Abstract
Prostate cancer (PCa) metastases and hematopoietic stem cells (HSCs) frequently home to the bone marrow where they compete to occupy the same HSC niche. We have also shown that under conditions of hematopoietic stress, HSCs secrete the bone morphogenetic proteins (BMPs)-2 and BMP-6 that drives osteoblastic differentiation from mesenchymal precursors. Because it is not known, we examined if metastatic PCa cells can alter regulation of normal bone formation by HSCs and hematopoietic progenitor cells (HPCs). HSC/HPCs isolated from mice bearing non-metastatic and metastatic tumor cells were isolated and their ability to influence osteoblastic and osteoclastic differentiation was evaluated. When the animals were inoculated with the LNCaP C4-2B cell line which produces mixed osteoblastic and osteolytic lesions in bone, HPCs but not HSCs were able to induced stromal cells to differentiate down an osteoblastic phenotype. Part of the mechanism responsible for this activity was the production of BMP-2. On the other hand, when the animals were implanted with PC3 cells that exhibits predominantly osteolytic lesions in bone, HSCs derived from these animals were capable of directly differentiating into tartrate-resistant acid phosphatase (TRAP) positive osteoclasts through an interleukin-6 (IL-6) mediated pathway. These studies for the first time identify HSC/HPCs as novel targets for future therapy involved in the bone abnormalities of PCa.
Keywords: Hematopoietic stem cells, osteoblast, osteoclast, differentiation, disseminated tumor cells
Introduction
Prostate cancer (PCa) is the second leading cause of cancer related death in men. Bone is the preferred metastatic site of advanced PCa (1–3). PCa cells that reach the bone marrow can stimulate osteoblasts or osteoclasts leading to either the production of poorly woven bone (osteoblastic lesions) or bone loss (osteolytic lesions) (4, 5). PCa cells secrete many factors that directly or indirectly alter osteoblast and osteoclast activities that potentially may result in the skeletal phenotype of PCa observed clinically (6, 7). Yet the precise mechanisms that lead to whether a lesion will be predominantly osteoblastic or osteolytic have not clearly been defined. Therefore understanding the mechanisms that control the differentiation and activity of osteoblasts/osteoclasts is of central importance in the area of bone metastasis.
Work by our group and others have shown that the metastatic process is functionally similar to the homing behavior of hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs) to the bone marrow (8, 9). A combination of markers c-kit and sca-1 has been usually used for identifying murine HSCs (Lin− CD48− CD41− Sca-1+ cKit+ (LSK HSCs) (10) Recently discovery of SLAM family protein (CD150) as a selection marker for HSCs provide a new strategy for HSC enrichment (CD150+ Lin− CD48− CD41− Sca-1+ cKit+ (SLAM HSCs) (10, 11). It has been suggested that SLAM HSCs are more primitive HSCs compared to LSK HSCs (10, 11). SLAM and LSK HSCs reside in specialized compartments within the bone marrow termed the “stem cell niche” which control HSC quiescence and self-renewal (12, 13). More recently, we have shown that metastatic cells shed from a primary PCa tumor directly target the HSC niche and compete with HSCs for occupancy of the niche (14). These disseminated tumor cells (DTCs) may remain in a dormant/quiescent state for extended periods in the niche (15–17). In addition, we have shown that HSCs can regulate mesenchymal fate by inducing osteoblastic precursors to commit to the osteoblastic lineage (18). Once in the niche, whether disseminated PCa disrupt the ability of HSCs to regulate normal bone formation is not known.
To determine if disseminated PCa disrupts the ability of HSCs and HPCs to regulate the normal bone phenotype, HSCs/HPCs from the bone marrow of tumor bearing animals were co-cultured with mixed bone marrow stromal cells (BMSCs). Our data suggest that HPCs derived from animals implanted with PCa cell lines that form osteoblastic lesions in bone stimulated osteoblastic differentiation of BMSCs in vitro through the BMP-2 signaling. Interestingly, HSCs derived from animals implanted with PCa cell lines that produce a predominantly osteolytic lesion in bone differentiated into TRAP positive osteoclastic cells through IL-6 signaling. These data demonstrated that the disseminated PCa cells indirectly regulate the bone phenotype at metastatic site by directing HSC/HPCs.
Materials and Methods
Cell culture
The PC3 (CRL-1435) human PCa cell line and RAW 264.7 mouse monocytic cell line were obtained from the American Type Culture Collection (Rockville, MD). The metastatic human PCa subline LNCaP C4-2B was originally isolated from a lymph node of a patient with disseminated bony and lymph node involvement (19). Normal human non-metastatic prostate epithelial control cell lines (Non Met CNTR)) were obtained from patients undergoing prostatectomy in accordance with the Investigation Review Board of University of Michigan. The tissue was collected from a distal location from the tumor (within the prostate). This cell line is morphologically and pathologically distinct from the tumor. PCa cell lines and control cells were cultured in RPMI 1640 (Invitrogen, Grand Island, NY) and DMEM (Invitrogen), respectively. RAW 264.7 cells were cultured in low glucose DMEM (Invitrogen). All cultures were supplemented with 10% (v/v) fetal bovine serum (FBS, Invitrogen) and 1% (v/v) penicillin-streptomycin (Invitrogen) and maintained at 37°C, 5% CO2, and 100% humidity.
Tumor implantation
All experimental procedures were approved by the University of Michigan Committee for the Use and Care of Animals (UCUCA). Male 5- to 7-week-old SCID mice (CB.17. SCID; Taconic, Germantown, NY) were implanted s.c. with 2 × 105 cells (PCa cell line or control cell) within sterile collagen scaffolds (3 × 3× 3 mm3; Gel foam; Pharmacia and Upjohn) in the mid-dorsal region of each mouse (n=5). Animals implanted with scaffolds alone during surgery were kept as negative controls (Surgical control). After 3 weeks, animals were sacrificed and bone marrow cells from the mice were isolated.
Isolation of HSCs
Tumor bearing animals were euthanized and the bone marrow cells were flushed from the femurs and tibias with Hanks buffered salt solution (HBSS, Invitrogen) supplemented with 2% (v/v) FBS. HSCs were isolated as previously described (14, 18). The bone marrow cells were incubated first with a biotinylated anti-Lin (CD5, CD45R [B220], CD11b, Gr-1 [Ly- 6G/C], and Ter-119) antibody cocktail (Miltenyi Biotec, Auburn, CA) for 10 minutes at 4°C, and then stained with an antibody cocktail of allophycocyanin-conjugated anti–Sca-1 (clone D7; eBioscience, San Diego, CA), PE/Cy7-conjugated anti–c-Kit (clone 2B8; BioLegend), PE-conjugated anti-CD150 (clone TC15-12F12.2; BioLegend, San Diego, CA), FITC-conjugated anti-CD41 (clone MWReg30; BD Biosciences, Bedford, MA), FITC-conjugated anti-CD48 (clone BCM-1; BD Biosciences) and FITC-conjugated anti-biotin antibodies (Miltenyi Biotec) for another 20 minutes at 4°C. HSCs were sorted on a BD FACS Aria I flow cytometer by gating on HSCs (i.e., CD150+ Lin− CD48− CD41− Sca-1+ cKit+ (SLAM HSCs) or Lin−CD48− CD41− Sca-1+ cKit+ (LSK HSCs). Lin− CD48− CD41−Sca-1+ progenitor cells (HPCs) were isolated from mouse bone marrow after lineage depletion followed by magnetic cell sorting (Mouse Sca-1 selection Kit, EasySep, Stem cell Technologies Inc., Vancouver, BC). A representative FACS plot to confirm the specificity of antibodies and a typical FACS plot of the recovered cells are presented in supplemental online Fig. 1 and 2 (S1 and S2).
Bone marrow stromal cells (BMSCs)
Marrow obtained from the femur and tibia of C57BL6 (Charles River Laboratories, Wilmington, MA) animals were used to generate stromal cells. After flushing the marrow with α-MEM medium supplemented with 2% (v/v) FBS, cells were cultured in α-MEM containing 10% (v/v) FBS and 1% (v/v) penicillin-streptomycin. After 4 days, nonadherent cells were removed and fresh media were replaced. Once confluent, the cells were passaged 2–3 times with trypsin.
The collection of PCa conditioned medium
For preparing conditioned media, PCa cells were cultured in RPMI 1640 (Invitrogen) supplemented with 10% (v/v) FBS and 1% (v/v) penicillin-streptomycin under a humidified atmosphere of 5% CO2 at 37 °C to 90% confluency. Cells were then washed with PBS and cultured in serum free media for 24 h. The conditioned media was concentrated 10X using centrifugal filters (Ultracel-3K, Amicon Ultra, Millipore, Billerica, MA) and stored in −80°C until use. For standardization, total protein in the conditioned medium was measured by a Bradford microassay (Bio-Rad Laboratories, Hercules, CA).
Osteoblastic differentiation assays
HPCs (2×103 cells) or HSCs (200 cells) isolated from tumor-bearing mice were placed in the top chambers of 24-well Transwell™ plates (0.4 μM, polycarbonate, Corning Life Sciences, Lowell, MA). BMSCs were plated in the bottom chambers at a final density of ~ 2 × 104/cm2 in α-MEM containing 10% heat inactivated FBS, antibiotics, 10 mM β-glycerol phosphate and 10 μg/ml L-ascorbic acid (Sigma-Aldrich, St.Louis, MO). After 2 weeks, the cell matrix were fixed with 10% (v/v) normal buffered formalin and stained with 2% (v/v) Alizarin red (Sigma-Aldrich). Quantification of the staining density was analyzed using NIS Elements software (Nikon Instruments Inc, USA) as an index of mineralization. In some case, monoclonal anti-mouse Noggin antibodies or corresponding IgG (5ng/ml) were added every other day to the co-cultures (R&D Systems, Minneapolis, MN). After 2 weeks, osteoblastic differentiation from BMSCs was evaluated by real-time Reverse-Transcription–polymerase chain reaction (qRT-PCR).
Osteoclastic differentiation assays
Three types of differentiation assays were performed. First, mixed bone marrow mononuclear cells (BMMCs) were evaluated for their ability to differentiate into osteoclasts following treatment with HSCs derived from tumor-bearing animals in a Transwell assay. After 2 weeks, the cell matrix were fixed with 10% (v/v) normal buffered formalin and stained for TRAP (K-ASSAY, Kamiya Biomedical Company, Seattle, WA). Colonies (CFU-OC) of >50 cells were counted under a light microscope (10X). Secondly, the ability of test conditions to influence osteoclast differentiation from pre-osteoclasts was examined. Here, HSCs from tumor bearing animals were cultured in direct contact with murine RAW264.7 cells in the presence of RANKL for 4 days and stained for TRAP. In the final assay cells of hematopoietic origin recovered from tumor bearing animals (SLAM HSCs/LSK HSCs,) were cultured in the presence or absence of PCa-conditioned medium (standardized by total protein, 20 μg), RANKL (50 ng/ml, Peprotech Inc., Rocky Hill, NJ), M-CSF (50 ng/ml, Peprotech Inc.) or in combination. After nine days the cells were fixed in 10% formalin and stained for TRAP. In some cases, the cultures were treated with anti IL-6 mAb or corresponding IgG (1 μ/ml, BD BioScience).
RNA analysis and Real-Time Reverse-Transcription–Polymerase Chain Reaction
Total RNA was harvested from cells using RNeasy Mini or Micro Kit (Qiagen, Valencia, CA). First strand cDNA synthesis and real-time Reverse-Transcription–polymerase chain reaction (qRT-PCR) were performed following the manufacturer’s directions (Applied Biosystems, Foster City, CA) or using Message Booster cDNA synthesis kit when evaluating mRNA levels from isolated HSCs (Epicenter Biotechnologies, Madison, WI). TaqMan pre-developed assay reagents (FAM/MGB probe; Applied Biosystems) were used for detection of mouse BMP-2, BMP-6, IL-6, BSP, Dlx5, Msx2 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Universal mouse reference RNA (Stratagene, La Jolla, CA) was used to generate a relative standard curve. Real-time detection of the PCR products was performed using an ABI PRISM 7700 sequence detector (Applied Biosystems). Expression levels were calculated based on a standard curve, and normalized to GAPDH. Cycle numbers of analyzed genes are presented in supplemental Table 1.
Statistical analysis
Results were presented as mean ± standard error of the mean (SEM). Significance of the difference between two measurements was determined by unpaired Student’s t test. Statistical differences between the means for the different groups were evaluated with Instat 4.0 (GraphPAD software) using one-way analysis of variance (ANOVA). Values of p<0.01 were considered significant.
Results
Disseminated osteoblastic PCa cells target HPCs to drive osteoblast phenotype of BMSCs by modulating BMP-2 response
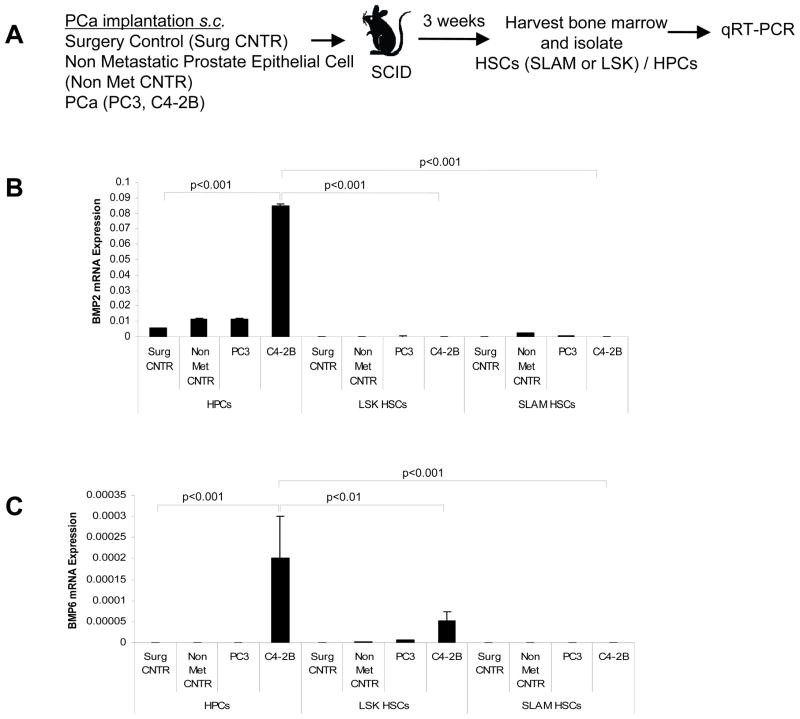
In previous work we demonstrated that under conditions of stress (20% blood volume loss and 5-Flurouracil), HSCs and HPCs activate produce BMP-2 and BMP-6 which is able to activate osteoblastic differentiation from mesenchymal stem cells (18). To determine whether disseminated PCa are also capable of inducing changes in the bone marrow microenvironment perhaps through HSC or HPC intermediaries, we used a micrometastasis model in which DTCs from primary tumors can be established in the marrow (20). Thereafter, HSCs and HPCs were isolated from the bone marrow of tumor-bearing mice and analyzed for the expression of BMP-2 and BMP-6 by qRT-PCR (Fig. 1A). BMP-2 and BMP-6 mRNA levels were significantly higher in the HPCs isolated from animals implanted with the C4-2B cell line compared to HPCs isolated from the control treated (surgical control and non metastatic control cells implanted) or PC3 implanted mice (Fig. 1B and 1C). However, BMP-2 mRNA expression was virtually unchanged under any conditions in which HSCs (SLAM or LSK) were examined (Fig. 1B and 1C).
Figure 1. Disseminated PCa alters BMP expression in HSC/HPCs.
(A) Experimental outline. HSCs (CD150+ Lin− CD48− CD41− Sca-1+ cKit+ (SLAM HSCs) or Lin− CD48− CD41− Sca-1+ cKit+ (LSK HSCs)/HPCs (Lin− CD48− CD41−Sca1+) from tumor bearing animals were isolated. Total RNA was extracted and subjected to qRT-PCR for BMP-2 and BMP-6. Relative expression of mRNA was calculated for (B) BMP-2 and (C) BMP-6 and normalized to GAPDH. Data represent mean ± SD performed in triplicate in 3 independent experiments. Level of significance (p<0.001 and p<0.01) were calculated compared to the surgical control treated group.
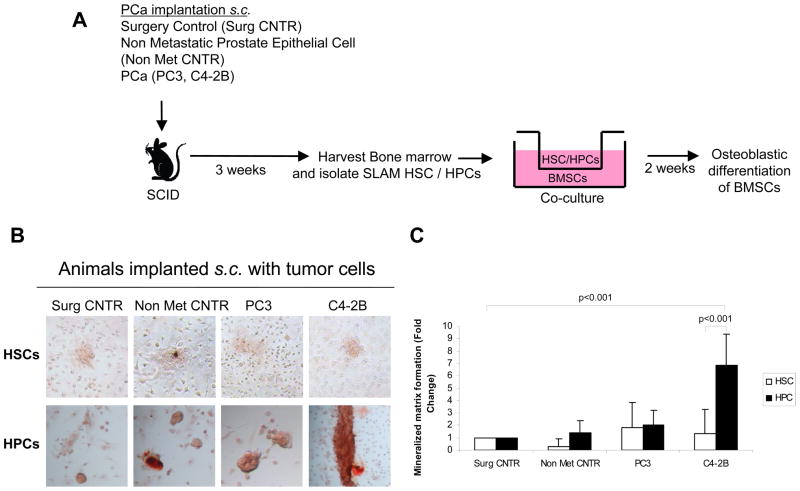
To validate that BMPs produced by HPCs can regulate mesenchymal fate, co-cultures were established by placing the HSC/HPCs in the top chamber and mixed BMSCs in the bottom chamber of a dual chamber plates under osteogenic conditions. Following a two-week culture period, the osteoblastic differentiation of BMSCs was analyzed (Fig. 2A). To determine the mineralization of BMSCs, the extracellular matrices were stained with alizarin red. HPCs obtained from animals implanted with C4-2B cells enhanced mineralized matrix formation compared to HPCs isolated from animals implanted with PC3 or controls, while HSCs did not enhance any mineralization (Fig. 2B and 2C). A control study was performed by co-culturing PC3 or C4-2B cells with BMSCs. PCa cells couldn’t induce mineralization when co-culture with BMSCs (Supplemental Fig. 3A). To further substantiate if BMPs derived from HPCs regulate BMSC fate, co-culture investigations were treated with neutralizing antibody to BMPs (Noggin). After two weeks, the osteoblastic phenotype was examined by qRT-PCR for the expression of osteoblast specific markers Dlx5, BSP and Msx2. As expected, HPCs derived from osteoblastic C4-2B tumor-bearing animals expressed enhanced levels of osteoblast specific markers compared to HPCs derived from controls or PC3 tumor-bearing animals (Fig. 2D,E, F). Interestingly, Noggin prevented the increase in the expression of osteoblastic markers when HPCs were co-cultured with BMSCs isolated from C4-2B tumor-bearing animals (Fig. 2D, E, F). These data suggest that PCa may target HPCs to induce osteoblastic differentiation of BMSCs.
Figure 2. HPCs from osteoblastic tumor bearing animals increase mineralization of BMSCs.
(A) HSC/HPCs were isolated from tumor bearing animals and co-cultured with BMSCs in osteogenic media. (B) Images of monolayer cultures incubated in osteogenic media for 2 weeks stained with Alizarin Red, showing substantial staining in the C4-2B (osteoblastic) HPC group compared to other tumor groups and compared to HSCs derived from PCa tumor bearing groups. (C) The amount of alizarin staining in the mineralized matrix was quantified using NIS Elements software and normalized to cell number. Fold change was calculated compared to surgical control treated group. (D, E, F) HPCs from osteoblastic tumor bearing animals regulate mesenchymal fate through BMPs. Coculture investigations were established by placing HPCs derived from tumor bearing animals in the top chamber of a dual culture plate and BMSCs in the bottom well in the presence of neutralizing antibody to BMPs or an isotype matched IgG control (5ng/ ml). Osteoblast phenotype was examined by RT-PCR for osteoblast specific markers, (D) BSP, (E) Dlx5 and (F) Msx2. Data represent mean ± SD fold change performed in triplicate in 2 independent cultures. The data indicate that HPCs from osteoblastic tumor bearing animals directed the formation of osteoblast differentiation and BMP neutralizing antibody significantly reduced the osteoblast differentiation from HPCs isolated from osteoblastic tumor bearing animals. (p<0.02, p<0.01 and p<0.001).
HSCs derived from PCa bearing animals may regulate osteoclastic differentiation from mixed marrow mononuclear cells but not from preosteoclasts
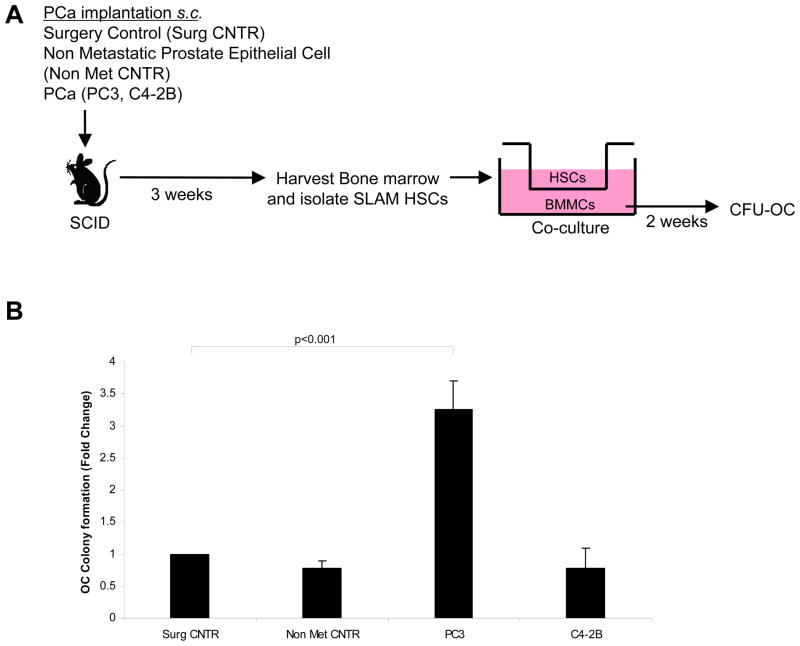
Thus far the results show that DTCs from osteoblastic tumor cell lines could stimulate BMP-2 production by HPCs (but not HSCs) and stimulate osteoblastic induction of BMSCs. Since osteoblastic and osteoclastic activities are often coupled, we next determined the ability of DTCs to influence osteoclastic activities. For these studies co-cultures were established by placing the recovered SLAM HSCs or HPCs in the top chamber of a dual culture well plate, with mixed marrow mononuclear cells in the bottom well. After 2 weeks the phenotype of the mixed marrow mononuclear cells were evaluated for the ability to generate osteoclast colonies (Fig. 3A). A significant increase in the number of progenitors with osteoclastic potential were detected in SLAM HSCs isolated from animals harboring PC3 tumors compared with SLAM HSCs isolated from animals implanted with C4-2B tumors (Fig. 3B). HPCs isolated from tumor bearing animals were however unable to influence osteoclast development of mixed marrow cells (Data not shown). A control study was performed by co-culturing PC3 or C4-2B cells with preosteoclastic RAW264.7 cells in the presence of RANKL. PCa cells could not induce TRAP positive cells when co-culture with RAW264.7 cells (Supplemental Fig. 3B). These data suggest that HSCs respond to the presence of different DTCs in a way that reflects the bone phenotype of the parental PCa cells implanted.
Figure 3. HSCs from osteolytic PCa implanted animals induce osteoclastic colony forming units from BMMCs.
(A) Experimental outline. Co-cultures were established by placing SLAM HSCs (CD150+ Lin− CD48− CD41− Sca-1+ cKit+) derived from animals bearing s.c. tumors or control animals in the top chamber of a dual culture well plate, and mixed mononuclear bone marrow cells in the bottom well. After 2 weeks the phenotype of the resulting colonies were stained for TRAP to visualize osteoclast colonies (CFU-OC). (B) Number of TRAP stained cells/culture. Significant increase in CFU-OCs was noted in the presence of HSCs derived from PC3 tumor-bearing animals vs. surgical control animals. Data represent mean ± SD fold change performed in triplicate in 3 independent cultures. Significance (p<0.001) were calculated compared to surgical control treated group.
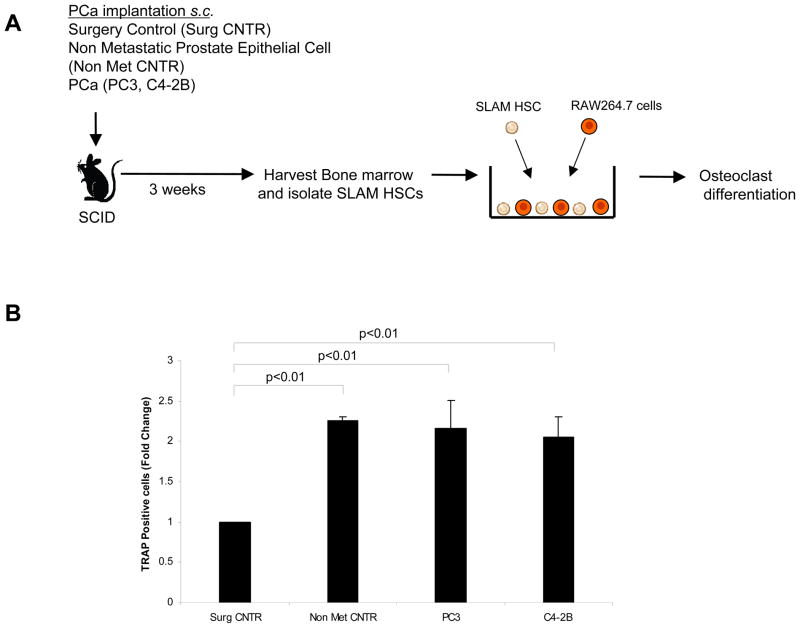
In order to determine whether SLAM HSCs isolated from tumor-bearing animals could stimulate preosteoclasts to differentiate into osteoclasts, direct cell-to-cell co-cultures were established between SLAM HSCs and the murine preosteoclastic RAW264.7 cells (Fig. 4A). SLAM HSCs isolated from non metastatic control, PC3 and C4-2B cell implanted animals but not from surgical control animals, stimulated multinucleation (>3 nuclei) of TRAP positive cells from RAW264.7 cells (Fig. 4B). There was however no differences in number of osteoclasts produced (Fig. 4B). These data suggest that HSCs may not be primed to regulate cells already committed to osteoclast lineages.
Figure 4. HSCs from PCa tumor bearing animals induce same extent of osteoclast differentiation from existing preosteoclasts.
(A) Experimental conditions. SLAM HSCs were isolated from tumor bearing animals and co-cultured with preosteoclastic RAW 246.7 cells. After the culture conditions, the cultures were stained with TRAP and evaluated for multinucleation. (B) Number of osteoclasts/culture. The data indicate that culturing HSCs derived from PCa implanted animals with preosteoclastic cells increased the multinucleated TRAP positive cells (>3nuclei) from RAW 264.7 cells compared to surgical control (p<0.01). Data represent mean ± SD performed in triplicate in 2 independent experiments. No significant differences in osteoclast formation from HSCs derived from osteoblastic vs. osteolytic tumors were obtained.
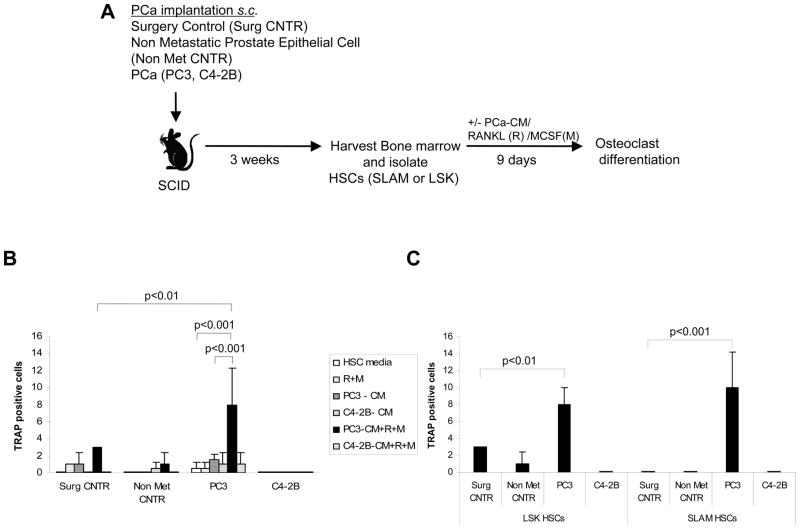
Disseminated osteolytic PCa cells directly differentiate HSCs into osteoclasts
The ability of HPCs to differentiate into osteoclastic cells has been reported in previous studies (21). Our finding that SLAM HSCs influence osteoclastic differentiation from mixed marrow populations was intriguing and prompted us to explore whether DTCs from PCa can induce osteoclastic differentiation directly from HSCs (LSK or SLAM). Therefore we first isolated LSK HSCs from animals bearing tumor cells and were treated in vitro with PCa conditioned medium for 9 days (Fig. 5A). Initial studies suggested that conditioned medium from PC3 or C4-2B cells alone was insufficient to drive osteoclast differentiation from LSK HSCs. Hence the osteoclastic differentiation factors RANKL and M-CSF were included in the studies (Fig. 5B). RANKL and M-CSF alone were not sufficient to generate osteoclasts from LSK HSCs under our culture conditions (Fig. 5B). However conditioned medium derived from PC3 cells in the presence of RANKL and M-CSF activated osteoclast formation directly from LSK HSCs that were isolated from PC3 tumor-bearing animals but not from controls or C4-2B implanted animals (Fig. 5B). To further dissect the stem cell population that could differentiate into osteoclasts in an environment with DTCs, bone marrow cells were isolated from tumor bearing animals by FACS by gating on Lin− CD48− CD41− Sca1+cKit+ (LSK HSCs) and CD150+ Lin− CD48− CD41− Sca-1+ cKit+ (SLAM HSCs) which constitute 0.01% and 0.001% of total bone marrow cells, respectively. Each of these populations was cultured in the presence of conditioned medium derived from PC3 cells as well as RANKL and M-CSF. The numbers of osteoclasts generated by the SLAM HSCs were same as those produced by LSK HSCs (Fig. 5C). These observations suggest that DTCs from PC3 implanted animals are able to instruct or prime HSCs to differentiate into TRAP positive osteoclasts.
Figure 5. HSCs from osteolytic PC3 bearing animals differentiated directly into osteoclasts.
(A) Experimental outline. HSCs (CD150+ Lin− CD48− CD41− Sca-1+ cKit+) (SLAM)and Lin− CD48− CD41−Sca-1+ cKit+ (LSK) were isolated from tumor bearing animals and differentiated into osteoclast in the presence or absence of PCa-conditioned medium (PC3-CM and C4-2B-CM), RANKL/M-CSF or in combination. (B) Number of TRAP+ cells after culture. LSK HSCs from osteolytic PC3 tumor bearing animals could direct the formation of osteoclast differentiation in the presence of PC3-conditioned medium (PC3-CM), M-CSF (M) and RANKL (R). Significant differences from the surgical control group (p<0.01) or HSCs derived from PC3 implanted animals treated with either vehicle alone or PC3 conditioned medium alone (p<0.001) were calculated. (C) Evaluation of the ability of different HSC populations to differentiate into osteoclasts. Osteoclast differentiation ability of SLAM HSCs and LSK HSCs from osteolytic PC3 tumor-bearing animals were compared in the presence of PC3 conditioned medium with RANKL and M-CSF. Data represent mean ± SD performed in triplicate in 3 independent experiments. Significant differences from surgical controls were established at p<0.01 and p<0.001
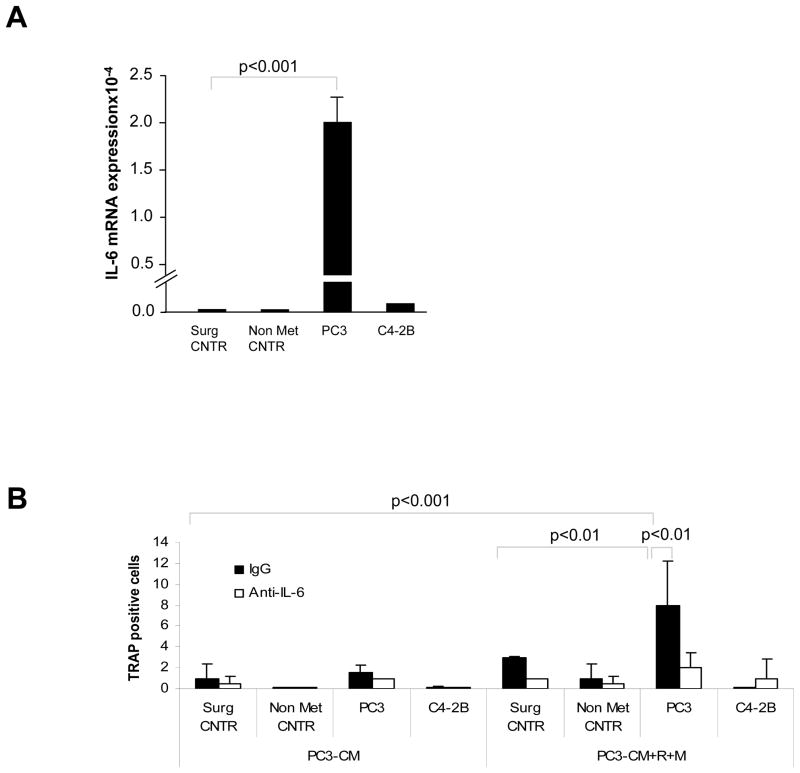
Osteoclast differentiation of HSCs is driven by IL-6 secreted from osteolytic PCa
Many cancer cell types that metastasize to the bone secrete high levels of IL-6 (22). Likewise, other cancer cell types stimulate the surrounding stromal cells to release copious amounts of this cytokine (23, 24). Deregulation of cytokine/growth factor production is also implicated in disorders of bone homeostasis such as osteoporosis and osteopetrosis (25). We therefore postulated that IL-6 may play a significant role in regulating the ability of DTCs to mediate HSC differentiation into osteoclasts. Accordingly, qRT-PCR was used to examine the extent to which HSCs isolated from tumor implanted animals express mRNA for IL-6. IL-6 mRNA was significantly expressed by HSCs (SLAM or LSK) isolated from animals implanted with PC3 tumor cells, but not controls or C4-2B implanted animals (Fig. 6A). To confirm and extend these findings, LSK HSCs from tumor-bearing animals were isolated and differentiated in the presence and absence of IL-6 blocking or control IgG antibodies to neutralize the activity of IL-6. A significant reduction in the production of TRAP positive colonies was observed under these experimental conditions (Fig. 6B). These data suggest that IL-6 participates in the osteoclastic differentiation of HSCs driven by DTCs.
Figure 6. Osteoclast differentiation is mediated partly through IL-6 secreted by osteolytic PCa.
(A) HSCs derived from osteolytic PC3-bearing animals express endogenous IL-6. HSCs (SLAM or LSK) isolated from tumor bearing animals were analyzed by qRT-PCR to evaluate IL-6 mRNA levels. (B) Effect of IL-6 on osteoclast differentiation. LSK HSCs were isolated from tumor bearing animals and differentiated into osteoclast in the presence or absence of PC3-conditioned medium (PC3-CM) or combination of PC3-CM, RANKL and M-CSF. For blocking studies IL-6 neutralizing antibody (or corresponding control IgG) was added to the treatment groups. Data represent mean ± SD performed in triplicate in 2 independent experiments. The data indicate that IL-6 neutralizing antibody significantly reduced the osteoclast differentiation from HSCs isolated from osteolytic PC3 tumor-bearing animals. (p<0.01 and p<0.001).
Discussion
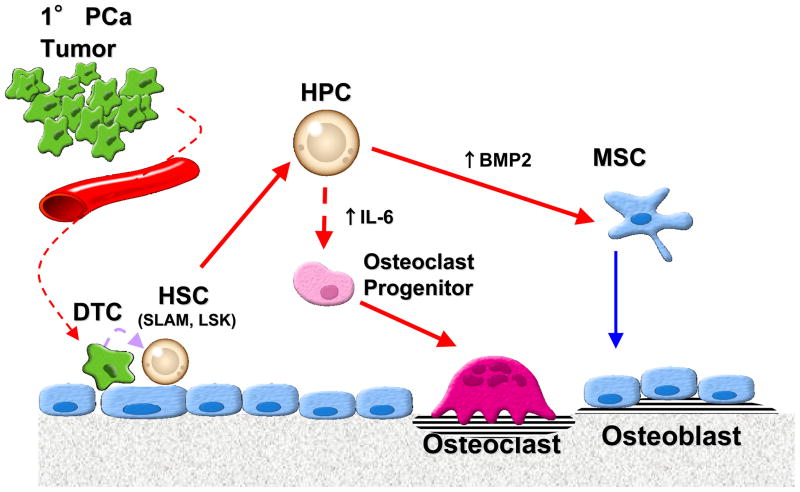
Our data suggest that metastatic PCa can disrupt the ability of HSCs to regulate normal bone formation (Fig. 7). HSCs isolated from PC3 implanted animals differentiated directly into TRAP expressing osteoclasts in the presence of PC3-CM, MCSF and RANKL, but not HSCs isolated from animals implanted with C4-2B cells or control implanted animals. In part, autocrine production of IL-6 possibly by the HSCs themselves is central to facilitating HSCs ability to form osteoclasts. On the other hand, more mature hematopoietic cells including HPCs isolated from animals implanted with C4-2B cells which generate a predominantly osteoblastic bone phenotype showed enhancement of osteoblastic differentiation possibly through the production of BMP-2.
Figure 7. Experimental Model: Disseminated tumor cells (DTC) alter hematopoietic stem cell function to regulate bone phenotype.
At early stage of dissemination process, osteoblastic PCa activate BMP production by HPCs that induce osteoblastic differentiation from mixed bone marrow stromal cells. At the same time, osteolytic PCa activate HSCs to direct the differentiation of osteoclasts through IL-6 pathway. Although further study to exclude the paracrine effects or the effects from DTCs will be needed, the activation of HSCs/HPCs by PCa may be a molecular mechanism for the PCa bone phenotype.
Due to the complexity and diversity of factors produced by the PCa cells in marrow, the heterogeneity of bone metastases in a given patient is dramatic (26, 27). PCa cells express several factors associated with bone remodeling which are closely linked to an osteoblastic response or osteoclastic response (28). BMPs are osteoinductive morphogens critical for skeletal development (29, 30). PCa cells are known to produce BMPs which are believed to play a major role in the osteoblastic bone response to tumor. Furthermore, PCa cells express receptors for the BMPs, which may serve in an autocrine fashion to induce SMAD1 signaling and over expression of osteoprotegerin (31). Recent studies have shown that BMP-2 and BMP-6 stimulate the invasive capability of PCa cells (32). Therefore, inhibition of the BMPs may have clinical relevance (33, 34). In our experiments we observed that the osteogenic capability of HPCs could be mediated by production of BMP-2. HSCs derived from animals with disseminated PCa cells in the marrow formed osteoblastic colonies but there was not a significant difference between osteoblastic colonies formed from osteoblastic (C4-2B) or osteolytic (PC3) tumors. These results suggest a critical role of BMP2 and HPCs in the context of osteoblastic phenotype in PCa.
Part of the mechanism whereby PCa may direct osteoclastic differentiation by HSCs is through IL-6. Several investigators have observed increases in serum markers reflective of bone resorption in men with PCa with bone lesions that are predominately osteoblastic in nature, and often several times the levels observed in individuals with predominantly osteolytic bone metastases, such as breast cancer (35–37). IL-6 has been demonstrated to stimulate osteoclastogenesis in RANKL dependent and independent mechanism (38–40). Recently, interest in using serum IL-6 as a specific prognostic factor for PCa has emerged (41, 42). In addition to serum IL-6 levels, the concentration of soluble receptor to IL-6 (sIL-6R) in the serum may also help to predict the aggressiveness of cancer metastasis and the level of bone destruction (43, 44). When cancer cells metastasize to the bone, increased IL-6 may be endogenously expressed or produced by both the cancer cells and the osteoblasts, as an inflammatory response to the cancer cells. IL-6 then stimulates various types of stromal cells in the bone in the area of the metastasis, to increase the expression of RANKL and M-CSF by osteoblasts which in turn, activate the osteoclast differentiation cascade. Once this occurs, osteoclast activity becomes deregulated and reduces bone integrity. In our experiments we observed that PC3 conditioned medium alone was not sufficient to induce osteoclastic differentiation from HSCs. At the same time PC3-conditioned medium in the presence of RANKL and M-CSF potentiated the osteoclast formation from HSCs isolated from osteolytic PC3 tumor bearing animals. Moreover, HSCs isolated from PC3 implanted animals express endogenous IL-6 and the subsequent activation of autocrine mechanism of IL-6 may lead to osteoclast differentiation
We have shown that PCa compete with HSCs for the stem cell niche and occupy the niche in order to facilitate metastasis (14). The major role of the niche is to limit cell division and therefore maintain stem cells with pluripotent potential (12, 45). Once stem cells or their immediate progeny leave the niche they begin to proliferate and ultimately differentiate, and in fact we observed that in men with metastatic PCa bone disease there is a higher frequency of HPCs in their blood than in age matched or men newly diagnosed with localized PCa (14). We determined the number of osteoblasts and osteoclasts in the long bone obtained from tumor-bearing animals. No significant differences between the treatment groups were observed (Supplemental Figure 4) indicating that our studies represent early events during metastasis. Although further study will be needed, the present study suggests that once the HSCs are out of the niche it may affect mesenchymal fate. The concept that once HSCs are evicted by PCa out of the niche where they may be primed to differentiate into osteoclasts may have immediate and long term consequences. First, the generation of osteoclasts in the local bone environment may release a number of growth factors sequestered in bone matrix, most notably TGF-β which stimulate tumor growth. Second, by expanding the marrow space within bone by activating endosteal resorption, additional space for tumor growth may occur within the confines of a tightly restricted tissue compartment. Combined, the process of expanding the space for growth, as well as the liberation of factors that may feed cancer growth directly is often called a “vicious cycle” (46, 47).
In the present study we also compared osteoclastic differentiation potential of early hematopoietic cells expressing Lin− CD48− CD41− Sca1+cKit+ (LSK HSCs) or CD150+ Lin− CD48− CD41− Sca-1+ cKit+ (SLAM HSCs) and found a similar extent of osteoclast differentiation in the presence of PC3 conditioned medium, RANKL and M-CSF. To date, the earliest identified osteoclast progenitor in bone marrow has been shown to express c-Kit+/c-Fms−/CD11b−/RANK− cell (21). Interestingly, the CD150+ Lin− CD48− CD41− Sca-1+ cKit+ cells isolated from surgical control or control tumor implanted animals did not generate osteoclasts even in the presence of RANKL and M-CSF and/or PCa conditioned media. Yet when isolated from animals with disseminated PC3 in their marrow, differentiation into osteoclasts was possible. This raises the possibility that the CD150+ Lin− CD48− CD41− Sca-1+ cKit+ cells may express RANKL or receptors that make these cells responsive to osteoclastic induction.
Bone is one of the most common sites of PCa metastases with ~ 80% of men with metastatic disease having bone involvement. Typically, these lesions result in increased but uncontrolled bone formation. Clinically, pain, compression of the spinal cord, fracture and in effective hematopoiesis result from disseminated PCa cells in the marrow (48). Current paradigms suggest that the osteosclerosis of PCa is the result of soluble factors produced by PCa cells that induce osteoblastic differentiation of mesenchymal stem cells, prevent apoptosis of osteoblasts or their precursors, and inhibit osteoclastic differentiation or activation. Others have suggested that PCa may mimic osteoblasts and may produce bone-like tissues directly by a process termed osteomimicry (28, 32). Data from our group suggest an alternative and important hypothesis that metastatic PCa disrupts the ability of HSCs and/or HPCs to regulate normal bone metabolism. The concept that HSCs or HPCs directly participate in the generation of the skeletal phenotype of PCa directly challenges the existing paradigms but suggest that these cells may serve as new therapeutic targets of the bone microenvironment that may be worth considering to ameliorate the effects of bone metastases.
Supplementary Material
Acknowledgments
We thank the University of Michigan Flow Cytometry Core and the Imaging Core for their expertise. This work is directly supported by the National Cancer Institute (CA093900, K.J.P. and R.S.T., CA163124, Y.S., K.J.P. and R.S.T.), the Department of Defense (Y.S., K.J.P. and R.S.T.), and the Prostate Cancer Foundation (Y.S., K.J.P. and R.S.T.). K.J.P. receives support as an American Cancer Society Clinical Research Professor, NIH SPORE in prostate cancer grant P50 CA69568, and the Cancer Center support grant P30 CA46592.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed
References
- 1.Walczak JR, Carducci MA. Prostate cancer: a practical approach to current management of recurrent disease. Mayo Clin Proc. 2007 Feb;82(2):243–9. doi: 10.4065/82.2.243. [DOI] [PubMed] [Google Scholar]
- 2.Keller ET, Dai J, Escara-Wilke J, Hall CL, Ignatoski K, Taichman RS, et al. New trends in the treatment of bone metastasis. J Cell Biochem. 2007 Dec 1;102(5):1095–102. doi: 10.1002/jcb.21540. [DOI] [PubMed] [Google Scholar]
- 3.Taichman RS, Loberg RD, Mehra R, Pienta KJ. The evolving biology and treatment of prostate cancer. J Clin Invest. 2007 Sep;117(9):2351–61. doi: 10.1172/JCI31791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002 Aug;2(8):584–93. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 5.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004 Apr 15;350(16):1655–64. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 6.Logothetis CJ, Lin SH. Osteoblasts in prostate cancer metastasis to bone. Nat Rev Cancer. 2005 Jan;5(1):21–8. doi: 10.1038/nrc1528. [DOI] [PubMed] [Google Scholar]
- 7.Suva LJ, Griffin RJ, Makhoul I. Mechanisms of bone metastases of breast cancer. Endocr Relat Cancer. 2009 Sep;16(3):703–13. doi: 10.1677/ERC-09-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001 Mar 1;410(6824):50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 9.Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002 Mar 15;62(6):1832–7. [PubMed] [Google Scholar]
- 10.Chen J, Ellison FM, Keyvanfar K, Omokaro SO, Desierto MJ, Eckhaus MA, et al. Enrichment of hematopoietic stem cells with SLAM and LSK markers for the detection of hematopoietic stem cell function in normal and Trp53 null mice. Exp Hematol. 2008 Oct;36(10):1236–43. doi: 10.1016/j.exphem.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005 Jul 1;121(7):1109–21. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 12.Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008 Dec 12;135(6):1118–29. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 13.Yin T, Li L. The stem cell niches in bone. J Clin Invest. 2006 May;116(5):1195–201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. 2011 Apr 1;121(4):1298–312. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiozawa Y, Pedersen EA, Patel LR, Ziegler AM, Havens AM, Jung Y, et al. GAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow niche. Neoplasia. 2010 Feb;12(2):116–27. doi: 10.1593/neo.91384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003 Apr 1;100(7):3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clarke MF, Fuller M. Stem cells and cancer: two faces of eve. Cell. 2006 Mar 24;124(6):1111–5. doi: 10.1016/j.cell.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Jung Y, Song J, Shiozawa Y, Wang J, Wang Z, Williams B, et al. Hematopoietic stem cells regulate mesenchymal stromal cell induction into osteoblasts thereby participating in the formation of the stem cell niche. Stem Cells. 2008 Aug;26(8):2042–51. doi: 10.1634/stemcells.2008-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu TT, Sikes RA, Cui Q, Thalmann GN, Kao C, Murphy CF, et al. Establishing human prostate cancer cell xenografts in bone: induction of osteoblastic reaction by prostate-specific antigen-producing tumors in athymic and SCID/bg mice using LNCaP and lineage-derived metastatic sublines. Int J Cancer. 1998 Sep 11;77(6):887–94. doi: 10.1002/(sici)1097-0215(19980911)77:6<887::aid-ijc15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 20.Havens AM, Pedersen EA, Shiozawa Y, Ying C, Jung Y, Sun Y, et al. An in vivo mouse model for human prostate cancer metastasis. Neoplasia. 2008 Apr;10(4):371–80. doi: 10.1593/neo.08154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arai F, Miyamoto T, Ohneda O, Inada T, Sudo T, Brasel K, et al. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J Exp Med. 1999 Dec 20;190(12):1741–54. doi: 10.1084/jem.190.12.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giri D, Ozen M, Ittmann M. Interleukin-6 is an autocrine growth factor in human prostate cancer. Am J Pathol. 2001 Dec;159(6):2159–65. doi: 10.1016/S0002-9440(10)63067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ara T, Song L, Shimada H, Keshelava N, Russell HV, Metelitsa LS, et al. Interleukin-6 in the bone marrow microenvironment promotes the growth and survival of neuroblastoma cells. Cancer Res. 2009 Jan 1;69(1):329–37. doi: 10.1158/0008-5472.CAN-08-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas RJ, Guise TA, Yin JJ, Elliott J, Horwood NJ, Martin TJ, et al. Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocrinology. 1999 Oct;140(10):4451–8. doi: 10.1210/endo.140.10.7037. [DOI] [PubMed] [Google Scholar]
- 25.Barille S, Collette M, Bataille R, Amiot M. Myeloma cells upregulate interleukin-6 secretion in osteoblastic cells through cell-to-cell contact but downregulate osteocalcin. Blood. 1995 Oct 15;86(8):3151–9. [PubMed] [Google Scholar]
- 26.Roudier MP, True LD, Higano CS, Vesselle H, Ellis W, Lange P, et al. Phenotypic heterogeneity of end-stage prostate carcinoma metastatic to bone. Hum Pathol. 2003 Jul;34(7):646–53. doi: 10.1016/s0046-8177(03)00190-4. [DOI] [PubMed] [Google Scholar]
- 27.Shah RB, Mehra R, Chinnaiyan AM, Shen R, Ghosh D, Zhou M, et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res. 2004 Dec 15;64(24):9209–16. doi: 10.1158/0008-5472.CAN-04-2442. [DOI] [PubMed] [Google Scholar]
- 28.Koeneman KS, Yeung F, Chung LW. Osteomimetic properties of prostate cancer cells: a hypothesis supporting the predilection of prostate cancer metastasis and growth in the bone environment. Prostate. 1999 Jun 1;39(4):246–61. doi: 10.1002/(sici)1097-0045(19990601)39:4<246::aid-pros5>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 29.Hogan BL. Bone morphogenetic proteins in development. Curr Opin Genet Dev. 1996 Aug;6(4):432–8. doi: 10.1016/s0959-437x(96)80064-5. [DOI] [PubMed] [Google Scholar]
- 30.Reddi AH. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotechnol. 1998 Mar;16(3):247–52. doi: 10.1038/nbt0398-247. [DOI] [PubMed] [Google Scholar]
- 31.Brubaker KD, Corey E, Brown LG, Vessella RL. Bone morphogenetic protein signaling in prostate cancer cell lines. J Cell Biochem. 2004 Jan 1;91(1):151–60. doi: 10.1002/jcb.10679. [DOI] [PubMed] [Google Scholar]
- 32.Dai J, Keller J, Zhang J, Lu Y, Yao Z, Keller ET. Bone morphogenetic protein-6 promotes osteoblastic prostate cancer bone metastases through a dual mechanism. Cancer Res. 2005 Sep 15;65(18):8274–85. doi: 10.1158/0008-5472.CAN-05-1891. [DOI] [PubMed] [Google Scholar]
- 33.Haudenschild DR, Palmer SM, Moseley TA, You Z, Reddi AH. Bone morphogenetic protein (BMP)-6 signaling and BMP antagonist noggin in prostate cancer. Cancer Res. 2004 Nov 15;64(22):8276–84. doi: 10.1158/0008-5472.CAN-04-2251. [DOI] [PubMed] [Google Scholar]
- 34.Feeley BT, Krenek L, Liu N, Hsu WK, Gamradt SC, Schwarz EM, et al. Overexpression of noggin inhibits BMP-mediated growth of osteolytic prostate cancer lesions. Bone. 2006 Feb;38(2):154–66. doi: 10.1016/j.bone.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 35.Berruti A, Tucci M, Mosca A, Tarabuzzi R, Gorzegno G, Terrone C, et al. Predictive factors for skeletal complications in hormone-refractory prostate cancer patients with metastatic bone disease. Br J Cancer. 2005 Sep 19;93(6):633–8. doi: 10.1038/sj.bjc.6602767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrioli R, Rossi S, Caniggia M, Pozzessere D, Messinese S, Sabatino M, et al. Analysis of biochemical bone markers as prognostic factors for survival in patients with hormone-resistant prostate cancer and bone metastases. Urology. 2004 Feb;63(2):321–6. doi: 10.1016/j.urology.2003.09.044. [DOI] [PubMed] [Google Scholar]
- 37.Noguchi M, Yahara J, Noda S. Serum levels of bone turnover markers parallel the results of bone scintigraphy in monitoring bone activity of prostate cancer. Urology. 2003 May;61(5):993–8. doi: 10.1016/s0090-4295(02)02583-9. [DOI] [PubMed] [Google Scholar]
- 38.Mizutani K, Sud S, McGregor NA, Martinovski G, Rice BT, Craig MJ, et al. The chemokine CCL2 increases prostate tumor growth and bone metastasis through macrophage and osteoclast recruitment. Neoplasia. 2009 Nov;11(11):1235–42. doi: 10.1593/neo.09988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kudo O, Sabokbar A, Pocock A, Itonaga I, Fujikawa Y, Athanasou NA. Interleukin-6 and interleukin-11 support human osteoclast formation by a RANKL-independent mechanism. Bone. 2003 Jan;32(1):1–7. doi: 10.1016/s8756-3282(02)00915-8. [DOI] [PubMed] [Google Scholar]
- 40.Novotny NM, Markel TA, Crisostomo PR, Meldrum DR. Differential IL-6 and VEGF secretion in adult and neonatal mesenchymal stem cells: role of NFkB. Cytokine. 2008 Aug;43(2):215–9. doi: 10.1016/j.cyto.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 41.Kuroda K, Nakashima J, Kanao K, Kikuchi E, Miyajima A, Horiguchi Y, et al. Interleukin 6 is associated with cachexia in patients with prostate cancer. Urology. 2007 Jan;69(1):113–7. doi: 10.1016/j.urology.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 42.George DJ, Halabi S, Shepard TF, Sanford B, Vogelzang NJ, Small EJ, et al. The prognostic significance of plasma interleukin-6 levels in patients with metastatic hormone-refractory prostate cancer: results from cancer and leukemia group B 9480. Clin Cancer Res. 2005 Mar 1;11(5):1815–20. doi: 10.1158/1078-0432.CCR-04-1560. [DOI] [PubMed] [Google Scholar]
- 43.Nakchbandi IA, Mitnick MA, Lang R, Gundberg C, Kinder B, Insogna K. Circulating levels of interleukin-6 soluble receptor predict rates of bone loss in patients with primary hyperparathyroidism. J Clin Endocrinol Metab. 2002 Nov;87(11):4946–51. doi: 10.1210/jc.2001-011814. [DOI] [PubMed] [Google Scholar]
- 44.Chalaris A, Garbers C, Rabe B, Rose-John S, Scheller J. The soluble Interleukin 6 receptor: generation and role in inflammation and cancer. Eur J Cell Biol. 2011 Jun-Jul;90(6–7):484–94. doi: 10.1016/j.ejcb.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 45.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4(1–2):7–25. [PubMed] [Google Scholar]
- 46.Clines GA, Guise TA. Molecular mechanisms and treatment of bone metastasis. Expert Rev Mol Med. 2008;10:e7. doi: 10.1017/S1462399408000616. [DOI] [PubMed] [Google Scholar]
- 47.Guise TA, Mohammad KS, Clines G, Stebbins EG, Wong DH, Higgins LS, et al. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res. 2006 Oct 15;12(20 Pt 2):6213s–6s. doi: 10.1158/1078-0432.CCR-06-1007. [DOI] [PubMed] [Google Scholar]
- 48.Coleman RE. Skeletal complications of malignancy. Cancer. 1997 Oct 15;80(8 Suppl):1588–94. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.