Abstract
The Anaphase-Promoting Complex/Cyclosome (APC) is a ubiquitin ligase required for exit from mitosis. We previously showed that Tosyl Arginine Methyl Ester (TAME) inhibits APC-dependent proteolysis by competing with the C-terminal IR-tail of the APC activator Cdc20 for APC binding. Here we show that in the absence of APC substrates, TAME ejects Cdc20 from the APC by promoting Cdc20 auto-ubiquitination in its N-terminal region. Cyclin B1 antagonizes TAME's effect by promoting binding of free Cdc20 to the APC and suppressing Cdc20 auto-ubiquitination. Nevertheless, TAME stabilizes cyclin B1 in Xenopus extract by two mechanisms. First, it reduces the kcat of the APCCdc20/cyclin B1 complex without affecting the Km, slowing the initial ubiquitination of unmodified cyclin B1. Second, as cyclin B1 becomes ubiquitinated, it loses its ability to promote Cdc20 binding to the APC in the presence of TAME. As a result, cyclin B1 ubiquitination terminates before reaching the threshold necessary for proteolysis.
Introduction
The Anaphase-Promoting Complex/Cyclosome (APC/C or APC) is a multi-subunit ubiquitin ligase that initiates anaphase and mitotic exit by ubiquitinating substrates including cyclin B1 and securin to target them for degradation by the 26S proteasome1. We identified Tosyl-L-Arginine Methyl Ester (TAME) as a small molecule that stabilizes APC substrates in mitotic Xenopus extract2. In this system, TAME binds to the APC and prevents loading of Cdc203, an essential activator of mitotic APC4. As expected, a prodrug of TAME arrests human cells in metaphase3. However, in this context, proTAME does not induce significant dissociation of Cdc20 from the APC. Furthermore, proTAME-induced mitotic arrest is highly dependent on the Spindle Assembly Checkpoint (SAC)3, a pathway that inhibits APC activation in response to improperly attached kinetochores. Together, these findings suggest that TAME might inhibit APC function through mechanisms that are more complex than simple blockade of Cdc20 loading.
The APC activators Cdc20 or Cdh1 bind reversibly to the APC in a regulated manner, and are themselves subject to regulation by APC-dependent ubiquitination and proteolysis4. The activators help recruit substrates to the APC and may also directly stimulate catalytic activity of the ligase5,6. Three distinct interactions help recruit the activators to the APC. The N-terminal domain of the activator contains the conserved C-box, which is thought to interact with the Apc2 subunit7,8. The C-terminus of the activator contains a conserved isoleucine-arginine motif (IR-tail) that interacts with Cdc27 and perhaps additional TPR-containing subunits of the APC9,10. Finally, the activators contain a WD40-repeat domain that folds into a β-propeller structure and interacts with APC substrates11-14. Electron microscopy of the APC revealed that a core APC subunit (Apc10/Doc1) and the WD40 domain of the activator form a co-receptor for the destruction box, a degron present in APC substrates7,15. The ability of APC substrates to form a bridging interaction between the APC and the activator may explain why substrates can promote activator protein binding to the ligase16-18.
TAME structurally mimics the IR-tail of Cdc20/Cdh1 and competes with the IR-tail for the same binding site on the APC3. This effect is sufficient to prevent Cdc20 from binding to the APC in mitotically-arrested Xenopus extracts, which lack APC substrates, but is not sufficient to block Cdc20 binding in somatic cells, which contain abundant APC substrates as well as an intact SAC signaling pathway3. In this context, it is not clear how TAME inhibits the APC without inducing Cdc20 dissociation. Another mystery of the role of the IR-tail is that budding yeast expressing Cdc20ΔIR in place of endogenous Cdc20 show no abnormality in proliferation8, although Cdc20 is an essential gene. The exact nature of the defect in APC activity as a consequence of the loss of the IR-tail interaction, and how cells might compensate for such a defect, remain poorly understood.
Here we describe the precise mechanism by which TAME inhibits APC activation. Surprisingly, TAME actively promotes Cdc20 dissociation from the APC in Xenopus extracts by inducing Cdc20 auto-ubiquitination, which is suppressed by binding of APC substrates such as cyclin B1. However, in this context, TAME induces a catalytic defect in the APCCdc20/cyclin B1 complex that slows the initial ubiquitination of unmodified cyclin B1. More importantly, TAME causes substrate ubiquitination to terminate prematurely, such that substrates do not become ubiquitinated sufficiently to be recognized by the proteasome.
Results
TAME-induced Cdc20 dissociation requires APC activity
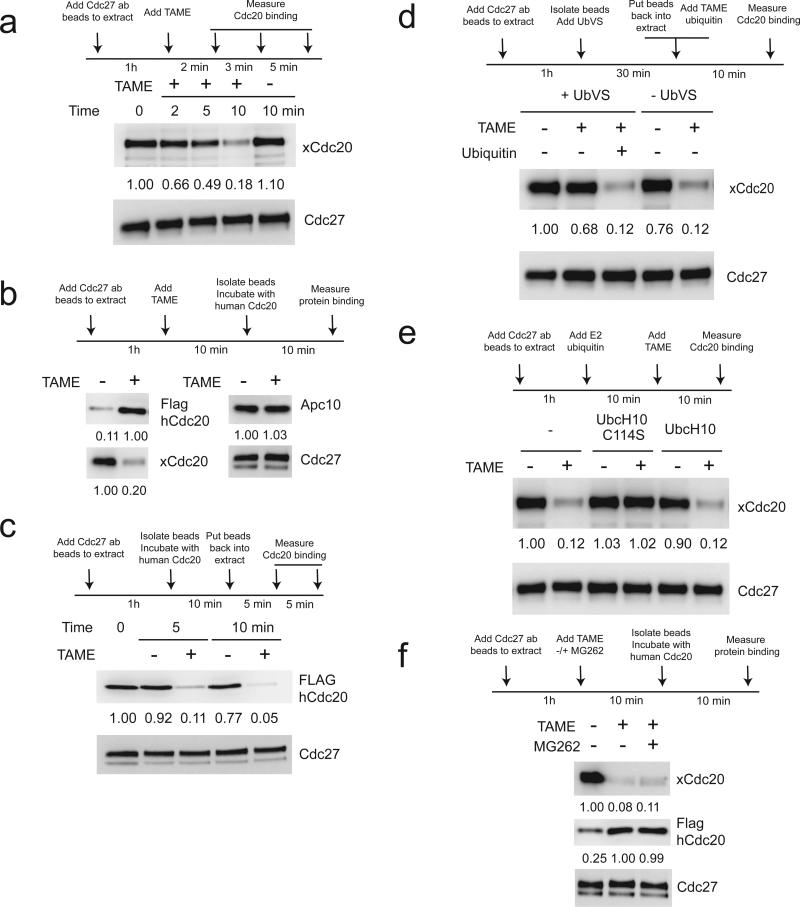
We previously showed that TAME inhibits the loading of free Cdc20 onto purified APC3. Surprisingly, we found that TAME also caused rapid disappearance of pre-bound Cdc20 from the APC when added to mitotic Xenopus extract (Fig. 1a). The loss of Cdc20 signal likely reflects dissociation of Cdc20 from the APC, because the APC from TAME-treated extract could be loaded with a significantly larger amount of in vitro-translated Cdc20 (Fig. 1b). Binding of Apc10, which also harbors an IR-tail, was not affected by TAME (Fig. 1b). To measure the effect of TAME on the dissociation rate of Cdc20, we took advantage of the fact that Xenopus extract contains a free pool of Cdc20 that is sufficient to outcompete the binding of exogenous Flag-tagged human Cdc20 (Supplementary Results, Supplementary Fig. 1a). We preloaded APC with Flag-Cdc20 and added the complex to Xenopus extract, and found that TAME strongly accelerated the rate of Cdc20 dissociation (Fig. 1c). Interestingly, TAME did not have this effect in interphase extract or when the mitotic APCCdc20 was resuspended in buffer (Supplementary Fig. 1b,c). Furthermore, this effect required free ubiquitin, as inhibiting ubiquitin recycling with ubiquitin vinyl sulfone (UbVS)19, an irreversible inhibitor of deubiquitinating enzymes, blocked the ability of TAME to induce Cdc20 dissociation (Fig. 1d). Addition of ubiquitin restored TAME-induced Cdc20 dissociation (Fig. 1d). A dominant negative mutant of UbcH10 (C114S)20, but not WT UbcH10, also suppressed TAME's ability to induce Cdc20 dissociation (Fig. 1e). Cdc20 dissociation did not require proteasome-dependent degradation, as the proteasome inhibitor MG262 altered neither TAME-induced disappearance of endogenous Cdc20 nor the amount of Flag-Cdc20 that could be reloaded onto the APC (Fig. 1f). Together these findings suggest that TAME-induced Cdc20 dissociation requires APC-dependent ubiquitination but not proteolysis.
Figure 1. TAME-induced Cdc20 dissociation from the APC in mitotic Xenopus extract requires APC-dependent ubiquitination.
(a) TAME induces rapid loss of Cdc20 from the APC in mitotic Xenopus extract. Immunoprecipitated APC on Cdc27 antibody beads (APC beads) was incubated in mitotic extract treated with TAME (200 μM). Numbers represent the relative band intensity of Cdc20 normalized to Cdc27. (b) TAME induces dissociation of Cdc20 but not Apc10 from the APC. APC beads from mitotic extract +/- TAME were incubated with in vitro-translated Flag-tagged human Cdc20. (c) TAME actively promotes dissociation of Cdc20 from the APC. APC beads were washed with high salt XB buffer to remove endogenous Cdc20 and re-loaded with in vitro-translated Flag-tagged human Cdc20. The beads were then resuspended in mitotic extract +/-TAME for the indicated period of time. (d) TAME-induced Cdc20 dissociation is ubiquitin-dependent. APC beads were incubated in mitotic extract +/- 20 μM ubiquitin vinyl sulfone (UbVS) and other components (200 μM TAME or 20 μM ubiquitin) as indicated for 10 min. (e) TAME-induced Cdc20 dissociation is suppressed by dominant negative UbcH10 (C114S mutant). APC beads were incubated in mitotic extract treated with UbcH10 or the C114S mutant (5 μM), ubiquitin (20 μM) and TAME (200 μM) for 10 min. (f) TAME-induced Cdc20 dissociation does not require proteasomal degradation. APC beads loaded with in vitro-translated Flag-tagged human Cdc20 were incubated in mitotic extract treated with TAME or MG262 (both at 200 μM).
TAME induces Cdc20 dissociation via Cdc20 ubiquitination
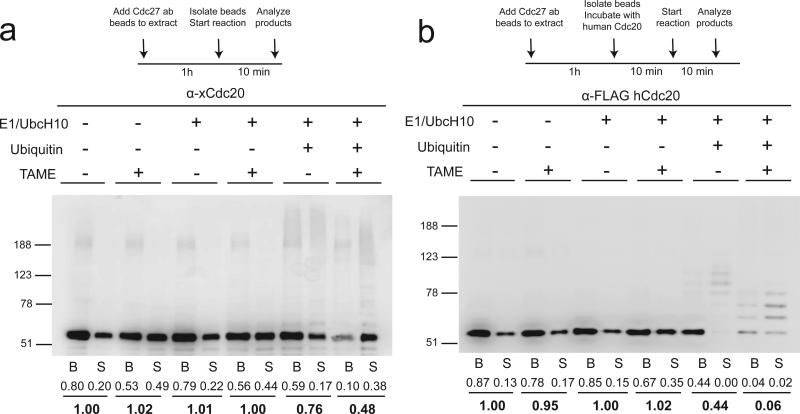
Since Cdc20 is known to undergo auto-ubiquitination21,22, we tested whether TAME can induce Cdc20 dissociation by affecting Cdc20 ubiquitination in a reconstituted system. In the absence of ubiquitination components, the majority of Cdc20 remained bound to the APC in the presence of TAME (Fig. 2a), consistent with the slow dissociation rate observed in buffer (Supplementary Fig. 1c). However, in the presence of ubiquitination components, only 10% of unmodified Cdc20 remained bound to the APC in the presence of TAME, with the ubiquitinated form existing predominantly in the supernatant (Fig. 2a and Supplementary Fig. 2a for darker exposure). Dominant negative UbcH10 did not support Cdc20 dissociation (Supplementary Fig. 2b), confirming that ubiquitin transfer is critical. Similar results were obtained with APC loaded with in vitro-translated human Flag-Cdc20, although the mass of ubiquitin conjugates assembled on human Cdc20 was lower than for the endogenous Xenopus protein (Fig. 2b and Supplementary Fig. 2c for darker exposure). Because ubiquitinated Cdc20 was not detected efficiently by the Flag antibody, we deubiquitinated the material in the supernatant using the catalytic domain of Usp2, and confirmed that TAME treatment doubles the amount of ubiquitinated Cdc20 present in the supernatant (Supplementary Fig. 2d). Adding E1 and UbcH10 but no ubiquitin modestly enhanced TAME's ability to induce Cdc20 dissociation compared to adding TAME alone (Fig. 2b), for reasons that are unclear, although this effect is due to E1 but not UbcH10 (Supplementary Fig. 2e). A lower dose (20 μM) of TAME produced similar though more modest effects (Supplementary Fig. 2f). Consistent with these findings, a nonubiquitinatable mutant of Cdc20, in which all lysine residues were changed to arginine (Cdc20K-less)23, was resistant to TAME-induced ubiquitination and dissociation in the reconstituted system (Supplementary Fig. 2g) and in Xenopus extract (Supplementary Fig. 2h). However, these results were complicated by the fact that Cdc20K-less binds the APC more tightly than WT Cdc20 (compare untreated lanes in Figs. 2a, b to Supplementary Fig. 2g). Together these findings indicate that TAME-induced Cdc20 dissociation can be reconstituted in the presence of an active ubiquitin conjugation pathway.
Figure 2. TAME induces Cdc20 dissociation from the APC by promoting Cdc20 ubiquitination.
(a) TAME-induced Cdc20 dissociation can be reconstituted in vitro with immunopurified mitotic APCCdc20, E1 (250 nM), UbcH10 (2 μM) and ubiquitin (150 μM). Immunoprecipitated APCCdc20 was incubated with various components as shown for 10 min. Cdc20 was analyzed by Western blot separately in the bead-bound (B) and the supernatant (S) fractions. Numbers represent the relative band intensity of the unmodified Cdc20. (b) TAME-induced Cdc20 dissociation can be reconstituted in vitro with immunopurified mitotic APC and in vitro-translated human Cdc20. The same assay was performed as in a, except that the APC was first washed with high salt buffer to remove Cdc20 and then re-loaded with in vitro-translated human Cdc20.
We next tested whether TAME can induce ubiquitination and dissociation of Cdh1, the interphase APC activator. Our previous study showed that TAME inhibits Cdh1 binding to the APC less efficiently than it inhibits Cdc20 binding3. We loaded interphase APC with in vitro-translated Cdh1 and tested its behavior in the reconstituted ubiquitination system. In the absence of TAME, Cdc27 instead of Cdh1 became the target of auto-ubiquitination (Supplementary Fig. 2i). Addition of TAME promoted much less ubiquitination and dissociation of Cdh1 compared to Cdc20 (Supplementary Fig. 2i). As expected, TAME did not induce rapid dissociation of pre-bound Cdh1 in the extract (Supplementary Fig. 2j). We therefore conclude that TAME induces ubiquitination-dependent dissociation of Cdc20 but not of Cdh1.
Sites of ubiquitination Cdc20 causing dissociation
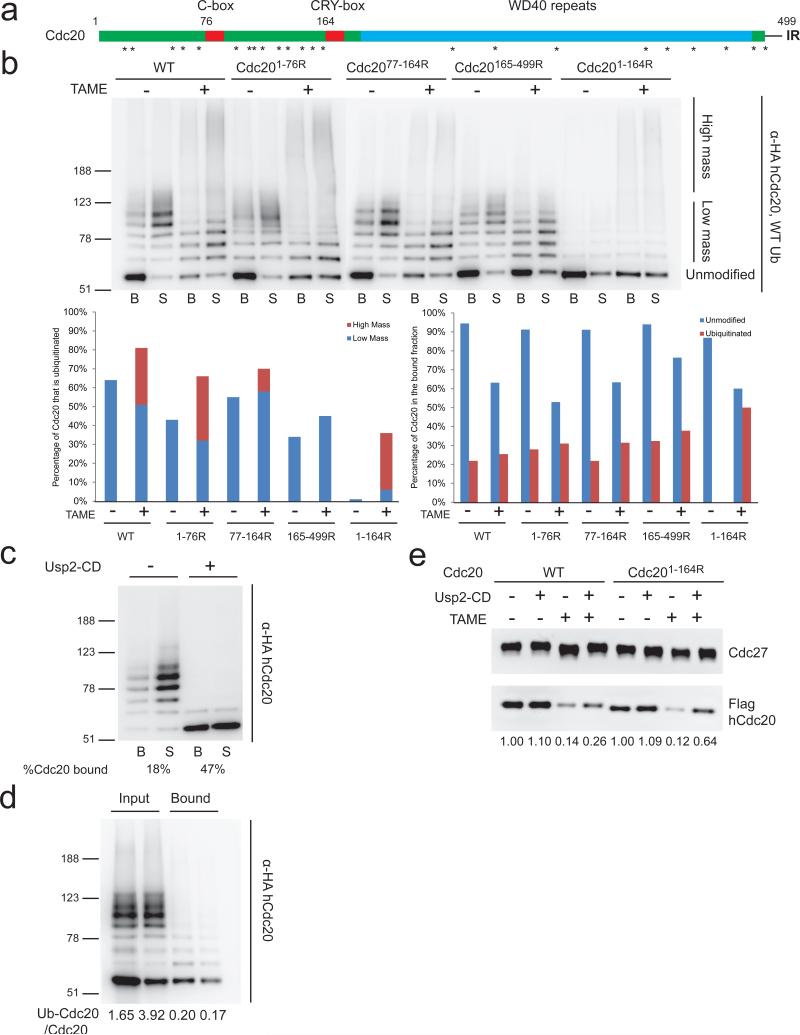
To identify lysine residues that are critical for Cdc20 ubiquitination and dissociation, we generated a series of mutants in which lysines in different regions of Cdc20 were mutated to arginine (Fig. 3a). We chose the C-box (DRYIP; residues 77-81) and the CRY-box (CRYIP; residues 165-169) as landmarks for the mutants and used a human Cdc20 construct with an N-terminal double-HA tag, which allowed better detection of the ubiquitinated species. In the absence of TAME, we observed comparable extents of ubiquitination of WT, Cdc201-76R, Cdc2077-164R and Cdc20165-499R, whereas ubiquitination of Cdc201-164R was severely compromised (Fig. 3b and Supplementary Fig. 3a), suggesting that ubiquitination of Cdc20 occurs predominantly upstream of the CRY-box. Addition of TAME increased the fraction of Cdc20 that became ubiquitinated for all forms of Cdc20 (Fig. 3b), suggesting that TAME enhances the susceptibility of Cdc20 to auto-ubiquitination, both in the N-terminal (residues 1-164) and the C-terminal regions (165-499). However, TAME had distinct effects on the conjugation of ubiquitin to these two regions of Cdc20. TAME reduced the mass of the ubiquitin conjugates in the N-terminal region, as evident in its effects on the Cdc20165-499R mutant, whereas it enhanced formation of high mass conjugates in the C-terminal region, as evident in its effects on the Cdc201-164R mutant (Fig. 3b). For the other forms of Cdc20, TAME also enhanced the formation of high mass ubiquitin conjugates while reducing formation of low mass ubiquitin conjugates (Fig. 3b). Experiments with methylated ubiquitin, which supports only multiple-monoubiquitination, revealed that many lysines in the N-terminal domain become ubiquitinated and that TAME reduces the number of lysines that are ubiquitinated in this region, as revealed by analysis of the Cdc20165-499R mutant. In contrast, analysis of the Cdc201-164R mutant revealed that little methylated ubiquitin was conjugated to the C-terminal domain unless TAME was present and that methylated ubiquitin suppressed formation of high molecular weight conjugates, confirming that ubiquitin chains form principally on lysines in the C-terminal domain (Supplementary Fig. 3b). Together these results suggest that whereas TAME increases the fraction of Cdc20 susceptible to ubiquitination, it alters the distribution of ubiquitin, reducing the number of ubiquitin molecules that can be added to the N-terminal region (residues 1-164) while promoting ubiquitination in the C-terminal region (residues 165-499).
Figure 3. Ubiquitination upstream of the CRY-box of Cdc20 reduces its binding affinity for the APC.
(a) Schematic illustration of human Cdc20. Asterisks indicate lysine residues. (b) Ubiquitination/dissociation of Cdc20 requires lysines in the N-terminal region. In vitro ubiquitination assays were performed with APC re-loaded with double HA-tagged human WT or Cdc20 mutants as described in Fig. 2b. (c) Deubiquitination of ubiquitinated Cdc20 increases its affinity for the APC. Supernatant from an in vitro Cdc20 ubiquitination assay containing ubiquitinated Cdc20 was divided into two aliquots and one aliquot was incubated with the catalytic domain of Usp2 (Usp2-CD). Both aliquots were then re-incubated with a fresh batch of high salt-washed APC to assess the binding of Cdc20. (d) Unmodified Cdc20 outcompetes ubiquitinated Cdc20 for binding to the APC. Ubiquitinated Cdc20 (Ub-Cdc20) was generated as in (c) and mixed with reticulocyte lysate containing unmodified Cdc20 at different ratios. The mixture was then incubated with high salt-washed APC and Cdc20 binding was assessed by Western blot. (e) Cdc201-164R is more resistant to TAME-induced dissociation than WT Cdc20. APC beads loaded with WT or Cdc201-164R were incubated in mitotic extract +/- TAME for 10 min. A fraction of the beads were then treated with Usp2-CD.
We next analyzed how ubiquitination of Cdc20 correlated with its binding to the APC. Whereas unmodified Cdc20 existed predominantly in the bound fraction in the absence of TAME, a fraction dissociated into the supernatant upon the addition of TAME (Fig. 3b). In contrast, ubiquitinated Cdc20 preferentially dissociated into the supernatant regardless of the presence of TAME, with the only exception being the high mass conjugates observed with Cdc201-164R (Fig. 3b). These results suggest that ubiquitination upstream of the CRY-box may promote Cdc20 dissociation, whereas ubiquitination downstream of the CRY box may have little effect on Cdc20 binding to the APC. To test directly whether ubiquitination of Cdc20 affects its affinity for the APC, we collected supernatants from Cdc20 ubiquitination reactions performed without TAME. We deubiquitinated one portion of the products, and then incubated the proteins with the APC to measure rebinding of Cdc20. Whereas ubiquitinated Cdc20 existed predominantly in the supernatant, 47% of the deubiquitinated Cdc20 was able to bind to the APC (Fig. 3c). To directly compare the binding properties of ubiquitinated Cdc20 and unmodified Cdc20, we mixed different amounts of ubiquitinated Cdc20 and unmodified Cdc20 with the APC. We found that unmodified Cdc20 bound to the APC much more readily than ubiquitinated Cdc20 (Fig. 3d and Supplementary Fig. 3c), further supporting the idea that ubiquitination of Cdc20 causes its dissociation from the APC.
We next tested whether ubiquitination of Cdc20 in its N-terminal region is specifically required for TAME-induced dissociation of Cdc20 in Xenopus extract. We incubated APC loaded with WT Cdc20 or Cdc201-164R in TAME-treated extract, isolated the beads, and treated them with deubiquitinating enzyme to accurately quantitate the amount of bound Cdc20. We found that a significantly larger fraction of Cdc201-164R remained associated with the APC compared to WT Cdc20 (Fig. 3e). Importantly, as opposed to Cdc20K-less, which binds the APC unusually tightly, Cdc201-164R and WT Cdc20 showed comparable binding affinities for the APC (Supplementary Fig. 3d). Taken together, we conclude that ubiquitination upstream of the CRY-box promotes TAME-induced Cdc20 dissociation.
Cyclin B1 suppresses TAME-induced Cdc20 dissociation
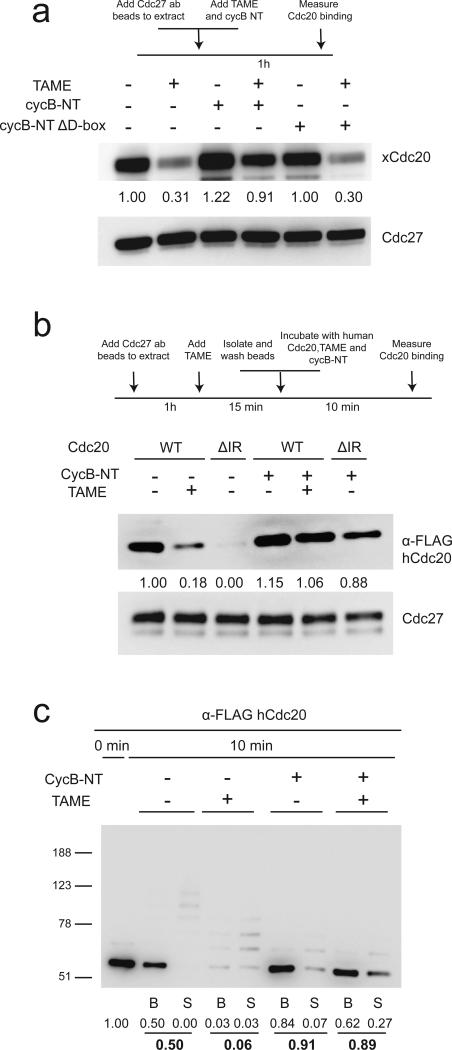
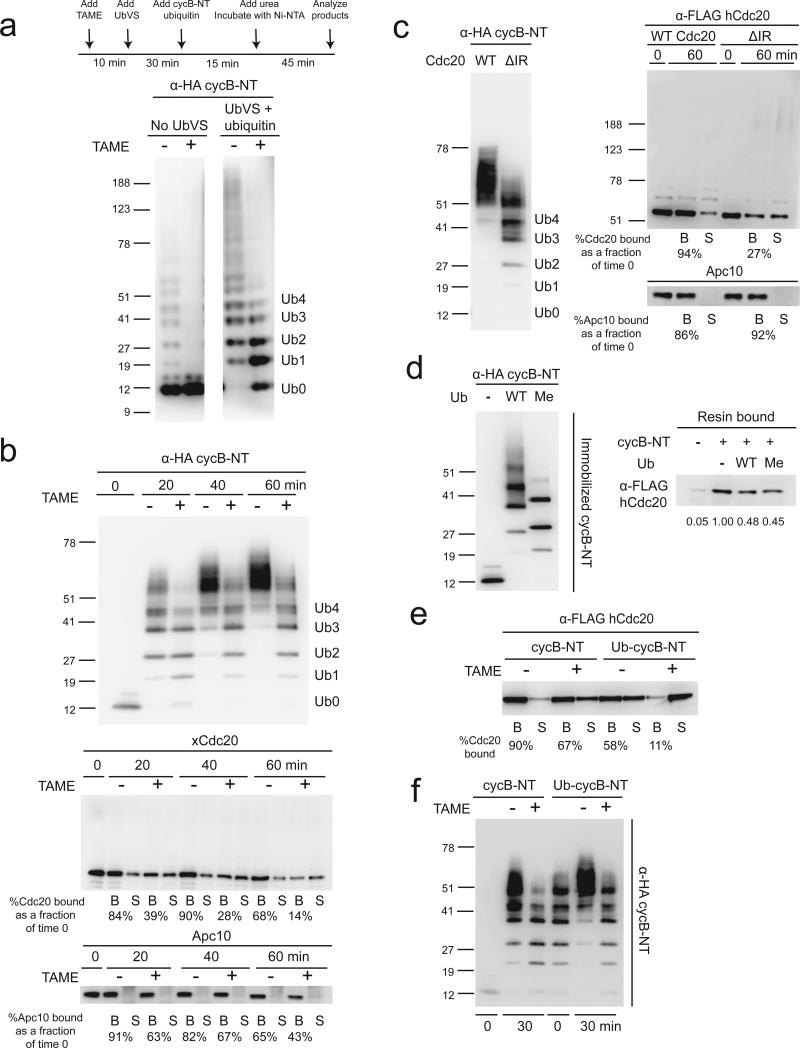
Although TAME efficiently reduces Cdc20 binding to the APC in mitotically-arrested Xenopus extracts, which lack endogenous APC substrates, the effect is lost in human cells, which contain abundant APC substrates3. Because APC substrates enhance the binding of Cdh1 to the APC16-18, we examined whether substrates can suppress TAME-induced Cdc20 dissociation. We found that adding a cyclin B1 N-terminal fragment (cycB-NT) to mitotic Xenopus extract enhanced binding of Cdc20 to the APC in a destruction-box dependent manner, both in the absence and presence of TAME (Fig. 4a). In a reconstituted system with purified APC and in vitro-translated Cdc20, we found that TAME addition or IR-tail deletion strongly prevented the binding of free Cdc20 to the APC, but that this effect could be suppressed by co-adding cycB-NT (Fig. 4b). These effects did not rely on Cdc20 ubiquitination, as the reticulocyte lysate system lacks the components required to support Cdc20 ubiquitination (Supplementary Fig. 4a). CycB-NT also promoted the binding of free Cdh1 to the APC in the presence of TAME (Supplementary Fig. 4b). When supplemented with ubiquitination components, we found that CycB-NT strongly suppressed Cdc20 ubiquitination both in the presence and absence of TAME (Fig. 4c). A similar result was obtained with APCCdc20 isolated from Xenopus extract (Supplementary Fig. 4c). Interestingly, the presence of cycB-NT also suppressed Cdh1-dependent ubiquitination of Cdc27 (Supplementary Fig. 4b). Together these results show that APC substrates can promote activator binding to the APC when the IR-tail interaction is perturbed, and furthermore indicate that APC substrates have priority over Cdc20 to accept ubiquitin.
Figure 4. CycB-NT promotes Cdc20 binding to the APC and suppresses Cdc20 ubiquitination.
(a) Cyclin B-NT increases the steady-state level of Cdc20 bound to the APC in non-treated or TAME-treated mitotic Xenopus extract. A cyclin B1 N-terminal fragment (CycB-NT, 6 μM) or the same fragment without the D-box (CycB-NT ΔD-box, 6 μM) and TAME (200 μM) were added to mitotic extract as indicated. APC was immunoprecipitated from the extract and protein levels were analyzed by Western blot. (b) CycB-NT promotes Cdc20 binding to the APC in the absence of the IR-tail. APC cleared of Cdc20 by TAME-treatment was incubated with in vitro-translated WT Cdc20 or Cdc20ΔIR for 10 minutes. CycB-NT (2 μM) and TAME (200 μM) were added as indicated. (c) CycB-NT suppresses Cdc20 ubiquitination. The same assay was performed as in Fig. 2b in the presence or absence of 500 nM cycB-NT.
TAME reduces the kcat without altering the Km of APCCdc20
These results raise a paradox: how does TAME stabilize APC substrates in mitotic extract if substrates suppress TAME-induced Cdc20 dissociation? To resolve this paradox, we first tested whether TAME affects the initial stage of substrate ubiquitination by measuring the initial velocity of cycB-NT mono-ubiquitination in the presence of methylated ubiquitin (Supplementary Fig. 5a,b and Table 1). We found that TAME reduced the kcat of APCCdc20 by ~63% but had little effect on the Km. Reducing the concentration of TAME to 20 μM inhibited kcat by 53%, consistent with the binding affinity of TAME for the APC3 (Supplementary Fig. 5c). Repeating the experiment with WT ubiquitin yielded similar results (Supplementary Fig. 5d), consistent with a previous report showing that the initial stage of ubiquitination catalyzed by APC and UbcH10 is multiple monoubiquitination24. In these experiments, we pre-incubated the APC with TAME before adding substrate and reaction components, which caused some dissociation of Cdc20 (Supplementary Fig. 5e). To avoid this complication, we pre-equilibrated APCCdc20 with TAME and cycB-NT before adding other ubiquitination components, thereby preserving Cdc20 binding to the APC (Supplementary Fig. 5e). Nevertheless, we still observed a ~55% reduction in kcat (Supplementary Fig. 5f), suggesting that TAME slows kcat even when the binding of Cdc20 to the APC is not reduced. An IR peptide consisting of the C-terminal 20 residues of Xenopus Cdc20 also reduced kcat without a significant effect on Km (Supplementary Fig. 5g, h). These results suggest that TAME reduces the rate of transfer of the first several molecules of ubiquitin without affecting the affinity of the Cdc20/Apc10 co-receptor for the substrate.
Table 1.
Summary of kinetics data
| Experiment 1 | Experiment 2 | Experiment 3 | Average ± SEM | |
|---|---|---|---|---|
| Km –TAME, nM | 228 | 215 | 241 | 228 ± 8 |
| Km +TAME, nM | 226 | 210 | 289 | 242 ± 24 |
| kcat –TAME/kcat +TAME | 31.5% | 42.0% | 36.6% | 36.7 ± 3.0% |
TAME prematurely terminates cyclin B1 ubiquitination
The ability of TAME to reduce the kcat of APC-dependent ubiquitination is unlikely to fully explain the complete stabilization of cycB-NT observed in Xenopus extract, as a 50% reduction in kcat would be expected to slow degradation proportionately. Surprisingly, TAME fully inhibited the ubiquitination of cycB-NT in Xenopus extract, as only the unmodified form of the substrate could be observed (Fig. 5a). To test whether deubiquitination of the substrate is important for this effect, we repeated the experiment in extract treated with UbVS and added ubiquitin. Under this condition, accumulation of oligo-ubiquitinated cycB-NT was observed in TAME-treated extract (Fig. 5a). However, a cycB-NT-luciferase reporter was stable under this condition (Supplementary Fig. 6a), suggesting that this degree of ubiquitination is not sufficient to promote degradation by the proteasome.
Figure 5. TAME causes premature termination of cycB-NT ubiquitination.
(a) TAME reduces cycBNT ubiquitination level in mitotic extract. CycB-NT was isolated from mitotic extract treated with ubiquitin vinyl sulfone (20 μM) and ubiquitin (20 μM) (+UbVS) or buffer (-UbVS) +/- TAME (200 μM) as indicated and detected by anti-HA blot. (b) TAME reduces cycB-NT ubiquitination level in a reconstituted ubiquitination assay, which is accompanied by loss of Cdc20 from the APC. A reconstituted ubiquitination assay was performed with immunopurified APCCdc20 and 500 nM cycB-NT +/- TAME. Quantification of Cdc20 and Apc10 levels bound to the beads is shown. (c) TAME's effect on cycB-NT ubiquitination can be recapitulated with Cdc20ΔIR. The same assay as in b was performed with APC reloaded with WT Cdc20 or Cdc20ΔIR. (d) Ubiquitinated cycB-NT has reduced binding affinity for Cdc20. Unmodified or ubiquitinated cycB-NT was immobilized on an HA-antibody resin and then incubated with in vitro-translated Flag-Cdc20. Resin bound cycB-NT and Cdc20 were analyzed by anti-HA and anti-Flag Western blot, respectively. (e) Ubiquitinated cycB-NT cannot promote Cdc20 binding to the APC in the presence of TAME. Unmodified or ubiquitinated cycB-NT was mixed with in vitro-translated Flag-Cdc20 and then incubated with APC +/- TAME. The binding of Cdc20 was assessed by anti-Flag Western blot. (f) TAME prevents further ubiquitination of partially ubiquitinated cycB-NT. Unmodified or ubiquitinated cycB-NT, along with ubiquitination reaction components, were mixed with in vitro-translated Flag-Cdc20 and then incubated with APC +/- TAME.
We next tested whether TAME's ability to reduce the mass of ubiquitin conjugates on cycB-NT could be reconstituted in a purified system. In the presence of TAME, cycB-NT ubiquitination was slowed (compare 20 min +/- TAME) and stopped prematurely (compare 40 to 60 min +/- TAME) (Fig. 5b). The cessation of cycB-NT ubiquitination was accompanied by a significant reduction in Cdc20 binding to the APC, and Apc10 to a lesser extent (Fig. 5b). This finding was surprising given that cycBNT should promote Cdc20 binding to the APC in the presence of TAME (Fig. 4c). When we repeated the experiment with dominant negative UbcH10, Cdc20 and APC10 binding to the APC were preserved, indicating that ubiquitin transfer somehow reduces the ability of cycB-NT to promote Cdc20 binding to the APC (Supplementary Fig. 6b). Importantly, APCCdc20ΔIR displayed the same defect in cycB-NT ubiquitination as APCCdc20 treated with TAME, without any perturbation of Apc10 binding (Fig. 5c). Binding of Cdc20ΔIR to the APC was maintained in the presence of dominant negative UbcH10 (Supplementary Fig. 6c). We observed similar TAME-induced defects in cycB-NT ubiquitination with APC loaded with Cdc201-164R or Cdc20K-less, indicating that ubiquitination of Cdc20 is unlikely to explain the defect in substrate ubiquitination (Supplementary Fig. 6d, e).
We hypothesized that ubiquitination of cycB-NT might reduce its ability to promote Cdc20 binding to the APC. If so, once Cdc20 dissociates from the APC during the assay, it would not be reloaded onto the APC in the presence of TAME, terminating the reaction. To test this model, we measured the binding of immobilized unmodified or ubiquitinated cycB-NT to Cdc20 and found that ubiquitinated cycB-NT has a reduced affinity for Cdc20 (Fig. 5d). This effect was also observed with conjugates containing methylated ubiquitin, suggesting that multiple mono-ubiquitination of cycB-NT is sufficient to reduce its binding affinity for Cdc20 (Fig. 5d). Ubiquitinated cycB-NT was also defective in its ability to promote Cdc20 binding to the APC (Fig. 5e). One prediction from these observations is that the APC should be unable to conjugate additional ubiquitin molecules to pre-ubiquitinated cycB-NT in the presence of TAME, due to the inability of pre-ubiquitinated cycB-NT to promote Cdc20 binding to the APC. This was indeed the case, as the APC was able to convert all the unmodified cycB-NT into ubiquitin conjugates in the presence of TAME, but it failed to further ubiquitinate pre-ubiquitinated cycBNT in the presence of TAME (Fig. 5f). In contrast, further ubiquitination of pre-ubiquitinated cycB-NT occurred in the absence of TAME (Fig. 5f). Taken together, we conclude that ubiquitinated cycB-NT cannot promote Cdc20 binding to the APC in the presence of TAME due to its reduced binding affinity for Cdc20, causing premature termination of ubiquitination.
UBE2S destabilizes cyclin B1 in TAME-treated extract
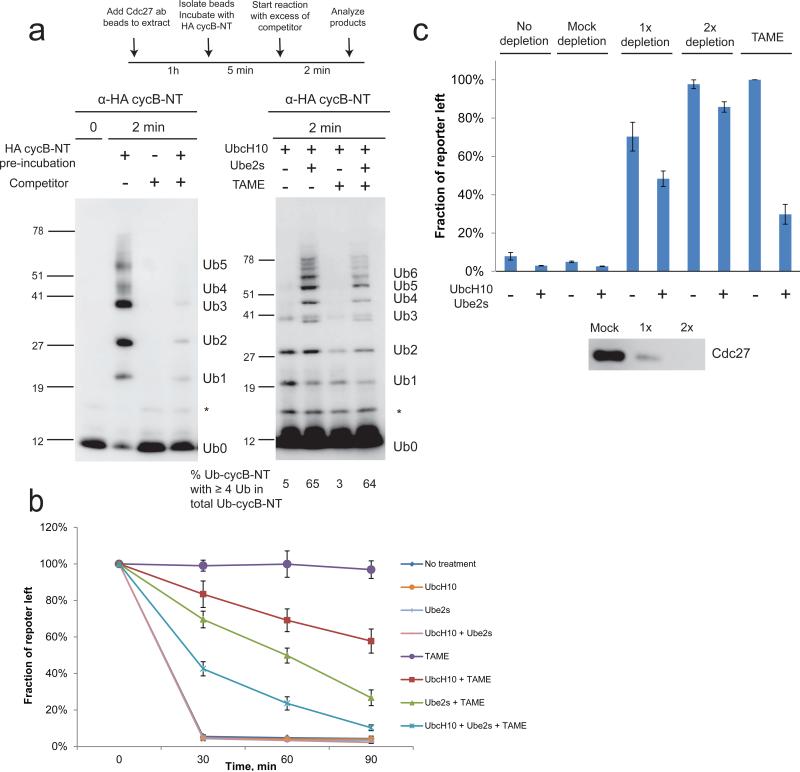
Our data support a model in which the processivity of ubiquitination may influence the ability of TAME to stabilize APC substrates. In this model, the APC first generates partially ubiquitinated substrates that are proteolysis-incompetent. In the absence of TAME, these substrates can rebind APCCdc20 and become further ubiquitinated such that they can be recognized by the proteasome. However, in the presence of TAME, these partially ubiquitinated substrates cannot promote Cdc20 binding to the APC, and instead become deubiquitinated. The presence of TAME therefore creates a futile cycle of ubiquitination and deubiquitination that occurs below the threshold of ubiquitination required for proteolysis. A prediction of this model is that increasing the concentration of the E2 enzyme may achieve a higher degree of ubiquitination in the first binding cycle, and thereby convert futile ubiquitination to ubiquitination sufficient for proteolysis. UBE2S is an E2 enzyme that promotes processive K11-specific ubiquitin chain elongation on APC substrates25-27. We therefore tested whether UBE2S could increase the mass of ubiquitin conjugates in a single substrate binding cycle in the presence of TAME, using conditions similar to those reported previously28,29. We pre-incubated the APC with HA-tagged cycB-NT, and then added excess untagged competitor in the presence of ubiquitination components, to limit the observed ubiquitination to the pre-bound substrate (Fig. 6a). Adding the untagged competitor simultaneously with HA-tagged cycB-NT completely abrogated ubiquitination, confirming that unlabeled substrate was present in sufficient excess to prevent rebinding of labeled substrate (Fig. 6a). With UbcH10 alone, <5% of ubiquitinated cycB-NT reached the threshold of proteolysis (4 ubiquitin molecules) in a single binding cycle (Fig. 6a). When 2 μM UBE2S was co-added with UbcH10, >60% of ubiquitinated cycB-NT reached the threshold of proteolysis (Fig. 6a). TAME reduced the total amount of ubiquitinated cycB-NT (Fig. 6a), consistent with a reduced kcat, but did not alter the fraction of ubiquitinated cycB-NT reaching the threshold of proteolysis, indicating that UBE2S can processively elongate the ubiquitin chains on cycB-NT in a single binding cycle regardless of the presence of TAME. Consistent with this finding, adding 2 μM UBE2S to TAME-treated mitotic Xenopus extract significantly promoted degradation of the cyclin-luciferase reporter. Adding 2 μM UbcH10 had a weaker effect, likely because UbcH10 is less effective in building high mass ubiquitin conjugates (Fig. 6b). Because UBE2S is incapable of nucleating ubiquitin chains25-27, adding excess UBE2S to the extract may delay chain nucleation and degradation by competitively inhibiting the charging of UbcH10 by E1. This problem should be overcome by co-adding UbcH10 and UBE2S. Indeed, co-addition of 2 μM UbcH10 and UBE2S strongly rescued degradation of the reporter in TAME-treated extract (Fig. 6b). In contrast, adding these E2s did not efficiently rescue degradation in Xenopus extract in which proteolysis was inhibited to a similar extent by APC depletion (Fig. 6c), indicating that an overall depletion of APC activity cannot be as efficiently rescued by E2 supplementation. These results suggest that a significant fraction of APC activity is preserved in TAME-treated extract, although substrates are not degraded due to futile cycles of ubiquitination and deubiquitination occurring below the threshold for proteolysis. Our findings indicate that this defect can be rescued by enhancing the rate of ubiquitin transfer in the first substrate binding cycle.
Figure 6. UBE2S extends ubiquitin chains on cycB-NT in the presence of TAME in a single substrate binding cycle and promotes substrate degradation in TAME-treated extract.
(a) UBE2S extends ubiquitin chains on cycB-NT in the presence of TAME in a single substrate binding cycle. APC beads were pre-incubated with HA-tagged cycB-NT and then mixed with an excess of untagged competitor and the ubiquitination reaction mixture for 2 min. UbcH10/UBE2S (2 μM) was added as indicated. The fraction of ubiquitinated cycB-NT carrying at least 4 ubiquitin molecules was quantitated. (b) UBE2S promotes substrate degradation in TAME-treated extract. Mitotic extract was incubated with UbcH10 (2 μM), UBE2S (2 μM), ubiquitin (20 μM) and TAME (200 μM) for 10 min as indicated and then the luciferase reporter was added. Samples were collected at indicated time points and the reporter level was measured by luminometer. Error bar: SEM from 3 independent experiments. (c) UBE2S and UbcH10 rescue substrate degradation much less effectively in APC-depleted extract than in TAME-treated extract. APC-depleted or TAME-treated extracts were supplemented with UbcH10 (2 μM), UBE2S (2 μM), ubiquitin (20 μM) and the luciferase reporter, as indicated. Samples were collected at 60 min and the reporter level was measured by luminometer. Results were normalized to the value obtained under the +TAME/-E2 condition, which was set arbitrarily at 100%. Error bar: SEM from 3 independent experiments.
Discussion
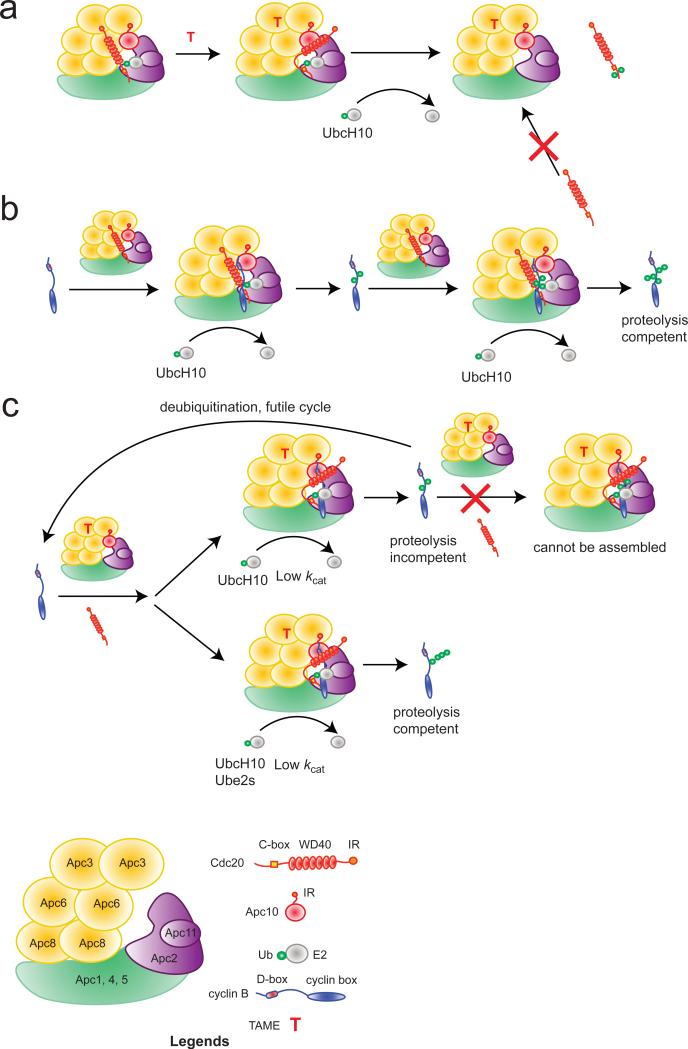
Here we have characterized the precise mechanism by which TAME inhibits the APC. In the absence of APC substrates, TAME reduces Cdc20 binding to the APC in two ways (Fig. 7a): it inhibits the binding of free Cdc20 to the APC and promotes dissociation of pre-bound Cdc20 by inducing Cdc20 auto-ubiquitination. APC substrates suppress both aspects of TAME's mechanism. However, the APCCdc20/substrate/TAME complex remains vulnerable to Cdc20 dissociation as the substrate becomes ubiquitinated, preventing the substrate from reaching the threshold of ubiquitination necessary for proteolysis. The defect in degradation can be partially rescued by increasing the concentration of a processive chain-elongating E2, although the rescue is not complete, likely because the APCCdc20-substrate-TAME complex has a reduced kcat (Fig. 7b,c).
Figure 7. Schematic illustration of TAME's mechanism.
(a) In the absence of substrate, TAME promotes ubiquitination and dissociation of pre-bound Cdc20 and blocks the binding of free Cdc20 to the APC. (b) Substrate (cyclin B1) requires multiple APC binding cycles to become sufficiently ubiquitinated for proteolysis in a UbcH10-mediated reaction. (c) TAME reduces the kcat of the APCCdc20/substrate complex. In a UbcH10-mediated reaction, the partially ubiquitinated substrate cannot undergo further ubiquitination because it fails to promote Cdc20 binding to the APC in the presence of TAME. Instead, it is reversed to the unmodified state by deubiquitination. In a UBE2S-mediated reaction, substrate becomes sufficiently ubiquitinated for proteolysis in a single binding cycle in the presence of TAME.
The phenomenon of TAME-induced Cdc20 dissociation provides new insights into the mechanism of Cdc20 auto-ubiquitination, a process that is not well understood. Two reports suggest that Cdc20 auto-ubiquitination is required to disassemble the MCC21,22, but these studies did not identify the sites of ubiquitination on Cdc20 nor study the process in the absence of the MCC. We found that Cdc20 auto-ubiquitination occurs predominantly upstream of the CRY-box in the absence of TAME. This can be explained by the recent EM structure of yeast APC/Cdh1, in which the N-terminal region of Cdh1 is in the close proximity of the Apc2/11 complex, the site of E2 recruitment7. TAME enhances the susceptibility of Cdc20 to auto-ubiquitination, in both its N-terminal and the C-terminal regions. By disengaging the IR-tail docked onto Cdc27/Apc3, TAME may cause a shift in the position of Cdc20 towards the Apc2/11 complex, making additional lysine residues in Cdc20 more accessible to the charged E2. However, TAME also reduces the number of ubiquitin molecules that can be added to the N-terminal region of Cdc20 before it dissociates from the APC. This effect can be explained by our finding that ubiquitination of Cdc20 in its N-terminal region reduces the binding affinity of Cdc20 for the APC. Previously, we showed that TAME and a C-box-containing fragment of Cdc20 cooperatively inhibit the binding of Cdc20 to the APC3. Hence the loss of the IR-interaction caused by TAME may cooperate with the effect of ubiquitination near the C-box, thereby promoting premature Cdc20 dissociation and reducing the efficiency of further ubiquitination. The lysine side chains in the N-terminal region of Cdc20 may make individually transient but collectively important interactions with the APC that facilitate auto-ubiquitination of these sites on a pre-bound Cdc20 molecule. Once ubiquitination occurs, the interaction would be permanently lost, thereby compromising binding between Cdc20 and the APC. This idea is consistent with a recent report showing that acetylation of lysines in the N-terminal region of Cdc20 inhibits its interaction with the APC30, as both ubiquitination and acetylation neutralize the positive charge on the lysine side chain. Interestingly, there are fewer lysines upstream of the C-box in Xenopus Cdh1 (1) compared to Cdc20 (8), which may explain why Cdh1 shows less susceptibility to auto-ubiquitination.
When the SAC is active, Cdc20 is highly susceptible to auto-ubiquitination21,31 and is degraded by the proteasome23,31-33. Whether Cdc20 ubiquitination and proteolysis maintain the SAC by limiting Cdc20 levels, or promote SAC inactivation by triggering MCC disassembly, is under debate21-23. One recent study suggests that p31 promotes SAC inactivation by stimulating auto-ubiquitination and proteolysis of Cdc20, leading to disassembly of the APC/MCC complex to allow the loading of free Cdc2031. Interestingly, proTAME prevents inactivation of the SAC and stabilizes Cdc20 in cells3. Since TAME reduces the number of ubiquitin molecules that can be added to the N-terminal region of Cdc20 before it dissociates from the APC, it is possible that proTAME prevents Cdc20 ubiquitination from reaching the threshold required for MCC disassembly or for Cdc20 proteolysis. However, the relevance of N-terminal ubiquitination of Cdc20 in SAC inactivation has not been established and C-terminal ubiquitination of Cdc20 has been observed in the context of the MCC34.
TAME's ability to inhibit Cdc20 binding and promote Cdc20 autoubiquitination are antagonized by binding of substrate. This phenomenon may explain why TAME induces efficient dissociation of Cdc20 from the APC in mitotically arrested Xenopus extract, which lacks APC substrates, yet shows little effect on Cdc20 binding in somatic cells, where the presence of APC substrates and pseudosubtrates35 may promote Cdc20 binding and antagonize Cdc20 auto-ubiquitination. The ability of cycB-NT to promote Cdc20 loading is consistent with the D-box co-receptor model in which the D-box of the substrate bridges the core subunit Apc10 and Cdc20, providing an additional interaction site between Cdc20 and the APC that can compensate for the loss of the IR-interaction7,15,18 (Fig. 7b). The fact that cycB-NT suppresses Cdc20 auto-ubiquitination suggests that in the APCCdc20/cycB-NT complex, lysines on cycB-NT are in a more favorable position to accept ubiquitin from the E2 and effectively “shield” lysines on Cdc20 from ubiquitination. The ability of substrate to suppress Cdc20 auto-ubiquitination appears evolutionarily conserved, as similar results in budding yeast were reported while this manuscript was under revision36.
The ability of cycB-NT to rescue Cdc20 binding and suppress Cdc20 auto-ubiquitination was difficult to reconcile with the fact that TAME fully stabilizes cycB-NT in Xenopus extract. Our data reveal that TAME-induced stabilization of cycB-NT is achieved through two effects. First, the APCCdc20-cycB-NT-TAME complex has a reduced kcat, which slows the rate of the addition of the first few ubiquitin molecules to cycB-NT. However, the Km of the reaction is unaffected, suggesting that the IR-tail is dispensable for proper formation of the co-receptor. This finding is consistent with a previous report showing that mutations in Cdc27 that inhibit IR-tail binding do not affect the residence time of the substrate on the APCCdh1 (ref. 18). Second, TAME makes Cdc20 binding to the APC highly dependent on substrate. This has little consequence for the initial stage of ubiquitination, because unmodified substrate can efficiently promote Cdc20 binding. However, later stages of ubiquitination are strongly inhibited, because ubiquitinated cycB-NT cannot promote Cdc20 binding to the APC in the presence of TAME. Therefore, in a physiological system such as Xenopus extract, instead of being further ubiquitinated, the substrate is deubiquitinated rather than degraded. This results in a futile cycle of ubiquitination and deubiquitination that occurs below the threshold of ubiquitination required for proteolysis (Fig. 7c). Processivity of ubiquitination depends on the binding affinity of the substrate as well as the availability and kinetic properties of E2 enzymes29. The fact that TAME strongly stabilizes cycB-NT in Xenopus extract implies that the inherently less processive UbcH10 may play a dominant role in the destruction of APC substrates in Xenopus extract, which is supported by our observation that UBE2S is not required for efficient proteolysis of cyclin B1 in Xenopus extracts19. Other systems such as somatic cells may express higher levels UBE2S, which may limit TAME's ability to stabilize cyclin B1. Indeed, proTAME only transiently delays mitotic exit in SAC-deficient human cells3. In budding yeast, the E2 enzyme Ubc1 promotes processive ubiquitin chain extension after the initial mono-ubiquitination or short chain formation by Ubc437. The availability of Ubc1 may explain why budding yeast expressing Cdc20ΔIR show no abnormality in proliferation8.
Supplementary Material
Acknowledgments
We thank Jonathan Pines for providing the Cdc20K-less construct and Wade Harper for critically reading the manuscript. This work was supported by NIH grant GM66492 to RWK.
Methods
Depletion of ubiquitin by ubiquitin vinyl sulfone (UbVS)
Mitotic Xenopus extract (Supplementary Methods) was treated with 20 μM ubiquitin vinyl sulfone (Boston Biochem, U-202, >98% by HPLC) at RT for 30 min on a shaker19.
In vitro translation of Cdc20/Cdh1
For PCR product-based transcription/translation of Flag-Cdc20/Cdh1 (Supplementary Methods), 3 μl of PCR product, 1 μl of methionine (1 mM), 1 μl of TNT T7 PCR enhancer and 5 μl of water were mixed with 40 μl TNT T7 Quick Master Mix (Promega, L1170) and incubated at 30°C for 90 min. For plasmid-based translation of HA-tagged Cdc20, 2 μl of plasmid (0.5 μg/μl), 1 μl of methionine (1 mM) and 7 μl of water were mixed with 40 μl TNT SP6 Quick Master Mix (Promega L2080) and incubated at 30°C for 90 min.
In vitro ubiquitination assay
Interphase Xenopus egg extract was driven into mitosis by addition of non-degradable MBP-cyclin B1Δ90 (1 μM). APC was then immunoprecipitated from the extract with Cdc27 antibody (Santa Cruz, sc-9972, AF3.1) covalently coupled to protein A beads (Bio-Rad, 156-0006, Supplementary Methods). For a single IP, 2 μg antibody was bound to 5 μl beads and mixed with 100 μl extract for 1 h at 4°C. For experiments with endogenous Cdc20, the beads were washed with ubiquitin chain buffer (UCB, 20 mM Tris, 100 mM KCl, 2 mM ATP and 2.5 mM MgCl2, pH 7.7) + 0.1% IGEPAL CA-630 (Sigma I-8896) 3 times. The ubiquitination mixture was prepared by adding 250 nM E1 (Boston Biochem, E-304, >95% by SDS-PAGE), 2 μM his-tagged UbcH10 (purified with Ni-NTA resin, >95% by SDS-PAGE), 150 μM ubiquitin (Boston Biochem, U-100H, >95% by SDS-PAGE), 500 nM cycB-NT (>90% by SDS-PAGE, if needed) and 0.1% IGEPAL CA-630 to the ubiquitin chain buffer 30 min prior to the reaction. The reaction was started by mixing 5 μl beads with 10 μl of the ubiquitination mixture and left on a shaker at RT. For experiments with in vitro-translated Cdc20, APC beads were washed with high salt XB (500 mM KCl, 0.1 mM CaCl2, 1 mM MgCl2 and 10 mM HEPES, pH 7.7) 3 times to remove endogenous Cdc20 and then low salt XB (same as high salt XB but with 100 mM KCl) + 0.1% IGEPAL CA-630 3 times. Reticulocyte lysate containing Cdc20 was diluted 5-fold with XB + 0.1% IGEPAL CA-630 and 1 μM okadaic acid potassium salt (VWR, 89149-000, >98% by HPLC). The beads were mixed with the diluted reticulocyte lysate (50 μl lysate per 5 μl of beads) and left on a shaker at RT for 10 min. The beads were then washed with UCB + 0.1% IGEPAL CA-630 3 times and the reaction was performed as described above for endogenous Cdc20. To assist the loading of Cdc20ΔIR, 2 μM of cycB-NT was added to the lysate. For Western blots, Flag-HRP antibody was from Sigma (A8592), HA-HRP antibody was from Roche Applied Science (12013819001, 3F-10), Xenopus Cdc20 antibody (sc-53399) and Apc10 antibody (sc-20989) were from Santa Cruz, and Cdc27 antibody was from BD Transduction Laboratories (610593).
Deubiquitination of Cdc20
Supernatants from Cdc20 ubiquitination reactions were divided into 2 equal aliquots. The catalytic domain of Usp2 (Usp2-CD, Boston Biochem, E-506, >95% by HPLC) was added to one aliquot at 500 nM and EDTA (1 mM) and DTT (5 mM) were added to both aliquots. The mixtures were incubated at 37°C for 1 h and desalted with Zeba micro desalt spin columns (Thermo, 87764) following manufacturer's protocol. UbcH10 C114S was added to the mixture at 20 μM to prevent re-ubiquitination. Both aliquots were then incubated with APC beads pre-washed with high salt XB at RT for 10 min.
Isolation of ubiquitinated cyclin B1 N-terminal fragment from mitotic Xenopus egg extract
An N-terminal HA-tagged and C-terminally His-tagged human cyclin B1 N-terminal fragment (cycB-NT; aa1-88 from cyclin B1) was added to mitotic Xenopus egg extract at 250 nM and incubated at RT for 15 min. The extract was then quickly mixed with 8 volumes of 20 mM HEPES, 100 mM KCl, 9M urea, pH 7.7. To re-isolate cycB-NT, the mixture was incubated with Ni-NTA resin (Qiagen) on a rotator at RT for 45 min.
Kinetic analysis of APCCdc20 reactions
Ubiquitination mixture was prepared as described with the exception that 60 μM methylated ubiquitin (Boston Biochem, U-502, >95% by SDS-PAGE) was used in place of WT ubiquitin. CycB-NT was diluted into the mixture as a two-fold dilution series from 1 μM to 62.5 nM prior to the reaction. For each reaction, 2.5 μl APCCdc20 beads were mixed with 20 μl ubiquitination mixture and left on a shaker at RT for 30 seconds. The reaction was stopped by adding 7 μl hot 4× lithium dodecyl sulfate (LDS) sample buffer (Invitrogen NP0007) containing 100 μM DTT. Images were acquired as described below, and band intensities of the mono-, di-, and tri-ubiquitinated cycB-NT were quantitated with Image J and fitted to a hyperbolic equation by nonlinear regression. For single binding cycle assays, 5 μl APCCdc20 beads were mixed with 2 μl 500 nM HA-tagged cycB-NT for 5 min. A non-tagged sea urchin cycB-NT fragment was added to the ubiquitination mixture at 15 μM and 20 μl of the mixture was added to the beads. The reaction was stopped at 2 min as described above.
Quantification of Western blot
All Western blots were analyzed with a Fuji LAS-3000 CCD imager. Images were then opened with Image J v1.43u and individual lanes were selected with the lane selection tool (Image J-Analyze-Gel-Select lanes). The lanes were then plotted (Image J-Analyze-Gel-Plot lanes) to obtain a distribution of pixel intensity of each lane with bands represented as peaks in the plot. The areas under peaks that correspond to bands of interest were selected with the line tool and then integrated with the wand tool of Image J. The linearity of quantitation was confirmed by quantitating blots loaded with known amounts of proteins.
Additional methods information, including Xenopus extract preparation and construction of Cdc20 mutants, is provided in the Supplementary Methods.
Footnotes
Author contributions
X.Z. and R.W.K. designed the experiments; X.Z. performed the experiments, and X.Z. and R.W.K. wrote the manuscript.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Peters JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol. 2006;7:644–656. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- 2.Verma R, et al. Ubistatins inhibit proteasome-dependent degradation by binding the ubiquitin chain. Science. 2004;306:117–120. doi: 10.1126/science.1100946. [DOI] [PubMed] [Google Scholar]
- 3.Zeng X, et al. Pharmacologic inhibition of the anaphase-promoting complex induces a spindle checkpoint-dependent mitotic arrest in the absence of spindle damage. Cancer Cell. 2010;18:382–395. doi: 10.1016/j.ccr.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pesin JA, Orr-Weaver TL. Regulation of APC/C activators in mitosis and meiosis. Annu Rev Cell Dev Biol. 2008;24:475–499. doi: 10.1146/annurev.cellbio.041408.115949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayes MJ, et al. Early mitotic degradation of Nek2A depends on Cdc20-independent interaction with the APC/C. Nat Cell Biol. 2006;8:607–614. doi: 10.1038/ncb1410. [DOI] [PubMed] [Google Scholar]
- 6.Kimata Y, Baxter JE, Fry AM, Yamano H. A role for the Fizzy/Cdc20 family of proteins in activation of the APC/C distinct from substrate recruitment. Mol Cell. 2008;32:576–583. doi: 10.1016/j.molcel.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 7.da Fonseca PC, et al. Structures of APC/C(Cdh1) with substrates identify Cdh1 and Apc10 as the D-box co-receptor. Nature. 2011;470:274–278. doi: 10.1038/nature09625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thornton BR, et al. An architectural map of the anaphase-promoting complex. Genes Dev. 2006;20:449–460. doi: 10.1101/gad.1396906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Passmore LA, et al. Doc1 mediates the activity of the anaphase-promoting complex by contributing to substrate recognition. EMBO J. 2003;22:786–796. doi: 10.1093/emboj/cdg084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vodermaier HC, Gieffers C, Maurer-Stroh S, Eisenhaber F, Peters JM. TPR subunits of the anaphase-promoting complex mediate binding to the activator protein CDH1. Curr Biol. 2003;13:1459–1468. doi: 10.1016/s0960-9822(03)00581-5. [DOI] [PubMed] [Google Scholar]
- 11.Burton JL, Solomon MJ. D box and KEN box motifs in budding yeast Hsl1p are required for APC-mediated degradation and direct binding to Cdc20p and Cdh1p. Genes Dev. 2001;15:2381–2395. doi: 10.1101/gad.917901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hilioti Z, Chung YS, Mochizuki Y, Hardy CF, Cohen-Fix O. The anaphase inhibitor Pds1 binds to the APC/C-associated protein Cdc20 in a destruction box-dependent manner. Curr Biol. 2001;11:1347–1352. doi: 10.1016/s0960-9822(01)00399-2. [DOI] [PubMed] [Google Scholar]
- 13.Kraft C, Vodermaier HC, Maurer-Stroh S, Eisenhaber F, Peters JM. The WD40 propeller domain of Cdh1 functions as a destruction box receptor for APC/C substrates. Mol Cell. 2005;18:543–553. doi: 10.1016/j.molcel.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 14.Schwab M, Neutzner M, Mocker D, Seufert W. Yeast Hct1 recognizes the mitotic cyclin Clb2 and other substrates of the ubiquitin ligase APC. EMBO J. 2001;20:5165–5175. doi: 10.1093/emboj/20.18.5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buschhorn BA, et al. Substrate binding on the APC/C occurs between the coactivator Cdh1 and the processivity factor Doc1. Nat Struct Mol Biol. 2011;18:6–13. doi: 10.1038/nsmb.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burton JL, Tsakraklides V, Solomon MJ. Assembly of an APC-Cdh1-substrate complex is stimulated by engagement of a destruction box. Mol Cell. 2005;18:533–542. doi: 10.1016/j.molcel.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 17.Eytan E, Moshe Y, Braunstein I, Hershko A. Roles of the anaphase-promoting complex/cyclosome and of its activator Cdc20 in functional substrate binding. Proc Natl Acad Sci U S A. 2006;103:2081–2086. doi: 10.1073/pnas.0510695103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matyskiela ME, Morgan DO. Analysis of activator-binding sites on the APC/C supports a cooperative substrate-binding mechanism. Mol Cell. 2009;34:68–80. doi: 10.1016/j.molcel.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dimova N, et al. APC/C-mediated multiple monoubiquitination provides an alternative degradation signal for cyclin B1. Nat Cell Biol. doi: 10.1038/ncb2425. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Townsley FM, Aristarkhov A, Beck S, Hershko A, Ruderman JV. Dominant-negative cyclin-selective ubiquitin carrier protein E2-C/UbcH10 blocks cells in metaphase. Proc Natl Acad Sci U S A. 1997;94:2362–2367. doi: 10.1073/pnas.94.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reddy SK, Rape M, Margansky WA, Kirschner MW. Ubiquitination by the anaphase-promoting complex drives spindle checkpoint inactivation. Nature. 2007;446:921–925. doi: 10.1038/nature05734. [DOI] [PubMed] [Google Scholar]
- 22.Stegmeier F, et al. Anaphase initiation is regulated by antagonistic ubiquitination and deubiquitination activities. Nature. 2007;446:876–881. doi: 10.1038/nature05694. [DOI] [PubMed] [Google Scholar]
- 23.Nilsson J, Yekezare M, Minshull J, Pines J. The APC/C maintains the spindle assembly checkpoint by targeting Cdc20 for destruction. Nat Cell Biol. 2008;10:1411–1420. doi: 10.1038/ncb1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirkpatrick DS, et al. Quantitative analysis of in vitro ubiquitinated cyclin B1 reveals complex chain topology. Nat Cell Biol. 2006;8:700–710. doi: 10.1038/ncb1436. [DOI] [PubMed] [Google Scholar]
- 25.Garnett MJ, et al. UBE2S elongates ubiquitin chains on APC/C substrates to promote mitotic exit. Nat Cell Biol. 2009;11:1363–1369. doi: 10.1038/ncb1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williamson A, et al. Identification of a physiological E2 module for the human anaphase-promoting complex. Proc Natl Acad Sci U S A. 2009;106:18213–18218. doi: 10.1073/pnas.0907887106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu T, et al. UBE2S drives elongation of K11-linked ubiquitin chains by the anaphase-promoting complex. Proc Natl Acad Sci U S A. 2010;107:1355–1360. doi: 10.1073/pnas.0912802107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pierce NW, Kleiger G, Shan SO, Deshaies RJ. Detection of sequential polyubiquitylation on a millisecond timescale. Nature. 2009;462:615–619. doi: 10.1038/nature08595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rape M, Reddy SK, Kirschner MW. The processivity of multiubiquitination by the APC determines the order of substrate degradation. Cell. 2006;124:89–103. doi: 10.1016/j.cell.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 30.Kim HS, et al. SIRT2 Maintains genome integrity and suppresses tumorigenesis through regulating APC/C activity. Cancer Cell. 2011;20:487–499. doi: 10.1016/j.ccr.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varetti G, Guida C, Santaguida S, Chiroli E, Musacchio A. Homeostatic control of mitotic arrest. Mol Cell. 2011;44:710–720. doi: 10.1016/j.molcel.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 32.Ge S, Skaar JR, Pagano M. APC/C- and Mad2-mediated degradation of Cdc20 during spindle checkpoint activation. Cell Cycle. 2009;8:167–171. doi: 10.4161/cc.8.1.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan J, Chen RH. Spindle checkpoint regulates Cdc20p stability in Saccharomyces cerevisiae. Genes Dev. 2004;18:1439–1451. doi: 10.1101/gad.1184204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mansfeld J, Collin P, Collins MO, Choudhary JS, Pines J. APC15 drives the turnover of MCC-CDC20 to make the spindle assembly checkpoint responsive to kinetochore attachment. Nat Cell Biol. 2011;13:1234–1243. doi: 10.1038/ncb2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burton JL, Solomon MJ. Mad3p, a pseudosubstrate inhibitor of APCCdc20 in the spindle assembly checkpoint. Genes Dev. 2007;21:655–667. doi: 10.1101/gad.1511107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foe IT, et al. Ubiquitination of Cdc20 by the APC occurs through an intramolecular mechanism. Curr Biol. 2011;21:1870–1877. doi: 10.1016/j.cub.2011.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodrigo-Brenni MC, Morgan DO. Sequential E2s drive polyubiquitin chain assembly on APC targets. Cell. 2007;130:127–139. doi: 10.1016/j.cell.2007.05.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.