Abstract
The aim of this retrospective study is to describe changes of seizure frequency in epilepsy patients who participated in the Andrews/Reiter behavioral intervention for epilepsy. For this uncontrolled retrospective study, data was extracted from patients’ medical journals. Intention-to-treat-analyses were restricted to patients with sufficient documentation supporting a diagnosis of probable or definite epilepsy. Main outcome variable was a comparison of mean seizure frequency at baseline and towards completion of the program. The seizure frequency of 30 (50%%) patients showed a clinically meaningful improvement (>50% reduction of seizures) towards the end of the intervention. Twenty-two (37%) patients became seizure-free at the end of the intervention. In summary, a clinically meaningful reduction in reported seizure frequency was observed in epilepsy patients who received the Andrews/Reiter intervention for epilepsy. Prospective trials are needed to further investigate the program’s efficacy and to study epileptic seizure triggers.
Keywords: Seizure Warning, Aura Interruption, Seizure Triggers, Seizure Self-Control, Cognitive Behavioral Therapy, Counseling, Relaxation, Self-Efficacy
INTRODUCTION
Uncertainty about when an epileptic seizure will occur may lead to high levels of anxiety in the epilepsy population. 1 This does not only apply to drug-refractory patients, concerns over recurring seizures may diminish the quality of life even in patients with well-controlled epilepsy. 2
Behavioral interventions intend to decrease the possible impact of seizure precipitants by providing instructions for prevention or interruption of seizures and may help epilepsy patients regain a sense of self-control. Therefore they have been proposed as adjunctive or alternative treatments to pharmaceutical and/or surgical treatment options. 3–5
The majority of epilepsy patients report at least one non-specific seizure precipitant (or, “seizure inducing factor”, i.e. physiologic factors that affect the whole brain and temporarily lower the seizure threshold). 6 Reflex epilepsy on the other hand is characterized by seizures that are precipitated by a specific identifiable factor or “seizure trigger” which may activate hyperexcitable cortex close to a seizure focus and could trigger epileptic activity in susceptible patients. 7 Electro-clinical evidence suggests the existence of complex cognitive or emotional “seizure triggers”. 8–10
It has been suggested that certain human brain regions represent emotion-category specific activity at a supramodal level. 11 Previous observations with the Andrews/Reiter approach suggested that specific emotions may function as potential endogenous triggers for epileptogenic foci in specific hemispheres (e.g. anger for left-hemispheric, and fear for right-hemispheric foci). 12 It is possible that the physiology that underlies the genesis of seizures in the case of emotional seizure triggers may function similarly to the model of reflex epilepsies.
Most epilepsy patients report at least one warning sign (prodrome or aura) prior to a seizure. 13 Many patients report being able to successfully prevent and interrupt their seizures by various self-developed strategies. 14
The intervention developed by Andrews and Reiter 15 may represent the most comprehensively developed cognitive behavioral approach to epilepsy 16 and benefits from 30 years of experience providing epilepsy-specific counseling. The purpose of the present study was to systematically analyze the extensive long-term data that is available from a patient population treated consistently with the Andrews/Reiter therapeutic approach. This retrospective study examines changes of seizure frequency in epilepsy patients who received a short, intensive version of the Andrews/Reiter intervention. In the following, a short description of the intervention is given.
The intensive Andrews/Reiter program began with a three-day assessment of individual epilepsy characteristics and potential seizure triggers presented by each patient. The assessment then led to an individualized treatment plan. The implementation of the treatment plan by the patient was supervised by the therapist using weekly phone calls or email communication. The initial patient assessment and the development and supervision of the treatment plan of all patients were conducted by the epilepsy counselor Dr. Donna Andrews. A neurologist’s evaluation prior to the initial patient assessment was done by Dr. Joel Reiter (on all patients seen before 2005) or other neurologists (on later patients).
On the first day, a detailed history was taken in order to explore a patient’s personal and social development, future goals and to identify potential emotional or other seizure triggers. The Wechsler Adult Intelligence Scale (WAIS) or Wechsler Intelligence Scale for Children (WISC) was used for intellectual assessment and to help identify cognitive changes and localize a seizure focus. The MMPI or MMPI for Adolescents (MMPI-A), and the House-Tree-Person Test were used for individual psychological assessment, and the identification of potential subconscious issues. On the second day, deep diaphragmatic breathing was introduced as a technique to prevent and interrupt impending seizures. EEG (four electrodes placed bilaterally on temporal and frontal cortex) and EMG (measuring forehead muscle tension) biofeedback was also used once to confirm to patient and therapist the patient’s ability to induce a state of awake relaxation and to allow a patient to observe the correspondence between subjective states of relaxation and measurable changes of EEG and EMG activity. For each patient, an individualized treatment plan was formulated. Treatment plans included specific daily tasks: reading the workbook Taking Control of Your Epilepsy, 15 recording seizure occurrences in seizure logs, journaling and the practice of deep relaxation by using a CD. 17
For continuation at home, patients were asked to keep daily journals of their activities to allow for the identification of recurring specific (e.g. fear, anger, situational settings, and sensory triggers) and unspecific precipitants (e.g. sleep deprivation, hypoglycemia) and warning signs of a seizure. Patients were provided guidance by the therapist in the identification of trigger sensitivities and coping strategies. Coping strategies included strict avoidance, limited amounts of exposure or the application of desensitizing strategies. Depending on the endogenous or exogenous nature of a precipitant, limitation strategies required internal or external modifications. They were implemented by setting small and realistic weekly goals. In addition, the daily practice of relaxation was intended to decrease chronically raised levels of stress and deep, diaphragmatic breathing was used as a tool in trigger situations to prevent or arrest impending seizures.
METHODS
Data Sources
Between 1980 and 2010, 2504 patients were treated by Dr. Andrews using the Andrews/Reiter cognitive behavioral intervention. A 3 day intensive version of the intervention was offered to patients who were not living in commuting distance of the private practice setting. All participants in the intensive intervention signed an informed consent form to authorize the use of their information for research purposes. After 1996, the intensive intervention followed a uniform protocol. To select a homogenous, representative group for this retrospective study, data was obtained of all 135 non-local patients who followed the Andrews/Reiter intensive program’s uniform protocol between 1996 and 2009.. To ensure patient confidentiality, no personal identifying information was obtained. All aspects of this retrospective study were reviewed and approved by the Andrews/Reiter Research Board and the OHSU Institutional Review Board.
Data was obtained from patient files that included copies of prior medical records, a neurologist evaluation, the report and treatment plan of epilepsy counselor Dr. Andrews, copies of the patients’ journals and seizure logs, extracts from e-mail communications and reports made by patients during their phone consultations with Dr. Andrews. Data about self-reported seizure warning signs and emotion- and stressor-related seizure precipitants was obtained in order to assess a patient’s awareness of these at the beginning of the intervention. Data about seizure occurrences and the interruption of seizures during the aura stage were obtained to allow for the long term analysis of seizure frequency. All increases, decreases and discontinuations of previously prescribed anticonvulsive medications and introductions of newly prescribed anticonvulsive medications were recorded in order to limit the analysis of seizure frequency to periods of unchanged medication. To assess the pretreatment seizure frequency and to evaluate the variability of seizure frequency prior to the beginning of the intervention, the maximum and minimum seizure frequency and the duration of transient seizure remissions before onset of treatment were obtained.
Data Analysis
The data in this study consisted of patient self-reports, which were provided by patients in pursuit of getting well rather than to contribute to a scientific evaluation of the program. The availability of test results and medical reports was often limited by a patient’s lack of financial resources. Dealing with occasional information gaps posed a challenge for data analysis and required a careful methodological approach that will be described in the following.
1. Reliability of Epilepsy Diagnosis
As a first step of data analysis, seizure semiology, narratives of diagnostic tests (Electroencephalography (EEG), Magnetic Resonance Imaging (MRI), Computed Tomography (CT) and EEG-Video-Telemetry (CCTV-EEG)), risk factors and response to anticonvulsant medication were reviewed by an epileptologist who was not involved in patient treatment to assign a certainty score to each patient. Epileptiform EEG abnormalities, epileptogenic MRI/CT findings, a reliable semiology, and a response to anticonvulsant medication were each assigned one point. A positive inpatient CCTV-EEG was assigned three points. Thus, the maximum possible diagnostic certainty score was seven. A patient with a diagnostic certainty score of three points or more was considered a patient with definite epilepsy, and a patient with one or two points was considered a patient with probable epilepsy.
In addition, the probability of additional non-epileptic spells (NES) was determined using a combination of the Minnesota Multiphasic Personality Inventory (MMPI) Hysteria (Hy) Score, the duration of seizures and the presence of epileptiform abnormalities in a routine EEG. This method has been described to have an overall accuracy of 86% for predicting NES in the inpatient setting, compared to 74% for EEG alone. 18 The main concern with additional NES was that their presence would confound seizure frequency outcomes. Therefore, a patient with a probability of NES greater than 30% was considered as a patient with probable additional NES.
2. Baseline Measurement
The change in seizure frequency from baseline was the primary outcome in this study. The baseline was obtained by averaging the seizure count over the first two four-week intervals at the beginning of treatment.
3. Outcome Measures
Two seizure outcome measures were used in this study: change in seizure frequency and count of seizure free weeks.
The primary outcome measure was obtained by comparing the baseline with the seizure frequency during the final months of treatment. The baseline seizure count per four weeks was compared with each of the last two four-week intervals of consecutive documentation. Outcomes were categorized as follows: seizure free (100% decrease), much improved (90% to 100% decrease), improved (≥ 50% to 90% decrease), minimal change (<50% decrease) and worsened (increase of seizures), or “insufficient documentation” for those 17 patients (28%) who did not provide sufficient documentation for this step of analysis (i.e. less than 8 weeks of consecutive documentation each at the beginning and at the end of the intervention). As a separate measure, each patient’s category of outcome was retraced for as long as consistent recording was available to measure the duration of any improvement or other change. Duration of improvement or change was calculated as a median number of months for each category of outcome.
As a secondary seizure outcome measure, seizure-free weeks were determined to allow an assessment of meaningful change even for some patients with insufficient seizure documentation. The total available documentation from each patient was divided into equal first, second and third portions and the percentage of seizure-free weeks of all documented weeks was determined during the first and the last third. The mean difference was calculated in points of percent (pp), and a paired t-test (one-tailed) was performed to determine if the mean change was significant, using p=0.05 as significance level. Seven patients (12%) who had provided insufficient documentation for the primary seizure outcome measure could be assessed in the secondary outcome measure of seizure free weeks. These seven had provided a mean duration of 18 weeks of documentation. In contrast, another ten patients (17%) did not provide any seizure logs and therefore could not be included in this analysis.
Changes in anticonvulsant medication
Only seizure data from periods with unchanged or decreased anticonvulsant dosage were used for analysis. In eight cases, only the seizure data prior to a dosage change of drug was used for analysis, and in one case, only the seizure data after a dosage change at the beginning of treatment was used for analysis.
4. Data Inclusion Criteria
In order to restrict data analysis to patients with a reliable diagnosis of epilepsy and interpretable seizure data, the following data criteria had to be met by each patient to be included in this retrospective study:
-
1
A minimum diagnostic certainty score of 1.
-
2
A probability of additional NES < 30%.
-
3.1
No history of relapsing remissions, i.e. one or more transient seizure remissions that lasted one year or longer.
-
3.2
At least 3 seizures prior to behavioral treatment.
-
3.3
At least one seizure event during each of the first two four-week intervals at the beginning of treatment.
Summary: Excluded Patients
Of 135 patients, the data of 75 patients did not meet the inclusion criteria and was therefore excluded from further analyses:
-
1
Eight patients were excluded because of a diagnostic certainty score of 0.
-
2
Twenty-nine patients were excluded due to a probability of additional NES > 30%.
-
3.1
Nine patients were excluded because they had reported a transient seizure remission of ≥ 1 year prior to the beginning of the intervention.
-
3.2
Three patients were excluded because they had reported less than three seizures prior to the beginning of the intervention.
-
3.3
Twenty-six patients were excluded because they had documented less than one seizure during each of the first two four-week intervals at the beginning of treatment.
Of the remaining 60 patients who met the inclusion criteria, 20 (33%) were categorized as patients with a definite diagnosis of epilepsy due to a diagnostic certainty score of ≥ 3.
RESULTS
Characteristics of the Patient Population
Thirty-one patients (52%) had been experiencing complex partial seizures with a potential of secondary generalization for at least five years (ILAE criteria). Thirty-four (57%) patients had failed at least two antiepileptic drugs (AED) before the beginning of treatment and could be considered drug refractory patients. 19 Only three patients (5%) had never taken any medication before entering the program. Six patients (10%) had undergone resective epilepsy surgery. An overview of the composition of the whole patient population and the different diagnostic certainty groups is given in Table 1.
Table 1. Patient population.
Patient population by age, gender, IQ, diagnostic details and treatment of epilepsy prior to and at the beginning of the program, given as the mean and standard deviation (SD) or as total patient number (and in percent) for each diagnostic certainty group. The information concerning unilateral and bilateral focal impairment was not available in all cases with partial seizures.
| All Patients N = 60 |
Definite Epilepsy N = 20 |
Probable Epilepsy N = 40 |
|
|---|---|---|---|
| Age, Mean Years ± SD | 28 ± 12 | 28 ± 13 | 27 ± 11 |
| Mean IQ ± SD | 129 ± 19 | 128 ± 21 | 130 ± 17 |
| MeanYrs since 1st spell± SD | 17 ± 12 | 16 ± 12 | 18 ± 12 |
| Mean N° of AEDs ± SD | 1.7 ± 0.9 | 1.9 ± 0.8 | 1.5 ± 0.8 |
| Mean N° of AEDTrials ± SD | 3.1 ± 2.2 | 3.8 ± 2.5 | 2.9 ± 1.9 |
| Failed at least 2 AED Trials | 34 (57%) | 13 (65%) | 21 (52%) |
| Resective Epilepsy Surgery | 6 (10%) | 1 (5%) | 5 (12%) |
| Gender | |||
| Male | 30 (50%) | 10 (50%) | 20 (50%) |
| Female | 30 (50%) | 10 (50%) | 20 (50%) |
| Type of Seizure Disorder | |||
| Primary Generalized | 9 (15%) | 1 (6%) | 8 (20%) |
| Partial | 51 (85%) | 19(95%) | 32 (80%) |
| Type of Partial Epilepsy | N = 51 | N = 19 | N = 32 |
| Secondarily generalized | 38 (75%) | 16( 84%) | 22 (69%) |
| Lateralization | |||
| Unilateral | 18 (35%) | 7 (37%) | 11 (34%) |
| Bilateral | 22 (43%) | 8 (42%) | 14 (43%) |
Identification of Triggers and Seizure Warning Signs during Initial Assessment
During the initial patient assessment, 39 (65%) patients identified at least one specific physiologic risk factor for seizures, and 51 (85%) patients were able to identify at least one emotional, social or sensory stimulus as a potential seizure trigger under guidance of the therapist (Table 2). Physiologic risk factors were reported by 10 (50%) patients with definite epilepsy and by 29 (73%) patients with probable epilepsy. Emotional and/or social stimuli were reported by 16 (80%) patients with definite epilepsy and by 33 (82%) patients with probable epilepsy. Sensory stimuli were reported by 2 (10%) patients with definite epilepsy and by 7 (18%) patients with probable. At least one early seizure warning sign (prodrome or aura) was reported by 17 (85%) of patients with definite epilepsy and by 32 (80%) patients with probable epilepsy. Except for two patients, all patients identified either a seizure trigger or a trigger warning during the initial assessment.
Table 2. Reported Seizure Precipitants.
Reported seizure precipitants: physiologic risk factors for seizures and stimulus-related seizure triggers which were identified by patients under guidance of the therapist during the initial assessment. The number of patients who identified each factor, trigger or category, and the relative percentages of all 60 patients are given.
|
Physiologic risk factors (39 pts/65%) |
Stimulus-related triggers (51 pts/85%) |
|||
|
Chemical factors (23 pts/38%) |
Body functions (35 pts/58%) |
Emotional stimuli (47 pts/78%) |
Social stimuli (11 pts/18%) |
Sensory stimuli (9 pts/15%) |
|
| ||||
|
Hormonal (Menses) (13/22%) |
Sleep (Fatigue/lack of sleep) (31/52%) |
Stress (24/40%) |
Relationships (Tension, conversations) (3/5%) |
Visual (flashing lights, computer screens) (5/8%) |
|
Food (Skipping meals/excess) (8/13%) |
Exercise (5/8%) |
Fear (Startle, panic, fear of seizures) (17/28%) |
Work (little support, overworking) (7/12%) |
Auditory (Noise, rhythms, telephone) (4/7%) |
|
Substance use (Alcohol or caffeine) (4/7%) |
Respiration (Hypoventilation or Hyperventilation) (2/3%) |
Anger (or frustration) (15/25%) |
Family (Tension, conversations) (3/5%) |
Heat (4/7%) |
|
Missing meds (4/7%) |
Urination (Urination or urge) (1/2%) |
Anxiety (or worry) (14/23%) |
School (Exams, overambition) (1/2%) |
Smell (Molds, lack of fresh air) (1/2%) |
|
Fever (2/3%) |
Self-image issues (Self-doubt, neg. thoughts etc.) (6/10%) |
Past Trauma (Flashbacks) (1/2%) |
Touch (1/2%) |
|
|
Excitement/Agitation (6/10%) |
||||
Outcome
Twenty-nine patients (this represents 59% of the patients who had identified seizure warning signs during initial assessment) reported the interruption of seizures during the course of the intervention (Table 3).
Table 3. Interrupted Seizures.
Reports of seizure interruptions as documented by patients during the the intervention are shown as percent of all reported seizures and for each diagnostic certainty group.
| All Patients | Definite Patients | Probable Patients | |
|---|---|---|---|
| Identified Seizure Warning during Initial Assessment | 49 pts (82%) | 17 pts (85%) | 20 pts (75%) |
| Documented Interruption of Seizures during Course of the Intervention | 29 pts (48%) | 9 pts (45%) | 20 pts (50%) |
| Median Seizures reported as interrupted, [%] | 19% | 17% | 20% |
The seizure frequency of about half of all patients showed a clinically meaningful improvement (>50% reduction of seizures) towards the end of the intervention. About one third of all patients were seizure-free by the end of the program and one third of the patients had not provided sufficient long documentation for this analysis (Figure 1). Of those patients who did provide sufficient documentation, 51% (22 patients) were seizure-free by the end of the intervention and 79% (30 patients) showed a clinically meaningful improvement of seizure frequency. The median time between first and last report was 2.5 years, ranging from 6 months to 13 years in this group of patients.
Figure 1.
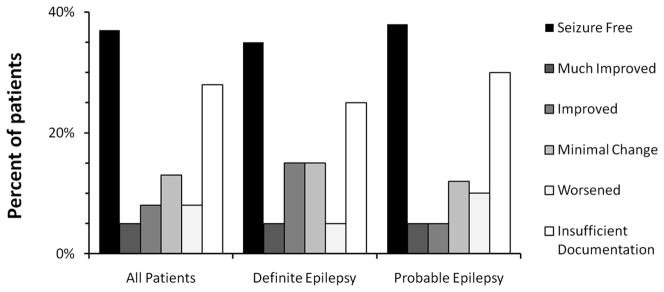
Changes of seizure frequency over the course of the intervention. Results are given in % and for each group of diagnostic certainty. Outcomes were categorized as seizure free = 100% decrease, much improved = 90% to 100% decrease, improved = ≥50% to 90% decrease, minimal change = <50% decrease or worsened = increase of seizures.
In those patients who were seizure-free by the end of the intervention, the duration of documented complete freedom from seizures had lasted for a median of 2.2 years, ranging from three months to eight years (Figure 2).
Figure 2.
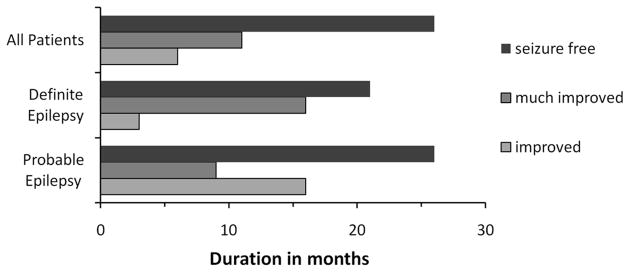
Documented duration of improvement of seizure frequency given in median months and for each diagnostic certainty group.
For all patients who had provided seizure logs, the mean increase of seizure free weeks from the first to the last third of the program was significantly greater than zero (mean change = 22.9 points of percentage (pp), p = 0.000). In 12 patients (20%), the individual increase exceeded 60 points of percentage (Table 4).
Table 4. Changes in percentages of seizure-free weeks.
Changes in percentages of seizure-free weeks from the first third of the program to the last third of the program and their p-values derived from a paired t-test (one-tailed), given for all 50 patients (83%) who provided seizure documentation during the the intervention by diagnostic certainty group.
| All Patients | Definite Epilepsy | Probable Epilepsy | |
|---|---|---|---|
| N = 50 | N = 17 | N = 33 | |
| Seizure-free weeks first third [%] | 46.0% | 43.6% | 42.8% |
| Seizure-free weeks last third [%] | 68.9% | 61.6% | 70.2% |
| Mean Change [pp] | 22.9 | 18.0 | 27.4 |
| SD [pp] | 33.2 | 27.9 | 33.3 |
| p-value | 0.000 | 0.009 | 0.000 |
Special Patient Groups
Patients with special conditions including children, patients with nocturnal seizures, primary epilepsy and those with an IQ ≤ 80 showed similar improvements of their seizure frequency as the larger group of patients (Table 5). Numbers were too small for statistical analysis.
Table 5. Patients with special conditions.
| Age < 17 years | Nocturnal Seizures | Primary Generalized Epilepsy | IQ ≤ 80 | |
|---|---|---|---|---|
| N = 11 | N = 8 | N = 9 | N = 2 | |
| Seizure-free | 4 | 4 | 4 | 1 |
| Much improved | 0 | 0 | 0 | 0 |
| Improved | 1 | 0 | 1 | 0 |
| Minimal change | 1 | 1 | 0 | 1 |
| Worsened | 0 | 0 | 0 | 0 |
| Insufficient Documentation | 5 | 3 | 4 | 0 |
Summary of pertinent Patient Characteristics and Results
Forty of the 60 patients included in this retrospective analysis had been experiencing complex partial seizures on a monthly basis for a median of 18 years and were medically refractor; 32 of these patients also experienced occasional secondary generalization. Except for 2 patients, all of them had identified at least one seizure precipitant or seizure warning sign during the initial days of assessment. Thirteen (43%) of all 30 patients who had provided sufficient seizure documentation, were seizure-free at the end of the intervention: five patients with definite epilepsy and eight patients with probable epilepsy. In five of these patients, the medication dosage was reduced by about two thirds. Five of the total 13 drug-refractory patients with complex partial seizures who were seizure free by the end of the intervention had bilateral seizure onsets and were therefore no candidates for epilepsy surgery.
DISCUSSION
The purpose of this retrospective intention-to-treat analysis was a systematic study of the long-term data that was available from an epilepsy population treated with the Andrews/Reiter comprehensive cognitive behavioral approach.
Data Limitations
The patient inclusion criteria that had been applied in this study were developed to restrict the analysis to patients with interpretable data. The limited availability of test results and medical reports for establishing a patient’s diagnostic certainty increased the chances that a patient was not categorized correctly. However, in our scoring system this would only have led to an underestimation of diagnostic certainty. Our method of the prediction of the probability of additional non-epileptic events was developed in a different setting and has natural limitations. In spite of our efforts to exclude unreliable data, the possibility of spontaneous fluctuations or false negative self-reports of seizure occurrence could not be ruled out. Insufficient seizure documentation limited our ability to analyze the data.
Outcome Measures
This study was not able to evaluate a patient’s compliance with the program. However, it is quite possible that lack documentation in seizure logs may have resulted from limited compliance. A future, prospective trial should pay detailed attention to compliance with the various elements of this program in order to evaluate the correlation between an individual patient’s motivation and outcome and to assess which elements of the program are most crucial for the program’s success. A recently completed prospective pilot study of the Andrews/Reiter intervention found that program compliance was required for seizure reduction. 20
Even though journal entries and e-mail communications contained many personal references illustrating an improved quality of life, no objective health related quality of life questionnaires had been used. As a result, no direct assessment of changes in quality of life was feasible.
A suitable control group was not available for this retrospective study. Therefore, it was not possible to evaluate to what extent any patient-expectation influenced the outcome. However, the characteristics of patients who were seizure-free at the end of the intervention can be discussed in regard to prognostic relevance as found in the published literature. Chronic refractoriness usually follows a progressive or remission-relapse course. 21 To avoid potential false positive results, we aimed to exclude patients showing a remission-relapse pattern from this study. A terminal seizure remission rate of 15% has been observed in a group of drug-refractory patients only treated with medication. 22 The terminal remission rate of 32% (14 patients) in the drug-refractory patient population in this study was much higher, suggesting a real effect of the intervention.
Self-reported Seizure Triggers and Warning Signs
Introspective phenomena such as emotional or social seizure triggers and the arrest of seizures in the aura stage played an important role in this intervention. Naturally this type of information is subjective. The reported seizure precipitants and seizure warnings were reported by the patients and evaluated under guidance of the Andrews/Reiter therapist, and in most cases were subsequently utilized to develop personalized strategies for seizure interruption. The Andrews/Reiter therapist operates with the assumption that with careful observation and analysis one or more precipitants can be found for any seizure.
Specific and unspecific, endogenous and exogenous precipitants (Table 2) may converge in a sensation that patients unspecifically refer to as “stress”. However, a comprehensive resolution in the context of behavioral therapy requires a careful analytical approach. The terminology that is used in the medical literature for the categorization of psychological seizure precipitants is not yet sufficiently developed for this purpose. It would make sense to operate with psychological models that suggest an approach of regarding emotions primarily as indicators of underlying cognitive processes (as found e.g. in the computational belief-desire theory of emotions). 23 Such views may render emotions accessible to therapeutic cognitive behavioral modification and resolution.
A future investigation of the interictal, preictal and ictal electroencephalographic and other (e.g. intracranial blood flow) correlates of introspective phenomena described in this study using CCTV-EEG would be desirable and might allow for a better understanding of how cognition, emotions and other subjective experiences might interact with the physiologic processes which initiate or interrupt epileptic seizures.
Special patient groups
Our observations agree with previous studies which suggest that cognitive behavioral interventions have been successfully used by patients younger than 17, 3 patients with primary epilepsy, 24 patients with nocturnal seizures 25 and patients with an IQ less than 80 (not yet studied). However, due to the small number of patients within each subgroup a statistical analysis was not feasible. Prospective and controlled studies with more homogeneous study groups will be needed for further clarification.
This study did not differentiate the prevalence of warning signs and seizure precipitants in regard to age groups, gender and syndromes, these have been described elsewhere. 13, 26
Epilepsy-specific Counseling
It has been observed that general psychological counseling is not sufficient to reduce seizures in epilepsy patients. 27 In contrast, the Andrews/Reiter approach provides tools for epilepsy specific counseling and utilizes an individualized and epilepsy-specific interpretation of the WAIS/WISC and the MMPI/MMPI-A. WAIS/WISC findings can provide an understanding of a patient’s individual cognitive challenges in light of the location of respective seizure foci. In a similar fashion, a careful interpretation of MMPI results can help patients to identify emotional challenges which may function as potential seizure triggers.
Several studies have recently addressed isolated methodological aspects which have long been integral parts of the Andrews/Reiter approach. E.g., such studies encourage the supervision of epilepsy self-management programs with telephone calls, 28 individual goal-setting by epilepsy patients, 29 the potential value of questioning life stressors as triggers for the onset of epilepsy 30 and slow breathing exercises. 31 It is likely that the most effective behavioral approach will combine many or all of these elements into a comprehensive method such as the Andrews/Reiter approach studied here.
Conclusion
Despite the methodological limitations of this study, our data suggests that comprehensive behavioral approaches such as the Andrews/Reiter approach studied merit consideration as a treatment of either drug-refractory, nonsurgical candidates or motivated epilepsy patients seeking an alternative or adjunctive therapeutic options. Future prospective trials are needed to substantiate the efficacy of the Andrews/Reiter method, to prioritize its components and investigate the mechanisms by which individual factors may trigger epileptic seizures and by which patients are enabled to prevent the occurrence of seizures by reducing seizure triggers and interrupting impending seizures. One can hope that behavioral approaches to epilepsy will be studied in more detail and eventually will be incorporated into standard care for epilepsy patients.
Highlights.
Seizure frequency in epilepsy patients participating in the A/R intervention was retrospectively analyzed.
Thirty (50%) patients had more than 50% reduction, 22 (37%) patients became seizure-free.
The incorporation of behavioral approaches into standard care for epilepsy patients merits consideration.
Acknowledgments
The authors thank Donna J. Andrews for providing the documentation necessary to complete this study, allowing the observation of the treatment process itself and sharing her knowledge, the Andrews/Reiter Board, for allowing us to obtain data of patients who had engaged in the intensive Andrews/Reiter treatment, and Joel Reiter, for providing access to work-space and data storage. This publication was made possible with support from grant number K23 AT01993 by NIH NCCAM to S.M.E.
Footnotes
Disclosures of Conflicts of Interest
None of the authors has a conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Snyder M. Stressors, coping mechanisms, and perceived health in persons with epilepsy. Int Disabil Stud. 1990;12:100–103. doi: 10.3109/03790799009166261. [DOI] [PubMed] [Google Scholar]
- 2.Stevanovic D. Health-related quality of life in adolescents with well-controlled epilepsy. Epilepsy Behav. 2007;10:571–575. doi: 10.1016/j.yebeh.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Dahl J, Melin L, Leissner P. Effects of a behavioral intervention on epileptic seizure behavior on epileptic seizure behavior and paroxysmal activity: A systematic replication of three cases of children with intractable epilepsy. Epilepsia. 1988;29:172–183. doi: 10.1111/j.1528-1157.1988.tb04415.x. [DOI] [PubMed] [Google Scholar]
- 4.Schmid-Schoenbein C. Improvement of seizure control by psychological methods in patients with intractable epilepsies. Seizure. 1998;7:261–270. doi: 10.1016/s1059-1311(98)80017-4. [DOI] [PubMed] [Google Scholar]
- 5.Wolf P, Okujava N. Possibilities of non-pharmacological conservative treatment of epilepsy. Seizure. 1999;8:45–52. doi: 10.1053/seiz.1998.0243. [DOI] [PubMed] [Google Scholar]
- 6.Nakken O, Solaas M, Kjeldsen M, Friis M, Pellock J, Corey L. Which seizure-precipitating factors do patients with epilepsy most frequently report? Epilepsy Behav. 2005;6:85–89. doi: 10.1016/j.yebeh.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Ferlazzo E, Zifkin B, Andermann E, Andermann F. Cortical triggers in generalized reflex seizures and epilepsies. Brain. 2005;128:700–710. doi: 10.1093/brain/awh446. [DOI] [PubMed] [Google Scholar]
- 8.Matsuka H, Takahashi T, Sasaki M, Matrumoto K, Yoshida S, Numachi Y, Saito H, Ueno T, Sato M. Neuropsychological EEG activation in patients with epilepsy. Brain. 2000;123:318–330. doi: 10.1093/brain/123.2.318. [DOI] [PubMed] [Google Scholar]
- 9.Bonanni E, Pizzanelli C, Maestri M, Fabbrini M, Galli R, Murri L. Seizures induced by nursery thymes and children’s games. Seizure. 2004;13:282–283. doi: 10.1016/S1059-1311(03)00078-5. [DOI] [PubMed] [Google Scholar]
- 10.Woods RJ, Gruenthal M. Cognition-induced epilepsy associated with specific emotional precipitants. Epilepsy Behav. 2006;9:360–362. doi: 10.1016/j.yebeh.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 11.Peleen MV, Atkinson AP, Vuilleumier P. Supramodal representation of perceived emotions in the human brain. J Neurosci. 2010;30:10127–10134. doi: 10.1523/JNEUROSCI.2161-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrews DJ, Reiter JM, Schonfeld W, Kastl A, Denning P. A neurobehavioral treatment for unilateral complex partial seizure disorders: a comparison of right- and left- hemisphere patients. Seizure. 2000;9:189–97. doi: 10.1053/seiz.1999.0375. [DOI] [PubMed] [Google Scholar]
- 13.Pinikahana J, Dono J. Age and gender differences in initial symptoms and precipitant factors of epileptic seizures: an Australian study. Epilepsy Behav. 2009;16:231–239. doi: 10.1016/j.yebeh.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Lee SA, No YJ. Perceived self-control of seizures in patients with uncontrolled partial epilepsy. Seizure. 2005;14:100–105. doi: 10.1016/j.seizure.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Andrews DJ, Reiter JM, Janis C. A workbook for patients and professionals. Santa Rosa: The Basics Publishing Company; 1987. Taking control of your epilepsy. [Google Scholar]
- 16.Elsas SM. Epilepsy. In: Oken BS, editor. Complementary Treatments in Neurology. London: The Parthenon Publishing Group; 2004. pp. 265–277. [Google Scholar]
- 17.Jackson T. Relaxation and stress management. Palos Verdes, CA: Inner Health Inc; 1992. [Google Scholar]
- 18.Storzbach D. MMPI-2: Improved prediction of non-epileptic seizures with combined MMPI and EEG measures. Epilepsia. 2000;41:332–337. doi: 10.1111/j.1528-1157.2000.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 19.Kwan P, Arzinmanogou Berg AT, Brodie MJ, Hauser AW, Mathern G, Moshe SL, Perucca E, Wiebe S, French J. Definition of drug resistant epilepsy; Consensus proposal by the ad hoc task force of the ILAE commission on therapeutic strategies. Epilepsia. 2010 doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 20.Elsas SM, Gregory WL, White G, Navarro G, Salinsky MC, Andrews DJ. Auta interruption: the Andrews/Reiter intervention may reduce seizures and improve quality of life – a pilot trial. Epilepsy Behav. 2011 doi: 10.1016/j.yebeh.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 21.Beleza P. Refractory epilepsy: a clinically oriented review. Eur Neurol. 2009;62:65–71. doi: 10.1159/000222775. [DOI] [PubMed] [Google Scholar]
- 22.Callaghan BC, Anand K, Hesdorffer D, Hauser WA, French JA. Likelihood of seizure remission in an adult population with refractory epilepsy. Ann Neurol. 2007;62:311–3. doi: 10.1002/ana.21166. [DOI] [PubMed] [Google Scholar]
- 23.Reisenzein R. Emotional experience in the computational belief-desire theory of emotions. Emotion Review. 2009;1:214–222. [Google Scholar]
- 24.Martinovic Z. Adjunctive behavioral treatment in adolescents and young adults with juvenile myoclonic epilepsy. Seizure. 2001;10:42–47. doi: 10.1053/seiz.2000.0479. [DOI] [PubMed] [Google Scholar]
- 25.Müller B. Psychological approaches to the prevention and inhibiton of nocturnal epileptic seizures: A meta-analysis of 70 case studies. Seizure. 2001;10:13–33. doi: 10.1053/seiz.2000.0486. [DOI] [PubMed] [Google Scholar]
- 26.Frucht MM, Quigg M, Schwaner C. Fountain NB Distribution of seizure precipitants among epilepsy syndromes. Epilepsia. 2000;41:1534–1539. doi: 10.1111/j.1499-1654.2000.001534.x. [DOI] [PubMed] [Google Scholar]
- 27.Feldmann RG, Norman LP. Identity of emotional triggers in epilepsy. The journal of nervous and mental disease. 1976;162:345–353. doi: 10.1097/00005053-197605000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Dilorio C, Reisinger EL, Yeager KA, McCarty F. A telephone-based self-management program for people with epilepsy. Epilepsy Behav. 2009;14:232–236. doi: 10.1016/j.yebeh.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 29.Walker ER, Wexler B, Dilorio C, Excoffery C, Mc Carty F, Yeager KA. Content and characteristics of goals created during a self-management intervention for people with epilepsy. J Neurosci Nurs. 2009;41:312–21. doi: 10.1097/jnn.0b013e3181b6bec5. [DOI] [PubMed] [Google Scholar]
- 30.Koutsogiannopoulos S, Adelson F, Lee V, Andermann F. Stressors at the onset of adult epilepsy:implications for practice. Epileptic Disord. 2009;11:42–47. doi: 10.1684/epd.2009.0236. [DOI] [PubMed] [Google Scholar]
- 31.Yuen AW, Sander JW. Can slow breathing exercises improve seizure control in people with refractory epilepsy? A hypothesis. Epilepsy Behav. 2010;18:331–334. doi: 10.1016/j.yebeh.2010.05.019. [DOI] [PubMed] [Google Scholar]


