Abstract
Ca2+-mediated mitochondrial permeability transition (mPT) is the final common pathway of stress-induced cell death in many major pathologies, but its regulation in intact cells is poorly understood. Here we report that the mitochondrial carrier SCaMC-1/SLC25A24 mediates ATP-Mg2−/Pi2− and/or HADP2−/Pi2− uptake into the mitochondria after an increase in cytosolic [Ca2+]. ATP and ADP contribute to Ca2+ buffering in the mitochondrial matrix, resulting in desensitization of the mPT. Comprehensive gene expression analysis showed that SCaMC-1 overexpression is a general feature of transformed and cancer cells. Knockdown of the transporter led to vast reduction of mitochondrial Ca2+ buffering capacity and sensitized cells to mPT-mediated necrotic death triggered by oxidative stress and Ca2+ overload. These findings revealed that SCaMC-1 exerts a negative feedback control between cellular Ca2+ overload and mPT-dependent cell death, suggesting that the carrier might represent a novel target for cancer therapy.
Keywords: adenine nucleotides, ATP-Mg/Pi carriers, calcium, mitochondrial permeability transition pore, oxidative stress, cancer
ATP generation by aerobic glycolysis is indispensable for survival and proliferation of many tumour cell types, associated with resistance to stress-induced apoptotic and necrotic death.1, 2 In many cell death models, mitochondrial permeability transition (mPT) has a central role in permeabilizing the inner mitochondrial membrane (IMM), leading to necrotic cell death.3 The mPT is triggered by the formation of a large (cut-off ∼1.5 kDa), non-specific pore (mPTP) in the IMM under conditions of Ca2+ overload and/or oxidative stress. mPTP opening dissipates the mitochondrial membrane potential (Δψ) and can be associated with mitochondrial swelling, particularly under in vitro conditions.4 Although the molecular composition of the mPTP is still debated, pharmacological and genetic evidence strongly supports its implication in cell death in a wide range of pathologies.5 Accordingly, resistance of tumour cells to death could be explained by decreased probability of mPTP opening. Indeed, it has been suggested that Bcl-2 overexpression or reduction of Bax/Bak expression can mediate protective effect by diminishing mitochondrial Ca2+ ([Ca2+]m) load from the endoplasmic reticulum (ER) Ca2+ store.6 In addition, direct modification of putative mPTP components, such as hexokinase-II binding to the voltage-dependent anion channel (VDAC)7 and dephosphorylation of cyclophilin-D (CyP-D),8 have also been shown to reduce the sensitivity of mPTP formation to elevations of [Ca2+]m. However, modification of the mPT is not essential for mediating cancer cell protection,9 and no intrinsic mitochondrial factor has been described so far contributing to the development of resistance to mPT in cancer cells.
The net mitochondrial content of adenine nucleotides is central to the regulation of Ca2+-induced mPT in isolated organelles, but the underlying mechanisms remained unclear.10 The adenine nucleotide translocator (ANT) of the IMM exchanges ATP4− for ADP3−, but does not change the total matrix ATP/ADP/AMP content. The net content of adenine nucleotides may rather be determined by the ATP-Mg/Pi transporter, which mediates a reversible, electroneutral exchange of ATP-Mg2− or HADP2− for HPO42−, stimulated by extra-mitochondrial Ca2+.11 Recently, the genes encoding this transporter have been identified. There are four paralogues in mammals, SCaMC-1/SLC25A24, SCaMC-2/SLC25A25, SCaMC-3/SLC25A23 and SCaMC-3-like/SLC25A41.12, 13 The transporter consists of a C-terminal domain comprising six transmembrane helices homologous to the mitochondrial carrier proteins,14 and an N-terminal domain with Ca2+-binding EF hands,15 which confers Ca2+ sensitivity to the carrier.11, 16 These properties of the carrier prompted us to investigate the role it might have in regulating the Ca2+ retention capacity (CRC) of mitochondria and mPTP formation in intact cells.
Here we report that SCaMC-1 is the dominant isoform of the ATP-Mg/Pi carrier in cancer cells and is highly overexpressed in a series of in vivo tumours and cell lines. We found that cytosolic Ca2+ ([Ca2+]cyt)-mediated uptake of ATP/ADP by SCaMC-1 increases intra-mitochondrial Ca2+ buffering, and thus contributes to the resistance to mPT in tumour cells.
Results and Discussion
SCaMC-1 is highly expressed in tumours and cancer cell lines, and mediates Ca2+-dependent ATP-Mg and ADP uptake into mitochondria
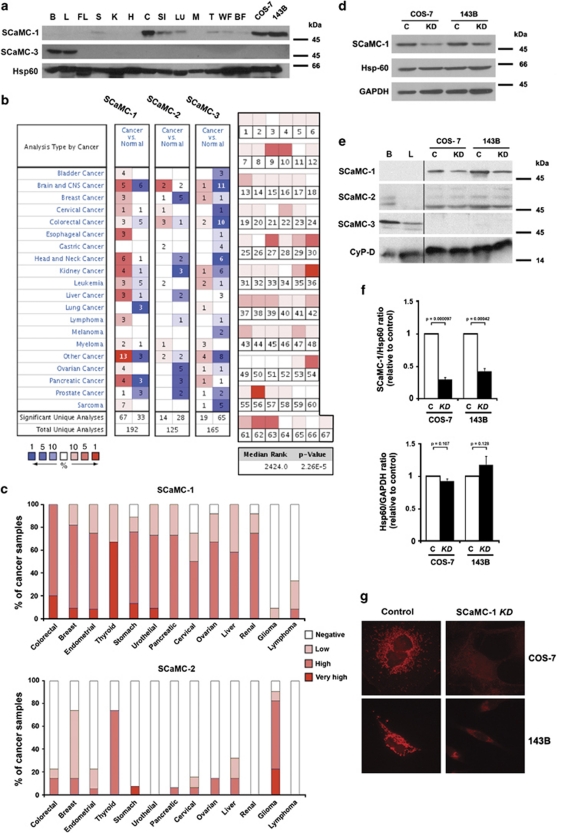
First, using western blotting, immunofluorescence and in silico approaches we showed that SCaMC-1 is the dominant and highly expressed isoform of ATP-Mg/Pi carrier family in a wide range of tumours, cancer cell lines and highly proliferative immortalized cells (Figures 1a–c and Supplementary Figure S1). Therefore, in order to study the role of SCaMC-1 in mitochondrial adenine nucleotide transport and cancer cell fate, we generated stable SCaMC-1-knockdown (SCaMC-1-KD) cell lines by using COS-7 and 143B parental lines (Figures 1d–g). In the SCaMC-1-KD COS-7 and 143B clones, expression was reduced to 29.6±3.8% and 41.7±5.0%, respectively (Figure 1f).
Figure 1.
Expression of SCaMC isoforms in normal and tumour tissues. Generation of SCaMC-1-KD cell lines. (a) Immunoblot analysis of SCaMC-1 and SCaMC-3 expression in total homogenates from mouse tissues and from COS-7 and 143B cells. Antibodies against Hsp60 were used as loading control. B, brain; L, liver; FL, fetal liver; S, spleen; K, kidney; H, heart; C, colon; SI, small intestine; LU, lung; M, muscle; T, testis; WF, white fat; BF, brown fat. (b) Disease summary for the SCaMC family (SCaMC-1, SCaMC-2, SCaMC-3) analysed by the Oncomine database (www.oncomine.org). The table shows the number of significant unique analyses across the whole Oncomine database (including 93 data sets curated for cancer versus normal analysis), with overexpression (red) or under-expression (blue). Cell colour is determined by the best gene rank percentile for the analyses within the cell. SCaMC-1 shows an overexpression/under-expression ratio of 67/33 from 192 analyses, in contrast to 14/28 from 125 and 19/65 from 165 for SCaMC-2 and SCaMC-3, respectively. A threshold significance value of 0.05 was used including setting to ‘ALL' for fold change, gene rank thresholds and data types. For links to the analyses see Supplementary Information. Right panel: Comparison of SCaMC-1/SLC25A24 expression across 67 cancer versus normal analyses, including overexpression (mRNA) and copy-number gain (DNA). The rank for a gene is the median rank for that gene across each of the analyses. The P-value of the median-ranked analysis is shown. For references and link to analysis see Supplementary Information. (c) Protein expression profiles of SCaMC-1 (upper panel) and SCaMC-2 (lower panel) obtained from the Human Protein Atlas database (www.proteinatlas.org) based on immunohistochemistry of cancer samples.40 The colour determines the percentage of cancer samples in the database with a given expression level. SCaMC-3 is not available in the database. (d–f) Immunoblot to detect SCaMC-1 and other SCaMC isoforms in total homogenates (d) or isolated mitochondria (e) from COS-7 and 143B control (C) and SCaMC-1-KD (KD) cells. Mouse brain (B) and liver (L) mitochondria are also shown in panel e. Antibodies against Hsp60, GAPDH or CyP-D were used as loading control. (f) Quantification of expression of SCaMC-1 or Hsp60 as compared with GAPDH (mean±S.E.M., n=5). (g) Immunofluorescence in COS-7 and 143B control and SCaMC-1-KD cells using anti-SCaMC-1 antibodies
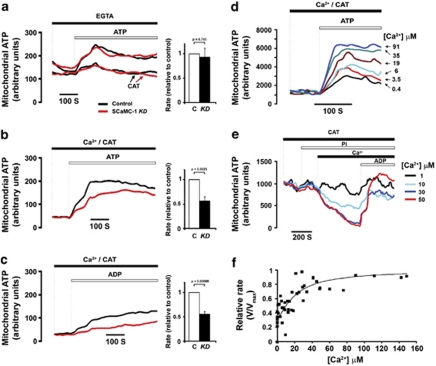
Next, in order to evaluate mitochondrial adenine nucleotide transport in the parental and SCaMC-1-KD clones, cells were transiently or stably transfected with mitochondrial matrix-targeted luciferase to measure mitochondrial ATP levels in digitonin-permeabilized cells or isolated mitochondria.17 After cell permeabilization in an intracellular (IC) buffer supplemented with luciferin, the added ATP was imported into the mitochondria (Figure 2). Under Ca2+-free conditions uptake of ATP was completely inhibited by 10 μM carboxyatractyloside (CAT), indicating that it was mediated entirely by ATP/ADP exchange through ANT. Ca2+-independent ATP import was identical in parental and SCaMC-1-KD cells (Figure 2a). However, in the presence of 100 μM Ca2+, ATP was rapidly imported into the mitochondrial matrix even in the presence of CAT (Figure 2b). The transport was activated by extra-mitochondrial Ca2+, as 200 nM ruthenium red (RR) was present in all the experiments to prevent Ca2+ uptake through the mitochondrial Ca2+ uniporter (MCU). Under these conditions, ADP uptake was also observed, as measured after its conversion to ATP owing to oxidative phosphorylation in the matrix (Figure 2c and Supplementary Figure S2A).16 Chelation of Mg2+ (with 1 mM EDTA) reduced the ATP uptake rate (not shown). The dependence of CAT-insensitive ATP transport on extra-mitochondrial [Ca2+] was determined on isolated mitochondria by using a mitochondrial luciferase-expressing COS-7 cell line, showing half-maximal activation at [Ca2+]=12.7±5.3 μM (Figures 2d and f). Pi addition reversed the activity of the transporter (Figure 2e), with a similar Ca2+ dependence. These results confirmed that these cells express a bona fide Ca2+-dependent ATP-Mg/Pi carrier, mediating the exchange of ATP-Mg and free ADP to Pi.18
Figure 2.
Ca2+-dependent/CAT-insensitive adenine nucleotide uptake through SCaMC-1. (a–c) 143B cells transiently expressing mitochondrial luciferase17 were permeabilized and 100 μM luciferin was added to report mitochondrial ATP levels. The black traces indicate control cells, whereas the red traces indicate SCaMC-1-KD cells. (a) ANT activity in the absence of Ca2+: The medium contained 100 μM EGTA and 1 mM ATP was added when indicated. The traces marked by arrows were obtained from cells treated with 10 μM CAT. (b and c) SCaMC-1 activity: The medium contained 100 μM Ca2+ and 10 μM CAT, and 1 mM ATP (b) or 2 mM ADP (c) was added when indicated. The bar charts indicate the transport rate of SCaMC-1-KD (KD) cells as compared with controls (C) (mean±S.E.M., n=6). (d–f) To determine the Ca2+ sensitivity of SCaMC-1-mediated ATP/ADP transport, mitochondrial ATP changes were measured in isolated mitochondria from COS-7 cells stably expressing mitochondrial luciferase after addition of 100 μM luciferin. (d) The medium contained 10 μM CAT and free Ca2+ at the indicated concentrations, and 1 mM ATP was added when indicated. (e) Efflux of ATP from mitochondria was triggered by addition of 10 mM Pi followed by different Ca2+ concentrations. The efflux was reverted at the end of the experiment by addition of 1 mM ADP. (f) Ca2+ activation of CAT-insensitive ATP transport. Transport rates were calculated from the slopes in panel d after ATP addition and fit to the following equation: V=V0+[(Vmax−V0) × [Ca2+]N)/(S0.5N+[Ca2+]N)] (where V is transport activity at each [Ca2+] V0 is the basal transport rate at [Ca2+]≈0; Vmax is the maximal activity; N is the hill coefficient; and S0.5 is the Ca2+ concentration, which generates half-maximal transport activity). Pooled data from six independent experiments are shown
Importantly, the CAT-insensitive/Ca2+-dependent ATP-Mg/ADP transport was strongly reduced in SCaMC-1-KD cells (Figures 2b and c; ATP: to 56±8% and ADP: to 55±5% of the control rate) to an extent similar to the reduction of SCaMC-1 expression, demonstrating that SCaMC-1 mediates ATP-Mg/Pi transporter activity facilitated by extra-mitochondrial [Ca2+].
SCaMC-1 protects cancer cells from oxidative stress-induced death
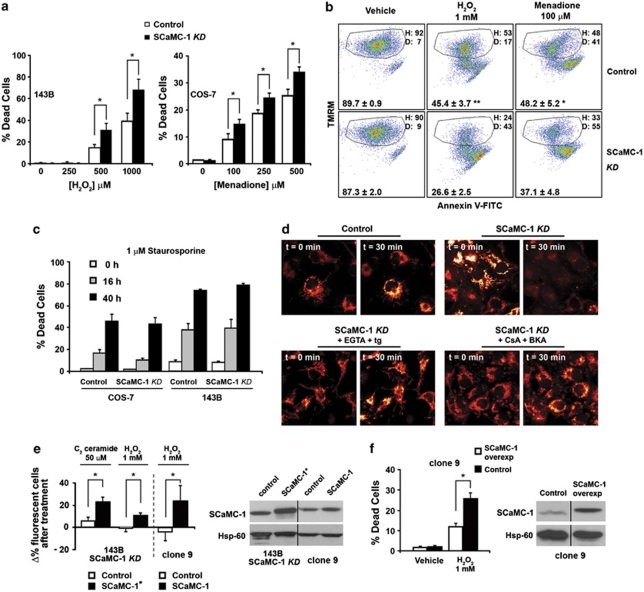
High expression levels of SCaMC-1 in tumours and cancer cell lines suggested that the carrier confers selective advantage to proliferation either by altering cellular metabolism or by mediating protection against cell death. We observed no effect on cellular energy metabolism and proliferation (see Supplementary Figure S3). Thus next, in order to test whether SCaMC-1 has a role in modulating cell death pathways, control and SCaMC-1-KD cells were exposed to (i) oxidative stress by H2O2 or menadione treatment, leading to mitochondrial Ca2+ overload, mPT and necrosis; or (ii) staurosporine, triggering the intrinsic pathway of apoptosis. As shown in Figures 3a–c, reduced expression of SCaMC-1 rendered cells more susceptible to oxidative stress-induced cell death (Figures 3a and b), whereas it had no effect on apoptosis induced by staurosporine (Figures 3c and Supplementary Figure S4A). These data indicated that high expression levels of SCaMC-1 confer resistance specifically to mPT-dependent cell death.
Figure 3.
. SCaMC-1 selectively protects from oxidative stress-induced cell death. (a) Left panel: Control and SCaMC-1-KD 143B cells were incubated with H2O2 for 6 h and cell death was evaluated by confocal microscopy after 1 μM calcein-AM and 2 μM PI staining. Right panel: Control and SCaMC-1-KD COS-7 cells were incubated with menadione for 2 h and cell death was evaluated by flow cytometry after PI staining (mean±S.E.M., n=6 and 5, respectively; *P<0.05). (b) Control and SCaMC-1-KD 143B cells were incubated with 1 mM H2O2 for 3.5 h or with 100 μM menadione for 1 h and cell death was evaluated by flow cytometry after TMRM and Annexin-V–FITC staining. A representative experiment is shown where H indicates the percentage of healthy cells (positive for TMRM and negative for Annexin-V, delimited by the quadrant) and D indicates the percentage of dead cells (negative for TMRM and positive for Annexin-V). The number at the bottom of each panel indicates the percentage (mean±S.E.M., n=6) of healthy cells. **P=0.00031; *P=0.014. (c) Control and SCaMC-1-KD COS-7 and 143B cells are equally sensitive to 1 μM staurosporine-induced cell death. Cells were treated with the drug for 0, 16 or 40 h, and death was evaluated by flow cytometry after PI and Annexin-V–FITC staining. (d) SCaMC-1-KD cells are more vulnerable to phototoxicity-induced oxidative stress. Control and SCaMC-1-KD COS-7 cells were loaded with 100 nM TMRM; illuminated with 10% laser power of the 543 nm He–Ne line of the confocal system; and collapse of mitochondrial ΔΨ was monitored over time. Lower left panels: SCaMC-1-KD cells were treated with 100 μM EGTA and 1 μM thapsigargin. Lower right panels: Cells treated with 5 μM CsA and 50 μM BKA. The figure shows representative images of at least three independent experiments. (e) Sensitivity of SCaMC-1-overexpressing 143B SCaMC-1-KD cells (left panel) and liver clone-9 cells (right panel) to mPT-dependent cell death. Cells were transiently transfected with GFP, or with GFP and SCaMC-1, and exposed to C2 ceramide or H2O2 for 6 h. The bar shows the change in the percentage of GFP fluorescent cells (as compared with total cell number) after the treatment.21 An increase in this percentage as compared with control transfections indicates protection by SCaMC-1. The right panel shows the increase in SCaMC-1 expression after the transfection. SCaMC-1*: Rescue construct with synonymous mutations (see the Supplementary Materials and Methods). (f) Control and stable SCaMC-1-overexpressing liver clone-9 cells were incubated with H2O2 for 3 h and cell death was evaluated by flow cytometry after PI staining (mean±S.E.M., n=3; *P<0.05). The right panel shows the increase in SCaMC-1 expression in the stable SCaMC-1-overexpressing clone
In order to directly demonstrate the effect of SCaMC-1 silencing on mPT in intact cells, we studied Ca2+- and reactive oxygen species (ROS)-mediated mitochondrial depolarization by confocal microscopy, and a previously established model of phototoxicity.19 After tetramethylrhodamine methyl ester (TMRM) loading, cells were illuminated with high laser power, which leads to local generation of ROS in the mitochondria. ROS-mediated Ca2+ release from the ER results in mitochondrial Ca2+ overload and mPT, visualized by loss of TMRM. As shown in Figure 3d, illumination of parental COS-7 cells had no effect on the mitochondrial membrane potential (ΔΨm), whereas it led to complete loss of ΔΨm in SCaMC-1-KD cells. The effect was Ca2+-dependent, as it was completely prevented in Ca2+-free medium (in the presence of 100 μM EGTA) and by depleting the internal Ca2+ stores using thapsigargin (1 μM). Moreover, addition of cyclosporin-A (CsA) and bongkrekic acid (BKA), inhibitors of mPT,20 prevented mitochondrial depolarization in SCaMC-1-KD cells, confirming that it was indeed due to mPT.
Finally, to test whether the high level of SCaMC-1 expression is indeed responsible for protection against mPT-dependent cell death, we (i) rescued the expression of SCaMC-1 in SCaMC-1-KD 143B cells, using a mutant cDNA containing synonymous mutations (SCaMC-1*), and (ii) overexpressed the protein in liver clone-9 cells with low levels of endogenous SCaMC-1 (Supplementary Figure S1). Re-expression of SCaMC-1 in SCaMC-1-KD 143B cells (Figure 3e), as well as its overexpression in both a transient (Figure 3e) or a stable (Figure 3f) manner in clone-9 cells rendered cells more resistant to H2O2- or C2 ceramide-induced cell death.21 These results confirmed that SCaMC-1 mediates protection against mPT-induced cell death.
SCaMC-1-mediated ATP/ADP uptake controls mitochondrial Ca2+ buffering capacity
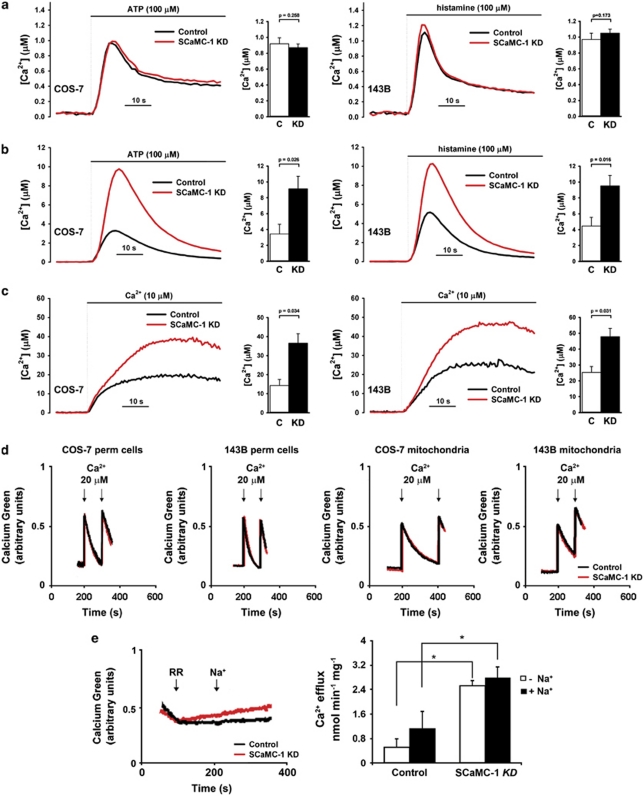
Elevation of mitochondrial matrix free [Ca2+] is the most potent inducer of mPT. To determine the effect of SCaMC-1 on cellular and mitochondrial Ca2+ handling in intact cells, we measured agonist-evoked cytosolic and mitochondrial Ca2+ transients in cells transiently transfected with a cytosolic aequorin (cytAEQ) or a mitochondrial aequorin (mtAEQwt) probe, respectively.22
After reconstitution of the probe with the aequorin cofactor coelenterazine, COS-7 cells were stimulated with 100 μM ATP, an agonist acting on G-protein-coupled receptors and leading to the production of inositol-1,4,5-trisphosphate (IP3). The consequent Ca2+ release from the IC stores induced a cytosolic Ca2+ transient, which was identical in control and SCaMC-1-KD cells (Figure 4a, left panel). However, intriguingly, mitochondrial Ca2+ uptake was increased almost three times in SCaMC-1-KD cells as compared with controls (Figure 4b, left panel). Similar results were obtained when parental and SCaMC-1-KD 143B cells were stimulated with 100 μM histamine (Figures 4a and b, right panels).
Figure 4.
SCaMC-1 knock-down reduces mitochondrial Ca2+ buffering in intact cells and isolated mitochondria. Agonist-induced Ca2+ increases in the cytosol (a) and mitochondria (b) of cells transiently expressing cytAEQ and mtAEQwt probes, respectively. Ca2+ signals were induced by ATP in COS-7 and by histamine in 143B cells (black, controls; red, SCaMC-1-KD). The bar charts show quantification of results in control (C) and SCaMC-1-KD cells (KD, mean±S.E.M., n=3). (c) [Ca2+]m of COS-7 (left panel) and 143B (right panel) permeabilized cells after addition of 10 μM (free [Ca2+]) Ca2+ to the IC buffer. The bar charts show quantification of results in control (C) and SCaMC-1-KD cells (KD, mean±S.E.M., n=3). (d) Ca2+ uptake rate in permeabilized cell and isolated mitochondria, measured by using the Calcium-Green 5N free salt as an extra-mitochondrial Ca2+ indicator. A 20-μM (free [Ca2+]) Ca2+ pulse was added where indicated (black, controls; red, SCaMC-1-KD). (e) Ca2+ efflux was measured by using the Calcium-Green 5N free salt in the extra-mitochondrial space of isolated 143B mitochondria, in the presence of 1 mM ATP, followed by loading with 320 nmol of Ca2+ per mg of protein. Where indicated, 200 nM RR was added to inhibit Ca2+ uptake, followed by addition of 10 mM NaCl to stimulate the Na+/Ca2+ exchanger (black, controls; red, SCaMC-1-KD). The bar chart shows quantification of Na+-independent (white bars) and Na+-dependent (black bars) Ca2+ efflux rates in control and SCaMC-1-KD mitochondria (mean±S.E.M., n=3; *P<0.05)
Mitochondrial free [Ca2+] is determined by the balance between Ca2+ influx into and Ca2+ extrusion from the organelle, as well as by Ca2+ buffering in the matrix. Thus, in order to characterize the mechanism by which SCaMC-1 activity affects [Ca2+]m, we determined mitochondrial Ca2+ influx and efflux rates in isolated mitochondria or permeabilized cells. When measured in the mitochondrial matrix in permeabilized cells expressing the low-affinity mitochondrially targeted aequorin probe (mtAEQmut), addition of the same amount of extra-mitochondrial Ca2+ (10 μM) caused significantly greater elevation in [Ca2+]m in both COS-7 and 143B SCaMC-1-KD cells as compared with their parental counterpart (Figure 4c). However, when measured in the extra-mitochondrial space using the fluorescent Ca2+-sensitive dye Calcium-Green, mitochondria from both the parental and SCaMC-1-KD cells took up the same amount of Ca2+ with the same rate (Figure 4d). Ca2+ efflux was measured after inhibition of Ca2+ uptake using RR. In that situation, the rate of Ca2+ efflux depends only on the matrix free [Ca2+]. After loading 143B mitochondria with Ca2+ in the presence of 1 mM ATP-Mg, RR was added. Figure 4e shows that efflux of Ca2+ (both Na+-independent and Na+-dependent) was more rapid in SCaMC-1-KD mitochondria than in controls, indicating (i) higher free [Ca2+] after Ca2+ load in mitochondria lacking SCaMC-1-mediated ATP uptake and (ii) normal activity of the efflux machineries. These results show that increased [Ca2+]m in the mitochondria of SCaMC-1-KD cells is not the consequence of increased Ca2+ uptake or reduced Ca2+ efflux, but rather reflects a reduced Ca2+ buffering capacity in the matrix.
Ca2+ buffering in the mitochondrial matrix is principally achieved by the formation of insoluble Ca2+-Pi precipitates.23 A higher level of Ca2+ precipitation in control mitochondria could be explained if the adenine nucleotides transported by SCaMC-1 facilitate the precipitation of Ca2+ and Pi in the matrix, as has been suggested previously.24 Formation of Ca2+-Pi precipitates can be measured by the apparent mitochondrial contraction (an increase in light scattering measured as absorbance at 540 nm) immediately after Ca2+ uptake.23, 25 Indeed, mitochondria of 143B cells showed apparent contraction when Ca2+ was added to the medium, which was inhibited by the addition of RR, preventing Ca2+ entry through the MCU. Moreover, the initial increase in absorbance in the presence of ATP-Mg was significantly higher in the mitochondria of control than that of SCaMC-1-KD 143B cells (0.041±0.006 absorbance units per minute compared with 0.018±0.003, respectively, mean±S.E.M., n=3, P=0.048; not shown). These results suggest a higher efficiency of Ca2+-Pi precipitate-mediated Ca2+ buffering in the presence of SCaMC-1.
SCaMC-1 increases mitochondrial CRC and reduces the probability of Ca2+-mediated mPTP opening
Regulation of Ca2+-dependent mPTP opening by low micromolar concentrations of ATP/ADP is well established,26 and is mediated by a nucleotide-induced switch in the conformation of ANT. However, higher concentrations of adenine nucleotides, in the physiological millimolar range, are also able to prevent mPTP formation.27 Thus we tested whether Ca2+-mediated uptake of ATP-Mg/ADP by SCaMC-1 can affect mitochondrial CRC, a measure of the amount of Ca2+ required in order to trigger mPT.
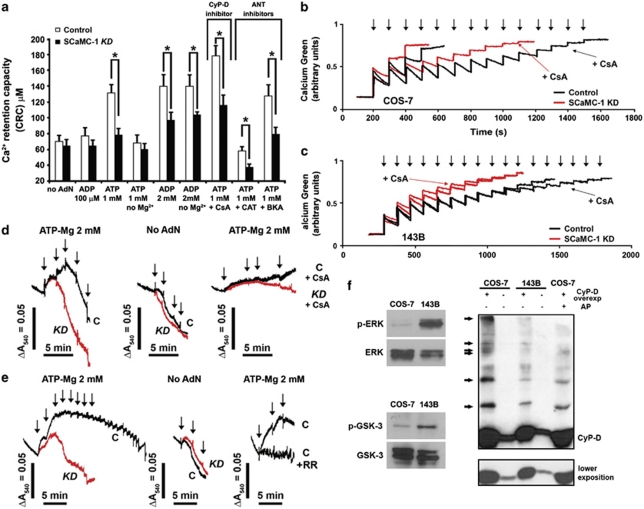
CRC was studied by monitoring Ca2+ uptake into mitochondria by using Calcium-Green to measure [Ca2+] in the buffer. Permeabilized cells were treated with sequential Ca2+ pulses and Ca2+ uptake into mitochondria was measured as a decrease in fluorescence. mPTP opening was detected as a steady increase of fluorescence owing to Ca2+ release from mitochondria. Other organelles, such as the ER, do not contribute significantly to Ca2+ uptake, as RR completely inhibited uptake (not shown). As summarized in Figure 5a, permeabilized control and SCaMC-1-KD COS-7 cells show similar mitochondrial CRCs both in the absence of adenine nucleotides and in the presence of low ADP (100 μM). In both lines, 100 μM ADP increased the CRC only slightly (less than 10% on COS-7 cells (P>0.05); 20% in 143B cells (P<0.05); not shown), probably by interacting with the ANT and triggering the ‘m' conformation of the carrier, as demonstrated previously in liver and brain mitochondria.20 Most importantly, however, in the presence of 1 mM ATP or 2 mM ADP, control COS-7 or 143B cells showed a pronounced increase in the threshold for mPTP opening as compared with SCaMC-1-KD cells, whose CRC was practically identical to that measured in the presence of 100 μM ADP. The presence of Mg2+ was essential for the protective effect of ATP, but not for that of ADP, consistently with the substrate specificity of SCaMC-1. The same results were obtained in isolated mitochondria from COS-7 or 143B cells (Figures 5b and c), as mitochondria from control cells are able to take up more Ca2+ in the presence of 1 mM ATP or 2 mM ADP than SCaMC-1-KD mitochondria before release of accumulated Ca2+ by mPTP opening.
Figure 5.
SCaMC-1 reduces the Ca2+ sensitivity of mPT in isolated mitochondria and permeabilized cells. Ca2+-mediated mPT in control (black traces) and SCaMC-1-KD (red traces) COS-7 (a, b and d) and 143B cell (c and e) mitochondria. (a) Quantification of CRC after administration of subsequent Ca2+ pulses (20 μM each) to control (white bars) and SCaMC-1-KD (black bars) permeabilized COS-7 cells (0.15 mg total protein per millilitre). CRC was determined in the presence or absence of adenine nucleotides, Mg2+ and inhibitors. The Ca2+ concentration required to open the mPTP is shown (mean±S.E.M., n=6–16). (b and c) CRC was measured in isolated mitochondria in the presence of 1 mM ATP (b) or 2 mM ADP (c) by using the Ca2+-sensitive probe Calcium-Green 5N free salt in the extra-mitochondrial space. Experiments started by addition of mitochondria, followed by repetitive Ca2+ pulses (arrows, 20 μM each). The traces marked by a long arrow are in the presence of 5 μM CsA. (d and e) Swelling of isolated mitochondria after subsequent Ca2+ pulses (arrows, 40 μM each) was measured as a decrease in light scattering (as absorbance at 540 nm) in the presence (left panel) or absence (middle panel) of 2 mM ATP. Traces in the right panel were recorded in the presence of 2 mM ATP and either 5 μM CsA (d) or 200 nM RR (e). (f) The ERK pathway is constitutively active in 143B cells, but not in COS-7 cells, and controls CyP-D phosphorylation. Left panels: Immunoblot against phospho-ERK and total ERK, and phospho-GSK-3β and total GSK-3β, in total homogenates from COS-7 and 143B cells. Right panel: Immunoblot against CyP-D in total homogenates from control or FLAG-CyP-D8 overexpressing COS-7 and 143B cells after Mn2+-Phos-tag SDS-PAGE.39 Phosphorylated proteins are shifted upwards in the gel compared with unphosphorylated proteins. The same bands were observed when anti-FLAG antibodies were used, confirming the specificity of the bands (not shown). CyP-D phosphorylation is greatly decreased if the extracts are treated with alkaline phosphatase (AP) for 30 min (far right lane)
mPTP opening can also be studied by monitoring mitochondrial swelling. Figure 5d shows that Ca2+ addition to COS-7 mitochondria in the presence of ATP initially caused contraction, while further additions led to swelling (a decrease in absorbance). The number of Ca2+ additions needed to trigger swelling in the presence of 2 mM ATP was always higher in control mitochondria as compared with SCaMC-1-KD mitochondria. On the other hand, swelling was identical in the absence of nucleotides. Similar results were obtained in 143B isolated mitochondria (Figure 5e).
In the presence of 5 μM CsA, an inhibitor of mPT, the CRC was enhanced in permeabilized cells and isolated COS-7 mitochondria (Figures 5a and b), and mitochondrial swelling was inhibited (Figure 5d), demonstrating that the effects observed were due to mPTP opening. CsA produced these effects in both control and SCaMC-1-KD mitochondria, but differences between both lines in the presence of 1 mM ATP were still observed. On the other hand, 5 μM CAT, a stimulator of mPT, greatly reduced the CRC in both control and SCaMC-1-KD permeabilized cells, but a difference between the two cell lines in the presence of 1 mM ATP was still observed (Figure 5a). The increase in CRC by 1 mM ATP in the presence of CAT in control cells proves that the effect of high concentrations of adenine nucleotides was indeed independent of the ANT, as had been suggested previously.27 Furthermore, in the presence of 5 μM BKA, another ANT inhibitor, the differences between both lines obtained at 1 mM ATP were still present (Figure 5a).
Interestingly, 143B permeabilized cells and isolated mitochondria were largely unaffected by CsA (Figure 5c and Supplementary Figure S4B). This is not due to a lower expression of CyP-D (Figure 1e), the regulatory component of the pore that is targeted by CsA, an argument, which is used to explain the CsA insensibility of brain mitochondria.28 An alternative explanation is that 143B are tumour cells, whereas COS-7 are merely immortalized cells. Tumour cells show a constitutive activation of the ERK pathway, which has been suggested to dephosphorylate and inactivate CyP-D,8 and, thus, although present, CyP-D may be inactive in 143B mitochondria. In order to study this possibility, first the phosphorylation state of CyP-D was assayed by the Mn2+-Phos-tag SDS-PAGE technique. In FLAG-CyP-D-overexpressing COS-7 cells, several shifted CyP-D bands were observed, demonstrating the phosphorylated status of the protein (Figure 5f). Importantly, these bands were almost completely missing in FLAG-CyP-D-overexpressing 143B cells in spite of equal expression of CyP-D. In addition, 143B cells showed a constitutive phosphorylation of ERK, whereas COS-7 cells did not (Figure 5f). In this pathway, ERK was shown to phosphorylate and inactivate GSK-3β, the putative CyP-D kinase. Indeed, we found that that GSK-3β was phosphorylated in 143B cells but not in COS-7 cells (Figure 5f). These results suggest that, in 143B cells, as a result of a constitutively active ERK/GSK-3β pathway, CyP-D is mostly dephosphorylated and thus inactive. Conversely, in COS-7 cells CyP-D is partially phosphorylated and active owing to lack of ERK activation.
In summary, these results provide evidence that adenine nucleotides regulate mPTP opening independently of the ANT, through a lower affinity site, reflecting the activity of the ATP-Mg/Pi carrier. Modification of the mitochondrial adenine nucleotide content by the ATP-Mg/Pi carrier regulates the CRC and mPTP opening, probably by modifying the formation of Ca2+-Pi precipitates and thus altering the Ca2+ buffering capacity of the mitochondrial matrix.
Concluding Remarks
Altogether, our data fully support a model where SCaMC-1 is responsible for a cytoprotective mechanism by mediating (i) ATP/ADP uptake in the mitochondrial matrix, triggered by increases of [Ca2+]cyt; (ii) increased mitochondrial Ca2+ buffering capacity owing to elevated adenine nucleotide levels; and (iii) desensitization of Ca2+-mediated mPT. This pathway represents a novel mechanism promoting cancer cell survival under stress conditions.
High levels of SCaMC-1 were previously found by screening approaches in colorectal carcinomas,29 breast cancer30 and in several cancer cell lines.15, 31 Our in silico studies confirmed and extended these findings to 67 different tumour types where SCaMC-1 expression significantly exceeded its expression in their normal tissue counterpart. Importantly, this pattern was specific to the SCaMC-1 isoform, which has an order of magnitude higher transport activity than SCaMC-3.18 This suggested that the benefit conferred by SCaMC-1 overexpression implies a high rate of transport of adenine nucleotides in the mitochondrial matrix under conditions of pathologically elevated [Ca2+]cyt. The S0.5 for Ca2+ of SCaMC-1 was found to be 12.7 μM, adequate to respond to changes in the [Ca2+]cyt that are transmitted to the mitochondria through the MCU during Ca2+ overload both under physiological and pathological conditions. Indeed, SCaMC-1 expression was not essential for cellular proliferation, rather was required for protection against oxidative stress- and Ca2+ overload-induced cell death. This resembles the effect of Bcl-2 overexpression,32 Bax and Bak downregulation,6 Bad phosphorylation,33 hexokinase-II binding to mitochondria7 and constitutive activation of the ERK pathway.8 Distinct from some of these studies, however, is that the role of SCaMC-1 was exclusive to mPT-driven cell death, as no effect was observed on cell death induced by staurosporine. Altogether, selective overexpression of SCaMC-1 over normal tissues and its specific role in protection from oxidative stress-induced cell death indicate that selective ablation of SCaMC-1 function might represent a novel strategy to abolish tumour growth in a wide range of cancers.
Low concentrations of adenine nucleotides (≈100 μM) inhibit mPTP opening in isolated liver and brain mitochondria,26 most likely by conformational changes triggered by binding to high-affinity sites (Kd ≈ 5–10 μM) of the ANT.20 However, adenine nucleotides in the millimolar, physiological range also show potent mPT inhibition, even in the presence of ANT inhibitors.10, 27, 34 The site of this ANT-independent protection remained unexplained.35 Here we found that SCaMC-1, which shows binding sites for adenine nucleotides in this affinity range (Kd ≈0.2–0.5 mM), is able to mediate this effect.
While the mechanism by which matrix adenine nucleotides protect from mPT is not completely understood, our data clearly suggest that altered mitochondrial Ca2+ handling has a major role in the effect. The lack of ATP/ADP uptake in the mitochondria of SCaMC-1-KD cells led to a vast increase in free matrix [Ca2+] after activation of cellular Ca2+ signalling pathways, as compared with cells with high SCaMC-1 expression levels.
Accordingly, we propose that the SCaMC-1-mediated adenine nucleotide load in the mitochondrion buffers matrix free [Ca2+], preventing mitochondrial Ca2+ overload during oxidative stress and preventing mPT induction. As a major route of mitochondrial Ca2+ buffering, Ca2+ and Pi form amorphous Ca3(PO4)2 precipitates in the matrix.23, 25 It has long been suggested that presence of adenine nucleotides in the mitochondrial matrix is essential for this process, either by stabilizing the Ca2+-Pi deposits or by priming their precipitation.24 As a result, mitochondrial Ca2+-Pi precipitates contain adenine nucleotides,36 and ATP may account for as much as 7% of the dry weight of some Ca2+-Pi precipitates.37 Interestingly, Ca2+-Pi precipitates in isolated mitochondria also contain Mg2+ when Ca2+ loading was performed in the presence of ATP, but not in the presence of ADP,25 which matches the substrate specificity of the ATP-Mg/Pi carriers.11, 18
The protective effect of increasing amounts of matrix Ca2+-Pi precipitates has already been observed, particularly in cell death models mediated entirely by Ca2+ overload, such as glutamate excitotoxicity in primary neurons.38 Our findings extend these observations to cancer cells, and provide direct demonstration of the effect of SCaMC-1 knockdown-mediated adenine nucleotide depletion on mitochondrial Ca2+ handling.
Materials and Methods
Cell culture, transfection, generation of SCaMC-1-KD and mitochondrial luciferase-expressing clones
COS-7 cells, liver clone-9 cells and 143B osteosarcoma cells were cultured in Dulbecco's modified Eagle's medium supplemented with 5% inactivated fetal bovine serum (Invitrogen, Carlsbad, CA, USA) at 37 °C in a 5% CO2 atmosphere. For transfections, the LipofectAMINE reagent (Invitrogen) was used as described previously.15 Details of the constructs and generation of SCaMC-1-KD clones, cells overexpressing SCaMC-1 or cells stably expressing mitochondrially targeted luciferase are described under Supplementary Materials and Methods.
Immunofluorescence and western blotting
Immunofluorescent stainings were performed as described previously.15 Anti-SCaMC-1 antibodies were diluted 1 : 400. Fluorescence microscopy was performed by using an Axiovert epifluorescence microscope (Carl Zeiss, Göttingen, Germany) at a nominal magnification of × 100.
For western blotting, total homogenates and mitochondrial-enriched fractions were obtained from cells and mouse tissues as described15 and analysed by western blotting (for details see Supplementary Materials and Methods). To determine the phosphorylation status of CyP-D, Mn2+-Phos-tag SDS-PAGE was performed.39 Polyacrylamide gels were supplemented with 200 μM MnCl2 and the Phos-tag reagent (Nard Institute, Amagasaki, Japan). Phosphorylated proteins bind to the Phos-tag, and thus are delayed in the gel and separated from unphosphorylated proteins. After electrophoresis, the gels were washed with transfer buffer containing 1 mM EDTA and then with transfer buffer without EDTA according to the manufacturer's protocol, before transfer to PVDF membranes. The membranes were probed with CyP-D antibodies.
Measurements of mitochondrial Ca2+ uptake and swelling on isolated mitochondria or permeabilized cells
Mitochondria were isolated by homogenization and differential centrifugation according to standard protocols. [Ca2+]m uptake was measured in the presence of the Ca2+-sensitive fluorescent probe Calcium-Green 5N (0.1 μM, excitation 506 nm, emission 532 nm) using digitonin-permeabilized cells or isolated mitochondria (for details see Supplementary Materials and Methods).
Measurement of cellular respiration and Δψm
Cell respiration was measured using a Seahorse XF24 Extracellular Flux Analyzer (Seahorse Bioscience, Billerica, MA, USA; for details see Supplementary Materials and Methods). Δψm was measured using the fluorescent lipophilic cationic dye TMRM. Cells were stained with 200 nM TMRM for 10 min and red fluorescence was measured by flow cytometry.
Ten percent laser power of the 543 He–Ne laser line on the Zeiss 510 confocal system was used to induce mPT, which was detected by loss of TMRM signal, as described previously.19 Cells were pre-incubated with 100 nM TMRM for 20 min at 37 °C in a modified Krebs–Ringer buffer (KRB; 135 mM NaCl, 5 mM KCl, 1 mM MgSO4, 0.4 mM K2HPO4, 1 mM CaCl2, 5.5 mM glucose, 20 mM HEPES, pH 7.4). TMRM was then present throughout the subsequent measurements. Confocal images were obtained using a Zeiss 510 LSM/META system, using a × 40 oil-immersion objective. For detection a 560-nm long-pass filter was used.
Luminescent detection of cellular [Ca2+] and [ATP]
[Ca2+]cyt and [Ca2+]m in intact cells were measured 48 h after transient transfection with cytAEQ and mtAEQwt probes as described previously.22 To measure mitochondrial ATP levels in permeabilized cells, experiments were performed 24 h after transient transfection with mitochondrial luciferase.17 For experimental details see Supplementary Materials and Methods.
Cell death detection
Cells were incubated with H2O2, menadione or staurosporine for the time periods indicated in the figure legends and cell death was evaluated by previously described methods:5, 7, 8, 21 (i) by microscopy after loading with 1 μM calcein-AM to stain live cells and 2 μM PI to stain the nuclei of dead cells; (ii) by flow cytometry after loading with 2 μM PI to stain the nuclei of dead cells; (iii) by flow cytometry after incubation with 200 nM TMRM to detect mitochondrial depolarization and FITC-conjugated Annexin-V (Sigma, St. Louis, MO, USA) to detect phosphatidylserine exposure on the cell surface; and (iv) by flow cytometry using the Annexin-V–FITC Apoptosis Detection kit (Sigma).
Acknowledgments
We thank Dr. María Sánchez-Aragó, Dr. Laura Formentini, Laura Sánchez-Cenizo and María Royo for help, reagents and comments; Dr. Claudia Castillo for help with data analysis; and Alejandro Arandilla for technical assistance. We also thank Dr. Paolo Bernardi and Dr. Andrea Rasola for the supply of plasmid pcDNA3-FLAG-CyP-D. This work was supported by grants from Ministerio de Educación y Ciencia (BFU2008-04084/BMC), Comunidad de Madrid (S-GEN-0269-2006 MITOLAB-CM), the European Union (LSHM-CT-2006-518153), and CIBERER (an initiative of the ISCIII) to JS; by grants from the ISCIII (PI080610) to AdelA; and by an institutional grant from the Fundación Ramón Areces to the Centro de Biología Molecular Severo Ochoa. GS was supported by Parkinson's UK (G-0905). JT is a recipient of a fellowship from Comunidad de Madrid.
Glossary
- [Ca2+]cyt
cytosolic [Ca2+]
- [Ca2+]m
mitochondrial [Ca2+]
- ANT
adenine nucleotide translocator
- BKA
bongkrekic acid
- CAT
carboxyatractyloside
- CRC
Ca2+ retention capacity
- CsA
cyclosporin-A
- CyP-D
cyclophilin-D
- cytAEQ
cytosolic aequorin
- ER
endoplasmic reticulum
- IC
intracellular
- IMM
inner mitochondrial membrane
- IP3
inositol-1,4,5-trisphosphate
- MCU
mitochondrial Ca2+ uniporter
- mPT
mitochondrial permeability transition
- mPTP
mitochondrial permeability transition pore
- mtAEQmut
low-affinity mitochondrial aequorin
- mtAEQwt
mitochondrial aequorin
- RR
ruthenium red
- ROS
reactive oxygen species
- VDAC
voltage-dependent anion channel
- ΔΨ
mitochondrial membrane potential
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by R Gottlieb
Supplementary Material
References
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer's Achilles' heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- Di Lisa F, Bernardi P. Mitochondria and ischemia-reperfusion injury of the heart: fixing a hole. Cardiovasc Res. 2006;70:191–199. doi: 10.1016/j.cardiores.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- Chiara F, Castellaro D, Marin O, Petronilli V, Brusilow WS, Juhaszova M, et al. Hexokinase II detachment from mitochondria triggers apoptosis through the permeability transition pore independent of voltage-dependent anion channels. PLoS One. 2008;3:e1852. doi: 10.1371/journal.pone.0001852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasola A, Sciacovelli M, Chiara F, Pantica B, Brusilow WS, Bernardi P. Activation of mitochondrial ERK protects cancer cells from death through inhibition of the permeability transition. Proc Natl Acad Sci USA. 2010;107:726–731. doi: 10.1073/pnas.0912742107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AW, Szabadkai G. Ca2+ transfer from the ER to mitochondria: channeling cell death by a tumor suppressor. Dev Cell. 2011;19:789–790. doi: 10.1016/j.devcel.2010.11.013. [DOI] [PubMed] [Google Scholar]
- Zoratti M, Szabò I. The mitochondrial permeability transition. Biochim Biophys Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- Aprille JR. Mechanism and regulation of the mitochondrial ATP-Mg/P(i) carrier. J Bioenerg Biomembr. 1993;25:473–481. doi: 10.1007/BF01108404. [DOI] [PubMed] [Google Scholar]
- Satrústegui J, Pardo B, del Arco A. Mitochondrial transporters as novel targets for intracellular calcium signaling. Physiol Rev. 2007;87:29–67. doi: 10.1152/physrev.00005.2006. [DOI] [PubMed] [Google Scholar]
- Traba J, Satrústegui J, del Arco A. Characterization of SCaMC-3-like/slc25a41, a novel calcium-independent mitochondrial ATP-Mg/Pi carrier. Biochem J. 2009;418:125–133. doi: 10.1042/BJ20081262. [DOI] [PubMed] [Google Scholar]
- del Arco A, Satrústegui J. New mitochondrial carriers: an overview. Cell Mol Life Sci. 2005;62:2204–2227. doi: 10.1007/s00018-005-5197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Arco A, Satrústegui J. Identification of a novel human subfamily of mitochondrial carriers with calcium-binding domains. J Biol Chem. 2004;279:24701–24713. doi: 10.1074/jbc.M401417200. [DOI] [PubMed] [Google Scholar]
- Traba J, Froschauer EM, Wiesenberger G, Satrústegui J, del Arco A. Yeast mitochondria import ATP through the calcium-dependent ATP-Mg/Pi carrier Sal1p, and are ATP consumers during aerobic growth in glucose. Mol Microbiol. 2008;69:570–585. doi: 10.1111/j.1365-2958.2008.06300.x. [DOI] [PubMed] [Google Scholar]
- Poncet D, Pauleau AL, Szabadkai G, Vozza A, Scholz SR, Le Bras M, et al. Cytopathic effects of the cytomegalovirus-encoded apoptosis inhibitory protein vMIA. J Cell Biol. 2006;174:985–996. doi: 10.1083/jcb.200604069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiermonte G, De Leonardis F, Todisco S, Palmieri L, Lasorsa FM, Palmieri F. Identification of the mitochondrial ATP-Mg/Pi transporter. Bacterial expression, reconstitution, functional characterization, and tissue distribution. J Biol Chem. 2004;279:30722–30730. doi: 10.1074/jbc.M400445200. [DOI] [PubMed] [Google Scholar]
- Duchen MR. Mitochondria and Ca(2+)in cell physiology and pathophysiology. Cell Calcium. 2000;28:339–348. doi: 10.1054/ceca.2000.0170. [DOI] [PubMed] [Google Scholar]
- Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-Dyson E, Di Lisa F, et al. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 2006;273:2077–2099. doi: 10.1111/j.1742-4658.2006.05213.x. [DOI] [PubMed] [Google Scholar]
- Szabadkai G, Simoni AM, Chami M, Wieckowski MR, Youle RJ, Rizzuto R. Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol Cell. 2004;16:59–68. doi: 10.1016/j.molcel.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Chiesa A, Rapizzi E, Tosello V, Pinton P, de Virgilio M, Fogarty KE, et al. Recombinant aequorin and green fluorescent protein as valuable tools in the study of cell signalling. Biochem J. 2001;355:1–12. doi: 10.1042/0264-6021:3550001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG, Chalmers S. The integration of mitochondrial calcium transport and storage. J Bioenerg Biomembr. 2004;36:277–281. doi: 10.1023/B:JOBB.0000041753.52832.f3. [DOI] [PubMed] [Google Scholar]
- Carafoli E, Rossi CS, Lehninger AL. Uptake of adenine nucleotides by respiring mitochondria during active accumulation of Ca++ and phosphate. J Biol Chem. 1965;240:2254–2261. [PubMed] [Google Scholar]
- Kristián T, Pivovarova NB, Fiskum G, Andrews SB. Calcium-induced precipitate formation in brain mitochondria: composition, calcium capacity, and retention. J Neurochem. 2007;102:1346–1356. doi: 10.1111/j.1471-4159.2007.04626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, et al. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gizatullina ZZ, Chen Y, Zierz S, Gellerich FN. Effects of extramitochondrial ADP on permeability transition of mouse liver mitochondria. Biochim Biophys Acta. 2005;1706:98–104. doi: 10.1016/j.bbabio.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Kristián T, Gertsch J, Bates TE, Siesjö BK. Characteristics of the calcium-triggered mitochondrial permeability transition in nonsynaptic brain mitochondria: effect of cyclosporin A and ubiquinone O. J Neurochem. 2000;74:1999–2009. doi: 10.1046/j.1471-4159.2000.0741999.x. [DOI] [PubMed] [Google Scholar]
- Chen JS, Chen KT, Fan CW, Han CL, Chen YJ, Yu JS, et al. Comparison of membrane fraction proteomic profiles of normal and cancerous human colorectal tissues with gel-assisted digestion and iTRAQ labeling mass spectrometry. FEBS J. 2010;277:3028–3038. doi: 10.1111/j.1742-4658.2010.07712.x. [DOI] [PubMed] [Google Scholar]
- Chen YW, Chou HC, Lyu PC, Yin HS, Huang FL, Chang WS, et al. Mitochondrial proteomics analysis of tumorigenic and metastatic breast cancer markers. Funct Integr Genomics. 2011;11:225–239. doi: 10.1007/s10142-011-0210-y. [DOI] [PubMed] [Google Scholar]
- Fountoulakis M, Schlaeger EJ. The mitochondrial proteins of the neuroblastoma cell line IMR-32. Electrophoresis. 2003;24:260–275. doi: 10.1002/elps.200390022. [DOI] [PubMed] [Google Scholar]
- Pinton P, Ferrari D, Rapizzi E, Di Virgilio F, Pozzan T, Rizzuto R. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. EMBO J. 2001;20:2690–2701. doi: 10.1093/emboj/20.11.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy SS, Madesh M, Davies E, Antonsson B, Danial N, Hajnóczky G. Bad targets the permeability transition pore independent of Bax or Bak to switch between Ca2+-dependent cell survival and death. Mol Cell. 2009;33:377–388. doi: 10.1016/j.molcel.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen T, Lagace CJ, Modica-Napolitano JS, Aprille JR. Permeability transition in rat liver mitochondria is modulated by the ATP-Mg/Pi carrier. Am J Physiol Gastrointest Liver Physiol. 2003;285:G274–G281. doi: 10.1152/ajpgi.00052.2003. [DOI] [PubMed] [Google Scholar]
- Chinopoulos C, Adam-Vizi V. Mitochondrial Ca(2+) sequestration and precipitation revisited. FEBS J. 2010;277:3637–3651. doi: 10.1111/j.1742-4658.2010.07755.x. [DOI] [PubMed] [Google Scholar]
- Weinbach EC, von Brand T. The isolation and composition of dense granules from Ca++-loaded mitochondria. Biochem Biophys Res Commun. 1965;19:133–137. [Google Scholar]
- Becker GL, Chen CH, Greenawalt JW, Lehninger AL. Calcium phosphate granules in the hepatopancreas of the blue crab Callinectes sapidus. J Cell Biol. 1974;61:316–326. doi: 10.1083/jcb.61.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivovarova NB, Andrews SB. Calcium-dependent mitochondrial function and dysfunction in neurons. FEBS J. 2010;277:3622–3636. doi: 10.1111/j.1742-4658.2010.07754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa T, Saito T, Asada A, Fukunaga K, Hisanaga S. Quantitative measurement of in vivo phosphorylation states of Cdk5 activator p35 by Phos-tag SDS-PAGE. Mol Cell Proteomics. 2010;9:1133–1143. doi: 10.1074/mcp.M900578-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlén M, Björling E, Agaton C, Szigyarto CA, Amini B, Andersen E, et al. A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol Cell Proteomics. 2005;4:1920–1932. doi: 10.1074/mcp.M500279-MCP200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.