Abstract
The human malaria parasite Plasmodium falciparum causes the most deadly parasitic disease worldwide, necessitating the development of interventions that block infection. Yet, preclinical assays to measure inhibition of infection date from the 1980’s and are based on microscopy. Here, we describe the development of a simple flow cytometric assay that can be used to quantitatively assess P. falciparum sporozoite infection in vitro in low and medium throughput. We demonstrate the utility of this assay for assessing both drug inhibition of infection and measuring efficacy of antibodies in blocking parasite infection. This methodology will aid in assessing functional antibody responses to vaccination and novel drugs that prevent mosquito-to-man transmission of malaria.
Keywords: malaria, assay development, flow cytometry
Malaria presents a significant global health burden affecting 300–500 million people annually and resulting in approximately 800,000 deaths world-wide [1]. After transmission by an infected Anopheles mosquito, the parasite traverses skin cells and enters a blood vessel. It then travels quickly through the blood stream to the liver, exits the blood stream by traversing the endothelium, and infects hepatocytes. The parasite then grows and replicates within hepatocytes, evading detection by the host, and ultimately spawns tens of thousands of daughter merozoites, which are released into the blood stream, infect red blood cells, and result in symptomatic infection [2]. Because of the absence of clinical symptoms during liver-stage infection, targeting interventions at this stage is an obvious goal.
Efforts over the past decades have led to several vaccine candidates currently in clinical development. Whereas some vaccination strategies, such as vectored antigens aim to elicit cellular responses, other approaches including recombinant subunit approaches and Virus-like particle administration induce primarily humoral responses. Whole sporozoite vaccination approaches, which confer the highest level of protection in animal models of malaria and in humans [3–5] induce both cellular and antibody responses [6]. However, to date, the most advanced malaria vaccine is a subunit vaccine based on a fusion between the Hepatitis B surface antigen and the parasites’ circumsporozoite protein (CSP), RTS,S [7], which was recently the subject of a Phase III trial. This trial demonstrated up to 50% efficacy against clinical malria [8], yet linking protection to the functionality of antibody responses has been difficult [9–10]. Although the recognition of antibody titers elicited during vaccination are routinely monitored by enzyme-linked immunosorbent assay (ELISA), this assay cannot distinguish antibody binding and functional, neutralizing responses. In order to correlate an antibody response with protection, the function of antibodies that are elicited by vaccination must be monitored.
In addition to monitoring efficacy of antibodies in blocking invasion, an efficient methodology to monitor sporozoite-blocking drugs is critical. No drugs that block sporozoite invasion are currently available and the only widely-available drug that kills early liver stages is primaquine, which cannot be used in patients with glucose 6-phosphate dehydrogenase (G6PD) deficiency [11], the most common described enzyme deficiency worldwide [12]. With so little known about sporozoite targets that could block invasion, a medium throughput assay for drug screens is essential.
To date, assaying P. falciparum pre-erthrocytic stages has been challenging in anything other than low-throughput. Although the technical details of current methodologies vary somewhat, primarily three assays are used for the evaluation of sporozoite infection. Most simply, in an assay that strictly measures gliding motility of sporozoites, parasites (incubated with drug, sera or control) are deposited on a glass slide coated with bovine serum albumin (BSA). Since sporozoites shed CSP when they glide, trails of sporozoite gliding can be visualized using an antibody to CSP and a fluorescent microscope. Although straightforward, this approach is limited because quantifying trails is subjective. One solution may be to automate analysis through advanced image analysis techniques, however, the inconsistent shape and length of trails would likely make algorithm development for this task extremely challenging. Alternatively, because of the biological property of sporozoites to traverse through cells creating holes in their membranes along the way, a second assay that monitors the ability of sporozoites to wound cells was more recently developed [13].
Finally, the monitoring of sporozoite to liver-stage transition is traditionally monitored by incubating sporozoites with susceptible hepatocytes for a variable amount of time, then visualizing parasites by microscopy. In this assay, inhibition can be induced by functional-antibody blocking or drug. When incubation time is short (1–3 hours), this is referred to as an inhibition of sporozoites infection (ISI), whereas when incubation time is longer (1–7 days) the assay is referred to as inhibition of liver stage development assay (ILSDA). Historically, this assay was performed using horseradish peroxidase-conjugated antibodies [14], but more recently the assay has been adapted to utilize fluorescent microscopy. During early time points, sporozoites that do not successfully infect hepatocytes often adhere to the surface of the slide or the target cells, so two color staining with a parasite-specific antibody is used to distinguish between sporozoites inside hepatocytes and sporozoites outside of cells. This assay is limited by throughput and operator-biased, as operators must determine between sporozoites inside and outside of the cell by microscopic inspection. An overview of the relevant assays is provided in Table 1.
Table 1.
Benefits and Disadvantages of four techniques to measure efficacy drugs and antibodies directed at pre-erthrocytic stages of malaria.
| Assay | Pros | Cons |
|---|---|---|
| Visual inspection of gliding trails | Straight forward | Non-quantitative, subjective, low-throughput |
| Traversal of Hepatocytes by sporozoites | Straight forward, can be assessed by microscopy or flow cytometry | Does not give information regarding parasite invasion, only ability of parasites to wound cells |
| ISI by immunoflourescent microscopy | Straight forward, low-througput | Introduction of human bias, low-throughput |
| ILSDA immunoflourescent microscopy | Straight forward, gives information about development | Introduction of human bias, low-throughput requires antibody specific to invaded parasite which is not available for all Plasmodium species. |
| Flow cytometric-based invasion/development | Straight forward, unbiased, medium-throughput, compatible with multiplexing | Instrumentation required |
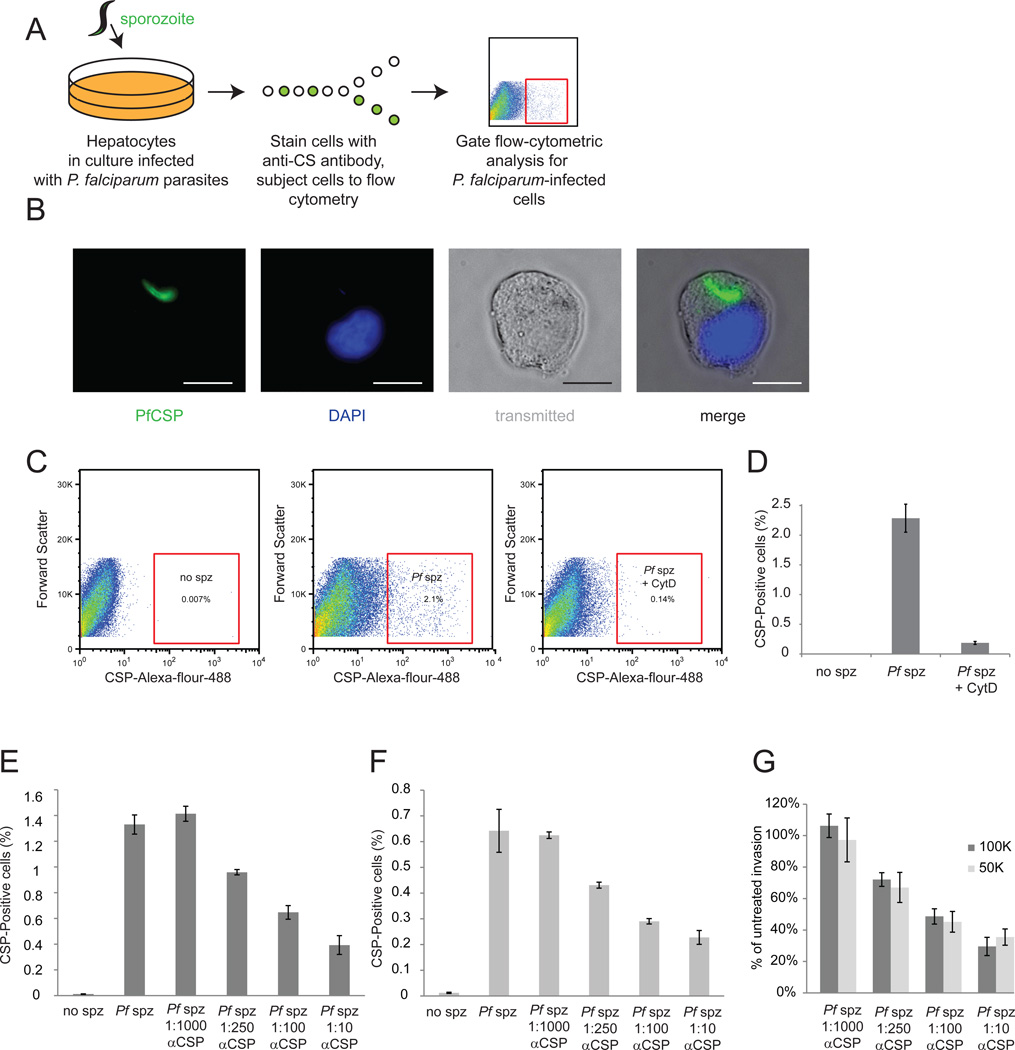
To address these challenges, we adapted a flow-cytometry-based technique to quantitatively monitor sporozoite invasion. In this assay, we first grow HC04 hepatocytes in culture, which have been previously demonstrated to support P. falciparum in vitro invasion and development [15]. We then infect these cells with P. falciparum sporozoites, and allow the parasites to establish intracellular residence for 90 minutes. After this time, cells are detached, fixed and permeabilized, and then stained with a monoclonal antibody (2A10) to P. falciparum CSP, and subjected to flow cytometric analysis. Here, we monitor the percentage of hepatocytes that are infected with parasites by first selecting live hepatocytes by their forward and side scatter, then identifying hepatocytes with high levels of CSP (Figure 1A, B).
Figure 1. Flow-cytrometric analysis is efficient at monitoring P. falciparum liver-stage infection.
(A) Schematic describing methodology used. HC04 hepatocytes are grown in culture then infected with P. falciparum sporozoites. Cells are detached, fixed, permeablized and stained with a monoclonal antibody against circumsporozoite protein (CSP). Infected cells are identified by flow cytometric analysis. (B) Image of an infected cell isolated by FACS. Parasite CSP is stained green, and the host cell nucleus is visualized by DAPI in blue. Scale bar is 10 µm. (C) Scatter plots representing gating strategy for identifying infected cells. Live hepatocytes are first selected by forward and side scatter (not shown) and then hepatocytes with high levels of CSP selected. (D) P. falciparum sporozoites were incubated for 30 minutes with or without Cytochalasin D, and then used to infect HC04 hepatocytes. Cytochalasin D significantly inhibits parasite invasion. 100K (E) or 50K (F) P. falciparum sporozoites were incubated for 30 min without or with various dilutions of 2A10 CSP antibody, and then used to infect HC04 hepatocytes. Antibody pre-incubation with sporozoites decreases invasion rates in a dose-dependent manner. (G) Inhibition rates do not vary depending on invasion rates. To determine invasion rates, antibody-blocked rate was divided by untreated P. falciparum invasion rate for both 100,000 sporozoites (dark grey bars) and 50,000 sporozoites (light grey bars)
To first ensure that the flow cytometer only identifies infected cells as CSPhigh, these cells were isolated by fluorescence-activated cell sorting (FACS) and visualized using fluoresence microscopy. Upon visual inspection, we saw approximately 90% of cells had intracellular parasites, which present as very early liver-stages, and retain the elongated sporozoite shape (Figure 1C). We next assessed the ability of this technique to monitor sporozoite drug inhibition. As a proof-of-concept, we treated sporozoites with Cytochalasin D, a small molecule that inhibits actin polymerization in the sporozoite, eliminating its ability to invade. Cytochalasin D dramatically decreased invasion (Figure 1D). Further, to measure the ability of this methodology to assess quantitative changes in invasion due to antibody blocking, we incubated P. falciparum sporozoites without or with four concentrations of purified monoclonal antibody to CSP. We found that invasion of sporozoites was inhibited in a concentration-dependent manner, which was consistent when multiplicities of infection (MOI) varied (Figure 1E–G). The development of efficient, quantitative, unbiased methodology for monitoring P. falciparum invasion will not only aid in the evaluation of drug candidates that block parasite invasion, but also assist in the evaluation of functional antibody responses in clinical settings.
Although we and others have previously described FACS-based methodologies for quantitation of infection with rodent malaria parasites that express fluorescent proteins [16–19], this requires the challenging development of transgenic parasites in P. falciparum and is not easily applicable alternative parasite strains or field isolates. The assay we describe is also useful for parasites such as Plasmodium vivax, that cannot currently be genetically manipulated. Our assay is highly reproducible, behaves dynamically at different MOIs and can be standardized between laboratories. Thus, our work broadly expands the application for sporozoite invasion-blocking assays. Since vaccine candidates are usually based on one or few parasite genotypes, yet a vaccinated individual may come in contact with a variety of parasites in the field, the ability to assess functionality of antibodies elicited during vaccination in a range of parasite genotypes is a distinct advantage. The medium-throughput, quantitative, and single-cell monitoring capacity of flow-cytometric analysis is extremely well-suited for analyses of large numbers of precious clinical samples only available in small quantities.
Materials and Methods
Cell Lines and Culture
We thank the Walter Reed Army Institute for Research for HC04 cells. Cells were maintained in DMEM complete media (Dulbecco's Modified Eagle Medium (Cellgro, Manassas, VA), supplemented with 10% FBS (Sigma-Aldrich, St. Louis, MO), 100 IU/mL penicillin (Cellgro), 100µg/mL streptomycin (Cellgro), 2.5 µg/mL fungizone (HyClone/ Thermo Fisher, Waltham, MA) and 5 µg/mL gentamicin (BioWhittaker/ Lonza, Basel, Switzerland)), and split 1–2 times weekly.
Mosquito Rearing and Sporozoite Production
For P. falciparum sporozoite production, we produced NF54 gametocytes as described previously [20]. We isolated salivary gland sporozoites according to standard procedures at days 14 or 15 post blood meal.
Quantification of infected cells by FACS
Cells were cultured as described above. 3.75×105 cells were plated in each well of a 24-well plate, and infected with 5×104 or 505 P. falciparum sporozoites. 90 minutes after infection, cells were trypsinized, and then fixed with Perm/Fix buffer (BD Biosciences). Cells were blocked in Perm/Wash buffer (BD Biosciences) supplemented with 2% BSA. Additional staining steps were performed in Perm/Wash buffer alone. We stained cells using anti-sera to circumsporozoite protein at 25°C then washed several times and antibodies were visualized with the use of AlexaFlour-488 goat anti-mouse secondary antibody (Invitrogen). Parasitized hepatocytes were identified by flow cytometry using a BD-LSRII flow cytometer (BD Biosciences). Sorting of CS-positive hepatocytes was carried out with a Cytopeia Influx Cell Sorter using the Spigot Operating Software Version 5.0.3.1 (Cytopeia). Flow cytometric analysis was performed using FlowJo software (TreeStar). All experimental conditions were tested in biological triplicate. All data is representative of three independent experiments.
Acknowledgements
We are grateful to Heather S. Kain, William W. Benz, Mark F. Kennedy and Jen C.C. Hume of the Seattle Biomed insectary facility for mosquito and sporozoite production. We thank Hieu Nguyen for technical assistance with FACS. A.K. is a recipient of a NRSA Ruth L. Kirschstein National Research Service Award (F32 AI091129), which has partially funded this work. Additionally, this work has been funded by a contract from the Department of Defense (W81XWH-11-2-0184).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaughan AM, Aly AS, Kappe SH. Malaria parasite pre-erythrocytic stage infection: gliding and hiding. Cell Host Microbe. 2008;4:209–218. doi: 10.1016/j.chom.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nardin E, Zavala F, Nussenzweig V, Nussenzweig RS. Pre-erythrocytic malaria vaccine: mechanisms of protective immunity and human vaccine trials. Parassitologia. 1999;41:397–402. [PubMed] [Google Scholar]
- 4.Hoffman SL, Goh LM, Luke TC, Schneider I, Le TP, Doolan DL, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185:1155–1164. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 5.Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature. 1967;216:160–162. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- 6.Herrington D, Davis J, Nardin E, Beier M, Cortese J, Eddy H, et al. Successful immunization of humans with irradiated malaria sporozoites: humoral and cellular responses of the protected individuals. Am J Trop Med Hyg. 1991;45:539–547. doi: 10.4269/ajtmh.1991.45.539. [DOI] [PubMed] [Google Scholar]
- 7.Gordon DM, McGovern TW, Krzych U, Cohen JC, Schneider I, LaChance R, et al. Safety, immunogenicity, and efficacy of a recombinantly produced Plasmodium falciparum circumsporozoite protein-hepatitis B surface antigen subunit vaccine. J Infect Dis. 1995;171:1576–1585. doi: 10.1093/infdis/171.6.1576. [DOI] [PubMed] [Google Scholar]
- 8.Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, Conzelmann C, et al. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med. 2011;365:1863–1875. doi: 10.1056/NEJMoa1102287. [DOI] [PubMed] [Google Scholar]
- 9.Agnandji ST, Fendel R, Mestre M, Janssens M, Vekemans J, Held J, et al. Induction of Plasmodium falciparum-specific CD4+ T cells and memory B cells in Gabonese children vaccinated with RTS,S/AS01(E) and RTS,S/AS02(D) PLoS One. 2011;6:e18559. doi: 10.1371/journal.pone.0018559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olotu A, Lusingu J, Leach A, Lievens M, Vekemans J, Msham S, et al. Efficacy of RTS,S/AS01E malaria vaccine and exploratory analysis on anti-circumsporozoite antibody titres and protection in children aged 5–17 months in Kenya and Tanzania: a randomised controlled trial. Lancet Infect Dis. 2011;11:102–109. doi: 10.1016/S1473-3099(10)70262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirkman HN, Crowell BB. Molecular deficiency of glucose-6-phosphate dehydrogenase in primaquine sensitivity. Nature. 1963;197:286–287. doi: 10.1038/197286a0. [DOI] [PubMed] [Google Scholar]
- 12.Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008;371:64–74. doi: 10.1016/S0140-6736(08)60073-2. [DOI] [PubMed] [Google Scholar]
- 13.Mota MM, Pradel G, Vanderberg JP, Hafalla JC, Frevert U, Nussenzweig RS, et al. Migration of Plasmodium sporozoites through cells before infection. Science. 2001;291:141–144. doi: 10.1126/science.291.5501.141. [DOI] [PubMed] [Google Scholar]
- 14.Hollingdale MR, Nardin EH, Tharavanij S, Schwartz AL, Nussenzweig RS. Inhibition of entry of Plasmodium falciparum and P. vivax sporozoites into cultured cells; an in vitro assay of protective antibodies. J Immunol. 1984;132:909–913. [PubMed] [Google Scholar]
- 15.Karnasuta C, Pavanand K, Chantakulkij S, Luttiwongsakorn N, Rassamesoraj M, Laohathai K, et al. Complete development of the liver stage of Plasmodium falciparum in a human hepatoma cell line. Am J Trop Med Hyg. 1995;53:607–611. doi: 10.4269/ajtmh.1995.53.607. [DOI] [PubMed] [Google Scholar]
- 16.Tarun AS, Peng X, Dumpit RF, Ogata Y, Silva-Rivera H, Camargo N, et al. A combined transcriptome and proteome survey of malaria parasite liver stages. Proc Natl Acad Sci U S A. 2008;105:305–310. doi: 10.1073/pnas.0710780104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mikolajczak SA, Aly AS, Dumpit RF, Vaughan AM, Kappe SH. An efficient strategy for gene targeting and phenotypic assessment in the Plasmodium yoelii rodent malaria model. Mol Biochem Parasitol. 2008;158:213–216. doi: 10.1016/j.molbiopara.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Natarajan R, Thathy V, Mota MM, Hafalla JC, Menard R, Vernick KD. Fluorescent Plasmodium berghei sporozoites and pre-erythrocytic stages: a new tool to study mosquito and mammalian host interactions with malaria parasites. Cell Microbiol. 2001;3:371–379. doi: 10.1046/j.1462-5822.2001.00117.x. [DOI] [PubMed] [Google Scholar]
- 19.Prudencio M, Rodrigues CD, Ataide R, Mota MM. Dissecting in vitro host cell infection by Plasmodium sporozoites using flow cytometry. Cell Microbiol. 2008;10:218–224. doi: 10.1111/j.1462-5822.2007.01032.x. [DOI] [PubMed] [Google Scholar]
- 20.Mikolajczak SA, Sacci JB, Jr, De La Vega P, Camargo N, VanBuskirk K, Krzych U, et al. Disruption of the Plasmodium falciparum liver-stage antigen-1 locus causes a differentiation defect in late liver-stage parasites. Cell Microbiol. 2011;13:1250–1260. doi: 10.1111/j.1462-5822.2011.01617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]