Abstract
Objective
Identify the etiology of elevated B12 in autoimmune lymphoproliferative syndrome (ALPS).
Design
Peripheral blood of ALPS patients with elevated B12 and controls were evaluated.
Results
Total and holo-haptocorrin (HC) levels were 26- and 23-fold higher in ALPS patients, respectively. No abnormal B12-binding proteins were found. Western blot revealed HC in lymphocyte lysates only from ALPS patients.
Conclusion
Elevated concentrations of B12 found in ALPS patients were due to increased lymphocyte expression of HC.
Keywords: ALPS, vitamin B12, cobalamin, germline, haptocorrin, transcobolamin
Introduction
Autoimmune lymphoproliferative syndrome (ALPS) is an immune disease characterized by lymphoproliferation, accumulation of double-negative T cells (DNT, TCRalpha/beta+ CD3+CD4−CD8−), abnormal apoptosis, and increased autoimmunity and lymphoma [1, 2]. Most patients have been genetically classified as having germline (ALPS-FAS) or somatic (ALPS-sFAS) FAS/APO-1 mutations [3]. Patients with ALPS have a number of laboratory abnormalities, including elevated vitamin B12 levels most prevalent in ALPS-FAS, ALPS-sFAS and ALPS-U (genetic defect unknown) [3].
B12 (Cobalamin) is an essential micronutrient that plays a fundamental role in cell division and one-carbon metabolism. Serum B12 is bound to both transcobalamin (TC) and haptocorrin (HC). These proteins circulate in blood partly saturated (holo) and partly unsaturated (apo) with B12 [4]. TC saturated with B12 (holo-TC) constitutes 6 to 20% of endogenous circulating B12. In contrast, HC binds ~80–94% of the endogenous plasma B12 (holo-HC), and is largely saturated with B12 or analogues [5]. We sought to determine the source of elevated B12 in patients with ALPS-FAS germline mutation.
Methods
Subjects
We studied 28 patients with ALPS-FAS germline mutation, 18 relatives and 8 healthy controls. Peripheral blood was obtained from subjects with signed informed consent under Institution Review Board approved protocols of the National Institute of Allergy and Infectious Diseases.
Vitamin B12
Serum B12 was measured on the IMMULITE 2000 analyzer (Diagnostic Products Corp., Los Angeles, CA). Total homocysteine and methymalonic acid (MMA) concentrations were measured by gas chromatography-mass spectrometry at Mayo Medical Laboratory, Rochester, MN. Holo-TC was evaluated using an RIA (Axis-Shield, Norton, MA). B12-HC was calculated from the difference between total B12 and holo-TC levels. Serum from ALPS and control subjects were incubated with excess 57[Co]cyanocobalamin (10.5 Ci/mL; MP Biomedical, Solon, OH) and fractionated with a SMART system (GE Life Sciences, Pittsburgh, PA) with a Superdex 200 HR column (1.0 x 30cm) as described by Nexo et al [6]. Fractions (400μL) were collected and assayed for B12 with a gamma counter and total TC and HC by ELISA.
Western Blot
Separation of granulocytes and lymphocytes was performed using dextran/ficoll separation. Protein was run on a 4%–12% gel (Expedeon, San Diego, CA) and then transferred to a nitrocellulose membrane. Haptocorrin (HC; Abcam, Cambridge, MA) in the lymphocyte lysates was detected with chicken anti-HC IgY antibodies (Abcam) and then probed for actin as a loading control.
Results and Discussion
In this study, we examined B12, haptocorrin and transcobalamin in patients with ALPS, first-degree relatives and healthy controls. The mean B12 concentrations were significantly different among controls (274 pmol/L; N=8), relatives (500 pmol/L; N=18), and patients (3900 pmol/L; N=28) (P<0.0001). Serum B12 concentrations were significantly higher in ALPS patients compared to controls and healthy relative groups (P<0.0001). Although B12 levels were higher in ALPS relatives compared to controls, these differences were not statistically significant. Whereas elevated B12 levels are not typically observed in lymphoproliferative disorders, there have been reports in patients with disseminated neoplasia, myeloproliferative disorders, and hypereosinophilic syndrome [7]: none of these co-morbid conditions applied to our subjects. Also, high B12 concentrations are associated with renal failure [8] and hepatic disease [7]: the ALPS patients in this study showed normal renal and hepatic function. Previous studies have shown that some patients given cyano- or hydroxycobalamin have developed antibodies to TC, leading to markedly increased serum TC and B12 [9]. In the ALPS patients, however, serum holo-TC concentrations were within reference intervals, with only slight increases in total TC in the presence of high B12 concentrations.
We observed no significant difference in mean serum holo-TC concentrations among the three groups (P=0.93), with concentrations in controls, ALPS relatives, and ALPS patients of 81, 86, and 85 pmol/L, respectively. In contrast, we found significant differences in mean holo-HC concentrations among the three groups (P<0.0001). The mean holo-HC concentration in this part of serum B12 in ALPS patients (3810 pmol/L) was significantly higher than controls (194 pmol/L) and ALPS relatives (414 pmol/L), whereas there was no significant difference in holo-HC concentrations between controls and ALPS relatives.
We evaluated whether there was an association between total B12 concentrations and concentrations of B12 bound to TC or HC in serum. We found no correlation between the total serum B12 and holo-TC concentrations for controls (r=0.18), ALPS relatives (r=0.29), and ALPS patients (r=0.51). However, there were strong positive correlations between holo-HC concentrations and total B12 concentrations in all three groups of individuals studied (r=0.93, 0.99 and 1.0 for the control, ALPS relatives and ALPS patients, respectively).
The functional B12 status of a patient can be determined by the activities of the B12 dependent enzymes. Therefore, the plasma concentrations of homocysteine and methylmalonic acid, which are the substrates of methionine synthase and methylmalonyl CoA mutase, respectively, have a diagnostic value in tracing a functional deficiency of B12 [10]. Cell damage can directly lead to reduced concentrations of intracellular B12, thus significantly elevated plasma B12 can be associated with a functional B12 deficiency. This condition can also result from the increased binding of B12 to HC, which has an inhibitory effect on B12 binding to TC, the physiologic transport protein required for intracellular uptake. The paradox of high plasma B12 and elevated homocysteine and/or MMA has been described [10], but we found normal levels of MMA and homocysteine in ALPS patients and control subjects, respectively; confirming that these individuals are not functionally deficient in B12, a finding consistent with the normal transcobalamin levels in both these groups. Furthermore, the Superdex column elution profile from patients and controls did not show any unusual B12 binding protein.
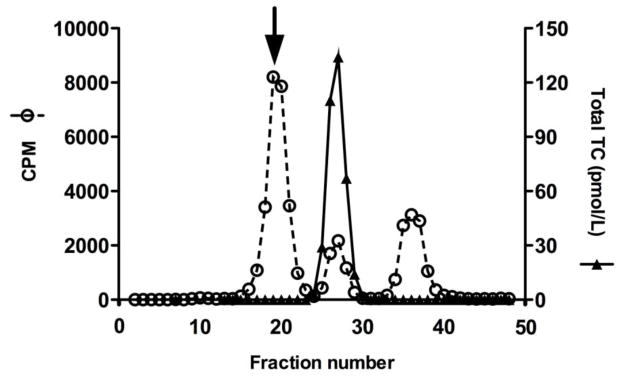
Two sets of experiments were performed to clarify whether the part of serum B12 not accounted for by holo-TC represented B12 bound to haptocorrin. First, we examined by gel filtration the occurrence of unsaturated haptocorrin, and employing labeled cobalamin we observed a major peak of unsaturated binding protein eluting similar to haptocorrin (Fig. 1). Second, we measured the amount of holo and total HC in six patients to find mean and [range] values of 15600 [854–30400] and 21700 [855–42900] pmol/L, respectively. The values are considerably higher than those observed for a reference population of 240–680 for holo-HC and 250–840 for total HC
Figure 1.
Distribution of B12 among the B12 binding proteins. Superdex 200 HR gel filtration of serum from ALPS-FAS patient. The primary y-axis displays the 57Co-cyanocobalamin activity in counts per minute (cpm; open circles, dashed line). The secondary y-axis displays the serum level of total TC (closed triangles, solid line). An arrow shows a major peak of unsaturated binding protein eluting similar to haptocorrin by gel filtration.
Finally we explored the origin of the increased level of haptocorrin. Here we show the novel finding of haptocorrin in lymphocytes from patients with ALPS and high serum B12 levels. Western blot analysis revealed the expected presence of haptocorrin in granulocytes from both control and ALPS-FAS subjects (data not shown); however, lymphocytes showed the presence of haptocorrin only from ALPS patients (Fig. 2). No difference was observed when lymphocyte lysates were probed for transcobalamin. Hence, lymphocytes may be synthesizing and releasing haptocorrin but not transcobalmin into serum, which may partly account for the high B12 in affected ALPS subjects.
Figure 2.
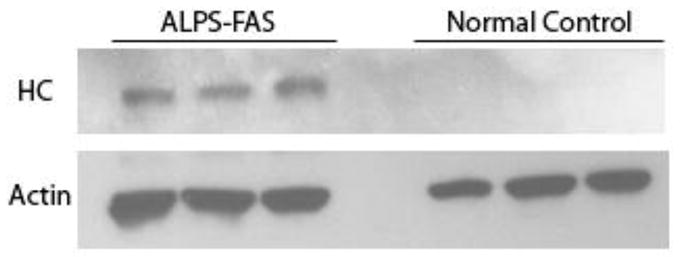
Western blot showing haptocorrin in ALPS-FAS lymphocytes but not in control lymphocytes. Lanes 1–3 represent three discrete ALPS-FAS patients and lanes 4–6 represent three discrete healthy controls.
A substantial amount of knowledge with regard to the occurrence and production of HC has accumulated during the past three decades, but there are still major questions that remain. Most importantly, HC is still a protein with an unexplained function characterized by its ability to bind B12 analogues, but the significance of this in relation to human pathophysiology is unclear. In patients with ALPS-FAS germline mutation, plasma levels of B12 can be elevated, up to 15 times the upper limit of the reference interval, and with an even larger increase in the level of HC. The increase in HC appears to be related to an elevated production of HC by lymphocytes. When HC is released from these cells it binds B12 liberated from various tissues and the expanded granulocyte pool. The possibility that the concentration of HC has prognostic value in affected ALPS subgroups warrants further investigation. Additional studies are also required to elucidate the mechanism for the increased synthesis and secretion of haptocorrin by circulating leukocytes in ALPS, particularly, double-negative T cells.
Highlights.
Vitamin B12 levels were significantly increased in ALPS-FAS patients.
Serum total and holo-haptocorrin levels were elevated in ALPS-FAS patients.
No abnormal serum vitamin B12-binding proteins were present in ALPS-FAS patients.
Serum transcobalamin levels were not elevated in ALPS-FAS and control subjects.
Haptocorrin were found in lymphocyte lysates of ALPS but not control subjects.
Acknowledgments
This research was supported by the intramural programs of the National Institute of Allergy and Infectious Diseases and the Warren Grant Magnuson Clinical Center.
Abbreviations
- ALPS
autoimmune lymphoproliferative syndrome
- DNT
double negative T cells
- HC
haptocorrin
- Hcy
homocysteine
- TC
transcobalamin
- B12
vitamin B12 or cobalamin
- MMA
methylmalonic acid
Footnotes
Conflict of interest statement
The authors declare that they have no conflict of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sneller MC, Wang J, Dale JK, Strober W, Middelton LA, Choi Y, et al. Clincal, immunologic, and genetic features of an autoimmune lymphoproliferative syndrome associated with abnormal lymphocyte apoptosis. Blood. 1997;89:1341–8. [PubMed] [Google Scholar]
- 2.Straus SE, Jaffe ES, Puck JM, Dale JK, Elkon KB, Rosen-Wolff A, et al. The development of lymphomas in families with autoimmune lymphoproliferative syndrome with germline Fas mutations and defective lymphocyte apoptosis. Blood. 2001;98:194–200. doi: 10.1182/blood.v98.1.194. [DOI] [PubMed] [Google Scholar]
- 3.Oliveira JB, Bleesing JJ, Dianzani U, Fleisher TA, Jaffe ES, Lenardo MJ, et al. Revised diagnostic criteria and classification for the autoimmune lymphoproliferative syndrome (ALPS): report from the 2009 NIH International Workshop. Blood. 2010;116:e35–40. doi: 10.1182/blood-2010-04-280347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Markle HV. Cobalamin. Crit Rev Clin Lab Sci. 1996;33:247–356. doi: 10.3109/10408369609081009. [DOI] [PubMed] [Google Scholar]
- 5.Hall CA. Transcobalamins I and II as natural transport proteins of vitamin B12. J Clin Invest. 1975;56:1125–31. doi: 10.1172/JCI108187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nexo E, Christensen AL, Petersen TE, Fedosov SN. Measurement of transcobalamin by ELISA. Clin Chem. 2000;46:1643–9. [PubMed] [Google Scholar]
- 7.Ermens AA, Vlasveld LT, Lindemans J. Significance of elevated cobalamin (vitamin B12) levels in blood. Clin Biochem. 2003;36:585–90. doi: 10.1016/j.clinbiochem.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Carmel R, Vasireddy H, Aurangzeb I, George K. High serum cobalamin levels in the clinical setting--clinical associations and holo-transcobalamin changes. Clin Lab Haematol. 2001;23:365–71. doi: 10.1046/j.1365-2257.2001.00134.x. [DOI] [PubMed] [Google Scholar]
- 9.Skouby AP, Hippe E, Olesen H. Antibody to transcobalamin II and B12 binding capacity in patients treated with hydroxocobalamin. Blood. 1971;38:769–74. [PubMed] [Google Scholar]
- 10.Miller JW, Garrod MG, Allen LH, Haan MN, Green R. Metabolic evidence of vitamin B-12 deficiency, including high homocysteine and methylmalonic acid and low holotranscobalamin, is more pronounced in older adults with elevated plasma folate. Am J Clin Nutr. 2009;90:1586–92. doi: 10.3945/ajcn.2009.27514. [DOI] [PMC free article] [PubMed] [Google Scholar]