Abstract
During the course of both canine and human aging, the mitral valve remodels in generally predictable ways. The connection between these aging changes and the morbidity and mortality that accompany pathologic conditions has not been made clear. By exploring work that has investigated the specific valvular changes in both age and disease, with respect to the cells and the extracellular matrix found within the mitral valve, heretofore unexplored connections between age and myxomatous valve disease can be found. This review addresses several studies that have been conducted to explore such age and disease related changes in extracellular matrix, valvular endothelial and interstitial cells, and valve innervation, and also reviews attempts to correlate aging and myxomatous disease. Such connections can highlight avenues for future research and help provide insight as to when an individual diverts from an aging pattern into a diseased pathway. Recognizing these patterns and opportunities could result in earlier intervention and the hope of reduced morbidity and mortality for patients.
Keywords: Extracellular matrix, remodeling, valvular endothelial cells, valvular interstitial cells, innervation
Introduction
Myxomatous mitral valve degeneration (MMVD) causes severe valvular regurgitation and ultimately death for millions of dogs around the world.1,2 In humans, this disease is the primary cause of mitral valvular regurgitation leading to the need for surgical valve repair 3. For humans afflicted with MMVD, it has been observed that the mean patient age is 60 years old. As opposed to calcific aortic valve disease, which has a clearly demonstrated association with aging,4 MMVD in humans appears distinct from age-related changes.5–7 This review will describe the age-related changes in mitral valve composition and material behavior and contrast these changes with those found in MMVD, with a particular emphasis on comparisons of human, porcine, and canine mitral valves.
Gross and micro-anatomy of the mitral valve
The canine mitral valve complex, similar to human and other mammalian species, consists of the annulus, two leaflets (anterior and posterior) of different shape and size, numerous chordae tendineae, and the papillary muscles (Fig. 1A).8,9 The function of the mitral valve is to direct the blood flow from the left atrium to the left ventricle during diastole and to prevent the backflow of blood into the left atrium during systole. In order to do so, the mitral valve complex requires all of its components, together with the adjacent atrial and ventricular muscle, to work in a synchronized fashion.10,11
Figure 1.
(A) Top (atrial) view of the mitral valve in the closed (left) and open (right) position. (B) Bottom (ventricular) view of the mitral valve, cut open at the commissures to display the posterior (left) and anterior (right) leaflets. Dotted white line demarcates leaflet center from free edge. Chordae may have basal or marginal insertion into leaflet. Scale bar = 1cm. Reprinted from Grande-Allen KJ, Calabro A, Gupta V, Wight TN, Hascall VC, Vesely I. Glycosaminoglycans and proteoglycans in normal mitral valve leaflets and chordae: association with regions of tensile and compressive loading. Glycobiology. 2004;14(7):621–33 with permission from Oxford Journals.
The mitral valve leaflets each consist of four layers that differ from each other in extracellular matrix (ECM) makeup and functionality. From top to bottom, the layers are the atrialis, spongiosa, fibrosa, and ventricularis. The fibrosa, the thickest layer of the valve, is comprised of dense, circumferentially aligned collagen, in contrast to the loose collagen found within the atrialis, spongiosa, and ventricularis.12 The dense collagen of the fibrosa provides the mitral valve leaflets with tensile strength, whereas the looser collagen and high glycosaminoglycan (GAG) content in the other layers provides the leaflets with compressive strength. The atrialis, the layer located on the inflow side of the mitral valve, has an abundance of the protein elastin, which allows the valve to undergo considerable stretch and then recoil back to its original undeformed shape during the cardiac cycle.13 The spongiosa contains a high concentration of the GAG hyaluronan (HA) and hydrated chondroitin/dermatan sulfate proteoglycans (PGs), which also provide compressive strength for the valve.14 The ventricularis layer of the mitral valve leaflets is very thin compared with other layers and consists of elastic fibers and regularly spaced, circumferentially oriented collagen fibers. The regions containing the highly aligned collagen is also rich in small leucine-rich proteoglycans (SLRPs) that connect to and provide mechanical support for the collagen fibrils.13
In addition to the mitral valve leaflets, the chordae tendineae are a critical component to the functioning of the mitral valve apparatus. These chordae connect the underside of the valve leaflets to the papillary muscles along the left ventricular wall. As the left ventricle contracts during systole, the papillary muscles contract as well and the chordae in turn provide mechanical support to the mitral valve leaflets, helping them maintain closure during the highly pressurized left ventricular systole.15 In order to withstand the high tensile stress applied to the chordae, they are comprised of highly organized collagen fibers that are orientated in the direction of load 15. As in the leaflet, these regions of highly aligned collagen are also rich in SLRPs, such as decorin and biglycan. The GAGs found within the chordae are predominantly those found on the SLRPs, and demonstrate an abundance of iduronate and 4-sulfated-N-actylgalactosamine.15 The chordae also contain a small distribution of elastic fibers in their outer sheath.16
The mitral valve leaflets also vary in makeup and function from the annulus to the free edge. For example, there is a distinct difference between the so-called “rough zone” and “clear zone” of the anterior leaflet. Historically, the “rough zone” has referred to those parts of the leaflet that contain chordal attachments, while the “clear zone” has referred to regions without chordal attachment. In our previous studies of mitral valve leaflets we have defined these regions as the mitral valve free edge (MVF) and the center of the anterior leaflet (MVAC), respectively (Fig. 1B). The MVAC maintains the tensile integrity of the valve;14 compared with the MVF, the MVAC contains a thicker fibrosa layer and has a lower concentration of GAGs.14,17 The specific characteristics of the GAGs also varies between these two regions, e.g., the MVAC contains relatively less unsulfated, 6-sulfated, and 4-sulfated glucuronate than the MVF.14 In contrast, the MVF, which is more suited to bearing compressive loading, has a much higher concentration of HA than does the MVAC, mainly because the free edge has a much larger spongiosa component compared to the MVAC.14 The composition of the mitral valve posterior leaflet (also known as the mural leaflet), which experiences a compressive load during the cardiac cycle,18 closely resembles that of the other major compressive load-bearing region, the MVF.15
Due to the lack of comprehensive studies on normal valvular aging in dogs, it becomes necessary to look to studies conducted on other species in order to provide some insight into aging and its relation to MMVD. However, it is important to note the anatomical and therefore potential histological differences between species when doing so. For example, it has been reported that larger animals have a distinct increase in the branching (first-order and second order) of chordae when compared to the hearts of smaller animals.8 The need to support the larger valve cusp area of the larger animals seems to be the logical explanation for this structural arrangement. Variations in shapes of the anterior leaflet (also known as the aortic or septal leaflet) have been also been reported when comparing human and canine mitral valves.8 Human anterior leaflets have a clear line demarcating the boundary of the appositional area, the area of leaflet in contact during valve closure.9 In dogs, the absence of a clear line of demarcation, together with higher muscle content in both mitral cusps than found in human valves, has lead to the speculation that canine mitral valves demonstrate a different closing mechanism.9 Humans and dogs also have different angles between the plane of the atrioventricular orifice in relation to the axis extending from the ventricular apex through the middle of the aortic valve.8 However, the significance of this finding is unknown and could be related to the 90° angle difference in anteroposterior axis orientation relative to the gravitational field between dogs (a quadruped mammal) and bipedal humans.
Aging and Cell-Mediated Remodeling of the Extracellular Matrix
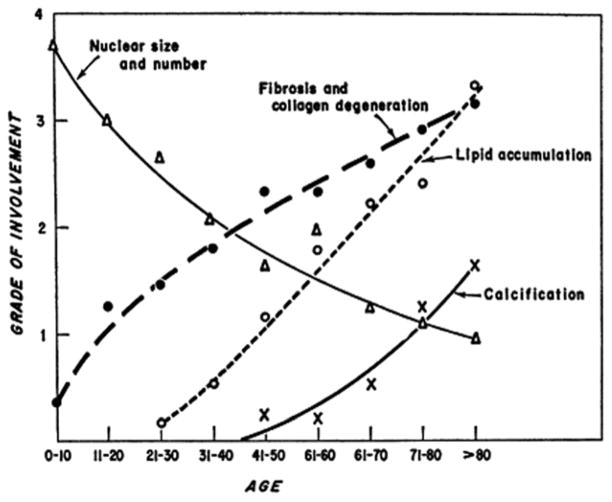
Studies of human and porcine mitral valves have shown the effect of aging on ECM and microstructural composition as well as the effects of these changes on valve function. With age, there is a reduction in cell density throughout the valve, across all layers. The valvular interstitial cells (VICs) that remain are increasingly associated with an “activated” remodeling phenotype of α-smooth muscle actin (α-SMA), co-localized with enzymes involved in collagen synthesis and degradation in porcine valves.19 Microstructurally, the layers become more delineated with advancing age,19 and the PGs decorin and biglycan show greater expression throughout the valve. These PGs colocalize with collagen and aid in collagen fibrillogenesis, lending further support that collagen remodeling occurs throughout aging.14 In contrast, with aging, the amount of elastin decreases throughout the valve, particularly for individuals over the age of 50 years.14,20 In addition, with advancing age, the incidence of lipid accumulation and calcification in valves increases throughout the human population (Fig. 2).21
Figure 2.
Changes in mitral valve with age averaged across 200 mitral valve samples. Trends include decreasing cellularity and increasing fibrosis and collagen degradation, lipid accumulation and calcification. Reprinted from Sell, S., Scully, R. E. (1965). Aging changes in the aortic and mitral valves: histologic and histochemical studies, with observations on the pathogenesis of calcific aortic stenosis and calcification of the mitral annulus. Am J Pathol, 46 (3), 345–365 with permission from Elsevier.
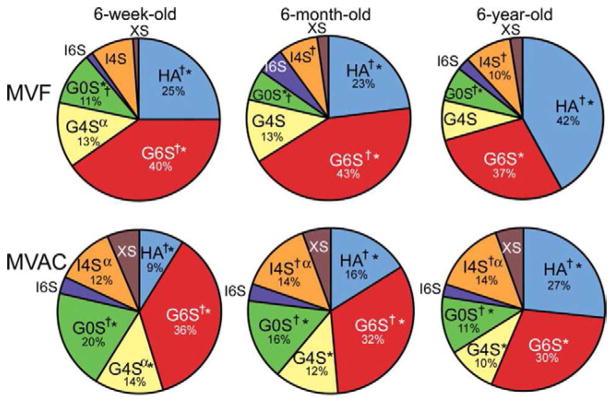
With respect to types of GAGs present in the mitral valve, with age there is a decrease in unsulfated and 6-sulfated glucuronate, as well as a decrease in the ratio of chondroitin sulfate to dermatan sulfate.14 Interestingly, the total amount of HA remains fairly constant with age in porcine mitral valves, although HA comes to represent an increasing percentage of the total GAGs in the MVF due to a reduction in 4-sulfated and 6-sulfated GAGs.14 In the MVAC, there is no age-related decrease in total GAGs or in HA, but there is an age-related increase in 6-sulfated glucoronate and 4-sulfated iduronate (Figs. 3–4).14
Figure 3.
Proportion of glycosaminoglycans (GAGs) in the MVAC and MVF of 6-week-old, 6-month-old, and 6-year old porcine valves, as calculated from FACE. Statistically significant differences (p < 0.05) are labeled † (significant differences in MVAC and MVF at a given age) or * (significant differences across ages in a given region (MVAC or MVF)). α represents a lower p-value (p < 0.03) for one set of comparisons (I4S) between ages in a given region (MVF). Significance was not displayed for averaged data (i.e., difference between MVF and MVAC averaged across all ages). MVF = mitral valve (MV) free edge; MVAC = MV anterior center; G0S = unsulfated glucuronate; G4S = 4-sulfated glucuronate; G6S = 6-sulfated glucuronate; I4S = 4-sulfated iduronate; I6S = 6-sulfated iduronate; XS = di- and tri-sulfated glucuronate / iduronate. Only GAGs that comprise >10% are labeled. Modified from Stephens EH, Chu CK, Grande-Allen KJ. Valve proteoglycan content and glycosaminoglycan fine structure are unique to microstructure, mechanical load and age: relevance to an age-specific tissue-engineered heart valve. Acta Biomaterial. 2008;4(5):1148–1160 with permission from Elsevier.
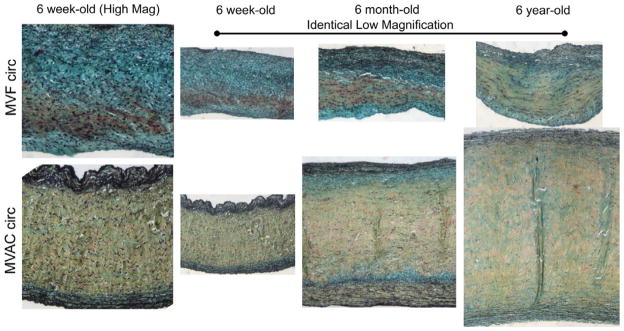
Figure 4.
Movat-stained circumferential sections of the MVAC and MVF of 6-week-old, 6-month-old, and 6-year-old porcine valves. Both high magnification (column 1) and low magnification (columns 2 – 4) are shown. All images within a magnification type (high vs. low) are the same magnification to allow comparison of leaflet and layer thickness across age and leaflet region. Movat pentachrome staining preferentially stains different ECM elements certain colors (yellow = aligned collagen, black = elastic fibers, and green/blue = PGs / GAGs). Comparing regions, the MVAC fibrosa comprised a larger proportion of the valve than in the MVF. Across ages (regardless of region), an increase with collagen with increasing age was noted, especially in the fibrosa and ventricularis. Scale bar = 200 mm. Modified from Stephens EH, Jonge N de, McNeill MP, Durst C a, Grande-Allen KJ. Age-related changes in material behavior of porcine mitral and aortic valves and correlation to matrix composition. Tissue Eng A. 2010;16(3):867–78 with permission from Elsevier.
Aging and Mechanical Behavior
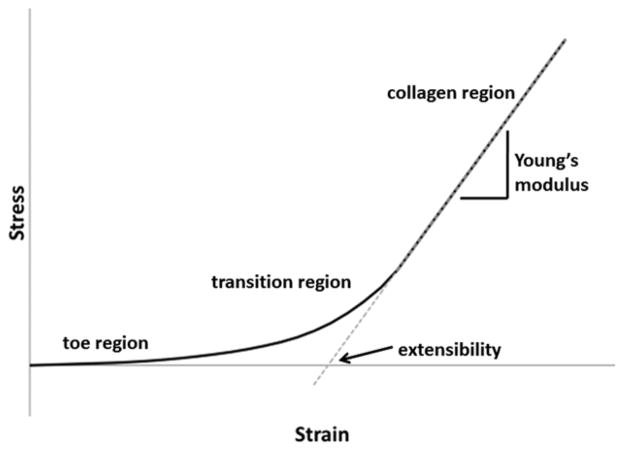
With respect to mechanical behavior, the overall stiffness of the valve increases in both the radial and circumferential directions as a function of age. Accompanying these age-related changes are a reduction in extensibility22 and an increase in stress relaxation.17 Extensibility can be generally described as the magnitude that a tissue can stretch before the collagen fibers become fully uncrimped and bear load (Fig. 5). Stress relaxation is an indicator of the viscoelastic (time-dependent) behavior of the tissue; an increase in stress-relaxation indicates that there is a substantial rearrangement of the extracellular matrix that progresses for some time after the tissue is stretched. These altered material properties can be generally explained by thickening of the collagen-rich fibrosa layer as well as the increase in collagen production and remodeling that occurs throughout aging.17,19 It is also likely that there is collagenous reinforcement of elastic fibers with age, which would reduce extensibility. There is an increase in the radius of curvature of the transition region of the valve leaflet load-deformation curve with age, which we speculate is due to increased collagen alignment and crosslinking in the valve14,17 as well as greater abundance of PGs and GAGs in the collagenous fibrosa layer. Indeed, the SLRPs decorin and biglycan, both of which become more abundant with age in valves, bind to and form bridges between collagen fibrils. Therefore, increased transmission of hemodynamic forces to collagen and the other underlying matrix proteins via interconnecting PGs could be responsible for the age-related changes in these mechanical properties.14,17
Figure 5.
Representative stress-strain curve seen in mechanical testing of valves in tension (blue line). The first linear portion of the bi-linear curve is called the toe region. It corresponds to the region where collagen is crimped and elastic fibers dominate the material properties of the valve. The second linear portion can be referred to as the collagen region because it corresponds to the region where collagen is fully uncrimped and is dominating the material properties. The slope of this region of the curve is defined as the Young’s modulus or stiffness of the material. One can determine a value for the extensibility by extrapolating the collagen region backwards using the Young’s modulus as the slope (dashed red line) to where it crosses the x-intercept. The extensibility corresponds to the degree of crimping of collagen in the valve. The curved area between these two regions is the transition region. It corresponds to the region in which collagen is actively uncrimping. The sharpness of this transition, defined by the radius of transition curvature, corresponds to the alignment and crosslinking present in the collagen in the tissue.
Aging and Innervation
Over the past several years, the recognition that mammalian mitral valve leaflets have well developed nerve plexuses,23,24 consisting of different transmitter-specific subpopulations of nerves, has challenged the conventional concept of mitral valve as a passive flap. The nerve plexus of nerves is most dense in the basal zone of the valve adjacent to the fibrous annulus ring,25 and becomes finer and more branched towards the free margin of the cusp. It has been shown in human mitral valves that the nerve density within the anterior leaflet is two fold greater than in the posterior leaflet and that the nerve fibers are closely associated with different types of VICs.26 Together with contractile cell types found within the valve (activated VICs and cardiomyocytes from the annulus), the nerve plexuses form a model for an active valve that is capable of contraction and relaxation, fine tuning its motion during each cardiac cycle.27 Giving support to this claim, experiments in dogs have shown that autonomic stimulation results in the valve better able to withstand pressure-induced displacement of the leaflets into the left atrium.28
With increasing age, there is a loss of innervation in the mitral valves of guinea pigs, mice and rats.29,30 In rats, both sensory and motor type of nerves were markedly diminished in older animals.31 There was no difference in mitral valve nerve density between young dogs aged 6–12 months and adult dogs aged 5 years, but there was a significant reduction in mitral valve nerve density in dogs above 10 years of age.25
Aging and Valvular Cells
The valve leaflets are covered with a single layer of valvular endothelial cells (VECs) that is continuous with the endocardium of the heart. The VECs are believed to coordinate with the VICs located in the leaflet interior to maintain the ECM structure and contribute to the mechanical function of the valve. Although VICs and VECs demonstrate distinctly different phenotypes in the postnatal mitral valve, they share a common embryonic origin. During the earliest stage of valve development, the GAG-rich cardiac cushions arise within the atrioventricular canal and outflow tract of the looped heart tube. Certain endothelial cells on the surface of these cardiac cushions migrate into the interior of the cushion through a process referred to as endothelial-mesenchymal transformation (EMT) and ultimately become the VICs;32 it is believed that the underlying myocardium regulates the EMT. It does so through the regulation of growth factors including VEGF, TGF-β, NOTCH, and WNT, as well as extracellular matrix components, such as hyaluronan, which regulate pathways that direct endothelial cells towards mesenchymal transformation.33 Once activated for mesenchymal transformation, the cells within these cushion regions then initiate ECM remodeling and later development of the valve leaflets.34 In contrast, the VECs do not undergo EMT during normal aging (Fig. 6).32 However, recent experiments indicate that some adult VECs retain the ability to differentiate in a manner similar to EMT while in a diseased state.35
Figure 6.
Graphic overview of heart development and endothelial-mesenchymal transformation (EMT). As the heart tube develops it contains three layers, an inner lining of endothelial cells, a middle separating layer of ECM referred to as cardiac jelly, and an outer layer of myocardium. As valves form, a subset of the endothelial cells undergo EMT by delaminating, differentiating, and then migrating into the cardiac jelly. Then, in a process that is poorly understood, local swellings of cardiac jelly and mesenchymal cells (cardiac cushions) undergo remodeling and form heart valves. Reprinted from Armstrong, E. J., & Bischoff, J. (2004). Heart valve development: endothelial cell signaling and differentiation. Circ Res, 95(5), 459–470 with permission from Wolters Kluwer Heath.
Although age-related changes in VECs have been investigated infrequently, scanning electron microscopy has been used to demonstrate denudation of the endothelium in some areas of the diseased valves of older dogs (9–15 years) with regional pleomorphism in adjacent areas of intact endothelium.36 In addition, it has been shown in human valves that fetal VECs (second and third trimesters) express significantly higher amounts (p<0.001) of SMemb, matrix metalloproteinase-1 (MMP-1), MMP-13, and cell adhesion molecules ICAM-1 and VCAM-1 when compared to adult VECs, which had relatively negligible expression of these proteins.37
Myxomatous changes alter valve motion, resulting in abnormal closure that, taken together with the changing in hemodynamic force due to regurgitation, may cause or contribute to endothelial damage.38 Damage to the VEC layer could influence the synthesis and release of vasoactive mediators that in turn interact with subendothelial matrix tissue. One such potent vasoconstrictor is endothelin, which also has the potential to stimulate proliferation of fibroblasts and increase collagen production. Indeed, an increase in endothelin receptor density has been found in the distal end of aged canine mitral leaflets (mean age 12 years) showing myxomatous changes.39
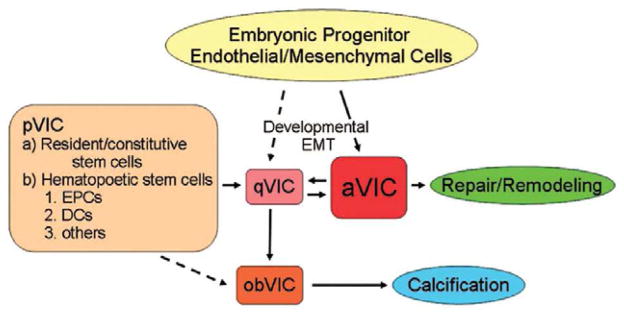
VICs are the predominant cells found in all layers of mitral valve and are considered to be primarily responsible for maintaining valve structure and function. VICs are a heterogeneous and dynamic population of cells that have a range of distinct cellular phenotypes.40,41 This plasticity of VIC phenotype appears to be crucial in heart valve development, remodeling and repair, and progression of diseased states – all instances in which the myofibroblast cell type predominates.42,43 Liu et al. has proposed that there are five identifiable VIC phenotypes during different stages of mitral valve development, remodeling, and disease. These five are embryonic progenitor endothelial/mesenchymal cells (EMT stage), quiescent VICs (normal fibroblast like), activated VICs (diseased myofibroblast like), progenitor VICs (stem cell like) and osteoblastic VICs (Fig. 7).44 Despite such characterizations, the current understanding of VIC biology remains very limited and information on the aging aspect of VIC biology is particularly rare. An early investigation of mitral valves from humans aged 5 days to 89 years reported a gradual age-related decrease in VIC number and nuclei size, notably after the fifth decade of life.21 High densities of VICs were found in fetal mitral valves from human, sheep and pig.19,45 In pigs, the highest VIC densities were found in the first trimester, after which there was a decrease in cell numbers throughout fetal developmental and postnatal aging. Interestingly, distinctions between layers are evident as early as the first trimester, with the spongiosa layer having a lower cell density than the outer leaflet layers.19 During fetal valve development, most VICs stain positive for vimentin, α-SMA, and the collagenase MMP-13, and are thus considered to have an activated myofibroblast phenotype, the cell phenotype most involved in tissue remodeling.19 Throughout development and into adulthood, there is a significant reduction in the numbers of α-SMA positive VICs and a relatively greater number of the normal quiescent fibroblast phenotype in humans.37
Figure 7.
The functions of VICs can be organized into five major phenotypes: embryonic progenitor endothelial / mesenchymal cells, quiescent VICs (qVICs), activated VICs (aVICs), stem cell-derived progenitor VICs (pVICs), and osteoblastic VICs (obVICs). Through the process of EMT, embryonic progenitor endothelial / mesenchymal cells differentiate into aVICs and qVICs. aVICs are capable migration, proliferation, and ECM synthesis. qVICs remain quiescent until the heart valve is injured, at which point they transition to aVICs and aid in the repair and remodeling process. In addition, they can differentiate into obVICs in certain conditions. pVICs are derived from bone marrow. They are present in the bone marrow, in circulation, and in the heart valve. They are an additional source of aVICs in adulthood. obVICs respond to osteogenic and chondrogenic factors and promote valve calcification. Hatched arrows represent hypothesized transitions for which there is currently not solid evidence. Other abbreviations used: EPCs = Endothelial Progenitor Cells, DCs = Dendritic cells. Reprinted from Liu AC, Joag VR, Gotlieb AI. The Emerging Role of Valve Interstitial Cell Phenotypes in Regulating Heart Valve Pathobiology. Am J Pathol. 2007;171(5):1407–1418 with permission from Elsevier.
Myxomatous mitral valve degeneration
MMVD is the most common cardiac disease in the dog.46 The disease is also referred to as Mitral valve endocardiosis (MVE) or Chronic valvular disease, and affects many different species, including pigs47–49 and humans in addition to dogs. In dogs, unlike humans, the prevalence and severity of the disease is found to be closely age dependent.50 However, as described later, some breeds of dog are more predisposed to the disease than others, suggesting a likely inherited component to the disease.51,52 Various reports have estimated the occurrence of MMVD as ranging from 21% to 89% in dogs.46,53,54 This wide range might be attributed to the disparate clinical sample selection of the groups of dogs, which could be affected by factors such as sample pool size, age, and breed.
MMVD involves a complex connective tissue degenerative process with little or no inflammatory reaction. Histologically, MMVD is manifested by excessive destruction and derangement of the valve stroma with loss of collagen bundle organization and accumulation of PGs and GAGs in the leaflets and chordae.7,16,55,56 In addition, elastic fibers are increased in number, but appear to be disrupted and granular in nature.56 In dogs, the pathology is grossly recognized by the presence of greyish-white, smooth, glistening nodules or plaque-like elevations, also referred to as lesions, often situated in the area of apposition on the atrial surface of the leaflets.57 In humans, a much larger region of the tissue is affected by myxomatous degeneration, which may extend throughout the leaflet and chordae. Normal healthy mitral valve leaflets are thin, pliable, translucent and soft. In contrast, diseased valve leaflets and chordae become opaque and thickened; they are more extensible and less stiff than normal leaflets.58 The structural changes due to myxomatous degeneration in the leaflets are not always uniformly distributed, but have long been identified as the most important factors contributing to mitral valve dysfunction in dogs.46,54,57,59,60 The rough zone of the leaflet on the ventricular side, especially where the tendinous chords attach, is particularly prone to myxomatous degeneration.61 At later stages of this disease, gross distortion of the apposition line of the leaflets can be caused by the development of interchordal hoodings,62 also referred to as parachute change. The middle section of the leaflet, which withstands the strongest pressure loading during the cardiac cycle,63 is less susceptible to myxomatous degeneration in dogs. In humans with MMVD, the mid-section of the leaflets is frequently involved, especially the middle scallop of the posterior (mural) leaflet with changes observed in 88% of patients.6,64
Both dogs and humans with MMVD demonstrate phenotypic changes in VICs from a quiescent fibroblast to an activated myofibroblast (α-SMA positive).42,59,65–67 Many of these cells also express stem/progenitor cell markers CD-34 and/or CD-117.42,68,69 In the early stages of MMVD in dogs, α-SMA positive cells are found to congregate toward the atrialis edge, then extend more deeply into the tissue.70 At later stage of this disease, α-SMA positive cells also accumulated in the distal ventricular side, presumably as a result of regurgitation-driven forces.65 Moreover, VICs staining positive for α-SMA were frequently co-localized with expression of TGF-β1 and TGF-β3. Interestingly, in the overtly myxomatous area of canine valves, α-SMA positive cells were uncommon, as was TGF-β3 expression, although TGF-β1 was strongly expressed.71 There was also a reduction in vimentin-positive cells in myxomatous areas of canine valves,65 compared with normal valve regions. Cell density in myxomatous areas was not significantly altered between different grades of MMVD or with canine age, downplaying a role for cell recruitment or cell proliferation in this condition in dogs.72 In human, myxomatous mitral valves demonstrate an increase in certain catabolic enzymes and other phenotypic markers, including MMPs, cathepsins, SMemb, and interleukin-1β, that are associated with “activated myofibroblasts.”42
MMPs and tissue inhibitors of metalloproteinases (TIMPs) both play an important role in the remodeling of ECM, both in aging and in MMVD. For example, both MMP-2 and MMP-9 are increased in myxomatous mitral valves.73 In addition, it was shown that the expression of MMP-2 decreased and the expression of MMP-14 and of TIMP-2 and -3 increased with increased MMVD severity and age,74 with the decrease in MMP-2 attributed to increased abundance of its inhibitor TIMP-3.75,76
In dogs, an increase in the severity of valvular damage in MMVD corresponds to a worsening of the clinical disability.1 MMVD leads to regurgitation of blood across the closed mitral valve during ventricular systole. The lesions of MMVD represent a gradual process and may cause no detectable clinical signs during early stage of structural change. Indeed, valve closure in the early stages may appear normal. However, progression of the disease will lead to insufficient coaptation of the leaflets, increasing regurgitation of blood back into the atria, and in the late stage of the disease, dilation of the left ventricle and mitral annulus occurs, as well as jet lesions and ruptured chordae in some cases. As a consequence, these lesions will lead to mitral systolic murmurs, and in severe cases, congestive heart failure. This disease progression is similar to MMVD in humans in terms of the pathological change and the clinical outcome.77 In humans, however, displacement of mitral valve leaflets into the atrium can be less common,62,78 and the frequency of chordal rupture is very high.6
Due to considerable variation in the size of the normal heart valve in different breeds of dogs, a visual scoring method is widely used to classify the diseased valve lesions into one of four grades (Table 1). The grading system is easy to apply and can provide useful information on the pathogenesis of the disease as each grade represents a stage in the development of the disease. However, this grading system requires a familiarization with all the manifestations of the disease and is somewhat subjective. Although MMVD in humans shares several of these changes, no similar classification system exists for human patients, partly because of the difficulty of identifying patients experiencing the early stages of the disease.
Table 1.
| Lesion Grade | Nodules (Location) | Leaflets (Location) | Chordae | Valvular Competence |
|---|---|---|---|---|
| 1 | Few, small oedamatous (apposition) | Areas of diffuse opacity (basal) | Unchanged | Maintained |
| 2 | Multiple, grayish-white, coalesce (apposition) | Areas of diffuse opacity (throughout) | Unchanged | Maintained |
| 3 | Increased size, plaque- like (apposition) | Defined areas of opacity (basal) | Thickened at junction with leaflets | Some incompetent |
| 4 | Greyish white, plaque- like elevations (apposition) | Grossly distorted; ballooning overgrowth (closure line) | Thickened, can be stretched/ruptured | Majority incompetent |
Comparison of Age Related Changes and Disease
Mitral valve disease begins during the first third of a dog’s life, frequently producing mitral and occasionally tricuspid insufficiency during the middle years. The degree of valvular malformation caused by this disease is fairly well tolerated in some individuals up to 9 years of age or older.53 Although there is no comparable grading system in human medicine, the age distribution in dogs is comparable with the prevalence of mitral valve disease in human patients. The prevalence of mitral valve prolapse (MVP) is low in young children, but increases from childhood to adolescence, a change that is concomitant with the growth spurt of adolescence.79 Most reported human patients requiring mitral valve repair surgery or replacement tend to be middle-aged,80 with mean age at 56 years old.64,81,82 This age distribution suggests that while MMVD might not be a part of normal aging, aging and growth have a significant impact on disease progression.
A favored hypothesis at present is that MMVD is a response to repeated impact of the leaflet edges.62 Myxomatous degeneration then alters the apposition zone and valve motion resulting in abnormal closure. In addition, the changes in motion and apposition cause regurgitation that then impacts the distribution of hemodynamic forces and ultimately results in further tissue damage.38 An observation that links this hypothesis to the aging process is the fact that the heart rate, and thus the frequency of mitral leaflet coaptation impacts, increases with age in certain breeds. This greater frequency of impact could augment the progression of MMVD in older dogs.83 The location and the histology of the thickened foci on leaflet edges supports this hypothesis, but the sequence of events and the time course is still unknown.
In view of the high prevalence of MMVD in the aged canine population and that its clinical symptom, mitral regurgitation, represents the most important geriatric heart disease, some veterinarians and researchers have classified MMVD as a geriatric disease.50,84,85 Indeed, given the slow progression of this disease, most affected dogs are clinically asymptomatic for some time. Other authors, however, have claimed that canine MMVD is a naturally acquired disease.62,70,86,87 Regardless of classification, increasing age has a marked effect on prevalence and severity in canine MMVD as well as human MVP.88 For this reason as well as the histological similarities between dogs and humans, canine MMVD has been proposed as a naturally occurring model of human MVP.62
As described earlier, important ECM changes that occur during the aging process include remodeling of collagen, altered GAG and PG composition, and decreased elastin. Moreover, progressive thickening of the leaflet cusps was a notable change during the aging process.20,89 Given the similarity between these histological and gross findings and those of MMVD in dogs it is possible that a link exists between MMVD and normal aging of dogs. MMVD might, in fact, be an extreme or abnormal amplification of a normal aging process; perhaps aged “normal” subjects in a human study would be classified as abnormal in a veterinary context. Furthermore, in human patients with MMVD, the mitral valve leaflets and chordae are thicker, and the ECM derangements are significantly more pronounced, compared with age-matched control human valves.7
Using his lesion grading system, Whitney found the prevalence and severity of mitral valve lesions correlated with increase in age, based on a postmortem study of 200 dogs.57 When combining all four grades, he found lesions in 37% at 0–4 years of age, 80% at 5–8 years of age, 93% at 9–12 years of age and 100% at 13–16 years of age. Type 1 lesions (15%) were found predominantly in dogs under 5 years old. Type 2 and 3 lesions (49%) were common in dogs 9–12 years old, whereas Type 3 and 4 lesions (88%) were predominately confined to dogs 13 years and over. The more advanced Type 3 and 4 lesions were found in 24% of the dogs under 9 years old, in comparison to 58% of dogs over 9 years old; the majority of dogs with Type 3 and 4 lesions showed evidence of congestive heart failure.57 A modification of Whitney’s lesion grading strategy was proposed by Kogure.54 Instead of 4 types of lesions, Kogure categorized the mitral lesions into 3 groups. Group I corresponded to Whitney’s types 1 and 2, whereas Group II is similar to Whitney’s type 3 and Group III resembled Whitney’s type 4. Among the 64 dogs with mitral valve lesion studied by Kogure, 30 (47%) dogs with a median age of 2 years old were classified into Group I; 22 (34%) dogs with a median age of 4 years old were in Group II, and 12 (19%) dogs with a median age of 10 years old were in Group III.54 The result was parallel to Whitney’s finding that the severity of lesions increased with age. Grading lesions as 1–4, corresponding to Whitney’s Type I-IV, is widely performed in the veterinary cardiology community. These two comprehensive studies have demonstrated the structural change in canine mitral valves is an age dependent process.
Effect of Breed and Genetic Inheritance
Dogs of smaller breeds, including Cavalier King Charles spaniels (CKCS), Chihuahuas, Cocker spaniels, Dachshunds and Miniature schnauzers, are particularly predisposed to MMVD,90 even though dogs of smaller breeds generally live much longer than dogs of larger breeds.91 It is well documented that certain highly predisposed breeds like the CKCS can show signs of MMVD as early as one to two years of age, with average initial diagnosis at 6.5 years old. This age is much lower than the average age of initial diagnosis of 12 years in other breeds.51,92 Because of this breed association, it has long been thought that the disease is inherited.52 Echocardiographic studies of mitral regurgitation in CKCS and Dachshunds have been conducted to confirm a parent to offspring inheritable relationship,93–95 while other studies lend support that MMVD is an autosomal dominant complex polygenic trait with variable penetrance.94 A breeding program aimed at reducing the prevalence of MMVD in the Swedish CKCS population started in 2000, but thus far it has been unsuccessful.2 An inherited form of the disease is seen in human Marfan syndrome patients who develop myxomatous mitral degeneration at an early age. However, Marfan syndrome is caused by the mutation a single gene encoding for the extracellular structural protein fibrillin.96–98 Some cases of mitral valve disease in humans, specifically familial cases of apparently autosomal dominant or X-linked inheritance of MVP or MMVD, have been traced to the expression of certain genes,99–102 although there does not appear to be a clear, universal genetic link between all cases. The specfic loci identified thus far include are on chromosome 11 (11p15.4),103 13 (13q31–32),103 chromosome 16 (16p11-p12),101,103 and the X chromosome (Xq28).100 The underlying genetic defects are not yet known for chromosomes 11, 13, and 16, but there appears to be some linkages to defects in filamin A in certain families with MVP with X-linked inheritance.103
Conclusions and future directions
It has proven challenging to distinguish the effects of normal aging from myxomatous mitral valve disease for both human and canine mitral valves. Human heart valves, both normal and diseased, are becoming more challenging to study for many reasons, such as legislation concerning human subjects protections and the asymptomatic nature of the early stages of valve disease. Canine valves also present a challenge because of the high incidence of the disease. Regardless of these hurdles, it appears that there is a distinct relationship between age and MMVD, although this relationship differs between humans and dogs. This distinction may be due to the focus upon breed-specific studies of MMVD in dogs. Indeed, age does not appear to be the only factor in the development of MMVD; as shown in studies of breed-specific and familial MMVD, there is likely a role for genetic inheritance. In contrast with MMVD, it is unclear whether age is a factor in other diseases that affect dogs, such as the less common myxomatous lesions on the tricuspid and aortic valves104 or in the relationships between heartworm disease, pulmonary hypertension, and mitral valve lesions.53,105 It is evident that significant research remains in addressing the challenges of mitral valve disease in many mammalian species. One of the most promising directions appears to be focusing on the biology of valvular cells from myxomatous and unaffected regions of mitral valves106,107 from dogs of all ages. Other studies have addressed the role of mechanical stimulation on the production of GAGs and PGs by mitral VICs grown from either leaflets or chordae15,108,109 as well as the impact of growth factors on mitral VIC migration and wound healing,110,111 all of which are relevant to MMVD. These in-vitro approaches, together with studies that focus on certain proteins and their signaling pathways, are expected to provide insight into connections between aging and MMVD and to provide genetic linkages to disease mechanisms. Given the purported role of repeated mechanical impacts in initiating and driving myxomatous degeneration, it may be useful to employ in-vitro mechanical stimulation bioreactor approaches to investigate the mechanobiology of these cells and their ability to remodel the ECM. These efforts should reveal new information about the biology of this integral component of the cardiovascular system and provide critical insight into the development of novel therapies for this devastating disease.
Table 2.
Summary of Changes Seen in Aging and Myxomatous Disease
| Property | Age Related Changes | Disease Related Changes |
|---|---|---|
| Mechanical behavior and gross anatomy | Reduced extensibility and increased elastic modulus Thickening of leaflet cusps |
Increased extensibility and reduced strength Both: Leaflets become opaque and thickened (especially along coaptation edge) Dogs: Greyish-white, smooth glistening nodules / plaques on atrial surface of leaflets and in rough zone Humans: Larger region affected throughout leaflets and chordae |
|
| ||
| Layers (microstructure) | Thickening of fibrosa layer Layers more delineated |
Disruption of atrialis layer, expansion of spongiosa layer and destruction of fibrosa layer |
|
| ||
| Cells | VICs more activated (remodeling phenotype) Decrease in cell density |
VICs and myofibroblasts activated phenotype Cell density (DNA concentration) unaltered |
| Collagen and MMPs | Increased production and remodeling Reduced crimp |
Mbr1>Loss of fiber bundle organization but increase in immature collagen MMP-2 and -9 increased MMP-2 decreased MMP-14, TIMP-2 and -3 increased with severity and age |
| Elastin | Decreases Increase in collagen reinforcement of elastic fibers |
Increase in number but appear more granular |
| PGs and GAGs | Increased abundance in fibrosa layer Decorin and biglycan greater expression Altered expression of certain sulfated GAGs Increase of chondroitin sulfate / dermatan sulfate ratio |
Accumulate in leaflets and chordae |
Abbreviations
- α-SMA
α-smooth muscle actin
- CKCS
Cavalier King Charles spaniels
- EMT
endothelial-mesenchymal transformation
- ECM
extracellular matrix
- GAG
glycosaminoglycan
- HA
hyaluronan
- MMP
matrix metalloproteinase
- MVP
mitral valve prolapse
- MVF
mitral valve free edge
- MVAC
mitral valve center of the anterior leaflet
- MMVD
myxomatous mitral valve degeneration
- PG
proteoglycan
- SLRP
small leucine-rich proteoglycan
- TIMP
tissue inhibitors of metalloproteinase
- VEC
valvular endothelial cell
- VIC
valvular interstitial cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Häggström J, Höglund K, Borgarelli M, Haggstrom J, Hoglund K. An update on treatment and prognostic indicators in canine myxomatous mitral valve disease. J Small Anim Pract. 2009 September;50(Suppl 1):25–33. doi: 10.1111/j.1748-5827.2009.00800.x. [DOI] [PubMed] [Google Scholar]
- 2.Lundin T, Kvart C. Evaluation of the Swedish breeding program for cavalier King Charles spaniels. Acta Vet Scand. 2010;52:54–59. doi: 10.1186/1751-0147-52-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boudoulas H, Sparks EE, Wooley CF. Mitral valvular regurgitation: etiology, pathophysiologic mechanisms, clinical manifestations. Herz. 2006;31(1):6–13. doi: 10.1007/s00059-006-2777-y. [DOI] [PubMed] [Google Scholar]
- 4.Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29(3):630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 5.Grande-Allen KJ, Griffin BP, Calabro A, Ratliff NB, Cosgrove DM, Vesely I. Myxomatous mitral valve chordae. II: Selective elevation of glycosaminoglycan content. J Heart Valve Dis. 2001;10(3):325–332. discussion 332–333. [PubMed] [Google Scholar]
- 6.Hayek E, Gring CN, Griffin BP. Mitral valve prolapse. Lancet. 2005;365(9458):507–518. doi: 10.1016/S0140-6736(05)17869-6. [DOI] [PubMed] [Google Scholar]
- 7.Grande-Allen KJ, Griffin BP, Ratliff NB, Cosgrove DM, Vesely I. Glycosaminoglycan profiles of myxomatous mitral leaflets and chordae parallel the severity of mechanical alterations. J Am Coll Cardiol. 2003;42(2):271–277. doi: 10.1016/s0735-1097(03)00626-0. [DOI] [PubMed] [Google Scholar]
- 8.Frater RW, Ellis FH., Jr The anatomy of the canine mitral valve, with notes on function and comparisons with other mammalian mitral valves. J Surg Res. 1961;1:171–178. doi: 10.1016/s0022-4804(61)80039-5. [DOI] [PubMed] [Google Scholar]
- 9.Walmsley R. Anatomy of human mitral valve in adult cadaver and comparative anatomy of the valve. Br Heart J. 1978;40(4):351–366. doi: 10.1136/hrt.40.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho SY. Anatomy of the mitral valve. Heart. 2002;88(Suppl 4):iv5–10. doi: 10.1136/heart.88.suppl_4.iv5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanani M, Anderson RH. The anatomy of the mitral valve: a retrospective analysis of yesterday’s future. J Heart Valve Dis. 2003;12(5):543–547. [PubMed] [Google Scholar]
- 12.Kunzelman K, Cochran R, Murphree S, Ring W, Verrier E, Eberhart R. Differential Collagen Distribution in the Mitral Valve and its influence on biomech. behavior. J Heart Valve Dis. 1993;2(2):236–244. [PubMed] [Google Scholar]
- 13.Latif N, Sarathchandra P, Taylor PM, Antoniw J, Yacoub MH. Localization and pattern of expression of extracellular matrix components in human heart valves. J Heart Valve Dis. 2005;14(2):218–227. [PubMed] [Google Scholar]
- 14.Stephens EH, Chu CK, Grande-Allen KJ. Valve proteoglycan content and glycosaminoglycan fine structure are unique to microstructure, mechanical load and age: relevance to an age-specific tissue-engineered heart valve. Acta Biomater. 2008;4(5):1148–1160. doi: 10.1016/j.actbio.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grande-Allen KJ, Calabro A, Gupta V, Wight TN, Hascall VC, Vesely I. Glycosaminoglycans and proteoglycans in normal mitral valve leaflets and chordae: association with regions of tensile and compressive loading. Glycobiology. 2004;14(7):621–633. doi: 10.1093/glycob/cwh076. [DOI] [PubMed] [Google Scholar]
- 16.Akhtar S, Meek KM, James V. Ultrastructure abnormalities in proteoglycans, collagen fibrils, and elastic fibers in normal and myxomatous mitral valve chordae tendineae. Cardiovasc Pathol. 1999;8(4):191–201. doi: 10.1016/s1054-8807(99)00004-6. [DOI] [PubMed] [Google Scholar]
- 17.Stephens EH, de Jonge N, McNeill MP, Durst Ca, Grande-Allen KJ. Age-related changes in material behavior of porcine mitral and aortic valves and correlation to matrix composition. Tissue Eng Part A. 2010;16(3):867–878. doi: 10.1089/ten.tea.2009.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunzelman KS, Reimink MS, Cochran RP. Flexible versus rigid ring annuloplasty for mitral valve annular dilatation: a finite element model. J Heart Valve Dis. 1998;7(1):108–116. [PubMed] [Google Scholar]
- 19.Stephens EH, Grande-Allen KJ. Age-related changes in collagen synthesis and turnover in porcine heart valves. J Heart Valve Dis. 2007;16(6):672–682. [PubMed] [Google Scholar]
- 20.McDonald PC, Wilson JE, McNeill S, Gao M, Spinelli JJ, Rosenberg F, Wiebe H, McManus BM. The challenge of defining normality for human mitral and aortic valves: geometrical and compositional analysis. Cardiovasc Pathol. 2002;11(4):193–209. doi: 10.1016/s1054-8807(01)00102-8. [DOI] [PubMed] [Google Scholar]
- 21.Sell S, Scully RE. Aging changes in the aortic and mitral valves: histologic and histochemical studies, with observations on the pathogenesis of calcific aortic stenosis and calcification of the mitral annulus. Am J Pathol. 1965;46(3):345–365. [PMC free article] [PubMed] [Google Scholar]
- 22.Christie GW, Barratt-Boyes BG. Age-dependent changes in the radial stretch of human aortic valve leaflets determined by biaxial testing. Ann Thorac Surg. 1995;60(2 Suppl):S156–S158. doi: 10.1016/0003-4975(95)00219-b. discussion S159. [DOI] [PubMed] [Google Scholar]
- 23.Williams TH. Mitral and Tricuspid Valve Innervation. Br Heart J. 1964;26:105–115. doi: 10.1136/hrt.26.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams TH, Folan JC, Jew JY, Wang YF. Variations in atrioventricular valve innervation in four species of mammals. Am J Anat. 1990;187(2):193–200. doi: 10.1002/aja.1001870208. [DOI] [PubMed] [Google Scholar]
- 25.Culshaw GJ, French AT, Han RI, Black A, Pearson GT, Corcoran BM. Evaluation of innervation of the mitral valves and the effects of myxomatous degeneration in dogs. Am J Vet Res. 2010;71(2):194–202. doi: 10.2460/ajvr.71.2.194. [DOI] [PubMed] [Google Scholar]
- 26.Marron K, Yacoub MH, Polak JM, Sheppard MN, Fagan D, Whitehead BF, de Leval MR, Anderson RH, Wharton J. Innervation of human atrioventricular and arterial valves. Circulation. 1996;94(3):368–375. doi: 10.1161/01.cir.94.3.368. [DOI] [PubMed] [Google Scholar]
- 27.Williams TH, Jew JY. Is the mitral valve passive flap theory overstated? An active valve is hypothesized. Medical Hypotheses. 2004;62(4):605–611. doi: 10.1016/j.mehy.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Curtis MB, Priola DV. Mechanical properties of the canine mitral valve: effects of autonomic stimulation. American Journal of Physiology. 1992;262(1 Pt 2):H56–H62. doi: 10.1152/ajpheart.1992.262.1.H56. [DOI] [PubMed] [Google Scholar]
- 29.Folan-Curran J, Wang YF, Jew JY, Williams TH. The terminal innervation patterns in young and old guinea pig heart valves: a quantitative analysis using acetylcholinesterase staining. Exp Gerontol. 1994;29(5):543–552. doi: 10.1016/0531-5565(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 30.Jew JY, Williams TH. Innervation of the mitral valve is strikingly depleted with age. Anat Rec. 1999;255(3):252–260. doi: 10.1002/(SICI)1097-0185(19990701)255:3<252::AID-AR2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 31.Jew JY, Fink CA, Williams TH. Tyrosine hydroxylase- and nitric oxide synthase-immunoreactive nerve fibers in mitral valve of young adult and aged Fischer 344 rats. J Auton Nerv Syst. 1996;58(1–2):35–43. doi: 10.1016/0165-1838(95)00106-9. [DOI] [PubMed] [Google Scholar]
- 32.Combs MD, Yutzey KE. Heart valve development: regulatory networks in development and disease. Circ Res. 2009;105(5):408–421. doi: 10.1161/CIRCRESAHA.109.201566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butcher JT, Markwald RR. Valvulogenesis: the moving target. Philos Trans R Soc Lond B Biol Sci. 2007;362(1484):1489–1503. doi: 10.1098/rstb.2007.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eisenberg LM, Markwald RR. Molecular regulation of atrioventricular valvuloseptal morphogenesis. Circ Res. 1995;77(1):1–6. doi: 10.1161/01.res.77.1.1. [DOI] [PubMed] [Google Scholar]
- 35.Bischoff J, Aikawa E. Progenitor Cells Confer Plasticity to Cardiac Valve Endothelium. J Cardiovasc Transl Res. 2011 doi: 10.1007/s12265-011-9312-0. [DOI] [PubMed] [Google Scholar]
- 36.Corcoran BM, Black A, Anderson H, McEwan JD, French A, Smith P, Devine C. Identification of surface morphologic changes in the mitral valve leaflets and chordae tendineae of dogs with myxomatous degeneration. Am J Vet Res. 2004;65(2):198–206. doi: 10.2460/ajvr.2004.65.198. [DOI] [PubMed] [Google Scholar]
- 37.Aikawa E, Whittaker P, Farber M, Mendelson K, Padera RF, Aikawa M, Schoen FJ. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: implications for postnatal adaptation, pathology, and tissue engineering. Circulation. 2006;113(10):1344–1352. doi: 10.1161/CIRCULATIONAHA.105.591768. [DOI] [PubMed] [Google Scholar]
- 38.Stein PD, Wang CH, Riddle JM, Sabbah HN, Magilligan DJ, Jr, Hawkins ET, Magilligan DJ. Scanning electron microscopy of operatively excised severely regurgitant floppy mitral valves. Am J Cardiol. 1989;64(5):392–394. doi: 10.1016/0002-9149(89)90543-2. [DOI] [PubMed] [Google Scholar]
- 39.Mow T, Pedersen HD. Increased endothelin-receptor density in myxomatous canine mitral valve leaflets. J Cardiovasc Pharmacol. 1999;34(2):254–260. doi: 10.1097/00005344-199908000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Blevins TL, Carroll JL, Raza AM, Grande-Allen KJ. Phenotypic characterization of isolated valvular interstitial cell subpopulations. J Heart Valve Dis. 2006;15(6):815–822. [PubMed] [Google Scholar]
- 41.Mulholland DL, Gotlieb AI. Cardiac Valve Interstitial Cells: Regulator of Valve Structure and Function. Cardiovasc Pathol. 1997;6(3):167–174. doi: 10.1016/s1054-8807(96)00115-9. [DOI] [PubMed] [Google Scholar]
- 42.Rabkin E, Aikawa M, Stone JR, Fukumoto Y, Libby P, Schoen FJ. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation. 2001;104(21):2525–2532. doi: 10.1161/hc4601.099489. [DOI] [PubMed] [Google Scholar]
- 43.Merryman WD, Youn I, Lukoff HD, Krueger PM, Guilak F, Hopkins RA, Sacks MS. Correlation between heart valve interstitial cell stiffness and transvalvular pressure: implications for collagen biosynthesis. Am J Physiol Heart Circ Physiol. 2006;290(1):H224–H231. doi: 10.1152/ajpheart.00521.2005. [DOI] [PubMed] [Google Scholar]
- 44.Liu AC, Joag VR, Gotlieb AI. The Emerging Role of Valve Interstitial Cell Phenotypes in Regulating Heart Valve Pathobiology. Am J Pathol. 2007;171(5):1407–1418. doi: 10.2353/ajpath.2007.070251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rabkin-Aikawa E, Farber M, Aikawa M, Schoen FJ. Dynamic and reversible changes of interstitial cell phenotype during remodeling of cardiac valves. J Heart Valve Dis. 2004;13(5):841–847. [PubMed] [Google Scholar]
- 46.Buchanan JW. Chronic valvular disease (endocardiosis) in dogs. Adv Vet Sci Comp Med. 1977;21:75–106. [PubMed] [Google Scholar]
- 47.Amoresano A, Amedeo S, D’andrea G, Siciliano R, Gagna C, Castagnaro M, Marino G, Guarda F. N-Linked glycans of proteins from mitral valves of normal pigs and pigs affected by endocardiosis. FEBS J. 2000;267(5):1299–1306. doi: 10.1046/j.1432-1327.2000.01090.x. [DOI] [PubMed] [Google Scholar]
- 48.Gagna C, Meier D, Ru G, Pospischil A, Guarda F. Pathology of mitral valve in regularly slaughtered pigs: an abattoir survey on the occurrence of myxoid degeneration (endocardiosis), fibrosis and valvulitis. Zentralbl Veterinarmed A. 1998;45(6–7):383–395. doi: 10.1111/j.1439-0442.1998.tb00841.x. [DOI] [PubMed] [Google Scholar]
- 49.Castagnaro M, Amedeo S, Bertolotto A, Manzardo E, Riccio A, Guarda F. Morphological and biochemical investigations of mitral valve endocardiosis in pigs. Res Vet Sci. 1997;62(2):121–125. doi: 10.1016/s0034-5288(97)90132-6. [DOI] [PubMed] [Google Scholar]
- 50.Guglielmini C. Cardiovascular diseases in the ageing dog: diagnostic and therapeutic problems. Vet Res Commun. 2003;27 (Suppl 1):555–560. doi: 10.1023/b:verc.0000014216.73396.f6. [DOI] [PubMed] [Google Scholar]
- 51.Beardow AW, Buchanan JW. Chronic mitral valve disease in cavalier King Charles spaniels: 95 cases (1987–1991) J Am Vet Med Assoc. 1993;203(7):1023–1029. [PubMed] [Google Scholar]
- 52.Lewis T, Swift S, Woolliams JA, Blott S. Heritability of premature mitral valve disease in Cavalier King Charles spaniels. Vet J. 2011;188(1):73–76. doi: 10.1016/j.tvjl.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 53.Detweiler DK, Patterson DF. The prevalence and types of cardiovascular disease in dogs. Ann N Y Acad Sci. 1965;127(1):481–516. doi: 10.1111/j.1749-6632.1965.tb49421.x. [DOI] [PubMed] [Google Scholar]
- 54.Kogure K. Pathology of chronic mitral valvular disease in the dog. Nippon Juigaku Zasshi. 1980;42(3):323–335. doi: 10.1292/jvms1939.42.323. [DOI] [PubMed] [Google Scholar]
- 55.Whittaker P, Boughner DR, Perkins DG, Canham PB. Quantitative structural analysis of collagen in chordae tendineae and its relation to floppy mitral valves and proteoglycan infiltration. Br Heart J. 1987;57(3):264–269. doi: 10.1136/hrt.57.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tamura K, Fukuda Y, Ishizaki M, Masuda Y, Yamanaka N, Ferrans VJ. Abnormalities in elastic fibers and other connective-tissue components of floppy mitral valve. Am Heart J. 1995;129(6):1149–1158. doi: 10.1016/0002-8703(95)90397-6. [DOI] [PubMed] [Google Scholar]
- 57.Whitney JC. Observations on the effect of age on the severity of heart valve lesions in the dog. J Small Anim Pract. 1974;15(8):511–522. doi: 10.1111/j.1748-5827.1974.tb06529.x. [DOI] [PubMed] [Google Scholar]
- 58.Barber JE, Kasper FK, Ratliff NB, Cosgrove DM, Griffin BP, Vesely I. Mechanical properties of myxomatous mitral valves. J Thorac Cardiovasc Surg. 2001;122(5):955–962. doi: 10.1067/mtc.2001.117621. [DOI] [PubMed] [Google Scholar]
- 59.Black A, French AT, Dukes-McEwan J, Corcoran BM. Ultrastructural morphologic evaluation of the phenotype of valvular interstitial cells in dogs with myxomatous degeneration of the mitral valve. Am J Vet Res. 2005;66(8):1408–1414. doi: 10.2460/ajvr.2005.66.1408. [DOI] [PubMed] [Google Scholar]
- 60.Whitney JC. Cardiovascular pathology. J Small Anim Pract. 1967;8(8):459–465. doi: 10.1111/j.1748-5827.1967.tb04575.x. [DOI] [PubMed] [Google Scholar]
- 61.Pomerance A. Ballooning deformity (mucoid degeneration) of atrioventricular valves. Br Heart J. 1969;31(3):343–351. doi: 10.1136/hrt.31.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pedersen HD, Häggström J. Mitral valve prolapse in the dog: a model of mitral valve prolapse in man. Cardiovasc Res. 2000;47(2):234–43. doi: 10.1016/s0008-6363(00)00113-9. [DOI] [PubMed] [Google Scholar]
- 63.Sacks MS, He Z, Baijens L, Wanant S, Shah P, Sugimoto H, Yoganathan AP. Surface strains in the anterior leaflet of the functioning mitral valve. Ann Biomed Eng. 2002;30(10):1281–1290. doi: 10.1114/1.1529194. [DOI] [PubMed] [Google Scholar]
- 64.Fornes P, Heudes D, Fuzellier JF, Tixier D, Bruneval P, Carpentier A. Correlation between clinical and histologic patterns of degenerative mitral valve insufficiency: a histomorphometric study of 130 excised segments. Cardiovasc Pathol. 1999;8(2):81–92. doi: 10.1016/s1054-8807(98)00021-0. [DOI] [PubMed] [Google Scholar]
- 65.Han RI, Black A, Culshaw GJ, French AT, Else RW, Corcoran BM. Distribution of myofibroblasts, smooth muscle–like cells, macrophages, and mast cells in mitral valve leaflets of dogs with myxomatous mitral valve disease. Am J Vet Res. 2008;69(6):763–769. doi: 10.2460/ajvr.69.6.763. [DOI] [PubMed] [Google Scholar]
- 66.Darke PG. Valvular incompetence in cavalier King Charles spaniels. Vet Rec. 1987;120(15):365–366. doi: 10.1136/vr.120.15.365. [DOI] [PubMed] [Google Scholar]
- 67.Soini Y, Satta J, Määttä M, Autio-Harmainen H. Expression of MMP2, MMP9, MT1-MMP, TIMP1, and TIMP2 mRNA in valvular lesions of the heart. J Pathol. 2001;194(2):225–231. doi: 10.1002/path.850. [DOI] [PubMed] [Google Scholar]
- 68.Barth PJ, Köster H, Moosdorf R. CD34+ fibrocytes in normal mitral valves and myxomatous mitral valve degeneration. Pathol Res Pract. 2005;201(4):301–304. doi: 10.1016/j.prp.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 69.Veinot JP, Prichett-Pejic W, Song J, Waghray G, Parks W, Mesana TG, Ruel M. CD117- positive cells and mast cells in adult human cardiac valves--observations and implications for the creation of bioengineered grafts. Cardiovasc Pathol. 2006;15(1):36–40. doi: 10.1016/j.carpath.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 70.Disatian S, Ehrhart EJ, Zimmerman S, Orton EC. Interstitial cells from dogs with naturally occurring myxomatous mitral valve disease undergo phenotype transformation. J Heart Valve Dis. 2008;17(4):402–411. discussion 412. [PubMed] [Google Scholar]
- 71.Aupperle H, März I, Thielebein J, Schoon H-A. Expression of transforming growth factor- beta1, -beta2 and -beta3 in normal and diseased canine mitral valves. J Comp Pathol. 2008;139(2–3):97–107. doi: 10.1016/j.jcpa.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 72.Han RI, Black A, Culshaw G, French AT, Corcoran BM. Structural and cellular changes in canine myxomatous mitral valve disease: an image analysis study. J Heart Valve Dis. 2010;19(1):60–70. [PubMed] [Google Scholar]
- 73.Togashi M, Tamura K, Nitta T, Ishizaki M, Sugisaki Y, Fukuda Y. Role of matrix metalloproteinases and their tissue inhibitor of metalloproteinases in myxomatous change of cardiac floppy valves. Pathol Int. 2007;57(5):251–259. doi: 10.1111/j.1440-1827.2007.02096.x. [DOI] [PubMed] [Google Scholar]
- 74.Aupperle H, Thielebein J, Kiefer B, März I, Dinges G, Schoon H-A. An immunohistochemical study of the role of matrix metalloproteinases and their tissue inhibitors in chronic mitral valvular disease (valvular endocardiosis) in dogs. Vet J. 2009;180(1):88–94. doi: 10.1016/j.tvjl.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 75.Aupperle H, Thielebein J, Kiefer B, Marz I, Dinges G, Schoon HA, Schubert A. Expression of genes encoding matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) in normal and diseased canine mitral valves. J Comp Pathol. 2009;140(4):271–277. doi: 10.1016/j.jcpa.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 76.Oyama MA, Chittur SV. Genomic expression patterns of mitral valve tissues from dogs with degenerative mitral valve disease. Am J Vet Res. 2006;67(8):1307–1318. doi: 10.2460/ajvr.67.8.1307. [DOI] [PubMed] [Google Scholar]
- 77.Cheng TO. Mitral valve prolapse--some historical facts. Int J Cardiol. 2006;112(2):264. doi: 10.1016/j.ijcard.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 78.Pomerance A, Whitney JC. Heart valve changes common to man and dog: a comparative study. Cardiovasc Res. 1970;4(1):61–66. doi: 10.1093/cvr/4.1.61. [DOI] [PubMed] [Google Scholar]
- 79.Hickey AJ, Wilcken DE. Age and the clinical profile of idiopathic mitral valve prolapse. Br Heart J. 1986;55(6):582–586. doi: 10.1136/hrt.55.6.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zuppiroli A, Rinaldi M, Kramer-Fox R, Favilli S, Roman MJ, Devereux RB. Natural history of mitral valve prolapse. Am J Cardiol. 1995;75(15):1028–1032. doi: 10.1016/s0002-9149(99)80718-8. [DOI] [PubMed] [Google Scholar]
- 81.Davies MJ, Moore BP, Braimbridge MV. The floppy mitral valve. Study of incidence, pathology, and complications in surgical, necropsy, and forensic material. Br Heart J. 1978;40(5):468–481. doi: 10.1136/hrt.40.5.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rahimtoola SH. The year in valvular heart disease. J Am Coll Cardiol. 2004;43(3):491–504. doi: 10.1016/j.jacc.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 83.Haidet GC. Effect of age on cardiovascular responses to static muscular contraction in beagles. J Appl Physiol. 1992;73(6):2320–2327. doi: 10.1152/jappl.1992.73.6.2320. [DOI] [PubMed] [Google Scholar]
- 84.Hamlin RL. Geriatric heart diseases in dogs. Vet Clin North Am Small Anim Pract. 2005;35(3):597–615. doi: 10.1016/j.cvsm.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 85.Borgarelli M, Haggstrom J. Canine degenerative myxomatous mitral valve disease: natural history, clinical presentation and therapy. Vet Clin North Am Small Anim Pract. 2010;40(4):651–663. doi: 10.1016/j.cvsm.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 86.Bernay F, Bland JM, Häggström J, Baduel L, Combes B, Lopez A, Kaltsatos V. Efficacy of spironolactone on survival in dogs with naturally occurring mitral regurgitation caused by myxomatous mitral valve disease. J Vet Intern Med. 2010;24(2):331–341. doi: 10.1111/j.1939-1676.2009.0467.x. [DOI] [PubMed] [Google Scholar]
- 87.Häggström J, Boswood a, O’Grady M, Jöns O, Smith S, Swift S, Borgarelli M, Gavaghan B, Kresken J-G, Patteson M, Ablad B, Bussadori CM, Glaus T, Kovacevi3 a, Rapp M, Santilli Ra, Tidholm a, Eriksson a, Belanger MC, Deinert M, Little CJL, Kvart C, French a, Rønn-Landbo M, Wess G, Eggertsdottir aV, O’Sullivan ML, Schneider M, Lombard CW, Dukes-McEwan J, Willis R, Louvet a, DiFruscia R. Effect of pimobendan or benazepril hydrochloride on survival times in dogs with congestive heart failure caused by naturally occurring myxomatous mitral valve disease: the QUEST study. J Vet Intern Med. 2008;22(5):1124–35. doi: 10.1111/j.1939-1676.2008.0150.x. [DOI] [PubMed] [Google Scholar]
- 88.Davies MJ, Moore BP, Braimbridge MV. The floppy mitral valve. Study of incidence, pathology, and complications in surgical, necropsy, and forensic material. Br Heart J. 1978;40(5):468–481. doi: 10.1136/hrt.40.5.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sahasakul Y, Edwards WD, Naessens JM, Tajik AJ. Age-related changes in aortic and mitral valve thickness: implications for two-dimensional echocardiography based on an autopsy study of 200 normal human hearts. Am J Cardiol. 1988;62(7):424–430. doi: 10.1016/0002-9149(88)90971-x. [DOI] [PubMed] [Google Scholar]
- 90.Olsen LH, Martinussen T, Pedersen HD. Early echocardiographic predictors of myxomatous mitral valve disease in dachshunds. Vet Rec. 2003;152(10):293–297. doi: 10.1136/vr.152.10.293. [DOI] [PubMed] [Google Scholar]
- 91.Greer KA, Canterberry SC, Murphy KE. Statistical analysis regarding the effects of height and weight on life span of the domestic dog. Res Vet Sci. 2007;82(2):208–214. doi: 10.1016/j.rvsc.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 92.Pedersen HD, Lorentzen KA, Kristensen BO. Echocardiographic mitral valve prolapse in cavalier King Charles spaniels: epidemiology and prognostic significance for regurgitation. Vet Rec. 1999;144(12):315–320. doi: 10.1136/vr.144.12.315. [DOI] [PubMed] [Google Scholar]
- 93.Olsen LH, Mortensen K, Martinussen T, Larsson LI, Baandrup U, Pedersen HD. Increased NADPH-diaphorase activity in canine myxomatous mitral valve leaflets. J Comp Pathol. 2003;129(2–3):120–130. doi: 10.1016/s0021-9975(03)00019-7. [DOI] [PubMed] [Google Scholar]
- 94.Olsen LH, Fredholm M, Pedersen HD. Epidemiology and inheritance of mitral valve prolapse in Dachshunds. J Vet Intern Med. 1999;13(5):448–456. doi: 10.1892/0891-6640(1999)013<0448:eaiomv>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 95.Swenson L, Haggstrom J, Kvart C, Juneja RK. Relationship between parental cardiac status in Cavalier King Charles spaniels and prevalence and severity of chronic valvular disease in offspring. J Am Vet Med Assoc. 1996;208(12):2009–2012. [PubMed] [Google Scholar]
- 96.Chou HT, Shi YR, Hsu Y, Tsai FJ. Association between fibrillin-1 gene exon 15 and 27 polymorphisms and risk of mitral valve prolapse. J Heart Valve Dis. 2003;12(4):475–481. [PubMed] [Google Scholar]
- 97.Weyman AE, Scherrer-Crosbie M. Marfan syndrome and mitral valve prolapse. J Clin Invest. 2004;114(11):1543–1546. doi: 10.1172/JCI23701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nasuti JF, Zhang PJ, Feldman MD, Pasha T, Khurana JS, Gorman JH, 3rd, Gorman RC, Narula J, Narula N. Fibrillin and other matrix proteins in mitral valve prolapse syndrome. Ann Thorac Surg. 2004;77(2):532–536. doi: 10.1016/S0003-4975(03)01584-4. [DOI] [PubMed] [Google Scholar]
- 99.Levine R, Slaugenhaupt S. Molecular genetics of mitral valve prolapse. Curr Opin Cardiol. 2007;22(3):171–175. doi: 10.1097/HCO.0b013e3280f3bfcd. [DOI] [PubMed] [Google Scholar]
- 100.Kyndt F, Schott JJ, Trochu JN, Baranger F, Herbert O, Scott V, Fressinaud E, David A, Moisan JP, Bouhour JB, Le Marec H, Bénichou B. Mapping of X-linked myxomatous valvular dystrophy to chromosome Xq28. Am J Hum Genet. 1998;62(3):627–632. doi: 10.1086/301747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Disse S, Abergel E, Berrebi A, Houot aM, Le Heuzey JY, Diebold B, Guize L, Carpentier A, Corvol P, Jeunemaitre X. Mapping of a first locus for autosomal dominant myxomatous mitral-valve prolapse to chromosome 16p11.2-p12.1. Am J Hum Genet. 1999;65(5):1242–1251. doi: 10.1086/302624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Grau JB, Pirelli L, Yu P-J, Gallowaya C, Ostrer H. The genetics of mitral valve prolapse. Clin Genet. 2007;72(4):288–95. doi: 10.1111/j.1399-0004.2007.00865.x. [DOI] [PubMed] [Google Scholar]
- 103.Lardeux A, Kyndt F, Lecointe S, Marec HL, Merot J, Schott J-J, Tourneau TL, Probst V. Filamin-A-Related Myxomatous Mitral Valve Dystrophy: Genetic, Echocardiographic and Functional Aspects. J Cardiovasc Transl Res. 2011 doi: 10.1007/s12265-011-9308-9. [DOI] [PubMed] [Google Scholar]
- 104.Machida N, Hoshi K, Kobayashi M, Katsuda S, Yamane Y. Cardiac myxoma of the tricuspid valve in a dog. J Comp Pathol. 2003;129(4):320–324. doi: 10.1016/s0021-9975(03)00049-5. [DOI] [PubMed] [Google Scholar]
- 105.Sasaki Y, Kitagawa H, Hirano Y. Relationship between pulmonary arterial pressure and lesions in the pulmonary arteries and parenchyma, and cardiac valves in canine dirofilariasis. J Vet Med Sci. 1992;54(4):739–744. doi: 10.1292/jvms.54.739. [DOI] [PubMed] [Google Scholar]
- 106.Obayashi K, Miyagawa-Tomita S, Matsumoto H, Koyama H, Nakanishi T, Hirose H. Effects of transforming growth factor-β3 and matrix metalloproteinase-3 on the pathogenesis of chronic mitral valvular disease in dogs. Am J Vet Res. 2011;72(2):194–202. doi: 10.2460/ajvr.72.2.194. [DOI] [PubMed] [Google Scholar]
- 107.Heaney AM, Bulmer BJ, Ross CR, Schermerhorn T. A technique for in vitro culture of canine valvular interstitial cells. J Vet Cardiol. 2009;11(1):1–7. doi: 10.1016/j.jvc.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 108.Gupta V, Barzilla JEE, Mendez JSS, Stephens EHH, Lee ELL, Collard CDD, Laucirica R, Weigel PHH, Grande-Allen KJJ. Abundance and location of proteoglycans and hyaluronan within normal and myxomatous mitral valves. Cardiovasc Pathol. 2009;18(4):191–197. doi: 10.1016/j.carpath.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Blevins TL, Peterson SB, Lee EL, Bailey AM, Frederick JD, Huynh TN, Gupta V, Grande-Allen KJ. Mitral valvular interstitial cells demonstrate regional, adhesional, and synthetic heterogeneity. Cells Tissues Organs. 2008;187(2):113–122. doi: 10.1159/000108582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Han L, Gotlieb AI. Fibroblast growth factor-2 promotes in vitro mitral valve interstitial cell repair through transforming growth factor-β/Smad signaling. Am J Pathol. 2011;178(1):119–127. doi: 10.1016/j.ajpath.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu AC, Gotlieb AI. Transforming growth factor-beta regulates in vitro heart valve repair by activated valve interstitial cells. Am J Pathol. 2008;173(5):1275–1285. doi: 10.2353/ajpath.2008.080365. [DOI] [PMC free article] [PubMed] [Google Scholar]