Abstract
Objectives.
Gerontological research suggests that depressive symptoms show antecedent and consequent relations with late-life disability. Less is known, however, about how depressive symptoms change with the progression of disability-related processes and what factors moderate such changes.
Methods.
We applied multiphase growth models to longitudinal data pooled across 4 Swedish studies of very old age (N = 779, M age = 86 years at disability onset, 64% women) to describe change in depressive symptoms prior to disability onset, at or around disability onset (the measurement wave at which assistance in personal activities of daily living was first recorded), and postdisability onset.
Results.
Results indicate that, on average, depressive symptoms slightly increase with approaching disability, increase at onset, and decline in the postdisability phase. Age, study membership, being a woman, and multimorbidity were related to depressive symptoms, but social support emerged as the most powerful predictor of level and change in depressive symptoms.
Discussion.
Our findings are consistent with conceptual notions implicating disability-related factors as key contributors to late-life change and suggest that contextual and psychosocial factors play a pivotal role for how well people adapt to late-life challenges.
Keywords: Disability, Growth models, Late life, Oldest old, Successful aging
FUNCTIONAL changes and disablement processes are often closely intertwined with psychosocial risk and protective factors (Verbrugge & Jette, 1994). Empirical studies linking depressive symptoms and disability have primarily focused on antecedent–consequent relations, with depressive symptoms predicting subsequent disability (Bruce, Seeman, Merrill, & Blazer, 1994; Li & Conwell, 2009; Penninx, Leveille, Ferrucci, van Eijk, & Guralnik, 1999; Tinetti, Inouye, Gill, & Doucette, 1995; and for a review, see Schillerstrom, Royall, & Palmer, 2008), disability onset predicting subsequent increases in depressive symptoms (Chang et al., 2009; Covinsky et al., 2010; Roberts, Kaplan, Shema, & Strawbridge, 1997; Weinberger, Raue, Meyers, & Bruce, 2009; Zeiss, Lewinsohn, Rohde, & Seeley, 1996), or even bidirectional associations (Ormel, Rijsdijk, Sullivan, van Sonderen, & Kempen, 2002).
Associations between changes in depressive symptoms and disability are also studied via process-oriented perspectives promoting the idea that in late life, changes in depression and disability are closely intertwined (Taylor & Lynch, 2004). The Disablement Process model (Verbrugge & Jette, 1994) suggests that the path from disease to disability has a bidirectional relationship with many psychological factors, including depression. Specifically, the model states that acute and chronic diseases may eventually lead to impairment in body systems, and when these impairments worsen, functional abilities become constrained. Individuals become disabled when they no longer function independently. Finally, at all phases, this underlying disablement process is likely accompanied by systematic changes in psychological factors, including depression.
Observing disablement as a process by which depression changes requires repeated measurements of both depression and disability along with the use of a disability-related time metric. Prior studies have utilized various time metrics to study different types of developmental processes (Li & Schmiedek, 2002; Ram, Gerstorf, Fauth, Zarit, & Malmberg, 2010; Sliwinski, Hofer, Hall, Buschke, & Lipton, 2003; Wohlwil, 1973). For example, when longitudinal data are tracked in relation to birth, the time variable “age” serves as a proxy for progressive “aging-related” processes (Alwin, Hofer, & McCammon, 2006; Hertzog & Nesselroade, 2003). When tracked in relation to death, the variable “time-to-death” serves as a proxy for progressive mortality-related processes (Diehr, Williamson, Burke, & Psaty, 2002; Gerstorf, Ram, Estabrook, et al., 2008; Gerstorf, Ram, Fauth, Schupp, & Wagner, 2009; Gerstorf, Ram, Röcke, et al. 2008; Ghisletta, McArdle, & Lindenberger 2006; Johansson et al., 2004; Sliwinski et al., 2006; Thorvaldsson, Hofer, & Johansson, 2006; Wilson, Beck, Bienias, & Bennett, 2007; Wilson, Beckett, Bienias, Evans, & Bennett, 2003). Similarly, when tracked in relation to the onset of disability, the variable “time-to/from-disability” serves as a proxy for progressive disability-related processes (Ram et al., 2010). Our objective here is to pursue a process-oriented approach and examine how depressive symptoms change vis-à-vis a disablement time metric.
The Role of Demographic Characteristics, Cognitive Function, Health, and Social Support
Several factors posited to influence disability-related processes are also related to the level or progression of depressive symptoms. These variables include demographic characteristics (such as age, gender, education, widowhood, and living in an institution) and psychosocial risk or protective factors, including cognitive ability, health, and social support.
Specifically, chronological age is associated with higher prevalence of depressive symptoms (Mirowsky & Ross, 1992). Relative to men, women experience steeper increases in depressive symptoms over time (Yang & George, 2005) and are more prone to suffer from major depression (Blazer, 2003). More education protects against increases in depression late in life (Yang & George, 2005); however, widowhood status predicts higher depressive symptoms (Bennett, 1997; Lucas, Clark, Georgellis, & Diener, 2003), and the transition into an institution and continued use of long-term care are predictive of increases in depressive symptoms, even after controlling for certain physical and cognitive aspects of health (Pot, Deeg, Twisk, Beekman, & Zarit, 2005).
Associations have also been reported between cognitive performance (particularly memory recall) and both number of depressive symptoms (Rosenberg, Mielke, Xue, & Carlson, 2010) and time spent in a depressive episode (Gorwood, Corruble, Falissard, & Goodwin, 2008). In a similar vein, multiple indicators of health including specific diseases (e.g., lung disease, arthritis, cardiovascular disease, cancer) and the presence of multiple health conditions, or multimorbidity, predict greater depressive symptoms, even after controlling for demographic and psychosocial covariates (Bisschop, Kriegsman, Deeg, Beekman, & van Tilburg, 2004; Lee, Choi, & Lee, 2001; Palinkas, Wingard, & Barrett-Connor, 1990; Williamson & Schulz, 1992). Finally, social factors predict the progression of depression and disability, typically serving as a protective role for both. Greater levels of support are associated with fewer depressive symptoms (Glass, De Leon, Bassuk, & Berkman, 2006; Yang & George, 2005), and longitudinal studies of disability find that the presence of depression in nondisabled older adults predicts an increased risk of future disability onset, partially because of the intermediary role of fewer social interactions (Penninx et al., 1999). In sum, social support is a central mediator of relations between disability and depressive symptoms (Taylor & Lynch, 2004; Yang, 2006).
The Current Study
We address two main research questions. First, how do depressive symptoms change along a time-to/from-disability time axis that serves as a proxy for the progression of disability-related processes? We apply multiphase growth models to longitudinal data obtained from older adults to describe changes in depressive symptoms in the years prior to the onset of disability, those occurring at or around disability onset (i.e., the occasion of measurement at which disability was first reported), and those proceeding in the years immediately following disability. The hedonic adaptation model (Brickman & Campbell, 1971) suggests that initially people’s well-being decreases when they experience misfortune, but afterward, they adapt and habituate to the situation, and they no longer feel unhappy. Following such a model, we hypothesize that, on average, depressive symptoms increase as individuals approach disability, increase rapidly at disability onset (Chang et al., 2009), and then adjust back toward prior levels.
Second, are between-person differences in disability-related change in depressive symptoms linked to extra- and intra-individual factors? Drawing from the Disablement Process model (Verbrugge & Jette, 1994), we expect the extent of disability-related change to be moderated by demographic characteristics (age, gender, education, widowhood, and living in an institution), cognitive function (memory ability), health (multimorbidity), and social support. In particular, we hypothesize that higher education, memory ability, and social support serve as resources people can draw from in times of need and should thus be related to more favorable disability-related trajectories.
METHOD
To examine change in depressive symptoms along a disablement time metric, we pooled data across four multidisciplinary population-based studies of aging in Sweden: GENDER (Gold, Malmberg, McClearn, Pedersen, & Berg, 2002), OCTO (Johansson & Zarit, 1995), OCTO-TWIN (McClearn et al., 1997), and NONA (Fauth, Zarit, Malmberg, & Johansson, 2007). Each of the studies involves at least three waves of longitudinal data collected at approximately two-year intervals (4 years for GENDER) over 12 years from individuals aged 70 to 100+ years who were living in either ordinary housing or institutional housing (total N = 1,793). Design by overlapping sets of researchers, and consistency of sampling procedures, measures, and protocols allowed for straightforward pooling of the data. To account for potential study differences, a variable denoting the original study for the individual (“study membership”) is included as a control variable in the analyses. The original studies were each approved by research ethics committees at Linköping University (NONA and OCTO) and the Karolinska Institute (OCTO-Twin and GENDER) in Sweden.
Participants and Procedure
Participants in OCTO and NONA were selected randomly from the population registry containing the names and birth dates of all residents in the municipality of Jönköping, Sweden. This region includes rural, suburban, and urban settings and is considered representative of the variety of living situations throughout the nation. In OCTO, people initially aged 84, 86, 88, and 90 years were identified and recruited. In NONA, initial ages were 86, 90, and 94 years. Participants in OCTO-TWIN and GENDER were recruited from population-based twin registries that list all instances of multiple births in the country. OCTO-TWIN is a representative sample of all intact, same-sex twin pairs (mono- and dizygotic) aged 80+ years at baseline. GENDER is a representative sample of unlike-sex twins aged 70–79 years at baseline. Relative to representative samples of same-aged singletons, twin samples identified from the twin registry were similar in vitality, well-being, physical and cognitive functioning, and health utilization (Simmons et al., 1997). Across the studies, more than 80% of initially contacted individuals agreed to participate.
Included in our analyses were all 779 participants who had experienced, at any point during the course of data collection, some form of disability, defined as needing assistance in one or more personal activities of daily living (PADL) tasks (Guralnik, Alecxih, Branch, & Wiener, 2002; Seeman, Bruce, & McAvay, 1996; see “Measures” section later for more details). As one would expect, relative to the remaining participants, our disabled subsample was older at baseline assessment, M = 84.26, SD = 5.12 vs. M = 80.79, SD = 6.16; F (1, 1,755) = 159.4, had slightly less education, M = 6.95, SD = 2.17 vs. M = 7.23, SD = 2.23; F (1, 1,579) = 6.5, reported more depressive symptoms, M = 0.65, SD = 0.50 vs. M = 0.51, SD = 0.44; F (1, 1,438) = 34.66, but did not differ in gender representation (p > .10). Table 1 provides an overview of sample characteristics separately for the four studies.
Table 1.
Sample Characteristics Across the Four Studies
| Study |
||||||||
| GENDER |
OCTO-TWIN |
OCTO |
NONA |
|||||
| Start year | 1995 | 1990 | 1987 | 1999 | ||||
| Years of follow-up | 4 | 8 | 6 | 4 | ||||
| Occasions | 2 | 5 | 4 | 3 | ||||
| N at T1 | 112 | 335 | 228 | 104 | ||||
| M (SD) | n (%) | M (SD) | n (%) | M (SD) | n (%) | M (SD) | n (%) | |
| Depressive symptoms T1 | 52.16 (9.36) | 48.75 (9.97) | 55.28 (10.54) | 50.73 (10.07) | ||||
| Time-to-disability T1 | −3.61 (2.94) | −1.90 (2.31) | −0.40 (1.04) | −0.93 (1.26) | ||||
| Age T1 | 75.20 (2.61) | 83.83 (3.29) | 86.95 (2.28) | 89.51 (3.24) | ||||
| Years of education | 7.41 (2.17) | 7.16 (2.42) | 6.49 (1.79) | 6.79 (1.89) | ||||
| Women | 60 (54) | 221 (66) | 147 (64) | 73 (70) | ||||
| Widowed | 31 (28) | 181 (54) | 136 (60) | 71 (68) | ||||
| Institutionalized (predisability) | 15 (13) | 115 (34) | 84 (37) | 49 (47) | ||||
| Institutionalized (disability onset) | 14 (13) | 114 (34) | 84 (37) | 49 (47) | ||||
| Institutionalized (postdisability) | 28 (25) | 188 (56) | 126 (55) | 68 (65) | ||||
| Memory (predisability) | 51.54 (13.62) | 41.12 (17.46) | 40.15 (16.20) | 42.50 (15.05) | ||||
| Memory (disability onset) | 35.55 (26.20) | 35.67 (20.40) | 39.24 (16.77) | 38.51 (18.21) | ||||
| Memory (postdisability) | 35.55 (26.12) | 39.41 (18.90) | 41.24 (15.58) | 40.67 (16.85) | ||||
| Multimorbidity (predisability) | 2.15 (1.35) | 1.75 (1.41) | 0.39 (0.64) | 1.18 (1.13) | ||||
| Multimorbidity (disability onset) | 2.15 (1.35) | 1.74 (1.41) | 0.39 (0.64) | 1.11 (1.11) | ||||
| Multimorbidity (postdisability) | 2.35 (1.43) | 2.18 (1.55) | 0.57 (0.74) | 1.11 (1.11) | ||||
| Social support (predisability) | 50.39 (11.20) | 45.97 (12.42) | 44.17 (11.23) | 47.57 (10.41) | ||||
| Social support (disability onset) | 46.99 (16.30) | 44.49 (13.47) | 43.65 (11.63) | 42.68 (17.55) | ||||
| Social support (postdisability) | 46.89 (16.13) | 45.65 (10.23) | 42.89 (9.67) | 42.98 (16.36) | ||||
Note: Scores for depressive symptoms standardized to a T metric (M = 50; SD = 10) using the pooled sample of all participants at baseline assessment T1 as the reference (N = 1,440).
Measures
Depressive symptoms.—
Across studies and occasions, depressive symptoms were measured using either the full 20-item Center for Epidemiological Studies-Depression scale (Radloff, 1977) or a 10-item short version (OCTO study). For consistency across studies, we used the sum of the common 10 items as our measure of depressive symptoms, which in preliminary analyses was highly correlated with the 20-item sum score (>.85 for subsamples tested). We note that 1 of the 10 depression items (I felt lonely) overlapped in content with the construct of subjective support (a moderator variable). The analyses for the current study were first run with this item included and then rerun with this item removed. The substantive pattern of results did not differ across the two approaches. Internal consistency of the 10-item scale was high; Cronbach’s α ≥.79 across studies.
Demographics.—
Sociodemographic characteristics included age group, gender, years of education, marital status (widowed or not), and institutionalization. To capture possible nonlinear associations, “age group” was coded into three categories, age 70–79, 80–89, and 90+ years. “Institutionalization” indicated at each measurement wave whether the participant lived in ordinary housing or in an institution (e.g., nursing home or service apartment). Service apartments are residential care facilities similar to assisted living facilities in the United States, and past Swedish studies consider both nursing facilities and service apartments as examples of institutionalized care (Jensen, Lundin-Olsson, Nyberg, & Gustafson, 2002). To accommodate and control for possible differences between samples (GENDER, OCTO, OCTO-Twin, and NONA), “study membership” was included as an additional covariate.
“Memory,” specifically, scores on a delayed memory recall task, were used as an indicator of individuals’ cognitive function. These subtest scores of the Memory In Reality test (Johansson, 1988/1989) asked participants to recall 10 words that had been presented 30 min earlier.
“Multimorbidity,” the presence of multiple diseases and medical conditions, was used as an overall indicator of physical health. Reports were obtained by nurse interviewers who reviewed medical information with participants and, when necessary, family informants (Gold et al., 2002). Such self-reports of medical conditions are often consistent with physicians’ diagnoses (Simpson et al., 2004). Disease diagnoses included arthritis, hip fracture, osteoporosis, stroke, heart attack, chest pain/angina, diabetes, asthma, coughing with yellow phlegm, malignant tumor, and Parkinson’s disease. We calculated multimorbidity as the number of diseases present.
“Social support” was assessed in all studies as the sum of five items (Cronbach’s α ≥.75 across studies). Four items were drawn from the UCLA Loneliness Scale (Russell, 1982; see Hartshorne, 1993 for information on construct validity) pertaining to the extent to which participants felt they had someone to talk to and the extent to which they felt they were part of a circle of friends and supplemented with a reverse-coded fifth item measuring global loneliness.
Time-to/from-disability.—
Disability was assessed using standard self-report measures of PADLs (Katz, Ford, Moskowitz, Jackson, & Jaffe, 1963) with reliability and validity comparable to objective indicators of functional ability (Crawford, Jette, & Tennstedt, 1997; Zarit, Johansson, & Berg, 1993). Four PADL items were included across all four studies: individuals’ ability to bathe, dress, toilet, and feed oneself, each measured on a 4-point scale ranging from 0 (completely independent) to 3 (unable to do the activity at all). Following usual procedures, we defined the onset of disability as the first wave of measurement at which difficulties were reported in one or more PADL tasks (Guralnik et al., 2002; Seeman et al., 1996). “Time-to/from-disability” was calculated for each observation as the time-to or time-from the date on which disability was first reported. Negative scores indicate occasions obtained in the years prior to disability onset (predisability), a 0 indicates the point of disability onset, and positive scores indicate occasions obtained in the years after disability onset (postdisability). Of the 1,848 total observations, 1,238 observations or 67% were provided prior to or at the onset of disability and another 610 observations or 33% were provided at or after the onset of disability (for overview, see Table 2).
Table 2.
Descriptive Statistics for Depressive Symptoms Over the Time Metric of Time-To/From-Disability
| Depressive symptoms |
|||
| Time-to/from-disability | # of observations | M | SD |
| −8 | 21 | 48.73 | 6.77 |
| −7 | 32 | 50.13 | 8.85 |
| −6 | 33 | 49.14 | 9.23 |
| −5 | 30 | 50.69 | 10.05 |
| −4 | 120 | 47.14 | 8.46 |
| −3 | 78 | 49.57 | 9.54 |
| −2 | 183 | 48.25 | 10.13 |
| −1 | 50 | 48.10 | 9.96 |
| 0 | 691 | 51.51 | 11.45 |
| 1 | 123 | 51.34 | 11.05 |
| 2 | 208 | 48.53 | 11.70 |
| 3 | 80 | 49.42 | 11.07 |
| 4 | 110 | 47.53 | 12.02 |
| 5 | 31 | 50.06 | 9.93 |
| 6 | 36 | 45.66 | 11.90 |
| 7 | 14 | 53.56 | 11.19 |
| 8 | 8 | 43.82 | 10.73 |
Notes: N = 779 who provided 1,848 observations. The occasion column indicates the years before, at, and after the onset of disability, with 0 representing the measurement occasion where the onset of disability was first noted. The −1 through −8 occasions represent the predisability phase (−1 indicating a measurement occasion taken 1 year prior to the onset of disability). The 1 through 8 occasions represent the postdisability phase (1 indicating a measurement occasion taken 1 year after the onset of disability). Scores for depressive symptoms standardized to a T metric (M = 50; SD = 10) using the pooled sample of all participants at baseline assessment T1 as the reference (N = 1,440).
Note that individuals in our subsample may have become disabled at a point between baseline and the measurement wave in which it was first identified in the data collection, or they may have been disabled prior to entry to the study. Thus, the “point of onset” in our analyses contains some measurement error. Although past studies have used similar methods of identifying disability onset (e.g., Ferrucci et al., 1996; Lucas, 2007), we note that the resulting potential for misalignment of this method confounds changes occurring directly after the onset of disability (for individuals for whom the onset date is precise) with those that occurred some months or years later (for individuals for whom the onset date is imprecise). To accommodate the possible confounds associated with the onset of disability having occurred prior to participants’ enrollment in the study, we allowed for differences in three aspects of disability-related development. Specifically, we coded longitudinal data with respect to time-to/from-disability, and whether the data were obtained either prior to or postdisability onset (predisabilityti = 1 or = 0, respectively). Allowing for discrete shifts in level of depressive symptoms between these two phases accommodates, in part, the mixing of individuals who may have lived with disability for varying amounts of time in the postdisability period.
Data Analysis
In a first step, we used a multiphase growth model to describe disability-related within-person change in depressive symptoms (see Ram & Grimm, 2007). Specifically, we described between-person differences in three components of within-person change: (a) change across the predisability years, (b) at the point of disability onset (the first wave of measurement in which PADL impairment was reported), and (c) change across the postdisability years. The multiphase growth model was specified as.
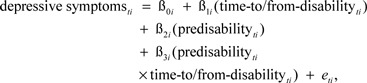 |
(1) |
where person i’s level of depressive symptoms at time t, depressive symptomsti, is a function of an individual-specific intercept parameter, β0i, representing individuals’ level of depressive symptoms at disability onset; an individual-specific slope parameter, β1i, capturing rates of linear disability-related change during the postdisability phase (when predisabilityti = 0); an individual-specific parameter, β2i, capturing discrete differences in level of depressive symptoms between pre- and postdisability phases; a second individual-specific slope parameter, β3i, capturing differences in the rate of linear disability-related change between the pre- and postdisability phases; and residual error, eti. Following standard growth modeling and model selection procedures, (e.g., McArdle & Nesselroade, 2003; Ram & Grimm, 2007; Singer & Willett, 2003), individual-specific intercepts and slopes (βs from the Level 1 model given in Equation 1) were modeled as
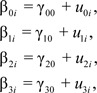 |
(2a) |
(i.e., Level 2 model), where between-person differences, u0i, u1i, u2i, and u3i were assumed to be multivariate normal distributed, correlated with each other, and uncorrelated with the residual errors, eti, the variance/covariance matrix of which was modeled as diagonal.
Subsequently, potential moderators were added into the model at the between-person level (Level 2). Specifically, the demographic variables (age group, gender, education, widowhood, institutionalization, study membership), cognition (memory recall), health (multimorbidity), and social support were added as predictors of β0i, β1i. β2i, and β3i. Of particular interest was whether these variables were associated with differences in the level of depressive symptoms at disability onset and rates of pre- and postdisability-related changes. To map person-level differences in the potentially time-varying covariates (institutionalization, memory, multimorbidity, and social support) to the person-level differences in change trajectories, observations were summarized across each of the three “phases.” For example, repeated observations of an individual’s memory obtained prior to disability were averaged to obtain a person-level predisability memory score for use as a predictor of predisability change, the memory score obtained at the point of disability onset was used as the predictor of the intercept, and repeated observations obtained after onset were averaged to obtain a person-level score for use as a predictor of postdisability change (for the binary institutionalization measure, we used the maximum rather than the average). This phase-specific parsing allowed us to effectively accommodate the time-varying nature of the moderators within the modeling framework.
Models were estimated using SAS (Proc Mixed) with incomplete data accommodated under missing at random assumptions at the within-person level and, to retain longitudinal data, missing completely at random at the between-person level (Little & Rubin, 1987). All person-level predictors (including categorical variables), except for study membership, were centered to obtain effect coding for the final model parameter estimates.
RESULTS
Changes in Depressive Symptoms Over Time-To/From-Disability
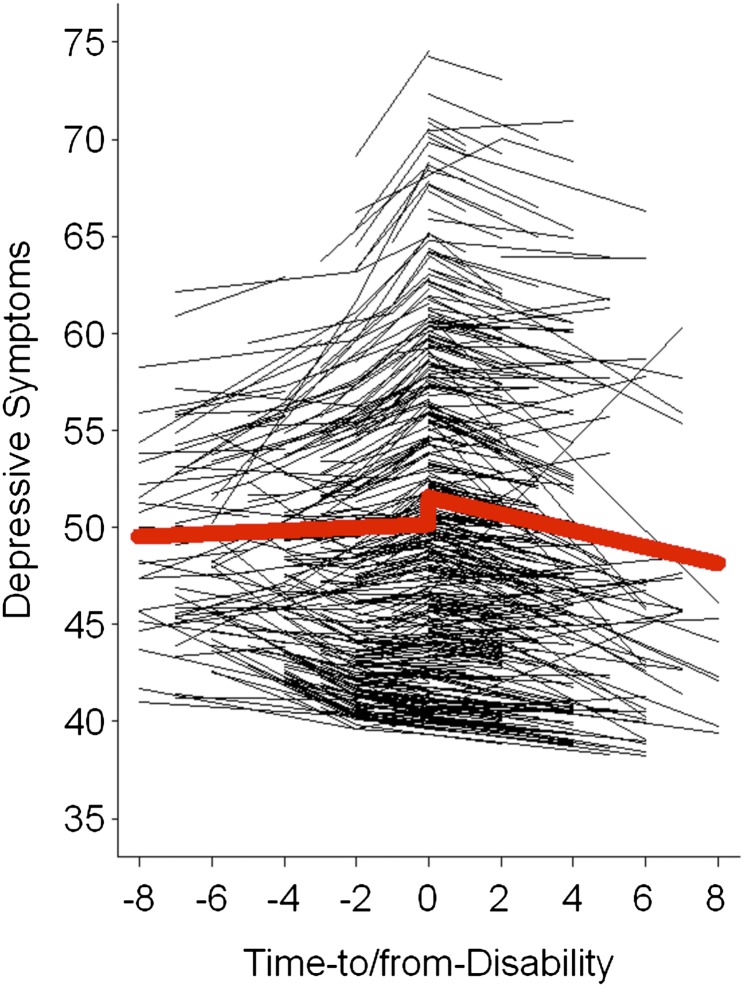
To address our first research question, we modeled between-person differences in change in depressive symptoms along the time-to/from-disability onset time metric, describing if and how depressive symptoms changed in relation to disablement-related processes. Estimated parameters include the prototypical level (γ00) and discrete shift in depressive symptoms at disability onset (γ20), postdisability rate of change in depressive symptoms (γ10), and difference between pre- and postdisability rates of change (γ30). Results are given in Table 3. The prototypical individual’s depressive symptoms declined γ10 = −0.43 T-units per year after disability onset. This postdisability rate of change in depressive symptoms was significantly different than the rate of change prior to onset, where the prototypical individuals’ change in depressive symptoms was characterized by a slight increase, γ10 + γ30 = −0.43 + 0.51 = 0.08 T-units per year. Differences in average levels of depressive symptoms between entry to γ00 + γ20 = 51.54 − 1.39 = 50.15 and exit from γ00 = 51.54 the point of onset of disability were not significant (p = .06, but were needed to accommodate the data structure (n = 444 or 57% of participants were only observed at or after the onset of disability). Predicted prototypical and individual trajectories are shown in Figure 1. Significant between-person differences were noted in estimated levels of depressive symptoms at disability onset, σ2u0 = 87.09, in rates of change for the postdisability phase, σ2u1 = 1.16, and in the differences in change between post-and predisability phases, σ2u3 = 4.03. Variability in the discrete differences in level of depressive symptoms between entry and exit from onset point, σ2u2, were tested but were not significant and were thus removed from the final model.
Table 3.
Growth Models for Depressive Symptoms Over Time-To/From-Disability
| Depressive symptoms | |
| Parameter | Estimate (SE) |
| Fixed effects | |
| Intercept, γ00 | 51.54* (0.42) |
| Time-to/from disability, γ10 | −0.43* (0.15) |
| Predisability, γ20 | −1.39 (0.74) |
| Predisability × Time-To/From-disability, γ30 | 0.51* (0.24) |
| Random effects | |
| Variance intercept, | 87.09* (6.82) |
| Variance Time-to/from-disability, | 1.16* (0.49) |
| Variance Predisability × Time-to/from-disability, | 4.03* (1.96) |
| Covariance, σu0u1 | −2.96 (1.72) |
| Covariance, σu0u3 | 11.59* (2.76) |
| Covariance, σu1u3 | −1.88 (1.05) |
| Residual, | 41.71* (4.46) |
| Residual, | 47.85* (4.41) |
| Residual, | 46.86* (5.34) |
| Residual, | 38.51* (7.04) |
| Residual, | 87.05* (18.26) |
| Pseudo R2 | .129 |
| −2LL | 13,551 |
| AIC | 13,581 |
Notes: Unstandardized estimates and standard errors are presented. Intercept is centered at disability onset; slope or rate of change is scaled in T-units per year. N = 779 who provided 1,848 observations. Scores for depressive symptoms standardized to a T metric (M = 50, SD = 10) using the pooled sample of all oldest old participants at baseline assessment T1 as the reference (N = 1,440). AIC = Akaike Information Criterion; −2LL = −2 log likelihood, relative model fit statistics. The Pseudo R2 was obtained from comparing the residual within-person variance from the conditional model, including the time-to/from-disability as the time variable with that obtained from an unconditional or intercept-only model (see Snijders & Bosker, 1999, pp. 99–105).
*p < .05.
Figure 1.
The figure graphically illustrates predicted average change (thick line) and individual changes (thin lines) for depressive symptoms over time-to/from disability. On average, depressive symptoms increased slightly with approaching disability, increased considerably at disability onset (i.e., first wave of measurement in which a participant needed assistance in personal activities of daily living), and declined after disability had set in.
The Role of Demographic Characteristics, Cognitive Function, Health, and Social Support
To address our second research question, we tested whether demographic characteristics (age group and study membership—with OCTO as reference category, gender, education, and living in an institution), memory, multimorbidity, and social support variables were independently related to interindividual differences in disability-related change in depressive symptoms. Results are reported in Table 4, with significant effects (p < .05) highlighted.
Table 4.
Growth Model for Depressive Symptoms Over Time-To/From-Disability: The Effect of Covariates
| Depressive symptoms |
||
| Parameter | Estimate | SE |
| Fixed effects | ||
| Intercepta, γ00 | 51.81* (0.35) | |
| Time-to/from disability (TtfD)b, γ10 | −0.22 (0.18) | |
| Predisability, γ20 | −1.03 (0.79) | |
| Predisability × TtfDb, γ30 | 0.49 (0.37) | |
| Aged 70–79, γ01 | 3.61 (2.59) | |
| Aged 90+, γ02 | 1.09 (1.00) | |
| GENDER study γ03 | −1.98 (2.89) | |
| OCTO-TWIN study γ04 | −8.81* (0.88) | |
| NONA study γ05 | −4.67* (1.19) | |
| Women γ06 | 1.56* (0.73) | |
| Education, γ07 | 0.06 (0.16) | |
| Widowhood, γ08 | 0.51 (0.72) | |
| Institutionalization, γ09 | 1.04 (0.70) | |
| Memory, γ010 | 0.04 (0.04) | |
| Multimorbidity, γ011 | 0.77* (0.27) | |
| Social support, γ012 | −0.40* (0.03) | |
| TtfD × Aged 70–79, γ11 | −0.57 (1.00) | |
| TtfD × Aged 90+, γ12 | −0.04 (0.53) | |
| TtfD × GENDER study γ13 | −0.08 (1.17) | |
| TtfD × OCTO-Twin study γ14 | −0.78 (0.42) | |
| TtfD × NONA study γ15 | −0.75 (0.72) | |
| TtfD × Women γ16 | −0.07 (0.35) | |
| TtfD × Education, γ17 | 0.05 (0.07) | |
| TtfD × Widowhood, γ18 | −0.42 (0.32) | |
| TtfD × Institutionalization, γ19 | 0.15 (0.24) | |
| TtfD × Memory, γ110 | 0.01 (0.01) | |
| TtfD × Multimorbidity, γ111 | 0.07 (0.10) | |
| TtfD × Social Support, γ112 | −0.06* (0.01) | |
| Predisability × TtfD × Aged 70–79, γ31 | 2.53 (1.47) | |
| Predisability × TtfD × Aged 90+, γ32 | 0.76 (0.97) | |
| Predisability × TtfD × GENDER study γ33 | −1.76 (1.70) | |
| Predisability × TtfD × OCTO-Twin study γ34 | −0.31 (0.72) | |
| Predisability × TtfD × NONA study γ35 | 0.45 (1.12) | |
| Predisability × TtfD × Women γ36 | 0.33 (0.48) | |
| Predisability × TtfD × Education, γ37 | −0.10 (0.10) | |
| Predisability × TtfD × Widowhood, γ38 | 0.03 (0.45) | |
| Predisability × TtfD × Institutionalization, γ39 | 0.09 (0.34) | |
| Predisability × TtfD × Memory, γ310 | 0.02 (0.02) | |
| Predisability × TtfD × Multimorbidity, γ311 | −0.06 (0.13) | |
| Predisability × TtfD × Social Support, γ312 | 0.02 (0.02) | |
| Random effects | ||
| (Residual) variance intercept, | 43.48* (5.54) | |
| (Residual) variance TtfD, | 1.32* (0.52) | |
| (Residual) variance predisability × TtfD, | 3.14** (2.01) | |
| Covariance, σu0u1 | −1.97 (1.39) | |
| Covariance, σu0u3 | 4.49* (2.17) | |
| Covariance, σu1u3 | −1.97 (1.08) | |
| Residual, | 38.15* (3.92) | |
| Residual, | 49.66* (4.18) | |
| Residual, | 44.83* (5.15) | |
| Residual, | 44.88* (7.50) | |
| Residual, | 86.11* (18.49) | |
| −2LL | 13,067 | |
| AIC | 13,169 | |
Notes: Participants in the OCTO study served as the reference category. Unstandardized estimates and standard errors are presented. N = 779 who provided 1,848 observations. Scores for depressive symptoms standardized to a T metric (M = 50, SD = 10) using the pooled sample of all oldest old participants at baseline assessment T1 as the reference (N = 1,440). AIC = Akaike Information Criterion; −2LL = −2 log likelihood, relative model fit statistics. The change in Pseudo R2 was obtained from comparing the residual within-person variance from the conditional model, including the time-to/from-disability as the time variable with that obtained from an unconditional or intercept-only model (see Snijders & Bosker, 1999, pp. 99–105).
Intercept is centered at disability onset.
Slope or rate of change is scaled in T-units per year.
*p < .05; **p = .06.
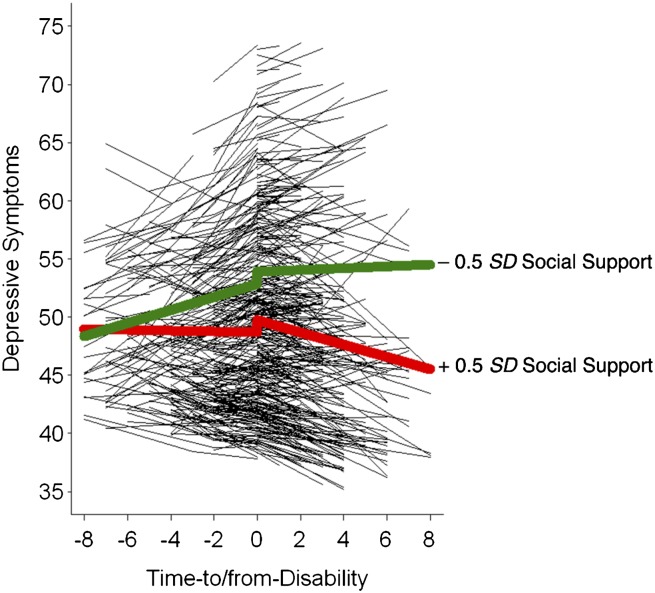
Differences in level of depressive symptoms at onset were related to study membership, with the OCTO-TWIN (γ04 = − 8.81) and NONA samples (γ05 = − 4.67) reporting significantly lower levels than the OCTO sample. Of substantive interest were that being a woman (γ06 = 1.56) and higher levels of multimorbidity (γ011 = 0.77) were associated with higher level of depressive symptoms, and higher levels of social support (γ012 = − 0.40) were associated with lower levels of depressive symptoms. Differences in social support were also related to linear rates of change in the postdisability phase (γ112 = −0.06). These associations did not differ across predisability and postdisability phase. That is, the noted associations were present (or not) in both phases. In sum, setting aside the covariates dealing with study design effects (e.g., OCTO-TWIN and NONA study membership), the significant effect of note was that higher levels of social support were associated with steeper post disability-related declines in depressive symptoms. Figure 2 illustrates the protective effect of social support for depressive symptoms change along the time-to/from-disability metric.
Figure 2.
The figure shows the protective effect of social support for change in depressive symptoms along the time-to/from-disability metric. Participants with low social support (−0.5 SD) evinced predisability-related increases in depressive symptoms, whereas socially embedded participants (+0.5 SD) remained relatively stable followed by postdisability decreases in depressive symptoms.
Discussion
Disability onset, or the point at which a person needs assistance in performing PADLs, marks an important transition point in the disablement continuum. Consistent associations are often found between late-life depressive symptoms and disability outcomes. Recent research utilizes approaches in which individuals are assessed prior to a challenge (disability or some other serious health event), at the point of challenge, and after challenge so as to examine patterns of event-related change and adaptation (Helson, George, & John, 2009; Lucas, 2007). Our objective here was to apply multiphase growth models to describe how depressive symptoms change in the years prior to the onset of disability, at or around disability onset, and after disability onset. We also examined the role of demographic characteristics, memory, multimorbidity, and social support as possible moderators of disability-related change in depressive symptoms.
Changes in Depressive Symptoms Over Time-To/From-Disability
Our results revealed that, on average, our sample of older Swedes’ depressive symptoms increased slightly with approaching disability, increased further at the onset of disability, and declined thereafter. Utilizing a time variable that allowed for modeling of disability-related change in depressive symptoms, our study provides compelling support for earlier reports that depressive symptoms change, at least in part, in relation to disablement processes. Although the Disablement Process model recognizes that some individuals face a rapid onset of PADL impairments (as in the case of a stroke), the model also posits that others go through a predisability phase involving a slow accumulation of chronic conditions, impairments, and limitations leading up to the onset of disability (Verbrugge & Jette, 1994). In the latter case, it is possible that persons in this predisability phase recognize the concurrent loss of control and independence that they are experiencing as they become more physically limited, or they may anticipate the looming need for care in the future, both of which may trigger decreased well-being.
Although not reliably different than zero (γ20 = − 1.39, p = .06), there was some indication, also supported by past research, that depressive symptoms change immediately at the point of disability onset. Although disablement is typically a continuous process, Yang and George (2005) reported that the onset of disability was a more important predictor of increases in depressive symptoms than was level of disability. Chang and colleagues (2009) emphasized that the onset of PADL impairment is associated with an immediate development of depressive symptoms. These results suggest that the transition into a disabled state may trigger a rise of depressive symptoms, a finding mirrored in studies reporting that negative health events are associated with immediate declines in well-being (Helson et al., 2009; Lucas, 2007; Shmotkin, 2005). We highlight that an alternative explanation for the noted change at the point of onset, however, is a measurement error. For participants who were disabled when entering the study, we took their first point of measurement to represent the onset of disability. It may well be that for some of these participants, the true transition into a disabled condition occurred prior to baseline measurement. As a consequence, increased depressive symptoms at onset may not necessarily reflect a recent shift into the disabled state but higher levels of depressive symptoms that are characteristic of this particular group. Both of the above explanations support the idea that being in a disabled state corresponds with higher levels of depressive symptoms. However, only the first explanation suggests that this increase is carried by the transition into the need for PADL assistance.
In the phase following disability onset, we observed declines in depressive symptoms. Such a pattern may indicate that self-regulatory processes are still effective in this age group, allowing for psychological readjustment and increased acceptance of one’s disability. Our finding that there is a period of adaptation in well-being after exposure to the challenge of disability onset supports prior studies on challenges imposed by health constraints (Helson et al., 2009) and disability (Lucas, 2007). These studies reported that well-being declined around the time of the event, followed by periods of readjustment and improvements. Our findings also fit the theory of hedonic adaptation, which suggests that people are negatively affected by unfortunate circumstances but typically adjust to the situation by returning to a more positive state of well-being (Brickman & Campbell, 1971).
The Role of Demographic Characteristics, Cognitive Function, Health, and Social Support
Describing change in depressive symptoms along the disablement process, we identified vast between-person variability in individuals’ trajectories. To explore factors contributing to those differences, we tested whether demographic, memory, multimorbidity, and social support variables served as risk or protective factors at all three phases of the model, allowing some individuals to maintain few depressive symptoms, despite approaching disability onset and/or dealing with existing impairment. Results indicated differences among the various studies of origin. In particular, participants from OCTO-Twin and NONA reported fewer depressive symptoms at disability onset relative to those in GENDER and OCTO. Likely, each study tapped a unique subset of the population, with there being evidence of some differences among the subpopulations or some differential sampling strategies among studies. In either case, the effects of other variables are in essence statistically adjusted for these differences. Our findings that being a woman, higher multimorbidity, and lower social support were each predictive of reporting more depressive symptoms at disability onset corresponds with the literature on predictors of late-life depressive symptoms (Bisschop et al., 2004; Blazer, 2003; Glass et al., 2006; Lee et al., 2001; Palinkas et al., 1990; Yang & George, 2005). The current study indicates that these same associations are maintained when examining depressive symptoms from a disablement process perspective.
Greater social support was associated with fewer depressive symptoms at disability onset and more pronounced decline in depressive symptoms postdisability, suggesting that social resources serve as protective factors in the disablement process. Our finding is consistent with earlier reports that older adults with poorer social support have a higher risk of suffering from late-life depressive symptoms (Bisschop et al., 2004; Lee et al., 2001; Palinkas et al., 1990). For example, Golden and colleagues (2009) reported that loneliness and social networks accounted for 70% of the prevalence of depressed mood. Our study adds to the literature by considering how depressive symptoms change in relation to the longitudinal course of disablement. In this disablement process framework, our results suggest that social support remains a pivotal resource people can draw from as they experience the onset disability and manage these functional limitations. However, we note that although the analyses in our paper capture the dynamic nature of depressive symptoms as they change over time, social support was utilized here as a static predictor variable within each phase (averaged at each phase). Past research has suggested that the approach to or transition into disability may cooccur with changes in social roles as well as changes in identity and/or decreases in self-confidence, all of which affect late-life depressive symptoms (Aneshensel, Frerichs, & Huba, 1984; Yang & George, 2005). Therefore, in addition to testing predictors of well-being as static at each of the three model phases, it may be informative to test time-varying predictors within each disablement process phase.
Limitations and Outlook
We use the time-to/from-disability time metric as a simplified proxy for the disablement process, much in the way that time-since-birth (chronological age) and time-to-death are used as proxies for age-related or mortality-related processes (Ram et al., 2010; Sliwinski et al., 2003). We recognize that in “real life,” individuals may become impaired in PADL temporarily and then recover into a nondisabled state and that for some individuals, the onset of disability is acute (e.g., a debilitating stroke), whereas for others, it is a lengthy process that is not easily demarcated by an exact date of onset. Therefore, we see the use of our time-to/from-disability metric as a simplified representation of the true intricacies that underlie disability-related processes. We recommend that future analyses seek ways to incorporate more complex modeling of time structures, including multiple episodes of onset and recovery. We also emphasize that time from disability onset is not an indicator of the extent to which a person is impaired, thereby precluding any inferences regarding disability severity. Similarly, the time-to-death metric, used in many gerontological studies, is not an indicator of severity of health problems; rather it anchors time in a way that is better at representing underlying mortality processes, as compared with age, or the time from birth metric (Ram et al., 2010).
As an additional limitation, we recognize that the current samples are in some ways representative of old–old and oldest-old populations (they include individuals living at home and in institutions). At the same time, population segments with the most severe physical or cognitive impairment were probably not included in the original studies. Either these lowest functioning individuals were selected out of the study (or analyses) due to their inability to provide self-report data within a wave of measurement or they had selective attrition (refusal, inability, or death). Therefore, the trajectories of depressive symptoms observed in the current study might not generalize to the frailest subset of the older population.
Finally, our measure of social support assessed self-reported “perceived support.” It is possible that individuals with more depressive symptoms are biased toward perceiving less support or, alternatively, that people with few depressive symptoms are biased toward reporting more support. Although we tried to keep the social support and depressive symptoms scales as independent as possible (see “Method” section), self-reported social support scales still exhibit conceptual overlap with (lack of) depressive symptoms.
In closing, we emphasize that disability and depressive symptoms are closely intertwined in late life, and we suggest that research gains a better understanding of changes in depressive symptoms (and perhaps related topics) when these changes are examined using a disablement process framework. We have presented findings that, on average, depressive symptoms change as disability progresses. Our results also revealed substantial between-person differences in depressive symptoms levels and rates of change within this disability context. We suggest that variables such as social support buffer the negative effects of disability onset. Finally, we encourage future inquiry into late-life depressive symptoms in the disablement context and particularly call for testing additional correlates to uncover risk and protective factors in this process, particularly factors that are amenable to intervention.
Funding
The authors gratefully acknowledge the support provided by the National Institute on Aging (RC1-AG035645, R21-AG032379, R21-AG033109, and R03 AG028471) and the Penn State Center for Population Health and Aging. The OCTO and NONA studies were funded by the European Union (QLK6-CT-2001-02283), the Research Board in the County Council of Jönköping, and the Research Council in the Southeast of Sweden (FORSS). The OCTO-TWIN study was funded by National Institute on Aging (AG-08861). The GENDER study was funded by the MacArthur Foundation Research Network on Successful Aging, the Axel and Margaret Axson Johnson's Foundation, the Swedish Council for Social Research, and the Swedish Foundation for Health Care Sciences and Allergy Research.
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. The authors would also like to extend their gratitude to Stig Berg, who was an instrumental leader in the collection of the Swedish data sets and whose research career contributed significantly to the current study. Special thanks also to Steven Zarit and Gerald McClearn from Penn State University and to Boo Johansson from the University of Göteborg, Sweden, for their immense leadership roles on the collection and management of the original Swedish data sets. Finally, we thank all members of the research teams at the Institute for Gerontology at Jönköping University in Sweden, the Center for Developmental and Health Genetics at the Pennsylvania State University, and the Division of Genetic Epidemiology at the Karolinska Institute in Stockholm, Sweden, for their design and collection of the original data.
References
- Alwin DF, Hofer SM, McCammon RJ. Modeling the effects of time: Integrating demographic and developmental perspectives. In: Binstock RH, George LK, editors. Handbook of aging and the social sciences. 6th ed. New York, NY: Elsevier; 2006. pp. 20–38. [Google Scholar]
- Aneshensel CS, Frerichs RR, Huba GJ. Depression and physical illness: A multiwave, nonrecursive causal model. Journal of Health and Social Behavior. 1984;25:350–371. doi:10.2307/2136376. [PubMed] [Google Scholar]
- Bennett KM. Widowhood in elderly women: The medium- and long-term effects on mental and physical health. Mortality. 1997;2:137–148. doi:10.1080/713685857. [Google Scholar]
- Bisschop MI, Kriegsman DM, Deeg DJ, Beekman AT, van Tilburg W. The longitudinal relation between chronic disease and depression in older persons in the community: The Longitudinal Aging Research Amsterdam. Journal of Clinical Epidemiology. 2004;57:187–194. doi: 10.1016/j.jclinepi.2003.01.001. doi:10.1016/j.jclinepi.2003.01.001. [DOI] [PubMed] [Google Scholar]
- Blazer DG. Depression in late life: Review and commentary. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. 2003;58:249–265. doi: 10.1093/gerona/58.3.m249. doi:10.1093/gerona/58.3.M249. [DOI] [PubMed] [Google Scholar]
- Brickman P, Campbell DT. Hedonic relativism and planning the good society. In: Appley M, editor. Adaptation-level theory. New York, NY: Academic Press; 1971. pp. 287–305. [Google Scholar]
- Bruce ML, Seeman TE, Merrill SS, Blazer DG. The impact of depressive symptomotology on physical disability: MacArthur studies of successful aging. American Journal of Public Health. 1994;84:1796–1799. doi: 10.2105/ajph.84.11.1796. doi:10.2105/AJPH.84.11.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M, Phillips C, Coppin AK, van der Linden M, Ferrucci L, Fried L, Guralnik JM. An association between incident disability and depressive symptoms over 3 years of follow-up among older women: The Women’s Health and Aging Study. Aging Clinical and Experimental Research. 2009;21:191–197. doi: 10.1007/bf03325228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covinsky KE, Yaffe K, Lindquist K, Cherkasova E, Yelin E, Blazer DG. Depressive symptoms in middle age and the development of later-life functional limitations: The long-term effect of depressive symptoms. Journal of the American Geriatrics Society. 2010;58:551–556. doi: 10.1111/j.1532-5415.2010.02723.x. doi:10.1111/j.1532-5415.2010.02723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford SL, Jette AM, Tennstedt SL. Test-retest reliability of self-reported disability measures in older adults. Journal of the American Geriatrics Society. 1997;45:338–341. doi: 10.1111/j.1532-5415.1997.tb00950.x. [DOI] [PubMed] [Google Scholar]
- Diehr P, Williamson J, Burke GL, Psaty BM. The aging and dying processes and the health of older adults. Journal of Clinical Epidemiology. 2002;55:269–278. doi: 10.1016/s0895-4356(01)00462-0. doi:10.1016/S0895-4356(01)00462-0. [DOI] [PubMed] [Google Scholar]
- Fauth EB, Zarit SH, Malmberg B, Johansson B. Physical, cognitive, and psychosocial variables from the disablement process model predict patterns of change in disability for the oldest-old. The Gerontologist. 2007;47:613–624. doi: 10.1093/geront/47.5.613. doi:10.1093/geront/47.5.613. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Guralnik JM, Simonsick E, Salive ME, Corti C, Langlois J. Progressive versus catastrophic disability: A longitudinal view of the disablement process. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. 1996;51:123–130. doi: 10.1093/gerona/51a.3.m123. doi:10.1093/gerona/51A.3.M123. [DOI] [PubMed] [Google Scholar]
- Gerstorf D, Ram N, Estabrook R, Schupp J, Wagner GG, Lindenberger U. Life satisfaction shows terminal decline in old age: Longitudinal evidence from the German Socio-Economic Panel Study (SOEP) Developmental Psychology. 2008;44:1148–1159. doi: 10.1037/0012-1649.44.4.1148. doi:10.1037/0012-1649.44.4.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstorf D, Ram N, Fauth E, Schupp J, Wagner GG. Between-person disparities in the progression of late-life well-being. In: Antonucci TC, Jackson JS, editors. Annual review of gerontology and geriatrics. 2009. (Vol. 29, Life course perspectives on late-life health inequalities, pp. 205–232). New York, NY: Springer. [Google Scholar]
- Gerstorf D, Ram N, Röcke C, Lindenberger U, Smith J. Decline in life satisfaction in old age: Longitudinal evidence for links to distance-to-death. Psychology and Aging. 2008;23:154–168. doi: 10.1037/0882-7974.23.1.154. doi:10.1037/0882-7974.23.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghisletta P, McArdle JJ, Lindenberger U. Longitudinal cognition-survival relations in old and very old age: 13-year data from the Berlin Aging Study. European Psychologist. 2006;11:204–223. doi:10.1027/1016-9040.11.3.204. [Google Scholar]
- Glass TA, De Leon CFM, Bassuk SS, Berkman LF. Social engagement and depressive symptoms in late life: Longitudinal findings. Journal of Aging and Health. 2006;18:604–628. doi: 10.1177/0898264306291017. doi:10.1177/0898264306291017. [DOI] [PubMed] [Google Scholar]
- Gold C, Malmberg B, McClearn GE, Pedersen N, Berg S. Gender and health: A study of unlike sex twins. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2002;57:168–176. doi: 10.1093/geronb/57.3.s168. doi:10.1093/geronb/57.3.S168. [DOI] [PubMed] [Google Scholar]
- Golden J, Conroy R, Bruce I, Denihan A, Greene E, Kirby M, Lawlor BA. Loneliness, social support networks, mood and wellbeing in community-dwelling elderly. International Journal of Geriatric Psychiatry. 2009;24:694–700. doi: 10.1002/gps.2181. doi:10.1002/gps.2181. [DOI] [PubMed] [Google Scholar]
- Gorwood P, Corruble E, Falissard B, Goodwin G. Toxic effects of depression on brain function: Impairment of delayed recall and the cumulative length of depressive disorder in a large sample of depressed outpatients. American Journal of Psychiatry. 2008;165:731–739. doi: 10.1176/appi.ajp.2008.07040574. doi:10.1176/appi.ajp.2008.07040574. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Alecxih L, Branch LG, Wiener JM. Medical and long-term care cost when older persons become more dependent. American Journal of Public Health. 2002;92:1244–1245. doi: 10.2105/ajph.92.8.1244. doi:10.2105/AJPH.92.8.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorne TS. Psychometric properties and confirmatory factor analysis of the UCLA Loneliness Scale. Journal of Personality Assessment. 1993;61:182–195. doi: 10.1207/s15327752jpa6101_14. doi:10.1207/s15327752jpa6101_14. [DOI] [PubMed] [Google Scholar]
- Helson RM, George LG, John OP. Challenge episodes over middle age: A person-centered study of aging well in poor health. Journal of Research in Personality. 2009;43:323–334. doi:10.1016/j.jrp.2008.12.004. [Google Scholar]
- Hertzog C, Nesselroade JR. Assessing psychological change in adulthood: An overview of methodological issues. Psychology and Aging. 2003;18:639–657. doi: 10.1037/0882-7974.18.4.639. doi:10.1037/0882-7974.18.4.639. [DOI] [PubMed] [Google Scholar]
- Jensen J, Lundin-Olsson L, Nyberg L, Gustafson Y. Falls among frail older people in residential care. Scandinavian Journal of Public Health. 2002;30:54–61. doi:10.1080/140349401753481592. [PubMed] [Google Scholar]
- Johansson B. The MIR-memory in reality test. Stockholm, Sweden: Psykologiforlaget AB; 1988/1989. [Google Scholar]
- Johansson B, Hofer SM, Allaire JC, Maldonado-Molina M, Piccinin AM, Berg S, McClearn GE. Change in cognitive capabilities in the oldest-old: The effects of proximity to death in genetically related individuals over a six-year period. Psychology and Aging. 2004;19:145–156. doi: 10.1037/0882-7974.19.1.145. doi:10.1037/0882-7974.19.1.145. [DOI] [PubMed] [Google Scholar]
- Johansson B, Zarit SH. Prevalence and incidence of dementia in the oldest-old: A longitudinal study of a population-based sample of 84–90-year-olds in Sweden. International Journal of Geriatric Psychiatry. 1995;10:359–366. doi:10.1002/gps.930100504. [Google Scholar]
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness and the aged. The index of ADL: A standardized measure of biological and psychosocial function. Journal of the American Medical Association. 1963;185:914–923. doi: 10.1001/jama.1963.03060120024016. doi:10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- Lee Y, Choi K, Lee YK. Association of comorbidity with depressive symptoms in community-dwelling older persons. Gerontology. 2001;47:254–262. doi: 10.1159/000052809. doi:10.1159/000052809. [DOI] [PubMed] [Google Scholar]
- Li LW, Conwell Y. Effects of changes in depressive symptoms and cognitive functioning on physical disability in home care elders. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. 2009;64:230–236. doi: 10.1093/gerona/gln023. doi:10.1093/gerona/gln023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Schmiedek F. Age is not necessarily aging: Another step towards understanding the “clocks” that time aging. Gerontology. 2002;48:5–12. doi: 10.1159/000048917. doi:10.1159/000048917. [DOI] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical analysis with missing data. New York, NY: Wiley; 1987. [Google Scholar]
- Lucas RE. Long-term disability is associated with lasting changes in subjective well-being: Evidence from two nationally representative longitudinal studies. Journal of Personality and Social Psychology. 2007;92:717–730. doi: 10.1037/0022-3514.92.4.717. doi:10.1037/0022-3514.92.4.717. [DOI] [PubMed] [Google Scholar]
- Lucas RE, Clark AE, Georgellis Y, Diener E. Reexamining adaptation and the set point model of happiness: Reactions to changes in marital status. Journal of Personality and Social Psychology. 2003;84:527–539. doi: 10.1037//0022-3514.84.3.527. doi:10.1037/0022-3514.84.3.527. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Nesselroade JR. Growth curve analysis in contemporary psychological research. In: Schinka JA, Velicer WF, editors. Handbook of psychology: Research methods in psychology. Vol. 2. Hoboken, NJ: Wiley; 2003. pp. 447–480. [Google Scholar]
- McClearn G, Johansson B, Berg S, Ahern F, Nesselroade J, Pedersen N, Plomin R. Substantial genetic influence on cognitive abilities in twins 80+ years old. Science. 1997;276:1560–1563. doi: 10.1126/science.276.5318.1560. doi:10.1126/science.276.5318.1560. [DOI] [PubMed] [Google Scholar]
- Mirowsky J, Ross CE. Age and depression. Journal of Health and Social Behavior. 1992;33:187–205. doi:10.2307/2137349. [PubMed] [Google Scholar]
- Ormel J, Rijsdijk FV, Sullivan M, van Sonderen E, Kempen GIJM. Temporal and reciprocal relationship between IADL/ADL disability and depressive symptoms in late life. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2002;57:338–348. doi: 10.1093/geronb/57.4.p338. doi:10.1093/geronb/57.4.P338. [DOI] [PubMed] [Google Scholar]
- Palinkas LA, Wingard DL, Barrett-Connor E. Chronic illness and depressive symptoms in the elderly: A population-based study. Journal of Clinical Epidemiology. 1990;43:1131–1141. doi: 10.1016/0895-4356(90)90014-g. doi:10.1016/0895-4356(90)90014-G. [DOI] [PubMed] [Google Scholar]
- Penninx BWJH, Leveille S, Ferrucci L, van Eijk JTM, Guralnik JM. Exploring the effect of depression on physical disability: Longitudinal evidence from the established populations for epidemiologic studies of the elderly. American Journal of Public Health. 1999;89:1346–1352. doi: 10.2105/ajph.89.9.1346. doi:10.2105/AJPH.89.9.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pot AM, Deeg DJ, Twisk JW, Beekman AT, Zarit SH. The longitudinal relationship between the use of long-term care and depressive symptoms in older adults. Gerontologist. 2005;45:359–369. doi: 10.1093/geront/45.3.359. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. doi:10.1177/014662167700100306. [Google Scholar]
- Ram N, Gerstorf D, Fauth E, Zarit SH, Malmberg B. Aging, disablement, and dying: Using time-as-process and time-as-resources metrics to chart late-life change. Research in Human Development. 2010;7:1–17. doi: 10.1080/15427600903578151. doi:10.1080/15427600903578151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram N, Grimm KJ. Using simple and complex growth models to articulate developmental change: Matching theory to method. International Journal of Behavioral Development. 2007;31:303–316. doi:10.1177/0165025407077751. [Google Scholar]
- Roberts RE, Kaplan GA, Shema SJ, Strawbridge WJ. Prevalence and correlates of depression in an aging cohort: The Alameda County Study. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 1997;52:252–258. doi: 10.1093/geronb/52b.5.s252. doi:10.1093/geronb/52B.5.S252. [DOI] [PubMed] [Google Scholar]
- Rosenberg P, Mielke M, Xue Q, Carlson M. Depressive symptoms predict incident cognitive impairment in cognitive healthy older women. American Journal of Geriatric Psychiatry. 2010;18:204–211. doi: 10.1097/JGP.0b013e3181c53487. doi:10.1097/JGP.0b013e3181c53487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. The measurement of loneliness. In: Peplau LA, Perlman D, editors. Loneliness: A sourcebook of current theory, research and therapy. New York, NY: Wiley; 1982. [Google Scholar]
- Schillerstrom JE, Royall DR, Palmer RF. Depression, disability and intermediate pathways: A review of longitudinal studies in elders. Journal of Geriatric Psychiatry and Neurology. 2008;21:183–197. doi: 10.1177/0891988708320971. doi:10.1177/0891988708320971. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Bruce ML, McAvay GJ. Social network characteristics and onset of ADL disability: MacArthur studies of successful aging. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 1996;51:191–200. doi: 10.1093/geronb/51b.4.s191. doi:10.1093/geronb/51B.4.S191. [DOI] [PubMed] [Google Scholar]
- Shmotkin D. Happiness in the face of adversity: Reformulating the dynamic and modular bases of subjective well-being. Review of General Psychology. 2005;9:291–325. doi:10.1037/1089-2680.9.4.291. [Google Scholar]
- Simmons SF, Ljungquist B, Johansson B, Plomin R, Zarit SH, McClearn GE. Selection bias in samples of older twins? A comparison between octogenarian twins and singletons in Sweden. Journal of Aging and Health. 1997;9:553–567. doi: 10.1177/089826439700900407. doi:10.1177/089826439700900407. [DOI] [PubMed] [Google Scholar]
- Simpson CF, Boyd CM, Carlson MC, Griswold ME, Guralnik JM, Fried LP. Agreement between self-report of disease diagnoses and medical record validation in disabled older women: Factors that modify agreement. Journal of the American Geriatrics Society. 2004;52:123–127. doi: 10.1111/j.1532-5415.2004.52021.x. doi:10.1111/j.1532.5415.2004.52021.x. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York, NY: Oxford University Press; 2003. [Google Scholar]
- Sliwinski MJ, Hofer SM, Hall C, Buschke H, Lipton RB. Modeling memory decline in older adults: The importance of preclinical dementia, attrition, and chronological age. Psychology and Aging. 2003;18:658–671. doi: 10.1037/0882-7974.18.4.658. doi:10.1037/0882-7974.18.4.658. [DOI] [PubMed] [Google Scholar]
- Sliwinski MJ, Stawski RS, Hall RB, Katz M, Verghese J, Lipton RB. Distinguishing pre-terminal and terminal cognitive decline. European Psychologist. 2006;11:172–181. doi:10.1027/1016-9040.11.3.172. [Google Scholar]
- Snijders TAB, Bosker RJ. Multilevel analysis: An introduction to basic and advanced multilevel modeling. London, UK: Sage; 1999. [Google Scholar]
- Taylor MG, Lynch SM. Trajectories of impairment, social support, and depressive symptoms in later life. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2004;59:238–246. doi: 10.1093/geronb/59.4.s238. doi:10.1093/geronb/59.4.S238. [DOI] [PubMed] [Google Scholar]
- Thorvaldsson V, Hofer SM, Johansson B. Ageing and late life terminal decline: A comparison of alternative modeling approaches. European Psychologist. 2006;11:196–203. doi:10.1027/1016-9040.11.3.196. [Google Scholar]
- Tinetti ME, Inouye SK, Gill TM, Doucette JT. Shared risk factors for falls, incontinence, and functional dependence. Unifying the approach to geriatric syndromes. Journal of the American Medical Association. 1995;273:1348–1353. doi:10.1001/jama.1995.03520410042024. [PubMed] [Google Scholar]
- Verbrugge LM, Jette AM. The disablement process. Social Science and Medicine. 1994;38:1–14. doi: 10.1016/0277-9536(94)90294-1. doi:10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- Weinberger MI, Raue PJ, Meyers BS, Bruce ML. Predictors of new onset depression in medically ill, disabled older adults at one year follow up. American Journal of Geriatric Psychiatry. 2009;17:802–809. doi: 10.1097/JGP.0b013e3181b0481a. doi:10.1097/JGP.0b013e3181b0481a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson GM, Schulz R. Physical illness and symptoms of depression among elderly outpatients. Psychology and Aging. 1992;7:343–351. doi: 10.1037//0882-7974.7.3.343. doi:10.1037/0882-7974.7.3.343. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Beck TL, Bienias JL, Bennett DA. Terminal cognitive decline: Accelerated loss of cognition in the last years of life. Psychosomatic Medicine. 2007;69:131–137. doi: 10.1097/PSY.0b013e31803130ae. doi:10.1097/PSY.0b013e31803130ae. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Bienias JL, Evans DA, Bennett DA. Terminal decline in cognitive function. Neurology. 2003;60:1782–1787. doi: 10.1212/01.wnl.0000068019.60901.c1. doi:10.1212/01.WNL.0000068019.60901.C1. [DOI] [PubMed] [Google Scholar]
- Wohlwill JF. The study of behavioral development. Oxford, UK: Academic Press; 1973. [Google Scholar]
- Yang Y. How does functional disability affect depressive symptoms in late life? The role of perceived social support and psychological resources. Journal of Health and Social Behavior. 2006;47:355–372. doi: 10.1177/002214650604700404. doi:10.1177/002214650604700404. [DOI] [PubMed] [Google Scholar]
- Yang Y, George L. Functional disability, disability transitions, and depressive symptoms in late life. Journal of Aging and Health. 2005;17:263–292. doi: 10.1177/0898264305276295. doi:10.1177/0898264305276295. [DOI] [PubMed] [Google Scholar]
- Zarit SH, Johansson B, Berg S. Functional impairment and co-disability in the oldest old: A multidimensional approach. Journal of Aging and Health. 1993;5:291–305. doi:10.1177/089826439300500301. [Google Scholar]
- Zeiss AM, Lewinsohn PM, Rohde P, Seeley JR. The relationship of physical disease and functional impairment to depression in the elderly. Psychology and Aging. 1996;11:572–581. doi: 10.1037//0882-7974.11.4.572. doi:10.1037/0882-7974.11.4.572. [DOI] [PubMed] [Google Scholar]