Abstract
Aims
Recent research has revealed that early life trauma (ELS), including abuse (sexual and/or physical) and neglect, produce lasting changes in the CNS. We posited that cognitive deficits, often observed in psychiatric patients, result, in part, due to the neurobiological consequences of ELS. Additionally, we hypothesized that the nature and magnitude of cognitive deficits would differ according to the subtype of ELS experienced.
Method
The Cambridge Neuropsychological Test Automated Battery (CANTAB) was used to assess neurocognitive functioning in 93 subjects (60 with ELS and 33 without). In the patients with a history of ELS, 35% and 16.7%, respectively, met criteria for current major depression and PTSD.
Results
Significant associations between ELS status and CANTAB measures of memory and executive and emotional functioning were found.
Conclusions
These data suggest that exposure to ELS results in a cascade of neurobiological changes associated with cognitive deficits in adulthood that vary according to the type of trauma experienced.
Keywords: ELS, Cognition, Child Abuse
Introduction
There is evidence that trauma exposure early in life (referred to as early life stress or ELS) markedly increases the risk for major depression, bipolar disorder, schizophrenia, and post traumatic stress disorder (PTSD), all syndromes characterized by cognitive dysfunction. Numerous studies have observed deficits in executive functioning, attention and concentration, memory and processing speed in patients with depression (McClintock et al., 2010; Nebes et al., 2000), yet it is clear that not all depressed persons suffer such impairment (Porter et al., 2007), and trauma exposure may offer one explanation for variability in these findings. In addition to neuropsychological dysfunction, depressed patients are well known to display deficits in emotional processing (Elliott et al., 2002; Fales et al., 2008). Mounting evidence from functional neuroimaging studies suggests that neural circuit abnormalities underlie cognitive and emotional processing deficits in patients with major depressive disorder (MDD). Specifically, hypoactivity in the dorsal lateral prefrontal cortex, and the anterior cingulate cortex have been reported (Fales et al., 2008; Davidson et al., 2002). A growing body of literature suggests cognitive abnormalities including executive and attentional deficits, deficits in short-term and working memory for both visual and verbal material and psychomotor speed dysfunction characterize MDD even in young adulthood (Castaneda et al., 2008). Amongst, patients with MDD, executive and psychomotor impairments are more pronounced in the elderly, severely melancholic, psychotic and bipolar populations (Porter et al., 2007), Deficits in patients with MDD appear to persist even in the remitted state (Hasselbalch et al., 2010). Among patients with anxiety disorders in general, deficits in episodic memory and executive functioning have been found (Castaneda et al., 2008), but significant heterogeneity exists amongst these patients depending on their specific anxiety disorder subtype. For example, a relatively broader range of impairments in intellectual functioning, attention/working memory, processing speed, learning, and executive functioning have been found in patients with PTSD (Yehuda et al., 1995; Vasterling et al., 2002; Liberzon & Sripada, 2008). Functional and structural imaging correlates of cognitive deficits in PTSD have also been identified (Liberzon & Sripada, 2008; Woodward et al., 2009). There may be a bidirectional and temporal relationship between PTSD, the hippocampus and neuropsychological functioning in that structural and functional brain abnormalities may either precede the development of PTSD, or emerge after the onset of PTSD (Woodward et al., 2009; Vasterling & Verfaellie, 2009; Yehuda et al., 2007). However, no consistent link between hippocampal volume and cognition has emerged (Woodward et al., 2009) and alterations in hippocampal volumes may be a risk factor for the development of PTSD (Yehuda et al., 2007). Instead, a more fronto-striatal pattern of neuropsychological impairment has emerged (Twamley et al., 2009; Golier & Yuhuda, 2002) and implicates the importance of anterior brain regions such as the prefrontal cortex.
Trauma exposure can occur throughout the lifespan and the time-frame for exposure may have critical implications for lifelong functioning. Previous research has indicated that prenatal stress and stress throughout childhood both alter stress reactivity. Such abnormal development and subsequent increases in reactivity have been found to result in vulnerability to mood and anxiety disorders (Nemeroff, 2004). Nonhuman primate studies have indicated that sensitization to fear cues, corticotropin-releasing factor (CRF), neuronal hyperactivity and hypercortisolism may all result from early life stress (Coplan et al., 1996). Heim et al., (Heim et al., 2000) found that adult women with a history of ELS display increased sensitivity of the HPA axis and autonomic nervous system responses to stress. Twamley et al. (Twamley et al., 2009) investigated cognitive functioning in women with PTSD related to intimate partner violence and found that they demonstrated slower processing speed, poor reasoning performance, and dissociative symptoms proportionate to PTSD severity. Recent data suggests that the full impact of ELS on the HPA axis may not be observed until adolescence, but gender and timing of stress exposure are likely to modify neurobiological vulnerability to mood and anxiety disorders, yet more data is needed to determine precise critical periods for the maximum impact of trauma exposure (Anderson & Teicher, 2008; Heim & Nemeroff, 2009). Children with a history of ELS have been found to perform more poorly on intellectual, cognitive and achievement tasks and ELS exposure is broadly associated with both a high probability of neurocognitive dysfunction and real world functional impairment (Twamley et al., 2009; De Bellis et al., 2009). A range of disparate cognitive deficits have been associated with functional impairment in psychiatric patients across different neuropsychiatric conditions (Twamley et al., 2009; Wingo et al., 2009). Memory, executive functioning and psychomotor speed deficits have been uncovered by the majority of studies investigating neuropsychological dysfunction in patients with MDD (Porter et al., 2007), which is consistent with the fronto-striatal profile of cognitive impairments seen in other conditions where trauma-exposure may be an etiological factor, including PTSD and, perhaps, schizophrenia. Inconsistent findings may have arisen from the variation in potential etiological factors, including ELS, the influence of which has not been well characterized in investigations of neuropsychological functioning in MDD.
Only two previous studies (Majer et al., 2010; Pluck et al., 2011) have explicitly tested whether the cognitive deficits observed in abused or neglected children persist into adulthood. The Majer et al., (Majer et al., 2010) study excluded patients with both ELS and current depression in adulthood. The Pluck et al. (Pluck et al., 2011) study included a sample comprised exclusively of homeless individuals with subaverage IQs. Our current study examined the impact of ELS on relatively healthy, community dwelling adults via a battery of cognitive tests selected to measure the pattern of deficits observed in previous studies of PTSD and MDD. The present study included a predominantly female civilian population with a history of ELS as defined by a history of either sexual, physical, emotional abuse, or neglect. We hypothesized that deficits in memory, executive functioning, processing speed, and emotional processing would distinguish ELS patients from controls.
Method
Participants
The sample was comprised of 93 subjects (28 men and 65 women) 18–45 years old (M=29.83, SD=7.54 years) who had valid assessment data. Subjects were recruited as part of the Conte Center for the Psychobiology of Early-Life Trauma (MH58922) and included subjects with or without exposure to ELS before the age of 13 years and with or without a diagnosis of MDD. MDD was defined by a diagnosis of current MDD according to the Diagnostic and Statistical Manual for Mental Disorder 4th Edition (DSM-IV). For the present analysis, subjects were classified into those with and without a history of ELS on Childhood Trauma Questionnaire (CTQ) scores (First et al., 1997) as described below. Exclusion criteria were current unstable medical illness, lifetime history of psychosis or bipolar disorder, meeting DSM-IV criteria for alcohol or substance abuse within 6 months, heavy smoking (>20 cigarettes/day) or eating disorders within the past year. None of the participants was currently receiving psychotherapy or medications. All subjects were recruited from responses to advertising in local newspapers and in the public transportation system and screened for eligibility. Eligible subjects were invited and paid for their time if they participated. After description of the study to participants, written informed consent was obtained. The study was approved by the Institutional Review Board of Emory University School of Medicine.
General Procedure
Subjects were admitted as inpatients for 2.5 days to the Atlanta Clinical and Translational Science Institute, Emory University Hospital Clinical Interaction Site, which provides a standardized setting for clinical studies. For the diagnosis of lifetime and current MDD and other psychiatric disorders the Structured Clinical Interview for DSM-IV (SCID) (First et al., 1997) was administered to patients and controls. Depression symptom severity was measured using the 21-item Hamilton Depression Rating Scale (HAMD-21) (Williams, 1988). Exposure to ELS was assessed using the CTQ.
The CTQ is a self-report questionnaire that measures five categories of childhood trauma experience, including emotional, physical, and sexual abuse, and emotional and physical neglect. Each subscale is measured in 5 items rated on a 5-point Likert scale from 1 = “Never true” through 5 = “Very often true”. In addition, scores for none-low, low-moderate, moderate-severe, and severe-extreme exposure have been developed for each subscale. Good specificity and sensitivity of cut-off scores to classify maltreated subjects has been reported (Bernstein & Fink, 1998).
We used the moderate-severe cutoff scores for each subscale to classify subjects as positive for a history of childhood trauma in that category (Bernstein & Fink, 1998). Cases classified as positive on any one subscale were considered to have exposure to ELS, whereas cases classified as negative on all subscales were designated as not exposed to trauma. A total of 60 subjects were classified as positive and 33 were classified as negative for a history of ELS. On the morning of the first day of hospital admission, subjects completed the Cambridge Neuropsychological Testing Automated Battery (CANTAB) (Sahakian & Owen, 1992). The CANTAB has modules for several neurocognitive functions and processes, including psychomotor and motor speed, reasoning and planning abilities, memory and attention, and frontal, temporal and hippocampal dysfunction. All tests of the CANTAB are computerized and were presented on a touch screen. We utilized the following CANTAB modules: a.) Psychomotor Coordination and Motor Speed, b.) Reasoning and Planning Abilities, c.) Memory (Spatial Working Memory, Pattern Recognition Memory, Spatial Recognition Memory), and d.) Attention (Attention Shift and Sustained Attention). The CANTAB subtests included: Delayed Matching to Sample (DMS), a test of immediate and delayed perceptual matching; Intra – Extra Dimensional Set Shifting (IED), which assesses rule acquisition and attentional set shifting; the Reaction Time Task (RTI) which measures a participant’s speed of response; the Rapid Visual Information Processing task (RVP), a test of sustained visual attention; Stockings of Cambridge (SOC), a measure of spatial planning and motor control; the Spatial Working Memory task (SWM), a measure of working memory and strategy use that tests the participant’s ability to retain spatial information and manipulate objects in working memory; and the Affective Go-No Go (AGN) which assesses information processing bias and inhibitory control test for both positive and negative stimuli. For a more detailed description of all CANTAB subtests used in the present study and their outcome measures, see Table 1. We additionally employed the reading subtest of the Wide Range Achievement Test to approximate IQ (Wilkinson, 1993) and interpret CANTAB data. All of the cognitive tests in the battery required approximately 1 hour to administer.
Table 1.
CANTAB subtests
| Name | Task Demands | Dependent Variables |
|---|---|---|
| Delayed Matching to Sample (DMS) | Immediate and delayed perceptual matching |
|
| Intra – Extra Dimensional Set Shifting (IED) | Rule acquisition and attentional set shifting |
|
| The Reaction Time Task (RTI) | Speed of response |
|
| The Rapid Visual Information Processing task (RVP) | Sustained visual attention | Mean latency for correct responses |
| Stockings of Cambridge (SOC) | Spatial planning and motor control |
|
| The Spatial Working Memory task (SWM) | Working memory and strategy |
|
| The Affective Go-No Go (AGN) | Information processing bias and inhibitory control test | Number of (a)commissions and (b)omissions for
|
Statistical Analysis
Individuals (n=16) with a majority of missing data or missing response variables were removed from analysis. All analyses were conducted on the remaining 93 individuals. Two analyses were conducted using nonlinear canonical correlation analysis (Gifi, 1990) as implemented in the homals package for the R statistical language (deLeeuw & Mair, 2009). Traditional canonical correlations analysis (CCA) was not used because the basic assumption of CCA, i.e., independent sampling and multivariate normality of both sets of variables, was not satisfied. The first analysis aimed at determining how the CANTAB variables relate to abuse and neglect. The abuse and neglect variables (Abuse: emotional, physical, and/or sexual abuse and Neglect: emotional or physical neglect) were defined as the predictor variables and the cognitive dependent variables, presented in Table 4 below, were the criterion variables. Table 2 presents the demographic characteristics of the two samples, which did not differ statistically on age, education or premorbid intellectual functioning as measured by the WRAT-3. Performance on CANTAB subtests by participants with and without ELS is presented in Table 3. The second analysis aimed at determining how the CANTAB variables related to the different subtypes of abuse or neglect, namely, emotional, physical, or sexual. This analysis involved the same set of dependent variables as the first analysis, and the criterion variables were separated into the specific subtypes of abuse and neglect (i.e., emotional, physical, sexual) and these results are presented in Table 5.
Table 4.
Canonical loadings for dimensions labeled according to the type of ELS
| Dimensions | ||
|---|---|---|
| Abuse (.65) | Neglect (.18) | |
| Dependent Variables | ||
| DMS Total Correct | −0.17 | −0.05 |
| IED Stages Completed | −0.16 | 0.00 |
| IED Total Errors | 0.07 | −0.14 |
| SOC Problem Solved in Minimal Moves | 0.25 | −0.15 |
| SWM Total Errors | 0.10 | −0.14 |
| AGN Total Commissions for Positive Stimuli | −0.07 | 0.28 |
| AGN Total Omissions for Negative Stimuli | −0.07 | −0.21 |
| AGN Total Omissions for Neutral Stimuli | 0.01 | 0.13 |
Note. These loadings indicate which dependent variables have a significant influence on which canonical dimension, with significant influence defined as a loading with absolute value of 0.10 or greater. Canonical dimensions can be thought of as latent variables. For example, SOC Problem Solved in Minimal Moves has a significant influence on the dimensions or latent variables corresponding to Abuse and Neglect, with a larger influence on Abuse than Neglect. Its influence on Abuse is in a different direction relative to its influence on Neglect, as indicated by the signs of its loadings.
Table 2.
Sample Characteristics
| ELS Group | Control Group | |
|---|---|---|
| N (%) | N (%) | |
| Female | 27(45%) | (7) 20% |
| Emotional Abuse | 43 (72%) | |
| Physical Abuse | 38 (63%) | |
| Sexual Abuse | 34 (56%) | |
| Emotional Neglect | 34 (56%) | |
| Physical Neglect | 29 (17%) | |
| Current PTSD | 6 (9.3%) | |
| Current MDD | 21(35%) | |
| Mean(SD) | Mean(SD) | |
| Age | 30.20(7.38) | 29.15(7.88) |
| HAMD | 13(11) | 6.4(8.8) |
| WRAT-3 | 50.72(3.87) | 51.42(3.8) |
Table 3.
CANTAB performance data
| ELS | No ELS | |
|---|---|---|
| DMS Total Correct | ||
| N | 60 | 33 |
| Mean | 35.12 (4.98) | 36.97 (2.69) |
| Median (Range) | 36.00 (22.00) | 38.00 (10.00) |
|
| ||
| IED Stages Completed | ||
| N | 60 | 33 |
| Mean | 8.33 (0.98) | 8.69 (0.74) |
| Median | 9.00 (4.00) | 9.00 (2.00) |
|
| ||
| IED Total Errors | ||
| N | 60 | 33 |
| Mean | 23.59 (13.47) | 17.40 (10.55) |
| Median | 20.00 (55.00) | 13.00 (36.00) |
|
| ||
| RVP Total Hits | ||
| N | 60 | 33 |
| Mean | 19.05 (4.53) | 19.91 (4.86) |
| Median | 19.00 (18.00) | 21.00 (20.00) |
|
| ||
| SOC Problems Solved Minimal Moves | ||
| N | 60 | 33 |
| Mean | 8.66 (2.02) | 10.03 (1.98) |
| Median | 9.00 (9.00) | 11.00 (8.00) |
|
| ||
| SWM Total Errors | ||
| N | 60 | 33 |
| Mean | 26.69 (18.07) | 15.57 (16.12) |
| Median | 36.00 (73.00) | 11.00 (66.00) |
|
| ||
| AGN Omissions Positive | ||
| N | 60 | 33 |
| Mean | 5.66 (4.78) | 4.58 (2.68) |
| Median | 4.00 (25.00) | 4.00 (11.00) |
|
| ||
| AGN Commissions Positive | ||
| N | 60 | 33 |
| Mean | 8.49 (6.19) | 5.24 (4.19) |
| Median | 7.00 (31.00) | 4.00 (21.00) |
|
| ||
| AGN Omissions Negative | ||
| N | 60 | 33 |
| Mean | 3.88 (3.84) | 3.67 (3.37) |
| Median | 3.00 (18.00) | 3.00 (13.00) |
|
| ||
| AGN Total Omissions Neutral | ||
| N | 60 | 33 |
| Mean | 11.18 (8.95) | 6.58 (4.57) |
| Median | 7.00 (34.00) | 5.00 (16.00) |
Table 5.
Canonical loadings for dimensions labeled according to the type of abuse/neglect
| Dimensions | |||
|---|---|---|---|
| Emotional Abuse/Neglect (.67) | Sexual Abuse (−.64) | Physical Abuse/Neglect (−.37) | |
| Dependent Variables | |||
| DMS Total Correct | −0.15 | 0.05 | 0.05 |
| IED Stages Completed | −0.09 | 0.16 | 0.17 |
| RVP Total Hits | −0.05 | 0.05 | −0.14 |
| SOC Problem Solved in Minimum Moves | 0.26 | −0.10 | 0.15 |
| Spatial Working Memory Total Errors | 0.06 | −0.12 | 0.05 |
| AGN Total Omissions for Positive Stimuli | 0.03 | −0.02 | 0.10 |
| AGN Total Commissions for Positive Stimuli | −0.14 | −0.02 | 0.00 |
| AGN Total Omissions for Negative Stimuli | −0.05 | 0.07 | −0.11 |
Note. These loadings indicate which dependent variables have a significant influence on which canonical dimension, with significant influence defined as a loading with absolute value of 0.10 or greater. Canonical dimensions can be thought of as latent variables. For example, SOC Problem Solved in Minimal Moves has a significant influence on the dimensions or latent variables corresponding to all three types of abuse or neglect, with the largest influence on Emotional Abuse/Neglect. Its influence on Emotional or Physical Abuse/Neglect is in a different direction relative to its influence on Sexual Abuse, as indicated by the signs of its loadings.
Results
In order to examine demographic and symptom influences on cognitive performance, in addition to the effects of ELS, we performed regression analyses with each of the ten CANTAB variables as the dependent variables. In none of the regressions was the effect of depression, measured by the HAM-D-24 significant (all t<1.64, all p<.10). For age, there was only one variable in which older patients performed more poorly, RVIP total hits, t(88)= −3.50, p<.001. This association exceeds Bonferroni corrected (.05/10=.005) criteria for statistical significance. However, because the effects of age and depression were quite limited, we did not adjust the CANTAB variables for age or depression severity in the Canonical correlation analyses.
Abuse and Neglect
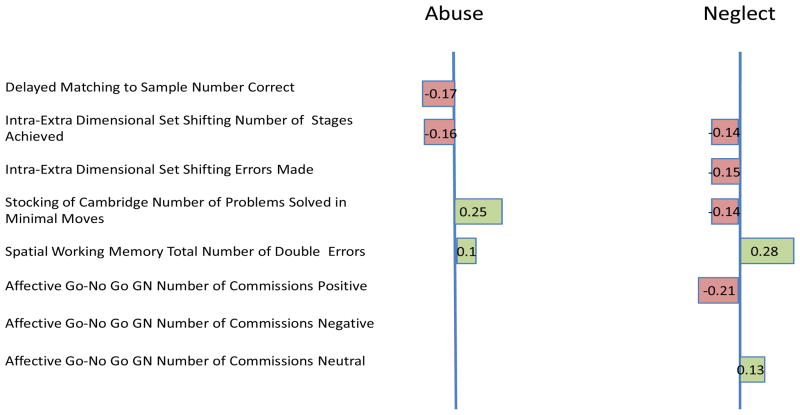
Table 4 presents the results of the nonlinear correlations. In the first analysis we specifically aimed to identify those CANTAB variables which most strongly related to either abuse or neglect. Two canonical dimensions (also referred to as roots or variates) based on CANTAB performance were found with eigenvalues of 0.243 and 0.134, respectively: The first appears to best relate to abuse (physical, emotional, sexual). The first canonical dimension was most strongly related to performance on Stockings of Cambridge (.25), Delayed Matching to Sample (−.17) and Intra-Extra dimensional set shift (−.16). The second dimension better relates to neglect (physical, emotional) and is most strongly influenced by commission (.27) and omission (−.20) errors on the Affective Go/No-Go with positive stimuli.
Emotional, Physical and Sexual Forms of ELS
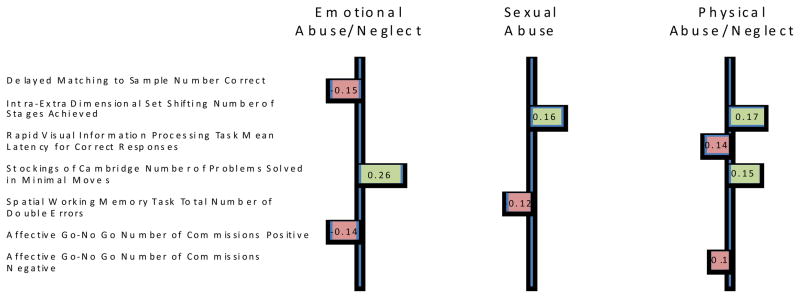
The analysis of subtypes of abuse and neglects identified three canonical dimensions and Table 5 presents the variable loadings for the three dimensions across both sets of variables. The first canonical dimension, with eigenvalue 0.250, best characterizes Emotional forms of ELS and is most strongly influenced by performance on the SOC (problems solved in minimal moves .26), DMS (total correct, −.15) and AGN task commission errors to positive stimuli (−.14). The second dimension, with eigenvalue 0.224, best characterizes sexual abuse and is also most strongly influenced by the Intra-Extra Dimensional Set Shift task (stages completed, .16) and Spatial Working Memory task (total errors, −.12). The third dimension, with eigenvalue 0.215 best characterizes physical forms of ELS and is most strongly influenced by IED (stages completed, .17), SOC (problem solved minimal moves, .15), and Rapid Visual Processing (total hits, −.14). Importantly due to overlapping group membership, both Emotional and Physical forms of ELS are strongly correlated with Dimension 1.
Discussion
The results clearly indicate that a history of ELS is associated with altered neurocognitive functioning. Using cognitive test performance, it was possible to discriminate patients with ELS from healthy controls. Two dimensions emerged from the analyses. Specifically, the first dimension included visual memory, executive functioning, and spatial working memory. This dimension was strongly associated with both abuse and neglect with abuse exerting a slightly stronger effect. The second dimension was additionally associated with emotional processing/inhibition; it was not significantly correlated with visual memory. Interestingly, the second dimension was most strongly associated with neglect. Due to overlapping group membership, these results do not suggest that abused children did not suffer from emotional processing deficits, but rather that there is a relatively stronger association of emotional processing impairments with neglect than abuse.
The second nonlinear canonical correlations were designed to examine differences between emotional, physical and sexual components of ELS. Three canonical dimensions emerged. The first dimension best characterized emotional forms of ELS (abuse/neglect) and was composed of visual memory, executive functioning, and emotional processing deficits. The second dimension best characterized sexual abuse and was most associated with executive functioning and spatial working memory deficits. The third dimension best characterized the physical forms of ELS (abuse/neglect) and was most strongly influenced by executive functioning, processing speed and emotional processing deficits. Thus, sexual abuse appears to be associated with an additional visual working memory deficit that was not associated with either physical or emotional forms of ELS. Taken together, relative deficits in visual memory, executive functioning and emotional processing distinguish individuals with a history of ELS from non-traumatized controls. Emotional processing and processing speed deficits appeared to be more pronounced in patients with a history of neglect. Although significantly associated with the root, the loadings were modest (<.2). Relatively more diverse executive functioning deficits, including deficits in spatial working memory, were found in patients with a history of sexual abuse. In the second analyses only emotional processing produced large (>.2) and significant root loadings on the first root (emotional abuse/neglect), with other significant associations being more modest (>.1).
Our finding that abuse (emotional and/or physical) and emotional (neglect and/or abuse) resulted in visual memory deficits may be at least partly explained by HPA axis alterations associated with ELS. CRF, released from the hypothalamus and transported to the anterior pituitary, stimulates the secretion of adrenocorticotropin which in turn stimulates glucocorticoid secretion from the adrenal cortex. This process is regulated by the circulating glucocorticoids that target the pituitary as well as prefrontal cortex and other CNS areas (Wingenfeld et al., 2011). Glucocorticoid receptors (GR) are particularly dense within the hippocampus, the brain region most essential to visual memory (de Kloet et al., 2005). Past research has indicated that glucocorticoid alterations result in disruptions in long-term potentiation and memory (Pavlides et al., 1996). Visual memory is a cognitive process dependent on long-term potentiation (and more similar in nature to long-term potentiation, as measured in animals, than other cognitive impairments we measured) and is supported by the hippocampus, which may explain why visual memory deficits develop and persist in individuals exposed to ELS.
However, GRs are also prominent in the prefrontal cortex. In fact, recent findings indicate GR are found in higher density in the prefrontal cortex than in the hippocampus (Grossman et al., 2006). This may explain why executive functioning, a cognitive process substantially supported by the prefrontal cortex, was found to be impaired across all subtypes of ELS. More extensive impairments with respect to working memory and other executive functions such as planning and processing speed were observed in patients with a history of ELS relative to controls. Greater GR density in the prefrontal cortex potentially may render this region more susceptible to the negative impact of stress. Further, the influence of glucocorticoids in this region has been found to result in part from dopaminergic and serotonergic alterations (Grossman et al., 2006). Given, that GRs act upon the frontal lobes via modulation of dopaminergic and serotonergic function, it is not surprising that a large proportion of individuals with a history of ELS go on to develop mood and/or anxiety disorders. These findings also provide a common underpinning for shared cognitive dysfunctions in mood and anxiety disordered patients. A stable and healthy mental state results, in part, from congruence and reasonably predictable interactions between experience and emotions, whereby pleasurable experiences and thoughts lead to positive emotions and painful ones trigger negative emotions (Post, 2000). Severe disruptions in these fundamental relationships likely occur when ELS produces stress during critical developmental time periods. This, in turn, produces lasting changes in stress reactivity and to the limbic and paralimbic regions of the brain resulting not only in psychiatric syndromes, but associated cognitive dysfunction.
There is ample evidence to support a strong relationship between depression and stressful life events, such as ELS (Kendler et al., 2004; Gatt et al., 2009). This relationship is believed to be moderated by genetic vulnerability (Gatt, et al., 2009). Specifically, brain-derived neurotrophic factor (BDNF) and specifically, polymorphisms in the BDNF Met alleles have also been found to increase the risk for depression in children exposed to abuse. Gatt et al. (Gatt et al., 2009) examined the interactions between BDNF Val66Met polymorphisms and early life stress and their ability to predict brain and/or arousal pathways to syndromal depression and anxiety. Their findings indicated that ELS exposure in conjunction with Met carrier status resulted in reduced grey matter volume (hippocampus and prefrontal cortex) as well as higher depression rates, which were in turn associated with poorer working memory. Although, genetic and brain imaging variables were not explicitly examined in the present study, our finding that ELS exposure was associated with working memory deficits is consistent with those described by Gatt et al. (Gatt et al., 2009). Thus, it is highly likely that genetic variables moderate the relationships between ELS and adult cognition observed in the present study, not only through HPA axis activity and reactivity, but also through CNS alterations in regions essential to these cognitive processes.
Limitations
Our study has several limitations. Although, the inclusion of both men and women may increase the generalizability of our findings, our sample included significantly more women than men. Further, endocrinological differences between men and women may mediate the relationship between ELS and adult cognition, but there is no evidence from our group to support that assertion (Heim & Nemeroff, 2001) at least as regards the HPA axis. Due to our current sample size limitations, we were unable to explicitly measure or control for any such differences according to gender. Another important limitation of the present study was the lack of an additional group of individuals exposed to ELS that did not develop subsequent depression or PTSD. Similarly, we were further limited by our lack of an MDD group without ELS. We were therefore unable to separately analyze the impact of mood disorders on cognition. Although, this was intended in the original design of the study we were unsuccessful in recruiting an adequate number of ELS exposed individuals who did not have a history of comorbid mood disorder with 73% of our ELS group with a past personal history of MDD, and 35% fulfilling criteria for MDD. Additionally, based on our limited sample size, it is possible that negative findings with respect to some of the trauma subtypes and neurocognitive domains measured may represent type II error. Larger studies with proportionate representation of men and women and individuals with ELS exposure but without current or past Axis I disorder are needed. Finally, it is possible the cognitive deficits observed in the present study did not result from ELS exposure but instead from preexisting differences in cognitive reserve that in turn render individuals more vulnerable to an Axis I disorder such as PTSD (Twamley et al., 2009).
Although, deficits observed in ELS groups on the CANTAB measures in the present study may reflect subtle deficits, the lack of relevant published norms on the CANTAB prevents further interpretation of these results. Future studies, would benefit from the inclusion of functional capacity measures in addition to standardized neuropsychological measures with appropriate published normative data in order to characterize the real-life impairment that may result from ELS. Our sample included relatively young individuals (mean age was approximately 30 years old); however, age may further exacerbate deficits that results from ELS. Further research with older participants may help to clarify the impact of age on our data. Our data suggested that ELS likely results in chronic, enduring cognitive deficits that may bestow additional vulnerability to subsequent cognitive dysfunction and mood disorder.
Conclusion
In summary, this is the first study to document altered neurocognitive functioning in patients with ELS and depression. Based on these preliminary findings, future studies of PTSD, depression and cognition should assess and control for the impact of ELS. Finally, in light of the potentially lasting impacts of ELS, early assessment and intervention efforts are needed to maximize the cognitive potential and mental health of children with a history of abuse.
Figure 1.
Explanatory variables with significant (nonlinear) associations with abuse and/or neglect. On the left are significant loadings on the dimension for Abuse, and on the right are significant loadings on the dimension for Neglect. For example, DMS Tot Correct has a significant loading on the dimension for Abuse, indicating that this measure has a significant association with Abuse status. DMS Tot Correct does not have a significant loading on the dimension for Neglect, indicating that this measure is not significantly associated with Neglect status. Due to the nonlinear nature of this analysis the sign of a given loading (negative or positive) does NOT indicate a positive or negative influence on Abuse or Neglect. The sign simply indicates that variables with positive loadings influence these dimensions in different ways relative to variables with negative loadings.
Figure 2.
Explanatory variables with significant (nonlinear) associations with different types of abuse and/or neglect. On the left are significant loadings on the dimension for Emotional, in the center are significant loadings on the dimension for Sexual, and on the right are significant loadings on the dimension for Physical. For example, DMS Tot Correct has a significant loading on the dimension for Emotional, indicating that this measure has a significant association with Emotional Abuse/Neglect. DMS Tot Correct does not have a significant loading on the dimensions for Sexual Abuse or Physical Abuse/Neglect, indicating that this measure is not significantly associated with either Sexual Abuse or Physical Abuse/Neglect. Due to the nonlinear nature of this analysis the sign of a given loading (negative or positive) does NOT indicate a positive or negative influence on the different types of abuse and/or neglect. The sign simply indicates that variables with positive loadings influence these dimensions in different ways relative to variables with negative loadings.
Acknowledgments
Funding Body
Support for this research was provided by a National Institute of Mental Health grant, MH-58922, which was awarded to Dr. Nemeroff.
Footnotes
Author Disclosure
Ethical Consideration
All participants were deemed to have the capacity to provide informed consent by study personnel. After a description of the study to participants, written informed consent was obtained. The study was approved by the Institutional Review Board of Emory University School of Medicine.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends in Neurosciences. 2008;31(4):183–91. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Bernstein D, Fink L. A retrospective self-report questionnaire and manual. San Antonio: The Psychological Corporation; 1998. Childhood trauma questionnaire. [Google Scholar]
- Castaneda AE, Annamari TH, Marttunen M, Suvisaari, Lonnqvist J. A review on cogntiive impaitments in depressive and anxiety disorders with a focus on young adults. Journal of Affective Disorders. 2008;106:1–27. doi: 10.1016/j.jad.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Andrews MW, Rosenblum LA, Owens MJ, Friedman S, Gorman JM, et al. Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: Implications for the pathophysiology of mood and anxiety disorders. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(4):1619–23. doi: 10.1073/pnas.93.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: Perspectives from affective neuroscience. Annual Review of Psychology. 2002;53(1):545–74. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Hooper SR, Spratt EG, Woolley DP. Neuropsychological findings in childhood neglect and their relationships to pediatric PTSD. Journal of the International Neuropsychological Society. 2009;15(06):868–78. doi: 10.1017/S1355617709990464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nature Reviews Neuroscience. 2005;6(6):463–75. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- de Leeuw J, Mair P. Gifi methods for optimal scaling in R: The package homals. Journal of Statistical Software. 2009;31(4):1–21. [Google Scholar]
- Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. The neural basis of mood-congruent processing biases in depression. Archives of General Psychiatry. 2002;59(7):597–604. doi: 10.1001/archpsyc.59.7.597. [DOI] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Rundle MM, Mintun MA, Snyder AZ, Cohen JD, et al. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biological Psychiatry. 2008;63(4):377–84. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV—clinical version (SCID-CV) (User’s Guide and Interview) Washington, D.C: American Psychiatric Press; 1997. [Google Scholar]
- Gatt JM, Nemeroff CB, Dobson-Stone C, Paul RH, Bryant RA, Schofield PR, et al. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Molecular Psychiatry. 2009;14(7):681–95. doi: 10.1038/mp.2008.143. [DOI] [PubMed] [Google Scholar]
- Gifi A. Nonlinear multivariate analysis. Chichester: John Wiley & Sons Ltd; 1990. [Google Scholar]
- Golier J, Yehuda R. Neuropsychological processes in post-traumatic stress disorder. Psychiatric Clinics of North America. 2002;25(2):295–315. doi: 10.1016/s0193-953x(01)00004-1. [DOI] [PubMed] [Google Scholar]
- Grossman R, Yehuda R, Golier J, McEwen B, Harvey P, Maria NS. Cognitive effects of intravenous hydrocortisone in subjects with PTSD and healthy control subjects. Annals of the New York Academy of Sciences. 2006;1071(1):410–21. doi: 10.1196/annals.1364.032. [DOI] [PubMed] [Google Scholar]
- Hasselbalch BJ, Knorr U, Kessing LV. Cognitive impairment in the remitted state of unipolar depressive disrder:a systematic review. Journal of Affective Disorders. 2010;134(1–3):20–31. doi: 10.1016/j.jad.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. Neurobiology of posttraumatic stress disorder. CNS Spectrums. 2009;14(1 Suppl 1):13–24. [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biological Psychiatry. 2001;49(12):1023–39. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. Journal of the American Medical Association. 2000;284(5):592–97. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn J, Prescott CA. The Interrelationship of Neuroticism, Sex, and Stressful Life Events in the Prediction of Episodes of Major Depression. American Journal of Psychiatry. 2004;161(4):631–36. doi: 10.1176/appi.ajp.161.4.631. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: A critical review. Progress in Brain Research. 2008;167:151–69. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- Majer M, Nater U, Lin J-M, Capuron L, Reeves W. Association of childhood trauma with cognitive function in healthy adults: A pilot study. BioMedCentral Neurology. 2010;10(1):61. doi: 10.1186/1471-2377-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock SM, Husain MM, Greer TL, Cullum CM. Association between depression severity and neurocognitive function in major depressive disorder: A review and synthesis. Neuropsychology. 2010;24(1):9–34. doi: 10.1037/a0017336. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Butters RD, Mulsant BH, Pollock BG, Zmuda MD, Houck PR, et al. Decreased working memory and processing speed mediate cognitive impairment in geriatric depression. Psycholological Medicine. 2000;30:679–91. doi: 10.1017/s0033291799001968. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. Neurobiological consequences of childhood trauma. Journal of Clinical Psychiatry. 2004;65:18–28. [PubMed] [Google Scholar]
- Pavlides C, Ogawa S, Kimura A, McEwen BS. Role of adrenal steroid mineralocorticoid and glucocorticoid receptors in long-term potentiation in the CA1 field of hippocampal slices. Brain Res. 1996;738(2):229–35. doi: 10.1016/s0006-8993(96)00776-7. [DOI] [PubMed] [Google Scholar]
- Pluck G, Kwang-Hyuk L, Rajan D, Macleod DC, Spence SA, Parks R. Neurobehavioural and cognitive function is linked to childhood trauma in homeless adults. British Journal of Clinical Psychology. 2011;51(1):33–45. doi: 10.1348/014466510X490253. [DOI] [PubMed] [Google Scholar]
- Post R. Neural substrates of psychiatric syndromes. In: Mesulum M-M, editor. Principles of Behavioral and Cognitive Neurology. 2. New York: Oxford University Press; 2000. [Google Scholar]
- Porter RJ, Bourke C, Gallagher P. Neuropsychological impairment in major depression: Its nature, origin and clinical significance. Australian and New Zealand Journal of Psychiatry. 2007;41(2):115–28. doi: 10.1080/00048670601109881. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Owen AM. Computerized assessment in neuropsychiatry using CANTAB. Journal of the Royal Society of Medine. 1992:399–402. [PMC free article] [PubMed] [Google Scholar]
- Twamley EW, Allard CB, Thorp SR, Norman SB, Hami Cissell S, Hughes Beradi K, et al. Cognitive impairment and functioning in PTSD related to intimate partner violence. Journal of International Neuropsychology Society. 2009;15(06):879–87. doi: 10.1017/S135561770999049X. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS. WRAT-3 Wide Range Achievement Test: Administration Manual. Wilmington, DE: Psychological Corporation; 1993. [Google Scholar]
- Williams JBW. A Structured Interview Guide for the Hamilton Depression Rating Scale. Archives of General Psychiatry. 1988;45(8):742–47. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- Wingo A, Harvey P, Baldessarini R. Neurocognitive impairment in bipolar disorder patients: functional implications. Bipolar Disorder. 2009;11(2):113–25. doi: 10.1111/j.1399-5618.2009.00665.x. [DOI] [PubMed] [Google Scholar]
- Wingenfeld K, Wolf S, Krieg J-C, Lautenbacher S. Working memory performance and cognitive flexibility after dexamethasone or hydrocortisone administration in healthy volunteers. Psychopharmacology. 2011:1–7. doi: 10.1007/s00213-011-2286-4. [DOI] [PubMed] [Google Scholar]
- Woodward SH, Kaloupek DG, Grande LJ, Stegman WK, Kutter CJ, Leskin L, et al. Hippocampal volume and declarative memory function in combat-related PTSD. Journal of the International Neuropsychological Society. 2009;15(06):830–39. doi: 10.1017/S1355617709990476. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Duke LM, Brailey K, Constans JI, Allain AN, Sutker PB. Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology. 2002;16(1):5–14. doi: 10.1037//0894-4105.16.1.5. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Verfaellie M. Symposium—Introduction: Posttraumatic stress disorder: A neurocognitive perspective. Journal of the International Neuropsychological Society. 2009;15(6):826–29. doi: 10.1017/S1355617709990683. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Keefe R, Harvey P, Levengood R, Gerber D, Geni J, et al. Learning and memory in combat veterans with posttraumatic stress disorder. American Journal of Psychiatry. 1995;152(1):137–39. doi: 10.1176/ajp.152.1.137. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Golier JA, Tischler L, Harvey PD, Newmark R, Yang RK, et al. Hippocampal volume in aging combat veterans with and without post-traumatic stress disorder: Relation to risk and resilience factors. Journal of Psychiatry Research. 2007;41(5):435–45. doi: 10.1016/j.jpsychires.2005.12.002. [DOI] [PubMed] [Google Scholar]