptin signaling were also positively correlated [226]. A recent report employing reverse chromatin precipitation in MCF-7 breast cancer cells further demonstrated that loading of HIF-1α on the proximal leptin promoter concurred with the recruitment of p300, the major HIF coactivator, suggesting that the HIF/p300 complex was involved in leptin transcription [227]. On the other hand, leptin could also upregulate HIF-1. We recently reported that leptin induced the increase of HIF-1α DNA binding activity to VEGF promoter [119]. Moreover, deletion analyses of VEGF promoter suggest that HRE binding sites are critical for leptin regulation of VEGF gene in mouse mammary tumor (MT) cells. Treatment of MT with HIF-1α shRNA completely abrogated leptin-mediated induction of VEGF protein, mRNA, promoter-luciferase activity and HIF-1α accumulation in MT nuclei [119]. These results suggest that leptin-induced VEGF transcription involves the enhanced ability of HIF-1α to bind HRE within the VEGF promoter in MT.
7.4. Other crosstalk signaling
7.4.1. ER signaling
Elevated lifetime estrogen exposure has been shown to be a major risk factor for cancer in hormone-dependent organs, particularly in breast and endometrium [228–229]. Estrogens are also important regulators of the development and progression of ER+ breast carcinoma [230–231]. ERs are known to regulate a huge number of genes affecting cancer proliferation and vascular function [232–233].
Earlier studies suggest that estrogen may influence leptin synthesis in a tissue- and cell type-specific fashion [234]. Jarde et al [199] examined the expression of leptin, Ob-R and ER in human primary breast cancer and adjacent non-cancerous tissue. Their findings suggest that Ob-R and ER are co-expressed in breast cancer indicating a possible interaction between leptin and estrogen systems to promote breast carcinogenesis [235]. Antiestrogens may stimulate the synthesis and release of leptin in the adipocytes. Moreover, tamoxifen therapy commonly used in ER+ breast cancer induces the levels of leptin. This effect of antiestrogens resembles that of the estrogen and consequently stimulation of leptin production can be added to the agonistic effects of some antiestrogens [236]. In addition, leptin can transactivate ER [237] and increase the expression of aromatase in MCF-7 cells [238], potentially contributing to the proliferation and progression of breast cancer. These leptin effects represent additional links between obesity and estrogen signaling that promotes the development of estrogen-responsive breast cancer.
7.4.2. Survivin
Survivin, a member of the inhibitor of apoptosis (IAP) family of proteins, regulates two essential cellular processes for cancer growth: inhibition of apoptosis and stimulation of cell proliferation. Survivin is highly expressed during fetal development and cancer development, but it is rarely found in normal healthy adult tissues [239–240]. Recently, Survivin, was identified as a novel target of Notch [239–240] and CD44 genes [241]. Notch directly activates at least one RPB-Jκ site in the Survivin promoter [242]. Activation of Notch resulted in direct transcriptional up-regulation of Survivin in ER–breast cancer cells [243–244]. This Notch-Survivin signaling axis may contribute to worse clinical outcome of basal-like breast cancer (TNBC) patients, and may open new prospects for individualized therapy of these recurrence-prone patients.
Leptin upregulated Survivin mRNA and protein expression in MCF-7 breast cancer cells [218, 245]. It seems leptin-induced pSTAT3 is involved in these effects as siRNA-STAT3 knockdown blocked leptin-induced upregulation of Survivin [218]. Moreover, leptin-induced transactivation of EGFR was also involved in leptin-mediated Notch1 and Survivin upregulation [73]. We recently reported that in breast cancer cells, leptin up-regulated Notch1-4/JAG1/Dll-4, Notch target genes: Hey2 and Survivin, together with IL-1 and VEGF/VEGFR-2 [71]. Blockade of Notch or IL-1R tI signaling inhibited leptin-induced Survivin as well as VEGF/VEGFR-2 expression. These data suggest leptin induction of Survivin is related to NILCO crosstalk [71].
7.4.3. Insulin and Adiponectin
Obesity and insulin resistance are risk factors for breast cancer and are associated with late-stage disease and poor prognosis. Thus, leptin actions may provide a biological mechanism by which obesity and insulin resistance are causally associated with breast cancer risk and poor prognosis [246]. Hyperinsulinemia could induce breast cancer progression through leptin-dependent mechanisms. In MDA-MB-231 cells, insulin up-regulates leptin by induction of Sp1- and HIF-1α mediated mechanisms. This process is partially regulated by the PI-3K and ERK1/2 pathways [247]. In contrast, adiponectin, the most abundant gene product in adipose tissue is a known anti-angiogenic and anti-breast cancer adipokine. Adiponectin levels are inversely correlated with body fat percentage in adults. This adipokine seems to have a protective role in the maintenance of insulin sensitivity and it is down-regulated by insulin [248]. Low serum adiponectin levels are significantly associated with an increased risk for breast cancer; tumors arising in women with low serum adiponectin levels are more likely to show a biologically aggressive phenotype [249]. Adiponectin and leptin have opposite roles in breast cancer cells and on their gene regulation. Adiponectin decreased leptin and Ob-Rt mRNA levels. In contrast, leptin significantly reduced the adiponectin receptor (AdipoR1) mRNA level. Similar contrasting results were found on the proliferation of MCF-7 cells (ER+). Moreover, microarray analyses showed that adiponectin and leptin have opposite effects on the expression of genes involved in cell cycle regulation [250].
8. Clinical Significance of leptin signaling in human breast cancer
8.1. Tumor marker and prognostic value of leptin signaling in breast cancer
In an earlier study, Ishikawa et al evaluated the expression of Ob-R, leptin, and clinicopathological features of breast cancer [52]. Protein expression of leptin and Ob-R was determined in 76 invasive ductal carcinomas and 32 samples of corresponding normal mammary glands. Distant metastasis was detected in 21 (34%) of 61 Ob-R+/leptin-overexpressed tumors, but was not detected in Ob-R−/non-leptin expressed tumors. (n=15, 100%; P < 0.05). In agreement with these data, leptin and Ob-R were found significantly overexpressed in primary (n=148) and metastatic breast cancer (n= 66) when compared to benign mammary lesions (n= 90) [167]. Moreover, high mRNA expression levels of leptin (99%) and leptin receptor (100%; Ob-Rb and ObRs) were found in breast cancer patients (n=322). Patients with elevated ObR-S expression had longer relapse-free survival (P = 0.008), whereas high ObR-L/ObR-S was associated with a shorter relapse-free survival (P = 0.05). These results indicate that leptin/Ob-R are biological markers of a more differentiated phenotype; and that ObR-S could be an independent prognostic factor [251]. Taken together, these data suggest that leptin and Ob-R might be good makers of breast cancer and may have potential value for prognosis. In contrast, the analysis of a large cohort of breast cancer patients (n=517) showed positive cytoplasmic immunoreactivity for leptin (39%) and Ob-R (79%) proteins that correlated with a high Ki-67 labeling index (P=0.019). Although there were no statistical significance, the rate of a leptin expression was higher for TNBC (P=0.08), no p53 overexpression (P=0.08) and no bcl-2 expression (P=0.38). No significant difference in overall survival was noted between the leptin/Ob-R expressing and non-expressing breast cancer patients [252]. However, the authors did not differentiate breast cancer expressing specific Ob-R isoforms as the antibody used (M-18; Santa Cruz Biotech.Inc.) can detect both Ob-Rb and Ob-Ra. Nevertheless, to date it is unknown if leptin/Ob-R markers would have independent prognostic value.
8.2. Leptin/Ob-R signaling as a therapeutic drug target
Extracellular activation of Ob-R is only achieved upon leptin binding to its extracellular region. Moreover, leptin only binds to Ob-R. Additionally, this family of receptors is only capable to bind leptin or leptin-modified peptides. These data highlight the potential use of leptin antagonists to block leptin binding to Ob-R and thereby, abrogating Ob-R signals [34].
We have previously demonstrated that inhibition of leptin signaling resulted in decreased growth of mammary tumors derived from mouse and humans [117–118]. A pegylated leptin peptide receptor antagonist (PEG-LPrA2) markedly reduced tumor growth and the expression of VEGF-A/VEGFR-2 in orthotopic mouse models of syngeneic and human breast cancer xenografts responsive or unresponsive (MDA-MB-231 cells; TNBC) to estrogens [117–118]. Interestingly, LPrA2-induced effects were more pronounced in vivo than in vitro. These results suggested that paracrine actions in stromal, endothelial, and/or inflammatory cells that may impact the growth of breast cancer. The relevance of these observations was underscored by the use of an orthotopic-syngeneic 4T1 breast cancer cell-model [117]. The mice treated with PEG-LPrA2 had diminished expression of VEGF-A/VEGFR-2, Ob-R, leptin, IL-1R tI, PCNA and cyclin D1 [118]. PEG-LPrA2 was not toxic and did not change body weight (BW) or appetite of a large number of normal lean (male and female) CD-1 and BALB/c mice during a several months. Similar data were later reported by another group using the same orthotropic xenograft model, (MDA-MB-231) but with a different leptin antagonist (Allo-aca) [253]. Subcutaneous delivery of this antagonist significantly extended mouse survival time for 1–2 weeks. While Allo-aca did not show systemic toxicity when tested for toxicity effects in normal CD-1 mice, it induced 6–10% body weight increase [253]. We further hypothesized that inhibition of leptin signaling could also be used in the prevention of human breast cancer. This hypothesis was tested in a 7,12-dimethylbenz[a]anthracene (DMBA).-induced breast cancer mouse model using lean or diet induced obesity (DIO)-mice fed with high-fat diet (54% Kcal from fat) [254]. Mice were treated with either one or two PEG-LPrA2 dose/weekly (i.v.; 50 μl/0.1 mM) one week before DMBA challenge. Breast cancer was only detected in control DIO-mice (21%; 3 of 14) receiving saline injections. High levels of leptin-targeted molecules: OB-R, IL-1R tI, VEGF/VEGFR-2, anti-apoptotic protein B-cell lymphoma 2 (Bcl-2), HIF-1α, NFκB and Notch (receptors, ligand and targeted genes) were found in DMBA-breast cancer tissues. Remarkably, PEG-LPrA2 (1 or 2 doses) completely prevented DMBA-breast cancer in DIO-mice (0%; 0 of 14) and reduced aryl hydrocarbon receptor (AhR) and Notch expression in mammary glands. Body weight and food intake of lean and DIO-mice treated for 32 weeks with PEG-LPrA2 were not affected. However, two-dose treatment decreased BW in DIO-mice [254]. These data suggest that inhibition of leptin signaling may serve as a novel adjuvant for prevention and treatment of breast cancer. The alarming increase of obesity incidence in the Western countries underscores the importance of these findings [243–244].
9. Conclusion and overall perspectives
High leptin level is a hallmark of obesity which has been correlated to incidence of several cancers including breast cancer. Leptin is an adipocyte-derived cytokine co-expressed with Ob-R by breast cancer cells. Leptin/Ob-R expression pattern correlates with metastasis and lower survival rate of breast cancer patients. In vitro and in vivo studies clearly demonstrated a role of leptin in mammary tumor development. Furthermore, leptin signaling and its crosstalks with many signaling pathways play critical roles in breast cancer cell growth, migration, invasion, angiogenesis and metastasis (see Fig 3). Importantly, inhibition of leptin signaling could be relevant for breast cancer prevention, especially for obese populations showing higher levels of leptin and incidence of breast cancer.
Better understanding of expression regulation, redundant functions and intracellular signaling pathway targets, as well as the complex crosstalk of leptin/Ob-R with other oncogenic signals in breast cancer cells will be essential to ensure rational use of treatment and development of new combinatory therapeutic possibilities. There are still many gaps to fill in the field of leptin signaling in breast cancer, especially the molecular mechanisms of leptin signaling-mediated regulation of BCSC. Novel opportunities could emerge from the discovery of leptin crosstalk with inflammatory and angiogenic cytokines (i.e., NILCO) and their links to obesity-related cancers and BCSC.
Acknowledgments
This work was partially funded by Grants from NIH/NCI 1SC1CA138658-03; and the Georgia Cancer Coalition Distinguished Cancer Scholar Award to R.R.G-P.; NIH/NCI 1R21CA153172-01A1 to MT-K, and facilities and support services at Morehouse School of Medicine (NIH RR03034 and 1C06 RR18386) and NIH/NCRR grant 1G-03.
Glossary
- 4T1 cells
mouse mammary cancer cell line
- Akt
protein kinase B
- ALDH
aldehyde dehydrogenase
- BALB/c
an albino, laboratory-bred strain of the house mouse
- Bcl-2
B-cell lymphoma 2
- Bcl-xl
B-cell lymphoma-extra large
- BCSC
breast cancer stem cells
- BMP
bone morphogenetic protein
- CSC
cancer stem cell
- CSL
CBF1/Su(H)/Lag-1
- Cyclin D1
kinase and regulator of cell cycle D1
- DAPT
N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester
- DLL-1
Delta-like 1
- DSL
Delta/Serrate/LAG-2
- E2
17β-estradiol
- EC
endothelial cells
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- EMT
epithelial-mesenchymal transformation
- EMT6, MMT
mouse mammary adenocarcinoma cell lines
- ER
estrogen receptor
- ERK 1/2
extracellular regulated kinase 1 and 2
- HIF-1α
hypoxia regulated factor-1 α
- HUVECs
human umbilical vein endothelial cells
- IGF-1
insulin like growth factor-1
- IL-1
interleukin-1
- IL-1R tI
interleukin-1 type I receptor
- JAK2
Janus kinase 2
- MAPK
mitogen activated protein kinase
- MCF-7
ER positive human breast cancer cell line
- MDA-MB-231
ER negative human breast cancer cell line
- mTOR
mammalian target of rapamycin
- NF-κB
eukaryotic nuclear transcription factor kappa B
- NICD
Notch intracellular domain
- NILCO
Notch, IL-1 and leptin crosstalk outcome
- LIF/LIFR
leukemia inhibitory factor and its receptor
- Ob-R
leptin receptor
- PEG-LPrA2
pegylated leptin peptide receptor antagonist 2
- PI-3K
phosphoinositide 3-kinase
- STAT3
signal transducer and activator of transcription 3
- VEGF
Vascular endothelial growth factor
- VEGFR-2
Vascular endothelial growth factor receptor 2 or KDR or Flk-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO. World Health Organization Fact Sheet for World Wide Prevalence of Obesity. 2006 http://www.who.int/mediacentre/factsheets/fs311/en/index.html.
- 2.Baskin ML, Ard J, Franklin F, Allison DB. Prevalence of obesity in the United States. Obes Rev. 2005;6:5–7. doi: 10.1111/j.1467-789X.2005.00165.x. [DOI] [PubMed] [Google Scholar]
- 3.Flegal KM, Ogden CL, Carroll MD. Prevalence and trends in overweight in Mexican-american adults and children. Nutr Rev. 2004;62:S144–148. doi: 10.1111/j.1753-4887.2004.tb00085.x. [DOI] [PubMed] [Google Scholar]
- 4.Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 5.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight, obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 6.Huxley R, Mendis S, Zheleznyakov E, Reddy S, Chan J. Body mass index, waist circumference and waist:hip ratio as predictors of cardiovascular risk--a review of the literature. Eur J Clin Nutr. 2010;64:16–22. doi: 10.1038/ejcn.2009.68. [DOI] [PubMed] [Google Scholar]
- 7.Travers ME, McCarthy MI. Type 2 diabetes and obesity: genomics and the clinic. Hum Genet. 2011;130:41–58. doi: 10.1007/s00439-011-1023-8. [DOI] [PubMed] [Google Scholar]
- 8.Kotsis V, Stabouli S, Papakatsika S, Rizos Z, Parati G. Mechanisms of obesity-induced hypertension. Hypertens Res. 2010;33:386–393. doi: 10.1038/hr.2010.9. [DOI] [PubMed] [Google Scholar]
- 9.Franssen R, Monajemi H, Stroes ES, Kastelein JJ. Obesity and dyslipidemia. Endocrinol Metab Clin North Am. 2008;37:623–633. viii. doi: 10.1016/j.ecl.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basen-Engquist K, Chang M. Obesity and cancer risk: recent review and evidence. Curr Oncol Rep. 2011;13:71–76. doi: 10.1007/s11912-010-0139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paz-Filho G, Lim EL, Wong ML, Licinio J. Associations between adipokines and obesity-related cancer. Front Biosci. 2011;16:1634–1650. doi: 10.2741/3810. [DOI] [PubMed] [Google Scholar]
- 13.Teucher B, Rohrmann S, Kaaks R. Obesity: focus on all-cause mortality and cancer. Maturitas. 2010;65:112–116. doi: 10.1016/j.maturitas.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 14.Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist. 2010;15:556–565. doi: 10.1634/theoncologist.2009-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prieto-Hontoria PL, Perez-Matute P, Fernandez-Galilea M, Bustos M, Martinez JA, Moreno-Aliaga MJ. Role of obesity-associated dysfunctional adipose tissue in cancer: a molecular nutrition approach. Biochim Biophys Acta. 2011;1807:664–678. doi: 10.1016/j.bbabio.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Bellows CF, Kolonin MG. Adipose tissue-derived progenitor cells and cancer. World J Stem Cells. 2010;2:103–113. doi: 10.4252/wjsc.v2.i5.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwaan HC, McMahon B. The role of plasminogen-plasmin system in cancer. Cancer Treat Res. 2009;148:43–66. doi: 10.1007/978-0-387-79962-9_4. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 19.Paracchini V, Pedotti P, Taioli E. Genetics of leptin and obesity: a HuGE review. Am J Epidemiol. 2005;162:101–114. doi: 10.1093/aje/kwi174. [DOI] [PubMed] [Google Scholar]
- 20.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer. 2011;11:886–895. doi: 10.1038/nrc3174. [DOI] [PubMed] [Google Scholar]
- 21.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 22.Han C, Zhang HT, Du L, Liu X, Jing J, Zhao X, Yang X, Tian B. Serum levels of leptin, insulin, and lipids in relation to breast cancer in china. Endocrine. 2005;26:19–24. doi: 10.1385/ENDO:26:1:019. [DOI] [PubMed] [Google Scholar]
- 23.Wu MH, Chou YC, Chou WY, Hsu GC, Chu CH, Yu CP, Yu JC, Sun CA. Circulating levels of leptin, adiposity and breast cancer risk. Br J Cancer. 2009;100:578–582. doi: 10.1038/sj.bjc.6604913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garofalo C, Surmacz E. Leptin and cancer. J Cell Physiol. 2006;207:12–22. doi: 10.1002/jcp.20472. [DOI] [PubMed] [Google Scholar]
- 25.Jarde T, Perrier S, Vasson MP, Caldefie-Chezet F. Molecular mechanisms of leptin and adiponectin in breast cancer. Eur J Cancer. 2011;47:33–43. doi: 10.1016/j.ejca.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Ray A, Cleary MP. Leptin as a potential therapeutic target for breast cancer prevention and treatment. Expert Opin Ther Targets. 2010;14:443–451. doi: 10.1517/14728221003716466. [DOI] [PubMed] [Google Scholar]
- 27.Ribeiro R, Lopes C, Medeiros R. Leptin and prostate: implications for cancer prevention--overview of genetics and molecular interactions. Eur J Cancer Prev. 2004;13:359–368. doi: 10.1097/00008469-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Wang SN, Lee KT, Ker CG. Leptin in hepatocellular carcinoma. World J Gastroenterol. 2010;16:5801–5809. doi: 10.3748/wjg.v16.i46.5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O’Rahilly S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341:879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 30.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 31.Leinninger GM. Location, location, location: the CNS sites of leptin action dictate its regulation of homeostatic and hedonic pathways. Int J Obes (Lond) 2009;33(Suppl 2):S14–17. doi: 10.1038/ijo.2009.66. [DOI] [PubMed] [Google Scholar]
- 32.Myers MG, Jr, Munzberg H, Leinninger GM, Leshan RL. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab. 2009;9:117–123. doi: 10.1016/j.cmet.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robertson SA, Leinninger GM, Myers MG., Jr Molecular and neural mediators of leptin action. Physiol Behav. 2008;94:637–642. doi: 10.1016/j.physbeh.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez RR, Leavis PC. A peptide derived from the human leptin molecule is a potent inhibitor of the leptin receptor function in rabbit endometrial cells. Endocrine. 2003;21:185–195. doi: 10.1385/ENDO:21:2:185. [DOI] [PubMed] [Google Scholar]
- 35.Imagawa K, Numata Y, Katsuura G, Sakaguchi I, Morita A, Kikuoka S, Matumoto Y, Tsuji T, Tamaki M, Sasakura K, Teraoka H, Hosoda K, Ogawa Y, Nakao K. Structure-function studies of human leptin. J Biol Chem. 1998;273:35245–35249. doi: 10.1074/jbc.273.52.35245. [DOI] [PubMed] [Google Scholar]
- 36.Fruhbeck G. Intracellular signalling pathways activated by leptin. Biochem J. 2006;393:7–20. doi: 10.1042/BJ20051578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friedman J. Leptin, leptin receptors, and the control of body weight. In: Blum WF, Kiess W, Rascher W, editors. Leptin—The Voice of Adipose Tissue. Heidelberg: JA Barth Verlag; 1997. pp. 3–22. [Google Scholar]
- 38.Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI. Identification, expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 39.Bazan JF. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci U S A. 1990;87:6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tartaglia LA. The leptin receptor. J Biol Chem. 1997;272:6093–6096. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- 41.Banks AS, Davis SM, Bates SH, Myers MG., Jr Activation of downstream signals by the long form of the leptin receptor. J Biol Chem. 2000;275:14563–14572. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- 42.Bjorbaek C, Uotani S, da Silva B, Flier JS. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem. 1997;272:32686–32695. doi: 10.1074/jbc.272.51.32686. [DOI] [PubMed] [Google Scholar]
- 43.Stahl N, Farruggella TJ, Boulton TG, Zhong Z, Darnell JE, Jr, Yancopoulos GD. Choice of STATs and other substrates specified by modular tyrosine-based motifs in cytokine receptors. Science. 1995;267:1349–1353. doi: 10.1126/science.7871433. [DOI] [PubMed] [Google Scholar]
- 44.Bjorbaek C, El-Haschimi K, Frantz JD, Flier JS. The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem. 1999;274:30059–30065. doi: 10.1074/jbc.274.42.30059. [DOI] [PubMed] [Google Scholar]
- 45.Masuzaki H, Ogawa Y, Sagawa N, Hosoda K, Matsumoto T, Mise H, Nishimura H, Yoshimasa Y, Tanaka I, Mori T, Nakao K. Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nat Med. 1997;3:1029–1033. doi: 10.1038/nm0997-1029. [DOI] [PubMed] [Google Scholar]
- 46.Smith-Kirwin SM, O’Connor DM, De Johnston J, Lancey ED, Hassink SG, Funanage VL. Leptin expression in human mammary epithelial cells and breast milk. J Clin Endocrinol Metab. 1998;83:1810–1813. doi: 10.1210/jcem.83.5.4952. [DOI] [PubMed] [Google Scholar]
- 47.Hu X, Juneja SC, Maihle NJ, Cleary MP. Leptin--a growth factor in normal and malignant breast cells and for normal mammary gland development. J Natl Cancer Inst. 2002;94:1704–1711. doi: 10.1093/jnci/94.22.1704. [DOI] [PubMed] [Google Scholar]
- 48.Jarde T, Caldefie-Chezet F, Damez M, Mishellany F, Perrone D, Penault-Llorca F, Guillot J, Vasson MP. Adiponectin and leptin expression in primary ductal breast cancer and in adjacent healthy epithelial and myoepithelial tissue. Histopathology. 2008;53:484–487. doi: 10.1111/j.1365-2559.2008.03121.x. [DOI] [PubMed] [Google Scholar]
- 49.Schaffler A, Scholmerich J, Buechler C. Mechanisms of disease: adipokines and breast cancer - endocrine and paracrine mechanisms that connect adiposity and breast cancer. Nat Clin Pract Endocrinol Metab. 2007;3:345–354. doi: 10.1038/ncpendmet0456. [DOI] [PubMed] [Google Scholar]
- 50.Vona-Davis L, Rose DP. Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr Relat Cancer. 2007;14:189–206. doi: 10.1677/ERC-06-0068. [DOI] [PubMed] [Google Scholar]
- 51.Yuan SS, Chung YF, Chen HW, Tsai KB, Chang HL, Huang CH, Su JH. Aberrant expression and possible involvement of the leptin receptor in bladder cancer. Urology. 2004;63:408–413. doi: 10.1016/j.urology.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 52.Ishikawa M, Kitayama J, Nagawa H. Enhanced expression of leptin and leptin receptor (OB-R) in human breast cancer. Clin Cancer Res. 2004;10:4325–4331. doi: 10.1158/1078-0432.CCR-03-0749. [DOI] [PubMed] [Google Scholar]
- 53.Uddin S, Bu R, Ahmed M, Abubaker J, Al-Dayel F, Bavi P, Al-Kuraya KS. Overexpression of leptin receptor predicts an unfavorable outcome in Middle Eastern ovarian cancer. Mol Cancer. 2009;8:74. doi: 10.1186/1476-4598-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoon Kim J, Lee SY, Myung SC, Kim YS, Kim TH, Kim MK. Clinical significance of the leptin and leptin receptor expressions in prostate tissues. Asian J Androl. 2008;10:923–928. doi: 10.1111/j.1745-7262.2008.00438.x. [DOI] [PubMed] [Google Scholar]
- 55.Ellerhorst JA, Diwan AH, Dang SM, Uffort DG, Johnson MK, Cooke CP, Grimm EA. Promotion of melanoma growth by the metabolic hormone leptin. Oncol Rep. 2010;23:901–907. doi: 10.3892/or_00000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Riolfi M, Ferla R, Del Valle L, Pina-Oviedo S, Scolaro L, Micciolo R, Guidi M, Terrasi M, Cetto GL, Surmacz E. Leptin and its receptor are overexpressed in brain tumors and correlate with the degree of malignancy. Brain Pathol. 2010;20:481–489. doi: 10.1111/j.1750-3639.2009.00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Howard JM, Beddy P, Ennis D, Keogan M, Pidgeon GP, Reynolds JV. Associations between leptin, adiponectin receptor upregulation, visceral obesity and tumour stage in oesophageal and junctional adenocarcinoma. Br J Surg. 2010;97:1020–1027. doi: 10.1002/bjs.7072. [DOI] [PubMed] [Google Scholar]
- 58.Cheng SP, Chi CW, Tzen CY, Yang TL, Lee JJ, Liu TP, Liu CL. Clinicopathologic significance of leptin and leptin receptor expressions in papillary thyroid carcinoma. Surgery. 2010;147:847–853. doi: 10.1016/j.surg.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 59.Uddin S, Bavi P, Siraj AK, Ahmed M, Al-Rasheed M, Hussain AR, Amin T, Alzahrani A, Al-Dayel F, Abubaker J, Bu R, Al-Kuraya KS. Leptin-R and its association with PI3K/AKT signaling pathway in papillary thyroid carcinoma. Endocr Relat Cancer. 2010;17:191–202. doi: 10.1677/ERC-09-0153. [DOI] [PubMed] [Google Scholar]
- 60.Kakarala M, Wicha MS. Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J Clin Oncol. 2008;26:2813–2820. doi: 10.1200/JCO.2008.16.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pang LY, Argyle DJ. Using naturally occurring tumours in dogs and cats to study telomerase and cancer stem cell biology. Biochim Biophys Acta. 2009;1792:380–391. doi: 10.1016/j.bbadis.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 62.Clarke MF. Self-renewal and solid-tumor stem cells. Biol Blood Marrow Transplant. 2005;11:14–16. doi: 10.1016/j.bbmt.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 63.Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med. 2011;17:313–319. doi: 10.1038/nm.2304. [DOI] [PubMed] [Google Scholar]
- 64.Chung AS, Lee J, Ferrara N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nat Rev Cancer. 2010;10:505–514. doi: 10.1038/nrc2868. [DOI] [PubMed] [Google Scholar]
- 65.Savarese TM, Strohsnitter WC, Low HP, Liu Q, Baik I, Okulicz W, Chelmow DP, Lagiou P, Quesenberry PJ, Noller KL, Hsieh CC. Correlation of umbilical cord blood hormones and growth factors with stem cell potential: implications for the prenatal origin of breast cancer hypothesis. Breast Cancer Res. 2007;9:R29. doi: 10.1186/bcr1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Korkaya H, Paulson A, Iovino F, Wicha MS. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene. 2008;27:6120–6130. doi: 10.1038/onc.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Korkaya H, Wicha MS. HER-2, notch, and breast cancer stem cells: targeting an axis of evil. Clin Cancer Res. 2009;15:1845–1847. doi: 10.1158/1078-0432.CCR-08-3087. [DOI] [PubMed] [Google Scholar]
- 68.Korkaya H, Paulson A, Charafe-Jauffret E, Ginestier C, Brown M, Dutcher J, Clouthier SG, Wicha MS. Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. PLoS Biol. 2009;7:e1000121. doi: 10.1371/journal.pbio.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou J, Wulfkuhle J, Zhang H, Gu P, Yang Y, Deng J, Margolick JB, Liotta LA, Petricoin E, 3rd, Zhang Y. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc Natl Acad Sci U S A. 2007;104:16158–16163. doi: 10.1073/pnas.0702596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pratt MA, Tibbo E, Robertson SJ, Jansson D, Hurst K, Perez-Iratxeta C, Lau R, Niu MY. The canonical NF-kappaB pathway is required for formation of luminal mammary neoplasias and is activated in the mammary progenitor population. Oncogene. 2009;28:2710–2722. doi: 10.1038/onc.2009.131. [DOI] [PubMed] [Google Scholar]
- 71.Guo S, Gonzalez-Perez RR. Notch, IL-1, leptin crosstalk outcome (NILCO) is critical for leptin-induced proliferation, migration and VEGF/VEGFR-2 expression in breast cancer. PLoS One. 2011;6(6):e21467. doi: 10.1371/journal.pone.0021467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guo S, Liu M, Gonzalez-Perez RR. Role of Notch and its oncogenic signaling crosstalk in breast cancer. Biochim Biophys Acta. 2011;1815:197–213. doi: 10.1016/j.bbcan.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Knight BB, Oprea-Ilies GM, Nagalingam A, Yang L, Cohen C, Saxena NK, Sharma D. Survivin upregulation, dependent on leptin-EGFR-Notch1 axis, is essential for leptin induced migration of breast carcinoma cells. Endocr Relat Cancer. 2011 doi: 10.1530/ERC-11-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 75.Leong KG, Karsan A. Recent insights into the role of Notch signaling in tumorigenesis. Blood. 2006;107:2223–2233. doi: 10.1182/blood-2005-08-3329. [DOI] [PubMed] [Google Scholar]
- 76.Radtke F, Raj K. The role of Notch in tumorigenesis: oncogene or tumour suppressor? Nat Rev Cancer. 2003;3:756–767. doi: 10.1038/nrc1186. [DOI] [PubMed] [Google Scholar]
- 77.Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 2008;10:R25. doi: 10.1186/bcr1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guo S, Gillespie C, Liu MG-PR. Autocrine stimulation of VEGFR-2 by leptin is associated with Notch signaling pathway and cancer stem cell marker expression. AACR 102 Annual Congress, #12-A-5965; 2012. [Google Scholar]
- 79.Zheng Q, Dunlap SM, Zhu J, Downs-Kelly E, Rich JN, Hursting SD, Berger NA, Reizes O. Leptin deficiency suppresses MMTV-Wnt-1 mammary tumor growth and abrogates tumor initiating cell survival. Endocr Relat Cancer. 2011 doi: 10.1530/ERC-11-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Feldman DE, Chen C, Punj V, Tsukamoto H, Machida K. Pluripotency factor-mediated expression of the leptin receptor (OB-R) links obesity to oncogenesis through tumor-initiating stem cells. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1114438109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Berrada N, Delaloge S, Andre F. Treatment of triple-negative metastatic breast cancer: toward individualized targeted treatments or chemosensitization? Ann Oncol. 2010;21(Suppl 7):vii30–35. doi: 10.1093/annonc/mdq279. [DOI] [PubMed] [Google Scholar]
- 82.McGlothen T, Gillespie C, Colbert L, Guo S, Gonzalez-Perez R. Leptin Signaling impacts on Notch and Wnt Crosstalk in Breast Cancer. CTRC-AACR San Antonio Breast Cancer Symposium. 2011 [Google Scholar]
- 83.Cao R, Brakenhielm E, Wahlestedt C, Thyberg J, Cao Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc Natl Acad Sci U S A. 2001;98:6390–6395. doi: 10.1073/pnas.101564798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sierra-Honigmann MR, Nath AK, Murakami C, Garcia-Cardena G, Papapetropoulos A, Sessa WC, Madge LA, Schechner JS, Schwabb MB, Polverini PJ, Flores-Riveros JR. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683–1686. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 85.Bouloumie A, Drexler HC, Lafontan M, Busse R. Leptin, the product of Ob gene, promotes angiogenesis. Circ Res. 1998;83:1059–1066. doi: 10.1161/01.res.83.10.1059. [DOI] [PubMed] [Google Scholar]
- 86.Paradis H, Gendron RL. LIF transduces contradictory signals on capillary outgrowth through induction of stat3 and (P41/43)MAP kinase. J Cell Sci. 2000;113(Pt 23):4331–4339. doi: 10.1242/jcs.113.23.4331. [DOI] [PubMed] [Google Scholar]
- 87.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 88.Soldi R, Mitola S, Strasly M, Defilippi P, Tarone G, Bussolino F. Role of alphavbeta3 integrin in the activation of vascular endothelial growth factor receptor-2. EMBO J. 1999;18:882–892. doi: 10.1093/emboj/18.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guo S, Colbert LS, Fuller M, Zhang Y, Gonzalez-Perez RR. Vascular endothelial growth factor receptor-2 in breast cancer. Biochim Biophys Acta. 2010;1806:108–121. doi: 10.1016/j.bbcan.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Siclari VA, Guise TA, Chirgwin JM. Molecular interactions between breast cancer cells and the bone microenvironment drive skeletal metastases. Cancer Metastasis Rev. 2006;25:621–633. doi: 10.1007/s10555-006-9023-1. [DOI] [PubMed] [Google Scholar]
- 91.Gonzalez RR, Devoto L, Campana A, Bischof P. Effects of leptin, interleukin-1alpha, interleukin-6, and transforming growth factor-beta on markers of trophoblast invasive phenotype: integrins and metalloproteinases. Endocrine. 2001;15:157–164. doi: 10.1385/ENDO:15:2:157. [DOI] [PubMed] [Google Scholar]
- 92.Gonzalez RR, Leavis P. Leptin upregulates beta3-integrin expression, interleukin-1beta, upregulates leptin and leptin receptor expression in human endometrial epithelial cell cultures. Endocrine. 2001;16:21–28. doi: 10.1385/ENDO:16:1:21. [DOI] [PubMed] [Google Scholar]
- 93.Heida NM, Leifheit-Nestler M, Schroeter MR, Muller JP, Cheng IF, Henkel S, Limbourg A, Limbourg FP, Alves F, Quigley JP, Ruggeri ZM, Hasenfuss G, Konstantinides S, Schafer K. Leptin enhances the potency of circulating angiogenic cells via src kinase and integrin (alpha)vbeta5: implications for angiogenesis in human obesity. Arterioscler Thromb Vasc Biol. 2010;30:200–206. doi: 10.1161/ATVBAHA.109.192807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schroeter MR, Leifheit M, Sudholt P, Heida NM, Dellas C, Rohm I, Alves F, Zientkowska M, Rafail S, Puls M, Hasenfuss G, Konstantinides S, Schafer K. Leptin enhances the recruitment of endothelial progenitor cells into neointimal lesions after vascular injury by promoting integrin-mediated adhesion. Circ Res. 2008;103:536–544. doi: 10.1161/CIRCRESAHA.107.169375. [DOI] [PubMed] [Google Scholar]
- 95.Huang CY, Yu HS, Lai TY, Yeh YL, Su CC, Hsu HH, Tsai FJ, Tsai CH, Wu HC, Tang CH. Leptin increases motility and integrin up-regulation in human prostate cancer cells. J Cell Physiol. 2011;226:1274–1282. doi: 10.1002/jcp.22455. [DOI] [PubMed] [Google Scholar]
- 96.Yang SN, Chen HT, Tsou HK, Huang CY, Yang WH, Su CM, Fong YC, Tseng WP, Tang CH. Leptin enhances cell migration in human chondrosarcoma cells through OBRl leptin receptor. Carcinogenesis. 2009;30:566–574. doi: 10.1093/carcin/bgp023. [DOI] [PubMed] [Google Scholar]
- 97.Hirai H, Karian P, Kikyo N. Regulation of embryonic stem cell self-renewal and pluripotency by leukaemia inhibitory factor. Biochem J. 2011;438:11–23. doi: 10.1042/BJ20102152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ferrara N, Winer J, Henzel WJ. Pituitary follicular cells secrete an inhibitor of aortic endothelial cell growth: identification as leukemia inhibitory factor. Proc Natl Acad Sci U S A. 1992;89:698–702. doi: 10.1073/pnas.89.2.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vasse M, Pourtau J, Trochon V, Muraine M, Vannier JP, Lu H, Soria J, Soria C. Oncostatin M induces angiogenesis in vitro and in vivo. Arterioscler Thromb Vasc Biol. 1999;19:1835–1842. doi: 10.1161/01.atv.19.8.1835. [DOI] [PubMed] [Google Scholar]
- 100.Sugiura S, Lahav R, Han J, Kou SY, Banner LR, de Pablo F, Patterson PH. Leukaemia inhibitory factor is required for normal inflammatory responses to injury in the peripheral and central nervous systems in vivo and is chemotactic for macrophages in vitro. Eur J Neurosci. 2000;12:457–466. doi: 10.1046/j.1460-9568.2000.00922.x. [DOI] [PubMed] [Google Scholar]
- 101.Vanderlocht J, Hendriks JJ, Venken K, Stinissen P, Hellings N. Effects of IFN-beta, leptin and simvastatin on LIF secretion by T lymphocytes of MS patients and healthy controls. J Neuroimmunol. 2006;177:189–200. doi: 10.1016/j.jneuroim.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 102.Gonzalez RR, Rueda BR, Ramos MP, Littell RD, Glasser S, Leavis PC. Leptin-induced increase in leukemia inhibitory factor and its receptor by human endometrium is partially mediated by interleukin 1 receptor signaling. Endocrinology. 2004;145:3850–3857. doi: 10.1210/en.2004-0383. [DOI] [PubMed] [Google Scholar]
- 103.Carino C, Olawaiye AB, Cherfils S, Serikawa T, Lynch MP, Rueda BR, Gonzalez RR. Leptin regulation of proangiogenic molecules in benign and cancerous endometrial cells. Int J Cancer. 2008;123:2782–2790. doi: 10.1002/ijc.23887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dinarello CA. Interleukin-1, interleukin-1 receptors and interleukin-1 receptor antagonist. Int Rev Immunol. 1998;16:457–499. doi: 10.3109/08830189809043005. [DOI] [PubMed] [Google Scholar]
- 105.Apte RN, Dotan S, Elkabets M, White MR, Reich E, Carmi Y, Song X, Dvozkin T, Krelin Y, Voronov E. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 2006;25:387–408. doi: 10.1007/s10555-006-9004-4. [DOI] [PubMed] [Google Scholar]
- 106.Perrier S, Caldefie-Chezet F, Vasson MP. IL-1 family in breast cancer: potential interplay with leptin and other adipocytokines. FEBS Lett. 2009;583:259–265. doi: 10.1016/j.febslet.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 107.Guo S, Fuller M, Gonzalez-Perez R. Regulation of VEGFR-2 by leptin-Notch signaling crosstalk in mammary cancer cells. Proceedings of the 101st Annual Meeting of the American Association for Cancer Research; Apr 17–21 (2010); p. Abstract #LB-365. [Google Scholar]
- 108.Zhou W, Guo S, Gonzalez-Perez R. Leptin regulates the IL-1 system in breast cancer [abstract]. Proceedings of the 101st Annual Meeting of the American Association for Cancer Research; Apr 17–21 (2010); p. Abstract nr 1715. [Google Scholar]
- 109.Kumar S, Kishimoto H, Chua HL, Badve S, Miller KD, Bigsby RM, Nakshatri H. Interleukin-1 alpha promotes tumor growth and cachexia in MCF-7 xenograft model of breast cancer. Am J Pathol. 2003;163:2531–2541. doi: 10.1016/s0002-9440(10)63608-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ljung L, Olsson T, Engstrand S, Wallberg-Jonsson S, Soderberg S, Rantapaa-Dahlqvist S. Interleukin-1 receptor antagonist is associated with both lipid metabolism and inflammation in rheumatoid arthritis. Clin Exp Rheumatol. 2007;25:617–620. [PubMed] [Google Scholar]
- 111.Reichlin S, Chen G, Nicolson M. Blood to brain transfer of leptin in normal and interleukin-1beta-treated male rats. Endocrinology. 2000;141:1951–1954. doi: 10.1210/endo.141.6.7459. [DOI] [PubMed] [Google Scholar]
- 112.Ueland T, Kristo C, Godang K, Aukrust P, Bollerslev J. Interleukin-1 receptor antagonist is associated with fat distribution in endogenous Cushing’s syndrome: a longitudinal study. J Clin Endocrinol Metab. 2003;88:1492–1496. doi: 10.1210/jc.2002-021030. [DOI] [PubMed] [Google Scholar]
- 113.Zhou W, Guo S, Gonzalez-Perez RR. Leptin pro-angiogenic signature in breast cancer is linked to IL-1 signalling. Br J Cancer. 2011;104:128–137. doi: 10.1038/sj.bjc.6606013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ferrara N. Vascular endothelial growth factor: molecular and biological aspects. Curr Top Microbiol Immunol. 1999;237:1–30. doi: 10.1007/978-3-642-59953-8_1. [DOI] [PubMed] [Google Scholar]
- 115.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 116.Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312:549–560. doi: 10.1016/j.yexcr.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 117.Gonzalez RR, Cherfils S, Escobar M, Yoo JH, Carino C, Styer AK, Sullivan BT, Sakamoto H, Olawaiye A, Serikawa T, Lynch MP, Rueda BR. Leptin signaling promotes the growth of mammary tumors and increases the expression of vascular endothelial growth factor (VEGF) and its receptor type two (VEGF-R2) J Biol Chem. 2006;281:26320–26328. doi: 10.1074/jbc.M601991200. [DOI] [PubMed] [Google Scholar]
- 118.Rene Gonzalez R, Watters A, Xu Y, Singh UP, Mann DR, Rueda BR, Penichet ML. Leptin-signaling inhibition results in efficient anti-tumor activity in estrogen receptor positive or negative breast cancer. Breast Cancer Res. 2009;11:R36. doi: 10.1186/bcr2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gonzalez-Perez RR, Xu Y, Guo S, Watters A, Zhou W, Leibovich SJ. Leptin upregulates VEGF in breast cancer via canonic and non-canonical signalling pathways and NFkappaB/HIF-1alpha activation. Cell Signal. 2010;22:1350–1362. doi: 10.1016/j.cellsig.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Garonna E, Botham KM, Birdsey GM, Randi AM, Gonzalez-Perez RR, Wheeler-Jones CP. Vascular endothelial growth factor receptor-2 couples cyclo-oxygenase-2 with pro-angiogenic actions of leptin on human endothelial cells. Plos one. 2011;6:e18823. doi: 10.1371/journal.pone.0018823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Aesoy R, Sanchez BC, Norum JH, Lewensohn R, Viktorsson K, Linderholm B. An autocrine VEGF/VEGFR2 and p38 signaling loop confers resistance to 4-hydroxytamoxifen in MCF-7 breast cancer cells. Mol Cancer Res. 2008;6:1630–1638. doi: 10.1158/1541-7786.MCR-07-2172. [DOI] [PubMed] [Google Scholar]
- 122.Beecken WD, Kramer W, Jonas D. New molecular mediators in tumor angiogenesis. J Cell Mol Med. 2000;4:262–269. doi: 10.1111/j.1582-4934.2000.tb00125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cirillo D, Rachiglio AM, la Montagna R, Giordano A, Normanno N. Leptin signaling in breast cancer: an overview. J Cell Biochem. 2008;105:956–964. doi: 10.1002/jcb.21911. [DOI] [PubMed] [Google Scholar]
- 124.Hausman GJ, Richardson RL. Adipose tissue angiogenesis. J Anim Sci. 2004;82:925–934. doi: 10.2527/2004.823925x. [DOI] [PubMed] [Google Scholar]
- 125.Ando S, Catalano S. The multifactorial role of leptin in driving the breast cancer microenvironment. Nat Rev Endocrinol. 2011 doi: 10.1038/nrendo.2011.184. [DOI] [PubMed] [Google Scholar]
- 126.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pahler JC, Tazzyman S, Erez N, Chen YY, Murdoch C, Nozawa H, Lewis CE, Hanahan D. Plasticity in tumor-promoting inflammation: impairment of macrophage recruitment evokes a compensatory neutrophil response. Neoplasia. 2008;10:329–340. doi: 10.1593/neo.07871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lambert KC, Curran EM, Judy BM, Milligan GN, Lubahn DB, Estes DM. Estrogen receptor alpha (ERalpha) deficiency in macrophages results in increased stimulation of CD4+ T cells while 17beta-estradiol acts through ERalpha to increase IL-4 and GATA-3 expression in CD4+ T cells independent of antigen presentation. J Immunol. 2005;175:5716–5723. doi: 10.4049/jimmunol.175.9.5716. [DOI] [PubMed] [Google Scholar]
- 129.Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, Klein AS, Bulkley GB, Bao C, Noble PW, Lane MD, Diehl AM. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12:57–65. [PubMed] [Google Scholar]
- 130.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 132.Blanco AM, Valles SL, Pascual M, Guerri C. Involvement of TLR4/type I IL-1 receptor signaling in the induction of inflammatory mediators and cell death induced by ethanol in cultured astrocytes. J Immunol. 2005;175:6893–6899. doi: 10.4049/jimmunol.175.10.6893. [DOI] [PubMed] [Google Scholar]
- 133.Zhong S, Ye WP, Xu PP, Feng E, Li H, Lin SH, Liu JY, Ma C, Lin YC. Aromatase expression in leptin-pretreated human breast pre-adipocytes is enhanced by zeranol and suppressed by (-)-gossypol. Anticancer Res. 2010;30:5077–5084. [PubMed] [Google Scholar]
- 134.Koda M, Sulkowska M, Kanczuga-Koda L, Jarzabek K, Sulkowski S. Expression of leptin and its receptor in female breast cancer in relation with selected apoptotic markers. Folia Histochem Cytobiol. 2007;45(Suppl 1):S187–191. [PubMed] [Google Scholar]
- 135.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, Heller R, Ellis LM, Karras J, Bromberg J, Pardoll D, Jove R, Yu H. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 137.Wang YC, He F, Feng F, Liu XW, Dong GY, Qin HY, Hu XB, Zheng MH, Liang L, Feng L, Liang YM, Han H. Notch signaling determines the M1 versus M2 polarization of macrophages in antitumor immune responses. Cancer Res. 2010;70:4840–4849. doi: 10.1158/0008-5472.CAN-10-0269. [DOI] [PubMed] [Google Scholar]
- 138.Kaler P, Augenlicht L, Klampfer L. Macrophage-derived IL-1beta stimulates Wnt signaling and growth of colon cancer cells: a crosstalk interrupted by vitamin D3. Oncogene. 2009;28:3892–3902. doi: 10.1038/onc.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jinushi M, Chiba S, Yoshiyama H, Masutomi K, Kinoshita I, Dosaka-Akita H, Yagita H, Takaoka A, Tahara H. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc Natl Acad Sci U S A. 2011;108:12425–12430. doi: 10.1073/pnas.1106645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jing Y, Han Z, Zhang S, Liu Y, Wei L. Epithelial-Mesenchymal Transition in tumor microenvironment. Cell Biosci. 2011;1:29. doi: 10.1186/2045-3701-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pischon T, Nothlings U, Boeing H. Obesity and cancer. Proc Nutr Soc. 2008;67:128–145. doi: 10.1017/S0029665108006976. [DOI] [PubMed] [Google Scholar]
- 142.Akther A, Khan KH, Begum M, Parveen S, Kaiser MS, Chowdhury AZ. Leptin: a mysterious hormone; its physiology and pathophysiology. Mymensingh Med J. 2009;18:S140–144. [PubMed] [Google Scholar]
- 143.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 144.Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 145.Boras-Granic K, Wysolmerski JJ. Wnt signaling in breast organogenesis. Organogenesis. 2008;4:116–122. doi: 10.4161/org.4.2.5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Prosperi JR, Goss KH. A Wnt-ow of Opportunity: Targeting the Wnt/beta-Catenin Pathway in Breast Cancer. Curr Drug Targets. 2010;11:1074–1088. doi: 10.2174/138945010792006780. [DOI] [PubMed] [Google Scholar]
- 147.Fenton JI, Lavigne JA, Perkins SN, Liu H, Chandramouli GV, Shih JH, Hord NG, Hursting SD. Microarray analysis reveals that leptin induces autocrine/paracrine cascades to promote survival and proliferation of colon epithelial cells in an Apc genotype-dependent fashion. Mol Carcinog. 2008;47:9–21. doi: 10.1002/mc.20357. [DOI] [PubMed] [Google Scholar]
- 148.Endo H, Hosono K, Uchiyama T, Sakai E, Sugiyama M, Takahashi H, Nakajima N, Wada K, Takeda K, Nakagama H, Nakajima A. Leptin acts as a growth factor for colorectal tumours at stages subsequent to tumour initiation in murine colon carcinogenesis. Gut. 2011 doi: 10.1136/gut.2010.235754. [DOI] [PubMed] [Google Scholar]
- 149.Gillespie C, Colbert L, McGlothen T, Guo S, Zhou W, Gonzalez-Perez R. DMBA Breast Cancer in Diet Induced Obesity (DIO) and Lean Mice is related to Leptin Signaling CTRC-AACR San Antonio Breast Cancer Symposium; 2011. [Google Scholar]
- 150.Kovall RA. More complicated than it looks: assembly of Notch pathway transcription complexes. Oncogene. 2008;27:5099–5109. doi: 10.1038/onc.2008.223. [DOI] [PubMed] [Google Scholar]
- 151.Danovi SA. Angiogenesis: Turning it down a Notch. Nature Reviews Cancer Nat Rev Cancer. 2008;8:572–573. [Google Scholar]
- 152.Polus AGJ, Piatkowska E, Dembinska-Kiec A. Differences in leptin, VEGF, and bFGF-induced angiogenic differentiation of HUVEC and human umbilical blood CD34+ progenitor cells. European Journal of Biochemistry. 2003:Abstract number: P4.1–67. [Google Scholar]
- 153.Lanier V, Gillespie C, McGlothen T, Dickson T, Guo S, G-PRR Leptin induces Notch in endothelial cells: role of VEGFR-2 transactivation. AACR 102 Annual Congress. 2012 [Google Scholar]
- 154.Tai W, Mahato R, Cheng K. The role of HER2 in cancer therapy and targeted drug delivery. J Control Release. 2010 doi: 10.1016/j.jconrel.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tzahar E, Yarden Y. The ErbB-2/HER2 oncogenic receptor of adenocarcinomas: from orphanhood to multiple stromal ligands. Biochim Biophys Acta. 1998;1377:M25–37. doi: 10.1016/s0304-419x(97)00032-2. [DOI] [PubMed] [Google Scholar]
- 156.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 157.Ray A, Nkhata KJ, Cleary MP. Effects of leptin on human breast cancer cell lines in relationship to estrogen receptor and HER2 status. Int J Oncol. 2007;30:1499–1509. [PubMed] [Google Scholar]
- 158.Soma D, Kitayama J, Yamashita H, Miyato H, Ishikawa M, Nagawa H. Leptin augments proliferation of breast cancer cells via transactivation of HER2. J Surg Res. 2008;149:9–14. doi: 10.1016/j.jss.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 159.Fiorio E, Mercanti A, Terrasi M, Micciolo R, Remo A, Auriemma A, Molino A, Parolin V, Di Stefano B, Bonetti F, Giordano A, Cetto GL, Surmacz E. Leptin/HER2 crosstalk in breast cancer: in vitro study and preliminary in vivo analysis. BMC Cancer. 2008;8:305. doi: 10.1186/1471-2407-8-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jernstrom H, Barrett-Connor E. Obesity, weight change, fasting insulin, proinsulin, C-peptide, and insulin-like growth factor-1 levels in women with and without breast cancer: the Rancho Bernardo Study. J Womens Health Gend Based Med. 1999;8:1265–1272. doi: 10.1089/jwh.1.1999.8.1265. [DOI] [PubMed] [Google Scholar]
- 161.Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 162.Irwin ML, McTiernan A, Bernstein L, Gilliland FD, Baumgartner R, Baumgartner K, Ballard-Barbash R. Relationship of obesity, physical activity with C-peptide, leptin, and insulin-like growth factors in breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2005;14:2881–2888. doi: 10.1158/1055-9965.EPI-05-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Johansson H, Gandini S, Guerrieri-Gonzaga A, Iodice S, Ruscica M, Bonanni B, Gulisano M, Magni P, Formelli F, Decensi A. Effect of fenretinide and low-dose tamoxifen on insulin sensitivity in premenopausal women at high risk for breast cancer. Cancer Res. 2008;68:9512–9518. doi: 10.1158/0008-5472.CAN-08-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fischer S, Hanefeld M, Haffner SM, Fusch C, Schwanebeck U, Kohler C, Fucker K, Julius U. Insulin-resistant patients with type 2 diabetes mellitus have higher serum leptin levels independently of body fat mass. Acta Diabetol. 2002;39:105–110. doi: 10.1007/s005920200027. [DOI] [PubMed] [Google Scholar]
- 165.Matsubara M, Maruoka S, Katayose S. Inverse relationship between plasma adiponectin and leptin concentrations in normal-weight and obese women. Eur J Endocrinol. 2002;147:173–180. doi: 10.1530/eje.0.1470173. [DOI] [PubMed] [Google Scholar]
- 166.Renehan AG, Harvie M, Howell A. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and breast cancer risk: eight years on. Endocr Relat Cancer. 2006;13:273–278. doi: 10.1677/erc.1.01219. [DOI] [PubMed] [Google Scholar]
- 167.Garofalo C, Koda M, Cascio S, Sulkowska M, Kanczuga-Koda L, Golaszewska J, Russo A, Sulkowski S, Surmacz E. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clin Cancer Res. 2006;12:1447–1453. doi: 10.1158/1078-0432.CCR-05-1913. [DOI] [PubMed] [Google Scholar]
- 168.Ozbay T, Nahta R. A novel unidirectional cross-talk from the insulin-like growth factor-I receptor to leptin receptor in human breast cancer cells. Mol Cancer Res. 2008;6:1052–1058. doi: 10.1158/1541-7786.MCR-07-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Saxena NK, Taliaferro-Smith L, Knight BB, Merlin D, Anania FA, O’Regan RM, Sharma D. Bidirectional crosstalk between leptin and insulin-like growth factor-I signaling promotes invasion and migration of breast cancer cells via transactivation of epidermal growth factor receptor. Cancer Res. 2008;68:9712–9722. doi: 10.1158/0008-5472.CAN-08-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Garofalo C, Sisci D, Surmacz E. Leptin interferes with the effects of the antiestrogen ICI 182,780 in MCF-7 breast cancer cells. Clin Cancer Res. 2004;10:6466–6475. doi: 10.1158/1078-0432.CCR-04-0203. [DOI] [PubMed] [Google Scholar]
- 171.Surmacz E. Function of the IGF-I receptor in breast cancer. J Mammary Gland Biol Neoplasia. 2000;5:95–105. doi: 10.1023/a:1009523501499. [DOI] [PubMed] [Google Scholar]
- 172.Steelman LS, Stadelman KM, Chappell WH, Horn S, Basecke J, Cervello M, Nicoletti F, Libra M, Stivala F, Martelli AM, McCubrey JA. Akt as a therapeutic target in cancer. Expert Opin Ther Targets. 2008;12:1139–1165. doi: 10.1517/14728222.12.9.1139. [DOI] [PubMed] [Google Scholar]
- 173.Wickenden JA, Watson CJ. Key signalling nodes in mammary gland development and cancer. Signalling downstream of PI3 kinase in mammary epithelium: a play in 3 Akts. Breast Cancer Res. 2010;12:202. doi: 10.1186/bcr2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sabbah M, Emami S, Redeuilh G, Julien S, Prevost G, Zimber A, Ouelaa R, Bracke M, De Wever O, Gespach C. Molecular signature and therapeutic perspective of the epithelial-to-mesenchymal transitions in epithelial cancers. Drug Resist Updat. 2008;11:123–151. doi: 10.1016/j.drup.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 175.Dillon RL, Muller WJ. Distinct biological roles for the akt family in mammary tumor progression. Cancer Res. 2010;70:4260–4264. doi: 10.1158/0008-5472.CAN-10-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cheng SP, Yin PH, Hsu YC, Chang YC, Huang SY, Lee JJ, Chi CW. Leptin enhances migration of human papillary thyroid cancer cells through the PI3K/AKT and MEK/ERK signaling pathways. Oncol Rep. 2011;26:1265–1271. doi: 10.3892/or.2011.1388. [DOI] [PubMed] [Google Scholar]
- 177.Uddin S, Bu R, Ahmed M, Hussain AR, Ajarim D, Al-Dayel F, Bavi P, Al-kuraya KS. Leptin receptor expression and its association with PI3K/AKT signaling pathway in diffuse large B-cell lymphoma. Leuk Lymphoma. 2010;51:1305–1314. doi: 10.3109/10428191003802365. [DOI] [PubMed] [Google Scholar]
- 178.Uddin S, Bavi PP, Hussain AR, Alsbeih G, Al-Sanea N, Abduljabbar A, Ashari LH, Alhomoud S, Al-Dayel F, Ahmed M, Al-Kuraya KS. Leptin receptor expression in Middle Eastern colorectal cancer and its potential clinical implication. Carcinogenesis. 2009;30:1832–1840. doi: 10.1093/carcin/bgp145. [DOI] [PubMed] [Google Scholar]
- 179.Shen Y, Wang Q, Zhao Q, Zhou J. Leptin promotes the immune escape of lung cancer by inducing proinflammatory cytokines and resistance to apoptosis. Mol Med Report. 2009;2:295–299. doi: 10.3892/mmr_00000099. [DOI] [PubMed] [Google Scholar]
- 180.Gao J, Tian J, Lv Y, Shi F, Kong F, Shi H, Zhao L. Leptin induces functional activation of cyclooxygenase-2 through JAK2/STAT3, MAPK/ERK, and PI3K/AKT pathways in human endometrial cancer cells. Cancer Sci. 2009;100:389–395. doi: 10.1111/j.1349-7006.2008.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Miyazaki T, Bub JD, Iwamoto Y. c-Jun NH(2)-terminal kinase mediates leptin-stimulated androgen-independent prostate cancer cell proliferation via signal transducer and activator of transcription 3 and Akt. Biochim Biophys Acta. 2008;1782:593–604. doi: 10.1016/j.bbadis.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 182.Saxena NK, Sharma D, Ding X, Lin S, Marra F, Merlin D, Anania FA. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res. 2007;67:2497–2507. doi: 10.1158/0008-5472.CAN-06-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Menon S, Manning BD. Common corruption of the mTOR signaling network in human tumors. Oncogene. 2008;27(Suppl 2):S43–51. doi: 10.1038/onc.2009.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Russell RC, Fang C, Guan KL. An emerging role for TOR signaling in mammalian tissue and stem cell physiology. Development. 2011;138:3343–3356. doi: 10.1242/dev.058230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ciuffreda L, Di Sanza C, Incani UC, Milella M. The mTOR pathway: a new target in cancer therapy. Curr Cancer Drug Targets. 2010;10:484–495. doi: 10.2174/156800910791517172. [DOI] [PubMed] [Google Scholar]
- 186.Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Lett. 2010;584:1287–1295. doi: 10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Attoub S, Noe V, Pirola L, Bruyneel E, Chastre E, Mareel M, Wymann MP, Gespach C. Leptin promotes invasiveness of kidney, colonic epithelial cells via phosphoinositide 3-kinase-,rho-, and rac-dependent signaling pathways. FASEB J. 2000;14:2329–2338. doi: 10.1096/fj.00-0162. [DOI] [PubMed] [Google Scholar]
- 188.Maya-Monteiro CM, Bozza PT. Leptin and mTOR: partners in metabolism and inflammation. Cell Cycle. 2008;7:1713–1717. doi: 10.4161/cc.7.12.6157. [DOI] [PubMed] [Google Scholar]
- 189.Sparks CA, Guertin DA. Targeting mTOR: prospects for mTOR complex 2 inhibitors in cancer therapy. Oncogene. 2010;29:3733–3744. doi: 10.1038/onc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ghayad SE, Cohen PA. Inhibitors of the PI3K/Akt/mTOR pathway: new hope for breast cancer patients. Recent Pat Anticancer Drug Discov. 2010;5:29–57. doi: 10.2174/157489210789702208. [DOI] [PubMed] [Google Scholar]
- 191.Hadad SM, Fleming S, Thompson AM. Targeting AMPK: a new therapeutic opportunity in breast cancer. Crit Rev Oncol Hematol. 2008;67:1–7. doi: 10.1016/j.critrevonc.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 192.Noh WC, Kim YH, Kim MS, Koh JS, Kim HA, Moon NM, Paik NS. Activation of the mTOR signaling pathway in breast cancer and its correlation with the clinicopathologic variables. Breast Cancer Res Treat. 2008;110:477–483. doi: 10.1007/s10549-007-9746-x. [DOI] [PubMed] [Google Scholar]
- 193.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 194.Prasad S, Ravindran J, Aggarwal BB. NF-kappaB and cancer: how intimate is this relationship. Mol Cell Biochem. 2010;336:25–37. doi: 10.1007/s11010-009-0267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 196.Takada Y, Ichikawa H, Badmaev V, Aggarwal BB. Acetyl-11-keto-beta-boswellic acid potentiates apoptosis, inhibits invasion, and abolishes osteoclastogenesis by suppressing NF-kappa B and NF-kappa B-regulated gene expression. J Immunol. 2006;176:3127–3140. doi: 10.4049/jimmunol.176.5.3127. [DOI] [PubMed] [Google Scholar]
- 197.Sethi G, Ahn KS, Sandur SK, Lin X, Chaturvedi MM, Aggarwal BB. Indirubin enhances tumor necrosis factor-induced apoptosis through modulation of nuclear factor-kappa B signaling pathway. J Biol Chem. 2006;281:23425–23435. doi: 10.1074/jbc.M602627200. [DOI] [PubMed] [Google Scholar]
- 198.Mattace Raso G, Esposito E, Iacono A, Pacilio M, Coppola A, Bianco G, Diano S, Di Carlo R, Meli R. Leptin induces nitric oxide synthase type II in C6 glioma cells. Role for nuclear factor-kappaB in hormone effect. Neurosci Lett. 2006;396:121–126. doi: 10.1016/j.neulet.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 199.Yeh WL, Lu DY, Lee MJ, Fu WM. Leptin induces migration and invasion of glioma cells through MMP-13 production. Glia. 2009;57:454–464. doi: 10.1002/glia.20773. [DOI] [PubMed] [Google Scholar]
- 200.Ogunwobi OO, Beales IL. Cyclo-oxygenase-independent inhibition of apoptosis and stimulation of proliferation by leptin in human colon cancer cells. Dig Dis Sci. 2007;52:1934–1945. doi: 10.1007/s10620-007-9784-6. [DOI] [PubMed] [Google Scholar]
- 201.Rouet-Benzineb P, Aparicio T, Guilmeau S, Pouzet C, Descatoire V, Buyse M, Bado A. Leptin counteracts sodium butyrate-induced apoptosis in human colon cancer HT-29 cells via NF-kappaB signaling. J Biol Chem. 2004;279:16495–16502. doi: 10.1074/jbc.M312999200. [DOI] [PubMed] [Google Scholar]
- 202.Fletcher S, Drewry JA, Shahani VM, Page BD, Gunning PT. Molecular disruption of oncogenic signal transducer and activator of transcription 3 (STAT3) protein. Biochem Cell Biol. 2009;87:825–833. doi: 10.1139/o09-044. [DOI] [PubMed] [Google Scholar]
- 203.Masciocchi D, Gelain A, Villa S, Meneghetti F, Barlocco D. Signal transducer and activator of transcription 3 (STAT3): a promising target for anticancer therapy. Future Med Chem. 2011;3:567–597. doi: 10.4155/fmc.11.22. [DOI] [PubMed] [Google Scholar]
- 204.Mankan AK, Greten FR. Inhibiting signal transducer and activator of transcription 3: rationality and rationale design of inhibitors. Expert Opin Investig Drugs. 2011;20:1263–1275. doi: 10.1517/13543784.2011.601739. [DOI] [PubMed] [Google Scholar]
- 205.Zhao M, Jiang B, Gao FH. Small Molecule Inhibitors of STAT3 for Cancer Therapy. Curr Med Chem. 2011 doi: 10.2174/092986711796957284. [DOI] [PubMed] [Google Scholar]
- 206.Bates SH, Kulkarni RN, Seifert M, Myers MG., Jr Roles for leptin receptor/STAT3-dependent and -independent signals in the regulation of glucose homeostasis. Cell Metab. 2005;1:169–178. doi: 10.1016/j.cmet.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 207.Ratke J, Entschladen F, Niggemann B, Zanker KS, Lang K. Leptin stimulates the migration of colon carcinoma cells by multiple signaling pathways. Endocr Relat Cancer. 2010;17:179–189. doi: 10.1677/ERC-09-0225. [DOI] [PubMed] [Google Scholar]
- 208.Catalano S, Giordano C, Rizza P, Gu G, Barone I, Bonofiglio D, Giordano F, Malivindi R, Gaccione D, Lanzino M, De Amicis F, Ando S. Evidence that leptin through STAT and CREB signaling enhances cyclin D1 expression and promotes human endometrial cancer proliferation. J Cell Physiol. 2009;218:490–500. doi: 10.1002/jcp.21622. [DOI] [PubMed] [Google Scholar]
- 209.Li L, Gao Y, Zhang LL, He DL. Concomitant activation of the JAK/STAT3 and ERK1/2 signaling is involved in leptin-mediated proliferation of renal cell carcinoma Caki-2 cells. Cancer Biol Ther. 2008;7:1787–1792. doi: 10.4161/cbt.7.11.6837. [DOI] [PubMed] [Google Scholar]
- 210.Saxena NK, Vertino PM, Anania FA, Sharma D. leptin-induced growth stimulation of breast cancer cells involves recruitment of histone acetyltransferases and mediator complex to CYCLIN D1 promoter via activation of Stat3. J Biol Chem. 2007;282:13316–13325. doi: 10.1074/jbc.M609798200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Pai R, Lin C, Tran T, Tarnawski A. Leptin activates STAT and ERK2 pathways and induces gastric cancer cell proliferation. Biochem Biophys Res Commun. 2005;331:984–992. doi: 10.1016/j.bbrc.2005.03.236. [DOI] [PubMed] [Google Scholar]
- 212.Yin N, Wang D, Zhang H, Yi X, Sun X, Shi B, Wu H, Wu G, Wang X, Shang Y. Molecular mechanisms involved in the growth stimulation of breast cancer cells by leptin. Cancer Res. 2004;64:5870–5875. doi: 10.1158/0008-5472.CAN-04-0655. [DOI] [PubMed] [Google Scholar]
- 213.Dieudonne MN, Machinal-Quelin F, Serazin-Leroy V, Leneveu MC, Pecquery R, Giudicelli Y. Leptin mediates a proliferative response in human MCF7 breast cancer cells. Biochem Biophys Res Commun. 2002;293:622–628. doi: 10.1016/S0006-291X(02)00205-X. [DOI] [PubMed] [Google Scholar]
- 214.Ogunwobi OO, Beales IL. The anti-apoptotic, growth stimulatory actions of leptin in human colon cancer cells involves activation of JNK mitogen activated protein kinase, JAK2 and PI3 kinase/Akt. Int J Colorectal Dis. 2007;22:401–409. doi: 10.1007/s00384-006-0181-y. [DOI] [PubMed] [Google Scholar]
- 215.Russo VC, Metaxas S, Kobayashi K, Harris M, Werther GA. Antiapoptotic effects of leptin in human neuroblastoma cells. Endocrinology. 2004;145:4103–4112. doi: 10.1210/en.2003-1767. [DOI] [PubMed] [Google Scholar]
- 216.Cascio S, Ferla R, D’Andrea A, Gerbino A, Bazan V, Surmacz E, Russo A. Expression of angiogenic regulators, VEGF, leptin, is regulated by the EGF/PI3K/STAT3 pathway in colorectal cancer cells. J Cell Physiol. 2009;221:189–194. doi: 10.1002/jcp.21843. [DOI] [PubMed] [Google Scholar]
- 217.Ren H, Zhao T, Wang X, Gao C, Wang J, Yu M, Hao J. Leptin upregulates telomerase activity and transcription of human telomerase reverse transcriptase in MCF-7 breast cancer cells. Biochem Biophys Res Commun. 2010;394:59–63. doi: 10.1016/j.bbrc.2010.02.093. [DOI] [PubMed] [Google Scholar]
- 218.Jiang H, Yu J, Guo H, Song H, Chen S. Upregulation of survivin by leptin/STAT3 signaling in MCF-7 cells. Biochem Biophys Res Commun. 2008;368:1–5. doi: 10.1016/j.bbrc.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 219.Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1) Mol Pharmacol. 2006;70:1469–1480. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- 220.Hirota K, Semenza GL. Regulation of angiogenesis by hypoxia-inducible factor 1. Crit Rev Oncol Hematol. 2006;59:15–26. doi: 10.1016/j.critrevonc.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 221.Li Y, Ye D. Cancer Therapy By Targeting Hypoxia-Inducible Factor-1. Curr Cancer Drug Targets. 2010 doi: 10.2174/156800910793605857. [DOI] [PubMed] [Google Scholar]
- 222.Li Z, Rich JN. Hypoxia and hypoxia inducible factors in cancer stem cell maintenance. Curr Top Microbiol Immunol. 2010;345:21–30. doi: 10.1007/82_2010_75. [DOI] [PubMed] [Google Scholar]
- 223.Grosfeld A, Andre J, Hauguel-De Mouzon S, Berra E, Pouyssegur J, Guerre-Millo M. Hypoxia-inducible factor 1 transactivates the human leptin gene promoter. J Biol Chem. 2002;277:42953–42957. doi: 10.1074/jbc.M206775200. [DOI] [PubMed] [Google Scholar]
- 224.Wu MH, Chen KF, Lin SC, Lgu CW, Tsai SJ. Aberrant expression of leptin in human endometriotic stromal cells is induced by elevated levels of hypoxia inducible factor-1alpha. Am J Pathol. 2007;170:590–598. doi: 10.2353/ajpath.2007.060477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Koda M, Sulkowska M, Wincewicz A, Kanczuga-Koda L, Musiatowicz B, Szymanska M, Sulkowski S. Expression of leptin, leptin receptor, and hypoxia-inducible factor 1 alpha in human endometrial cancer. Ann N Y Acad Sci. 2007;1095:90–98. doi: 10.1196/annals.1397.013. [DOI] [PubMed] [Google Scholar]
- 226.Koda M, Sulkowska M, Kanczuga-Koda L, Cascio S, Colucci G, Russo A, Surmacz E, Sulkowski S. Expression of the obesity hormone leptin and its receptor correlates with hypoxia-inducible factor-1 alpha in human colorectal cancer. Ann Oncol. 2007;18(Suppl 6):vi116–119. doi: 10.1093/annonc/mdm238. [DOI] [PubMed] [Google Scholar]
- 227.Cascio S, Bartella V, Auriemma A, Johannes GJ, Russo A, Giordano A, Surmacz E. Mechanism of leptin expression in breast cancer cells: role of hypoxia-inducible factor-1alpha. Oncogene. 2008;27:540–547. doi: 10.1038/sj.onc.1210660. [DOI] [PubMed] [Google Scholar]
- 228.Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med. 2001;344:276–285. doi: 10.1056/NEJM200101253440407. [DOI] [PubMed] [Google Scholar]
- 229.Henderson BE, Ross R, Bernstein L. Estrogens as a cause of human cancer: the Richard and Hinda Rosenthal Foundation award lecture. Cancer Res. 1988;48:246–253. [PubMed] [Google Scholar]
- 230.Bernstein L, Ross RK. Endogenous hormones and breast cancer risk. Epidemiol Rev. 1993;15:48–65. doi: 10.1093/oxfordjournals.epirev.a036116. [DOI] [PubMed] [Google Scholar]
- 231.Johnston SR. New strategies in estrogen receptor-positive breast cancer. Clin Cancer Res. 2010;16:1979–1987. doi: 10.1158/1078-0432.CCR-09-1823. [DOI] [PubMed] [Google Scholar]
- 232.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 233.Welboren WJ, Stunnenberg HG, Sweep FC, Span PN. Identifying estrogen receptor target genes. Mol Oncol. 2007;1:138–143. doi: 10.1016/j.molonc.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Henson MC, Castracane VD. Leptin: roles and regulation in primate pregnancy. Semin Reprod Med. 2002;20:113–122. doi: 10.1055/s-2002-32502. [DOI] [PubMed] [Google Scholar]
- 235.Jarde T, Caldefie-Chezet F, Damez M, Mishellany F, Penault-Llorca F, Guillot J, Vasson MP. Leptin and leptin receptor involvement in cancer development: a study on human primary breast carcinoma. Oncol Rep. 2008;19:905–911. [PubMed] [Google Scholar]
- 236.Marttunen MB, Andersson S, Hietanen P, Karonen SL, Koistinen HA, Koivisto VA, Tiitinen A, Ylikorkala O. Antiestrogenic tamoxifen and toremifene increase serum leptin levels in postmenopausal breast cancer patients. Maturitas. 2000;35:175–179. doi: 10.1016/s0378-5122(00)00121-3. [DOI] [PubMed] [Google Scholar]
- 237.Catalano S, Mauro L, Marsico S, Giordano C, Rizza P, Rago V, Montanaro D, Maggiolini M, Panno ML, Ando S. Leptin induces, via ERK1/ERK2 signal, functional activation of estrogen receptor alpha in MCF-7 cells. J Biol Chem. 2004;279:19908–19915. doi: 10.1074/jbc.M313191200. [DOI] [PubMed] [Google Scholar]
- 238.Catalano S, Marsico S, Giordano C, Mauro L, Rizza P, Panno ML, Ando S. Leptin enhances, via AP-1, expression of aromatase in the MCF-7 cell line. J Biol Chem. 2003;278:28668–28676. doi: 10.1074/jbc.M301695200. [DOI] [PubMed] [Google Scholar]
- 239.Altieri DC. New wirings in the survivin networks. Oncogene. 2008;27:6276–6284. doi: 10.1038/onc.2008.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ryan BM, O’Donovan N, Duffy MJ. Survivin: a new target for anti-cancer therapy. Cancer Treat Rev. 2009;35:553–562. doi: 10.1016/j.ctrv.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 241.Abdraboh ME, Gaur RL, Hollenbach AD, Sandquist D, Raj MH, Ouhtit A. Survivin Is a Novel Target of CD44-Promoted Breast Tumor Invasion. Am J Pathol. 2011;179:555–563. doi: 10.1016/j.ajpath.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 243.Lee CW, Raskett CM, Prudovsky I, Altieri DC. Molecular dependence of estrogen receptor-negative breast cancer on a notch-survivin signaling axis. Cancer Res. 2008;68:5273–5281. doi: 10.1158/0008-5472.CAN-07-6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Lee CW, Simin K, Liu Q, Plescia J, Guha M, Khan A, Hsieh CC, Altieri DC. A functional Notch-survivin gene signature in basal breast cancer. Breast Cancer Res. 2008;10:R97. doi: 10.1186/bcr2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Perera CN, Chin HG, Duru N, Camarillo IG. Leptin-regulated gene expression in MCF-7 breast cancer cells: mechanistic insights into leptin-regulated mammary tumor growth and progression. J Endocrinol. 2008;199:221–233. doi: 10.1677/JOE-08-0215. [DOI] [PubMed] [Google Scholar]
- 246.Rose DP, Komninou D, Stephenson GD. Obesity, adipocytokines, and insulin resistance in breast cancer. Obes Rev. 2004;5:153–165. doi: 10.1111/j.1467-789X.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 247.Bartella V, Cascio S, Fiorio E, Auriemma A, Russo A, Surmacz E. Insulin-dependent leptin expression in breast cancer cells. Cancer Res. 2008;68:4919–4927. doi: 10.1158/0008-5472.CAN-08-0642. [DOI] [PubMed] [Google Scholar]
- 248.Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R. Hormonal regulation of adiponectin gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2002;290:1084–1089. doi: 10.1006/bbrc.2001.6307. [DOI] [PubMed] [Google Scholar]
- 249.Miyoshi Y, Funahashi T, Kihara S, Taguchi T, Tamaki Y, Matsuzawa Y, Noguchi S. Association of serum adiponectin levels with breast cancer risk. Clin Cancer Res. 2003;9:5699–5704. [PubMed] [Google Scholar]
- 250.Jarde T, Caldefie-Chezet F, Goncalves-Mendes N, Mishellany F, Buechler C, Penault-Llorca F, Vasson MP. Involvement of adiponectin and leptin in breast cancer: clinical and in vitro studies. Endocr Relat Cancer. 2009;16:1197–1210. doi: 10.1677/ERC-09-0043. [DOI] [PubMed] [Google Scholar]
- 251.Revillion F, Charlier M, Lhotellier V, Hornez L, Giard S, Baranzelli MC, Djiane J, Peyrat JP. Messenger RNA expression of leptin and leptin receptors and their prognostic value in 322 human primary breast cancers. Clin Cancer Res. 2006;12:2088–2094. doi: 10.1158/1078-0432.CCR-05-1904. [DOI] [PubMed] [Google Scholar]
- 252.Kim HS. Leptin and leptin receptor expression in breast cancer. Cancer Res Treat. 2009;41:155–163. doi: 10.4143/crt.2009.41.3.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Otvos L, Jr, Kovalszky I, Riolfi M, Ferla R, Olah J, Sztodola A, Nama K, Molino A, Piubello Q, Wade JD, Surmacz E. Efficacy of a leptin receptor antagonist peptide in a mouse model of triple-negative breast cancer. Eur J Cancer. 2011;47:1578–1584. doi: 10.1016/j.ejca.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 254.Gillespie C, Guo S, Zhou W, G-PRR Leptin signaling disruption prevents DMBA-induced mammary tumors in lean and diet-induced-obesity (DIO) mice. AACR 102 Annual Congress. 2011:Abstract 825. [Google Scholar]
- 255.Aloulou N, Bastuji-Garin S, Le Gouvello S, Abolhassani M, Chaumette MT, Charachon A, Leroy K, Sobhani I. Involvement of the leptin receptor in the immune response in intestinal cancer. Cancer Res. 2008;68:9413–9422. doi: 10.1158/0008-5472.CAN-08-0909. [DOI] [PubMed] [Google Scholar]
- 256.Paik SS, Jang SM, Jang KS, Lee KH, Choi D, Jang SJ. Leptin expression correlates with favorable clinicopathologic phenotype and better prognosis in colorectal adenocarcinoma. Ann Surg Oncol. 2009;16:297–303. doi: 10.1245/s10434-008-0221-7. [DOI] [PubMed] [Google Scholar]
- 257.Wincewicz A, Koda M, Sulkowska M, Kanczuga-Koda L, Sulkowski S. Comparison of STAT3 with HIF-1alpha, Ob and ObR expressions in human endometrioid adenocarcinomas. Tissue Cell. 2008;40:405–410. doi: 10.1016/j.tice.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 258.Horiguchi A, Sumitomo M, Asakuma J, Asano T, Zheng R, Nanus DM, Hayakawa M. Increased serum leptin levels and over expression of leptin receptors are associated with the invasion and progression of renal cell carcinoma. J Urol. 2006;176:1631–1635. doi: 10.1016/j.juro.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 259.Wang SN, Chuang SC, Yeh YT, Yang SF, Chai CY, Chen WT, Kuo KK, Chen JS, Lee KT. Potential prognostic value of leptin receptor in hepatocellular carcinoma. J Clin Pathol. 2006;59:1267–1271. doi: 10.1136/jcp.2005.033464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Wang SN, Yeh YT, Yang SF, Chai CY, Lee KT. Potential role of leptin expression in hepatocellular carcinoma. J Clin Pathol. 2006;59:930–934. doi: 10.1136/jcp.2005.035477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Zhang YB, Fang M, He FL, Hua TF, Hu BD, Zhao H, Liu RY. Mechanism of regulation of leptin expression in human lung adenocarcinoma cells by hypoxia inducible factor-1alpha: a preliminary study. Zhonghua Yi Xue Za Zhi. 2008;88:2848–2853. [PubMed] [Google Scholar]