Abstract
Background
No prior reports documenting the safety and diagnostic yield of cardiac catheterization and endomyocardial biopsy (EMB) in heart transplant recipients include multicenter data.
Methods
Data on the safety and diagnostic yield of EMB procedures performed in heart transplant recipients were recorded in the Congenital Cardiac Catheterization Outcomes Project database at 8 pediatric centers over a 3 year period. Adverse events (AE) were classified according to a 5 level severity scale. Generalized estimating equation models identified risk factors for high severity adverse events (HSAE) (Levels 3-5) and non-diagnostic biopsy samples.
Results
A total of 2665 EMB cases were performed in 744 pediatric heart transplant recipients (median age 12 years [IQR: 4.8,16.7] and 54% male). AE occurred in 88 cases (3.3%), of which 28 (1.1%) were HSAE. AE attributable to EMB included tricuspid valve injury, transient complete heart block, and RBBB. Amongst 822 cases involving coronary angiography, 10 (1.2%) resulted in a coronary related AE. There were no myocardial perforations or deaths. Multivariable risk factors for HSAE included fewer prior catheterizations (p=0.006) and longer case length (p=<0.001). EMB yielded sufficient tissue for diagnosis in 99% of cases. Longer time since heart transplant was the most significant predictor of a non-diagnostic biopsy sample (p<0.001).
Conclusions
In the current era, cardiac catheterizations involving EMB can be performed in pediatric heart transplant recipients with a low AE rate and high diagnostic yield. Risk of HSAE is increased in early post-transplant biopsies and with longer case length. Longer time since heart transplant is associated with non-diagnostic EMB sample.
INTRODUCTION
Endomyocardial biopsy (EMB) remains the gold standard test for detection of acute cellular rejection (ACR) and antibody mediated rejection (AMR) in heart transplant recipients.(1, 2) As a result, surveillance for allograft rejection remains the most common indication for EMB in children.(3, 4) In addition, selective coronary angiography is an important tool for monitoring of coronary allograft vasculopathy (CAV).(5, 6)
Several single center case series have reported EMB high severity adverse event (HSAE) rates between 1 and 2%.(4, 7, 8) These reports are limited by the retrospective nature of the data collection, the long data collection periods (11 and 16 years), and the overrepresentation of patients with multiple biopsy cases. While studies of selective coronary angiography in children also suggest a favorable safety profile, all of these reports are limited to small, retrospective, single center studies.(9-11) The Congenital Cardiac Catheterization Outcomes Project (C3PO) database is the first prospectively collected database of selected patient characteristics, procedural characteristics, and AE data from 8 large pediatric cardiology centers. This database provided the opportunity to examine AE associated with catheterization procedures involving EMB in heart transplant recipients in the current era. Additional variables were added to the database in 2009 to assess rates of technical success and identify factors associated with insufficient biopsy samples. There are no previous pediatric or adult studies which comprehensively address the technical success rates of EMB.
METHODS
Population
The C3PO database is a multi-institutional database of patient and procedural characteristics collected at the time of all cardiac catheterizations performed at 8 pediatric cardiology centers (10). After IRB approval was obtained, data collection commenced in February 2007 at 6 centers, in April 2008 at one center, and in June 2009 at another. Based on the availability of prospectively collected data, the inclusion criteria for two overlapping populations were defined as follows:
To assess procedural safety, all consecutive cases collected in the C3PO database between February 1, 2007 and February 28, 2010 with an intervention code for a post transplant RV biopsy were analyzed as part of the safety cohort.
To assess technical success of EMB, all consecutive cases collected in the C3PO database between April 1, 2009 and February 28, 2010 with an intervention code for a post transplant RV biopsy were analyzed as part of the diagnostic yield cohort.
All procedures containing any code for endomyocardial biopsy were cross referenced with the physiologic diagnosis to ensure that all post-transplant biopsies were captured.
Exclusion Criteria
EMB cases performed at one of the original six centers prior to March 1, 2009 were excluded from the safety analysis as EMB cases were not reported to the C3PO database prior to that time.
Collected Data
Variables collected in the C3PO database since February 1, 2007 included: patient characteristics (age, weight, sex, diagnosis, time since transplant, number of prior catheterizations), case data (admission status, case type, type of anesthesia, corrected case length defined from sheath entry to sheath removal minus time spent addressing AE, fluoroscopy time, vascular access), and hemodynamic parameters (mixed venous saturation, cardiac index, left ventricular end diastolic pressure, inotropic support, use of extracorporeal membrane oxygenation (ECMO)).
In an effort to determine technical success rates of EMB cases in children and assess ability of the procedure to provide useful, diagnostic information, the following procedural characteristics were prospectively collected in the C3PO database beginning on April 1, 2009: Size (in Fr) and type of biopsy forceps used, number of biopsy attempts, number and adequacy of specimens obtained, and operator obtaining the biopsy (fellow, attending, or fellow and attending). The determination of whether or not a biopsy specimen was diagnostic was made at each institution in response to the prompt, “Result interpretable?” and entered into the C3PO database as a yes/no variable. The study plan defined a biopsy specimen as non-diagnostic if the pathologist was not able to grade the level of rejection or commented that non-diagnostic samples were obtained. Pathology practices at all of the participating centers were surveyed which confirmed that all centers require three evaluable pieces of myocardium in order to consider a EMB sample diagnostic of non-rejection, as suggested by the 2004 revision of the ISHLT guidelines.(2)
Adverse Event Data
AE data were collected at the time of the procedure and updated to include any late AE that were identified by the operating physician following the case, as previously described.(12) AE were defined as any anticipated or unanticipated event for which avoidable injury could have occurred, or did occur, potentially or definitely as a consequence of performing the catheterization case.(12) AE were classified according to severity and attributability (i.e. EMB, access, general catheterization, etc.) and reviewed by a minimum of two interventional cardiologists. Coronary angiography was not part of the original classification schema, therefore the AE description was used to identify events attributable to coronary angiography. Event severity was defined on a five point ordinal scale and further classified into low severity events (Levels 1 & 2) and high severity events (Levels 3, 4, & 5). Event severity levels are provided with examples in Table 1.(3, 12)
Table 1.
Adverse event severity level definitions with examples of adverse events which occurred during endomyocardial biopsy procedures.
| Severity Level | Description | Example |
|---|---|---|
| Level 1 – None | No harm, no change in condition, may have required monitoring to assess for potential change in condition with no intervention indicated. | Transient bradycardia during biopsy sampling. |
| Level 2 – Minor | Transient change in condition, not life threatening, condition returns to baseline, required monitoring, required minor intervention such as holding a medication or obtaining a laboratory test. | Transient ST-T wave changes after coronary angiography requiring further monitoring. |
| Level 3 – Moderate | Transient change in condition may be life threatening if not treated, condition returns to baseline, required monitoring, required intervention such as reversal agent, additional medication, transfer to the intensive care unit for monitoring, or moderate transcatheter intervention to correct condition. | Damage to the tricuspid valve apparatus that required unexpected hospitalization for observation. |
| Level 4 – Major | Change in condition, life threatening if not treated, change in condition may be permanent, may have required an intensive care unit admission or emergent readmission to hospital, may have required invasive monitoring, required interventions such as electrical cardioversion or unanticipated intubation, or required major invasive cases or transcatheter interventions to correct condition. | Myocardial perforation requiring emergent pericardiocentesis. |
| Level 5 – Catastrophic | Any death and emergent surgery or extracorporeal membrane oxygenation (ECMO) to prevent death with failure to wean from bypass support. | A patient who developed hemodynamic instability, was placed on ECMO and subsequently died. |
Primary Outcome Variables
The presence of any HSAE (Level 3, 4, or 5) was selected as the primary outcome variable for the safety cohort. Audits of the C3PO database have shown excellent case capture for these clinically relevant events.(12) Non-diagnostic EMB specimen was defined as the primary outcome variable for the diagnostic yield cohort.
Statistical Methods
Categorical variables are summarized with frequency and percentage. Continuous variables are displayed as mean ± standard deviation or median [interquartile range] depending upon the normality of the distribution. The impact of predictor variables on the primary outcome variables (HSAE and non-diagnostic EMB) was evaluated using generalized estimating equations (GEE) models. Although case characteristics were felt to carry an independent risk for an adverse event, patient characteristics for two procedures performed in the same subject could not be considered independent. GEE models allowed us to account for the non-independence of the patient specific characteristics. Multivariable modeling was performed for HSAE using forward stepwise selection; predictors were retained in the model if they remained statistically significant at the 0.05 level. Odds ratios and 95% confidence intervals are provided. Multivariable modeling was not performed for non-diagnostic EMB as the event rate was too low to support such a model. No corrections were made for multiple comparisons. Statistical analysis was performed using SAS (Cary, NC) statistical software.
RESULTS
Patient & Procedural Characteristics
Overall 2665 cardiac catheterizations involving an EMB were performed in 744 heart transplant recipients at 8 centers over 3 years. Patient characteristics and AE rates are summarized in Tables 2 and 3. The median age was 12.6 years old [IQR: 4.8, 16.7] with a median time since heart transplant of 1.5 years [0.3, 4.9]. Patients had undergone a median of 6 prior cardiac catheterization procedures and cases were elective 93% of the time. An additional catheter based intervention (CBI) was performed in 2.3% (n=63) of the cases. The additional interventions included angioplasty/stenting of a systemic vein (n=24), angioplasty/stenting of another vessel (n=16), atrial septal intervention (n=7), elective pericardiocentesis (n=6), elective pleurocentesis (n=4), RF ablation (n=3), and PFO closure (n=1). The hemodynamic profile of the transplant cohort fell within normal ranges for cardiac index, mixed venous saturation, and LVEDP or mean PCWp. It was rare for transplant patients to require inotropic support (n=70; 2.6%) or ECMO (n=11; 0.4%). Half of the EMB cases were performed with patients breathing spontaneously and mechanical ventilation was used in the other half. Amongst the subset of cases where diagnostic yield data were collected, the median number of biopsy attempts was 5 [4,7], and the median number of pieces obtained was also 5 [4,6]. Ninety-nine percent of the biopsy specimens were considered diagnostic by the reporting centers.
Table 2.
Patient and case characteristics. N (%) is provided for categorical variables and mean ± s.d. or median [IQR] is provided for continuous variables.
| Patient or Case Characteristic (n=2665) | |
|---|---|
| Patient Characteristic | |
| Patient Age | |
| < 1 year | 138 (5) |
| 1-9 years | 966 (36) |
| ≥ 10 years | 1561 (59) |
| Patient Weight | |
| < 4 kg | 22 (1) |
| 4-9 kg | 251 (9) |
| 10-19 kg | 563 (21) |
| ≥ 20 kg | 1829 (69) |
| Patient Sex: Male | 1447 (54) |
| Number of prior caths | 6 [0, 14] |
| Time since heart transplant (years) | 1.5 [0.3, 4.9] |
| Case Characteristic | |
| Admission Status: Elective | 2475 (93) |
| Case Type | |
| EMB | 1843 (69) |
| EMB plus coronary angiography | 822 (31) |
| Hemodynamic Parameters | |
| Cardiac index (L/min/m2) | 3.7 ± 1.2 |
| Mixed venous saturation (%) | 71 ± 7 |
| LVEDP or mean PCWp (mmHg) | 11 ± 4 |
| On inotropic support | 70 (2.6) |
| On ECMO | 11 (0.4) |
| Spontaneous respiration | 1354 (51) |
| Corrected Case Length (minutes) | 39 ± 29 |
| Fluoroscopy Time (minutes) | 10 ± 8 |
| Access | |
| Right internal jugular vein | 1265 (47) |
| Left internal jugular vein | 85 (3.2) |
| Right femoral vein | 928 (35) |
| Left femoral vein | 271 (10) |
| Right subclavian vein | 113 (4.2) |
| Left subclavian vein | 40 (1.5) |
| Other access site | 11 (0.4) |
| Right femoral artery | 741 (28) |
| Left femoral artery | 143 (5) |
| Number of biopsy attempts (n=1122) | 5 [4, 7] |
| Number of pieces obtained (n=1123) | 5 [4, 6] |
EMB, Endomyocardial biopsy; LVEDP, Left ventricular end-diastolic pressure; PCWp, Pulmonary capillary wedge pressure; ECMO, Extracorporeal membrane oxygenation;
Table 3.
A total of 94 AE occurred in 88 of 2665 EMB cases. Event characteristics are summarized below.
| Adverse Event Characteristic | All Levels n (%) | Severity Level (n) | ||||
|---|---|---|---|---|---|---|
| Level 1 (n=11) | Level 2 (n=53) | Level 3 (n=19) | Level 4 (n=11) | Level 5 (n=0) | ||
| Biopsy Related | 18 (19) | 5 | 6 | 6 | 1 | 0 |
| Atrial Arrhythmia | 9 (10) | 1 | 3 | 5 | — | — |
| Coronary Fistula | 0 (0) | — | — | — | — | — |
| Complete Heart Block, Transient | 2 (2) | — | — | 1 | 1 | — |
| RBBB/IVCD, New | 3 (3) | 3 | — | — | — | — |
| Tricuspid Valve Damage | 3 (3) | — | 3 | — | — | — |
| Myocardial Perforation | 0 (0) | — | — | — | — | — |
| Ventricular Arrhythmia | 1 (1) | 1 | — | — | — | — |
| Coronary Angiography Related | 10 (11) | 1 | 6 | 0 | 3 | 0 |
| Air Embolus | 3 (3) | 1 | 2 | — | — | — |
| Arrhythmia, Atrial or Ventricular | 2 (2) | — | 1 | — | 1 | — |
| Coronary Dissection | 1 (1) | — | — | — | 1 | — |
| ST Segment Changes/ Coronary Vasospasm | 4 (4) | — | 3 | — | 1 | — |
| Sedation or Airway Related | 19 (20) | 0 | 11 | 2 | 6 | 0 |
| Airway obstruction | 1 (1) | — | — | 1 | — | — |
| Anesthesia Problem | 2 (2) | — | 2 | — | — | — |
| Esophageal Hematoma | 1 (1) | — | 1 | — | — | — |
| Hypotension | 7 (7) | — | 5 | — | 2 | — |
| Hypoxia | 2 (2) | — | — | 1 | 1 | — |
| Lobar Collapse | 1 (1) | — | 1 | — | — | — |
| Respiratory Acidosis, pCO2 > 45 mmHg | 1 (1) | — | 1 | — | — | — |
| Respiratory Distress | 4 (4) | — | 1 | — | 3 | — |
| General Catheterization Related | 25 (27) | 2 | 17 | 5 | 1 | 0 |
| Allergic Reaction | 1 (1) | — | 1 | — | — | — |
| Atrial Arrhythmia | 6 (6) | — | 5 | 1 | — | — |
| Bradycardia, Sinus | 2 (2) | 1 | 1 | — | — | — |
| Cerebrovascular Accident, Embolic | 1 (1) | — | — | — | 1 | — |
| Equipment Problem, Broken Catheter or Wire | 2 (2) | — | 2 | — | — | — |
| Fever | 2 (2) | — | 1 | 1 | — | — |
| Heart Block, Resolved | 2 (2) | — | 2 | — | — | — |
| Hypoxia | 1 (1) | — | 1 | — | — | — |
| Imaging equipment problem | 3 (3) | 1 | 2 | — | — | — |
| Medication Error | 1 (1) | — | 1 | — | — | — |
| Peripheral Site Infection | 1 (1) | — | 1 | — | — | — |
| Seizure | 1 (1) | — | — | 1 | — | — |
| Ventricular Arrhythmia | 1 (1) | — | — | 1 | — | — |
| Vessel Trauma | 1 (1) | — | — | 1 | — | — |
| Access Related | 21 (22) | 2 | 13 | 6 | 0 | 0 |
| Bleeding after line removal | 1 (1) | — | 1 | — | — | — |
| Hemothorax | 1 (1) | — | — | 1 | — | — |
| Inadvertent arterial puncture | 1 (1) | — | 1 | — | — | — |
| Inadvertent sheath removal | 1 (1) | 1 | — | — | — | — |
| Local hematoma, Groin | 2 (2) | — | 1 | 1 | — | — |
| Local hematoma, Neck | 2 (2) | 1 | 1 | — | — | — |
| Pain, Right Lower Extremity | 1 (1) | — | 1 | — | — | — |
| Pneumothorax | 4 (4) | — | 1 | 3 | — | — |
| Rebleed after bandages applied | 6 (6) | — | 6 | — | — | — |
| Sheath placed outside vessel | 1 (1) | — | 1 | — | — | — |
| Systemic Arterial Thrombosis | 1 (1) | — | — | 1 | — | — |
| Angioplasty Related | 1 (1) | 1 | 0 | 0 | 0 | 0 |
| Balloon rupture with air embolus | 1 (1) | 1 | — | — | — | — |
AE, Adverse events; EMB, Endomyocardial biopsy; RBBB, Right bundle branch block; IVCD, Interventricular conduction delay;
AE Description for Post-Transplant Cardiac Catheterization
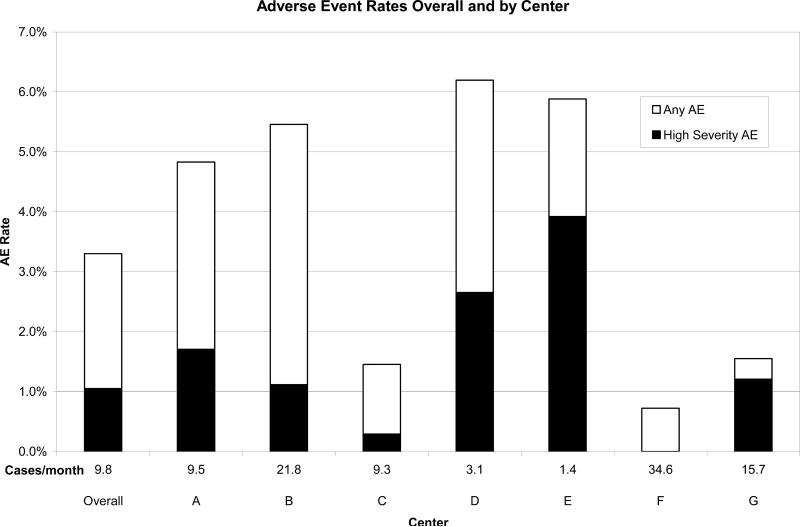
Ninety-four AE occurred during or following 88 (3.3%) catheterization cases. Multiple AE occurred during 6 cases. HSAE occurred during 1.1% of cases with center specific rates varying between 0% and 3.9% (Figure 1). There were a total of 11 Level 1 AE and 53 Level 2 AE recorded during the study period (Table 3). Level 1 events included air injected into the pulmonary circulation, transient bradycardia during biopsy sampling, and inadvertent loss of venous access during a sheath exchange. Level 2 events included coronary air embolus that did not require intervention, spontaneously resolving heart block, hematoma at the cannulation site, and arrhythmias which were not hemodynamically significant and resolved spontaneously. There were a total of 21 Level 3 AE which included complications such as thrombus on the tricuspid valve, coronary artery vasospasm requiring intra-arterial nitroglycerin administration, hypotension requiring fluid resuscitation, and transient heart block treated with temporary intracardiac pacing. Major AE (Level 4) occurred in 10 EMB cases and included events such as left main coronary artery dissection requiring surgical intervention (n=1) and all events requiring chest compressions (n=3) and mechanical support (n=3). All three patients requiring emergent mechanical support were non-elective cases added on due to clinical urgency. Two were supported with ECMO and the other required an intraaortic balloon pump. All survived after weaning from mechanical support.
Figure 1.
Rates of any AE and HSAE by center. Center volume (Cases per month) is noted above each center identifier. Data from center H are not displayed because of the low case volume.
Among these 94 events, a total of 18 AE (0.7% of cases) were classified as being biopsy related (Table 3). There were 3 cases of tricuspid valve damage without significant change in degree of regurgitation or need for surgical intervention. There were two patients with complete heart block which was treated with temporary pacing in one case and chest compressions in the other. Both patients had AV nodal recovery within minutes without sequelae. There were seven cases of supraventricular tachycardia noted during EMB, two cases of sinus bradycardia, and one case of non-sustained ventricular tachycardia. All of these arrhythmias resolved either spontaneously or with acute treatment in the catheterization laboratory. There were three cases of isolated QRS widening with a right bundle branch block pattern noted during biopsy which did not require treatment.
In 10 of 822 cases (1.2%) involving coronary angiography, an AE directly related to coronary angiography occurred. These included coronary air embolus(n=3), coronary vasospasm or ST segment changes (n=4), sinus bradycardia (n=1), and non-sustained VT (n=1). All of these events resolved within minutes without long term sequelae. There was one case of left main coronary dissection following angiography of normal coronaries during an elective outpatient procedure. The dissection was immediately recognized and was successfully repaired with a coronary artery bypass graft. No percutaneous coronary intervention procedures were performed during any cases involving EMB. Two low severity AE (air embolus due to balloon rupture and esophageal hematoma caused by a transesophageal echocardiogram performed during patent foramen ovale closure) and no HSAE were attributable to performance of other CBI.
Patient and Procedural Risk Factors for HSAE
In univariate analysis, HSAE were more likely among patients who had undergone fewer prior catheterizations and in cases that were non-elective, performed with mechanical ventilation, involving coronary angiography, and with longer case length (Table 4). In multivariable modeling, a history of fewer prior catheterization procedures (OR 1.1 for each 1 procedure decrease; p=0.006) and longer case length were associated with HSAE (OR 1.2 for each additional 10 minutes; p<0.001). In addition, there was an inverse relationship between the center specific rate of HSAE and average monthly case volume by linear regression analysis (R2=0.58; p=0.045).
Table 4. Patient and procedural risk factors for high severity adverse events during EMB procedures in heart transplant recipients.
N (%) is provided for categorical variables, median [interquartile range] is provided for ordinal/continuous variables, and mean ± s.d. is provided for normally distributed continuous variables. OR expresses risk of a HSAE calculated using generalized estimating equations (GEE) models.
| Demographic / Clinical Predictors | High Severity AE | Univariate | Multivariable Analysis | |||
|---|---|---|---|---|---|---|
| Any HSAE (n=28) | No HSAE (n=2634) | Odds Ratio (95% CI) | P value | Odds Ratio | P value | |
| Age | ||||||
| < 1 year | 3 (11) | 135 (5) | 2.2 (0.6, 8.3) | 0.21 | ||
| 1-9 years | 10 (36) | 956 (36) | 1.1 (0.5, 2.5) | 0.86 | ||
| ≥ 10 years | 15 (54) | 1546 (59) | 1.0 | — | ||
| Weight | ||||||
| < 10 kg | 4 (14) | 269 (10) | 1.5 (0.5, 4.3) | 0.49 | ||
| ≥ 10 kg | 24 (86) | 2368 (90) | 1.0 | — | ||
| Admission Status | ||||||
| Elective | 22 (79) | 2453 (93) | 1.0 | — | ||
| Urgent or Emergent | 6 (21) | 184 (7) | 3.6 (1.4, 9.3) | 0.007 | ||
| No. of prior caths; OR for each 1 unit decrease in No. of prior caths | 3 [0, 7] | 6 [0, 14] | 1.1 (1.0, 1.1) | 0.009 | 1.1 (1.0, 1.2) | 0.006 |
| Time since heart transplant (years); OR for each additional year since transplant | 3 [1, 5] | 2 [0, 5] | 1.0 (0.9, 1.2) | 0.49 | ||
| Case Type | ||||||
| EMB | 11 (39) | 1832 (69) | 1.0 | — | ||
| EMB plus coronary angiography | 17 (61) | 805 (31) | 3.5 (1.7, 7.3) | <0.001 | ||
| Airway & Sedation Management | ||||||
| Spontaneous respiration | 7 (25) | 1347 (51) | 1.0 | — | ||
| Other mechanical support with anesthesia | 21 (75) | 1290 (49) | 3.1 (1.3, 7.6) | 0.01 | ||
| Corrected Case Length (minutes); OR for each additional 10 minutes | 52 [36, 92] | 29 [20, 51] | 1.2 (1.1, 1.3) | <0.001 | 1.2 (1.1, 1.3) | <0.001 |
| Hemodynamic Parameters | ||||||
| Cardiac index (L/min/m2); OR for each 0.5 increase | 3.6 ± 1.4 | 3.7 ± 1.2 | 1.0 (0.8, 1.3) | 0.81 | ||
| Mixed venous saturation (%); OR for each 5% decrease | 70 ± 11 | 71 ± 7 | 1.1 (0.8, 1.6) | 0.45 | ||
| LVEDP or mean PCWp (mmHg) (n=1081); OR for each 2 mmHg decrease | 11 ± 3 | 11 ± 4 | 1.0 (0.9, 1.2) | 0.89 | ||
| On inotropic support? (%) | 2 (7) | 68 (3) | 2.9 (0.7, 12.9) | 0.16 | ||
HSAE, High severity adverse event; EMB, Endomyocardial biopsy; LVEDP, Left ventricular end-diastolic pressure; PCWp, Pulmonary capillary wedge pressure;
Procedural Success and Technical Characteristics
Patient and case characteristics for the 1138 biopsy cases used to assess technical procedural success were not significantly different from the cases included in the combined safety cohort. The majority of cases (98.7%) yielded sufficient endomyocardial tissue for pathologic interpretation, with only 15 cases resulting in a non-diagnostic biopsy specimen in 14 different patients (Table 5). A 6 Fr bioptome was used 48% of the time, while 5 Fr and 7 Fr bioptomes were used less frequently (35% and 16% respectively). Smaller bioptomes were used infrequently. The most commonly used bioptome was the Jawz™ (Argon Medical, Athens, TX) forceps in 40% of cases, while the Sparrow Hawk® (ATC Technologies, Woburn, MA) and Procure™ (St. Jude Medical, St. Paul, MN) bioptomes were used in 28% and 20% of cases respectively.
Table 5. Patient and Procedural Risk Factors for a Non-Diagnostic EMB Sample in Heart Transplant Recipients.
N (%) is provided for categorical variables, median [interquartile range] is provided for ordinal/continuous variables, and mean ± s.d. is provided for normally distributed continuous variables. Odds ratio expresses risk of obtaining a non-diagnostic sample calculated using generalized estimating equations (GEE) models.
| Demographic / Clinical Predictors | EMB Sample | Univariate Analysis | ||
|---|---|---|---|---|
| Non-Diagnostic Sample (n=15) | Diagnostic Sample (n=1123) | Odds Ratio | P value | |
| Age | ||||
| < 1 year | 0 (0) | 40 (4) | — | — |
| 1-9 years | 0 (0) | 386 (34) | — | — |
| ≥ 10 years | 15 (100) | 697 (62) | — | — |
| Weight | ||||
| < 10 kg | 0 (0) | 86 (8) | — | — |
| ≥ 10 kg | 15 (100) | 1037 (92) | — | — |
| Time since heart transplant (years); OR for each additional year since transplant | 8.6 [3.3, 10.6] | 1.6 [0.4, 4.5] | 1.2 (1.1, 1.2) | <0.001 |
| Admission Status | ||||
| Elective | 14 (93) | 1082 (96) | 1.0 | — |
| Urgent or Emergent | 1 (7) | 41 (4) | 1.9 (0.2, 14.2) | 0.54 |
| Airway & Sedation Management | ||||
| Spontaneous respiration | 11 (73) | 544 (48) | 1.0 | — |
| Other mechanical support with anesthesia | 4 (27) | 579 (52) | 0.3 (0.1, 1.1) | 0.08 |
| Hemodynamic Parameters | ||||
| Cardiac index (L/min/m2); OR for each 1 L/min/m2 decrease in CI | 3.0 ± 0.7 | 3.6 ± 1.1 | 2.2 (1.1, 4.3) | 0.02 |
| Access (n=13, 1095) | ||||
| Right or left femoral vein | 4 (31) | 471 (43) | 1.0 | — |
| Right or left internal jugular vein | 8 (61) | 586 (54) | 1.6 (0.5, 5.7) | 0.46 |
| Right or left subclavian vein | 1 (8) | 38 (3) | 3.1 (0.3, 29.8) | 0.33 |
| Case performed by | ||||
| Attending | 6 (40) | 752 (67) | 1.0 | — |
| Attending and fellow | 1 (7) | 67 (6) | 1.9 (0.2, 16.6) | 0.57 |
| Fellow | 8 (53) | 304 (27) | 3.3 (1.0, 10.5) | 0.04 |
| Type of bioptome | ||||
| Sparrow Hawk | 11 (73) | 312 (28) | 7.1 (1.9, 27.3) | 0.004 |
| Other | 4 (27) | 811 (72) | 1.0 | — |
| Size of bioptome | ||||
| 3, 4, or 5 Fr | 10 (67) | 398 (36) | 3.6 (1.1, 12.2) | 0.04 |
| 6 or 7 Fr | 5 (33) | 723 (64) | 1.0 | — |
| Number of biopsy attempts; OR for each additional attempt | 7 [6, 9] | 5 [4, 6] | 1.3 (1.2, 1.5) | <0.001 |
| Number of pieces obtained | 6 [5, 6] | 5 [4, 5] | 1.0 (1.0, 1.1) | 0.07 |
| Center performing the Biopsy | ||||
| Center B | 10 (67) | 193 (17) | 9.6 (2.9, 32.5) | <0.001 |
| All others | 5 (33) | 930 (83) | 1.0 | — |
EMB, Endomyocardial biopsy; CI, Cardiac Index; Fr, French;
Patient and Procedural Risk Factors for Non-Diagnostic Biopsy Specimens
In univariate analysis, patient specific risk factors for a non-diagnostic EMB specimen included longer time since heart transplantation, age greater than 10 years and lower cardiac index (Table 5). Procedure specific risk factors included more attempts at EMB, smaller bioptome size, use of a Sparrow Hawk® bioptome, and cases performed by cardiology fellows without attending assistance. A sub-analysis of the center with the largest number of non-diagnostic EMB specimens found longer time since heart transplantation, older age, and lower cardiac index were risk factors for a non-diagnostic EMB (data not shown).
Institutional Variation in Performance of Endomyocardial Biopsy
There are important variations in how endomyocardial biopsy is performed at different institutions. The busiest center performed 30% of the recorded biopsy cases while the three smallest centers each performed less than 5% of the biopsy cases (Table 6). The choice of anesthetic technique was largely related to the preference of the center performing the EMB with 2 centers performing > 70% of their cases under spontaneous respiration, three centers preferring general anesthesia in > 70% of cases, and 2 centers being evenly split. The site of venous vascular access was also largely center dependent. Although right internal jugular venous access was used in nearly half of the cases overall (47%), several centers (D and G) preferred neck access with combined right and left internal jugular access rates of 82% and 98% respectively. Even when femoral arterial access is necessary, some centers still prefer venous access from the neck.
Table 6. Post-Transplant EMB Case Characteristics by Center (n=2661).
N (%) is provided for categorical variables, median [interquartile range] is provided for ordinal/continuous variables, and mean ± s.d. is provided for normally distributed continuous variables. Center H performed 4 cases and was not included in the table.
| Patient and Case Characteristic | Center A | Center B | Center C | Center D | Center E | Center F | Center G |
|---|---|---|---|---|---|---|---|
| Number of cases | 352 | 806 | 344 | 113 | 51 | 415 | 580 |
| Average number of cases per month | 9.5 | 21.8 | 9.3 | 3.1 | 1.4 | 34.6 | 15.7 |
| Number of Patients | 153 | 131 | 82 | 39 | 19 | 160 | 157 |
| Admission status | |||||||
| Elective | 299 (85) | 707 (88) | 340 (99) | 110 (97) | 42 (82) | 411 (99) | 562 (97) |
| Urgent & Emergent | 53 (15) | 99 (12) | 4 (1) | 3 (3) | 9 (18) | 4 (1) | 18 (3) |
| Case Type | |||||||
| EMB | 224 (64) | 545 (68) | 227 (66) | 51 (45) | 25 (49) | 322 (78) | 445 (77) |
| EMB plus coronary angiography | 128 (36) | 261 (32) | 117 (34) | 62 (55) | 26 (51) | 93 (22) | 135 (23) |
| Airway & Sedation Management | |||||||
| Spontaneous respiration | 255 (72) | 483 (60) | 276 (80) | 23 (20) | 0 (0) | 252 (56) | 81 (14) |
| Access | |||||||
| Right or left femoral artery | 135 (38) | 269 (33) | 128 (37) | 77 (68) | 30 (59) | 102 (25) | 138 (24) |
| Right femoral vein | 171 (49) | 304 (38) | 227 (66) | 20 (18) | 29 (57) | 167 (40) | 8 (1) |
| Left femoral vein | 71 (20) | 83 (10) | 52 (15) | 8 (7) | 7 (14) | 38 (9) | 12 (2) |
| Right internal jugular vein | 36 (10) | 375 (47) | 39 (11) | 86 (76) | 16 (32) | 182 (44) | 530 (91) |
| Left internal jugular vein | 2 (1) | 11 (1) | 0 (0) | 7 (6) | 1 (2) | 24 (6) | 39 (7) |
| Right subclavian vein | 88 (25) | 25 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Other access | 15 (4) | 24 (3) | 6 (2) | 5 (4) | 1 (2) | 0 (0) | 0 (0) |
| Corrected Case Length (minutes) | 50 ± 30 | 33 ± 24 | 35 ± 21 | 64 ± 41 | 63 ± 29 | 31 ± 22 | 43 ± 34 |
| Fluoroscopy Time (minutes) | 13 ± 8 | 10 ± 8 | 7 ± 5 | 20 ± 17 | 15 ± 9 | 7 ± 6 | 8 ± 7 |
| Efficacy Information | |||||||
| Case performed by (n=1145) | |||||||
| Attending | 101 (88) | 86 (41) | 71 (61) | 37 (100) | 3 (25) | 306 (74) | 156 (66) |
| Attending and fellow | 4 (3) | 10 (5) | 2 (2) | 0 (0) | 1 (8) | 32 (8) | 19 (8) |
| Fellow | 10 (9) | 115 (54) | 44 (38) | 0 (0) | 8 (67) | 77 (18) | 63 (26) |
| Size of bioptome (n=1142) | |||||||
| 3 Fr | 0 (0) | 0 (0) | 0 (0) | 5 (14) | 0 (0) | 5 (1) | 0 (0) |
| 5 Fr | 23 (20) | 207 (99) | 20 (17) | 20 (54) | 1 (8) | 109 (26) | 21 (9) |
| 6 Fr | 9 (8) | 2 (1) | 96 (82) | 12 (32) | 11 (92) | 301 (73) | 114 (48) |
| 7 Fr | 82 (72) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 103 (43) |
| Type of bioptome (n=1145) | |||||||
| Sparrow Hawk | 115 (100) | 210 (99) | 1 (1) | 0 (0) | 0 (0) | 1 (0.2) | 0 (0) |
| Argon Jaws | 0 (0) | 1 (0.5) | 115 (98) | 28 (76) | 12 (100) | 297 (72) | 0 (0) |
| Cook | 0 (0) | 0 (0) | 1 (1) | 6 (16) | 0 (0) | 10 (2) | 0 (0) |
| St. Jude | 0 (0) | 0 (0) | 0 (0) | 3 (8) | 0 (0) | 1 (0.2) | 216 (91) |
| Schoulton | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 106 (26) | 0 (0) |
| Fehling | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 22 (9) |
| Number of biopsy attempts | 5 [5, 6] | 7 [6, 8] | 5 [4, 6] | 4 [3, 5] | 5 [4, 6] | 4 [4, 5] | 6 [5, 8] |
| Maximum number of attempts | 12 | 15 | 13 | 14 | 7 | 27 | 21 |
| Number of pieces obtained | 5 [5, 5] | 6 [5, 6] | 4 [4, 4] | 3 [3, 4] | 4 [4, 4] | 4 [4, 4] | 6 [5, 7] |
EMB, Endomyocardial biopsy; Fr, French;
The type of bioptome chosen was largely center dependent with most centers showing a preference for a single type of bioptome, although the specific type of bioptome varied considerably (Table 6). Center performing the biopsy was an important risk factor for a non-diagnostic biopsy specimen with an OR of 9.6 (CI 2.9-32.5) (p<0.001) for procedures performed at Center B. Center B utilized a 5 Fr bioptome exclusively.
DISCUSSION
This is the first multicenter report on the safety and diagnostic yield of cardiac catheterization procedures involving endomyocardial biopsy in pediatric heart transplant recipients. Overall, we found that catheterization is a safe procedure in pediatric heart transplant recipients with a HSAE rate of 1.1% and an overall AE rate of 3.3%. We found that fewer prior catheterizations and longer case length were associated with HSAE in multivariable analysis. We have also shown that centers with higher case volumes have a lower HSAE rate. Multiple studies have documented the utility of EMB in the diagnosis of post-transplant rejection.(1, 7, 13-21) Taken in combination with these prior studies, our results contribute vital information to making a risk-benefit decision regarding the use of EMB in the clinical management of pediatric heart transplant recipients.
Safety of Endomyocardial Biopsy and Coronary Angiography in Children
Several large single center case series and multiple smaller series have previously reported on the safety of EMB in children.(4, 7, 8, 22-26) Pophal et al. reported on 1000 consecutive EMB cases performed at a single institution, 85% of which were performed in heart transplant recipients. The overall incidence of serious complications from EMB was reported to be 1.9% with an overall cardiac perforation rate of 0.9%.(4) This number is similar to the numbers of AE reported in the two other large pediatric single center case series.(7, 8) All three large series are limited by retrospective collection of AE data, long study periods (10 to 16 years), and high mean case/patient ratios (5.2 to 16.1).(4, 7, 8) The current study adds to the overall understanding of the risks of catheterization procedures involving EMB in pediatric patients. By utilizing standard definitions and prospective data collection we were able to clearly define risk factors for HSAE including fewer prior catheterization procedures, longer case length, and performance at a center with lower EMB case volume. There were no cardiac perforations in this group of post-transplant patients and no increase in procedural risk associated with patient age or size contrary to prior studies.
The present study also highlights how the risk profile changes when coronary angiography is performed in conjunction with EMB. Coronary angiography was performed in 31% of the EMB cases in our series for the purpose of CAV screening. Complications of coronary angiography included left main coronary dissection, air embolus, and ST-T wave changes. While combining EMB with coronary angiography allows screening for both acute cellular rejection and CAV during the same procedure, it triples the risk of AE during the procedure. The addition of other CBI resulted in a similar HSAE rate to procedures without CBI (1.6% vs. 1.1%) and a marginally higher overall AE rate (4.8% vs. 3.3%). While additional CBI might be expected to confer additional risk, most appeared to be low risk interventions to address SVC stenosis. No percutaneous coronary interventions were reported in conjunction with EMB in our dataset.
Tricuspid regurgitation is a well reported complication of endomyocardial biopsy in adults.(19, 27-35) Prior reports have clearly associated severe tricuspid regurgitation with an increased number of EMB procedures and have suggested keeping the number of EMBs below 31.(33) A study of pediatric heart transplant recipients did not find an association between the number of EMBs and significant (moderate or severe) TR.(36) The lack of association between EMB and severe TR may be due to the relatively low number of biopsies performed in the pediatric transplant population (median of 6 biopsies [1, 18] in our study cohort). Three cases of tricuspid valve damage were noted by echocardiography in our cohort. While this number underestimates the true incidence of tricuspid valve damage following EMB, no patient required operative intervention for damage to the tricuspid valve within the timeframe of this study.
Damage to the AV conduction system remains an important concern when sampling from the RV septum. We report two cases of transient complete heart block which required either CPR or temporary ventricular pacing before the conduction system recovered. Sampling from the RV septum can also lead to damage to the right bundle branch, a clinically recognized EMB complication that is rarely reported in the literature.(37) Prior studies have shown an increasing prevalence of RBBB with increasing time after heart transplantation.(38) In our study there were three cases of transient complete RBBB or intermediate right bundle branch block. We speculate that repeated mechanical damage to the right bundle from EMB may explain the high prevalence of RBBB. There are no cases of permanent complete AV Block in our series and prior series report this as an extremely rare complication of EMB.(39, 40)
Diagnostic Yield of Endomyocardial Biopsy in Children
Our data show that 99% of the EMB cases yielded tissue that was adequate for pathologic interpretation. This is consistent with prior reports of the yield of endomyocardial biopsy in adults.(41-43) However it is significantly better than the 92% yield reported by Braunlin et al. in pediatric heart transplant patients.(14) We were able to identify longer time since heart transplant as a multivariable risk factor for a non-diagnostic biopsy specimen. Univariate risk factors for a non diagnostic procedure included whether the procedure was performed by a fellow, the use of a smaller bioptome, and use of a Sparrow Hawk® bioptome. Clear definition of these results has several important implications. First is that it may be beneficial to take more biopsy specimens in patients who are further out from heart transplant. These patients typically undergo EMB less frequently, thus making it vital to maximize yield during these cases. Our data suggest that there is recognition on the part of the operator that the biopsy specimens are inadequate since more biopsy attempts were made in patients with non-diagnostic biopsy specimens. In cases such as these, the allograft may have extensive fibrosis due to a combination of chronic rejection and/or repeated EMB attempts. Switching biopsy sites may lead to increased diagnostic yield. Better recognition of fibrosis within the gross biopsy specimens on the part of the operator would allow for more samples to be taken, and ultimately may decrease the chance of a non-diagnostic biopsy procedure. One of our study centers utilizes a pathologist to visualize all gross biopsy specimens in the catheterization laboratory with a reported 100% diagnostic yield and fewer biopsy samples taken.
The lower diagnostic yield in patients with a lower cardiac index is a concerning finding. These are typically the patients who are most in need of a diagnosis as lower cardiac index is one of the most consistent findings seen with high grade rejection.(18) In select patients, such as those with low cardiac output, prior non-diagnostic biopsy specimens, or patients more than 10 years out from transplant, it may be of benefit to have a pathologist evaluate the specimens in the catheterization laboratory to determine adequacy. This may help maximize both diagnostic yield and patient safety in this hemodynamically vulnerable group.
There are several limitations seen with the current study design. While pathologists at each of the centers reported that a minimum of three pieces of tissue are necessary to rule out rejection, pathologists may not clearly and consistently document the number of adequate samples submitted.(2) In this context, it is reassuring that a sub-analysis of the center with the largest number of non-diagnostic biopsies identified the similar risk factors to the overall cohort including longer time since heart transplantation and lower cardiac index. In addition, since different personnel (i.e. clinical fellows, nurse practitioners, and attending physicians) at each center adjudicated the adequacy of the sample based on a common definition, it is possible that the study definition was not uniformly applied. Despite these limitations, these data are the best available to identify risk factors for non-diagnostic EMB specimen.
CONCLUSION
We have found that cardiac catheterization and endomyocardial biopsy is a safe procedure in children after heart transplantation with a HSAE rate of 1.1% and an overall AE rate of 3.3%. Addition of coronary angiography increases the risk of adverse events during an EMB procedure. Finally, we have shown that EMB has an excellent technical success rate in children with 99% of the cases in our series yielding sufficient tissue for pathologic interpretation.
Acknowledgment of research support
A web-based application for data entry was developed in 2006 with funding support from the Children's Heart Foundation (Chicago, IL). The application was deployed on a Microsoft Internet Information Server obtained with funding support from the American Heart Association. The American Heart Association Physicians Roundtable Award (AHA-PRA) provides support for the project and career development plan for Dr. Bergersen (2006-2011). Dr. Daly received salary support from an NIH training grant (T32 HL07572) and the Children's Hospital Boston Cardiac Transplant and Education Fund while working on this study.
The authors would like to acknowledge Kimberlee Gauvreau, Sc.D. for her excellent statistical support and Gary Piercey for his help with C3PO database queries.
Appendix 1. Study Sites and Participants
Children's Hospital Boston (Lisa Bergersen, M.D., M.P.H., Michael Landzberg, M.D., Peter Lang, M.D., James Lock, M.D., Audrey Marshall, M.D., Doff McElhinney, M.D.)
Cincinnati Children's Hospital Medical Center (Robert Beekman, M.D, Russel Hirsch, M.D.)
Morgan Stanley Children's Hospital of New York Presbyterian (William Hellenbrand, M.D., Julie Vincent, M.D., Alejandro Torres, M.D.)
Nationwide Children's Hospital (John Cheatham, M.D., Ralf Holzer, M.D., Timothy Hoffman, M.D.)
St. Louis Children's Hospital (David Balzer, M.D., Susan Foerster, M.D., Ramzi Nicolas, M.D., Joshua Murphy, M.D.)
Rady Children's Hospital – San Diego (John Moore, M.D., Howaida El-Said, M.D.)
Pittsburgh Children's Hospital (Jacqueline Kreutzer, M.D., Sara Trucco, M.D., Brian Feingold, M.D., Susan Miller, M.D., Lee Berman, M.D.)
Oregon Health Sciences University (Grant Burch, M.D., Laurie Armsby, M.D.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Previous presentations: This work was presented as a poster at the American College of Cardiology Conference, April 3, 2010.
Disclaimers: None
DISCLOSURE STATEMENT
The authors have no other conflicts of interest to disclose.
REFERENCES
- 1.Wagner K, Oliver MC, Boyle GJ, et al. Endomyocardial biopsy in pediatric heart transplant recipients: a useful exercise? (Analysis of 1,169 biopsies). Pediatr Transplant. 2000;4:186–92. doi: 10.1034/j.1399-3046.2000.00100.x. [DOI] [PubMed] [Google Scholar]
- 2.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–20. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Bergersen L, Marshall A, Gauvreau K, et al. Adverse event rates in congenital cardiac catheterization - a multi-center experience. Catheter Cardiovasc Interv. 2010;75:389–400. doi: 10.1002/ccd.22266. [DOI] [PubMed] [Google Scholar]
- 4.Pophal SG, Sigfusson G, Booth KL, et al. Complications of endomyocardial biopsy in children. J Am Coll Cardiol. 1999;34:2105–10. doi: 10.1016/s0735-1097(99)00452-0. [DOI] [PubMed] [Google Scholar]
- 5.Baris N, Sipahi I, Kapadia SR, et al. Coronary angiography for follow-up of heart transplant recipients: insights from TIMI frame count and TIMI myocardial perfusion grade. J Heart Lung Transplant. 2007;26:593–7. doi: 10.1016/j.healun.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Zimmer RJ, Lee MS. Transplant coronary artery disease. JACC Cardiovasc Interv. 2010;3:367–77. doi: 10.1016/j.jcin.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Chin C, Akhtar MJ, Rosenthal DN, Bernstein D. Safety and utility of the routine surveillance biopsy in pediatric patients 2 years after heart transplantation. J Pediatr. 2000;136:238–42. doi: 10.1016/s0022-3476(00)70108-9. [DOI] [PubMed] [Google Scholar]
- 8.Cowley CG, Lozier JS, Orsmond GS, Shaddy RE. Safety of endomyocardial biopsy in children. Cardiol Young. 2003;13:404–7. [PubMed] [Google Scholar]
- 9.McManus BM, Waller BF, Jones M, Epstein SE, Roberts WC. The case for preoperative coronary angiography in patients with tetralogy of Fallot and other complex congenital heart diseases. Am Heart J. 1982;103:451–6. doi: 10.1016/0002-8703(82)90297-6. [DOI] [PubMed] [Google Scholar]
- 10.Vranicar M, Hirsch R, Canter CE, Balzer DT. Selective coronary angiography in pediatric patients. Pediatr Cardiol. 2000;21:285–8. doi: 10.1007/s002460010064. [DOI] [PubMed] [Google Scholar]
- 11.Schratz LM, Meyer RA, Schwartz DC. Serial intracoronary ultrasound in children: feasibility, reproducibility, limitations, and safety. J Am Soc Echocardiogr. 2002;15:782–90. doi: 10.1067/mje.2002.119911. [DOI] [PubMed] [Google Scholar]
- 12.Bergersen L, Gauvreau K, Jenkins KJ, Lock JE. Adverse event rates in congenital cardiac catheterization: a new understanding of risks. Congenit Heart Dis. 2008;3:90–105. doi: 10.1111/j.1747-0803.2008.00176.x. [DOI] [PubMed] [Google Scholar]
- 13.Balzer DT, Moorhead S, Saffitz JE, Huddleston CB, Spray TL, Canter CE. Utility of surveillance biopsies in infant heart transplant recipients. J Heart Lung Transplant. 1995;14:1095–101. [PubMed] [Google Scholar]
- 14.Braunlin EA, Shumway SJ, Bolman RM, et al. Usefulness of surveillance endomyocardial biopsy after pediatric cardiac transplantation. Clin Transplant. 1998;12:184–9. [PubMed] [Google Scholar]
- 15.Dixon V, Macauley C, Burch M, Sebire NJ. Unsuspected rejection episodes on routine surveillance endomyocardial biopsy post-heart transplant in paediatric patients. Pediatr Transplant. 2007;11:286–90. doi: 10.1111/j.1399-3046.2006.00650.x. [DOI] [PubMed] [Google Scholar]
- 16.Gradek WQ, D'Amico C, Smith AL, Vega D, Book WM. Routine surveillance endomyocardial biopsy continues to detect significant rejection late after heart transplantation. J Heart Lung Transplant. 2001;20:497–502. doi: 10.1016/s1053-2498(01)00236-4. [DOI] [PubMed] [Google Scholar]
- 17.Levi DS, DeConde AS, Fishbein MC, Burch C, Alejos JC, Wetzel GT. The yield of surveillance endomyocardial biopsies as a screen for cellular rejection in pediatric heart transplant patients. Pediatr Transplant. 2004;8:22–8. doi: 10.1046/j.1397-3142.2003.00115.x. [DOI] [PubMed] [Google Scholar]
- 18.Rosenthal DN, Chin C, Nishimura K, et al. Identifying cardiac transplant rejection in children: diagnostic utility of echocardiography, right heart catheterization and endomyocardial biopsy data. J Heart Lung Transplant. 2004;23:323–9. doi: 10.1016/S1053-2498(03)00209-2. [DOI] [PubMed] [Google Scholar]
- 19.Wong RC, Abrahams Z, Hanna M, et al. Tricuspid regurgitation after cardiac transplantation: an old problem revisited. J Heart Lung Transplant. 2008;27:247–52. doi: 10.1016/j.healun.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Zales VR, Crawford S, Backer CL, et al. Role of endomyocardial biopsy in rejection surveillance after heart transplantation in neonates and children. J Am Coll Cardiol. 1994;23:766–71. doi: 10.1016/0735-1097(94)90766-8. [DOI] [PubMed] [Google Scholar]
- 21.Kfoury AG, Hammond ME. Controversies in defining cardiac antibody-mediated rejection: need for updated criteria. J Heart Lung Transplant. 2010;29:389–94. doi: 10.1016/j.healun.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Leatherbury L, Chandra RS, Shapiro SR, Perry LW. Value of endomyocardial biopsy in infants, children and adolescents with dilated or hypertrophic cardiomyopathy and myocarditis. J Am Coll Cardiol. 1988;12:1547–54. doi: 10.1016/s0735-1097(88)80024-x. [DOI] [PubMed] [Google Scholar]
- 23.Lurie PR, Fujita M, Neustein HB. Transvascular endomyocardial biopsy in infants and small children: description of a new technique. Am J Cardiol. 1978;42:453–7. doi: 10.1016/0002-9149(78)90940-2. [DOI] [PubMed] [Google Scholar]
- 24.Schmaltz AA, Apitz J, Hort W, Maisch B. Endomyocardial biopsy in infants and children: experience in 60 patients. Pediatr Cardiol. 1990;11:15–21. doi: 10.1007/BF02239542. [DOI] [PubMed] [Google Scholar]
- 25.Shaddy RE, Bullock EA. Efficacy of 100 consecutive right ventricular endomyocardial biopsies in pediatric patients using the right internal jugular venous approach. Pediatr Cardiol. 1993;14:5–8. doi: 10.1007/BF00794836. [DOI] [PubMed] [Google Scholar]
- 26.Yoshizato T, Edwards WD, Alboliras ET, Hagler DJ, Driscoll DJ. Safety and utility of endomyocardial biopsy in infants, children and adolescents: a review of 66 procedures in 53 patients. J Am Coll Cardiol. 1990;15:436–42. doi: 10.1016/s0735-1097(10)80074-9. [DOI] [PubMed] [Google Scholar]
- 27.Aziz TM, Burgess MI, Rahman AN, Campbell CS, Deiraniya AK, Yonan NA. Risk factors for tricuspid valve regurgitation after orthotopic heart transplantation. Ann Thorac Surg. 1999;68:1247–51. doi: 10.1016/s0003-4975(99)00768-7. [DOI] [PubMed] [Google Scholar]
- 28.Bedanova H, Necas J, Petrikovits E, et al. Echo-guided endomyocardial biopsy in heart transplant recipients. Transpl Int. 2004;17:622–5. doi: 10.1007/s00147-004-0760-1. [DOI] [PubMed] [Google Scholar]
- 29.Chan MC, Giannetti N, Kato T, et al. Severe tricuspid regurgitation after heart transplantation. J Heart Lung Transplant. 2001;20:709–17. doi: 10.1016/s1053-2498(01)00258-3. [DOI] [PubMed] [Google Scholar]
- 30.Huddleston CB, Rosenbloom M, Goldstein JA, Pasque MK. Biopsy-induced tricuspid regurgitation after cardiac transplantation. Ann Thorac Surg. 1994;57:832–6. doi: 10.1016/0003-4975(94)90184-8. discussion 6-7. [DOI] [PubMed] [Google Scholar]
- 31.Kalra N, Copeland JG, Sorrell VL. Tricuspid regurgitation after orthotopic heart transplantation. Echocardiography. 2010;27:1–4. doi: 10.1111/j.1540-8175.2009.00979.x. [DOI] [PubMed] [Google Scholar]
- 32.Lewen MK, Bryg RJ, Miller LW, Williams GA, Labovitz AJ. Tricuspid regurgitation by Doppler echocardiography after orthotopic cardiac transplantation. Am J Cardiol. 1987;59:1371–4. doi: 10.1016/0002-9149(87)90922-2. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen V, Cantarovich M, Cecere R, Giannetti N. Tricuspid regurgitation after cardiac transplantation: how many biopsies are too many? J Heart Lung Transplant. 2005;24:S227–31. doi: 10.1016/j.healun.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Wiklund L, Caidahl K, Kjellstrom C, Nilsson B, Svensson G, Berglin E. Tricuspid valve insufficiency as a complication of endomyocardial biopsy. Transpl Int. 1992;5(Suppl 1):S255–8. doi: 10.1007/978-3-642-77423-2_81. [DOI] [PubMed] [Google Scholar]
- 35.Williams MJ, Lee MY, DiSalvo TG, et al. Biopsy-induced flail tricuspid leaflet and tricuspid regurgitation following orthotopic cardiac transplantation. Am J Cardiol. 1996;77:1339–44. doi: 10.1016/s0002-9149(96)00202-0. [DOI] [PubMed] [Google Scholar]
- 36.Ben Sivarajan V, Chrisant MR, Ittenbach RF, et al. Prevalence and risk factors for tricuspid valve regurgitation after pediatric heart transplantation. J Heart Lung Transplant. 2008;27:494–500. doi: 10.1016/j.healun.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Huang J, Yang YJ, Yin D, et al. [Safety analyses from 439 patients underwent endomyocardial biopsy via the right internal jugular vein approach]. Zhonghua Xin Xue Guan Bing Za Zhi. 2010;38:43–6. [PubMed] [Google Scholar]
- 38.Marcus GM, Hoang KL, Hunt SA, Chun SH, Lee BK. Prevalence, patterns of development, and prognosis of right bundle branch block in heart transplant recipients. Am J Cardiol. 2006;98:1288–90. doi: 10.1016/j.amjcard.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 39.Holzmann M, Nicko A, Kuhl U, et al. Complication rate of right ventricular endomyocardial biopsy via the femoral approach: a retrospective and prospective study analyzing 3048 diagnostic procedures over an 11-year period. Circulation. 2008;118:1722–8. doi: 10.1161/CIRCULATIONAHA.107.743427. [DOI] [PubMed] [Google Scholar]
- 40.Cui G, Kobashigawa J, Margarian A, Sen L. Cause of atrioventricular block in patients after heart transplantation. Transplantation. 2003;76:137–42. doi: 10.1097/01.TP.0000071933.14397.43. [DOI] [PubMed] [Google Scholar]
- 41.Hamour IM, Burke MM, Bell AD, Panicker MG, Banerjee R, Banner NR. Limited utility of endomyocardial biopsy in the first year after heart transplantation. Transplantation. 2008;85:969–74. doi: 10.1097/TP.0b013e318168d571. [DOI] [PubMed] [Google Scholar]
- 42.Ghadimi H, Tavangar SM. Histopathological findings in cardiac transplant recipients: an assessment after 10 years’ experience in Iran. Transplant Proc. 2005;37:4535–6. doi: 10.1016/j.transproceed.2005.10.106. [DOI] [PubMed] [Google Scholar]
- 43.Winters GL, Costanzo-Nordin MR. Pathological findings in 2300 consecutive endomyocardial biopsies. Mod Pathol. 1991;4:441–8. [PubMed] [Google Scholar]