Abstract
Background
We have shown that HB-EGF protects the intestines from injury in several different animal models including hemorrhagic shock and resuscitation (HS/R). The current study was designed to explore the mechanisms underlying the anti-inflammatory role of HB-EGF in preservation of gut barrier function after injury.
Methods
In vivo, HS/R was induced in wild type and neutropenic mice, with or without administration of HB-EGF, and intestinal permeability determined using the everted gut sac method. In vitro, cultured human umbilical vein endothelial cells (HUVEC) and freshly isolated human peripheral blood mononuclear cells (PMN) were used to determine the effects of HB-EGF on HUVEC-PMN adhesion, reactive oxygen species (ROS) production in PMN, adhesion molecule expression in HUVEC and PMN, and the signaling pathways involved.
Results
We found that administration of HB-EGF to normal mice led to preservation of gut barrier function after HS/R. Likewise, induction of neutropenia in mice also led to preservation of gut barrier function after HS/R. Administration of HB-EGF to neutropenic mice did not lead to further improvement in gut barrier function. In vitro studies showed that HB-EGF decreased neutrophil-endothelial cell (PMN-EC) adherence by down-regulating adhesion molecule expression in EC via the PI3K-Akt pathway, and by inhibiting adhesion molecule surface mobilization and reactive oxygen species (ROS) production in PMN.
Conclusions
These results indicate that HB-EGF preserves gut barrier function by inhibiting PMN and EC activation, thereby blocking PMN-EC adherence after HS/R in mice, and support the future use of HB-EGF in disease states manifested by hypoperfusion injury.
Introduction
Intestinal ischemia/reperfusion (I/R) injury is a common clinical event which occurs in many critical clinical situations including hemorrhagic shock and resuscitation (HS/R).1 Intestinal barrier dysfunction occurs with intestinal I/R injury, and triggers the systemic inflammatory response syndrome (SIRS) followed by multiple organ dysfunction syndrome (MODS),2–4 which remains the leading cause of death in critically ill patients.5 Neutrophil (PMN) - endothelial cell (EC) interactions play a vital role in the pathogenesis of intestinal I/R injury.6, 7 Once the neutrophil is activated by I/R-induced inflammation, it becomes a major source of released reactive oxygen species (ROS), proteases and inflammatory products.8, 9 These harmful products compromise the endothelial barrier integrity and intestinal barrier function,2, 5 and amplify the recruitment and activation of greater numbers of neutrophils into the effected intestine, thereby increasing the intestinal injury.10–13
Heparin-binding EGF-like growth factor (HB-EGF) was initially identified as a 22-kDa glycoprotein in the conditioned medium of cultured human macrophages, and was later found to be a member of the epidermal growth factor (EGF) family.14, 15 It is produced as a membrane-anchored precursor molecule (pro-HB-EGF) that undergoes extracelular proteolytic cleavage to yield the mature, secreted growth factor (sHB-EGF).16 HB-EGF binds to and activates the EGF receptor (EGFR/HER1/ErbB-1), ErbB-4 (HER4) and the HB-EGF-specific non-tyrosine kinase receptor N-arginine dibasic convertase (NRDc).17, 18 Mature HB-EGF also binds strongly to cell-surface heparan-sulfate proteoglycans, which enhances its binding to EGFR and its bioactivity in some cell types.17
Previous studies from our laboratory have shown that exogenous administration of HB-EGF protects the intestine from injury in animal models of superior mesenteric artery occlusion (SMAO),19 HS/R,20 and experimental necrotizing enterocolitis (NEC).21 Further studies revealed several mechanisms that contribute to the intestinal protective effects of HB-EGF: i) HB-EGF promotes intestinal epithelial cell (IEC) migration and restitution in a PI3K/Akt- and MEK/ERK1/2-dependent fashion,19 ii) HB-EGF promotes angiogenesis after injury via activation of PI3K, MAPK and eNOS in a VEGF-independent fashion,22 iii) exogenous HB-EGF prevents enterocyte apoptosis,21 and in response to injury, endogenous HB-EGF is induced and protects human intestinal epithelial cells from apoptosis though activation of MAPK and PI3K/AKT pathways,23 and iv) HB-EGF improves intestinal microcirculation and protects the intestinal microvasculature from injury via its effects on pericytes.20, 24, 25 In addition to these protective effects, recent studies suggest that HB-EGF is also an effective anti-inflammatory mediator, which is able to decrease neutrophil activation and ROS production,26 down-regulate the expression of inflammatory cell adhesion molecules and pro-inflammatory cytokines,27 decrease cytokine-induced production of nitric oxide and inducible nitric oxide synthase,28 and inhibit cytokine-induced NF-κB activation.29
Taken together, these important biological effects of HB-EGF play a major role in preserving gut barrier function after injury. It is possible that the dual protective actions of HB-EGF on both the intestinal mucosa and the intestinal microvasculature after I/R injury may be due to the ability of HB-EGF to inhibit PMN-EC interactions. To investigate this farther, the current study used both in vitro and in vivo models to examine the anti-inflammatory role of HB-EGF in preservation of gut barrier function, as well as to elucidate the underlying intracellular signaling pathways involved.
Materials and methods
Materials
Primary human umbilical vein endothelial cells (HUVEC), medium 200 and low serum growth supplements (LSGS) were from Cascade Biologics (Portland, OR, USA). Rabbit anti-mouse neutrophil antibody (AIA31140) was from Accurate Chemical & Scientific Corporation (Westbury, NY, USA). Cell-signaling inhibitors including the PI3K inhibitor LY294002, the EGFR tyrosine kinase inhibitors AG1478, the NFκB inhibitor MG132, and the Erk1/2 MAPK inhibitor UO126 were from Calbiochem (San Diego, CA, USA). Superoxide dismutase (SOD) and N-Formyl-L-methionyl-L-leucyl-L-phenylalanine (fMLP) were from Sigma-Aldrich (St. Louis, MO, USA). TRIzol reagent and the SuperScript first-strand synthesis system for RT-PCR kit were from Invitrogen (Carlsbad, CA, USA). Proteinase inhibitor cocktail and phosphatase inhibitor cocktail were from Thermo Scientific (Rockford, IL, USA). ECL advanced western blotting detection kit was from GE Healthcare (Little Chalfont, BUCKS, UK). Antibodies used for Western blotting including antibodies to PECAM-1 (sc-1506), E-selectin (sc-14011) and ICAM-1(sc-7891) were from Santa Cruz Biotechnologies (Santa Cruz, CA, USA). The antibody to CD11b (ab52478) was from Abcam (Cambridge, MA, USA). Peroxidase AffiniPure donkey anti-mouse IgG antibody (715-035-150), peroxidase AffiniPure donkey anti-rabbit IgG antibody (711-035-152) and peroxidase AffiniPure donkey anti- goat IgG antibody (705-035-147) were from Jackson ImmunoResearch (West Grove, PA, USA). The antibodies to Akt (9272) and phosphorlated Akt (9271s) were from cell signaling (Danvers, MA, USA). The anti-human CD11b APC conjugated antibody (17-0118-41) used for flow cytometry was from eBioscicence (San Diego, CA, USA). The antibody to E-selectin (560441) was from BD Biosciences (San Jose, CA, USA).
Mouse Model of Hemorrhagic Shock and Resuscitation
All animal procedures were approved by the Institutional Animal Care and Use Committee of the Research Institute at Nationwide Children’s Hospital (Protocol #00903AR). 8–12 week old male FVB mice (25–30g) were used. Randomly assigned mice were rendered neutropenic by an intraperitoneal (IP) injection of 0.2 ml undiluted rabbit anti-mouse neutrophil antibody (AIA31140; Accurate Chemical & Scientific Corporation, Westbury, NY) 12h prior to experimentation, as described,30 with control mice receiving 0.2 ml of non-specific rabbit serum IP. Neutropenia was confirmed by measurement of leukocyte counts in 0.2ml whole blood using a Sysmex XE-2100 automated hematology system (Sysmex America, Inc. Mundelein, IL).
Both neutropenic and non-neutropenic mice were exposed to either sham surgery or to HS/R. HS/R was performed as we previously described.31 Both femoral arteries were cannulated with PE10 tubing with the right catheter connected to a pressure monitor for monitoring of mean arterial pressure (MAP). Blood was withdrawn over 15 min via the left catheter to reduce MAP from 100±10 to 30–35 mmHg for 90 min. Mice were then resuscitated with the shed blood plus two times that volume of Ringer’s lactate solution infused over 30 min. Mice subjected to HS/R received either HB-EGF (1.2 mg/kg/dose in 100 μl) or the same volume of PBS via the left catheter upon reperfusion.
Determination of Intestinal Permeability
Small intestinal mucosal barrier function was assessed using the ex vivo isolated everted sac method as we described previously.31 Briefly, 6 cm segments of terminal ileum were harvested, inverted, and incubated in ice-cold Krebs’-Henseleit bicarbonate buffer (KHBB buffer) at pH 7.4. Fluorescein-isothiocyanate dextran (FD4, molecular weight: 4000 Da) was used as a permeability probe. The everted gut sacs were gently distended by injecting 0.4 ml of KHBB and suspending the sacs in KHBB buffer with added FD4 (60 μg/ml) for 30 min. The incubation medium was maintained at 37°C and was continuously bubbled with a gas mixture containing 95% O2 and 5% CO2. The gut length (L) and diameter (D) were measured, and the intraluminal KHBB buffer (FD4ser) was collected and measured (intraluminal volume). Both FD4muc and FD4ser were measured using a fluorescence spectrophotometer (SpectraMax Plus, Molecular Devices, CA). Gut permeability was expressed as the mucosal-to-serosal clearance of FD4 as follows:
Endothelial Cell Cultures
HUVEC were cultured at 37°C, 5% CO2 in medium 200 containing LSGS (complete medium).22 All experiments were performed in medium 200 containing 1% serum (starvation medium) and repeated at least three times. Where indicated, inhibitors including the PI3K inhibitor LY294002 (20 μM), the EGFR tyrosine kinase inhibitor AG1478 (500 nM), the NFκB inhibitor MG132 (40 μmol/L), the Erk 1/2 inhibitor UO126 (10 μM), or SOD (1000 U/ml) were added 30 min prior to the addition of growth factors. All experiments were performed on cells at passages 2 or 3.
Neutrophil Preparation
Human neutrophils were isolated from venous blood of 5 healthy adult volunteers by density gradient centrifugation with polymorphprep (Axis-Shield, Oslo, Norway). Donors were 29–49 years old (1 male Asian, 1 female Asian, 1 male Caucasian, 2 female Caucasian). Morphologic examination with Wrights-Giemsa staining confirmed that the purity of the neutrophil preparations was >97%.32 Where indicated, inhibitors were added 30 min prior to the addition of growth factors.
Neutrophil-Endothelial Cell (PMN-EC) Adhesion Assay
PMN-EC adhesion assays were performed as previously described.32 HUVEC monolayers were prepared in 24-well culture dishes. Neutrophils were labeled with calcein-AM. 32 HUVEC or PMN were pretreated with signal pathway inhibitors as indicated. Cells were then incubated with either HB-EGF (100 ng/ml) or the equivalent volume of phosphate-buffered saline (PBS) for 1 h at 37°C. HUVEC were exposed to anoxia (93% N 2/5% CO2/2% H2) for 1h followed by reoxygenation (74% N2/5% CO2/21% O2) for 4h or 12h (anoxia/reoxygenation, A/R). Control HUVEC were kept at normoxia at all times. Neutrophil suspensions (50 μl containing 1 × 106 cells/ml) were added to each well of HUVEC after reoxygenation of the EC. After 30 min of co-incubation, 200 μl of each supernatant was removed for assay. Cells were then gently washed with 200 μl PBS and lysed with 200 μl 2N NaOH. The numbers of adherent cells were calculated by measurement of fluorescent intensity using the following formula:
Measurement of ROS production in neutrophils
ROS production was measured using the cell-permeable fluorogenic probe 2′, 7′-dichlorodihydrofluorescin diacetate (DCFH-DA; Cell Biolabs, San Diego, CA, USA) as described previously.33 Freshly isolated human PMN were suspended at a concentration of 1×106 cells/ml in HBSS and incubated for 1 h with DCFH-DA (1mmol/l) at 37°C in the dark. Cells were then washed twice with HBSS by centrifugation for 5 min (400×g, 20°C). Neutrophil suspensions (100μl containing 1×105 cells) were transferred to 96 well plates and preincubated with or without signal pathway inhibitors or SOD. Cells were then incubated with either HB-EGF (100 ng/ml) or PBS for 1 h at 37°C. Neutrophils were inc ubated with fMLP (10−7 mol/l) to induce ROS generation.8 Fluorescence was measured using a fluorescence spectrophotometer (SpectraMax Plus, Molecular Devices, CA). Results were expressed as the fluorescence intensity in arbitrary units (AU).
Measurement of cell-surface adhesion molecule expression in neutrophils
Human neutrophils were pretreated with HB-EGF, SOD, or HB-EGF + SOD for 1h followed by the addition of fMLP (10−7 mol/l). Neutrophils were harvested 30 min, 1h or 4h after fMLP addition. After washing in PBS twice, neutrophils were resuspended in 100 μl PBS and labeled with APC conjugated antibody to detect CD11b cell membrane staining. After incubation on ice for 30 min, cells were washed with PBS and resuspended in 0.3 ml PBS. The neutrophil population was selected and analyzed using a flow cytometer (BD LSR2, BD Biosciences, San Jose, CA, USA). Results were expressed as the mean fluorescent intensity (MFI) of the positively labeled cells.
Western blot analysis
EC or PMN were lysed in RIPA buffer and clarified cytosolic extracts were subjected to SDS-PAGE followed by Western blot analysis using an ECL advanced Western blotting detection kit. Antibodies used for Western blotting including anti-PECAM-1 (sc-1506), anti-E-selectin (sc-14011), anti-ICAM-1(sc-7891), anti-phosphorlated Akt (9271s), anti-CD11b (ab52478). To confirm equal protein loading, membranes were stripped and probed with either a 1/3000 dilution of total Akt, or a 1/10,000 dilution of anti-β-actin antibody.
mRNA extraction and quantitative real time qRT-PCR analysis
RNA was isolated from HUVEC using TRIzol reagent. cDNA was generated from 500 ng total RNA using SuperScript frist-strand synthesis system for RT-PCR kit. qRT-PCR analysis was carried out using SyBrGreen real time RT-PCR. The fluorescent signal for target amplification was detected using an ABI7500 real time thermal cycler (Applied Biosystems, Foster City, CA, USA). Primers sequences were as follows: PECAM-1, 5′-GGAAAAGGCCCCAATACACTT-3′/3′-TAAAACGCGGTCCTGTTCCTC-5′; E-selectin, 5′-AGGACACTGGTCTGGCCTGCT-3′/3′-GGCAGCTGCTGGCAGGAACAA-5′; ICAM-1, 5′-TTGAACCCCACAGTCACCTAT-3′/3′-CCTCTGGCTTCGTCAGAATCA-5′. Values were normalised to glyceraldehyde-3-phosphate dehydrogenase.
NF kappa B DNA-binding activity assay
EC were treated with signal pathway inhibitors and then incubated with either HB-EGF (100 ng/ml) or PBS for 1h at 37°C. EC were then exposed to anoxia for 1h followed by reoxygenation for 4 or 12h. Preparation of nuclear lysates and the p65 subunit DNA binding activity assay was performed using the TransAM™ NF-κB p65 transcription factor assay kit (Active Motif, Carlsbad, CA) according to the manufacturer’s instructions.
Statistical analyses
The Student’s t-test or Wilcoxon two-sample test were used to compare the difference between two groups, and one-way ANOVA or Kruskal-Wallis test to compare the difference among multiple groups. ROS production of experimental groups was compared using a mixed model. P values less than 0.05 were considered statistically significant in all studies. Statistical analyses were performed using SAS software (SAS 9.2, SAS Institute, NC).
Results
Neutrophil depletion
The absolute neutrophil count of neutropenic mice was significantly less than that of non-neutropenic mice (59.5 ± 36.1 vs. 1494.2 ± 485.7 neutrophils/ml, p=0.0007). The platelet counts of neutropenic mice were also less than those of non-neutropenic mice (231.2 ± 55.4×103/mm3 vs. 563.0 ± 176.7×103/mm3, p=0.0016), however no neutropenic mice experienced abnormal bleeding. The hematocrits of neutropenic mice were not significantly different from those of non-neutropenic mice (39.3 ± 9.2% vs. 45.3 ± 6.2%, p=0.2033). All animals appeared healthy prior to surgical experimentation.
HB-EGF ameliorates intestinal barrier dysfunction in mice subjected to HS/R
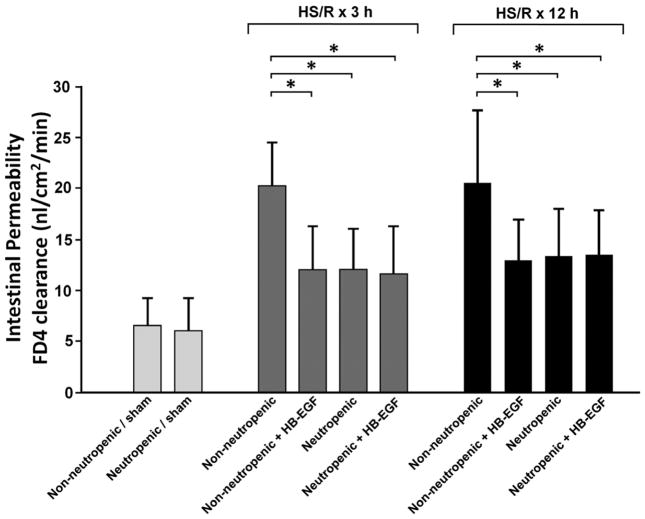
To determine whether the ability of HB-EGF to preserve gut barrier function is dependent upon neutrophils, we compared the effects of HB-EGF treatment in neutropenic and non-neutropenic mice, as determined by mucosal-to-serosal unidirectional clearance of FD4 using the everted gut sac method. All mice subjected to HS/R had significantly increased intestinal permeability at 3 and 12h of resuscitation compared to mice undergoing sham surgery (p<0.05) (Figure 1). HB-EGF treatment of non-neutropenic mice led to significantly decreased mucosal permeability at 3h of resuscitation (FD4 clearance 12.0 ± 4.4 vs. 20.2 ± 4.3 nl/min/cm2, p= 0.0015). Rendering mice neutropenic also led to lower mucosal permeability at 3h of resuscitation (FD4 clearance 12.1 ± 4.0 vs. 20.2 ± 4.3 nl/min/cm2, p= 0.002). Administration of HB-EGF to neutropenic mice did not lead to further improvement in intestinal permeability. Similar results were obtained at 12h of resuscitation.
Figure 1.
Intestinal permeability in mice subjected to HS/R. Non-neutropenic and neutropenic FVB mice were subjected to hemorrhagic shock (MAP maintained at 30–35 mmHg) for 90 min followed by reperfusion for 3 or 12 hours. HB-EGF (1.2 mg/kg/dose) was administered via the left femoral artery catheter at the end of the shock period. Intestinal permeability in the jejunum was determined by FD4 clearance using the everted gut sac method. Animals subjected to HS/R had n=8 per group; animals subjected to sham surgery had n=6 per group. *p<0.05, Student’s t-test for the comparison of 2 groups, one-way ANOVA for the comparison of multiple groups. Values shown represent mean ± SEM.
HB-EGF inhibits neutrophil-endothelial cell adhesion
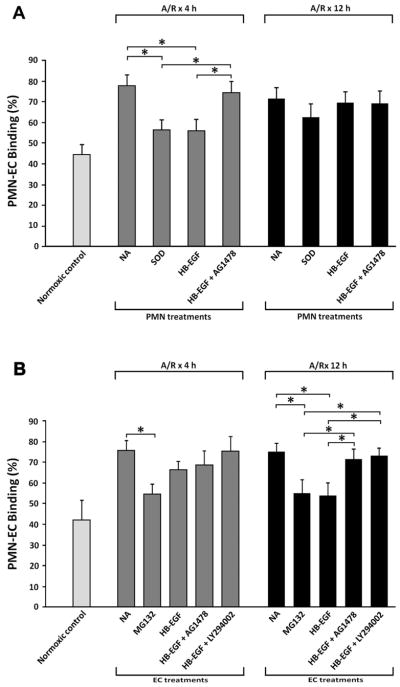
Since our in vivo results suggested that the protective effects of HB-EGF on gut barrier function are dependent upon the presence of neutrophils, and since PMN-EC interactions play major roles in the pathogenesis of intestinal injury after HS/R, 10 we next investigated the effects of HB-EGF on PMN-EC interactions in vitro. PMN-EC adherence was significantly increased when EC were exposed to A/R compared with EC grown under normoxic conditions (binding percentage 77.4 ± 5.6% vs. 41.9 ± 9.7% at 4h, p=0.0016; 74.9 ± 4.6% vs. 45.5 ± 5.3% at 12h, p=0.0063 ) (Figure 2A). Treatment of PMN with HB-EGF significantly decreased PMN-EC adherence 4h after A/R (binding percentage 56.2 ± 5.2% vs. 77.4 ± 5.6%, p=0.0084). The inhibitory effects of HB-EGF on PMN-EC adherence were reversed in the presence of AG1478. Treatment of PMN with SOD also significantly decreased PMN-EC adherence 4h after A/R.
Figure 2.
Effect of HB-EGF on PMN-EC adherence. Where indicated, HUVEC or PMN were pretreated with HB-EGF (100 ng/ml) for 1 h at 37°C. The antioxidant superoxide dismutase (SOD) or signal pathway inhibitors including the PI3K inhibitor LY294002 (20 μM), the NF-κB inhibitor MG132 (40 μmol/L), the EGFR phosphorylation inhibitor AG1478 (500 nM), or the MEK1/2 inhibitor UO126 (10mμM) were added to EC or PMN 30 min prior to HB-EGF addition. A) PMN-EC adherence after exposure of EC to A/R with various PMN treatments. B) PMN-EC adherence after exposure of EC to A/R with various EC treatments. These results reflect triplicate measurements obtained from three different experiments. *p<0.05, Student’s t-test.
Treatment of EC with HB-EGF significantly decreased PMN-EC adherence 12h after A/R (binding percentage 53.9 ± 6.2% vs. 74.9 ± 4.6%, p=0.0095) (Figure 2B). The inhibitory effect of HB-EGF on PMN-EC adherence was reversed in the presence of the EGFR inhibitor AG1478 or the PI3K inhibitor LY294002. Treatment of EC with the NFκB inhibitor MG132 also significantly decreased PMN-EC adherence 12 h after A/R. These results indicate that HB-EGF decreases PMN-EC adherence by affecting PMN and EC.
HB-EGF down-regulates ICAM-1, E-selectin and PECAM-1 expression in EC via the PI3K pathway
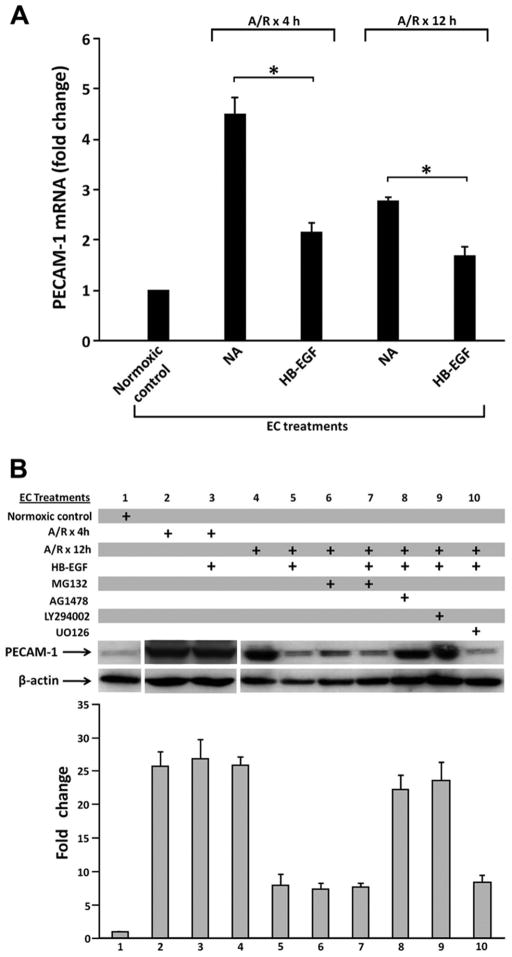
Since adhesion molecules mediate PMN-EC interactions, we next examined the effects of HB-EGF on adhesion molecule mRNA expression and protein production in EC. PECAM-1 mRNA expression was significantly increased upon exposure of EC to anoxia for 60 min followed by reoxygenation for 4 or 12h compared with EC maintained under normoxic conditions (fold change 4.5 ± 0.4 vs. 1 at 4h, p=0.0031; 3.0 ± 0.3 vs. 1 at 12h, p=0.0006) (Figure 3A). Treatment of EC with HB-EGF prior to A/R significantly reduced PECAM-1 mRNA expression 4 or 12h after A/R (fold change 2.1 ± 0.2 vs. 4.5 ± 0.4 at 4 h, p=0.0013; 1.6 ± 0.2 vs. 2.8 ± 0.1 at 12 h, p=0.005).
Figure 3.
HB-EGF down-regulates the expression of PECAM-1 in EC via the PI3K/Akt pathway. A) Inhibitory effect of HB-EGF on PECAM-1 mRNA expression as determined by real time RT-PCR in EC subjected to 30 min of anoxia followed by 4 h or 12 h of reoxygenation. *p< 0.05, Student’s t-test. B) Representative immunoblot analysis of PECAM-1 in EC subjected to A/R. Prior to A/R, EC were either untreated or were treated with LY294002 (20 μM), MG132 (40 μmol/L), AG1478 (500 nM) or UO126 (10mμM) for 30 min. Cells were then treated with HB-EGF for 1 h. The intensity of each PECAM-1 band was divided by the intensity of each β-actin band for quantification by densitometry as shown below. Densitometry data represent the mean ± SEM of three independent experiments.
We also examined ICAM-1, E-selectin and PECAM-1 protein levels in EC subjected to A/R. We found significantly increased PECAM-1 protein levels in EC 4 or 12h after A/R compared with normoxic controls (Figure 3B). HB-EGF treatment significantly suppressed the expression of PECAM-1 in EC 12h after A/R. To elucidate the signaling pathways involved, EC were treated with the NFκB inhibitor MG132, the EGFR inhibitor AG1478, the PI3K inhibitor LY294002 or the Erk1/2 MAPK inhibitor UO126 prior to HB-EGF treatment. We found that MG132, AG1478 or LY294002 could reverse the ability of HB-EGF to inhibit PECAM-1 protein levels in EC 12h after A/R, whereas UO126 had no effect. Treatment of EC with MG132 alone prior to A/R also decreased PECAM-1 protein levels EC 12h after A/R. Similar results for mRNA expression and protein production of ICAM-1 and E-selectin were also obtained (data not shown). These results suggest that the inhibitory effect of HB-EGF on EC adhesion molecule expression is dependent upon EGFR and NFκB activation and the PI3K/Akt pathway, but not the MAPK/Erk1/2 pathway.
HB-EGF inhibits A/R-induced NF-κB DNA-binding activity in EC via the PI3K/Akt pathway
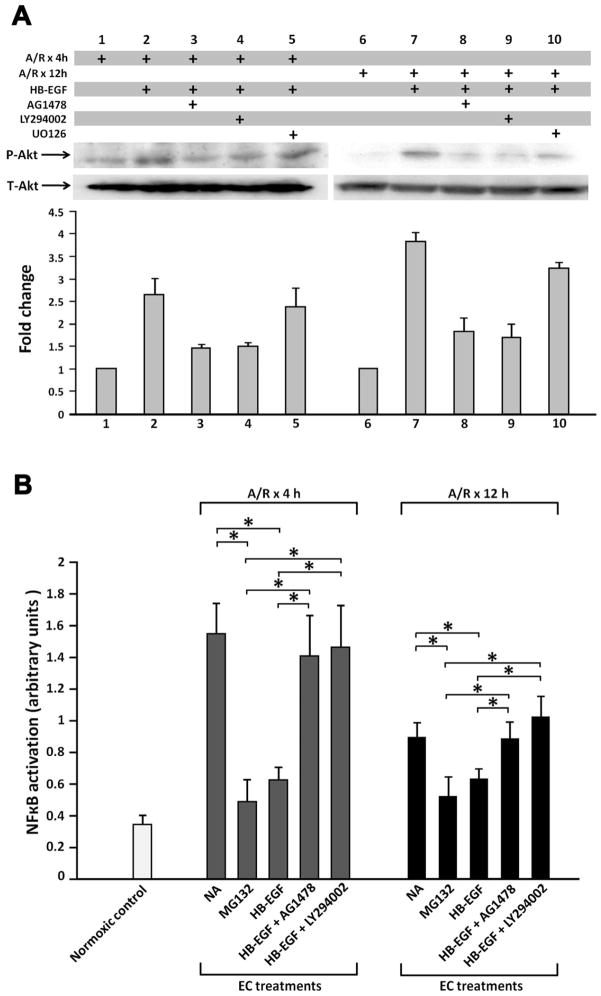
We previously demonstrated that HB-EGF blocks cytokine-activated NF-κB DNA-binding activity via the PI3K/Akt pathway in intestinal epithelial cells.34 The p65 subunit of NF-κB is responsible for transcription of E-selectin, ICAM-1 and PECAM-1.35–38 Since treatment of EC with the NF-κB inhibitor MG132 decreased PMN-EC binding (Figure 2) and decreased the expression of ICAM-1, E-selectin and PECAM-1 in EC (Figure 3), it is possible that HB-EGF exerts its anti-inflammatory effects by inhibiting NF-κB DNA-binding activity in EC. To further explore this, we examined the effect of HB-EGF on Akt phosphorylation and NF-κB DNA-binding activity in EC exposed to A/R. In EC exposed to 30 min of anoxia followed by 4 or 12h of reoxygenation, HB-EGF addition led to rapid phosphorylation of Akt which was inhibited by the PI3K inhibitor LY294002 or by the EGFR inhibitor AG1478 but not by the Erk1/2 MAPK inhibitor UO126 (Figure 4A), suggesting that HB-EGF-induced activation of Akt is PI3K dependent. NF-κB DNA-binding activity increased in EC 4 or 12h after A/R (binding activity at 4h 1.55 ± 0.20 vs. 0.34 ± 0.04, p=0.0005) (Figure 4B). HB-EGF blocked the binding activity of the p65 subunit of NF-κB in EC 4h (binding activity 0.63 ± 0.08 vs. 1.55 ± 0.20, p=0.0018) and 12h (binding activity 0.63 ± 0.06 vs. 0.89 ± 0.09, p=0.016) after A/R. This inhibitory effect of HB-EGF was reversed in the presence of AG1478 or LY294002. This suggests that HB-EGF inhibits A/R-induced NF-κB DNA-binding activity via activation of EGFR and the PI3K/Akt pathway in EC.
Figure 4.
HB-EGF activates Akt and inhibits NFκB DNA binding in EC via the PI3K/Akt pathway. EC were subjected to anoxia for 60 min followed by reoxygenation for 4 h or 12 h. EC were treated with LY294002 (20 μM) or AG1478 (500 nM) for 30 min prior to HB-EGF addition. A) PI3K-dependent activation of Akt in EC by HB-EGF. Cytosolic extracts were subjected to immunoblot analysis using phospho-specific Akt antibodies (p-Akt). Membranes were stripped and blotted with anti-total Akt (T-Akt) antibodies. Data are representative of three independent experiments. B) Effects of HB-EGF on NFκB DNA binding in EC. NFκB DNA binding activity in nuclear extracts of EC was assessed using a TransAMTM NFκB kit. The level of NFκB activation (p65 binding to its cognate DNA sequence) is expressed as absorbance at 450 nm. Results represent mean ± SEM of three separate experiments. *p< 0.05 Students t-test..
HB-EGF inhibits ROS production in human neutrophils
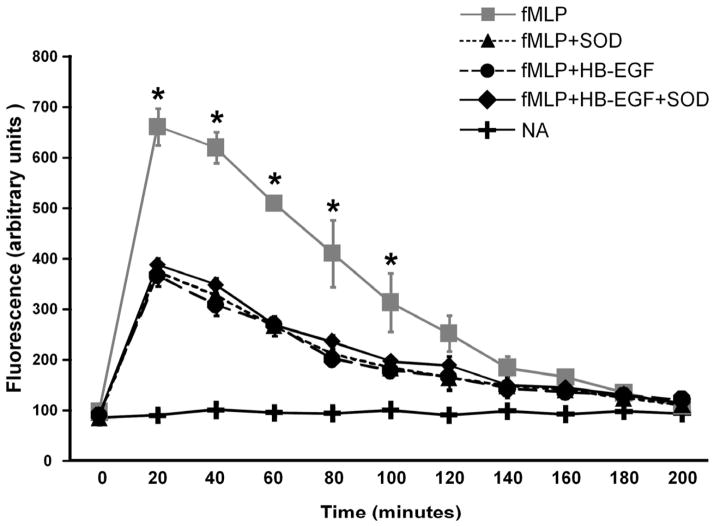
To examine the ability of HB-EGF to decrease ROS production in PMN, we used fMLP to induce ROS production and DCFH-DA to estimate the concentration of ROS. 33 Mixed modeling was used to analyze the global difference of ROS production from 20 min to 100 min among experimental groups. We found that treatment of PMN with HB-EGF inhibited fMLP-induced ROS production (Figure 5). SOD also decreased ROS production in activated neutrophils. In PMN treated with HB-EGF+SOD, ROS production was not further decreased. This suggests that HB-EGF is an anti-oxidant with the ability to decrease ROS production in activated human neutrophils.
Figure 5.
Effect of HB-EGF on neutrophil ROS production. Neutrophils (1×106/ml) were incubated in HBSS and labeled with 1mmol/l DCFH-DA for 1 h at 37°C in the dark. Neutrophil suspensions (100μl containing 1×105 cells) were transferred to 96 well plates, and incubated with or without signal pathway inhibitors or SOD (1000 U/ml). ROS production in neutrophils was induced by addition of fMLP (10−7 mol/l). Fluorescence was monitored every 20 min for 200 min after fMLP addition. Shown are representative recordings of 3 separate experiments. NA, no addition. *p< 0.05 compared to all other groups; the global difference of ROS production from 20 min to 100 min among experimental groups was analyzed using a mixed model.
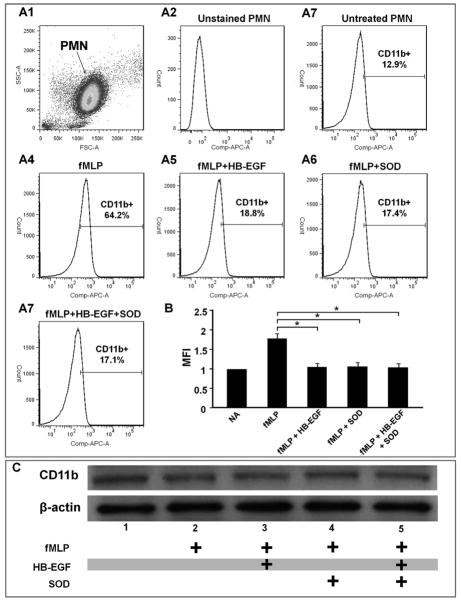
HB-EGF inhibits CD11b mobilization in activated human neutrophils
CD11b/CD18 is a member of the β2-integrin family. Most CD11b/CD18 glycoproteins are stored in granules that can be rapidly mobilized within minutes to the surface of activated neutrophils and monocytes by fusion of granule membranes with the cell membrane. 39 Since CD11b/CD18 and its ligand ICAM-1 on EC are important mediators of PMN-EC adherence, 40 we next examined the effect of HB-EGF on CD11b expression and mobilization in EC. Flow cytometric analyses revealed that CD11b on the cell surface of human PMN was significantly increased 30 min (Figure 6A, B) as well as 1 and 4h (data not shown) after fMLP addition. Addition of HB-EGF significantly decreased fMLP-induced cell-surface expression of CD11b. SOD also significantly decreased fMLP-induced cell-surface expression of CD11b. However, HB-EGF+SOD treatment did not lead to a further decrease in cell-surface CD11b expression. To determine whether HB-EGF inhibits CD11b transcription or mobilization, we examined total CD11b in PMN by immunoblot analysis of total cell extracts. The total amount of CD11b remained unchanged in PMN either with or without HB-EGF treatment 30 min after fMLP addition (Figure 6C). Similar results were obtained 1 and 4h after fMLP addition (data not shown).
Figure 6.
HB-EGF inhibits CD11b mobilization in activated human neutrophils. Freshly isolated human neutrophils were pretreated with either HB-EGF (100 ng/ml), SOD (1000 u/ml), or HB-EGF + SOD for 1 h and then activated by addition of fMLP (10−7 mol/L) for 30 min (shown), 1 h or 4 h (data not shown). PMN were then incubated with APC conjugated anti-human CD11b antibodies. Stained neutrophils were examined by flow cytometry and were gated for viable cells by forward and side scatter criteria. APC positive PMN and the mean fluorescent intensity (MFI) of the positive PMN are shown on the flow cytometric histograms. A1) Dot-plot of forward scatter (FSC) versus side angle light scatter (SSC) showing a main cell population of freshly isolated human PMN. 10,000 cells were analyzed, and the gated PMN comprised >95% of the total cells. A2-7 represent flow cytometric histograms of CD11b expression on the surface of: A2, unstained PMN indicating background fluorescence; A3) untreated PMN; A4) PMN treated with fMLP;.A5) PMN treated with HB-EGF + fMLP; A6) PMN treated with SOD. + fMLP; A7) PMN treated with HB-EGF + SOD + fMLP. B) Quantification of CD11b cell membrane expression. Results derived from histogram analyses are expressed as the mean fluorescence intensity (MFI; mean ± SEM). *p< 0.05, Student’s t-test. C) Immunoblot analysis of CD11b expression in total cell extracts 30 min after fMLP addition. Data are representative of three independent experiments.
Discussion
Intestinal I/R injury is associated with increased microvascular permeability, interstitial edema, impaired vasoregulation, inflammatory cell infiltration, and mucosal ulceration.1 Neutrophils have been implicated as an important mediator in intestinal I/R injury.3 Previous studies found accumulated neutrophils in the gut after I/R injury.1, 27 Neutrophil depletion was found to decrease the incidence of gastritis in primates and gastric bleeding in rats after HS/R, 41, 42 and improved postischemic hypoperfusion of the intestines in rats.10 In the current study, we used the strategy of neutrophil depletion to determine whether the intestinal cytoprotective effects of HB-EGF were dependent upon the presence of neutrophils. HB-EGF treatment of mice subjected to HS/R led to decreased intestinal permeability. Neutropenia provided the same level of gut barrier protection as did HB-EGF. However, the protective effects of HB-EGF treatment on gut barrier function was not synergistic with neutropenia, since neutropenia combined with HB-EGF treatment did not confer further improvement in gut barrier function. This observation suggests that the ability of HB-EGF to protect gut barrier function is dependent on the presence of neutrophils.
PMN-EC interactions play vitals roles in the pathogenesis of intestinal I/R injury.10 To examine PMN-EC interactions, an in vitro PMN-EC adhesion model was established.43 In this model, EC injured by A/R express various inflammatory mediators such as adhesion molecules, interleukins, growth factors, cytokines and chemokines,44 facilitating PMN-EC adherence. We found that treatment of PMN with HB-EGF significantly decreased PMN-EC adherence 4 h after A/R, and this effect was reversed with EGFR inhibition. Pretreatment of EC with HB-EGF significantly decreased PMN-EC adherence 12 h after A/R, and this effect was reversed in the presence of EGFR or PI3K inhibitors. These findings suggest that HB-EGF exerts its inhibitory effects on PMN-EC adherence via interaction with the EGFR and via the PI3K-Akt pathway.
PMN-EC adherence is mediated by a well orchestrated sequence of interactions between adhesion molecules on both EC and neutrophils.39 Some of these adhesion molecules including E-selectin, ICAM-1 and PECAM-1 are transcriptionally up-regulated once the PMN or EC are activated.39, 40 Others, including P-selectin, CD11b/CD18 and CD11c/CD18 are stored in intracellular granules that can be rapidly mobilized to the surface of EC or PMN by fusion of granule membranes with the cell membrane.39, 40 We found that HB-EGF treatment of EC led to inhibition of PMN-EC adherence at a late stage after A/R (12 h). However, HB-EGF treatment of PMN led to inhibition of PMN-EC adherence at an earlier stage after A/R (4 h in this study). In a previous study, we found that HB-EGF treatment of PMN began to inhibit PMN-EC adherence as early as 1 hour after A/R.32 These observations suggest that HB-EGF may regulate the expression of adhesion molecules on PMN and EC by different mechanisms.
Using similar PMN-EC adhesion assays, the transcription factor NF-κB was shown to be responsible for increased PMN-EC adherence 4 h after A/R by transcriptional upregulation of EC adhesion glycoproteins.43 The NF-κB/Rel family of transcription factors is composed of five distinct DNA-binding subunits - p50, p52, p65 (RelA), c-Rel, and Rel-B.43 Nuclear translocation of the activated p65 subunit was shown to increase significantly in EC within 30 min of A/R stimulation, and remained constant over 4 h.43 More importantly, the p65 subunit was shown to be responsible for the strong transcription activating potential of NF-κB, and binding sites for the p65 subunit was found in the promoter elements of E-selectin, ICAM-1 and PECAM-1. 35–38 In this study we found that treatment of EC with the NF-κB inhibitor MG132 led to decreased PMN-EC adherence 4 h and 12 h after A/R. HB-EGF treatment of EC decreased PMN-EC adherence, and down-regulated the mRNA expression and p;rotein production of E-selectin, ICAM-1 and PECAM-1 in EC 12 h after A/R. We found that p65 DNA binding was significantly increased 4 h and 12 h after exposure of EC to A/R, and that treatment of EC with HB-EGF inhibited p65 DNA at both of these time points. Furthermore, the inhibitory effects of HB-EGF on p65 DNA binding in EC, and the expression of the adhesion molecule PECAM 1 in EC, were reversed in the presence of inhibitors of EGFR inhibitor and PI3K. These findings suggest that the inhibition of NF-κB activation via the EGFR and PI3K pathway in EC is responsible for the inhibitory effects of HB-EGF on PMN-EC interactions.
Flow cytometric analysis of cell-surface membrane associated CD11b expression in PMN showed that CD11b was down-regulated by HB-EGF 30 min, 1 h and 4 h after fMLP addition to PMN. At the same time, Western blotting of cell extracts revealed that the total amount of CD11b in PMN remained unchanged. These findings suggest that HB-EGF inhibited the mobilization of CD11b to the cell surface rather than regulating the transcription of CD11b in PMN. ROS have been shown to be an important mediator of either CD11b/CD18 mobilization or transcription. Furthermore, PMN are the major source of ROS during inflammation. We showed that HB-EGF decreased ROS production in PMN in this study. These observations suggest that HB-EGF inhibits CD11b mobilization in PMN by decreasing ROS production in these cells. The signal pathways by which HB-EGF decreases ROS production in PMN remain unknown. We assessed possible signaling pathways by treating PMN with several signal pathway inhibitors, however the inhibitory effects of HB-EGF on PMN-EC adherence was only reversed by EGFR inhibition and not by inhibition of Erk1/2 or PI3K, suggesting that other signaling pathways must be involved in the anti-inflammatory effects of HB-EGF in PMN.
In conclusion, this study provides new insights into the anti-inflammatory role of HB-EGF in the preservation of gut barrier function after HS/R. This study demonstrates that the ability of HB-EGF to preserve gut barrier function is dependent upon the presence of neutrophils, that HB-EGF decreases PMN-EC adherence after A/R, that HB-EGF inhibits NF-κB activation via the PI3K pathway, that HB-EGF regulates the transcription of adhesion molecules in EC, and that HB-EGF inhibits adhesion molecule mobilization by decreasing ROS production in PMN. These findings further our knowledge regarding the mechanisms underlying the anti-inflammatory effects of HB-EGF, and support the clinical use of HB-EGF in the future to treat intestinal injuries associated with hypoperfusion/inflammatory states.
Acknowledgments
This work was financially supported by NIH R01 GM061193 (GEB).
List of Abbreviations
- A/R
anoxia and reoxygenation
- EGFR
epidermal growth factor receptor
- HB-EGF
heparin-binding EGF-like growth factor
- HS/R
hemorrhagic shock and resuscitation
- HUVEC
human umbilical vein endothelial cells
- ICAM-1
intercellular adhesion molecule 1
- I/R
ischemia/reperfusion
- MFI
mean fluorescent intensity
- MODS
multiple organ dysfunction syndrome
- PECAM-1
platelet/endothelial cell adhesion molecule 1
- PMN-EC
neutrophil-endothelial cell
- ROS
reactive oxygen species
- SIRS
systemic inflammatory response syndrome
- SMAO
superior mesenteric artery occlusion
Footnotes
Author Disclosure Statement
No competing financial interests exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kong S, Blennerhassett L, Heel K, McCauley R, Hall J. Ischaemia-reperfusion injury to the intestine. Aust N Z J Surg. 1998;68:554–61. doi: 10.1111/j.1445-2197.1998.tb02099.x. [DOI] [PubMed] [Google Scholar]
- 2.Thuijls G, de Haan J, Derikx J, Daissormont I, Hadfoune M, Heineman E, et al. Intestinal cytoskeleton degradation precedes tight junction loss following hemorrhagic shock. Shock. 2009;31:164–9. doi: 10.1097/SHK.0b013e31817fc310. [DOI] [PubMed] [Google Scholar]
- 3.Mallick I, Yang W, Winslet M, Seifalian A. Ischemia-reperfusion injury of the intestine and protective strategies against injury. Dig Dis Sci. 2004;49:1359–77. doi: 10.1023/b:ddas.0000042232.98927.91. [DOI] [PubMed] [Google Scholar]
- 4.Higuchi S, Wu R, Zhou M, Marini C, Ravikumar T, Wang P. Gut hyperpermiability after ischemia and reperfusion: attenuation with adrenomedullin and its binding protein treatment. Int J Clin Exp Pathol. 2008;1:409–18. [PMC free article] [PubMed] [Google Scholar]
- 5.Clark J, Coopersmith C. Intestinal crosstalk: a new paradigm for understanding the gut as the “motor” of critical illness. Shock. 2007;28:384–93. doi: 10.1097/shk.0b013e31805569df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olanders K, Sun Z, Börjesson A, Dib M, Andersson E, Lasson A, et al. The effect of intestinal ischemia and reperfusion injury on ICAM-1 expression, endothelial barrier function, neutrophil tissue influx, and protease inhibitor levels in rats. Shock. 2002;18:86–92. doi: 10.1097/00024382-200207000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Hayward R, Lefer A. Time course of endothelial-neutrophil interaction in splanchnic artery ischemia-reperfusion. Am J Physiol. 1998;275:H2080–6. doi: 10.1152/ajpheart.1998.275.6.H2080. [DOI] [PubMed] [Google Scholar]
- 8.Tsukimori K, Tsushima A, Fukushima K, Nakano H, Wake N. Neutrophil-derived reactive oxygen species can modulate neutrophil adhesion to endothelial cells in preeclampsia. Am J Hypertens. 2008;21:587–91. doi: 10.1038/ajh.2007.87. [DOI] [PubMed] [Google Scholar]
- 9.Zarbock A, Ley K. Neutrophil adhesion and activation under flow. Microcirculation. 2009;16:31–42. doi: 10.1080/10739680802350104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simpson R, Alon R, Kobzik L, Valeri C, Shepro D, Hechtman H. Neutrophil and nonneutrophil-mediated injury in intestinal ischemia-reperfusion. Ann Surg. 1993;218:444–53. doi: 10.1097/00000658-199310000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turnage R, Kadesky K, Rogers T, Hernandez R, Bartula L, Myers S. Neutrophil regulation of splanchnic blood flow after hemorrhagic shock. Ann Surg. 1995;222:66–72. doi: 10.1097/00000658-199507000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blake K, Carrigan S, Issekutz A, Stadnyk A. Neutrophils migrate across intestinal epithelium using beta2 integrin (CD11b/CD18)-independent mechanisms. Clin Exp Immunol. 2004;136:262–8. doi: 10.1111/j.1365-2249.2004.02429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zakaria el R, Garrison R, Kawabe T, Harris P. Role of neutrophils on shock/resuscitation-mediated intestinal arteriolar derangements. Shock. 2004;21:248–53. doi: 10.1097/01.shk.0000111824.07309.19. [DOI] [PubMed] [Google Scholar]
- 14.Higashiyama S, Abraham J, Miller J, Fiddes J, Klagsbrun M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 1991;251:936–9. doi: 10.1126/science.1840698. [DOI] [PubMed] [Google Scholar]
- 15.Besner G, Higashiyama S, Klagsbrun M. Isolation and characterization of a macrophage-derived heparin-binding growth factor. Cell Regul. 1990;1:811–9. doi: 10.1091/mbc.1.11.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goishi K, Higashiyama S, Klagsbrun M, Nakano N, Umata T, Ishikawa M, et al. Phorbol ester induces the rapid processing of cell surface heparin-binding EGF-like growth factor: conversion from juxtacrine to paracrine growth factor activity. Mol Biol Cell. 1995;6:967–80. doi: 10.1091/mbc.6.8.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishi E, Klagsbrun M. Heparin-binding epidermal growth factor-like growth factor (HB-EGF) is a mediator of multiple physiological and pathological pathways. Growth Factors. 2004;22:253–60. doi: 10.1080/08977190400008448. [DOI] [PubMed] [Google Scholar]
- 18.Nishi E, Prat A, Hospital V, Elenius K, Klagsbrun M. N-arginine dibasic convertase is a specific receptor for heparin-binding EGF-like growth factor that mediates cell migration. EMBO J. 2001;20:3342–50. doi: 10.1093/emboj/20.13.3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Assal O, Besner G. HB-EGF enhances restitution after intestinal ischemia/reperfusion via PI3K/Akt and MEK/ERK1/2 activation. Gastroenterology. 2005;129:609–25. doi: 10.1016/j.gastro.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Radulescu A, Chen Y, Besner G. HB-EGF Improves Intestinal Microcirculation after Hemorrhagic Shock. J Surg Res. 2010 doi: 10.1016/j.jss.2010.01.024. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng J, El-Assal O, Besner G. Heparin-binding epidermal growth factor-like growth factor reduces intestinal apoptosis in neonatal rats with necrotizing enterocolitis. J Pediatr Surg. 2006;41:742–7. doi: 10.1016/j.jpedsurg.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 22.Mehta V, Besner G. HB-EGF promotes angiogenesis in endothelial cells via PI3-kinase and MAPK signaling pathways. Growth Factors. 2007;25:253–63. doi: 10.1080/08977190701773070. [DOI] [PubMed] [Google Scholar]
- 23.Fang L, Li G, Liu G, Lee S, Aaronson S. p53 induction of heparin-binding EGF-like growth factor counteracts p53 growth suppression through activation of MAPK and PI3K/Akt signaling cascades. EMBO J. 2001;20:1931–9. doi: 10.1093/emboj/20.8.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu X, Radulescu A, Zorko N, Besner G. Heparin-binding EGF-like growth factor increases intestinal microvascular blood flow in necrotizing enterocolitis. Gastroenterology. 2009;137:221–30. doi: 10.1053/j.gastro.2009.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu X, Radulescu A, Chen C, James I, Besner G. Heparin-Binding EGF-Like Growth Factor Protects Pericytes from Injury. J Surg Res. 2010 doi: 10.1016/j.jss.2010.07.058. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuhn M, Xia G, Mehta V, Glenn S, Michalsky M, Besner G. Heparin-binding EGF-like growth factor (HB-EGF) decreases oxygen free radical production in vitro and in vivo. Antioxid Redox Signal. 2002;4:639–46. doi: 10.1089/15230860260220148. [DOI] [PubMed] [Google Scholar]
- 27.Xia G, Martin A, Besner G. Heparin-binding EGF-like growth factor downregulates expression of adhesion molecules and infiltration of inflammatory cells after intestinal ischemia/reperfusion injury. J Pediatr Surg. 2003;38:434–9. doi: 10.1053/jpsu.2003.50075. [DOI] [PubMed] [Google Scholar]
- 28.Lara-Marquez M, Mehta V, Michalsky M, Fleming J, Besner G. Heparin-binding EGF-like growth factor down regulates proinflammatory cytokine-induced nitric oxide and inducible nitric oxide synthase production in intestinal epithelial cells. Nitric Oxide. 2002;6:142–52. doi: 10.1006/niox.2001.0393. [DOI] [PubMed] [Google Scholar]
- 29.Mehta V, Besner G. Inhibition of NF-kappa B activation and its target genes by heparin-binding epidermal growth factor-like growth factor. J Immunol. 2003;171:6014–22. doi: 10.4049/jimmunol.171.11.6014. [DOI] [PubMed] [Google Scholar]
- 30.Manoury B, Nenan S, Guenon I, Lagente V, Boichot E. Influence of early neutrophil depletion on MMPs/TIMP-1 balance in bleomycin-induced lung fibrosis. Int Immunopharmacol. 2007;7:900–11. doi: 10.1016/j.intimp.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H, Radulescu A, Besner G. Heparin-binding epidermal growth factor-like growth factor is essential for preservation of gut barrier function after hemorrhagic shock and resuscitation in mice. Surgery. 2009;146:334–9. doi: 10.1016/j.surg.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rocourt D, Mehta V, Wu D, Besner G. Heparin-binding EGF-like growth factor decreases neutrophil-endothelial cell interactions. J Surg Res. 2007;141:262–6. doi: 10.1016/j.jss.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Guasti L, Marino F, Cosentino M, Cimpanelli M, Maio R, Klersy C, et al. Simvastatin treatment modifies polymorphonuclear leukocyte function in high-risk individuals: a longitudinal study. J Hypertens. 2006;24:2423–30. doi: 10.1097/01.hjh.0000251903.62804.77. [DOI] [PubMed] [Google Scholar]
- 34.Mehta V, Besner G. Heparin-binding epidermal growth factor-like growth factor inhibits cytokine-induced NF-kappa B activation and nitric oxide production via activation of the phosphatidylinositol 3-kinase pathway. J Immunol. 2005;175:1911–8. doi: 10.4049/jimmunol.175.3.1911. [DOI] [PubMed] [Google Scholar]
- 35.Edelstein L, Pan A, Collins T. Chromatin modification and the endothelial-specific activation of the E-selectin gene. J Biol Chem. 2005;280:11192–202. doi: 10.1074/jbc.M412997200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmitz M, Baeuerle P. The p65 subunit is responsible for the strong transcription activating potential of NF-kappa B. EMBO J. 1991;10:3805–17. doi: 10.1002/j.1460-2075.1991.tb04950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Botella L, Puig-Kröger A, Almendro N, Sánchez-Elsner T, Muñoz E, Corbí A, et al. Identification of a functional NF-kappa B site in the platelet endothelial cell adhesion molecule-1 promoter. J Immunol. 2000;164:1372–8. doi: 10.4049/jimmunol.164.3.1372. [DOI] [PubMed] [Google Scholar]
- 38.Winning S, Splettstoesser F, Fandrey J, Frede S. Acute hypoxia induces HIF-independent monocyte adhesion to endothelial cells through increased intercellular adhesion molecule-1 expression: the role of hypoxic inhibition of prolyl hydroxylase activity for the induction of NF-kappa B. J Immunol. 2010;185:1786–93. doi: 10.4049/jimmunol.0903244. [DOI] [PubMed] [Google Scholar]
- 39.Panes J, Granger D. Leukocyte-endothelial cell interactions: molecular mechanisms and implications in gastrointestinal disease. Gastroenterology. 1998;114:1066–90. doi: 10.1016/s0016-5085(98)70328-2. [DOI] [PubMed] [Google Scholar]
- 40.Zarbock A, Ley K. Mechanisms and consequences of neutrophil interaction with the endothelium. Am J Pathol. 2008;172:1–7. doi: 10.2353/ajpath.2008.070502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith S, Holm-Rutili L, Perry M, Grisham M, Arfors K, Granger D, et al. Role of neutrophils in hemorrhagic shock-induced gastric mucosal injury in the rat. Gastroenterology. 1987;93:466–71. doi: 10.1016/0016-5085(87)90907-3. [DOI] [PubMed] [Google Scholar]
- 42.Mileski W, Winn R, Vedder N, Pohlman T, Harlan J, Rice C. Inhibition of CD18-dependent neutrophil adherence reduces organ injury after hemorrhagic shock in primates. Surgery. 1990;108:206–12. [PubMed] [Google Scholar]
- 43.Kokura S, Rhoads C, Wolf R, Yoshikawa T, Granger D, Aw T. NF kappa b signaling in posthypoxic endothelial cells: relevance to E-selectin expression and neutrophil adhesion. J Vasc Res. 2001;38:47–58. doi: 10.1159/000051029. [DOI] [PubMed] [Google Scholar]
- 44.Yin J, Luo X, Yu W, Liao J, Shen Y, Zhang Z. Antisense oligodeoxynucleotide against tissue factor inhibits human umbilical vein endothelial cells injury induced by anoxia-reoxygenation. Cell Physiol Biochem. 2010;25:477–90. doi: 10.1159/000303053. [DOI] [PubMed] [Google Scholar]