Abstract
BACKGROUND
Transection of the secondary chordae on the anterior leaflet of the mitral valve to relieve leaflet tethering and reduce regurgitation is an experimentally proven procedure to correct functional mitral regurgitation. In this study, we sought to investigate if transecting the secondary chordae has an impact on the marginal chordal force on the same leaflet.
METHODS
Adult porcine mitral valves (N =8) were studied in a pulsatile heart simulator, in which the papillary muscle positions can be precisely positioned. The anterior marginal chordae were instrumented with miniature transducers to measure the chordal forces. Each valve was studied under baseline conditions, three different tethering conditions [apical, apical-lateral, apical-lateral-posterior], and following chordal cutting in the three tethering conditions. The temporal changes, the peak and average marginal chordal forces under each condition are reported.
RESULTS
Apical tethering increased marginal chordal force by an average 96% but remained unchanged after chordal cutting. With apical-lateral tethering, marginal chordal force increased by 210% from baseline, and further increased to 350% of baseline after chordal cutting. After apical-lateral-posterior tethering, the marginal chordal force increased to 335% of baseline before transection and by 548% after the transection.
CONCLUSIONS
Increase in marginal chordal force after secondary chordal cutting depends on the location of the papillary muscles and the extent of leaflet tethering. While, chordal cutting may not alter the valve mechanics under minimal leaflet tethering, it significantly impacts the mechanics when the leaflet tethering is more pronounced, which is typically seen in patients with functional mitral regurgitation.
Keywords: Mitral valve, Chordae Tendineae, Ischemic Mitral Regurgitation, Chordal Cutting, Valve Mechanics
INTRODUCTION
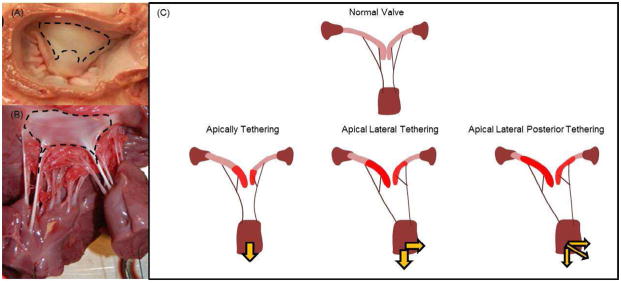
Functional mitral regurgitation (MR) is a secondary pathology in patients with dilated remodeled ventricles due to ischemic or non-ischemic etiologies1,2. Ventricular dilatation induced papillary muscle displacement results in apical tethering of the anterior and posterior leaflets resulting in MR. Mitral annuloplasty is currently used to repair MR by reducing the septal-lateral dimension of the mitral annulus, yet apical tethering of the leaflets persists after this procedure and is known to contribute to persistent or recurrent regurgitation3,4. Pronounced concavity of the anterior leaflet (sea gull shape) is vivid in these patients both before and after annuloplasty, and is associated with higher degrees of recurrence of the regurgitation5. The anterior strut chordae (ASC) of the mitral valve insert at the region of maximum leaflet bending, and are implicated in tethering the entire anterior leaflet surface between their insertion sites and the mitral annulus as shown in Figure 1.
Figure 1.
(A) Atrial surface of the anterior leaflet that has collagen fibers inserting from the anterior strut chordae tendineae; (B) Ventricular surface of the anterior leaflet with fibers from the strut chordae; (C) Different settings of anterior leaflet tethering for variety of papillary muscle positions
Transection of ASC to relieve anterior leaflet tethering was proposed as an adjunct technique to annuloplasty to correct MR and improve leaflet coaptation in these patients6–8. Though clinical experience with this procedure is limited, the hemodynamic efficacy of this technique in chronic ischemic animal models is documented and its potential role in diminishing left ventricular (LV) remodeling is emerging9. Favorable experimental results have encouraged clinical adoption of this procedure, but the impact of transecting the ASC - the main load bearing chordae- on mitral valve mechanics is in question. Specifically, the impact of anterior strut chordal cutting on the thinner marginal chordae on the anterior leaflet is currently unknown.
In a previous study, our group measured the chordal force distribution under different mitral valve tethering settings and reported that the anterior strut chordae of the mitral valve sustained the maximum loads during systole, while the anterior marginal chordae sustained loads an order of magnitude less than the strut chordae10. Subsequently, we also demonstrated that the strut chordae were structurally robust with a thick collagen core that is encapsulated in an elastin layer, and were highly extensible compared to the marginal chordae which were thin and least extensible compared to the other chordae11. Extending these studies, we sought to investigate the changes in the thinnest anterior marginal chordae before and after chordal cutting, under three distinct simulated clinical tethering conditions, in vitro.
2. MATERIALS & METHODS
2.1 Experimental Model
A pulsatile left heart simulator shown in Figure 2A, which allows precise dilatation of the mitral annulus, precise positioning of the papillary muscles in 3-dimensional space and quantitative and visual measurements on the valve was used in this study. This model was previously reported in investigating the integrated mechanisms of MR1,11,12, to study changes in chordal force balance with papillary muscle position10, to study the efficacy of chordal cutting to relieve anterior leaflet tenting4 and more recently was also validated against high temporal and spatial in vivo leaflet strain measurements13. In this model, instead of using a contractile and complex ventricular myocardial model, we used geared mechanisms that can precisely alter the papillary muscle position, to mimic adverse ventricular. A mitral annulus made of flexible silicone tubing, whose area/size can be altered using a spring mechanism and which is saddle shaped was used.
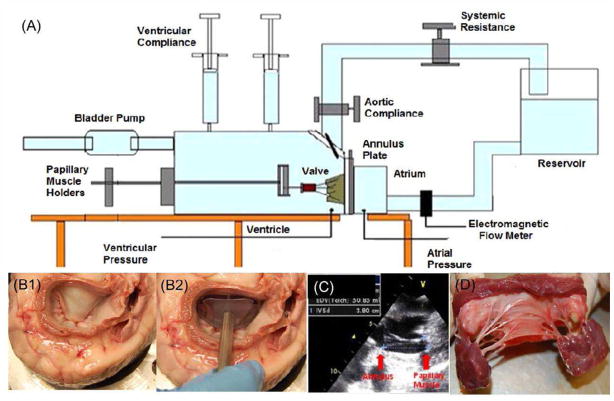
Figure 2.
(A) The ex vivo pulsatile left heart simulator in which chordal forces on the native mitral valve chordae tendineae can be measured, (B) Mitral valve leaflet and annular sizing using a sizer; (C) In vivo measurement of the annular tip to papillary muscle tip distance before explanting the mitral valve; (D) An explanted mitral valve with all the structures in tact
2.2 Mitral Valve Extraction and Preparation
Fresh porcine mitral valves were used due to their anatomical similarity to human mitral valves, and to be consistent with in vivo reports from other laboratories that used Yorkshire pigs. Use of normal human mitral valves would be ideal but it is challenging to obtain sufficient number of fresh transplant hearts for research purposes at our institution. Fresh pig hearts (~50–60kg pigs) were obtained from a local slaughter house (Hollifield Farms, Covington, GA) immediately upon sacrifice, and transported to the laboratory in ice-cold saline. After removing the pericardium and excising the left atrium, mitral valve sizing was performed by pressurizing the heart to 120mm Hg and the anterior leaflet size was measured using an Edwards Lifesciences Physio® ring sizer as shown in Figure 2B, and 28mm valves were selected. A 2D echo was performed to measure the distance of the papillary muscle tips from the mitral annulus in each valve, and recorded as shown in Figure 2C. The valves were extracted from the hearts leaving 5mm of atrial tissue proximal to the mitral annulus intact, and preserving the entire leaflet, chordal, and papillary muscle apparatus as shown in Figure 2D.
2.3 Experimental Preparation
Extracted mitral valves were mounted into the pulsatile simulator, by suturing the left atrial tissue onto the silicone annulus in a mattress fashion as shown in Figure 3A, and by mounting the papillary muscles onto the geared papillary muscle holders shown in Figure 3B. The silicone annulus can dilated from 28mm to 40mm and the papillary muscle holders can be displaced to a resolution of 0.9mm in the apical, posterior and lateral directions. Upon mounting the valve in the simulator, a solenoid timed bulb pump was attached to the ventricle and desired pressure and flow waveforms were generated.
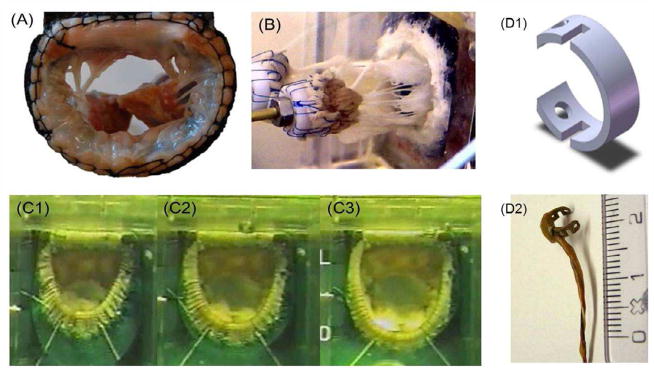
Figure 3.
(A) The extracted mitral valve sutured onto a silicone annulus using sutures; (B) The mitral valve mounted into the left heart simulator; (C) Photographs demonstrating the annular size increase that can be induced with the silicone annulus to simulated mitral annular dilatation; (D) The miniature C-ring force transducers developed with strain gauge technology to mount on the chordae tendineae to measure the forces acting on them throughout the cardiac cycle.
2.4 Chordal Force Measurement
Two marginal chordae on the anterior leaflet were then instrumented with miniature C-ring transducers shown in Figure 3D, which we previously used with success to measure mitral valve chordal forces. These C-ring force transducers consist of two strain gauges, one on the convex and one on the concave side of the ring structure, and by connecting the two gauges in a half bridge circuit the voltage drop across them could be measured. The voltage drop across the transducers was calibrated against measured loads before the experiment. Continuous hemodynamic monitoring was performed using differential pressure transducers (DP-09-40, Validyne Engineering, USA) and an electromagnetic flow probe (600 Model, 501D Analog Meter, Carolina Medical Electronics, USA) throughout the experiment. Continuous data monitoring and recording was performed using a high frequency data acquisition system (National Instruments PCMCIA DAQCard-1200, USA), and data acquisition was time to the cardiac cycle using a TTL synchronizing signal.
2.5 Imaging Protocol
Gated echocardiography acquisitions were performed using a 2D adult probe (Vivid 7, GE Healthcare) for all the experiments, and a 3D matrix array probe (Philips iE33, Philips Medical Systems, USA) was used in one experiment due to limited availability of a 3D ultrasound machine for research purposes at our institution. All images were acquired at 3.75MHz, at an imaging depth of 12mm; and echocardiographic images were acquired along the septal-lateral axis at the central and commissural cusps.
3. PROTOCOL
Experimental Protocol
The experimental protocol for each valve in this study is summarized in Figure 4A:
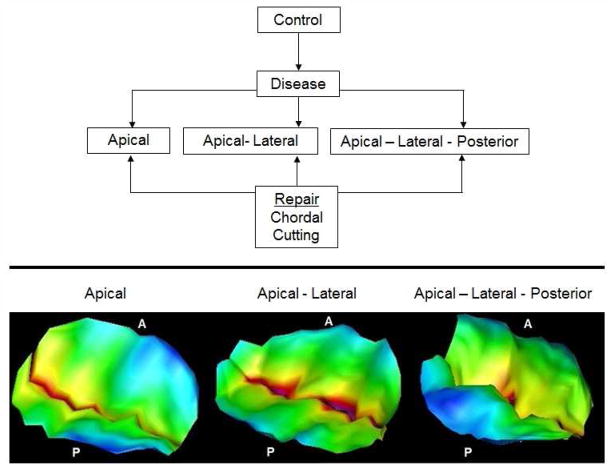
Figure 4.
(Top) Experimental protocol used in this study; (Bottom) Representative images of the mitral valve tethering obtained under different pathological mitral valve papillary muscle positions, reconstructed from 3D echocacrdiographs
Control/Baseline Conditions
The annulus was maintained at 28mm physiological size, and the papillary muscles were in their physiological state (based on the measurements made in the pressurized hearts) and each valve was studied under pulsatile hemodynamic conditions of 120 mmHg peak transmitral pressure, 5L/min cardiac output at 70 beats per minute.
FMR Conditions
The annulus was dilated to 36mm by increasing the silicone annular size as shown in Figure 3C. The papillary muscles were displaced by 10mm in the apical direction (Group 1) and measurements taken, followed by an additional 10mm in the lateral direction (Group 2), and by an additional 10mm in the posterior direction (Group 3). The order of simulating these geometries was randomized between different valves to avoid any systematic error due to procedure.
Anterior Strut Chordal Cutting
Following confirmation of leaflet tethering on ultrasound, the anterior strut chordae were transected using a miniature catheter that could be inserted into the experimental setup without disturbing the mitral valve. The insertion point of the chordae into the leaflet was isolated using a snare, and they were transected at the insertion point by taking care that no damage to the leaflet was made, which was ensured by visual examination.
Measurement Techniques
Chordal Force Measurements
Forces on the marginal chordae were measured over 15 consecutive cardiac cycles in each experimental condition, and the averaged temporal data and the peak force magnitudes are reported.
Statistical Analysis
All the measured endpoints are reported as mean ± standard deviation. The data were tested for normality using the Anderson-Darling Test in MINITAB 15. The experimental groups were compared using a paired t-test considering statistically significant difference at a p < 0.05 with a 95% confidence interval. A non-parametric Wilcoxon signed-rank test was conducted on the leaflet when data was not normally distributed.
4. RESULTS
Leaflet Tethering Pattern
To establish that the desired tethering pattern was achieved and that it was different between the three geometries, the leaflet shape was segmented from the echocardiographs and the tenting distance from the mitral annular plane was mapped and shown in Figure 4B. With apical displacement, the tethering was concentrated at the free edges of the two leaflets, with apical-lateral displacement the tethering was more pronounced but still focused near the free edges, and with apical-lateral-posterior displacement the tethering was diffused over the two leaflets but significantly higher on the anterior leaflet, producing the typical sea gull shape on one leaflet and slight prolapse on the other leaflet.
Temporal Marginal Chordal Forces Before Chordal Cutting
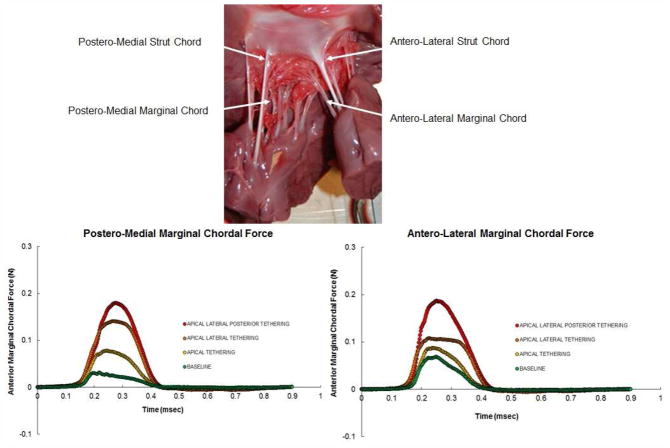
Figure 5 depicts the temporal changes in the marginal chordal forces (left and right anterior marginal chordae) under different tethering patterns, and are compared to the baseline. At baseline, a force trace that increased rapidly with early systole, relatively plateaued during early to mid-systole, and gradually reduced during late systole; similar to the characteristic force measurements reported by Salisbury et al14 in an animal model, but on the secondary chordae. With apical displacement of the papillary muscles, a rise in the peak force was measured, but the temporal trend remained similar to the baseline, if not identical. When the papillary muscles were moved laterally, the early systolic rise in the force was identical but a rather flat plateau was observed for rest of systole, followed by a rapid decline at end systole. With additional posterior displacement of the papillary muscles, the plateau was eliminated, with more or less a curved peak in the force, which involved a rapid increase to peak and then a rapid decline.
Figure 5.
(Top) A photograph of the ventricular surface of the mitral anterior leaflet depicting the insertion sites of the strut and marginal chordae. (Bottom – left) Left marginal chordal force at normal and different pathological papillary muscle positions before chordal cutting procedure. (Bottom – right) Right marginal chordal force at normal and different pathological papillary muscle positions before chordal cutting procedure.
Temporal Marginal Chordal Forces After Chordal Cutting
Figure 6 demonstrates a comparison between the marginal chordal force before and after chordal cutting under each of the PM positions for the left and right marginal chordae. After chordal cutting, the gradual decline or the plateau seen before chordal cutting was lost, with a rapid increase to peak, followed by a rapid fall in the force during systole. In comparison to the force trace measured before chordal cutting, increase in the peak force was observed only with apical-lateral and apical-lateral-posterior displacement, but not with apical displacement. The potential mechanisms of such change in comparison to those recorded before chordal cutting are detailed in subsequent sections.
Figure 6.
Changes in marginal chordal forces before and after chordal cutting in the left and right marginal chordae under different papillary muscle positions. (Row 1) Apical papillary muscle displacement depicting minimal changes in the measured chordal forces before and after chordal cutting; (Row 2) Apical lateral papillary muscle displacement depicting significant increase in the chordal force before and after chordal cutting in both the chordae; (Row 3) Apical lateral posterior papillary muscle displacement depicting significant changes in the chordal force before and after chordal cutting in both the chordae.
Peak Marginal Chordal Forces
In Table 1, the peak forces measured in each setting are stated, the percentage change compared to baseline and before chordal cutting, and an overall factor of safety are reported. In the left marginal chord, a 45% increase in peak force was measured after apical displacement compared to baseline, which after chordal cutting actually decreased to 17%. With apical-lateral displacement, an 80% increase in force was measured before chordal cutting, which increased to 208% after chordal cutting procedure. After apical-lateral-posterior displacement, a 210% increase in force was measured before chordal cutting, which subsequently increased to 367% after chordal cutting. In the right marginal chord, the increase in forces followed a similar trend but with higher magnitude changes. A 143% increase in peak force was measured after apical displacement compared to baseline, which after chordal cutting actually decreased to 119%. With apical-lateral displacement, an 341% increase in force was measured before chordal cutting, which increased to 491% after chordal cutting procedure. After apical-lateral-posterior displacement, a 459% increase in force was measured before chordal cutting, which subsequently increased to 728% after chordal cutting
Table 1.
Maximum marginal chordal forces measured under different papillary muscle positions in comparison to the baseline force measurements. The factor of safety is calculated as a ratio between the failure strength of the anterior marginal chord and the measured force in the experiments before and after chordal cutting
| Average Max Marginal Chordal Force – before CT cut (N) | % Change from Baseline | Factor of Safety (FOS) | Average Max Marginal Chordal Force –after CT cut (N) | % Change from Baseline | Factor of Safely (FOS) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Left | p-value | Right | P-value | Left | Right | Left | Right | Left | P value | Right | P value | Left | Right | Left | Right | |
| Apical PMD | 0.09±0.07 | 0.37 | 0.8±0.1 | 0.05 | 45 | 143 | 77.16 | 86.17 | 0.07±0.03 | 0.376 | 0.07±009 | 0.85 | 16.7 | 118.7 | 96.14 | 96.14 |
| Apical-Lateral PMD | 0.127+0.05 | 0.05 | 0.157±0.2 | 0.05 | 80 | 340.6 | 61.96 | 47.22 | 0.19±0.11 | 0.025 | 0.198±0.15 | 0.04 | 208.3 | 490.6 | 36.75 | 34.97 |
| Apical Lateral-Posterior PMD | 0.216±0.12 | 0.03 | 0.198±0.2 | 0.05 | 210 | 459.3 | 35.55 | 36.98 | 0.30±0.23 | 0.05 | 0.280±0.21 | 0.01 | 366.6 | 728.1 | 23.28 | 24.66 |
All p values are reported in comparison to the baseline
FOS = {(Failure Force of Anterior Marginal Chordae/ Measured Force on Anterior Marginal Chord) -1.}
5. DISCUSSION
In general, data from this study demonstrate that anterior strut chordal cutting increases the forces on the anterior marginal chordae in a papillary muscle position dependent manner. When the papillary muscles were displaced apically, no statistically significant increases in the marginal chordal force were measured before and after chordal cutting in either of the chordae. Anterior strut chordal transection is a hemodynamically proven technique to relieve mitral regurgitation in an ovine model of acute and chronic ischemia7,8. In a dilated ventricle, the strut chordae significantly tether the anterior leaflet, and their transection relieved the leaflet tethering and allowed better basal motion of towards the mitral annulus, and lateral motion towards the posterior leaflet. Though this is a hemodynamically and clinically appealing mechanism to restore valve competence in a tethered mitral valve, surgeons are skeptical about implementing this procedure in humans due to the notion that the smaller chordae tendineae may experience significantly high forces after the procedure. In this study, we attempt to provide a quantitative measurement of the increase in the marginal chordal force in comparison to the failure strength of the anterior marginal chord.
The baseline marginal chordal forces measured in this study fall within the range previously reported by Salisbury14, Nielsen15,16 and Jimenez et al.10, demonstrating the experimental accuracy of the techniques in the present study. Apical displacement of the papillary muscles did not increase the marginal chordal force significantly, both before and after chordal cutting, even though slight leaflet tethering was observed in the echocardiographs (Figure 4B). We speculate that when chordal cutting relieves the little tension that apical displacement imposes on the leaflet, the mobility of the leaflet is restored to physiological levels. Thus, physiological coaptation is restored, which literally mimics a native mitral valve, thus explaining the minimal change in the chordal forces. With lateral displacement it is reasonable to expect a significant rise in the leaflet free edge tension (photographs shown in supplementary information) and an equivalent reduction in its mobility. Thus, the marginal chordal forces increase only to the point till the free edge can move basally towards the mitral annulus, resulting in the force plateau, and then rapidly fall as the leaflet is pulled/pushed back to its diastolic configuration. However, when the papillary muscles are displaced more posteriorly, the tethering would have increased to a large enough magnitude that the leaflet has reduced mobility in its entirety (evident from figure 4), resulting in incomplete coaptation and a regurgitation orifice (shown in supplemental data). The leaflet thus tries to move basally towards the annulus driven by the ventricular pressure, but the tethering forces overpower it and let it move only marginally such that it cannot meet with the posterior leaflet. Thus, an increase in the force is expected to reach a rapid peak, without a plateau, and subsequently a rapid fall during late systole is expected. These changes in the leaflet mobility in some of these settings were previous reported as the case of “leaflet loitering” by the Miller group17.
Table 1 depicts the percentage changes in the marginal chordal forces from baseline and the factor of safety as a comparison to the failure force of the chordae. The data indicates that as the distance of the papillary muscle tips from the mitral annulus increased, so did the force in the anterior marginal chordae tendineae before and after chordal cutting and there is a significant fall in the factor of safety. However, it should be noted that the measured forces are significantly smaller than the failure load of 6.8 N required for rupture of the anterior marginal chordae reported by Sedransk et al18. Though the forces measured in this study seem much smaller than the failure load, it is known that increased stretch in mitral valve tissue can accelerate remodeling and degeneration of the tissue and potentially lead to failure at much lower loading conditions than those reported in literature. Pedersen et al. in an elegant study demonstrated that under porcine mitral valve leaflets subjected to chronic elevated load up regulated the expression of Endothelin-B receptors, which are known to regulate key apoptotic pathways in degenerative mitral valve disease19. Such degeneration may not be evident at the time of surgery, but can gradually build up resulting in catastrophic failure of the repair at a later stage in the patient’s life. Additionally, we also observed that the increase in marginal chordal forces were consistently higher in the right chordae than the left in these porcine valves, and measurements of chordal and papillary muscle lengths in pig hearts demonstrated that the right chordae were consistently shorter than the left (unpublished results). In the intact pig heart, the right papillary muscle is the postero-medial papillary muscle that is located on the inferior wall of the ventricle and closer to the mitral annular plane, than the left papillary muscle which is located on the antero-lateral position and slightly away from the mitral annular plane. Whether such a distinction is prevalent in human mitral valves is at best speculative, as there is no such information yet available in the literature. However, since we used the baseline measurements for each valve as its own control, this anatomical finding does not impact the overall end-point of this study.
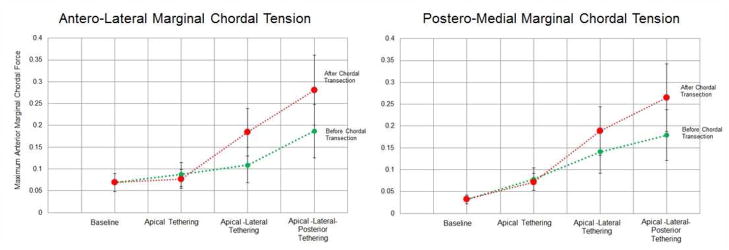
This ex vivo study for the first time allows quantitative assessment of chordal cutting on changes in the force distribution in the chordae tendineae. While these data suggest that under apical papillary muscle displacement, the changes in chordal forces are minimal, it is well known clinically that the ischemic dilated ventricle often remodels after mitral valve repair20. Thus if chordal cutting is adopted in patients with minimal tethering, and with apical PM displacement (such as due to non-ischemic dilated cardiomyopathy causing concentric hypertrophy); it should be first determined whether the ventricle may potentially remodel adversely after the repair. The authors do recognize that such predictive estimates of ventricular remodeling are challenging currently, and at best anecdotal, we are hopeful that current research efforts will lead us in that direction. In figure 7, we present the chordal forces on each valve with the papillary muscle positions as the main effectors, and demonstrate that the gradient of increase in marginal chordal force with evolving papillary muscle displacement was much higher after chordal cutting than before. It is important that these data should be considered within the ex vivo realm, as clinically it is impossible to predict if and how much the ventricle would remodel after the surgery. Potentially, echocardiographic measurement of the papillary muscle tip to mitral annular plane distance may be used as an indicator for selection of patients for chordal cutting procedure; or translocation of the transected secondary chordae to another position on the valve or implantation of neo-chordae for distributing the load should be considered. Further studies to relate the changes in marginal chordal forces in relation to different mitral valve geometric measurements are currently underway in large animal models of ischemic heart disease.
Figure 7.
Changes in the anterior marginal chordal force before and after chordal cutting with progressive change in the papillary muscle positions. As depicted in both figures, the slope of increase in the marginal chordal force after chordal cutting is significantly higher than before chordal cutting, in the situation that progressive displacement of the papillary muscles after chordal cutting is performed
6. LIMITATIONS
The experimental model used in this study, even though thoroughly validated against large animal models, has some inherent limitations. The lack of atrial and ventricular dynamics may impact the severity of mitral regurgitation, which we recognize, but for the scope of this study does not overshadow the model benefits. Mitral annular dynamics are not mimicked, but it was already shown in several animal models that the contractile motion of the annulus is eliminated after annuloplasty. Abnormalities in segmental ventricular wall motion or dyskinesis cannot be simulated in this model to mimic events involved in adverse remodeling of the ventricle, but their impact can be easily mimicked by altering the papillary muscle positions.
7. CONCLUSIONS
This first quantitative study on the impact of chordal cutting on valve mechanics demonstrates that significant increases in anterior marginal chordal forces can be expected after strut chordal transection, though in a papillary muscle position dependent manner. Quantification of the mitral valve annulus to papillary muscle tip distance may be useful in proper selection of patients for this procedure in the clinical setting.
Supplementary Material
Acknowledgments
Holifield farms for donating porcine hearts. M.P. was supported by an American Heart Association Fellowship (#0815159E) during the course of this study at Georgia Institute of Technology, L.G. was supported by a Georgia Tech Presidential Undergraduate Fellowship, and A.Y. and the research work were partially supported by NHLBI grant R01-HL090661.
ABBREVIATIONS
- MR
Mitral Regurgitation
- ASC
Anterior Strut Chordae
- AMC
Anterior Marginal Chordae
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.He S, Fontaine AA, Schwammenthal E, Yoganathan AP, Levine RA. Integrated mechanism for functional mitral regurgitation: leaflet restriction versus coapting force: in vitro studies. Circulation. 1997;96:1826–1834. doi: 10.1161/01.cir.96.6.1826. [DOI] [PubMed] [Google Scholar]
- 2.Komeda M, et al. Geometric determinants of ischemic mitral regurgitation. Circulation. 1997;96:II-128–133. [PubMed] [Google Scholar]
- 3.Anyanwu AC, Adams DH. Ischemic mitral regurgitation: recent advances. Curr Treat Options Cardiovasc Med. 2008;10:529–537. doi: 10.1007/s11936-008-0045-6. [DOI] [PubMed] [Google Scholar]
- 4.Calafiore AM, et al. Mitral valve repair for ischemic mitral regurgitation. Angiology. 2008;59:89S–92S. doi: 10.1177/0003319708321073. [DOI] [PubMed] [Google Scholar]
- 5.Nesta F, et al. Leaflet concavity: a rapid visual clue to the presence and mechanism of functional mitral regurgitation. J Am Soc Echocardiogr. 2003;16:1301–1308. doi: 10.1067/j.echo.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Sai-Sudhakar CB, Vandse R, Armen TA, Bickle KM, Nathan NS. Efficacy of chordal cutting in alleviating ischemic mitral regurgitation: insights from 3-dimensional echocardiography. J Cardiothorac Surg. 2007;2:39. doi: 10.1186/1749-8090-2-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Messas E, et al. Efficacy of chordal cutting to relieve chronic persistent ischemic mitral regurgitation. Circulation. 2003;108(Suppl 1):II111–115. doi: 10.1161/01.cir.0000087658.47544.7f. [DOI] [PubMed] [Google Scholar]
- 8.Messas E, et al. Chordal cutting: a new therapeutic approach for ischemic mitral regurgitation. Circulation. 2001;104:1958–1963. doi: 10.1161/hc4201.097135. [DOI] [PubMed] [Google Scholar]
- 9.Messas E, et al. Relief of mitral leaflet tethering following chronic myocardial infarction by chordal cutting diminishes left ventricular remodeling. Circ Cardiovasc Imaging. 2010;3:679–686. doi: 10.1161/CIRCIMAGING.109.931840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jimenez JH, Soerensen DD, He Z, Ritchie J, Yoganathan AP. Effects of papillary muscle position on chordal force distribution: an in-vitro study. The Journal of heart valve disease. 2005;14:295–302. [PubMed] [Google Scholar]
- 11.Ritchie J, Warnock JN, Yoganathan AP. Structural characterization of the chordae tendineae in native porcine mitral valves. Ann Thorac Surg. 2005;80:189–197. doi: 10.1016/j.athoracsur.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen SL, et al. Chordal force distribution determines systolic mitral leaflet configuration and severity of functional mitral regurgitation. J Am Coll Cardiol. 1999;33:843–853. doi: 10.1016/s0735-1097(98)00627-5. [DOI] [PubMed] [Google Scholar]
- 13.Sacks MS, et al. In-vivo dynamic deformation of the mitral valve anterior leaflet. Ann Thorac Surg. 2006;82:1369–1377. doi: 10.1016/j.athoracsur.2006.03.117. [DOI] [PubMed] [Google Scholar]
- 14.Salisbury PF, Cross CE, Rieben PA. Chorda Tendinea Tension. Am J Physiol. 1963;205:385–392. doi: 10.1152/ajplegacy.1963.205.2.385. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen SL, et al. Imbalanced chordal force distribution causes acute ischemic mitral regurgitation: mechanistic insights from chordae tendineae force measurements in pigs. J Thorac Cardiovasc Surg. 2005;129:525–531. doi: 10.1016/j.jtcvs.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen SL, et al. Mechanism of incomplete mitral leaflet coaptation--interaction of chordal restraint and changes in mitral leaflet coaptation geometry. Insight from in vitro validation of the premise of force equilibrium. J Biomech Eng. 2002;124:596–608. doi: 10.1115/1.1500741. [DOI] [PubMed] [Google Scholar]
- 17.Glasson JR, et al. Early systolic mitral leaflet “loitering” during acute ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 1998;116:193–205. doi: 10.1016/s0022-5223(98)70117-5. [DOI] [PubMed] [Google Scholar]
- 18.Sedransk KL, Grande-Allen KJ, Vesely I. Failure mechanics of mitral valve chordae tendineae. The Journal of heart valve disease. 2002;11:644–650. [PubMed] [Google Scholar]
- 19.Pedersen LG, et al. Increased expression of endothelin B receptor in static stretch exposed porcine mitral valve leaflets. Res Vet Sci. 2007;82:232–238. doi: 10.1016/j.rvsc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Pfeffer JM, Pfeffer MA, Fletcher PJ, Braunwald E. Progressive ventricular remodeling in rat with myocardial infarction. Am J Physiol. 1991;260:H1406–1414. doi: 10.1152/ajpheart.1991.260.5.H1406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.