Abstract
The amyloid precursor protein (APP) is one of the key proteins in Alzheimer’s disease (AD), as it is the precursor of amyloid β (Aβ) peptides accumulating in amyloid plaques. The processing of APP and the pathogenic features of especially Aβ oligomers have been analyzed in detail. Remarkably, there is accumulating evidence from cell biological and structural studies suggesting that APP and its mammalian homologs, the amyloid precursor-like proteins (APLP1 and APLP2), participate under physiological conditions via trans-cellular dimerization in synaptogenesis. This offers the possibility that loss of synapses in AD might be partially explained by dysfunction of APP/APLPs cell adhesion properties. In this review, structural characteristics of APP trans-cellular interaction will be placed critically in context with its putative physiological functions focusing on cell adhesion and synaptogenesis.
Keywords: Alzheimer disease, APLP-1, APLP-2, Dimerization, Cell adhesion, Synaptogenesis
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder, characterized by loss of neurons and synapses in brain regions critical for cognition and memory (reviewed in Selkoe 2002). Main pathological hallmarks found in AD brains are neurofibrillary tangles (NFT) and amyloid plaques (Haass and Mandelkow 2010). Amyloid plaques are extracellular protein deposits mainly composed of a short insoluble peptide termed amyloid β (Aβ), which is generated by proteolytic processing from the amyloid precursor protein (APP). APP is a type I transmembrane protein with a large extracellular domain and a short cytoplasmic tail. The extracellular domain is composed of two subdomains termed E1 and E2 that are interconnected by a highly acidic domain. The E1 domain is further subdivided into a growth factor-like domain (GFLD) and a copper binding domain (CuBD) (reviewed in Reinhard et al. 2005; Gralle and Ferreira 2007). Structural information is available for the E1 and E2 domain as well as the APP intracellular domain (AICD) and will be discussed later on.
APP is one member of a gene family with two additional homologs in mammals termed amyloid precursor-like proteins (APLP1 and APLP2). These proteins display high sequence homology and share a conserved domain structure, but the Aβ sequence is unique to APP. Alternative splicing of both APP and APLP2 gives rise to various isoforms with different expression patterns, whereas only one isoform of APLP1 has been detected so far (reviewed in Jacobsen and Iverfeldt 2009). The Kunitz protease inhibitor (KPI) domain is present in all splice variants of APLP2 and in the two longer splice forms of APP (APP751 and APP770), which are expressed only at low levels in neurons (Sandbrink et al. 1996). APP and APLP2 are ubiquitously expressed, whereas APLP1 expression is restricted to neurons (Slunt et al. 1994; Lorent et al. 1995; Walsh et al. 2007). Analysis of mouse models with genetic deletions of APP, APLP1 or APLP2 revealed a genetic redundancy between the family members since mice with single deletions were viable and fertile and showed only mild phenotypes (reviewed in Anliker and Muller 2006; Guo et al. 2011).
The proteolytic processing of APP by consecutive cleavage of β- and γ-secretases leading to the generation of Aβ peptides and release of the intracellular domain (AICD) is well understood (reviewed in Jacobsen and Iverfeldt 2009). However, despite extensive studies, the physiological functions of APP and its homologs remain still elusive. Based on genetic studies, many different functions have been proposed, including neuronal survival, cell migration, axonal transport as well as central and peripheral synaptogenesis, but so far the molecular mechanisms have remained unclear (reviewed in Zheng and Koo 2011). Some of these functions might be related to the extracellular secreted forms of APP (sAPP) either released by α- or β-secretase, functioning as a ligand of a so far not clearly identified receptor (Turner et al. 2003; Young-Pearse et al. 2008). However, other proposed functions of APP/APLPs, such as cell migration and synaptogenesis, could be explained by cell adhesion features of APP/APLPs, forming trans-cellular homo- and heterotypic dimers or oligomeric complexes (Soba et al. 2005).
Dimerization of APP family members
There is accumulating evidence that APP and its mammalian homologs are able to form homo- and heterodimers in cis and trans orientation at the cellular level. Protein interaction in cis seems to have an impact on APP processing and Aβ generation (Kaden et al. 2008; Eggert et al. 2009), whereas interaction in trans promotes cell adhesion and synaptogenesis (Soba et al. 2005; Wang et al. 2009b). In this review, we are focusing on structural aspects and physiological consequences of APP trans-dimerization.
It has been shown that APP homo- and heterodimerization with its mammalian homologs APLP1 and APLP2 promote cell adhesion by trans-cellular interaction in both S2 cells and mouse embryonic fibroblasts (MEF) (Soba et al. 2005). In co-immunoprecipitation studies, the well-conserved E1 domain was identified as the major interaction interface for dimerization, whereas deletion of the E2 domain had no effect on APP dimerization. The observed accumulation of APP and APLPs at sites of cell contact further indicates a direct trans-cellular interaction, a property that is even more pronounced for APLP1 and APLP2. Furthermore, APLP1 was shown to form trans-cellular interactions in human embryonic kidney (HEK293) cells as well, whereas trans-cellular interaction of APP and APLP2 could not be detected in this cell system (Kaden et al. 2009). In these cells, heterologously expressed APLP1 was particularly enriched at the cell surface, whereas both APP and APLP2 were mainly localized in intracellular compartments (Kaden et al. 2009). Thus, the discrepancy is most likely not due to different trans-interaction properties but is rather a consequence of different surface localization of the single APP family members heterologously expressed in kidney fibroblasts. However, these data suggest that surface localization of APP/APLPs is a major regulator of their cell adhesion features. Recently, the crystal structure of the whole E1 domain was resolved, indicating that the two constituting subdomains GFLD and CuBD interact tightly and form one functional entity (Dahms et al. 2010). It was further shown that addition of a defined heparin induced dimerization of the E1 domain. At least, a decasaccharide is required to bridge the positively charged surface area made up by two opposing GFLD. Therefore, it is tempting to speculate that extension of the oligosaccharide would lead to multimerization of APP resulting in the formation of tetramers and higher order oligomers. Since heparin is secreted under physiological conditions by mast cells mediating anticoagulant function, the binding of heparin by the E1 domain might also stay in context with the previously described anti-coagulant functions of APP and APLP2 (Xu et al. 2009). However, heparan sulfate proteoglycans (HSPG) are structurally related to heparin and are highly abundant components of the extracellular matrix (ECM). Thus, it is conceivable that binding of the E1 domain to HSPG might mediate APP-ECM interactions, as it is well described for other cell adhesion molecules (Kim et al. 2011). However, the in vivo relevance of heparin-induced APP dimerization remains elusive. Further investigations, such as introducing single amino acid substitutions in the heparin-binding domain or testing different substrates instead of heparin, will be necessary to further clarify the supposed mechanism of heparin-induced APP dimerization.
The E2 domain is an independently folded structural unit of the APP ectodomain consisting of two distinct coiled coil substructures connected by a continuous central helix (Wang and Ha 2004). It has been shown by analytical ultracentrifugation that the E2 domain can reversibly dimerize in solution, and structural data revealed an antiparallel orientation of the dimer. Remarkably, dimerization of the E2 domain is induced by heparin binding as well (Lee et al. 2011). However, in the absence of a ligand like heparin, the monomer thermodynamically predominates. Notably, antiparallel APP dimerization mediated by the E2 domain would bring adjacent cells in very close proximity (approx. 10 nm) as determined by ab initio reconstruction of molecular models from small angle X-ray scattering (SAXS) data (Gralle et al. 2006). Hence, it is rather unlikely that E2-mediated antiparallel dimerization of APP occurs at synapses, although it would be theoretically possible to cross the synaptic cleft (approx. 20–30 nm) if the polypeptide chain would be elongated to a maximal extent. Therefore, antiparallel dimerization mediated by the E2 domain is rather involved in specialized cell–cell contacts with minor distances between adjacent cells like gap junctions. Remarkably, comparison of the crystal structures of the APP and APLP1 E2 domain suggests a conserved antiparallel mode of dimerization within the APP protein family (Lee et al. 2011). However, further evidence supporting an antiparallel dimerization mediated by the E2 domain is still lacking. So far, in hemisynapse formation and in in vitro cell interaction assays performed with MEF, HEK293 and S2 cells, no indications for a relevant contribution of the E2 domain have been observed (Soba et al. 2005; Kaden et al. 2009; Wang et al. 2009b).
Taken together, there is conclusive evidence from cellular assays and structural information that APP and its mammalian homologs are able to form dimers in trans orientation at the cellular level and that dimerization promotes cell adhesion. However, taking into account that APP normally undergoes rapid proteolytic conversion by secretases once it reaches the plasma membrane, it will be important to understand how cell surface localization of the APP gene family is regulated. Notably, treatment of cells with phorbol esters like PMA (phorbol 12-myristate 13-acetate) leads to an increased processing of APP as well as APLPs, indicating that at least a small pool of APP/APLPs is stabilized at the cell surface and available for dimerization (Buxbaum et al. 1998; Lammich et al. 1999; Eggert et al. 2004). This is further supported by the estimation that under steady state levels, about 10% of APP is located at the plasma membrane (Kuentzel et al. 1993; Thinakaran and Koo 2008). Alternatively, APP/APLPs might not function as static cell adhesion molecules but are rather involved in more dynamic cell adhesion processes as for instance during neuronal development and/or synaptogenesis.
Physiological consequences of dimerization
APP dimerization in synaptogenesis
Synapses are specialized intercellular junctions that mediate transmission of information between neurons in the brain. Synaptic cell adhesion molecules (SAM) are connecting the pre- and postsynaptic terminals and mediate signaling across the synaptic cleft (Dalva et al. 2007). During synaptogenesis, synaptic cell adhesion molecules stabilize the initial and/or continuous contact between axons and dendrites and often mediate recruitment of additional synaptic proteins.
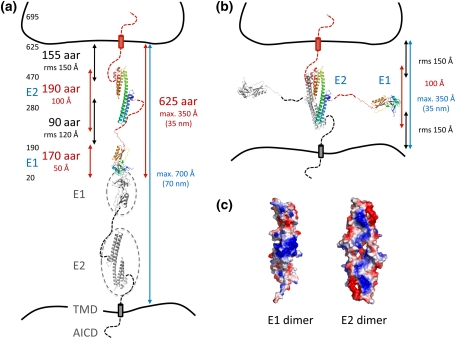
Does the APP family have similar features like common SAMs? Notably, APP/APLPs fulfill all essential preconditions: (1) They are localized to both the pre- and postsynaptic site (Kim et al. 1995; Lyckman et al. 1998; Hoe et al. 2009a). (2) Structural analysis shows that homo- and heterotypic interactions of APP/APLPs are able to span the entire synaptic cleft, mediated either by the E1 or, at maximal extension of the molecule, also by the E2 domain (Fig. 1). (3) APP expression in HEK293 cells cocultured with primary hippocampal neurons promotes hemisynapse formation (Wang et al. 2009b), similar to well-established SAMs as neuroligins or neurexins (Dean and Dresbach 2006). However, the synaptogenic effect of APP was considerably smaller (approx. 50%) compared to that of neuroligin, which might be explained by different expression levels. Notably, expression of deletion constructs lacking either the E1 or E2 domain revealed that the synaptogenic activity of APP primarily depends on the E1 domain (Wang et al. 2009b). These findings are supporting the idea from structural data and cell aggregation assays that the E1 domain is the major interaction interface for trans-dimerization at the synapse (Soba et al. 2005). This clearly classifies APP/APLPs as SAMs.
Fig. 1.
APP/APLP trans-dimerization. a Schematic illustration of the E1 domain-mediated APP dimer spanning intercellular space between, e.g., pre- and postsynaptic sites (APP695 numbering, aar amino acid residue). Structures of E1 [protein data bank (PDB) code 3KTM] and E2 (PDB code 1RW6) are shown as rainbow colored ribbons (blue to red: N- to C-terminus). E1 domain-mediated dimerization with a second APP molecule (gray subunit) is based on structural data of the E1 domain according to the PDB code 3KTM. Gray circles illustrate the positions of E1 and E2 domain in the APP structure. Sizes of unknown linker regions are estimated based on random coil geometry [rms root mean square, average end-to-end distance of a random coil polypeptide (Creighton 1993)]. b E2 domain-mediated APP dimer based on 1RW6. The estimated maximal length of the dimer is about half of the E1 dimer. Notably, taking available SAXS data into account, calculated rms distances might overestimate the maximal distance spanned by an APP trans-dimer under physiological conditions. c Surface potentials of the E1 and E2 dimers (blue positively charged, red negatively charged). Both dimers form continuous positively charged surface patches favorable for HSPG binding
This hypothesis is further corroborated by genetic studies on mice, which are complicated by the overlapping activities of the single APP family members. Mice deficient in both APP and APLP2 display defects at the neuromuscular junction (NMJ) with an aberrant apposition of presynaptic marker proteins with postsynaptic acetylcholine receptors and a reduced number of synaptic vesicles at presynaptic terminals (Wang et al. 2005). Additionally, tissue-specific deletion of APP in either presynaptic motor neurons or postsynaptic muscle cells results in similar neuromuscular synapse defects, suggesting that APP expression at both the presynaptic and the postsynaptic terminal is required for proper development of the NMJ (Wang et al. 2009b). Further, APP/APLP2 knockout mice expressing either sAPPα or sAPPβ, thus lacking the complete APP intracellular domain, exhibit impaired neuromuscular transmission and an aberrant apposition of pre- and postsynaptic sites at the NMJ, supporting the assumption that trans-synaptic adhesion of full length APP is required for proper synapse development (Li et al. 2010; Weyer et al. 2011).
However, the NMJ is a particular synapse since presynaptic motor neuron and postsynaptic muscle cells are separated by a basal lamina composed of several glycoproteins and proteoglycans (Patton 2003). Therefore, the synaptic cleft is considerably larger compared to synapses of the central nervous system. Nevertheless, based on structural information, a direct trans-cellular interaction of APP/APLPs is sufficient to span the entire distance of the synaptic cleft at the NMJ (Fig. 1). However, this interaction might be further stabilized or even partially mediated by components of the basal lamina, such as proteoglycans. Functional analyses at central nerve synapses are further complicated by the additional presence of APLP1, which is lacking in the muscle but is most likely present at the postsynaptic site of CNS synapses (Kim et al. 1995). Further, different excitatory or inhibitory synapses might be affected to different extents, depending on specific physiological conditions, as, e.g., stability of the synapse. Hence, defects caused by lack of APP/APLPs might be more subtle and, therefore, more difficult to detect.
Taken together, the currently available data strongly support the idea that APP acts as a SAM at central and peripheral synapses. In this context, APP, APLP1 and APLP2 might have diverse functions depending on different subcellular localization and pre- or postsynaptic expression as well as the propensity to form homo- and heterotypic dimers. Hence, expression of APP in HEK293 cells cocultured with primary hippocampal neurons of APP/APLP2 double knockout mice revealed that the AICD is dispensable at the presynaptic site, whereas both extracellular and intracellular domains are necessary for synaptogenesis at the postsynaptic site (Wang et al. 2009b). Interestingly, neurexin/neuroligin-induced synaptogenesis is supposed to be mediated by intracellular association partners as well (Biederer and Sudhof 2000), raising the possibility that the synaptogenic activity of APP is mediated by intracellular signaling events similar to other known SAMs.
APP in cell signaling
Due to structural similarities of the GFLD with other known growth factors and its growth promoting properties, APP was proposed to act as a cell surface receptor (Rossjohn et al. 1999). This idea was further supported by the observation that F-spondin, a neuronally secreted glycoprotein implicated in neuronal development, and reelin, an extracellular matrix protein essential for cortical development, are binding to the ectodomain of APP, thereby acting as potential APP ligands (Ho and Sudhof 2004; Hoe et al. 2009b). Moreover, APP undergoes strikingly similar processing as the Notch receptor, leading to the release of an intracellular domain that translocates to the nucleus and interacts with certain transcription factors to regulate expression of target genes involved in development (Bray 2006; Konietzko 2011). The AICD includes an evolutionary conserved YENPTY motif that is important for the interaction with several adaptor proteins, including Fe65, X11/MINT (munc interacting protein), JIP (JNK interacting protein), Numb and CASK (calcium/calmodulin-dependent serine protein kinase) (Jacobsen and Iverfeldt 2009). Interestingly, neurexin/neuroligin-induced synaptogenesis is supposed to be mediated by intracellular association with CASK and X11/MINT (Biederer and Sudhof 2000), indicating that the synaptogenic activity of APP is mediated by intracellular signaling events similar to other synaptic adhesion proteins. The interaction of X11/MINT with AICD seems to stabilize cellular APP, thus extending the half-life of the protein. Interestingly, a single point mutation in the YENPTY motif of APP (Y682G) diminished the interaction with X11/MINT and abolished these effects (Borg et al. 1998). In a recent study, a knockin mouse expressing APP with the same mutation (Y682G) was crossed to APLP2 knockout mice (Barbagallo et al. 2011). This results in postnatal lethality and neuromuscular synapse defects considerably similar to those observed in APP/APLP2 double knockout mice, indicating that tyrosine 682 (Y682) in the cytoplasmic domain of APP is indispensable for proper neuromuscular synapse formation and function. Remarkably, the Y682G mutation strongly reduced the interaction of X11/MINT with APP (Barbagallo et al. 2011), further supporting the idea that complex formation with X11/MINT and CASK is critical for APP function in synaptogenesis.
Of note, the interaction between APP and most adaptor proteins depends on phosphorylation of the AICD, either at threonine 668 (T668) or tyrosine 682 (Y682). Phosphorylation of T668 leads to structural rearrangements in the amino-terminal helix (αN) of the AICD (Ramelot and Nicholson 2001), resulting in reduced binding of the adaptor proteins as shown for Fe65 (Radzimanowski et al. 2008), the best investigated intracellular binding partner of APP/APLPs. Fe65 belongs to a gene family with the mammalian homologs Fe65 like 1 (Fe65L1) and Fe65 like 2 (Fe65L2) (McLoughlin and Miller 2008). Interestingly, Fe65 co-expression with APP/APLPs promotes cell surface localization of APP (Sabo et al. 1999, 2001), which in turn might enhance trans-cellular interaction of APP. In addition, genetic ablation of Fe65 gene family members in mice leads to learning deficits (Wang et al. 2004, 2009a). Moreover, Fe65 has been shown to regulate actin cytoskeleton dynamics as well as nuclear gene activation (Sabo et al. 2001; Cao and Sudhof 2001), linking APP to different intracellular signaling events possibly involved in synaptogenesis.
Although further work will be necessary to elucidate APP/APLP function, our current knowledge of the trans-cellular interaction properties of APP/APLPs encourage the hypothesis that APP/APLPs function as dynamic SAMs, probably not involved in static long-term stabilization of synapses, but rather in short-term signaling events involved in synaptogenesis.
Acknowledgments
We thank Luigina Hanke for administrative assistance and the Deutsche Forschungsgemeinschaft (DFG) for support of this work in context of the FOR1332 grant to SK (KI 819/5-1 and/6-1) and KW (WI 2649/2-1).
Conflict of interest
The authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Anliker B, Muller U. The functions of mammalian amyloid precursor protein and related amyloid precursor-like proteins. Neurodegener Dis. 2006;3(4–5):239–246. doi: 10.1159/000095262. [DOI] [PubMed] [Google Scholar]
- Barbagallo AP, Wang Z, Zheng H, D’Adamio L. A single tyrosine residue in the amyloid precursor protein intracellular domain is essential for developmental function. J Biol Chem. 2011;286(11):8717–8721. doi: 10.1074/jbc.C111.219873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederer T, Sudhof TC. Mints as adaptors. Direct binding to neurexins and recruitment of munc18. J Biol Chem. 2000;275(51):39803–39806. doi: 10.1074/jbc.C000656200. [DOI] [PubMed] [Google Scholar]
- Borg JP, Yang Y, De Taddeo-Borg M, Margolis B, Turner RS. The X11alpha protein slows cellular amyloid precursor protein processing and reduces Abeta40 and Abeta42 secretion. J Biol Chem. 1998;273(24):14761–14766. doi: 10.1074/jbc.273.24.14761. [DOI] [PubMed] [Google Scholar]
- Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7(9):678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- Buxbaum JD, Liu KN, Luo Y, Slack JL, Stocking KL, Peschon JJ, Johnson RS, Castner BJ, Cerretti DP, Black RA. Evidence that tumor necrosis factor alpha converting enzyme is involved in regulated alpha-secretase cleavage of the Alzheimer amyloid protein precursor. J Biol Chem. 1998;273(43):27765–27767. doi: 10.1074/jbc.273.43.27765. [DOI] [PubMed] [Google Scholar]
- Cao X, Sudhof TC. A transcriptionally [correction of transcriptively] active complex of APP with Fe65 and histone acetyltransferase Tip60. Science. 2001;293(5527):115–120. doi: 10.1126/science.1058783. [DOI] [PubMed] [Google Scholar]
- Creighton TE. Proteins: structures and molecular properties. 2. New York: W.H. Freeman; 1993. p. 177,. [Google Scholar]
- Dahms SO, Hoefgen S, Roeser D, Schlott B, Guhrs KH, Than ME. Structure and biochemical analysis of the heparin-induced E1 dimer of the amyloid precursor protein. Proc Natl Acad Sci USA. 2010;107(12):5381–5386. doi: 10.1073/pnas.0911326107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalva MB, McClelland AC, Kayser MS. Cell adhesion molecules: signalling functions at the synapse. Nat Rev Neurosci. 2007;8(3):206–220. doi: 10.1038/nrn2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C, Dresbach T. Neuroligins and neurexins: linking cell adhesion, synapse formation and cognitive function. Trends Neurosci. 2006;29(1):21–29. doi: 10.1016/j.tins.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Eggert S, Paliga K, Soba P, Evin G, Masters CL, Weidemann A, Beyreuther K. The proteolytic processing of the amyloid precursor protein gene family members APLP-1 and APLP-2 involves alpha-, beta-, gamma-, and epsilon-like cleavages: modulation of APLP-1 processing by n-glycosylation. J Biol Chem. 2004;279(18):18146–18156. doi: 10.1074/jbc.M311601200. [DOI] [PubMed] [Google Scholar]
- Eggert S, Midthune B, Cottrell B, Koo EH. Induced dimerization of the amyloid precursor protein leads to decreased amyloid-beta protein production. J Biol Chem. 2009;284(42):28943–28952. doi: 10.1074/jbc.M109.038646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralle M, Ferreira ST. Structure and functions of the human amyloid precursor protein: the whole is more than the sum of its parts. Prog Neurobiol. 2007;82(1):11–32. doi: 10.1016/j.pneurobio.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Gralle M, Oliveira CL, Guerreiro LH, McKinstry WJ, Galatis D, Masters CL, Cappai R, Parker MW, Ramos CH, Torriani I, Ferreira ST. Solution conformation and heparin-induced dimerization of the full-length extracellular domain of the human amyloid precursor protein. J Mol Biol. 2006;357(2):493–508. doi: 10.1016/j.jmb.2005.12.053. [DOI] [PubMed] [Google Scholar]
- Guo Q, Wang Z, Li H, Wiese M, Zheng H (2011) APP physiological and pathophysiological functions: insights from animal models. Cell Res. doi:10.1038/cr.2011.116 [DOI] [PMC free article] [PubMed]
- Haass C, Mandelkow E. Fyn-tau-amyloid: a toxic triad. Cell. 2010;142(3):356–358. doi: 10.1016/j.cell.2010.07.032. [DOI] [PubMed] [Google Scholar]
- Ho A, Sudhof TC. Binding of F-spondin to amyloid-beta precursor protein: a candidate amyloid-beta precursor protein ligand that modulates amyloid-beta precursor protein cleavage. Proc Natl Acad Sci USA. 2004;101(8):2548–2553. doi: 10.1073/pnas.0308655100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoe HS, Fu Z, Makarova A, Lee JY, Lu C, Feng L, Pajoohesh-Ganji A, Matsuoka Y, Hyman BT, Ehlers MD, Vicini S, Pak DT, Rebeck GW. The effects of amyloid precursor protein on postsynaptic composition and activity. J Biol Chem. 2009;284(13):8495–8506. doi: 10.1074/jbc.M900141200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoe HS, Lee KJ, Carney RS, Lee J, Markova A, Lee JY, Howell BW, Hyman BT, Pak DT, Bu G, Rebeck GW. Interaction of reelin with amyloid precursor protein promotes neurite outgrowth. J Neurosci. 2009;29(23):7459–7473. doi: 10.1523/JNEUROSCI.4872-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen KT, Iverfeldt K. Amyloid precursor protein and its homologues: a family of proteolysis-dependent receptors. Cell Mol Life Sci. 2009;66(14):2299–2318. doi: 10.1007/s00018-009-0020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaden D, Munter LM, Joshi M, Treiber C, Weise C, Bethge T, Voigt P, Schaefer M, Beyermann M, Reif B, Multhaup G. Homophilic interactions of the amyloid precursor protein (APP) ectodomain are regulated by the loop region and affect beta-secretase cleavage of APP. J Biol Chem. 2008;283(11):7271–7279. doi: 10.1074/jbc.M708046200. [DOI] [PubMed] [Google Scholar]
- Kaden D, Voigt P, Munter LM, Bobowski KD, Schaefer M, Multhaup G. Subcellular localization and dimerization of APLP1 are strikingly different from APP and APLP2. J Cell Sci. 2009;122(Pt 3):368–377. doi: 10.1242/jcs.034058. [DOI] [PubMed] [Google Scholar]
- Kim TW, Wu K, Xu JL, McAuliffe G, Tanzi RE, Wasco W, Black IB. Selective localization of amyloid precursor-like protein 1 in the cerebral cortex postsynaptic density. Brain Res Mol Brain Res. 1995;32(1):36–44. doi: 10.1016/0169-328X(95)00328-P. [DOI] [PubMed] [Google Scholar]
- Kim SH, Turnbull J, Guimond S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol. 2011;209(2):139–151. doi: 10.1530/JOE-10-0377. [DOI] [PubMed] [Google Scholar]
- Konietzko U (2011) AICD nuclear signaling and its possible contribution to Alzheimer’s disease. Curr Alzheimer Res [Epub ahead of print] [DOI] [PubMed]
- Kuentzel SL, Ali SM, Altman RA, Greenberg BD, Raub TJ. The Alzheimer beta-amyloid protein precursor/protease nexin-II is cleaved by secretase in a trans-Golgi secretory compartment in human neuroglioma cells. Biochem J. 1993;295(Pt 2):367–378. doi: 10.1042/bj2950367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammich S, Kojro E, Postina R, Gilbert S, Pfeiffer R, Jasionowski M, Haass C, Fahrenholz F. Constitutive and regulated alpha-secretase cleavage of Alzheimer’s amyloid precursor protein by a disintegrin metalloprotease. Proc Natl Acad Sci USA. 1999;96(7):3922–3927. doi: 10.1073/pnas.96.7.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Xue Y, Hu J, Wang Y, Liu X, Demeler B, Ha Y (2011) The E2 domains of APP and APLP1 share a conserved mode of dimerization. Biochemistry 50(24):5453. doi:10.1021/bi101846x [DOI] [PMC free article] [PubMed]
- Li H, Wang B, Wang Z, Guo Q, Tabuchi K, Hammer RE, Sudhof TC, Zheng H. Soluble amyloid precursor protein (APP) regulates transthyretin and Klotho gene expression without rescuing the essential function of APP. Proc Natl Acad Sci USA. 2010;107(40):17362–17367. doi: 10.1073/pnas.1012568107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorent K, Overbergh L, Moechars D, De Strooper B, Van Leuven F, Van den Berghe H. Expression in mouse embryos and in adult mouse brain of three members of the amyloid precursor protein family, of the alpha-2-macroglobulin receptor/low density lipoprotein receptor-related protein and of its ligands apolipoprotein E, lipoprotein lipase, alpha-2-macroglobulin and the 40,000 molecular weight receptor-associated protein. Neuroscience. 1995;65(4):1009–1025. doi: 10.1016/0306-4522(94)00555-J. [DOI] [PubMed] [Google Scholar]
- Lyckman AW, Confaloni AM, Thinakaran G, Sisodia SS, Moya KL. Post-translational processing and turnover kinetics of presynaptically targeted amyloid precursor superfamily proteins in the central nervous system. J Biol Chem. 1998;273(18):11100–11106. doi: 10.1074/jbc.273.18.11100. [DOI] [PubMed] [Google Scholar]
- McLoughlin DM, Miller CC. The FE65 proteins and Alzheimer’s disease. J Neurosci Res. 2008;86(4):744–754. doi: 10.1002/jnr.21532. [DOI] [PubMed] [Google Scholar]
- Patton BL. Basal lamina and the organization of neuromuscular synapses. J Neurocytol. 2003;32(5–8):883–903. doi: 10.1023/B:NEUR.0000020630.74955.19. [DOI] [PubMed] [Google Scholar]
- Radzimanowski J, Simon B, Sattler M, Beyreuther K, Sinning I, Wild K (2008) Structure of the intracellular domain of the amyloid precursor in complex with Fe65-PTB2. EMBO Rep 9(11):1134–1140 [DOI] [PMC free article] [PubMed]
- Ramelot TA, Nicholson LK. Phosphorylation-induced structural changes in the amyloid precursor protein cytoplasmic tail detected by NMR. J Mol Biol. 2001;307(3):871–884. doi: 10.1006/jmbi.2001.4535. [DOI] [PubMed] [Google Scholar]
- Reinhard C, Hebert SS, De Strooper B. The amyloid-beta precursor protein: integrating structure with biological function. EMBO J. 2005;24(23):3996–4006. doi: 10.1038/sj.emboj.7600860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossjohn J, Cappai R, Feil SC, Henry A, McKinstry WJ, Galatis D, Hesse L, Multhaup G, Beyreuther K, Masters CL, Parker MW. Crystal structure of the N-terminal, growth factor-like domain of Alzheimer amyloid precursor protein. Nat Struct Biol. 1999;6(4):327–331. doi: 10.1038/7562. [DOI] [PubMed] [Google Scholar]
- Sabo SL, Lanier LM, Ikin AF, Khorkova O, Sahasrabudhe S, Greengard P, Buxbaum JD. Regulation of beta-amyloid secretion by FE65, an amyloid protein precursor-binding protein. J Biol Chem. 1999;274(12):7952–7957. doi: 10.1074/jbc.274.12.7952. [DOI] [PubMed] [Google Scholar]
- Sabo SL, Ikin AF, Buxbaum JD, Greengard P. The Alzheimer amyloid precursor protein (APP) and FE65, an APP-binding protein, regulate cell movement. J Cell Biol. 2001;153(7):1403–1414. doi: 10.1083/jcb.153.7.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandbrink R, Masters CL, Beyreuther K. APP gene family. Alternative splicing generates functionally related isoforms. Ann N Y Acad Sci. 1996;777:281–287. doi: 10.1111/j.1749-6632.1996.tb34433.x. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298(5594):789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Slunt HH, Thinakaran G, Von Koch C, Lo AC, Tanzi RE, Sisodia SS. Expression of a ubiquitous, cross-reactive homologue of the mouse beta-amyloid precursor protein (APP) J Biol Chem. 1994;269(4):2637–2644. [PubMed] [Google Scholar]
- Soba P, Eggert S, Wagner K, Zentgraf H, Siehl K, Kreger S, Lower A, Langer A, Merdes G, Paro R, Masters CL, Muller U, Kins S, Beyreuther K. Homo- and heterodimerization of APP family members promotes intercellular adhesion. EMBO J. 2005;24(20):3624–3634. doi: 10.1038/sj.emboj.7600824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thinakaran G, Koo EH. Amyloid precursor protein trafficking, processing, and function. J Biol Chem. 2008;283(44):29615–29619. doi: 10.1074/jbc.R800019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner PR, O’Connor K, Tate WP, Abraham WC. Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog Neurobiol. 2003;70(1):1–32. doi: 10.1016/S0301-0082(03)00089-3. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Minogue AM, Sala Frigerio C, Fadeeva JV, Wasco W, Selkoe DJ. The APP family of proteins: similarities and differences. Biochem Soc Trans. 2007;35(Pt 2):416–420. doi: 10.1042/BST0350416. [DOI] [PubMed] [Google Scholar]
- Wang B, Hu Q, Hearn MG, Shimizu K, Ware CB, Liggitt DH, Jin LW, Cool BH, Storm DR, Martin GM. Isoform-specific knockout of FE65 leads to impaired learning and memory. J Neurosci Res. 2004;75(1):12–24. doi: 10.1002/jnr.10834. [DOI] [PubMed] [Google Scholar]
- Wang P, Yang G, Mosier DR, Chang P, Zaidi T, Gong YD, Zhao NM, Dominguez B, Lee KF, Gan WB, Zheng H. Defective neuromuscular synapses in mice lacking amyloid precursor protein (APP) and APP-Like protein 2. J Neurosci. 2005;25(5):1219–1225. doi: 10.1523/JNEUROSCI.4660-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ha Y. The X-ray structure of an antiparallel dimer of the human amyloid precursor protein E2 domain. Mol Cell. 2004;15(3):343–353. doi: 10.1016/j.molcel.2004.06.037. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang M, Moon C, Hu Q, Wang B, Martin G, Sun Z, Wang H. The APP-interacting protein FE65 is required for hippocampus-dependent learning and long-term potentiation. Learn Mem. 2009;16(9):537–544. doi: 10.1101/lm.1499309. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wang B, Yang L, Guo Q, Aithmitti N, Songyang Z, Zheng H. Presynaptic and postsynaptic interaction of the amyloid precursor protein promotes peripheral and central synaptogenesis. J Neurosci. 2009;29(35):10788–10801. doi: 10.1523/JNEUROSCI.2132-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyer SW, Klevanski M, Delekate A, Voikar V, Aydin D, Hick M, Filippov M, Drost N, Schaller KL, Saar M, Vogt MA, Gass P, Samanta A, Jaschke A, Korte M, Wolfer DP, Caldwell JH, Muller UC. APP and APLP2 are essential at PNS and CNS synapses for transmission, spatial learning and LTP. EMBO J. 2011;30(11):2266–2280. doi: 10.1038/emboj.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Previti ML, Nieman MT, Davis J, Schmaier AH, Van Nostrand WE. AbetaPP/APLP2 family of Kunitz serine proteinase inhibitors regulate cerebral thrombosis. J Neurosci. 2009;29(17):5666–5670. doi: 10.1523/JNEUROSCI.0095-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Pearse TL, Chen AC, Chang R, Marquez C, Selkoe DJ. Secreted APP regulates the function of full-length APP in neurite outgrowth through interaction with integrin beta1. Neural Dev. 2008;3:15. doi: 10.1186/1749-8104-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Koo EH. Biology and pathophysiology of the amyloid precursor protein. Mol Neurodegener. 2011;6(1):27. doi: 10.1186/1750-1326-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]