Conspectus
Over the last several decades, researchers have achieved remarkable progress in the field of organometallic chemistry. The development of metal-catalyzed cross-coupling reactions represents a paradigm shift in chemical synthesis, and today synthetic chemists can readily access carbon-carbon and carbon-heteroatom bonds from a vast array of starting compounds. Although we cannot understate the importance of these methods, the required pre-functionalization to carry out these reactions adds cost and reduces the availability of the starting reagents.
The use of C-H bond activation in lieu of pre-functionalization has presented a tantalizing alternative to classical cross-coupling reactions. Researchers have met the challenges of selectivity and reactivity associated with the development of C-H bond functionalization reactions with an explosion of creative advances in substrate and catalyst design. Literature reports on selectivity based on steric effects, acidity, and electronic and directing group effects are now numerous.
Our group has developed an array of C-H bond functionalization reactions that take advantage of a chelating directing group, and this Account surveys our progress in this area. The use of chelation control in C-H bond functionalization offers several advantages with respect to substrate scope and application to total synthesis. The predictability and decreased dependence on the inherent stereoelectronics of the substrate generally result in selective and high yielding transformations with broad applicability. The nature of the chelating moiety can be chosen to serve as a functional handle in subsequent elaborations.
Our work began with the use of Rh(I) catalysts in intramolecular aromatic C-H annulations, which we further developed to include enantioselective transformations. The application of this chemistry to the simple olefinic C-H bonds found in α,β-unsaturated imines allowed access to highly substituted olefins, pyridines, and piperidines. We observed complementary reactivity with Rh(III) catalysts and developed an oxidative coupling with unactivated alkenes. Further studies on the Rh(III) catalysts led us to develop methods for the coupling of C-H bonds to polarized π bonds such as those in imines and isocyanates. In several cases the methods that we have developed for chelation-controlled C-H bond functionalization have been applied to the total synthesis of complex molecules such as natural products, highlighting the utility of these methods in organic synthesis.
Keywords: C-H activation, rhodium, catalysis, total synthesis, transition metal
Introduction
The ability to directly transform unfunctionalized precursors into complex molecules is an important goal of synthetic organic chemistry. The use of transition metal catalysis in the elaboration of organic molecules has revolutionized the field of organic synthesis over the past several decades and has become an indispensible tool. Many of the most far-reaching advances in transition metal catalysis involve the coupling of two appropriately pre-functionalized precursors to give products in a predictable and highly selective manner. The predictable nature of these reactions, however, comes with a price. The necessity for prefunctionalization adds cost and reduces the availability of starting materials, in addition to producing potentially toxic waste streams. In light of the liabilities of traditional cross coupling reactions, the use C-H bond activation has become increasingly attractive to the synthetic chemist, and has enjoyed demonstrated success in the synthesis of complex molecules. However, by eliminating prefunctionalization, certain key challenges are introduced; namely selectivity and over-functionalization through the competing reactivity of multiple C-H bonds.
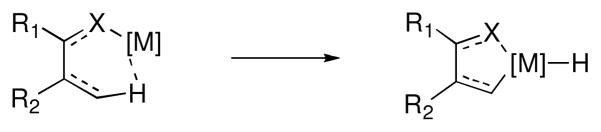
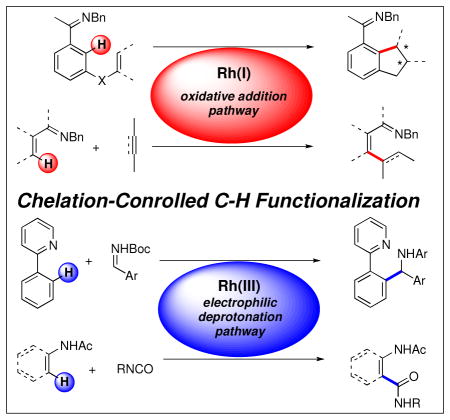
Accordingly, a great deal of effort in the past two decades has been devoted to the achievement of selectivity in C-H bond functionalization reactions. i,ii A rich array of catalysts have emerged that exploit subtle differences in the reactivity of specific C-H bonds within a molecule. One approach that has seen widespread use involves taking advantage of a chelating heteroatom to facilitate reactivity at a proximal site (Figure 1). With respect to functional group tolerance and efficiency, rhodium-based catalysts have emerged as leading candidates for this type of transformation, and over the past decade, our group has developed several methods for the efficient functionalization of sp2 C-H bonds that utilize this approach.
Figure 1.
Chelation-assisted C-H bond activation
Catalysis by Rh(I)
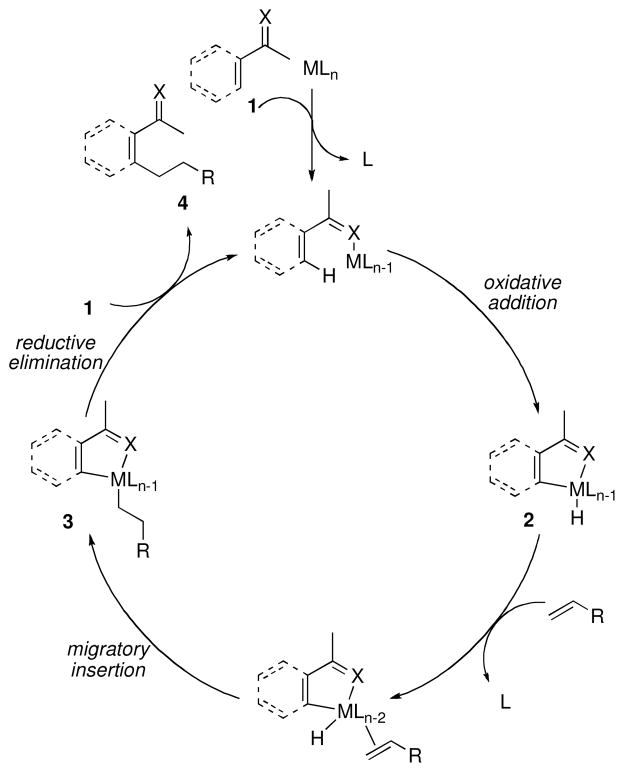
Early reports of Rh-catalyzed C-H bond functionalization reactions employed Rh(I) catalysts, which demonstrate reactivity that is mechanistically distinct from the Rh(III) pathways that will be discussed at the end of this review. A mechanism for chelation-assisted C-H bond functionalization, using alkylation as a representative example, is given in Scheme 1. Although the mechanistic details may vary from case to case, this classical pathway is similar for most chelation-driven processes. The initial coordination of the transition metal to the heteroatom of 1 followed by facile C-H bond activation provides the metallacyclic intermediate 2. Ligand exchange to coordinate the olefin, followed by hydride insertion into the alkene, gives 3. Reductive elimination from 3 forms a new C-C bond, and dissociation of the product 4 closes the catalytic cycle. In most C-H alkylation reactions, the reductive elimination step is rate limiting, and one of the beneficial effects of chelation is to stabilize 3 to facilitate reductive elimination.iii,iv
Scheme 1.
Mechanism of chelation-assisted C-H alkylation
Intramolecular Annulation of Arenes
Stemming from the seminal reports by Murai and coworkers on the intermolecular ortho-alkylation of aryl ketones with alkenes using a ruthenium catalyst,v Jun demonstrated that the scope and efficiency of this transformation could be greatly improved through the use of an imine directing group and a Rh-based catalyst such as Wilkinson’s catalyst.3a Despite these improvements in reactivity, the scope of imine-directed alkylation was still limited with respect to the olefinic component, and with few exceptions, only monosubstituted terminal olefins served as coupling partners. Our group therefore set out to demonstrate a more widespread applicability in the intramolecular annulation of arenes.
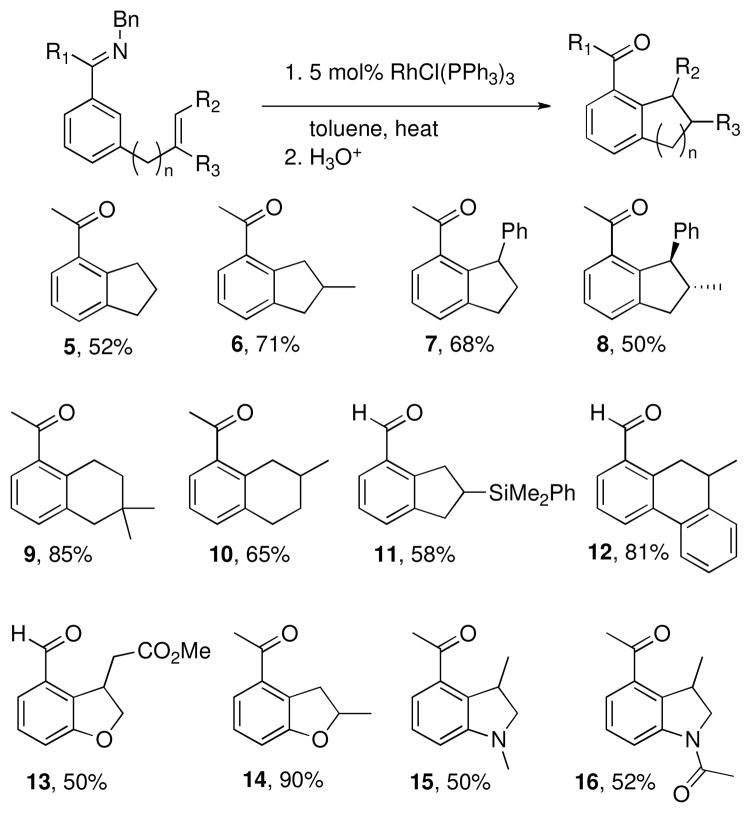
We demonstrated the annulation of aryl ketimines with mono (5), 1,1-di- (6), 1,2-di- (7), and even trisubstituted (8) olefins tethered to the meta position to provide the indane derivatives shown in Chart 1.vi In addition to indane derivatives, extensions of the tethered chain provided the tetralin derivatives 9 and 10. In the substrate used to produce tetralin 10, the double bond has the potential to isomerize to the internal position and then cyclize to give an indane derivative, but no evidence for the formation of this product was observed.
Chart 1.
Intramolecular alkylation of aryl imines
Aryl aldimines also reacted efficiently using Wilkinson’s catalyst (Chart 1, 11 – 13). Heteroatoms could also be incorporated into the tether, generating dihydrobenzofuran (13 and 14) and dihydroindole (15 and 16) derivatives, broadening the range of accessible structural motifs. This advancement in particular improves the potential applicability of C-H bond functionalization to pharmaceutical and industrial targets, where heterocycles are prominent.
Enantioselective Intramolecular Alkylation
In the realm of transition metal catalysis, the development of methods for the catalytic enantioselective functionalization of organic molecules is often emphasized as a means to produce chiral molecules that do not rely on the chiral pool or expensive auxiliaries. In contrast to other catalytic methods, enantioselective approaches to C-H bond functionalization has been slow to develop.vii The advancement of these methods is hindered, in part, by the substrate scope observed for C-H bond alkylation reactions where efficient reactivity is only seen with linear, α-olefins.
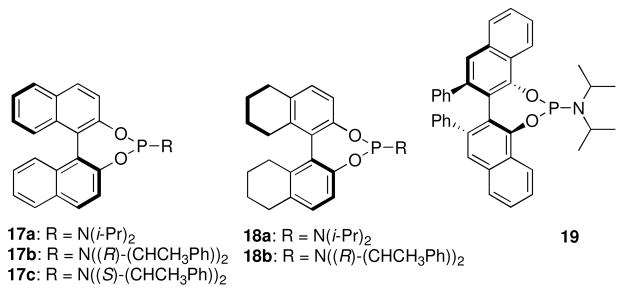
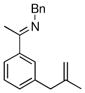
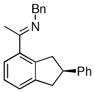
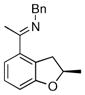
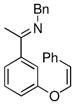
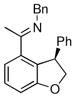
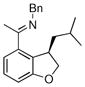
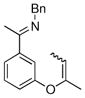
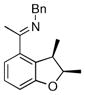
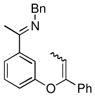
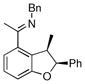
The tolerance of more highly substituted alkenes in intramolecular annulations provided a platform on which to develop catalytic systems for enantioselective C-H bond functionalization.viii Through the use of chiral phosphines, we hoped to bias the coordination of the rhodium-hydride complex to one diastereoface of the alkene, which would provide the stereocontrol required in the subsequent stereodetermining insertion step. Our investigation of this transformation led to the application of monodentate chiral phosphoramidite ligands for use in C-H bond functionalization reactions (Figure 2).ix These ligands, when combined with a Rh precatalyst, gave high conversion and selectivities in the cyclization of 1,2- and 1,1-di-, and trisubstituted olefins to give chiral indanes and benzofurans with both aryl and alkyl substituents (Table 1). N-Allylic indoles were also competent substrates (entry 3), though these challenging substrates required higher temperatures and gave modest ee’s (see also Scheme 4).
Figure 2.
Phosphoramidite ligands for enantioselective olefin hydroarylation
Table 1.
Enantioselective annulation of aryl imines
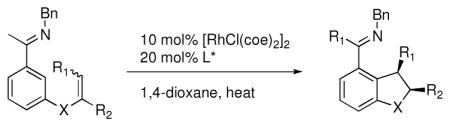
| ||||||
|---|---|---|---|---|---|---|
| Entry | Substrate | Product | L* | T (°C) | % Yield | % ee |
| 1 |
|
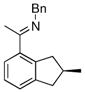
|
17b | 50 | 94 | 95 |
| 2 |
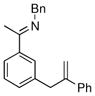
|
|
17c | 75 | 98 | 90 |
| 3 |
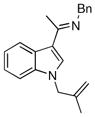
|
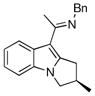
|
17c | 125 | 99 | 68 |
| 4 |
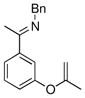
|
|
17c | 50 | 99 | 95 |
| 5 |
|
|
18a | 75 | 93 | 87 |
| 6 |
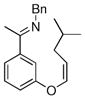
|
|
18a | 75 | 69 | 90 |
| 7 |
|
|
18b | 50 | 80 | 93 |
| 8 |
|
|
18b | 75 | 50 | 90 |
Yield of N-benzylimine product determined by 1H NMR using 2,6-dimethoxytoluene as an internal standard.
Ee’s determined after hydrolysis of products with silica gel or HCl/H2O-dioxane using chiral HPLC.
Scheme 4.
Enantioselective cyclization and synthesis of 27
Synthetic Applications
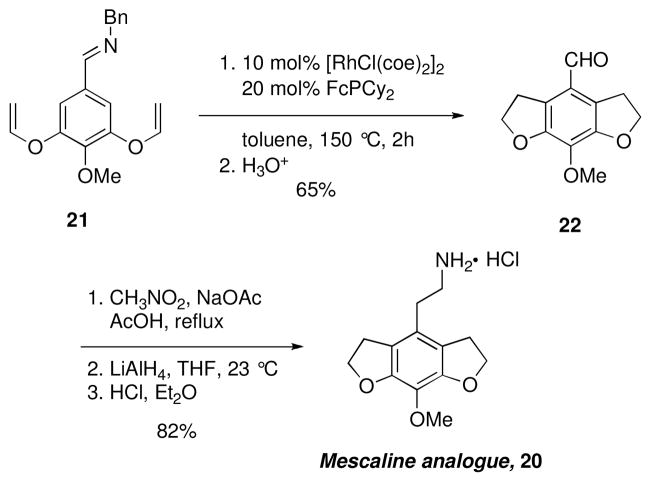
The total synthesis of natural products and biologically relevant molecules serves as a platform for the demonstration of the range and utility of catalytic methods in complex and less predictable situations. The Rh-catalyzed annulation of aryl imines has been utilized in the synthesis of several natural products and drug candidates. This transformation was first employed in a tandem alkylation reaction to produce 20, an analogue of mescaline with interesting biological properties (Scheme 2).x,xi The reaction of 21 using Wilkinson’s catalyst was sluggish and proceeded in low yields. However, electron-donating ligands were much more active in this transformation and the ferrocenyl-based ligand, (dicyclohexylphosphinyl)ferrocene (FcPCy2) produced the desired product 22 in good yield. The tricyclic aldehyde 22 could then be easily elaborated to give 20.
Scheme 2.
Synthesis of mescaline analogue 20
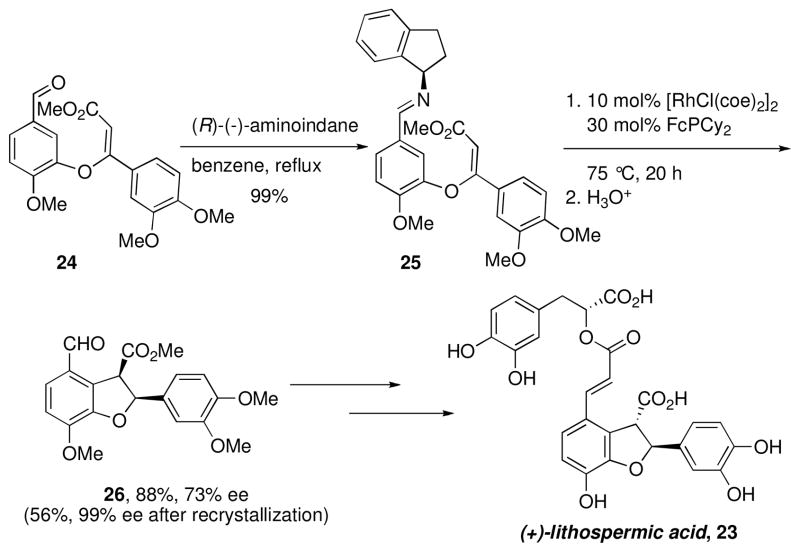
We next turned to the challenging structural motif found in the natural product (+)-lithospermic acid 23 (Scheme 3), requiring the stereoselective intramolecular ortho-alkylation of the densely functionalized trisubstituted alkene in aryl aldimine 25.xii Initial experiments on the use of chiral catalysts in the cyclization of 25 did not provide satisfactory yields and enantioselectivities, which prompted us to explore alternative approaches for accomplishing an asymmetric annulation.
Scheme 3.
Cyclization of 25 in the first synthesis of (+)-lithospermic acid
Because of the proximity of the substituent on the imine nitrogen to the metal center, the use of a chiral amine auxiliary that could later be cleaved by hydrolysis was an attractive alternative to enantioselective catalysis. Chiral non-racemic amines were condensed with aldehyde 24 and the resultant imines were tested for their efficiency and diastereoselectivity in the Rh-catalyzed cyclization (Scheme 3). The syn diastereomer 26 is formed in the Rh-catalyzed cyclization reaction through syn alkene insertion followed by reduction elimination proceeding with retention. This product then undergoes epimerization under basic conditions to the more thermodynamically favorable and desired anti-isomer. In the initial synthesis, the imine generated from (R)-aminoindane 25 provided the highest yield of cyclized product with good diastereoselectivity and resulted in the first synthesisxiii of this natural product in only ten steps from commercially available starting materials (Scheme 3).
In a subsequent study, the effect of substituents on the aminoindane auxiliary on the efficiency and stereoselectivity of the annulation reaction was evaluated.xiv Substituents at the 7-position provided superior results, with 7-fluoro-1-aminoindane leading to 26 in 70% yield and with 90% ee.
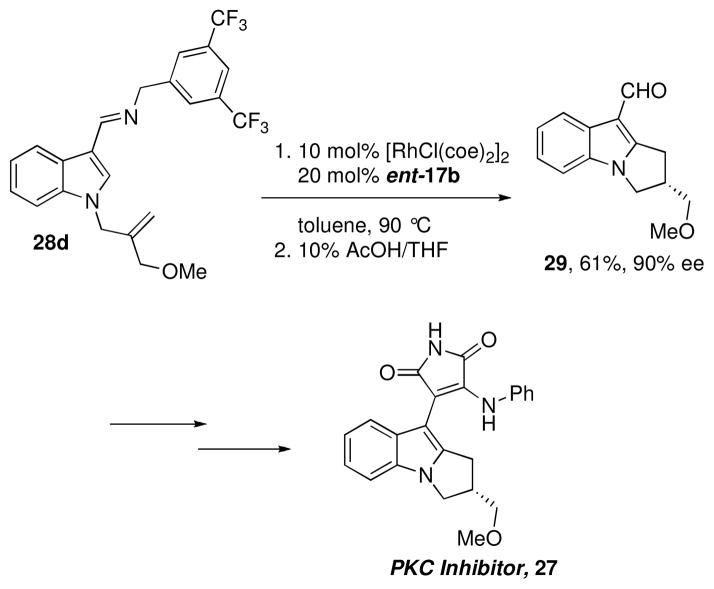
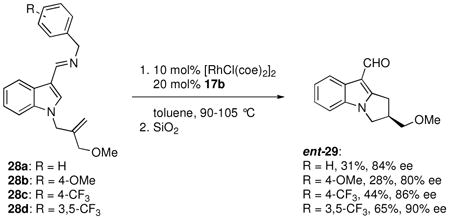
To demonstrate the power of the enantioselective annulation method in organic synthesis, we next embarked on a rapid synthesis of the tricyclic indole core of the protein kinase C inhibitor 27 (Scheme 4).xv We envisioned that the indole tricyclic core 29 could be generated by the enantioselective annulation of imine 28a, which could be prepared in just two steps from indole carboxaldehyde. Although the N-benzyl imine had routinely exhibited good reactivity in C-H bond functionalization reactions, 28a showed very poor conversion when the chiral phosphoramidite ligand 17b was used with a Rh pre-catalyst (eq 1). Optimization of this reaction through ligand and catalyst screening did not lead to an improvement in the observed reactivity, and so we began to consider substrate modifications that might lead to a more efficient reaction.
 |
(1) |
We envisioned that by reducing the electron-donating ability of the imine nitrogen, a more electron deficient metal center could be generated that would lower the barrier for reductive elimination, which has been shown to be rate limiting in C-H bond functionalization reactions.2,3c Indeed, use of the more electron-rich substrate 28b resulted in decreased reaction efficiency relative to 28a. Substrates with electron-withdrawing –CF3 substituents on the benzyl group, however, did show improved reaction efficiency, with the 3,5-bis-trifluoromethyl substituted substrate 28d providing ent-29 in 65% yield and 90% ee (eq 1). Although ligand 17b provided the enantiomer of the desired product 29, the enantiomer of 17b (ent-17b) is also commercially available and was used to produce 29 with the correct sense of induction (Scheme 4). The desired target 27 was produced in just four subsequent steps.
Chelation-Assisted Functionalization of Olefins
In comparison to the C-H bond functionalization reactions that have been developed with arenes, the reactivity of olefins remains underexplored. This is perhaps because the leading chelating moieties for Rh catalysis, namely the imine and carbonyl functionalities, serve to activate pendant olefins towards other reactions such as conjugate addition and polymerization.xvi
Early examples of Rh-catalyzed hydrovinylation of alkenes avoided the reactivity of α,β-unsaturated carbonyl derivatives by utilizing heterocyclic directing groups such as pyridine, imidazole, and oxazoline to afford inter-xvii and intramolecular8 alkylation. Jun was the first to report on the Rh-catalyzed alkylation of an α,β-unsaturated carbonyl derivative, though limited scope and isomeric product mixtures were observed.xviii
Based on our efforts in novel catalyst design for aromatic C-H alkylations, we pursued a method for α,β-unsaturated N-benzyl imine alkylation with substantially broadened scope and generality.xix Although Wilkinson’s catalyst was an ineffective catalyst for the alkylation of 30, electron-donating ligands such as PCy3 and FcPCy2 produced a much more efficient catalyst, allowing the temperature to be decreased and minimizing subsequent olefin isomerization. Using FcPCy2 as the ligand, the scope of the alkylation included olefins with alkyl (Table 2, entries 1 and 2), aryl (entry 3), ester (entries 4 and 5), and halo substituents (entry 6). In addition, the terminal alkyne tert-butylacetylene underwent hydrovinylation to give the dienal product (entry 7). Under acidic hydrolysis conditions, isomerization to the E isomer was generally seen (entries 2 and 5). However, concomitant hydrolysis and chromatography on activity III neutral alumina minimized isomerization to afford the trisubstituted Z enal products with good stereoselectivity (entries 1, 3–4, 6–7). Although the methacrolein-derived imine was the most efficient substrate, β-substituted aldimines also could be employed to generate the more synthetically challenging β,β-disubstituted tri- and tetrasubstituted enal products stereoselectively and in good yield (not shown). 19 At the current stage of development, more highly substituted alkenes do not couple efficiently. Overcoming this limitation would also enable a catalytic enantioselective method to be pursued.
Table 2.
Alkylation of α,β-unsaturated aldimines
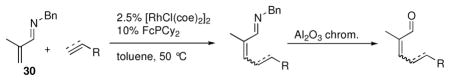
| ||||
|---|---|---|---|---|
| Entry | Alkene(yne) | Time (h) | Imine (Z:E) | Yield (Z:E)a |
| 1 |
|
12 | >95:5 | 91% (10:1) |
| 2b |
|
12 | >95:5 | 77% (5:>95) |
| 3 |
|
24 | 10:1 | 74% (5:1) |
| 4 |
|
4 | >95:5 | 78% (5:1) |
| 5b |
|
4 | >95:5 | 73% (5:>95) |
| 6 |
|
8 | >95:5 | 80% (10:1) |
| 7 |
|
4 | >95:5 | 86% (20:1) |
Isolated yields.
Crude imine was stirred at a concentration of 0.1M in a 5:5:2 solution of THF:acetic acid:H2O for 16 h prior to isolation.
Synthetic Applications
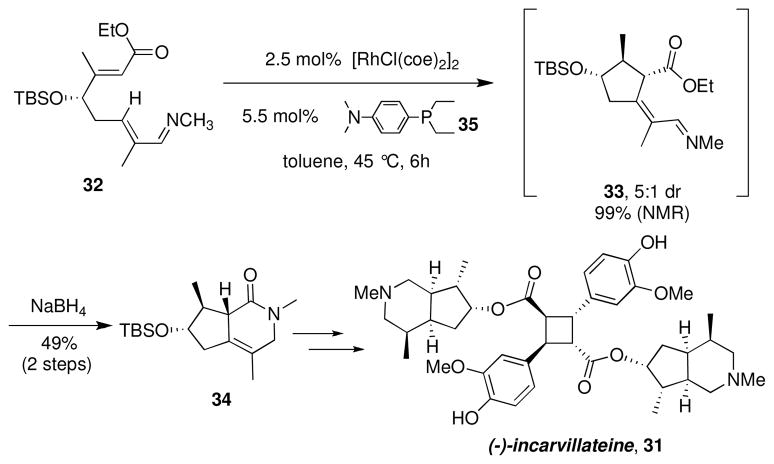
The synthetic utility of this method was demonstrated in an intramolecular alkylation of an α,β-unsaturated imine in the total synthesis of the potent analgesic (−)-incarvillateine 31 (Scheme 5).xx,xxi We envisioned that the piperidine functionality could be accessed very efficiently by employing an intramolecular β C-H bond alkylation of substrate 32. In particular, from substrate 32, syn alkene insertion and reductive elimination would exclusively provide the desired exocyclic double bond geometry and the requisite anti relationship of the methyl and ester functionalities.
Scheme 5.
Diastereoselective synthesis of (−)-incarvillateine
The requisite imine substrate 32 was prepared enantioselectively in just four steps from commercial reagents. We were particularly interested in the C-H bond functionalization of 32 because it represented the first example of diastereocontrol in intramolecular alkylation arising from a stereocenter on the tether. Ligand screening revealed that electron-donating phosphine ligands were best suited for the desired transformation, and use of the p-(N,N-dimethylaminophenyl)diethylphosphine ((DMAPh)PEt2, 35) ligand shown in Scheme 5 provided quantitative conversion to 33 with 5:1 diastereoselectivity.
To avoid the facile tautomerization and epimerization of compound 33, direct reduction of the crude imine product followed by lactamization provided lactam 34 as a single stereoisomer in 49% overall yield from acyclic precursor 32. By rapidly generating the piperidine core, (−)-incarvillateine 31 was prepared in only 11 steps and 15.4% overall yield.
Alkenylation Reactions
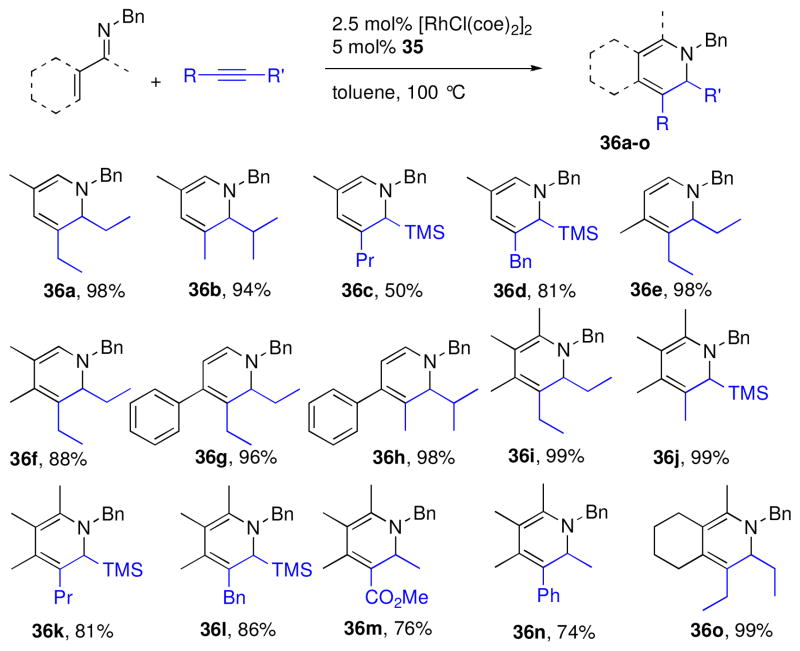
Although the reaction of 30 with tert-butylacetylene provided the product of β-alkenylation (Table 2, entry 7),19 reactions with internal alkynes or less bulky alkynes generated alkenylated products that underwent electrocyclization under the reaction conditions to yield 1,2-dihydropyridine (DHP) products (eq 2).4,19 This reactivity had been observed previously by Jun and coworkers with benzaldimine substrates,xxii and Odom had also reported a single example of a one-pot hydroamination/C-H alkenylation sequence that generated a 1,2-DHP from the N-phenyl imine of 1-acetylcyclohexene.xxiii
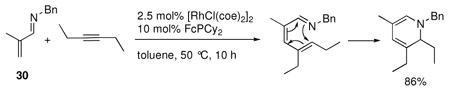 |
(2) |
Extensive catalyst optimization, including the design of novel ligands for C-H bond functionalization, revealed that (DMAPh)PEt2 ligand 35 (illustrated in Scheme 5) in a 1:1 ratio of L:Rh provided the highest yields of DHP products.4 Using these optimized conditions, imine 30 reacted cleanly with internal alkynes having alkyl (36a,b), silyl (36c,d), or benzyl (36d) substituents (Chart 2). Unsymmetrical alkynes typically reacted to give a single regioisomer provided one of the substituents was α-branched. In general, the more sterically bulky substituent is oriented proximal to the DHP nitrogen, with smaller substituents in the distal position. This supports a mechanism that includes hydrometalation of the alkyne as opposed to carbometalation. In addition to 30, β-substituted aldimines reacted to produce highly substituted DHP products in good yields. Ketimines were also very efficient substrates in this chemistry (36i–o), and furthermore exhibited clean conversion to DHP products with ester (36m) and aryl (36n) substituted alkynes.
Chart 2.
Dihydropyridine synthesis from imines and alkynesa
a Yields determined by NMR integration relative to 2,6-dimethoxytoluene as an internal standard.
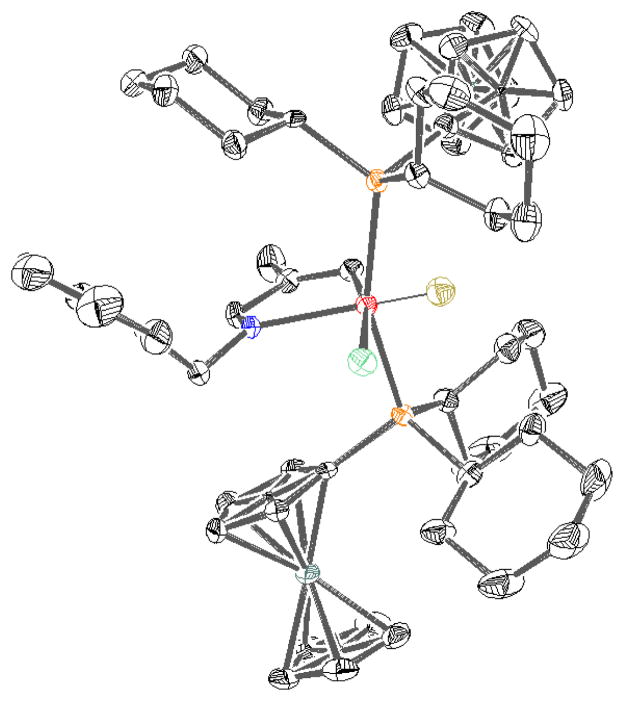
To gain an understanding of the mechanism of this class of reactions, stoichiometric quantities of imine 30, the Rh precatalyst, and FcPCy2 were combined and a new complex, 37, formed immediately upon combination of the reagents at room temperature (eq 3). The crystal structure of 37 shows an azametallacyclopentane formed by chelation and C-H activation of 30 to give a complex with distorted octahedral geometry (Figure 3). Re-dissolving isolated 37 in toluene-d8 or THF-d8 gives a 2:1 equilibrium mixture of 37:30. This demonstrates that C-H bond activation is facile and reversible under the reaction conditions, which is in agreement with deuterium labeling studies independently performed by Brookhart and Murai for related systems.3b,c
Figure 3.
X-ray crystal structure (ORTEP diagram) of 37 with thermal ellipsoids drawn at the 50% probability level. Hydrogen atoms distant from the metal center have been omitted for clarity.
It was further found that reducing the reaction temperature in the catalytic synthesis of 36a sufficiently slowed the electrocyclization step such that the azatriene intermediate could be observed to grow in and then decay when the reaction was monitored by NMR. This observation argues against a [4+2] cycloaddition-based mechanism.
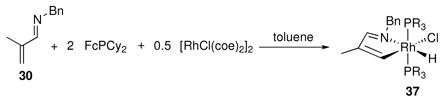 |
(3) |
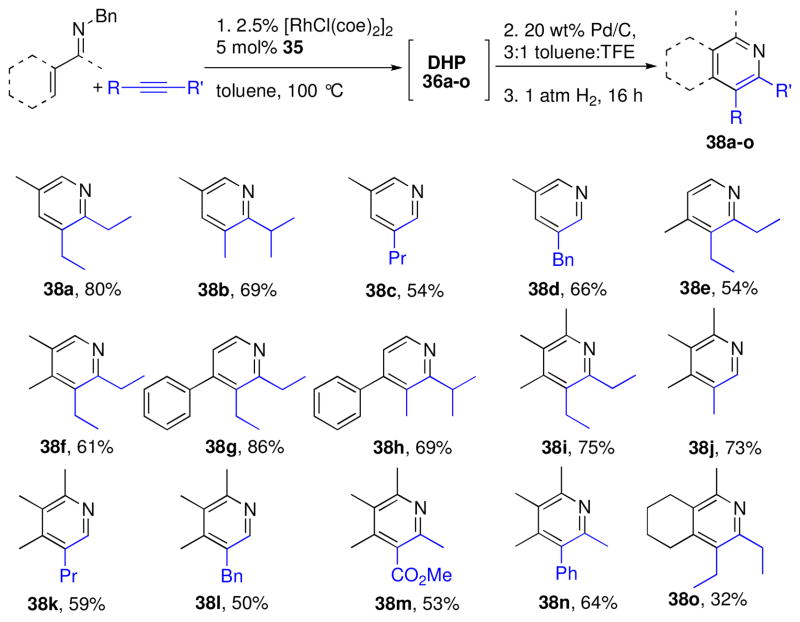
Although the high reactivity of dihydropyridines limits their isolation, these compounds have proven to be versatile intermediates for the synthesis of piperidines and pyridines, which are prevalent in natural products, drugs, and materials. We further developed the alkenylation/electrocyclization DHP synthesis to include a synthesis of highly substituted pyridine derivatives that would be difficult to selectively access using other approaches.xxiv To achieve this, an oxidation protocol was developed that enabled a one-pot synthesis of pyridines from imines and alkynes (Chart 3).4 Following DHP synthesis, the crude solution was subjected to catalytic oxidation using Pd/C and air, which generates the N-benzylpyridinium salt. Subsequent hydrogenolysis of the benzyl group under an atmosphere of H2 provided the pyridine derivatives in modest to high yields for the three step, one-pot protocol. In derivatives that displayed a silyl group α to the nitrogen (38c,d,k,l), desilylation occurred. The silyl functionality consequently served as a convenient blocking group that forced bulkier substituents distal to the pyridine nitrogen.
Chart 3.
One-pot synthesis of pyridines from imines and alkynes a
a Yields given are isolated yields based on starting imine.
A related synthesis of pyridines from ketoxime derivatives and alkynes using Wilkinson’s catalyst in which the DHP intermediates undergo aromatization through dehydration has also been reported, though this transformation was not demonstrated to proceed with aldoxime substrates.xxv
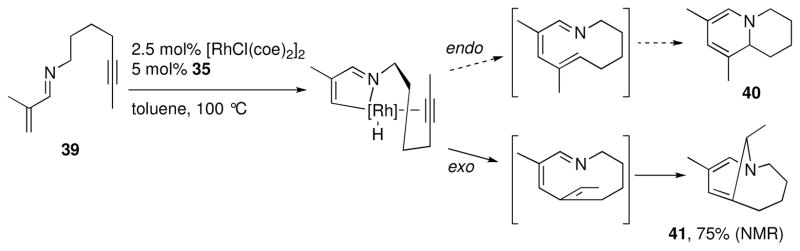
By applying the alkenylation chemistry to a substrate that has an alkyne tethered to the imine nitrogen (e.g. 39, Scheme 6), an interesting result was obtained.xxvi Depending on the direction of the migratory insertion step, two different products could be obtained, 40 or 41. Under all conditions explored, substrate 39 produced the highly strained bicyclic enamine 41 exclusively. Presumably, the formation of 40 is not observed because endo migratory insertion would lead to an unfavorable trans-double bond within the [6.3.0] metallacyclic fused ring intermediate.
Scheme 6.
Possible reaction pathways for 39
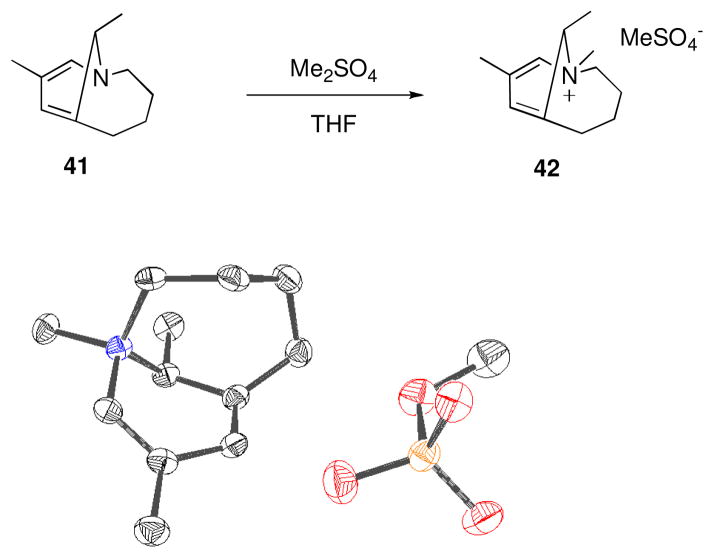
Enamine 41 possesses unique structural characteristics, namely a bridgehead enamine that lacks the proper geometry for conjugation and a bridgehead double bond in a nine-membered ring. Accordingly, 41 underwent methylation with dimethyl sulfate to provide the N-methylated product 42, as opposed to the C-alkylated product that is normally obtained from enamine alkylation. X-ray quality crystals of 42 were obtained, validating the highly strained structure of this bicycle (Figure 4).
Figure 4.
Methylation of 41. ORTEP diagram of 42 with thermal ellipsoids drawn at the 50% probability level.
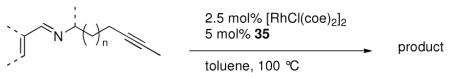
A series of substrates were prepared in order to evaluate the effect of changing substituents and tether length on this transformation (Table 3). Substrate 43, displaying a shorter tether, underwent alkenylation but the resulting azatriene decomposed without cyclizing, presumably due to the strain required to form a smaller ring with a bridgehead double bond. Substrate 44, with a methyl group α to the nitrogen on the tether chain, underwent alkenylation and slow electrocyclization with complete atropodiastereoselectivity to give a single product stereoisomer, 45. Substrates with β-substituents on the α,β-unsaturation (46 and 48) produced products with exocyclic double bonds. Isomerization likely occurs under the reaction conditions in order to relieve ring strain.
Table 3.
C-H Bond functionalization of imines with tethered alkynes
| |||
|---|---|---|---|
| Substrate | Time | Product | Yielda |
|
43 |
3 h |
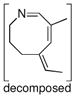
|
- |
|
44 |
9 days |
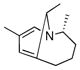 45 |
63% |
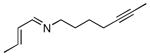 46 |
4 h |
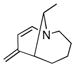 47 |
50% |
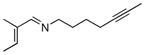 48 |
48 h |
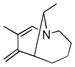 49 |
20% |
Isolated yields.
Catalysis by Rh(III) complexes
Insertion into alkenes
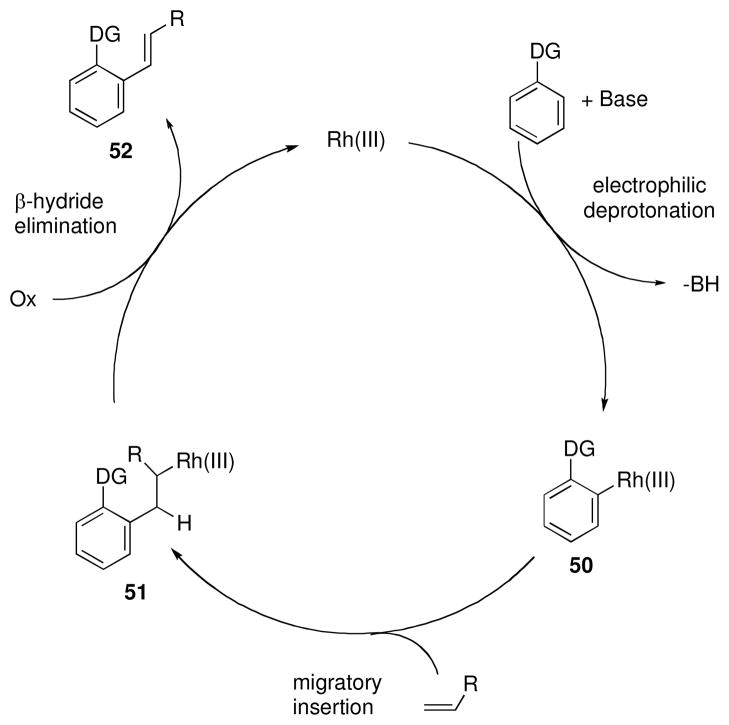
Complementary to the coupling with alkynes, similar alkenylated products could also be accessed through the oxidative coupling of alkenes using Rh(III) catalysts. In contrast to the Rh(I) complexes discussed previously, Rh(III) catalysts activate C-H bonds via an electrophilic deprotonation pathway to produce an aryl-Rh intermediate (50, Scheme 7).xxvii Insertion of an alkene into the newly formed bond generates an alkyl-Rh complex (51) that likely β-hydride eliminates to yield the Heck-type product (52). An oxidant then regenerates the Rh(III) catalyst.xxviii
Scheme 7.
Proposed mechanism for the Rh(III) catalyzed oxidative coupling of alkenes.
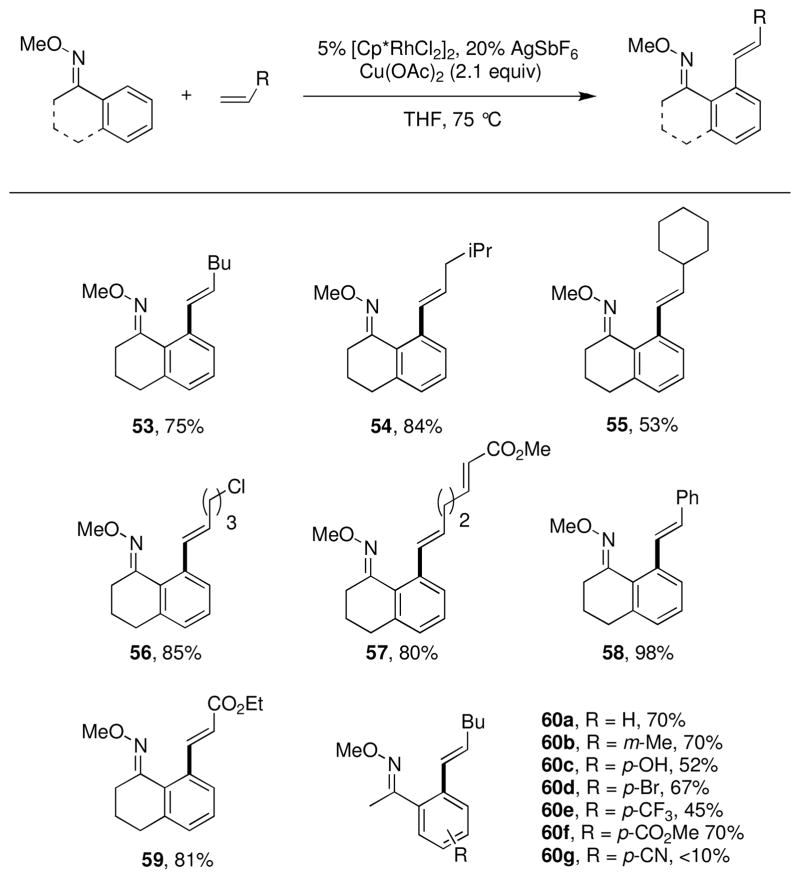
One limitation of these Heck-type couplings with Rh(III) and related Pd(II) catalystsxxix is that they only proceed with activated alkenes such as acrylates and styrenes.xxx We hoped to be able to expand the scope to include a broader range of olefins.xxxi Initial screens using methyl oximes and 1-hexene showed that the addition of a halide abstractor such as AgSbF6 and the solvent choice were important to obtain the desired reactivity. Under optimized conditions, a variety of aliphatic (53, 54, and 60), branched (55), and functionalized (56, 57, and 59) terminal alkenes could be oxidatively coupled in good yields (Chart 4). More highly substituted alkenes do not react under these conditions, potentially owing to the increased steric interactions encountered during migratory insertion. Future development in this area may benefit from the use of less sterically encumbered Rh(III) catalysts. In related work, Fagnou’s laboratory recently reported a Rh(III) catalyzed oxidative coupling of unactivated alkenes and alkynes with N-alkoxy amides to generate dihydroisoquinolones and isoquinolones, respectively.xxxii In this case, as opposed to an external oxidant, the increased oxidation state of the amide is exploited to provide the oxidized products.
Chart 4.
Substrate scope for oxidative coupling of alkenes
Insertion into polar π bonds
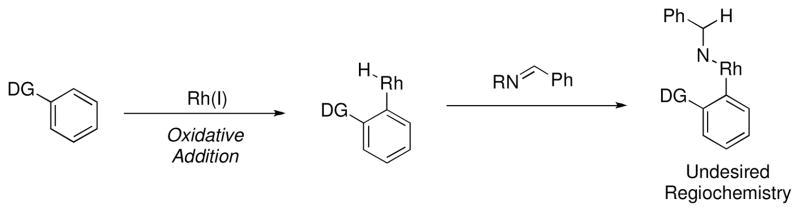
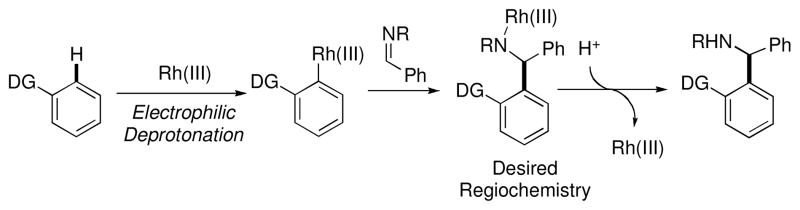
While Rh(I) catalysts have been shown to be well suited for couplings with alkenes and alkynes, analogous couplings with carbon-heteroatom π-bonds such as imines, have generally been less successful.xxxiii One reason for this limitation is that insertion of a prospective imine into the intermediate rhodium hydride is expected to occur with the regiochemistry opposite to that desired to form a C-C bond (Scheme 8).xxxiv To address this issue of regioselectivity we looked to Rh(III) catalysts. We hypothesized that imines may be able to insert across the Rh-arene bond generated from initial electrophilic deprotonation with the desired regiochemistry to form a C-C bond. Upon protonolysis, this should directly provide α-branched aryl amines and bypass traditional methods that require arene prefunctionalization (Scheme 9).
Scheme 8.
Regioselectivity of insertion of imines into a rhodium-hydride intermediate
Scheme 9.
Rh(III) catalyzed arylation of imines
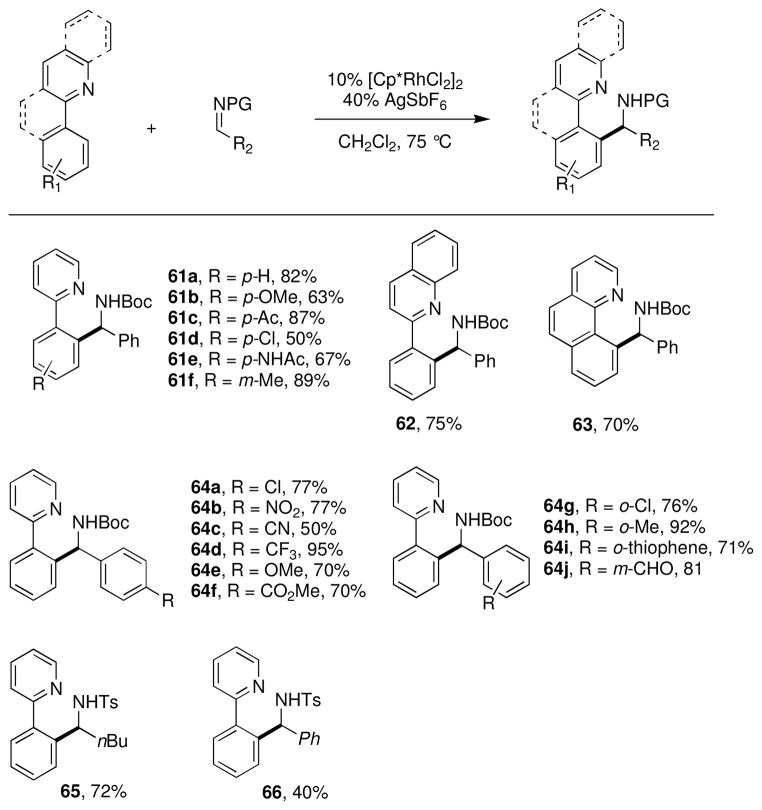
To test this, 2-phenylpyridine and the N-Boc imine of benzaldehyde were combined in the presence of the Rh(III) catalyst [Cp*RhCl2]2 (eq 4).xxxv,xxxvi During reaction optimization, the addition of AgSbF6 as a halide abstractor was found to be essential to achieve the desired reactivity, possibly by opening up the metal’s coordination sphere to allow for binding of both reaction partners and to increase the electrophilicity of the catalyst. The reaction is robust, accommodating electronically diverse 2-arylpyridines and N-Boc imines (Chart 5). Reactive functional groups such as esters (64f), amides (61e), aldehydes (64j), and aryl chlorides (61d, 64a and g) were well tolerated under the mild reaction conditions. It was also found that N-tosyl imines were suitable reaction partners. Notably, sensitive alkyl N-tosyl imines coupled in good yields (65). However, the yields for coupling of related aryl N-tosyl imines (66) were generally lower than with N-Boc imines. Further investigation into the reactivity of aryl N-tosyl imines showed that the coupling is reversible under the reaction conditions and the lower yield is due to a thermodynamic equilibrium (eq 5).
Chart 5.
Scope of 2-aryl pyridine alkylation with imines
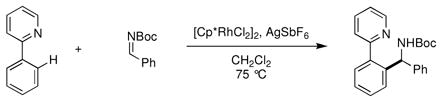 |
(4) |
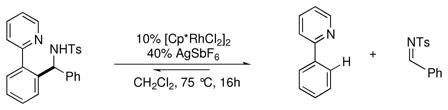 |
(5) |
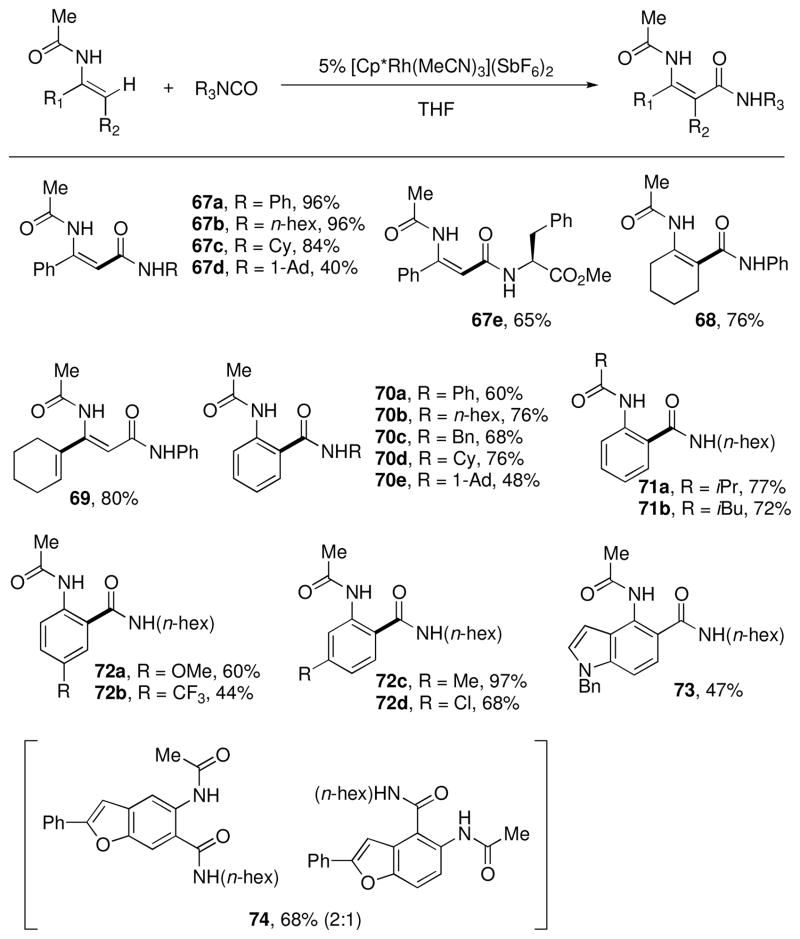
Further exploration into this mode of reactivity revealed that the pyridyl group also directed arylation of the polar carbon-nitrogen π-bond of isocyanates. Moreover, N-acyl amino groups also proved to be effective directing groups for the addition of both aryl and vinyl C-H bonds to isocyanates, which is particularly relevant to the synthesis of bioactive drugs and natural products (Chart 6).xxxvii Variously substituted enamides and anilides could be coupled with isocyanates with diverse steric and electronic properties to yield N-acyl β-enamine amides and anthranilamides, respectively. Furthermore, the same Rh(III) complex catalyzes the in-situ cyclodehydration of select N-acyl β-enamine amide products to pyrimidinones simply by increasing the reaction temperature (eq 6).
Chart 6.
Substrate scope of N-acyl directed additions of C-H bonds to isocyanates
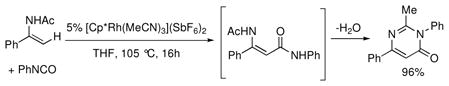 |
(6) |
A normal kinetic isotope effect (eq 7) was observed when initial rates are measured for deuterated and protiated 75. A normal kinetic isotope effect of comparable magnititude was also observed for a related anilide substrate (not shown). These observations are consistent with the C-H activation mechanism analogous to that proposed in Scheme 9 rather than direct π-bond addition of the enamine to the isocyanate, which should display an inverse kinetic isotope effect.xxxviii Further mechanistic investigation to confirm this inference is underway.
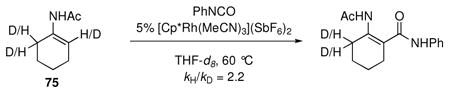 |
(7) |
Continued work is focused on broadening the scope of the Rh(III)-catalyzed transformations to include a wider range of directing groups and electrophiles, thereby expanding the impact of this chemistry. Additionally, the utility of the developed methods will directly be demonstrated through the synthesis of architecturally complex drugs and nautral products.
Conclusion
The rapid growth of the field of C-H bond functionalization over the last 20 years has established this mode of reactivity as a robust and efficient means for forming new chemical bonds in both simple and very complex targets. Further work towards new catalyst design and optimization promises to improve the selectivity and versatility of these transformations. The rich array of heteroatom directing groups, and the predictability of chelation effects, establishes this approach towards C-H functionalization as a highly effective tool for the synthetic chemist that will only further increase in utility as more efficient catalysts and new types of transformations are developed.
Acknowledgments
We gratefully acknowledge the following agencies for funding: National Institutes of Health (R01 GM069559 to J. A. E.) and the Director, Office of Energy Research, Office of Basic Energy Sciences, Chemical Sciences Division, U.S. Department of Energy (DE-AC02-05CH11231 to R. G. B.)
Biographies
Jonathan Ellman received his B.S. from the MIT and his Ph.D. from Harvard University under the direction of David Evans. He carried out postdoctoral research with Peter Schultz at the University of California at Berkeley, and in 1992, he became a member of the chemistry faculty at the same institution. In 2010 he relocated to the departments of pharmacology and chemistry at Yale University. His laboratory is engaged in the design of chemical tools for biological inquiry and in the development of new synthesis methods. He has collaborated with Robert Bergman since 2000 on the study of new C-H bond functionalization reactions
Robert Bergman received his B.A. from Carleton College in 1963 and his Ph.D. from the University of Wisconsin in 1966 under the direction of Jerome Berson, followed by postdoctoral work with Ronald Breslow at Columbia University. His independent career began at CalTech in 1967 and ten years later he joined the chemistry faculty of UC Berkeley. He has a longstanding interest in mechanistic chemistry and chemical reactivity; his early work on stoichiometric carbon-hydrogen bond activation reactions expanded into catalytic applications of C-H bond activation to organic synthesis in collaboration with Jonathan Ellman.
Denise Colby obtained a B.S. in Chemistry and Biochemistry and Molecular Biology in 2004 from Illinois State University under the mentorship of Timothy Lash. She then received her Ph.D. in 2008 from UC Berkeley working in the laboratories of Robert Bergman and Jonathan Ellman, followed by postdoctoral studies in the laboratories of Timothy Jamison at MIT. She is currently a research scientist at Gilead Sciences in California.
Andy Tsai received his B.S. in chemistry from the University of Michigan and his Ph.D. from UC Berkeley, under the direction of Jonathan Ellman and Robert Bergman. He is currently a postdoctoral researcher with William Roush at the Scripps Research Institute, Florida.
References
- i.We recently published an Accounts review on the separate topic of direct functionalization of nitrogen heterocycles by Rh-catalyzed C-H bond functionalization: Lewis JC, Bergman RG, Ellman JA. Direct Functionalization of Nitrogen Heterocycles via Rh-Catalyzed C-H Bond Activation. Acc Chem Res. 2008;41:1013–1025. doi: 10.1021/ar800042p.
- ii.For reviews on C-H bond functionalization, see the following and leading references therein: Crabtree RH. Introduction to Selective Functionalization of C-H Bonds. Chem Rev. 2010;110:575. doi: 10.1021/cr900388d.Colby DA, Bergman RG, Ellman JA. Rhodium-Catalyzed C-C Bond Formation via Heteroatom-Directed C-H Bond Activation. Chem Rev. 2010;110:624–655. doi: 10.1021/cr900005n.Bellina F, Rossi R. Transition Metal-Catalyzed Direct Arylation of Substrates with Activated sp3-Hybridized C-H Bonds and Some of Their Synthetic Equivalents with Aryl Halides and Pseudohalides. Chem Rev. 2010;110:1082–1146. doi: 10.1021/cr9000836.Lyons TW, Sanford MS. Palladium-Catalyzed Ligand-Directed C-H Functionalization Reactions. Chem Rev. 2010;110:1147–1169. doi: 10.1021/cr900184e.Davies HML, Du Bois J, Yu JQ. C-H Functionalization in Organic Synthesis. Chem Soc Rev. 2011;40:1855–1856. doi: 10.1039/c1cs90010b.
- 3.(a) Jun CH, Moon CW, Lee DY. Chelation-Assisted Carbon–Hydrogen and Carbon–Carbon Bond Activation by Transition Metal Catalysts. Chem Eur J. 2002;8:2422–2428. doi: 10.1002/1521-3765(20020603)8:11<2422::AID-CHEM2422>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]; (b) Kakiuchi F, Ohtaki H, Sonoda M, Chatani N, Murai S. Mechanistic Study of the Ru(H)2(CO)(PPh3)3-Catalyzed Addition of C-H Bonds in Aromatic Esters to Olefins. Chem Lett. 2001;30:918–919. [Google Scholar]; (c) Lenges CP, Brookhart M. Addition of Olefins to Aromatic Ketones Catalyzed by Rh(I) Olefin Complexes. J Am Chem Soc. 1999;121:6616–6623. doi: 10.1021/ja066509x. [DOI] [PubMed] [Google Scholar]; (d) Matsubara T, Koga N, Musaev D, Morokuma K. Density Functional Study on Highly ortho-Selective Addition of an Aromatic C-H Bond to Olefins Catalyzed by a Ru(H)2 (CO)(PR3)3 Complex. Organometallics. 2000;19:2318–2329. [Google Scholar]
- iv.Colby DA, Bergman RG, Ellman JA. Synthesis of Dihydropyridines and Pyridines from Imines and Alkynes via C-H Activation. J Am Chem Soc. 2008;130:3645–3651. doi: 10.1021/ja7104784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- v.Murai S, Kakiuchi F, Sekine S, Tanaka Y, Kamatani A, Sonoda M, Chatani N. Efficient Catalytic Addition of Aromatic Carbon-Hydrogen Bonds to Olefins. Nature. 1993;366:529–531. [Google Scholar]
- vi.(a) Thalji RK, Ahrendt KA, Bergman RG, Ellman JA. Annulation of Aromatic Imines via Directed C-H Bond Activation. J Org Chem. 2005;70:6775–6781. doi: 10.1021/jo050757e. [DOI] [PubMed] [Google Scholar]; (b) Thalji RK, Ahrendt KA, Bergman RG, Ellman JA. Annulation of Aromatic Imines via Directed C-H Activation with Wilkinson’s Catalyst. J Am Chem Soc. 2001;123:9692–9693. doi: 10.1021/ja016642j. [DOI] [PubMed] [Google Scholar]
- vii.(a) Davies HML, Manning JR. Catalytic C-H Functionalization by Metal Carbenoid and Nitrenoid Insertion. Nature. 2008;451:417–424. doi: 10.1038/nature06485. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kakiuchi F, Le Gendre P, Yamada A, Ohtaki H, Murai S. Atropselective Alkylation of Biaryl Compounds by Means of Transition Metal-Catalyzed C-H/Olefin Coupling. Tetrahedron: Asymmetry. 2000;11:2647–2651. [Google Scholar]; (c) Bosnich B, Wang X. Asymmetric Catalysis: Comparison of the Enantioselection of Rhodium-Catalyzed Intramolecular Hydrosilation and Hydroacylation. Organometallics. 1994;13:4131–4133. [Google Scholar]; (d) Han X, Widenhoefer RA. Platinum-Catalyzed Intramolecular Asymmetric Hydroarylation of Unactivated Alkenes with Indoles. Org Lett. 2006;8:3801–3804. doi: 10.1021/ol061441b. [DOI] [PubMed] [Google Scholar]; (e) Shi BF, Maugel N, Zhang YH, Yu JQ. Pd(II)-Catalyzed Enantioselective Activation of C(sp2)-H and C(sp3)-H Bonds Using Monoprotected Amino Acids as Chiral Ligands. Angew Chem Int Ed. 2008;47:4882–4886. doi: 10.1002/anie.200801030. [DOI] [PubMed] [Google Scholar]
- viii.For an early example of an enantioselective cyclization, see: Fujii N, Kakiuchi F, Yamada A, Chatani N, Murai S. Asymmetric Intramolecular C-H/Olefin Coupling: Asymmetric Cyclization Reactions of 1,5-Dienes Catalyzed by Rhodium Complexes. Chem Lett. 1997;26:425–426.
- ix.(a) Harada H, Thalji RK, Bergman RG, Ellman JA. Enantioselective Intramolecular Hydroarylation of Alkenes via Directed C-H Bond Activation. J Org Chem. 2008;73:6772–6779. doi: 10.1021/jo801098z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Thalji RK, Ellman JA, Bergman RG. Highly Efficient and Enantioselective Cyclization of Aromatic Imines via Directed C-H Bond Activation. J Am Chem Soc. 2004;126:7192–7193. doi: 10.1021/ja0394986. [DOI] [PubMed] [Google Scholar]
- x.Ahrendt KA, Bergman RG, Ellman JA. Synthesis of a Tricyclic Mescaline Analogue by Catalytic C-H Bond Activation. Org Lett. 2003;5:1301–1303. doi: 10.1021/ol034228d. [DOI] [PubMed] [Google Scholar]
- xi.A synthesis of 14 via a tandem aryl alkylation/Heck coupling: Alberico D, Lautens M. Palladium-Catalyzed Alkylation-Alkenylation Reactions: Rapid Access to Tricyclic Mescaline Analogues. Synlett. 2006:2969–2972.
- xii.O’Malley SJ, Tan KL, Watzke A, Bergman RG, Ellman JA. Total Synthesis of (+)-Lithospermic Acid by Asymmetric Intramolecular Alkylation via Catalytic C-H Bond Activation. J Am Chem Soc. 2005;127:13496–13947. doi: 10.1021/ja052680h. [DOI] [PubMed] [Google Scholar]
- xiii.Subsequent syntheses: Wang DH, Yu JQ. Highly Convergent Total Synthesis of (+)-Lithospermic Acid via a Late-Stage Intermolecular C-H Olefination. J Am Chem Soc. 2011;133:5767–5769. doi: 10.1021/ja2010225.Fischer J, Savage GP, Coster MJA. Concise Route to Dihydrobenzo[. b]furans: Formal Total Synthesis of (+)-Lithospermic. Acid Org Lett. 2011;13:3376–3379. doi: 10.1021/ol201130h.
- xiv.Watzke A, Wilson RM, O’Malley SJ, Bergman RG, Ellman JA. Asymmetric Intramolecular Alkylation of Chiral Aromatic Imines via Catalytic C-H Bond Activation. Synlett. 2007:2283–2389. [Google Scholar]
- xv.Wilson RM, Thalji RK, Bergman RG, Ellman JA. Enantioselective Synthesis of a PKC Inhibitor via Catalytic C-H Bond Activation. Org Lett. 2006;8:1745–1747. doi: 10.1021/ol060485h. [DOI] [PubMed] [Google Scholar]
- xvi.Early examples using Ru: Trost BM, Imi K, Davies IW. Elaboration of Conjugated Alkenes Initiated by Insertion into a Vinylic C-H Bond. J Am Chem Soc. 1995;117:5371–5372.Kakiuchi F, Sato T, Igi K, Chatani N, Murai S. The Ruthenium-Catalyzed Addition of β C-H Bonds in Aldehydes to Olefins. Chem Lett. 2001:386–387.and references therein.
- xvii.Lim YG, Kang JB, Kim YH. Rhodium(I)-Catalysed Alkylation of 2-Vinylpyridines with Alkenes as a Result of C-H Bond Activation. J Chem Soc, Perkin Trans. 1998;1:699. [Google Scholar]
- xviii.Jun C-H, Moon CW, Kim Y-M, Lee H, Lee JH. Chelation-Assisted β-Alkylation of α,β-Unsaturated Ketone using Rh(I) Catalyst and Dialkyl Amine. Tetrahedron Lett. 2002;43:4233–4336. [Google Scholar]
- xix.Colby DA, Bergman RG, Ellman JA. Stereoselective Alkylation of α,β-Unsaturated Imines via C—H Bond Activation. J Am Chem Soc. 2006;128:5604–5605. doi: 10.1021/ja0584931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xx.Tsai AS, Bergman RG, Ellman JA. Asymmetric Synthesis of (−)-Incarvillateine Employing an Intramolecular Alkylation via Rh-Catalyzed Olefinic C—H Bond Activation. J Am Chem Soc. 2008;130:6316–6317. doi: 10.1021/ja8012159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxi.Prior work: Ichikawa M, Takahashi M, Aoyagi S, Kibayashi C. Total Synthesis of (−)-Incarvilline, (+)-Incarvine C, and (−)-Incarvillateine. J Am Chem Soc. 2004;126:16553–16558. doi: 10.1021/ja0401702.
- xxii.Lim S-G, Lee JH, Moon CW, Hong J-B, Jun C-H. Rh(I)-Catalyzed Direct ortho-Alkenylation of Aromatic Ketimines with Alkynes and Its Application to the Synthesis of Isoquinoline Derivatives. Org Lett. 2003;5:2759–2761. doi: 10.1021/ol035083d. [DOI] [PubMed] [Google Scholar]
- xxiii.Cao C, Li Y, Shi Y, Odom AL. α,β-Unsaturated Imines from Titanium Hydroamination and Functionalization by Rhodium C–H Activation. Chem Commun. 2004:2002–2003. doi: 10.1039/b405620e. [DOI] [PubMed] [Google Scholar]
- xxiv.For a review on pyridine synthesis, see: Hill MD. Recent Strategies for the Synthesis of Pyridine Derivatives. Chem Eur J. 2010;16:12052–12062. doi: 10.1002/chem.201001100.
- xxv.Parthasarathy K, Jeganmohan M, Cheng C-H. Rhodium-Catalyzed One-Pot Synthesis of Substituted Pyridine Derivatives from α,β-Unsaturated Ketoximes and Alkynes. Org Lett. 2008;10:325–328. doi: 10.1021/ol7028367. [DOI] [PubMed] [Google Scholar]
- xxvi.Yotphan S, Bergman RG, Ellman JA. The Stereoselective Formation of Bicyclic Enamines with Bridgehead Unsaturation via Tandem C—H Bond Activation/Alkenylation/Electrocyclization. J Am Chem Soc. 2008;130:2452–2453. doi: 10.1021/ja710981b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxvii.(a) Li L, Brennessel WW, Jones WD. An Efficient Low-Temperature Route to Polycyclic Isoquinoline Salt Synthesis via C—H Activation with [Cp*MCl2]2 (M = Rh, Ir) J Am Chem Soc. 2008;130:12414–12419. doi: 10.1021/ja802415h. [DOI] [PubMed] [Google Scholar]; (b) Davies DL, Al-Duaij O, Fawcett J, Giardiello M, Hilton ST, Russell DR. Room-Temperature Cyclometallation of Amines, Imines and Oxazolines with [MCl2Cp*]2(M = Rh, Ir) and [RuCl2(p-cymene)]2. Dalton Trans. 2003;4132 [Google Scholar]
- xxviii.For a review see: Satoh T, Miura M. Oxidative Coupling of Aromatic Substrates with Alkynes and Alkenes under Rhodium Catalysis. Chem Eur J. 2010;16:11212–11222. doi: 10.1002/chem.201001363.For leading references, see: Patureau FW, Besset T, Glorius F. Rhodium-Catalyzed Oxidative Olefination of C-H Bonds in Acetophenones and Benzamides. Angew Chem Int Ed. 2011;50:1064–1067. doi: 10.1002/anie.201006222.
- xxix.For a review see: Chen X, Engle KM, Wang DH, Yu JQ. Palladium(II)-Catalyzed C-H Activation/C-C Cross-Coupling Reactions: Versatility and Practicality. Angew Chem Int Ed. 2009;48:5094–5115. doi: 10.1002/anie.200806273.
- xxx.For an oxidative coupling of 1-hexene see: Wang DH, Engle KM, Shi BF, Yu JQ. Ligand-Enabled Reactivity and Selectivity in a Synthetically Versatile Aryl C–H Olefination. Science. 2010;327:315–319. doi: 10.1126/science.1182512.
- xxxi.Tsai AS, Brasse M, Bergman RG, Ellman JA. Rh(III)-Catalyzed Oxidative Coupling of Unactivated Alkenes via C—H Activation. Org Lett. 2010;13:540–542. doi: 10.1021/ol102890k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guimond N, Gorelsky SI, Fagnou K. Rhodium(III)-Catalyzed Heterocycle Synthesis Using an Internal Oxidant: Improved Reactivity and Mechanistic Studies. J Am Chem Soc. 2011;133:6449–6457. doi: 10.1021/ja201143v. [DOI] [PubMed] [Google Scholar]
- xxxiii.For an intramolecular example, see: Fukutani T, Umeda N, Hirano K, Satoh T, Miura M. Rhodium-Catalyzed Oxidative Coupling of Aromatic Imines with Internal Alkynes via Regioselective C–H Bond Cleavage. Chem Commun. 2009:5141–5143. doi: 10.1039/b910198e.
- xxxiv.Shen Z, Khan HA, Dong VM. Rh-Catalyzed Carbonyl Hydroacylation: An Enantioselective Approach to Lactones. J Am Chem Soc. 2008;130:2916–2917. doi: 10.1021/ja7109025. [DOI] [PubMed] [Google Scholar]
- xxxv.Tsai AS, Tauchert ME, Bergman RG, Ellman JA. Rhodium(III)-Catalyzed Arylation of Boc-Imines via C—H Bond Functionalization. J Am Chem Soc. 2011;133:1248–1250. doi: 10.1021/ja109562x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxxvi.Li Y, Li B-J, Wang W-H, Huang W-P, Zhang X-S, Chen K, Shi Z-J. Rhodium-Catalyzed Direct Addition of Aryl C-H Bonds to N-Sulfonyl Aldimines. Angew Chem Int Ed. 2011;50:2115–2119. doi: 10.1002/anie.201007464. [DOI] [PubMed] [Google Scholar]
- xxxvii.Hesp KD, Bergman RG, Ellman JA. Expedient Synthesis of N-Acyl Anthranilamides and β-Enamine Amides by the Rh(III)-Catalyzed Amidation of Aryl and Vinyl C–H Bonds with Isocyanates. J Am Chem Soc. 2011:11430–11433. doi: 10.1021/ja203495c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxxviii.Anslyn EV, Dougherty DA. Modern Physical Organic Chemistry. University Science Books; California: 2006. [Google Scholar]