Abstract
Myxomatous mitral valve disease (MMVD) is the most commonly diagnosed cardiovascular disease in the dog accounting for more than 70% of all cardiovascular disease in dogs. As are most canine diseases with genetic underpinnings, risk of MMVD is greatly increased in a subset of breeds. What is uncommon is that the vast majority of the breeds at elevated risk for MMVD are small or toy breeds with average adult weights under 9 kg. These breeds appear to have little in common other than their diminutive size. In the following review we propose a number of mechanisms by which relatively unrelated small breeds may have developed a predisposition for chronic valvular disorders. Although factors such as age are key in the expression of MMVD, taking a comprehensive look at the commonalities, as well as the differences, between the susceptible breeds may assist in finding the causal variants responsible for MMVD and translating them to improved treatments for both dogs and humans.
Keywords: Canine genetics, degenerative valve disease, canine phenotype, dog breeds
Introduction
Myxomatous mitral valve disease (MMVD) is the most common congenital heart disease in dogs accounting for more than 70% of all canine heart disease1–3. The disease is chronic and progressive with initial signs, usually a heart murmur, developing after the age of six. Approximately 30% of dogs with MMVD progress to mitral regurgitation (MR) and eventually heart failure3. The incidence is particularly high in some breeds such as the Cavalier King Charles spaniel (CKCS) with as many as 90% developing MMVD by the age of 10 years3. Evidence from highly susceptible breeds such as the CKCS and dachshund shows a strong inherited component to the disease and suggests a polygenic mode of inheritance4–6. In fact, two loci have been recently associated with MMVD in the CKCS7. The breed specificity of MMVD incidence is not unusual for a genetic disease in dogs as the breed structure of the modern domestic dog can easily lend itself to sustaining a detrimental genetic mutation. Breeds are essentially closed populations, once a malicious mutation develops or is introduced it can readily expand throughout the population. As a result, the presence of an inherited disease in a small number of breeds is to be expected. What is unexpected in the case of MMVD is that the majority of affected breeds display an average adult weight of less than 9 kg8–10. Why is MMVD so much more prevalent in small breeds as opposed to large? What do all small dog breeds share that might generate the optimal conditions for MMVD to develop?
To approach the likely cause of the small dog – MMVD correlation we need first to consider what all small breeds have in common and assess how those commonalities could relate to heart disease. To best accomplish this we will break down the relationships into three sources of common ground: morphologic, genetic, and historic. Morphologic commonalities include overall body size but might also include skeletal shapes or components that are common in very small animals. Genetic similarities arise from the mutations that restrict growth. Aside from the genes under selection, MMVD-causing mutations may be carried with these genes through what is commonly referred to as “hitchhiking”. Historically, it is possible that all small dogs that are affected with MMVD share a unique common ancestor and have inherited a set of mutations from that common source that have increased susceptibility to MMVD. In this review we will assess each of these possibilities and how they may contribute to the overabundance of MMVD in small breed dogs.
Body Size and Heart Disease
According to the American Kennel Club standards, the dog breed average adult weight is 23 kg. Breeds averaging 9 kg and below are in the lowest 21% of average dog weights with approximately 43 breeds fitting the criterion. These breeds are also in the lowest 20% of dog heights with the tallest averaging 33 cm at the shoulder11. In 1985, Thrusfield and colleagues assessed the effect of breed and sex on valve disease in dogs by examining the case records of dogs treated at the University of Edinburgh. They identified 12 breeds with an increased incidence of heart valve incompetencies. Of these twelve, nine had average adult weights of 9 kg or less and 10 had average adult weights less than 14 kg. Recently, Fleming et al. examined 74,556 entries in the Veterinary Medical Database, a compilation of cases from 27 veterinary teaching hospitals in North America, for cause of death by breed10. A major cause of death is considered one that affects more than 10% of the breed. Based on this criterion they found that nearly 75% of breeds with average body weight of less than 9 kg report cardiovascular issues as a major cause of death compared to only 25% of breeds with average weights over 9 kg. Can there be a link between being small and developing heart disease?
In fact, height has been linked to a variety of heart diseases in human studies. In 1951, a study in the Journal of the American Medical Association examined the physical characteristics of 100 individuals with myocardial infarction prior to 40 years of age and found that they averaged 5 cm shorter than a similar control group12. Another study looking at a cohort of 7735 men in the United Kingdom in 1998 also showed a significant relationship between height and coronary heart disease13. In 2010 a meta-analysis was performed on a combined dataset of >3 million individuals. In this combined group the risk of cardiovascular death in short men and women was 50% higher than in tall men and women14. The short group was 5% smaller than the tall group in this analysis. In dogs the small breeds are 30% shorter at the shoulder than the average breed (<33 cm compared to 48 cm). If the correlation of height and heart disease observed in humans is true in dogs, it may be possible to attribute an overall increase in heart disease in small breeds to their size alone.
Though height has been associated with heart disease in general, the association does not account for the prevalence of valve disease compared to other forms of heart disease in the small breeds. Perhaps there are morphologic features common to the small breeds other than body weight or height. In 1981, Schutte et al. found that height and diameter of the chest was reduced in individuals with mitral valve prolapsed (MVP), the human disease that shares some of the components of MMVD, compared to unaffected individuals of the same overall body size15. Similar correlations have been made between MVP and genetic disorders with specific upper-body phenotypes such as scoliosis, Marfan’s syndrome, straight-back syndrome and funnel chest which may suggest that aspects of cardiac function are controlled by many of the same genes and pathways that shape the thorax. Alternately the shape of the chest may be directly responsible for the valve malformations. Raggi et al. measured the chest diameter, angulation of the mitral valve ring, and contact between the myocardium and the chest wall in over 150 MVP patients and concluded that the heart valve malformation is due to entrapment in the chest cavity16. In effect, disproportionate growth of the heart and chest cavity can cause distortion of the valve and lead to prolapse.
Unsynchronized morphological regulation has been observed in Insulin-like growth factor 1 (IGF1) negative mice. The body size of homozygous mutant mice is reduced to a third of the wild-type mice while the size of the heart is reduced by less than half 17. IGF1 also controls size variation in dogs and humans18–20. Given the extreme reduction in skeletal size in the smallest dog breeds, it would be interesting to measure the organ size and volume of the thorax in small and large dogs to see if the decrease is proportional or if the heart is being crowded in the ever decreasing chest cavities of small dogs.
Genetics of Body Size
It is possible that the shape and size of the small breed dogs is contributing directly to heart disease by restricting proper growth. Many centuries of selection for smaller size have resulted in a current range of body sizes in dogs that spans two degrees of magnitude with teacup sized toy breeds reaching an adult weight of less than 1 kg while a full-grown male mastiff can easily top 90 kg21. Is it not equally as possible that the same genes involved in skeletal growth are active in the developing heart? Multiple studies have been released that address the genetics of size variation in dogs from different viewpoints. In 2002, Chase et al. published a linkage study looking at the morphology of the Portuguese water dog (PWD)22. The PWD can range in size from 16 to 27 kg and 43 to 58 inches at the shoulder, allowing for segregation of a number of genes affecting overall body size. The authors obtained five different radiographs of >300 PWD and measured >70 aspects of skeletal shape and size. From these they calculated nine principal components (PCs), the first of which encompasses overall body size and accounts for ~45% of all variation in the breed. This PC is linked to two loci in the genome: one on canine chromosome 15 (CFA15) and a second on CFA37. The site on CFA15 is in the vicinity of the IGF1 gene which had been shown to affect body size in both mice and humans19,20. To further characterize the locus, Sutter and colleagues compared single-nucleotide polymorphism (SNP) allele frequencies across the region in small and large breeds of dog and found a selective sweep spanning the IGF1 gene in all small dogs. In fact, a single haplotype that was identical-by-descent was found in 93% of all dogs from breeds with adult weights averaging less than 10 kg. This study revealed two important facts about dogs: one, genes that affect major morphologic traits will be shared across multiple breeds, and two, all small dog breeds share at least one common ancestor that contributed the IGF1 allele.
This finding is particularly intriguing when we consider that the IGF1 gene has been implicated in cardiac development as well as body size23,24. While a loss of IGF1 expression is related to decreased body size, over expression of IGF1 leads to increased heart size by increasing the size of the cardiac myocytes. This response is under consideration as a means to treat heart failure by reducing the risk of myocardial ischemia25,26. As discussed earlier, IGF1 affects both skeletal growth and heart size, though the effects are not proportionate according to studies performed in mice17. Since it has been shown that IGF1 is a major contributor to reduction in body size in dogs, if the heart is not shrinking at the same scale in small dogs, this mutation alone could be responsible for over-crowding leading to the valve malformations16. In addition, IGF1 has a direct effect on heart growth which could lead to malformations if regulated improperly as would be expected under selection for small size.
Following the IGF1 findings, two additional studies were released looking at the genetics of body size across multiple breeds. In 2008 Jones et al. completed a low density SNP genome-wide association study (GWAS) in 148 dog breeds to look for major morphological control regions27. By assigning traits based on breed standards and photographs, the authors identified eight loci associated with height, weight, or a combination of the two. Five of these loci contained strong candidate genes for size such as IGF1, high mobility group AT-hook 2 (HMGA2), and insulin-like growth factor 2 mRNA binding protein 2 (IGF2BP2) (Table 1). Four out of five of the candidate genes identified for size control also affect heart development. For instance, Monzen et al. showed that HMGA2 affects cardiomyocyte differentiation and that a morpholino mediated knock down of the gene leads to improper heart formation in frog embryos28. Perhaps the most interesting gene in this group is SMAD family member 2 (SMAD2) which interacts directly with transforming growth factor beta (TGFβ)29. Recent studies show that TGFβ2, working through SMAD2/3, is required to achieve mature valve structure30. In addition, an increase in TGFβ signaling, identified by the correlated increase in SMAD2 expression, contributes to mitral valve degeneration in a mouse model of Marfan’s syndrome in which the mitral valves show increased leaflet length and thickness and folding conformation31. Given this correlation, a dog with altered SMAD2 expression due to selection for its growth retarding properties may also experience problems with cardiac valve development.
Table 1.
Breeds that may be at an increased risk for MMVD.
| Breed | Height (cm) |
Weight (kg) |
AKC Group |
Genetic Group |
Ref.a |
|---|---|---|---|---|---|
| Chihuahua | 15 | 2 | Toy | Toy | 1,5,6,7,9 |
| Maltese | 23 | 2 | Toy | Toyb | 1,5 |
| Yorkshire terrier | 15 | 3 | Toy | Terrier | 1 |
| Poodle - Toy | 25 | 4 | Toy | working | 1,6,7,9 |
| Papillon | 24 | 4 | Toy | Toy | 9 |
| Pekingese | 19 | 4 | Toy | Toy | 6,9 |
| Miniature pinscher | 28 | 5 | Toy | Toyb | 6,9 |
| Bolognese | 19 | 5 | n.d. | n.d. | 1 |
| Dachshund | 18 | 5 and 11c | Hound | Scenthound | 1,2,3,6,9 |
| Shih Tzu | 24 | 6 | Toy | Toy | 1 |
| Cairn Terrier | 25 | 6 | Terrier | Terrier | 6,9 |
| Miniature Schnauzer | 33 | 6 | Terrier | Terrierb | 7 |
| Bichon Frise | 25 | 6 | Toy | Toy | 1 |
| Cavalier King Charles Spaniel | 32 | 7 | Toy | Spaniel | 1,4,6,7,8,9 |
| Pug Dog | 33 | 7 | Toy | Toy | 1 |
| Miniature Poodle | 32 | 7 | Non-Sporting | Working | 1,6,7,9 |
| West Highland White Terrier | 28 | 8 | Terrier | Terrier | 1 |
| Fox terrier | 38 | 8 | Terrier | n.d. | 5,7 |
| Boston Terrier | 41 | 9 | Non-Sporting | Mastiff | 7,9 |
| Welsh terrier | 38 | 9 | Terrier | Mastiffb | 1 |
| Whippet | 48 | 10 | Hound | Sighthound | 6,9 |
| Bull terrier | 31 and 53c | 11 and 27c | Non-Sporting | Mastiff | 9 |
| American Cocker Spaniel | 37 | 12 | Sporting | Spaniel | 1,2,3 |
| Beagle | 36 | 12 | Hound | Scenthound | 1,2,6,9 |
| Standard Poodle | 38 | 26 | Non-Sporting | Working | 6,9 |
| German Shepherd Dog | 64 | 37 | Herding | Working | 8,9d |
| Great Dane | 81 | 66 | Working | Mountain | 9 |
1- Aupperle, 200955, 2-Buchanan, 199956, 3- Detweiler, 1965 1, 4- Egenvall, 200657, 5-Fleming, 201110, 6 – Thrusfield, 19859, 7- The Quest Trial58,59, 8-Inherited Diseases in Dogs (IDID) Database60, 9-Canine Inherited Disorders Database (CIDD)61.
Breed group determined by microsatellite analysis.
Sizes for miniature and standard versions of the breed.
German Shepherds are underrepresented in MVDD studies by Buchanan and Thrusfield.
More recently a large SNP dataset of ~50,000 markers was used in a multi-breed GWAS to identify genetic causes of morphologic traits. Boyko et al. examined 900 dogs from 80 diverse breeds to identify loci for size and shape32. They found six loci strongly associated with body size, two on the X chromosome and four on autosomes. Three of the four autosomal loci had been identified in at least one of the two earlier studies. The fourth on CFA4 was near the candidate gene stanniocalcin 2 (STC2) which has been shown to inhibit growth in mice33. Four of the six size associated loci were among the top six regions of the genome that displayed significantly increased fixation indices across the breeds. This indicates that size loci are under strong selection, corroborating the earlier assumption that genes affecting morphologic traits are largely shared across breeds.
Linkage Disequilibrium and Hitchhiking Genes
While the same genes can affect both body size and heart development, the act of selecting for particular traits can influence disease susceptibility by altering the genome architecture. Early proponents of mapping traits in dogs have assumed that the linkage disequilibrium (LD) found on dog chromosomes would be extensive due to the non-random aspects of dog husbandry. Beginning with the development of the breed clubs and competitive dog shows in the early 1800s, dog breeders have been required to contain their breeding program to only those dogs that have been officially recognized as members of the breed. The inevitable inbreeding that follows leads to an overall loss of heterozygosity (reviewed in Ostrander et al. 200034,and Parker et al. 2010 35). The heterogeneity within a breed is further lowered by the use of popular sires. Dogs that have performed well in competitions are selected to father large numbers of litters causing an over-representation of one set of alleles in later generations. Within dog breeds, LD is measured at 10–100 times the average length found in humans and varies depending on the breed and its demographic history36–38. Therefore, large stretches of each chromosome will remain unbroken from generation to generation within each breed.
In addition to the high levels of LD, canine chromosomes contain large tracts of homozygosity likely the result of selective pressures. When the draft sequence of the Boxer was completed in 2005, it was estimated that >60% of the genome lay within homozygous tracts that averaged over 6Mb in length37. Analyses of SNP genotyping data from at least 10 dogs each of 60 breeds found that individuals from all breeds showed 10–50 regions of homozygosity spanning more than 10 Mb32. In the majority of breeds, all individuals showed overlapping homozygous regions of at least 1 million bases. These stretches of homozygosity are likely the result of selection for traits within the breeds. When a trait is fixed within a breed a region of homozygosity or reduced heterozygosity is created around the selected mutation which is referred to as a selective sweep. This region can extend over 1 Mb within a breed depending on the age of the mutation under selection18,39. This means that any mutation within a gene or regulatory element within a million bases surrounding a selected mutation may become fixed within the population along with the desired trait and at the very least will increase in frequency in response to the selective pressures. Assuming that all small breeds are under selection for mutations within the seven regions that have been identified as size controlling loci in multiple GWAS, there is the potential for seven Mb of DNA to be shared by all of the breeds simply because they are all small in stature. These seven million bases contain, on average, 70 genes any one of which could contribute to an increased risk of heart defects in the breeds carrying them.
An interesting case can be found in the region of the STC2 gene which was identified as a candidate for small size32. STC2 has not been associated with heart development to date but it is located less than 80 kb from the gene NK2 homeobox 5 (NKX2–5). Given their proximity, these genes are likely in high LD with each other and within the range of an average selective sweep18,39,40. NKX2–5 works downstream of TGFβ and SMAD2 in a signaling pathway that controls cardiac valve formation41. Mutations in NKX2–5 have been associated with numerous congenital heart defects including mitral valve anomalies in humans42. A mutation affecting NKX2–5 could easily be swept along with the STC2 gene when it was under selective pressure at any point during the process of breeding small dogs.
Population Structure and Common Ancestors of Small Breeds
One final explanation for the over-representation or MMVD in small dogs could be common ancestry that is not directly related to size. If all small dogs stem from a common founder, MMVD may have been introduced at a very early stage of breed development and has reached relatively high rates due to reductions in the gene pools of most breeds after their development. A number of studies have shown that each breed carries a unique genetic pattern that is identifiable through analysis of markers scattered throughout the genome32,43,44. However, in addition to individual breeds, both microsatellite and SNP studies have grouped the breeds into clusters that largely correspond to specific morphologies, geographical origins, or historical occupations that are shared among the breeds in the cluster43,44, 45. The first of these groups is commonly referred to as the Ancient group. This group is comprised of breeds developed in Asia and Africa, and is distinct from the Modern breeds, which are largely of European origin. The Modern breeds are further divided into the Herding-Sighthound cluster, the Mastiff-Terrier cluster, the Mountain cluster, and the Hunting cluster based on microsatellites44. The Hunting cluster is the most diverse including not only gun dogs but also hounds, and a subset of terriers and working dogs. All toy breeds do not form a separate group but rather display a mixture of all five groups in different proportions.
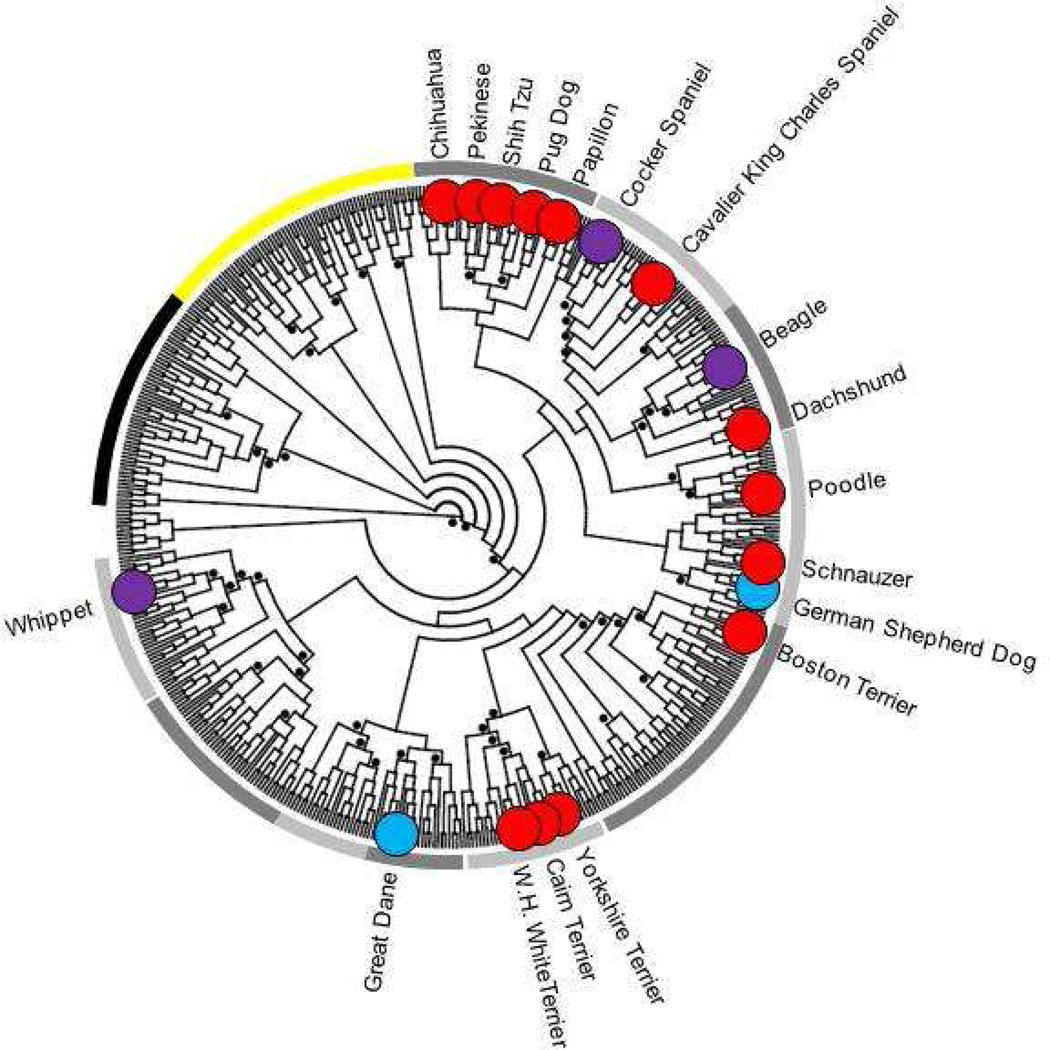
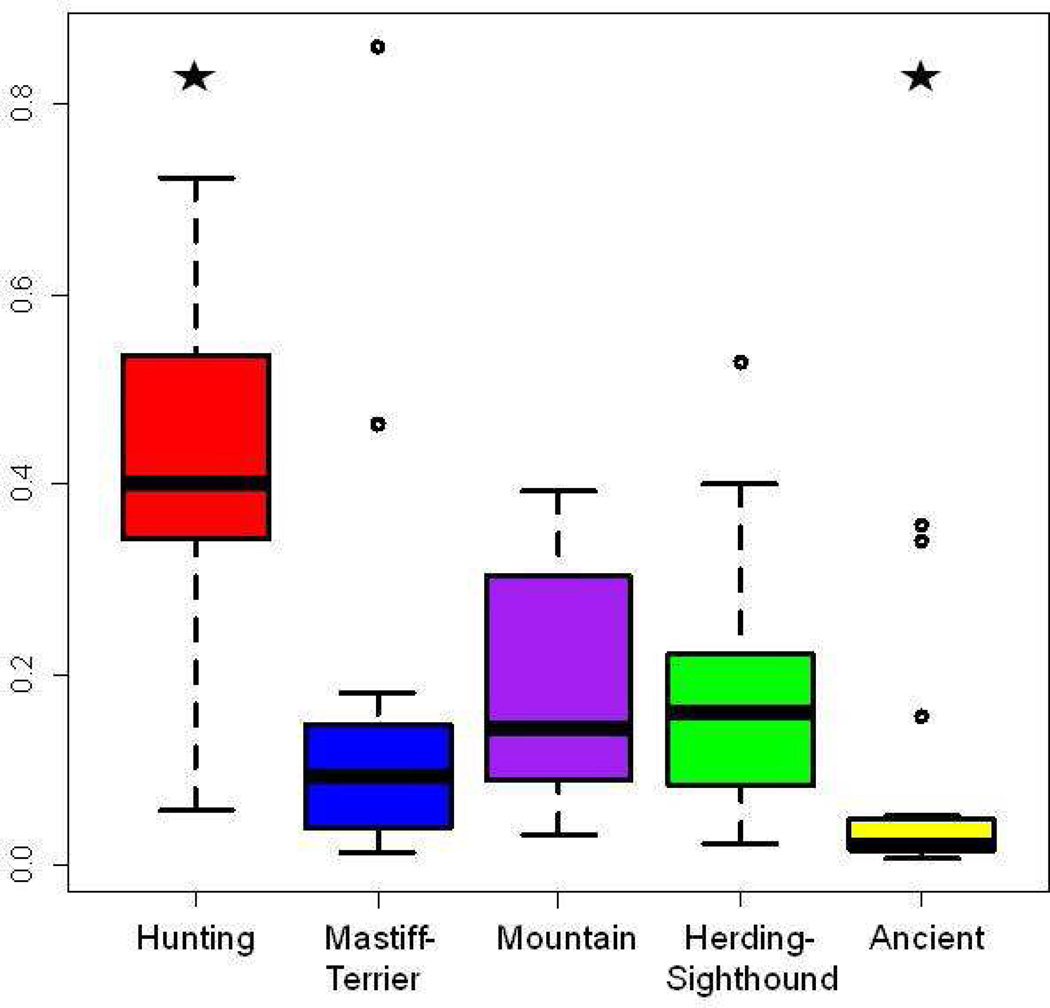
An analysis of ~50,000 SNPs genotyped in 80 breeds was used to develop a dendrogram of the breeds built on sharing of 15-SNP haplotypes45. This analysis effectively separated the individual breed types from the microsatellite analysis while maintaining the primary breed clusters. Based on haplotype sharing, the majority of toy dogs seem to form one cluster within the Modern group with the closest breeds belonging to the Hunting cluster. This branching is not statistically significant but these toy breeds appear more similar to each other than to the larger breeds in the study. The SNP haplotype analysis may have been able to pick out the sharing of selected loci that was not evident in microsatellite analyses. Although a subset of the toy dogs seem to show a common ancestral line, all breeds with an average adult weight of 9 kg or less, and susceptible to MMVD, are not toys. Many of these breeds are terriers, small hounds and spaniels, which come from nearly all regions of the breed tree (Figure 1). All of the breeds that are susceptible to MMVD are part of the Modern cluster, which comprises four of the five primary breed clusters44 and eight of the ten breed groups45. The microsatellite cluster analysis shows that Pekingese and the Shih Tzu gain a portion of their genome from the Asian cluster but they also are included in the Modern breed groups thus a common mutation is more likely to come from that portion of their heritage. Looking at the contribution of the five primary breed groups to the high risk breeds, the Hunting group is by far the most significant contributor with an average of 42% of their genomes coming from this ancestral cluster (Figure 2).
Figure 1.
Breeds at risk for MMVD trace back to an ancient common ancestor of the modern breeds. A neighbor joining tree based on 10-SNP haplotypes groups 80 dog breeds into approximately 10 breed clusters. The black arch indicates the position of wild canids on the tree and the yellow arch indicates the ancient breeds. All other branches comprise the modern breeds. These breeds are grouped in the following order clockwise from the top and indicated by alternating gray bands: toy, spaniel, scent hound, working, mastiff, terrier, mountain, retriever, herding, and sighthound. The positions of the MVDD at risk breeds are indicated by circles on the tree. Red circle=breed with an average adult weight less than 9 kg, purple circle=average adult weight between 9 and 14 kg, blue circle= average adult weight over 14 kg. The haplotype tree of breeds was originally published in Nature45.
Figure 2.
Distribution of proportion membership in five primary breed clusters by breeds at risk for MMVD. 132 breeds were clustered into five populations using 96 microsatellite markers as described by Parker et al.44. Twenty breeds that have been designated at risk were extracted and their genomic proportion in each breed cluster plotted. The stars at the top of the graph indicate significant differences in the distributions: comparing the contribution of Hunting to Mastiff, Mountain, Herding, and Ancient : P= 1.37×10−5, 7.16×10−6, 3.41×10−6, and 4.56×10−10 respectively. The contribution of the Ancient group was also significantly lower than the Mountain and the Sighthound clusters with P=0.000655 and 0.003587 respectively. Breed groups are listed below the graph. The y-axis shows percent membership in each breed group.
If we look individually at the at-risk breeds that are small but not considered toy dogs (i.e. the dachshund, Boston terrier, cocker spaniel), they have all gone through reductions in size during some period of their development. The Boston terrier was originally bred from English bulldogs and bull terriers, breeds that average 23 kg or more as adults where as the Boston averages only 9 kg with many members weighing less than 7 kg when full grown11. This indicates not only selection for size but outcrossing with much smaller breeds such as the pug.
The cocker spaniel is the smallest of the hunting spaniels and in the late 1700s averaged only 5–7 kg as an adult, placing it within the size range of today’s toy breeds46. The average body size of dogs from this breed has increased since then to the current 11–14 kg. However, since the cocker was divided into two breeds, the English and American, the American underwent additional changes creating “cute” features that made it the most popular breed in the United States for 16 years11,47. These features include a rounded skull and large eyes similar to modern toy dogs, likely indicating outcrossing in addition to selection.
The dachshund has lost ~4.5 kg of adult body weight since its development, and has undergone the creation of a miniature version, likely through outcrossing with miniature pinschers48. Other breeds have clearly created smaller versions over time, such as the miniature and toy poodles and the miniature schnauzer. These observations imply that the small susceptible breeds may have experienced recent introgression that has allowed the sharing of detrimental mutations responsible for MMVD susceptibility.
Conclusions
MMVD is the most common heart disorder found in dogs and is particularly evident in the smallest of breeds. We have considered a variety of mechanisms to explain the overrepresentation of small and toy breeds in the list of those most at risk for MMVD. One possibility is that smaller dogs have a larger heart to body size ratio than do larger dogs. This could be tested by measuring the dimensions of the heart and the over-all body size, including chest volume, on dogs of many sizes to determine if there is a significant difference in the ratio in the small breeds. Another option would be to look for small dog breeds that are not at risk for MMVD. For instance, the Brussels griffon is not on any of the published lists of breeds at increased risk of MMVD despite having an average adult weight of only nine pounds. Do they get MMVD? And if not, is there any difference in their dimensions that would account for the lack of the disease? Yet another possibility is to examine MMVD in a breed that has members both above and below 9 kg, such as the Boston terrier. A correlation between physical dimensions and development of the disease within a breed would help determine if body size is a primary cause of MMVD.
Many genes that affect skeletal growth and development also affect cardiac development. In addition, genes can be found near the growth loci that are required for normal cardiac valve formation. Because all small breeds carry many of the same mutations that create small size they will also carry linked genes that could increase susceptibility to MMVD. Selective pressures on these genes will make it difficult to map them in the smallest breeds because they will probably be nearing fixation. However, because size is a quantitative trait, there should be mid- to large-size dogs that carry at least one small size mutation. If these size-altering mutations are causing MMVD then the disease should appear at some small rate in the mid-sized breeds that carry them. Such breeds may prove invaluable in the search for the causal variant.
Many of the at-risk breeds share common ancestry from an early small dog that has transmitted susceptibility genes to its descendants. As the source of MVDD risk, this transmission could be identified through admixture mapping of the at-risk breeds with a dense set of markers and a large dataset of breed haplotypes. It is formally possible that each breed has developed a unique mutation that has become prevalent due to restrictive breeding practices. However, given the high incidence of the disease combined with similarity among susceptible breeds, it is more likely that shared loci contribute to the disease. None of the mechanisms listed above are mutually exclusive, and it is likely that further investigation will reveal that a combination of these characteristics proves causative for MMVD.
A final trait that is shared between small dogs that might contribute to the development of MMVD is extended lifespan. It is often noted that small dogs live longer, on average, than large dogs49–51. MMVD is reported to be a disease of the aging heart, as described in detail by Connell et al. in this issue 52. This has led to the speculation that small dogs are diagnosed more often merely because they live long enough for the disease to progress. While age is doubtless a contributor, lifespan is unlikely to be the sole reason for the increased appearance of MMVD in small dogs. For instance, symptoms of MMVD may not present until after the age of 9 years, but evidence of valvular dysfunction can be detected prior to 4-years of age8,52. This is well within the lifespan of any dog breed regardless of size49,53. Consider also that studies of cellular aging suggest that large dogs age quicker than small dogs51,54. If cellular aging and valvular aging are comparable, then the 10 year old heart of a small dog would be similar to the 6 year old heart of a giant dog and the disease would be diagnosed at an earlier age in the larger dog54. Finally, MMVD is diagnosed in large dog breeds such as the German shepherd and the Great Dane, a breed often considered to have one of the shortest predicted lifespans of all breeds53,54. Therefore, it would seem that lifespan alone cannot account for the presence of disease. Unfortunately, the data does not exist to effectively answer the question of size versus age. This would require a comprehensive database of prophylactic heart exams conducted on dogs of all breed sizes over a course of years to determine the true prevalence of the disease in each. Data from such an undertaking would be invaluable as it could also address questions regarding ages of onset of the disease and rates of progression in different breeds.
MMVD has a high cost for dogs and people alike. The disease worsens with age and can lead to congestive heart failure and mortality. The combination of genetic and morphologic traits correlated with MMVD in dogs provides a promising tool for dissecting the elements of the disease. When applied to molecular studies of the disease, these correlations should enhance both basic and translational progress toward understanding the cause of MMVD and improving treatment. We await the results of MMVD studies in the coming years with much anticipation. Though we have proposed many possibilities within this review, only time and experimental evidence will reveal the means by which MMVD has arisen in small breeds.
Table 2.
Genes within loci linked to body size in the dog and their possible associations to heart development.
| Chr | Mba | Gene | Size GWASb |
Cardiac Involvement |
L.G.c Cardiac Involvement |
Ref.d |
|---|---|---|---|---|---|---|
| 4 | 42.3 | STC2 | 3 | NKX2–5 – valve formation defects | 41 | |
| 7 | 46.7 | SMAD2 | 2,3 | Valve remodeling and development | 30 | |
| 10 | 11.4 | HMGA2 | 2,3 | Cardiogenesis | 28 | |
| 15 | 37 | SOCS2 | 2 | Interacts with IGF1R, controls heart growth | 62 | |
| 15 | 44.2 | IGF1 | 1,2,3 | Controls heart growth | 63 | |
| 34 | 21.4 | IGF2BP2 | 2 | SENP2 – embryonic heart defect | 64 | |
| 37 | 29.7 | OBSL1 | 1 | CHPF - valve development | 65 |
Acknowledgements
We thank Dr. Holly Beale for careful reading of the manuscript during preparation and thoughtful suggestions. We acknowledge the support of the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health. Finally, we thank the many dog owners and breeders who have graciously offered their dog’s participation in support of our efforts.
Abbreviations
- CKCS
Cavalier King Charles spaniel
- GWAS
Genome-wide association study
- LD
Linkage disequilibrium
- MMVD
Myxomatous mitral valve disease
- MR
Mitral regurgitation
- MVP
Mitral valve prolapse
- PC
Principal components
- SNP
Single-nucleotide polymorphism
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Detweiler DK, Patterson DF. The prevalence and types of cardiovascular disease in dogs. Ann N Y Acad Sci. 1965;127:481–516. doi: 10.1111/j.1749-6632.1965.tb49421.x. [DOI] [PubMed] [Google Scholar]
- 2.Haggstrom J, Hoglund K, Borgarelli M. An update on treatment and prognostic indicators in canine myxomatous mitral valve disease. J Small Anim Pract. 2009;50 Suppl 1:25–33. doi: 10.1111/j.1748-5827.2009.00800.x. [DOI] [PubMed] [Google Scholar]
- 3.Borgarelli M, Haggstrom J. Canine degenerative myxomatous mitral valve disease: natural history, clinical presentation and therapy. Vet Clin North Am Small Anim Pract. 2010;40:651–663. doi: 10.1016/j.cvsm.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Swenson L, Haggstrom J, Kvart C, et al. Relationship between parental cardiac status in Cavalier King Charles spaniels and prevalence and severity of chronic valvular disease in offspring. J Am Vet Med Assoc. 1996;208:2009–2012. [PubMed] [Google Scholar]
- 5.Olsen LH, Fredholm M, Pedersen HD. Epidemiology and inheritance of mitral valve prolapse in Dachshunds. J Vet Intern Med. 1999;13:448–456. doi: 10.1892/0891-6640(1999)013<0448:eaiomv>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 6.Pedersen HD, Lorentzen KA, Kristensen BO. Echocardiographic mitral valve prolapse in cavalier King Charles spaniels: epidemiology and prognostic significance for regurgitation. Vet Rec. 1999;144:315–320. doi: 10.1136/vr.144.12.315. [DOI] [PubMed] [Google Scholar]
- 7.Madsen MB, Olsen LH, Haggstrom J, et al. Identification of 2 Loci associated with development of myxomatous mitral valve disease in cavalier king charles spaniels. J Hered. 2011;102 Suppl 1:S62–S67. doi: 10.1093/jhered/esr041. [DOI] [PubMed] [Google Scholar]
- 8.Serfass P, Chetboul V, Sampedrano CC, et al. Retrospective study of 942 small-sized dogs: Prevalence of left apical systolic heart murmur and left-sided heart failure, critical effects of breed and sex. J Vet Cardiol. 2006;8:11–18. doi: 10.1016/j.jvc.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Thrusfield MV, Aitken CG, darke PG. Observations on breed and sex in relation to canine heart valve incompetence. Journal of Small Animal Practice. 1985;26:709–717. [Google Scholar]
- 10.Fleming JM, Creevy KE, Promislow DE. Mortality in north american dogs from 1984 to 2004: an investigation into age-, size-, and breed-related causes of death. J Vet Intern Med. 2011;25:187–198. doi: 10.1111/j.1939-1676.2011.0695.x. [DOI] [PubMed] [Google Scholar]
- 11.American Kennel Club. The Complete Dog Book. 19th Edition Revised ed. New York, NY: Howell Book House; 1998. [Google Scholar]
- 12.Gertler MM, Garn SM, White PD. Young candidates for coronary heart disease. J Am Med Assoc. 1951;147:621–625. doi: 10.1001/jama.1951.03670240005002. [DOI] [PubMed] [Google Scholar]
- 13.Wannamethee SG, Shaper AG, Whincup PH, et al. Adult height, stroke, and coronary heart disease. Am J Epidemiol. 1998;148:1069–1076. doi: 10.1093/oxfordjournals.aje.a009584. [DOI] [PubMed] [Google Scholar]
- 14.Paajanen TA, Oksala NK, Kuukasjarvi P, et al. Short stature is associated with coronary heart disease: a systematic review of the literature and a meta-analysis. Eur Heart J. 2010;31:1802–1809. doi: 10.1093/eurheartj/ehq155. [DOI] [PubMed] [Google Scholar]
- 15.Schutte JE, Gaffney FA, Blend L, et al. Distinctive anthropometric characteristics of women with mitral valve prolapse. Am J Med. 1981;71:533–538. doi: 10.1016/0002-9343(81)90196-0. [DOI] [PubMed] [Google Scholar]
- 16.Raggi P, Callister TQ, Lippolis NJ, et al. Is mitral valve prolapse due to cardiac entrapment in the chest Cavity? A CT view. Chest. 2000;117:636–642. doi: 10.1378/chest.117.3.636. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Zhou J, Powell-Braxton L, et al. Effects of Igf1 Gene Deletion on Postnatal Growth Patterns. Endocrinology. 1999;140:3391–3394. doi: 10.1210/endo.140.7.7045. [DOI] [PubMed] [Google Scholar]
- 18.Sutter NB, Bustamante CD, Chase K, et al. A single IGF1 allele is a major determinant of small size in dogs. Science. 2007;316:112–115. doi: 10.1126/science.1137045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker J, Liu JP, Robertson EJ, et al. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- 20.Woods KA, Camacho-Hubner C, Savage MO, et al. Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N Engl J Med. 1996;335:1363–1367. doi: 10.1056/NEJM199610313351805. [DOI] [PubMed] [Google Scholar]
- 21.Wilcox B, Walkowicz C. Atlas of Dog Breeds of the World. 5th ed. Neptune City, NJ: T.F.H. Publications; 1995. [Google Scholar]
- 22.Chase K, Carrier DR, Adler FR, et al. Genetic basis for systems of skeletal quantitative traits: Principal component analysis of the canid skeleton. Proc Natl Acad Sci U S A. 2002;99:9930–9935. doi: 10.1073/pnas.152333099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donath MY, Zapf J, Eppenberger-Eberhardt M, et al. Insulin-like growth factor I stimulates myofibril development and decreases smooth muscle alpha-actin of adult cardiomyocytes. Proc Natl Acad Sci U S A. 1994;91:1686–1690. doi: 10.1073/pnas.91.5.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donath MY, Gosteli-Peter MA, Hauri C, et al. Insulin-like growth factor-I stimulates myofibrillar genes and modulates atrial natriuretic factor mRNA in rat heart. Eur J Endocrinol. 1997;137:309–315. doi: 10.1530/eje.0.1370309. [DOI] [PubMed] [Google Scholar]
- 25.McMullen JR, Shioi T, Huang W-Y, et al. The Insulin-like Growth Factor 1 Receptor Induces Physiological Heart Growth via the Phosphoinositide 3-Kinase(p110α) Pathway. Journal of Biological Chemistry. 2004;279:4782–4793. doi: 10.1074/jbc.M310405200. [DOI] [PubMed] [Google Scholar]
- 26.Lisa M, Haleagrahara N, Chakravarthi S. Insulin-Like Growth Factor-1 (IGF-1) Reduces ischemic changes and increases circulating angiogenic factors in experimentally - induced myocardial infarction in rats. Vasc Cell. 2011;3:13. doi: 10.1186/2045-824X-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones P, Chase K, Martin A, et al. Single-nucleotide-polymorphism-based association mapping of dog stereotypes. Genetics. 2008;179:1033–1044. doi: 10.1534/genetics.108.087866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monzen K, Ito Y, Naito AT, et al. A crucial role of a high mobility group protein HMGA2 in cardiogenesis. Nat Cell Biol. 2008;10:567–574. doi: 10.1038/ncb1719. [DOI] [PubMed] [Google Scholar]
- 29.Gittenberger-de Groot AC, Azhar M, Molin DG. Transforming growth factor beta-SMAD2 signaling and aortic arch development. Trends Cardiovasc Med. 2006;16:1–6. doi: 10.1016/j.tcm.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Azhar M, Brown K, Gard C, et al. Transforming growth factor Beta2 is required for valve remodeling during heart development. Dev Dyn. 2011 doi: 10.1002/dvdy.22702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng CM, Cheng A, Myers LA, et al. TGF-beta-dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. J Clin Invest. 2004;114:1586–1592. doi: 10.1172/JCI22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyko AR, Quignon P, Li L, et al. A simple genetic architecture underlies morphological variation in dogs. PLoS Biol. 2010;8:e1000451. doi: 10.1371/journal.pbio.1000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gagliardi AD, Kuo EY, Raulic S, et al. Human stanniocalcin-2 exhibits potent growth-suppressive properties in transgenic mice independently of growth hormone and IGFs. Am J Physiol Endocrinol Metab. 2005;288:E92–E105. doi: 10.1152/ajpendo.00268.2004. [DOI] [PubMed] [Google Scholar]
- 34.Ostrander EA, Kruglyak L. Unleashing the canine genome. Genome Res. 2000;10:1271–1274. doi: 10.1101/gr.155900. [DOI] [PubMed] [Google Scholar]
- 35.Parker HG, Shearin AL, Ostrander EA. Man's best friend becomes biology's best in show: genome analyses in the domestic dog. Annu Rev Genet. 2010;44:309–336. doi: 10.1146/annurev-genet-102808-115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sutter NB, Eberle MA, Parker HG, et al. Extensive and breed-specific linkage disequilibrium in Canis familiaris. Genome Res. 2004;14:2388–2396. doi: 10.1101/gr.3147604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindblad-Toh K, Wade CM, Mikkelsen TS, et al. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature. 2005;438:803–819. doi: 10.1038/nature04338. [DOI] [PubMed] [Google Scholar]
- 38.Gray MM, Granka JM, Bustamante CD, et al. Linkage disequilibrium and demographic history of wild and domestic canids. Genetics. 2009;181:1493–1505. doi: 10.1534/genetics.108.098830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parker HG, VonHoldt BM, Quignon P, et al. An expressed fgf4 retrogene is associated with breed-defining chondrodysplasia in domestic dogs. Science. 2009;325:995–998. doi: 10.1126/science.1173275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cadieu E, Neff M, Quignon P, et al. Coat Variation in the Domestic Dog Is Governed by Variants in Three Genes. Science. 2009;326:150–153. doi: 10.1126/science.1177808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joziasse I, van de Smagt J, Smith K, et al. Genes in congenital heart disease: atrioventricular valve formation. Basic Research in Cardiology. 2008;103:216–227. doi: 10.1007/s00395-008-0713-4. [DOI] [PubMed] [Google Scholar]
- 42.Schott JJ, Benson DW, Basson CT, et al. Congenital heart disease caused by mutations in the transcription factor NKX2-5. Science. 1998;281:108–111. doi: 10.1126/science.281.5373.108. [DOI] [PubMed] [Google Scholar]
- 43.Parker HG, Kim LV, Sutter NB, et al. Genetic structure of the purebred domestic dog. Science. 2004;304:1160–1164. doi: 10.1126/science.1097406. [DOI] [PubMed] [Google Scholar]
- 44.Parker HG, Kukekova AV, Akey DT, et al. Breed relationships facilitate fine-mapping studies: a 7.8-kb deletion cosegregates with Collie eye anomaly across multiple dog breeds. Genome Res. 2007;17:1562–1571. doi: 10.1101/gr.6772807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vonholdt BM, Pollinger JP, Lohmueller KE, et al. Genome-wide SNP and haplotype analyses reveal a rich history underlying dog domestication. Nature. 2010;464:898–902. doi: 10.1038/nature08837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grossman A. The American Cocker Spaniel. Sun City, AZ: Doral Publishing; 2000. [Google Scholar]
- 47.Fergus C. Gun Dog Breeds; A Guide to Spaniels, Retrievers, and Pointing Dogs. New York, NY: Lynn & Burtrand, Publishing; 1992. [Google Scholar]
- 48.Schwartz I. Dachshund. Allenhurst, NJ: Kennel Club Books; 2003. [Google Scholar]
- 49.Greer KA, Canterberry SC, Murphy KE. Statistical analysis regarding the effects of height and weight on life span of the domestic dog. Research in Veterinary Science. 2007;82:208–214. doi: 10.1016/j.rvsc.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 50.Galis F, Van der Sluijs I, Van Dooren TJ, et al. Do large dogs die young? J Exp Zool B Mol Dev Evol. 2007;308:119–126. doi: 10.1002/jez.b.21116. [DOI] [PubMed] [Google Scholar]
- 51.Li Y, Deeb B, Pendergrass W, et al. Cellular proliferative capacity and life span in small and large dogs. J Gerontol A Biol Sci Med Sci. 1996;51:B403–B408. doi: 10.1093/gerona/51a.6.b403. [DOI] [PubMed] [Google Scholar]
- 52.Connell PS, Han RI, Grande-Allen J. Differentiating the Aging of the Mitral Valve from Human and Canine Myxomatous Degeneration. Journal of Veterinary Cardiology. 2012 doi: 10.1016/j.jvc.2011.11.003. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Egenvall A, Bonnett BN, Hedhammar A, et al. Mortality in over 350,000 insured Swedish dogs from 1995–2000: II. Breed-specific age and survival patterns and relative risk for causes of death. Acta Vet Scand. 2005;46:121–136. doi: 10.1186/1751-0147-46-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patronek GJ, Waters DJ, Glickman LT. Comparative longevity of pet dogs and humans: implications for gerontology research. J Gerontol A Biol Sci Med Sci. 1997;52:B171–B178. doi: 10.1093/gerona/52a.3.b171. [DOI] [PubMed] [Google Scholar]
- 55.Aupperle H, März I, Thielebein J, et al. Immunohistochemical characterization of the extracellular matrix in normal mitral valves and in chronic valve disease (endocardiosis) in dogs. Research in Veterinary Science. 2009;87:277–283. doi: 10.1016/j.rvsc.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 56.Buchanan JW. Prevalence of cardiovascular disorders. In: Fox P, Sisson D, Moise NS, editors. Textbook of canine and feline cardiology Principles and clinical practice. 2nd ed. Philadelpia: WB Saunders; 1999. pp. 457–470. [Google Scholar]
- 57.Egenvall A, Bonnett BN, Haggstrom J. Heart disease as a cause of death in insured Swedish dogs younger than 10 years of age. J Vet Intern Med. 2006;20:894–903. doi: 10.1892/0891-6640(2006)20[894:hdaaco]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 58.Ingelheim B. Quest trial: Resources for Owners, 2008; Information regarding a clinical trial for MMVD in dogs [Google Scholar]
- 59.Haggstrom J, Boswood A, O'Grady M, et al. Effect of pimobendan or benazepril hydrochloride on survival times in dogs with congestive heart failure caused by naturally occurring myxomatous mitral valve disease: the QUEST study. J Vet Intern Med. 2008;22:1124–1135. doi: 10.1111/j.1939-1676.2008.0150.x. [DOI] [PubMed] [Google Scholar]
- 60.Sargan D. IDID: inherited diseases in dogs: web-based information for canine inherited disease genetics. Mamm Genome. 2004;15:503–506. doi: 10.1007/s00335-004-3047-z. [DOI] [PubMed] [Google Scholar]
- 61.Crook A, Hill B, Dawson S. Canine Inherited Disorders Database. 1998 [PubMed] [Google Scholar]
- 62.Dey BR, Spence SL, Nissley P, et al. Interaction of human suppressor of cytokine signaling (SOCS)-2 with the insulin-like growth factor-I receptor. J Biol Chem. 1998;273:24095–24101. doi: 10.1074/jbc.273.37.24095. [DOI] [PubMed] [Google Scholar]
- 63.McMullen JR, Izumo S. Role of the insulin-like growth factor 1 (IGF1)/phosphoinositide-3-kinase (PI3K) pathway mediating physiological cardiac hypertrophy. Novartis Found Symp. 2006;274:90–111. discussion 111-117, 152-115, 272-116. [PubMed] [Google Scholar]
- 64.Kang X, Qi Y, Zuo Y, et al. SUMO-Specific Protease 2 Is Essential for Suppression of Polycomb Group Protein-Mediated Gene Silencing during Embryonic Development. Molecular Cell. 2010;38:191–201. doi: 10.1016/j.molcel.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peal DS, Burns CG, Macrae CA, et al. Chondroitin sulfate expression is required for cardiac atrioventricular canal formation. Developmental Dynamics. 2009;238:3103–3110. doi: 10.1002/dvdy.22154. [DOI] [PMC free article] [PubMed] [Google Scholar]