Summary
Objectives
The habitual chewing of betel quid (areca nut, betel leaf, tobacco) is estimated to occur among 600 million persons in Asia and the Asia-Pacific Region. Emerging data from rural Asia indicate that the betel quid is part of traditional medicine practices that promote its use for a wide range of ailments, including infectious disease. In the present study, we examined the association between betel quid, traditional medicine, and infectious disease outcomes.
Methods
For the purpose of a nationwide, interviewer-administered, cross-sectional survey of tobacco use (including betel quid), we conducted a stratified three-stage cluster sampling of 13 988 adults aged 18 years and older from all provinces of Cambodia.
Results
We found an association between the intensity of betel quid use and HIV/AIDS (odds ratio (OR) 2.06, 95% CI 1.09–3.89), dengue fever (OR 2.40, 95% CI 1.55–2.72), tuberculosis (OR 1.50, 95% CI 0.96–2.36), and typhoid (OR 1.48, 95% CI 0.95–2.30). These associations were even stronger in women – the primary users of betel quid in Cambodia. Multivariable analyses that controlled for age, gender, income, education, urban versus rural dwelling, receiving care from traditional medicine practitioners, and cigarette smoking did not alter the betel quid–infectious disease association.
Conclusions
Our findings raise the possibility of a role of betel quid use in the transmission of infectious disease through pathways such as immunosuppression, oral route of entry for a pathogen (i.e., through injury to the oral mucosa), and contamination (i.e., fecal–oral) of the betel quid ingredients.
Keywords: Smokeless tobacco, Epidemiology, Tuberculosis, HIV
Introduction
Betel quid use involves the chewing of areca nut (Areca catechu L. (Arecaceae)), betel leaf (Piper betle L. (Piperaceae)), slaked lime (calcium hydroxide), and tobacco, and is practiced by 600 million persons in Asia, South Asia, and Southeast Asia,1–3 and also among migrants from those regions living in Western nations.4 Large-scale prospective cohort studies in India and Taiwan have shown that betel quid use in women and men is associated with a 20–30% increase in the all-cause mortality rate.5,6 Some of the chronic diseases underlying this trend include (1) a well-known association with oral cancer, oral leukoplakia, and submucous fibrosis, which have primarily been attributed to the formation of carcinogenic N-nitroso compounds produced by the quid,7 and (2) a growing body of evidence of a link with cardiovascular disease and diabetes.8–10 Although a relationship between betel quid and periodontal infections has been reported,11 there is a paucity of data investigating a link between betel quid and infectious, communicable disease.
Qualitative and quantitative data are emerging from several nations indicating that the use of betel quid may be an integral part of cultural, familial, and traditional medicine practices.3,4,12,13 Specifically, the betel quid ingredients are known throughout Asia and South Asia for their medicinal effects as a stimulant, a digestive aid (specifically for the relief of morning sickness during pregnancy),12 an antiseptic, a de-worming agent, a pain reliever, and for the treatment of infectious disease.13 During a large-scale prevalence study of tobacco use in Cambodia (N = 13 988)12,14 in 2005–2006, we found that (1) about half the older women (>48 years) are habitual betel quid users, (2) one out of five women started the habit during pregnancy in response to morning sickness,15 and (3) by occupation the highest prevalence of chewing tobacco was among midwives (67.9%) and traditional healers (47.1%). These data raise the possibility that traditional medicine practices are influencing the initiation and use of the betel quid in Cambodia.
In the current analyses of this national sample of 13 988 adults in Cambodia,12 our aims were (1) to examine the association between habitual betel quid use and specific infectious diseases (HIV/AIDS, tuberculosis, dengue fever, typhoid), and (2) to examine the association between betel quid use and the use of traditional medicine services (i.e., traditional healer, traditional birth attendant, other faith healer) and to control for this association in the multivariable analysis of infectious diseases.
Methods
Study population
The study population has been described extensively elsewhere.12,14,16 Briefly, for the survey, we assembled a nationwide, representative sample of 13 988 adults aged 18 years and older. We worked with the National Institute of Statistics (Ministry of Planning, Cambodia) to conduct a stratified three-stage cluster sampling using the Cambodia General Population Census as a sampling frame.17 Specifically, we stratified the country into 17 sampling domains consisting of 12 individual provinces and five groups of similar provinces. Within each domain, we then randomly selected villages (we use the term ‘village’ to represent a rural village or urban area of comparable size) in multiple stages (by district and census block areas (representing 110 households)). We found that less than 3% declined to complete the final household survey. The final sample consisted of 13 988 adults.
Written informed consent was obtained from each subject and the protocols for the national survey and validation sub-studies were approved by the Institutional Review Board of Loma Linda University and the National Ethics Committee on Health (Ministry of Health) in Cambodia.
Questionnaire
Survey items were designed during focus groups,18 survey research training of Ministry of Health (Cambodia) personnel,16 and consultation with local non-governmental organizations (i.e., the Adventist Development and Relief Agency; ADRA) that had conducted tobacco surveys. The final survey was conducted in the local language (Khmer) after being translated using methods described by Flaherty et al.19
The 2005–2006 national prevalence survey was interviewer-administered (census workers from the National Institute of Statistics, Ministry of Planning, Cambodia) and included items on demographics, tobacco use (commercial cigarettes, hand-rolled cigarettes, chewing tobacco and betel quid, tobacco pipe), age at initiation of tobacco, reasons for starting to smoke, knowledge and attitudes about tobacco, smoking cessation, anthropometrics, diet, current health, women’s health, and media exposure. In addition to dichotomous measures of current betel quid use, the survey data allowed for an index of betel quid use (times per month) that was computed by: number of days per month chewing betel quid × number of times per day. In this context, a ‘time per day’ is defined as a session where the betel leaf, areca nut, slaked lime, and tobacco are combined into a packet and chewed and/or maintained in the gingival pocket. This index was further modified by a pictogram-derived weighting factor that allowed subjects to estimate (using pictures depicting weighed amounts of tobacco) the amount of tobacco that was added to the betel quid. This procedure has been described and validated elsewhere.12,20,21
Access to health care was assessed using an item that had been developed by the National Institute of Statistics, Ministry of Planning, Cambodia for ‘Demographic and Health’ and socioeconomic surveys during 2000–2007.17,22 These items classified health care options for the national sample into the following three categories: (1) public medical sector (national hospital, provincial referral hospital, district hospital, health center, health post, outreach), (2) private medical sector (private hospital, private clinic, home/office of trained health worker/nurse), and outside the medical sector (private pharmacy, store selling drugs, traditional healer (‘Kru Khmer’, ‘Magician’), traditional birth attendant, faith-based healer). These data were used to identify subjects who used traditional medicine practitioners (traditional healers, traditional birth attendants, other faith-based healers) when they were seeking health care.
Items on self-report of infectious disease and infectious disease symptoms were adapted from the ‘Demographic Health Surveys’ administered in 2000 and 2007.22 For each infectious disease (tuberculosis, dengue fever, HIV/AIDS, typhoid, malaria) subjects were asked whether a doctor or other health worker had ever diagnosed them with the disease. Symptoms were obtained using an item that asked about illness and/or health problems during the past 4 weeks.
Statistical analysis
Data analysis for this study needed to account for the stratified, multi-stage cluster sampling protocol described above. The 95% confidence intervals (95% CI) for prevalence, means, and odds ratios (OR) for tobacco use and health variables were calculated using a Taylor series linearized approach to compute between-cluster variance estimators that accounted for the intra-cluster correlation among subjects within the same village. Point estimates for prevalence, means, and OR were further adjusted by sample weights to account for different sampling fractions within each of the 17 domains described above. OR were derived from logistic regression models with dependent variables such as the use of traditional medicine and specific disease outcomes. The exposure variables tested in the model were for betel quid use (current use (yes/no), frequency of use of the quid, by subject’s estimate of weight of tobacco in the quid using a picture card). Confounders were included that changed the exposure point estimate to a meaningful extent. These statistical analyses were performed using SUDAAN software release 9.0 (RTI International, Research Triangle Park, NC, USA).
Results
The demographics of the study population (N = 13 988) have been published elsewhere.12,14 Briefly, the survey data indicated that the sample was more than 95% Khmer ethnicity, Buddhist, and had completed 12 years of education or less. By income and occupation, we found that 87% earned less than 2 US dollars (USD) per day, with farming and labor being the most common occupations (64%). We compared the demographics and health status of betel quid users and non-users (Table 1). We found that betel quid users tended to be older, female, rural dwelling, with lower income, less education, and higher rates of tuberculosis. The prevalence of using traditional medicine was slightly higher in users (14%) than non-users (11%). These differences all attained statistical significance (p < 0.05) in contingency table analysis (Chi-square for independence), with the exception of the higher rate of tuberculosis in betel quid users, which was marginal (p = 0.08).
Table 1.
Prevalence of selected demographic variables in a nationwide sample of 13 988 adults (aged 18 years and older) from Cambodia
| Variables | All (%) | Betel quid user (%) | Betel quid non-user (%) |
|---|---|---|---|
| Age (years)a | |||
| 18–25 | 26.7 | 1.5 | 29.5 |
| 26–36 | 23.2 | 8.6 | 24.8 |
| 37–48 | 25.5 | 24.9 | 25.6 |
| >48 | 24.6 | 65.0 | 20.2 |
| Gendera | |||
| Male | 43.6 | 4.4 | 48.0 |
| Female | 56.4 | 95.6 | 52.0 |
| Residencea | |||
| Rural | 83.0 | 93.8 | 81.8 |
| Urban | 17.0 | 6.2 | 18.2 |
| Ethnicitya | |||
| Khmer | 95.1 | 94.5 | 95.2 |
| Cham | 3.1 | 5.1 | 2.9 |
| Local tribeb | 0.6 | 0.3 | 0.7 |
| Otherc | 1.2 | 0.1 | 1.3 |
| Religiona | |||
| Buddhist | 95.6 | 94.6 | 95.8 |
| Muslim | 3.1 | 4.2 | 2.9 |
| Christian | 0.4 | 0.2 | 0.4 |
| Otherd | 0.9 | 1.0 | 0.8 |
| Educationa | |||
| 0–6 y | 74.3 | 97.8 | 71.6 |
| 7–12 y | 23.3 | 2.2 | 25.6 |
| 13–15 y | 1.4 | 0 | 1.6 |
| >15 y | 1.1 | 0 | 1.2 |
| Income per day (USD)a | |||
| <1 | 74.1 | 90.3 | 72.3 |
| 1–2 | 12.6 | 5.9 | 13.3 |
| >2–3 | 5.7 | 2.2 | 6.1 |
| >3 | 7.6 | 1.6 | 8.3 |
| Seek care from traditional medicine practitionera | |||
| Yes | 11.4 | 14.7 | 11.1 |
| No | 88.6 | 85.3 | 88.9 |
| Disease history | |||
| Tuberculosis | 2.9 | 4.0 | 2.8 |
| Typhoid fever | 4.9 | 4.1 | 4.9 |
| Dengue fever | 0.4 | 0.5 | 0.4 |
| HIV/AIDS | 0.5 | 0.8 | 0.5 |
| Malaria | 2.5 | 1.8 | 2.6 |
USD, US dollars.
p < 0.05 for a Chi-square test evaluating the null hypothesis of independence between the demographic variable and betel quid use.
Indigenous ‘hill tribes’ found throughout Southeast Asia.
Chinese, Vietnamese, Laos, Thai, Other.
Local or tribal religions (i.e., Animist).
Use of traditional medicine and betel quid use
In a logistic regression model with use of traditional medicine (defined as traditional healers (‘Kru Khmer’), traditional birth attendants, or other faith-based healers) as the outcome variable and age and betel quid use as covariates, we found that betel quid users were 41% more likely (OR 1.41, 95% CI 1.04–192) to use traditional medicine than non-users and that this effect persisted even after control for income, education, and rural residence. Since the prevalence of betel quid use in men is quite low (1.0%),12 we conducted an analysis of all subjects and of women separately. Among women, who are the major users of betel quid in Cambodia,12 we found betel quid users to be 51% (OR 1.51, 95% CI 1.06–2.16) more likely to use traditional medicine. After controlling for income, education, and rural status this effect was attenuated, albeit statistically significant. Based on these data, we decided to control for traditional medicine when investigating the relationship between betel quid use and infectious disease (Table 2).
Table 2.
Association between intensity of betel quid use (per 10 g tobacco added to each quid) and disease outcomes among 13 988 adults in a national sample from Cambodia (2005–2006)
| Disease | All | Women only | ||
|---|---|---|---|---|
| Age-adjusted OR (95% CI) |
Multivariablea OR (95% CI) |
Age-adjusted OR (95% CI) |
Multivariablea OR (95% CI) |
|
| Tuberculosis | 1.50 (0.96–2.36) p = 0.08 |
1.39 (0.87–2.31) p = 0.21 |
1.57 (0.97–2.55) p = 0.07 |
1.42 (0.85–2.37) p = 0.18 |
| Typhoid | 1.48 (0.95–2.30) p = 0.08 |
1.53 (0.99–2.34) p = 0.06 |
1.58 (1.03–2.44) p = 0.04 |
1.56 (1.01–2.41) p = 0.05 |
| HIV/AIDS | 2.06 (1.09–3.89) p = 0.03 |
2.04 (1.10–3.70) p = 0.02 |
2.03 (1.06–3.89) p = 0.03 |
2.06 (1.11–3.82) p = 0.02 |
| Dengue fever | 2.40 (1.55–2.72) p = 0.0001 |
2.58 (1.70–3.93) p < 0.0001 |
3.12 (2.17–4.19) p < 0.0001 |
3.44 (2.25–5.25) p < 0.0001 |
| Malaria | 0.86 (0.38–1.92) p = 0.71 |
1.49 (1.03–2.18) p = 0.04 |
1.60 (1.16–2.22) p = 0.004 |
1.58 (1.13–2.20) p = 0.008 |
OR, odds ratio; CI, confidence interval.
Adjusted for age and the following additional covariables: gender, income, education, urban/rural, use of traditional medicine healer, current smoking.
Betel quid use and infectious disease outcomes
In logistic regression models with specific disease outcomes (tuberculosis, typhoid fever, dengue fever, HIV/AIDS) as the dependent variable, we found that betel quid users were 2.6 times (OR 2.6, 95% CI 0.91–7.45) more likely to report a diagnosis of HIV/AIDS. Among women, a strong association was found for betel quid use and dengue fever (OR 3.01, 95% CI 1.01– 9.73). Among men, a strong association was found for betel quid use and tuberculosis (OR 4.43, 95% CI 1.66–11.86), but this was based on a small case series due to the low prevalence of use in men (eight tuberculosis cases among 63 betel quid users;168 tuberculosis cases among 6139 non-users).
In further analyses (Table 2) that explored the intensity of betel quid use (odds of disease per 10 g of tobacco added to each quid), we found an association with dengue fever (OR 2.40, 95% CI 1.55–2.72), HIV/AIDS (OR 2.06, 95% CI 1.09–3.89), typhoid (OR 1.48, 95% CI 0.95–2.30), and tuberculosis (OR 1.50, 95% CI 0.96–2.36). These findings persisted in the multivariable analysis where we controlled for age, poverty-related factors (education, income, urban/rural residence, seek care from traditional medicine), and current cigarette smoking. Among women (Table 2), the association with these diseases was even stronger and also included a betel quid–malaria association. The prevalence of use among men was too low for stratum-specific analysis.
Discussion
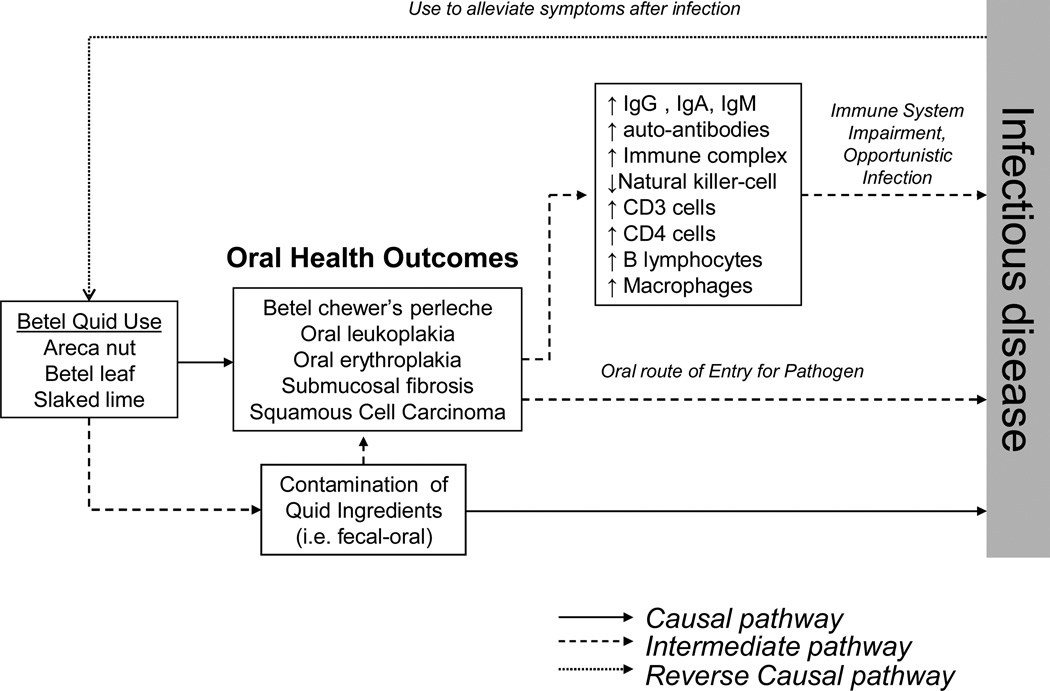
Our findings from a national sample of adults in Cambodia indicate an association between the intensity of habitual, daily betel quid use and HIV/AIDS, dengue fever, typhoid, and tuberculosis. These findings (Table 2) were even stronger among Cambodian women – a group for whom betel quid is the primary form of tobacco use. We have considered causal, intermediate, and reverse causal pathways to explain these findings (Figure 1).
Figure 1.
Multi-state model linking betel quid use to infectious disease through causal, intermediate, and reverse causal pathways.
Is habitual betel quid use a pre-disposing factor for opportunistic infectious disease?
Our findings link habitual betel quid use to disease caused by a range of viral and bacterial pathogens that are typically transmitted through mosquitoes (dengue fever, malaria), food and drink (typhoid), and person-to-person contact (HIV/AIDS, tuberculosis). We note that even though habitual betel quid use may not represent direct exposure to a pathogen, betel quid use could potentially increase the risk of infection through pathways such as providing an oral route of entry for the pathogen, immunosuppression, or due to transmission by the person-to-person contact that occurs in the preparation and chewing of betel quid (i.e., typically a group behavior among women of the Western Pacific Region).
Route of entry for pathogens and immunosuppression
Betel quid use could potentially increase the risk of developing infectious disease by providing an oral route of entry for pathogens through the constant injury to the oral mucosa. Specifically, Ling et al.11 found that betel quid chewers tended to exhibit significantly more bleeding on probing, gingival inflammation, and attachment loss – indicators of periodontal disease in this sample that were also linked to infection (i.e., Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis). Long-term betel quid use also provides an oral route of entry since it is associated with (1) a characteristic ‘betel chewer’s perlèche’ that consists of fissures at angles in the mouth due to constant exposure,23 (2) a characteristic oral leukoplakia in the gingival area, and (3) a submucosal fibrosis thought to be caused by the calcium hydroxide paste added to the quid. Among Cambodian female betel quid users, Reichart et al. found a very high prevalence of oral lesions (betel chewer’s mucosa (85.4%), oral leukoplakia (8.3%), leukoedema (37.5%), and oral lichen planus (4.2%)).24
Additionally, several studies have demonstrated that the submucosal fibrosis, oral leukoplakia, and oral erythroplakia that are commonly associated with betel quid, can impair a wide range of immune function parameters (Figure 1), which include but are not limited to: CD3 cells (T lymphocytes), CD4 cells (helper/inducer T lymphocytes), B lymphocytes, macrophages,25 natural killer cells,26 total leukocyte and lymphocyte counts,27 immunoglobulin (Ig) G, IgM, and IgA,28,29 circulating immune complex,30 auto-antibodies such as gastric parietal cell antibody (GPCA), thyroid microsomal antibody, anti-nuclear antibody (ANA), anti-reticulin antibody, and anti-smooth muscle antibody (SMA).31 Thus, under such a level of constant immunosuppression, it is biologically plausible that users experience an increased risk of opportunistic infection transmitted through other means (mosquito bite, food and drink, and risky sexual practices).
It is also noteworthy that the nicotine component from tobacco added to the quid may also induce immune suppression by affecting both humoral and cell-mediated immune responses,32–35 and additionally producing an altered immune response that is characterized by a decline in inflammation, a reduction in antibody response, and a decrease in T-cell receptor-mediated signaling.36 The arecoline component of the areca nut has also been shown in rodent models to have a possible immunomodulatory effect.37–39
Behaviors associated with betel quid preparation and chewing
Certain patterns of behavior associated with betel quid chewing in rural areas of the Western Pacific Region can potentially contribute to the transmission of infectious disease. In 200612 and 201140 national surveys of tobacco use, we reported that of the two million tobacco users in Cambodia, about 500 000 are women who chew a betel quid that consists of the areca nut, slaked lime, betel leaf, and loose tobacco. In contrast to South Asia, in rural areas of Cambodia the betel quid is not a pre-packaged commercial product, but rather is prepared by hand in such a way that the betel leaf is wrapped around the areca nut, slaked lime, and tobacco.2,5 This type of handling and preparation by users can promote fecal–oral transmission of typhoid bacteria when unwashed hands are involved in the preparation of the quid and/or insertion of the ‘quid’ into the gingival pocket. Direct contamination of betel quid ingredients (i.e., due to unsanitary conditions in storage) before preparation is also noteworthy. Lastly, it is noteworthy that many of the alkaloids (i.e., arecoline) found in the areca nut component of the betel quid are primarily parasympathetic in action and acutely stimulate glandular secretion (i.e., salivary, lacrimal) during a betel quid chewing session.41 Thus, betel quid chewing produces large volumes of saliva and frequent spitting of both the excess saliva and quid. Moreover, the communal environment of groups of chewers sitting in proximity to one another and spitting the excess saliva–quid mixture can increase person-to-person transmission of tuberculosis and other pathogens through contact with saliva droplets.
Betel quid chewing as a traditional medicine remedy: reverse causation?
We considered the possibility of ‘reverse causation’, where a betel quid–infectious disease relationship may be partly attributable to betel quid being used for medicinal purposes. In multivariable analyses (Table 2) where we controlled for the use of traditional medicine for the treatment of illness, the associations between betel quid and infectious disease indicated no important change in effect. Also, another argument against ‘reverse causation’ is that the associations with infectious disease depicted in Table 2 were for habitual, daily betel quid use and not for use during illness.
Despite this, we note that our survey does not measure behavioral patterns whereby after the onset of illness, betel quid use is increased among current users or becomes a reason for the initiation of use. The co-existence of causal, intermediate, and/or reverse causal pathways depicted in Figure 1 raises the possibility of a ‘vicious cycle’, where increased betel quid use post-infection (i.e., to alleviate symptoms) further suppresses immune function.
In recent key informant interviews in Cambodia that were published in 2008 in the national newspaper, a betel nut shop owner indicated that “I do not know if [betel quid] affects our health, but betel leaf and areca are important in curing typhoid”. The 63-year-old woman further indicated that betel quid ingredients “are Cambodian traditional medicine”.42 Such beliefs were also evident in our finding from the national survey data of Cambodia showing that one out of five women indicated that they started chewing betel quid during pregnancy as a relief for the symptoms of morning sickness.15 Some of this practice may relate to long-held beliefs among traditional medicine practitioners and herbalists that the areca nut-derived alkaloids (i.e., arecoline) are a remedy for relaxing the stomach and ileum.43
Do traditional medicine beliefs in Asia include the use of betel quid as prevention/treatment of infectious disease? In Asia and the Asia Pacific Region, traditional medicine practitioners have long touted the ‘betel leaf’ (leaf of the areca nut palm tree, Areca catechu) as a treatment for typhoid, cholera, tuberculosis, conjunctivitis, ear infection, boils, abscesses, and ringworm.44,45 The rationale for this treatment is a belief that essential oils in the betel leaf exhibit antibacterial, antiprotozoan, and antifungal properties.13 Traditional medicine in India also point to the vitamin and mineral content of the betel quid in the common claim that six betel leaves in combination with slaked lime (calcium hydroxide) have a vitamin and mineral content equivalent to 300 ml of cow milk.13
Implications for global epidemiology of betel quid use
Our findings from Cambodia indicate a strong association between betel quid use and infectious disease that is occurring among users who are predominantly non-smoking women – a trend that is found throughout the Western Pacific Region.2 In Southeast Asia (Cambodia,12 Myanmar,46 Thailand,47 and Vietnam48), betel quid is primarily used by middle-aged and older women (7–46% prevalence) and has been described as being a ‘rite of passage into womanhood’.4 Betel quid use in the Asia-Pacific Region (Palau, Papua New Guinea, and Malaysia) exceeds 50% among rural women.7,49–51 The potential co-aggregation of betel quid exposure and infectious disease in women of reproductive age in this region is an especially alarming scenario when considering the lethal consequences of transplacental transmission of the pathogen (i.e., HIV) and betel quid metabolites. Maternal betel quid use has been associated with a higher rate of stillbirth in cohort studies in India.52
When considering the infectious disease burden associated with betel quid use it is also important to note that in other parts of Asia (India, Pakistan, and Taiwan) the habit occurs in all adults, including males, and may occur together with cigarette smoking.2 Among the few men who did use betel quid in Cambodia we found a more than four-fold increase in the rate of tuberculosis. Recent national (India)53 and global54 estimates have strongly linked smoked tobacco to a significant burden of tuberculosis. Our findings raise the possibility that smokeless tobacco also contributes to this burden of tuberculosis.
Limitations
There are several limitations of this study that should be noted. The cross-sectional design does not allow us to entirely separate causal, intermediate, and non-causal scenarios for betel quid and infectious disease. Differentiating between these scenarios would require studies of betel quid use during disease and illness. Also useful would be the findings from prospective investigations of betel quid use and disease outcomes in the region. Measurement error in the survey measures of both the self-reported disease and the betel quid items should be mentioned. Our validation study of the tobacco items, however, showed excellent results.
Conclusions
Our findings indicate a strong association between betel quid use and infectious disease and underscore the need for studies in rural Southeast Asia that are designed to investigate bio-behavioral frameworks that include (1) oral health outcomes from betel quid use contributing to immune suppression that increases the risk of infectious disease, (2) oral health outcomes from betel quid use providing a route of entry for pathogens, (3) bacterial contamination (fecal–oral) of betel quid ingredients leading to transmission of infectious disease, (4) person-to-person transmission through contact with salivary droplets of infected users, and (5) changing and initiating patterns of betel quid use as part of traditional medicine remedies.
Acknowledgements
This study was funded by grant R01 TW05964-01 from the National Institutes of Health/Fogarty International Center (Asian Leadership Training for Tobacco Control Research).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: No conflict of interest to declare.
References
- 1.Trivedy CR, Craig G, Warnakulasuriya S. The oral health consequences of chewing areca nut. Addict Biol. 2002;7:115–125. doi: 10.1080/13556210120091482. [DOI] [PubMed] [Google Scholar]
- 2.Gupta PC, Warnakulasuriya S. Global epidemiology of areca nut usage. Addict Biol. 2002;7:77–83. doi: 10.1080/13556210020091437. [DOI] [PubMed] [Google Scholar]
- 3.Gupta PC, Ray CS, Sinha DN, Singh PK. Smokeless tobacco: a major public health problem in the SEA region: a review. Indian J Public Health. 2011;55:199–209. doi: 10.4103/0019-557X.89948. [DOI] [PubMed] [Google Scholar]
- 4.Pickwell SM, Schimelpfening S, Palinkas LA. ‘Betelmania’. Betel quid chewing by Cambodian women in the United States and its potential health effects. Western J Med. 1994;160:326–330. [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta PC, Ray CS. Epidemiology of betel quid usage. Ann Acad Med Singapore. 2004;33:31–36. [PubMed] [Google Scholar]
- 6.Lin WY, Chiu TY, Lee LT, Lin CC, Huang CY, Huang KC. Betel nut chewing is associated with increased risk of cardiovascular disease and all-cause mortality in Taiwanese men. Am J Clin Nutr. 2008;87:1204–1211. doi: 10.1093/ajcn/87.5.1204. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization/International Agency for Research on Cancer. Betel-quid and areca nut chewing and some areca-nut-derived nitrosamines. Vol. 85. Lyon, France: WHO/IARC; 2004. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. [Google Scholar]
- 8.Chung FM, Chang DM, Chen MP, Tsai JC, Yang YH, Shieh TY, et al. Areca nut chewing is associated with metabolic syndrome: role of tumor necrosis factor-alpha, leptin, and white blood cell count in betel nut chewing-related metabolic derangements. Diabetes Care. 2006;29:1714. doi: 10.2337/dc06-0628. [DOI] [PubMed] [Google Scholar]
- 9.Guh JY, Chen HC, Tsai JF, Chuang LY. Betel-quid use is associated with heart disease in women. Am J Clin Nutr. 2007;85:1229–1235. doi: 10.1093/ajcn/85.5.1229. [DOI] [PubMed] [Google Scholar]
- 10.Yen AM, Chiu YH, Chen LS, Wu HM, Huang CC, Boucher BJ, et al. A population-based study of the association between betel-quid chewing and the metabolic syndrome in men. Am J Clin Nutr. 2006;83:1153–1160. doi: 10.1093/ajcn/83.5.1153. [DOI] [PubMed] [Google Scholar]
- 11.Ling LJ, Hung SL, Tseng SC, Chen YT, Chi LY, Wu KM, et al. Association between betel quid chewing, periodontal status and periodontal pathogens. Oral Microbiol Immunol. 2001;16:364–369. doi: 10.1034/j.1399-302x.2001.160608.x. [DOI] [PubMed] [Google Scholar]
- 12.Singh PN, Yel D, Sin S, Sothy K, Lopez J, Job JS, et al. Tobacco use among adults in Cambodia: evidence for a tobacco epidemic among Cambodian women. Bull World Health Organ. 2009;87:905–912. doi: 10.2471/BLT.08.058917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guha P. Betel leaf: the neglected green gold of India. J Hum Ecol. 2006;19:87–93. [Google Scholar]
- 14.Rudatsikira EM, Knutsen SF, Job JS, Singh PN, Yel D, Montgomery SB, et al. Exposure to environmental tobacco smoke in the nonsmoking population of Cambodia. Am J Prev Med. 2008;34:69–73. doi: 10.1016/j.amepre.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Cumberland S, Ghent A, Singh PN, Bettcher D. Editorial: Cambodian women chew tobacco for morning sickness. Bull World Health Organ. 2009;87:885–886. [Google Scholar]
- 16.Ferry L, Job J, Knutsen S, Montgomery S, Petersen F, Rudatsikira E, et al. Mentoring Cambodian and Lao health professionals in tobacco control leadership and research skills. Tob Control. 2006;15:i42–i47. doi: 10.1136/tc.2005.015008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cambodia socio-economic survey, 2004. Phnom Penh, Cambodia: National Institute of Statistics; 2004. National Institute of Statistics, Ministry of Planning. [Google Scholar]
- 18.Singh PN, Sovann S, Sothy K, Job J, Rudatsikira E, Petersen F, et al. Society for Research on Nicotine and Tobacco. Prague, Czechoslovakia: 2005. Mar 20–23, Beliefs about smoke-free areas and tobacco use among Cambodian adults vary by gender. S45. [Google Scholar]
- 19.Flaherty JA, Gaviria FM, Pathak D, Mitchell T, Wintrob R, Richman JA, et al. Developing instruments for cross-cultural psychiatric research. J Nerv Ment Dis. 1988;176:257–263. [PubMed] [Google Scholar]
- 20.Singh PN, Yel D, Sovann S, Job J, Rudatsikira E, Petersen F, et al. Design, validation, and administration of a nationwide survey of adult tobacco use in Cambodia. P8870. The 13th World Conference on Tobacco OR Health. Building capacity for a tobacco-free world; July 12–15, 2006; Washington DC. [Google Scholar]
- 21.Singh PN, Sothy K, Yel D, Bunthy C, Nguyen D, Job J, et al. Validity and reliability of survey items and pictograms for use in a national household survey of tobacco use in Cambodia. S6. Society for Research on Nicotine and Tobacco Annual Meeting; February 15–18, 2006; Orlando, Florida. [Google Scholar]
- 22.National Institute of Public Health and National Institute of Statistics. Phnom Penh: Cambodia/ORC Macro; 2007. Results of the Cambodia demographic and health survey 2005. [Google Scholar]
- 23.Nelson BS, Heischober B. Betel nut: a common drug used by naturalized citizens from India, Far East Asia, and the South Pacific Islands. Ann Emerg Med. 1999;34:238–243. doi: 10.1016/s0196-0644(99)70239-8. [DOI] [PubMed] [Google Scholar]
- 24.Reichart PA, Schmidtberg W, Samaranayake LP, Scheifele C. Betel quid-associated oral lesions and oral Candida species in a female Cambodian cohort. J Oral Pathol Med. 2002;31:468–472. doi: 10.1034/j.1600-0714.2002.00009.x. [DOI] [PubMed] [Google Scholar]
- 25.Haque MF, Harris M, Meghji S, Speight PM. An immunohistochemical study of oral submucous fibrosis. J Oral Pathol Med. 1997;26:75–82. doi: 10.1111/j.1600-0714.1997.tb00025.x. [DOI] [PubMed] [Google Scholar]
- 26.Pillai MR, Balaram P, Kannan S, Sudha L, Nalinakumari KR, Hareendran NK, et al. Interferon activation of latent natural killer cells and alteration in kinetics of target cell lysis: clinical implications for oral precancerous lesions. Oral Surg Oral Med Oral Pathol. 1990;70:458–461. doi: 10.1016/0030-4220(90)90210-j. [DOI] [PubMed] [Google Scholar]
- 27.Pillai MR, Balaram P, Abraham T, Nair MK. Lymphocyte populations in premalignant lesions and cancer of the oral cavity. Neoplasma. 1987;34:469–479. [PubMed] [Google Scholar]
- 28.Shah N, Kumar R, Shah MK. Immunological studies in oral submucous fibrosis. Indian J Dent Res. 1994;5:81–87. [PubMed] [Google Scholar]
- 29.Gupta DS, Gupta M, Oswal RH. Estimation of major immunoglobulin profile in oral submucous fibrosis by radial immunodiffusion. Int J Oral Surg. 1985;14:533–537. doi: 10.1016/s0300-9785(85)80060-0. [DOI] [PubMed] [Google Scholar]
- 30.Remani P, Ankathil R, Vijayan KK, Haseena Beevi VM, Rajendran R, Vijayakumar T. Circulating immune complexes as an immunological marker in premalignant and malignant lesions of the oral cavity. Cancer Lett. 1988;40:185–191. doi: 10.1016/0304-3835(88)90009-2. [DOI] [PubMed] [Google Scholar]
- 31.Canniff JP, Harvey W, Harris M. Oral submucous fibrosis: its pathogenesis and management. Br Dent J. 1986;160:429–434. doi: 10.1038/sj.bdj.4805876. [DOI] [PubMed] [Google Scholar]
- 32.Geng Y, Savage SM, Johnson LJ, Seagrave J, Sopori ML. Effects of nicotine on the immune response. I. Chronic exposure to nicotine impairs antigen receptor-mediated signal transduction in lymphocytes. Toxicol Appl Pharmacol. 1995;135:268–278. doi: 10.1006/taap.1995.1233. [DOI] [PubMed] [Google Scholar]
- 33.Geng Y, Savage SM, Razani-Boroujerdi S, Sopori ML. Effects of nicotine on the immune response. II. Chronic nicotine treatment induces T cell anergy. J Immunol. 1996;156:2384–2390. [PubMed] [Google Scholar]
- 34.Johnston GA, Krogsgaard-Larsen P, Stephanson A. Betel nut constituents as inhibitors of gamma-aminobutyric acid uptake. Nature. 1975;258:627–628. doi: 10.1038/258627a0. [DOI] [PubMed] [Google Scholar]
- 35.Kalra R, Singh SP, Savage SM, Finch GL, Sopori ML. Effects of cigarette smoke on immune response: chronic exposure to cigarette smoke impairs antigen-mediated signaling in T cells and depletes IP3-sensitive Ca(2+) stores. J Pharmacol Exp Ther. 2000;293:166–171. [PubMed] [Google Scholar]
- 36.Sopori ML, Kozak W. Immunomodulatory effects of cigarette smoke. J Neuroimmunol. 1998;83:148–156. doi: 10.1016/s0165-5728(97)00231-2. [DOI] [PubMed] [Google Scholar]
- 37.Selvan RS, Rao AR. Influence of arecoline on immune system: III. Suppression of B cell-mediated immune response in mice after short-term exposure. Immunopharmacol Immunotoxicol. 1993;15:291–305. doi: 10.3109/08923979309026000. [DOI] [PubMed] [Google Scholar]
- 38.Selvan RS, Selvakumaran M, Rao AR. Influence of arecoline on immune system: II. Suppression of thymus-dependent immune responses and parameter of non-specific resistance after short-term exposure. Immunopharmacol Immunotoxicol. 1991;13:281–309. doi: 10.3109/08923979109019706. [DOI] [PubMed] [Google Scholar]
- 39.Selvan RS, Venkateswaran KS, Rao AR. Influence of arecoline on immune system: I. Short term effects on general parameters and on the adrenal and lymphoid organs. Immunopharmacol Immunotoxicol. 1989;11:347–377. doi: 10.3109/08923978909005375. [DOI] [PubMed] [Google Scholar]
- 40.Phallin Y. Cambodia: Dissemination workshop on National Adult Tobacco Survey of 2011. Southeast Asia Tobacco Control Alliance; 2011. [Google Scholar]
- 41.Boucher BJ, Mannan N. Metabolic effects of the consumption of Areca catechu. Addict Biol. 2002;7:103–110. doi: 10.1080/13556210120091464. [DOI] [PubMed] [Google Scholar]
- 42.Channyda C. Govt targets chewing tobacco. Phnom Penh Post. 2008 December 23; [Google Scholar]
- 43.Epstein D. The responses of the excised batrachian alimentary canal to certain autonomic drugs. Proceedings of the Physiological Society. 1930:i–v. [Google Scholar]
- 44.Chopra RN, Nayar SL, Chopra IC. Glossary of Indian medicinal plants. New Delhi: Council of Scientific and Industrial Research (CSIR); 1956. p. 194. [Google Scholar]
- 45.Khanra S. [Betel leaf based industry] (in Bengali) Nabanna Bharati. 1997;30:169. [Google Scholar]
- 46.Reichart PA, Way TH. Oral cancer and pre-cancer in Myanmar: a short review. J Oral Pathol Med. 2006;35:193–196. doi: 10.1111/j.1600-0714.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 47.Mougne C, MacLennan R, Atsana S. Smoking, chewing and drinking in Ban Pong. Northern Thailand. Soc Sci Med. 1982;16:99–106. doi: 10.1016/0277-9536(82)90430-0. [DOI] [PubMed] [Google Scholar]
- 48.Reichart PA, Nguyen XH. Betel quid chewing, oral cancer and other oral mucosal diseases in Vietnam: a review. J Oral Pathol Med. 2008;37:511–514. doi: 10.1111/j.1600-0714.2008.00669.x. [DOI] [PubMed] [Google Scholar]
- 49.Atkinson L, Chester IC, Smyth FG, Ten S. Oral cancer in New Guinea. A study in demography and etiology. Cancer. 1964;17:1289–1298. doi: 10.1002/1097-0142(196410)17:10<1289::aid-cncr2820171011>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 50.Barraclough S. Women and tobacco in Indonesia. Tob Control. 1999;8:327–332. doi: 10.1136/tc.8.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gan CY. Tobacco usage among rural Bajaus in Sabah, Malaysia. Southeast Asian J Trop Med Public Health. 1998;29:643–648. [PubMed] [Google Scholar]
- 52.Gupta PC, Subramoney S. Smokeless tobacco use and risk of stillbirth: a cohort study in Mumbai, India. Epidemiology. 2006;17:47–51. doi: 10.1097/01.ede.0000190545.19168.c4. [DOI] [PubMed] [Google Scholar]
- 53.Jha P, Jacob B, Gajalakshmi V, Gupta PC, Dhingra N, Kumar R, et al. A nationally representative case–control study of smoking and death in India. N Engl J Med. 2008;358:1137–1147. doi: 10.1056/NEJMsa0707719. [DOI] [PubMed] [Google Scholar]
- 54.Basu S, Stuckler D, Bitton A, Glantz SA. Projected effects of tobacco smoking on worldwide tuberculosis control: mathematical modelling analysis. BMJ. 2011;343:d5506. doi: 10.1136/bmj.d5506. [DOI] [PMC free article] [PubMed] [Google Scholar]