Abstract
Crohn’s disease and ulcerative colitis represent the two main forms of the idiopathic chronic inflammatory bowel diseases (IBD). Currently available blood and stool based biomarkers provide reproducible, quantitative tools which can complement clinical assessment to aid clinicians in IBD diagnosis and management. C-reactive protein and fecal based leukocyte markers can help the clinician distinguish IBD from non-inflammatory diarrhea and assess disease activity. The ability to differentiate between forms of IBD and predict risk for disease complications is specific to serologic tests including antibodies against Saccharomyces cerevisiae and perinuclear antineutrophil cytoplasmic proteins. Advances in genomic, proteomic and metabolomic array based technologies are facilitating the development of new biomarkers for IBD. The discovery of novel biomarkers which can correlate with mucosal healing or predict long term disease course has the potential to significantly improve patient care. This article reviews the uses and limitations of currently available biomarkers and highlights recent advances in IBD biomarker discovery.
Keywords: Ulcerative colitis, Crohn’s Disease, IBD, Biomarker, Tryptophan, C-reactive protein, ASCA, Calprotectin, indoleamine
INTRODUCTION
The two major forms of the chronic inflammatory bowel diseases (IBD) are Crohn’s disease and ulcerative colitis. Together these diseases affect over a million individuals in the United States with prevalence rates close to 200 per 100,000 in some populations.(1) Healthcare utilization by patients with IBD is high and disease associated morbidity is significant for those with progressive and difficult to manage disease.(2) While the etiology remains idiopathic, evidence suggests that the ongoing inflammation in IBD results from persistent overly aggressive inflammatory responses to a subset of commensal microorganisms in a genetically susceptible host with exposure to environmental triggers.(3–5) Genetic, clinical and animal model studies have revealed important roles in disease pathophysiology for host-microbe interactions, autophagy and several components of both the innate and adaptive immune systems.(3)
Crohn’s disease (CD) is characterized by discontinuous regions of intestinal inflammation most frequently involving the terminal ileum and colon, but can affect any part of the gastrointestinal tract from mouth to anus. Abdominal pain, weight loss and variable degrees of diarrhea are symptoms of CD. The inflammatory process of CD is transmural in nature and, as a result, potential disease complications include intestinal fibrosis, strictures and fistula formation. The inflammatory process of ulcerative colitis (UC) is limited to the mucosa and submucosa of the colon alone with disease almost invariably involving the rectum. Diarrhea, hematochezia, tenesmus and defecatory urgency are classic symptoms of active UC. Extraintestinal manifestations of IBD which may affect joints, eyes and/or the skin occur in up to 25% of patients. Disease activity is typically relapsing and remitting in both UC and CD. The disease course of CD is typically progressive. Persistent disease activity despite medical therapy or the development of disease complications frequently requires surgical intervention.
Frequent evaluative testing, often invasive, is routine for diagnosis and ongoing care of patients with IBD. The diagnosis of either CD or UC is based on clinical symptoms combined with typical findings on endoscopy, radiology and ultimately pathology. IBD care presents challenges to the most experienced clinicians in initial diagnosis, risk stratification for disease progression and therapy. In clinical trials, selection of the optimal patient population and monitoring their disease activity present additional challenges. Ongoing debate exists as to the ideal testing strategies to utilize for each of these tasks.
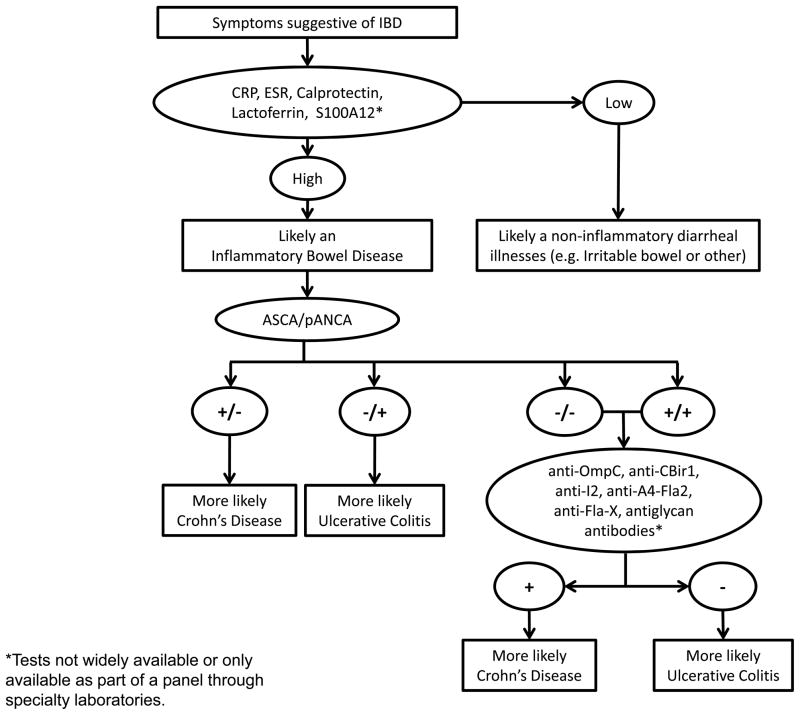
Biomarkers are currently used in conjunction with routine testing in clinical care of patients with IBD. Examined roles for biomarkers in IBD include support in making the IBD diagnosis, differentiation between CD and UC, determination of disease activity, risk stratification of patients for severity of disease course and in prediction of response to therapy. Most biomarkers used are not disease specific, but reflect generalized inflammation. The last decade has brought significant gains in insight to IBD genetics and pathogenesis. These insights have the potential to improve upon the utility of biomarkers currently in use. Based on the available literature, an algorithm for biomarker use in clinical care of IBD patients is depicted in figure 1. Development of an expanded repertoire of biomarkers for use in all aspects of clinical care of IBD patients and in the research of new therapies is needed. This review will summarize biomarkers currently used in clinical care, and highlight recent advances in field of IBD biomarker discovery.
Figure 1.
Use of biomarkers as adjunct to clinical evaluation for the diagnosis of IBD
HISTORICAL MEASUREMENT TOOLS OF IBD ACTIVITY
Methods of measuring disease activity in clinical trials have traditionally included patient symptoms, endoscopic appearance and basic laboratory markers. In CD, the Crohn’s disease activity index (CDAI) has been in use for greater than 30 years. Both subjective and objective markers of disease are incorporated into the CDAI including diarrhea, abdominal pain, general well-being, extra-intestinal manifestations, anti-diarrheal use, abdominal mass, hematocrit, and weight.(6) Symptoms are assessed over 7 days. The Harvey-Bradshaw index was developed after the CDAI, and includes only 5 of the 8 clinical parameters above (hematocrit, weight, and anti-diarrheal use are excluded). The HBI is measured over the last 24 hours prior to assessment and is a simpler method of CD activity assessment.(7) While there have been multiple indices developed for assessment of UC severity, the Mayo score and the Ulcerative Colitis Disease Activity Index (UCDAI) are the most commonly used. These two scoring systems have four components: stool frequency, rectal bleeding, endoscopic disease assessment, and the physician’s global assessment or rating of disease activity. The Mayo score also includes a patient’s functional assessment that is incorporated into the physician’s global assessment.(8) Various clinical trials have modified the definitions of clinical remission or response, while retaining the basic components of the Mayo score.(8) Health-related quality of life scales such as the inflammatory bowel disease questionnaire (IBDQ) and short from of the IBDQ (SIBDQ) have also been validated as measurements of bowel and systemic symptoms, as well as emotional and social functions for CD and UC.(9)
The use of clinical parameters alone to judge IBD disease activity can present challenges. Irritable bowel syndrome (IBS) type symptoms can sometimes confound study results which are been based on clinical disease indices alone. IBS type symptoms may account for the high placebo responses sometimes seen with CDAI use in trials.(10, 11) These high placebo rates lead to difficulty in demonstrating treatment efficacy, as evidenced by a recent trial of certolizumab in CD, where the use of biomarkers as objective measures of disease activity may have enhanced the ability to reach statistical significance.(12) In UC, mucosal healing can be assessed by flexible sigmoidoscopy; however, this test is invasive and adds significant cost to care. For both CD and UC, the indices currently in use are limited in their ability to predict long-term outcomes including surgery, hospitalization, relapse, and disability.(13–15) Thus, the inadequacies of currently used clinical indices of IBD activity highlight the need for biomarkers which can assist in providing objective assessments of treatment effects.
SERUM MARKERS OF ACUTE PHASE RESPONSE
C-Reactive Protein
C-reactive protein (CRP) is a protein produced by the liver in response to a variety of acute and chronic inflammatory conditions. Cytokines associated with active IBD [interleukin (IL)-6, tumor necrosis factor alpha (TNFα ) and IL-1β] stimulate increased production of CRP by hepatocytes over baseline levels which are typically less than 1mg/l.(16) During active IBD, levels can range from 5–200 mg/l depending on disease severity and the individual’s capacity to produce CRP. Such elevations of CRP are not specific to IBD as levels are also increased in various viral and bacterial infections, other autoimmune disorders, malignancy and other disorders resulting in tissue necrosis.(17) Positive features for CRP as a biomarker include that it is easily and reliably measured across diagnostic laboratories and has a short plasma half-life of ~19 hours which is determined by synthesis rather than degradation.(18)
Heterogeneity exists between individuals’ immune responses and elevations in CRP are more common in CD than in UC.(19) Potential explanations for this difference include the greater depth of inflammation and higher serum IL-6 found in CD verses UC.(20, 21) While most patients with active CD mount a CRP response, the level of CRP elevation varies both by individual and perhaps disease location. Florin et al found that ~10% of examined patients with active CD by clinical assessment were found to have a CRP of <10.(22) The phenotype of isolated ileal disease and low body mass index were found to be common in the low CRP producing group. However, analysis of a separate patient cohort did not confirm significance of this finding.(23) Gene polymorphisms have also been reported as an explanation for differences in CRP levels.(24)
Erythrocyte Sedimentation Rate
The rate at which red blood cells (RBC) migrate through the plasma over the period of 1 hour is termed ESR. ESR provides a crude, but rapid, assessment of the general acute phase response. When inflammation is present, pro-sedimentation factors, namely fibrinogen, cause RBCs to stick together and settle faster. Several factors influence the ESR including age, gender, anemia, blood dyscrasias and pregnancy.(25) Compared to CRP, the ESR peaks less rapidly and resolves more slowly in response to changes in inflammation, and has a smaller degree of change making it less suited to following changes in disease activity. Nonetheless, ESR has been and remains widely used as a biomarker of IBD activity.
Other Laboratory Markers
Separate from their hemostatic function, platelets have a recognized role in inflammatory processes.(26) Their relationship to IBD pathophysiology remains an area of active investigation.(27, 28) In active IBD, the platelet count may be elevated while the mean platelet volume is low. This finding led one group to suggest platelet count as useful in distinguishing IBD from infectious diarrhea.(29) While the normal level varies considerably, in practice the platelet count is routinely available on patients and, if elevated, may alert the clinician to ongoing inflammation.
Other serum based acute phase response tests are less useful or untested as biomarkers in IBD. The white blood cell count may be elevated though it is non-specific and may be influenced by therapies such as glucocorticoids. Serum albumin may be low with acute inflammation, but is also affected by nutritional status. Orosomucoid is another hepatocyte derived acute phase protein that has been shown to correlate with IBD activity(30); however, the long half-life (5 days) has limited its utility in practice. We will focus on the clinical utility of CRP, which is the most studied acute phase reactant in IBD.
CRP in the Differentiation of IBD from non-inflammatory GI conditions
For most individuals, after abscess and infection are excluded, CRP has value as a biomarker in distinguishing active CD from functional bowel disorders.(31) Serum CRP has a lower sensitivity in UC, thus it cannot be relied upon solely to exclude the diagnosis of this disorder.(32)
CRP in Evaluating Patients with known IBD
CRP has the ability to distinguish active from quiescent IBD and trends with mucosal healing. A retrospective analysis of Mayo Clinic data showed moderate or greater clinical disease severity (OR 4.5), active disease on colonoscopy (OR 3.5) and severe inflammation on histology (OR 10.6) to associate with an elevated CRP.(33) In the same study, 51% of UC patients with active disease by colonoscopy had an elevated CRP. In a follow up investigation from the same group, quantitative assessment by CT-enterography correlated with CRP when perienteric inflammation was included, but not inflammation limited to the small bowel wall.(34) In patients with symptoms of active CD and an elevated CRP, 86% exhibited evidence of inflammation at colonoscopy.(33) This suggests that CRP is additive to clinical assessment in the ability to predict active mucosal inflammation. Across studies however, CRP is less sensitive and has a lower correlation with mucosal inflammation by endoscopy than the stool based biomarkers calprotectin and lactoferrin.(32) This is true in both UC and CD patients. In studies including CD patients, results were generally reported without distinguishing patients by disease location (ileal vs colonic vs ileocolonic); however, one study noted that abnormal small bowel radiographic findings did not associate with CRP elevation.(33)
In patients on concomitant immunosuppressive therapy for CD, elevated CRP (>5) or elevated platelet count (>298) at time of azathioprine withdrawal was predictive of future infliximab failure.(35) Though not highly sensitive, several studies have shown CRP elevations to be predictive of recurrence with CD.(36–38) A recent study identified CRP levels to associate with response to infliximab therapy in CD. Early normalization of CRP correlated with long-term response and a sustained increase of CRP levels in patients on infliximab correlated with a loss of response to therapy.(39) In contrast, evaluations of ESR have shown only mixed results with regards to its ability to predict relapse.(37, 40)
SEROLOGIC MARKERS/ANTIBODIES
Immunologic biomarkers have been used individually and as biomarker panels in IBD care. Antibodies currently used cross-react with several bacterial and fungal antigens. The existence of these antibodies in IBD patients highlight the abnormal immune response which exists between host and commensal enteric microorganisms.
Anti-neutrophil cytoplasmic antibodies (ANCAs) and anti-Saccharomyces cerevisiae antibodies (ASCA)
ANCAs are found in a variety of immune conditions, such as Wegener’s granulomatosis and rheumatoid arthritis, as well as in UC. pANCA (perinulear ANCA) is found in 20–85% of UC patients, and 2–28% of CD patients.(41) ASCA binds mannose sequences in phosphopeptidomannan located in the cell wall of S. cervisiae (baker’s/brewer’s yeast). Candida albicans also has epitopes that bind ASCA.(42) ASCA is most prevalent in CD patients. ANCA and ASCA were the first utilized antibodies in the setting of IBD diagnosis.(41) In some estimates, ASCA is found in 39–76% of patients with CD, up to 15% of patients with UC, and 5% of healthy controls.(43) Of note, a positive ASCA has also been seen in patients with Behcet’s disease, celiac disease, autoimmune hepatitis, and primary biliary cirrhosis. ANCA positivity can be found in other forms of colitis, such as eosinophilic and collagenous colitis.(44)
Antibodies to outer membrane porin (Anti-OmpC), Flagellin (Anti-Cbir1), Pseudomonas flourescens -associated sequence I–2 (Anti-I2), and antibodies to Flagellin A4-Fla2 and Fla-X
Anti-OmpC is an antibody to an outer membrane protein isolated first from Eschericia coli. Adherent-invasive E. coli has been found in ileal CD lesions, and OmpC has been shown to be required for these organisms to thrive in the GI tract.(45, 46) Cbir1 is a flagellin related antigen that was initially identified in the enteric flora of mice, and has an ability to induce colitis in immunodeficient mice.(47) I2 is a bacterial DNA fragment that is a homolog of a bacterial transcription factor family. This sequence was identified in lamina propria mononuclear cells of active CD patients, and was later found to be associated with P. flourescens.(48, 49) Anti-OmpC, anti-CBir1 IgG, and anti-I2 IgA have a prevalence of approximately 50% in CD patients. (47, 48, 50) Their prevalence ranges from 5–11% in UC patients and 4–8% in healthy controls.(47, 48, 51) Flagellins A4-Fla2 and Fla-X are newly identified flagellins to which some CD patients are seropositive. In a two-year prospective cohort study of 252 patients with CD, 76% of whom had small bowel CD, 59% had antibodies to A4-Fla2 and 57% to Fla-X.(52) In a cross-sectional study, antibodies to flagellin A4-Fla2 and Fla-X were found in 29 and 26% of IBS patients, respectively.(53) These antibodies were more frequent in patients with post-infectious IBS. The prevalence of the remaining antimicrobial antibodies in IBS patients, immune-suppressed patients, and patients with gastrointestinal infections has not been adequately assessed.
Anticarbohydrate antibodies: antilaminaribioside carbohydrate IgG (ALCA), antichitobioside carbohydrate IgA (ACCA), anti-synthetic mannoside antibodies (AΣMA or AMCA)
ALCA, ACCA, and AMCA are novel antiglycan antibodies. They are similar to ASCA in that they are antibodies to sugars on the surface of microorganisms. ALCA and ACCA are associated with CD, and are found in 17–28% of CD patients, a rate lower than ASCA.(25) They may enhance testing sensitivity because they are positive in 34–44% of CD patients who were ASCA negative.(54, 55)
AΣMA are antibodies against synthetic oligomannose epitopes.(56) These antibodies were also found to be positive in 24% of patients with CD who were negative for ASCA, and had a lower sensitivity but higher specificity when compared to ASCA.(56) Anti-C, anti-chitin carbohydrate antibody and Anti-L, anti-laminarin carbohydrate antibody, are similar in their low sensitivity but relatively high specificity in CD patients, when compared with patients with UC.(57, 58)
Serologic markers in differentiation of IBD from non-inflammatory GI conditions, and UC from CD
A meta-analysis of 60 studies (3,841 UC and 4,019 CD patients) assessed the utility of ASCA and pANCA in IBD.(59) The presence of either pANCA or ASCA was able to differentiate between IBD and non-IBD with a sensitivity of 63% and a specificity of 93%, with low heterogeneity between studies. In this meta-analysis, ASCA+/ pANCA – test had a sensitivity of 55% and a specificity of 93% for CD. Sensitivity and specificity of the pANCA+ tests for UC were 55.3% and 88.5% respectively.(59) Of note, in patients with colonic CD, ASCA was less able to distinguish between CD and UC.(59) In a pediatric study, increased pANCA levels were seen in patients with UC or those with CD who had a UC-like pancolitis picture.(60) In practice, clinicians should recognize that false positives occur in patients with non-specific GI symptoms.(25)
The addition of anti-OmpC, anti-CBir1, and anti-I2 to ASCA and ANCA constituted the commercially available serologic panel (Serology 7, Prometheus, San Diego, CA). A study of 300 pediatric patients tested the use of this panel and yielded 67% sensitivity and 76% specificity from a cohort of patients referred for IBD evaluation (IBD prevalence of 38%).(61) Using this panel of 7 antibodies, there is sensitivity of 80% and a positive predictive value of 90% for CD, but only in a population with high CD prevalence.(62) Anti-A4-Fla2 and Anti-Fla-X can be useful in distinguishing CD from UC, as they were only positive in 6% of UC patients, as compared to 50–60% of CD patients.(52) These novel antibodies are part of a recently revised panel that includes additional serologic as well as genetic and inflammatory biomarkers relevant to human IBD (IBD sgi Diagnostic, Prometheus, San Diego, CA).(63)
In a study of 1225 IBD patients, 200 controls and 113 patients with other GI inflammation a combination of ASCA, pANCA and ALCA offered the best diagnostic accuracy.(64) As mentioned above, the antiglycan antibodies improve the specificity for CD, and may be useful in ASCA negative patients.(54, 55) Antibodies have a role in predicting phenotype progression of indeterminate colitis (IC) or IBD type unclassified (10% of colitis cases).(32) A prospective study following 97 patients with IC from 3 centers for a median of 9.9 years showed that ASCA + /pANCA− predicted CD (80%) and ASCA−/pANCA+ predicted UC (63.6%), but almost 50% of patients had neither antibody positive.(65) Anti-OmpC and anti-I2, when added to ASCA and ANCA, only provided a small benefit in diagnosis of CD or UC in patients with IC.(66) Interestingly, the novel AΣMA fared better than ASCA alone in predicting CD development in patients with IBD-type unclassified (sensitivity 45 vs 27%, specificity 100% vs 71%).(56)
Serologic markers in determining prognosis and predicting disease course in CD
In CD, 50% of patients have an uncomplicated course, when followed over 10–20 years. The remaining 50% develop stricturing or penetrating complications in the first 20 years, and require surgery in the first 6 months.(67) These patients at higher risk for complications may benefit from receiving more aggressive CD therapy upfront. Unfortunately most studies of serologic markers which aim to determine prognostication ability are performed in a cross-sectional manner; a study design that does not account for changes in the antibody profile over time.(32) In the previously mentioned meta-analysis of ASCA and ANCA, these antibodies predicted a more complex disease course inconsistently across different studies.(59) A study of 303 patients with CD by Mow et al showed that both antibody seropositivity and titer were associated with various complications. ASCA predicted small bowel disease, fibrostenosis, internal perforation and surgery. pANCA was not associated with any complications of CD. Anti-I2 was associated with increased fibrostenotic disease and small bowel surgery. Anti-Omp C was associated with stenosis, perforation, and small bowel surgery.(68) A separate study showed that antibodies to Cbir1 were associated with small bowel disease, internally penetrating disease, and fibrostenosis but not small bowel surgery.(69) Anti-A4-Fla2 and anti-FlaX were positively associated with a stricturing phenotype and small bowel disease location and negatively associated with an inflammatory CD phenotype.(52) Antiglycan antibodies have also been associated with the development of strictures and penetrating disease.(64) Prospective cohort studies following children from the time of CD diagnosis showed that 8.2%–9% of patients with one or more positive antibody (ASCA, anti-I2, anti-OmpC) had complications, compared to 2.3–2.7% with a negative serologic profile.(70, 71)
Serologic markers have been shown to be predictive of prognosis in research cohorts and cross sectional studies; however, their role in the management of the individual patient requires further study. Positive serologies occur in many CD patients who do not develop future complications. Furthermore, we do not yet have prospective studies which verify that early aggressive therapy changes long term outcomes in patients predicted to have a worse prognosis based on their serologic profile.(32)
FECAL BIOMARKERS
Fecal biomarkers are valuable in their specificity to the gastrointestinal tract. In the setting of mucosal inflammation inflammatory proteins, leukocyte products, and leukocytes themselves leak from a permeable mucosa.(25) The gold standard in this setting is the excretion of radiolabeled leukocytes in feces, which is cumbersome to use in everyday clinical practice.(72) The most frequently used fecal markers are calprotectin and lactoferrin.(32) They are inexpensive to perform and have demonstrated utility in diagnosing IBD, assessing disease activity, predicting disease relapse as well as response to therapy.(32) S100A12 is another fecal marker that has recently been studied and may be superior to the fecal markers currently used in IBD.(73)
Fecal calprotectin
Calprotectin is a 36 kilodalton protein that binds zinc and calcium and has antimicrobial effects.(74) It is resistant to bacterial degradation and stable in feces, for several days. Measurement in stool is by enzyme linked immunosorbent assays (ELISA).(74) Calprotectin makes up 50–60% of granulocyte cytosolic protein, and is released with cell death or activation, making it a sensitive marker of inflammation.(75) Other conditions with elevated fecal calprotectin include neoplasia, polyps, non-steroidal anti-inflammatory enteropathy, increasing age, celiac disease, microscopic colitis, allergic colitis, and infections.(21, 25, 76)
Fecal lactoferrin
Lactoferrin is an iron binding glycoprotein found in neutrophil granules, and possesses antimicrobial properties.(77) It is also measured by ELISA and is resistant to freeze-thaw cycles and degradation, facilitating its use as a laboratory test. Unlike calprotectin which can be produced by monocytes and possibly epithelial cells, lactoferrin is specific to neutrophils.(78)
Fecal S100A12
S100A12 is similar to calprotectin in its calcium-binding properties.(73) This protein activates NF-κB signal transduction and increases cytokine release.(25) S1000A12 is also detectable in serum, but the fecal assay is more sensitive and specific for IBD.(79)
Other fecal biomarkers are being investigated for use in IBD. While promise exists, thus far these alternatives have shown less consistent results, lower correlation to disease activity, and overlap between patients with active and inactive disease.(78, 80) These include lysozyme, leukocyte esterase, elastase, myeloperoxidase, TNF-alpha, IL1 B, IL4, IL 10, alpha 1 anti-trypsin, and alpha-2-macroglobulin.(25, 32, 74) M2-pyruvate kinase may be the most promising of these developing fecal biomarkers.(81)
Fecal markers in differentiating IBD from non-inflammatory diarrheal disorders
Fecal biomarkers are less invasive than endoscopy and imaging, and are attractive first step when there is a clinical suspicion of IBD. In a systematic review, fecal calprotectin had a high negative predictive value in differentiating IBD from IBS in symptomatic patients without a prior diagnosis, but a lower positive predictive value.(76) A meta-analysis evaluating fecal calprotectin (30 studies and 5,983 patients) showed a sensitivity and specificity of 89% and 81% when a cutoff of 50 μg/g was used.(82) Sensitivity and specificity for IBD changed to 98% and 91% in studies with a cutoff of 100 μg/g.(82) The ideal threshold above which IBD is more likely is unknown.(32) Another meta-analysis of 6 studies evaluated the use of fecal calprotectin in patients with a clinical suspicion of IBD and showed a sensitivity and specificity of 93% and 96% respectively. However depending on fecal calprotectin alone was shown to delay diagnosis in some adults due to false negative results.(83)
Fecal lactoferrin has a sensitivity and specificity of 80% and 82% respectively, when taking a weighted mean of 19 studies including 1001 patients, where IBD patients were compared to controls of IBS and other colonic diseases.(84) Both fecal lactoferrin and calprotectin have similar diagnostic accuracy in most studies (approximately 80%), and both were superior to CRP in a study including 42 UC (60% left sided) and 43 CD patients, with 24% of CD patients having left-sided colitis, and an additional 24% having colitis and/or ileitis.(85) S100A12 has a sensitivity and specificity of 86% and 96% in differentiating chronic IBD from IBS.(73)
Fecal markers in evaluating disease activity in patients with IBD
A summary of studies showing the correlation of fecal calprotectin with endoscopic scores of disease activity in IBD yielded correlation coefficients ranging between 0.48 and 0.83.(32) Fewer studies are available for lactoferrin, but there is still a positive correlation, with coefficients between 0.19 and 0.87.(32) Fecal biomarkers are particularly useful in patients who do not exhibit an elevated CRP in the setting of active inflammation.(25, 32)
There is a higher correlation of both fecal biomarkers with colonic rather than ileal disease, as seen in a study by Sipponen et al.(86) Fecal calprotectin is also more associated with the degree of inflammation rather than disease extent in a study of UC patients.(87) The sensitivity of fecal calprotectin and lactoferrin in identifying active disease based on endoscopy is 70–100%, with specificity between 44–100%.(32) Again, the cutoff concentration of calprotectin varied between studies, ranging from 50 μg/g to 200 μg/g.(32) Lactoferrin cutoff ranged from 7.5 to 10 μg/g.(32)
Fecal markers in assessment of response to therapy, mucosal healing and post-op recurrence
Fecal calprotectin levels decreased to <50 μg/g in UC and CD patients who achieved mucosal healing in response to medical therapy.(88) Fecal lactoferrin has similarly shown utility in monitoring patients on infliximab therapy.(89) In two small studies by Sipponen and colleagues, CD patients who responded to anti-TNF therapy had decreased lactoferrin and calprotectin levels, as opposed to patients without endoscopic improvement.(90, 91) There is currently no established threshold value of fecal markers that predicts mucosal healing.(32)
After ileocolonic resection in Crohn’s disease, fecal lactoferrin and calprotectin can identify postoperative recurrence.(92, 93) However since postoperative patients have higher baseline levels of the fecal biomarker, the tests’ sensitivity and specificity are reduced in evaluating this population.
Fecal markers in predicting disease relapse
Multiple studies have assessed the use of fecal calprotectin in the prediction of disease relapse in patients who are in clinical remission at the time of entry into the study. A study of 79 IBD patients by Costa showed that fecal calprotectin correlates better with disease relapse in UC than with CD, using a cutoff of 150 μg/g.(94) In CD and UC, there positive prediction value of 87% and 80% respectively, however, the negative predictive value was 90% in UC and only 43% in CD.(94) Tibble et al used a calprotectin cutoff of 50 μg/g, showing a sensitivity and specificity of predicting disease relapse of 90% and 83% respectively in a 12-month period.(95) There was a large range of duration of disease remission in some studies, ranging from 3 months to 36 months, and an increase in calprotectin earlier in remission may be a better predictor of disease relapse.(32, 96, 97)
INVESTIGATION OF FUTURE BIOMARKERS
The identification and testing of new IBD biomarkers is an area of active research. Methods of biomarker discovery include candidate based approaches as well as technology platform based approaches. These technology platforms can be used to both exploit and identify differences in genomics (genotyping and gene expression), proteomics and metabolomics. Newly identified biomarkers are then validated against control (or comparator) populations and/or across states of disease activity or disease phenotype.(98) Ideally new biomarkers will be easy to obtain, inexpensive to perform, reliably and quickly quantifiable across labs, and unaffected by co-morbid factors.(25) Ultimately the discovery and validation of these biomarkers depends on effective cross talk and collaboration between expert clinicians, innovative scientists and bioinformatics specialists.
Mucosal healing has been identified as a key therapeutic endpoint and ideally new blood or stool based biomarkers will be reflective of this process in order to provide the best predictive value for IBD.(13, 99, 100) A biomarker which correlates well with mucosal healing offers the greatest opportunity to predict long-term outcomes of patients with IBD. Several potential biomarkers in IBD are currently being investigated.
Metabolome Based Biomarkers
Comparative analysis of the metabolic profile in IBD patients and animal models represents an area of active investigation which has revealed several potential targets for evaluating IBD.(101) Some studies have targeted specific metabolites while others have used total metabolomic analysis. Urine, feces and colonic tissue have been evaluated from humans while urine, serum and colon tissue has been evaluated in mouse models of colitis. A specific study on human serum metabolics has not yet been published. While variability exists between studies, pathways which include amino acids and associated metabolic products linked to tissue inflammation or gut bacteria have thus far been elucidated. Further standardization of techniques and analyzed samples should improve accuracy of this technique which has potential to impact clinical practice.
We recently published findings on a metabolome based biomarker which exploits a component of the activated mucosal immune system in IBD that would be predicted to correlate with mucosal healing.(102) This approach will be detailed here for illustrative purposes and other potential future biomarkers are also discussed.
IDO1 mediated tryptophan catabolism pathway as a biomarker
Recent studies have identified a role for the enzyme indolemine 2,3 dioxygenase (IDO1) in intestinal inflammation and the inflammatory bowel diseases. IDO1 acts as the first and rate limiting step in tryptophan catabolism along the kynurenine pathway. This pathway has been identified as important in mediating immune tolerance via suppression of effector T-cell responses.(103) IDO1 expression is present in the gut at baseline, but is significantly elevated in the setting of intestinal inflammation.(102, 104) Proteomic and gene expression arrays studies found IDO1 to be among the most highly over-expressed genes in human IBD (105, 106), as well as in animal models of IBD.(107, 108) The functional importance of this enzyme in modifying colitis severity has now been evaluated using animal models. Inhibition of IDO1 worsens colitis severity while induction of IDO1 expression limits disease severity.(109–111)
Several lines of evidence support targeting of the IDO1 mediated tryptophan catabolism pathway as a potential biomarker of intestinal inflammation. Reduced serum tryptophan was reported in a small cohort of patients with Crohn’s disease, though at the time this finding was attributed to compromised absorption.(112) Another small study found elevated serum levels of both kynurenine and kynurenic acid in CD and UC patients compared to controls.(113, 114) Metabolomic profiling of the IL10−/− model of colitis identified elevation of urinary Xanthurenic acid.(115, 116) This finding led the investigators to identify the IDO1 metabolites kynurenine and 3-hydroxykynurenine (metabolic precursors of xanthurenic acid) as elevated in the plasma of these mice with colitis. Another group confirmed these findings and showed that urinary xanthurenic acid levels correlated with colitis severity.(117) Correspondingly, it has been shown that serum levels of tryptophan are reduced by nearly 80% in mice exposed to dextran sodium sulfate colitis.(118)
Using a targeted metabolic profiling approach we recently demonstrated the potential for utilizing the IDO1 pathway as a biomarker of disease activity in human Crohn’s disease.(102) IDO1 catabolizes tryptophan to kynurenine. In the setting of immune activation, the kynurenine/tryptophan (K/T) ratio is used as a surrogate marker for IDO1 activity. Using the K/T ratio rather than either alone limits potential bias related to differences in dietary intake of tryptophan.(119) This technique has been used to evaluate immune activation in several disorders including rheumatoid arthritis and lupus.(120, 121) In systematic evaluation of CD patients and a control population we found that serum tryptophan and the K/T ratio correlated with severity of CD activity as well as with the acute phase reactants ESR and CRP. The K/T ratio was useful in identifying patients with active colonic, ileo-colonic and isolated ileal disease. In a subgroup of CD patients, serial serum measurements were taken (once during active disease and once when remission was achieved). Here we found that as CD activity improved, tryptophan levels elevated while kynurenine levels and the K/T ratio lowered.
Mucosal healing was not evaluated as a component of our study. However, we did find that IDO1 was highly expressed in the colonic and small intestinal mucosa of patients with active CD. While most studies to date have focused on IDO1 expression within classical antigen presenting cells (macrophages and dendritic cells), in active IBD we identified strong IDO1 expression in the epithelium as well as likely antigen presenting cells of the lamina propria. The expression of IDO1 across several gut cell types along with the organs’ large surface area likely contributes to the profound changes in serum K/T ratios we identified in active CD compared to other autoimmune disorders.(102) High expression of IDO1 protein in active IBD has been confirmed by others.(104) Furthermore the normalization of mucosal IDO1 protein expression after treatment of active CD with infliximab has been also reported.(122) Thus, measurement of serum K/T ratios has well suited potential to serve as an objective surrogate marker of gut mucosal immune activation and biomarker for CD activity.
Gut microbe influence on metabolome
Williams and colleagues used urine based metabolic profiling with high-resolution nuclear magnetic resonance spectroscopy to compare IBD patients with controls.(123) The study focused on specific metabolites influenced by gut microbes. In CD patients verses control or UC patients, hippurate and 4-cresol sulfate levels were found to be significantly lower, while formate levels were significantly higher. However, dot plot analysis revealed notable overlap between all cohorts.
L-arginine in UC
Another amino acid, L-arginine (L-Arg), has recently been investigated in IBD. L-Arg has an important role in regulation of epithelial integrity and immune function. Hong and colleagues showed that serum levels of L-arginine are increased in patients with active UC and levels correlate with histologic disease severity.(124) It was suggested that L-Arg uptake by cells in the inflamed colon is defective which could contribute to UC pathogenesis. Metabolomic array studies have also identified elevated L-Arg in colonic mucosal specimens of UC patients and serum of an animal colitis model.(125, 126) Interestingly, L-Arg supplementation was also found to limit disease severity in an animal model of colitis.(127)
Gene expression profiling
Gene expression profiling is being examined as a predictive biomarker in human IBD. Since both CD and UC are both multi-genetic disorders with complex pathophysiology, it is more likely that a panel, rather than a single biomarker, may be better able to distinguish between disorders. A propriety DNA array assay termed IBDChip tests for ~100 gene mutations relevant to IBD and is currently being investigated for use as a predictor of clinical evolution, disease complication and response to certain pharmocotherapies.(128) Arijs and colleagues have shown that mucosal gene signatures can predict response to infliximab for both UC and colonic CD patients.(129, 130) However, these assays require endoscopy with biopsy to obtain tissue. A recent study has suggested that blood-based gene expression profiling may be able to differentiate IBD from non-inflammatory diarrhea. Burakoff et al used an Affymetrix GeneChip to examine whole blood gene expression in UC, CD and control subjects.(131) Using a supervised learning method, panels of four distinct genes were able to differentiate accurately between CD, UC and controls with diarrhea. These and other promising results will need to be further evaluated in a larger cohort of patients with varying levels of disease activity to ultimately determine its clinical utility.
Using transcription profiling of circulating T cells, a group from England reported that analogous CD8+ T cell transcriptional signatures was detectable in two unrelated autoimmune disorders.(132) Furthermore, the gene signature predicted disease prognosis in each. The expression profile identified genes involved in antigen-dependent T cell responses (both IL-7 and T-cell receptor initiated). These findings are now extended to CD and UC where the gene signatures effectively identified a patient cohort destined for frequently relapsing disease.(133) The equivalent correlation was not observed with CD4+ T cell gene expression, the T cell subset classically linked to disease pathogenesis. This gene expression signature had greater ability to predict need for escalation of therapy than standard clinical parameters (age <40, initial requirement for steroids and perianal involvement) or ASCA positivity. Confirmative studies will be needed, but this exciting development highlights the potential expression profiling in biomarker discovery.
Proteomic Array
Significant advances in proteomic array profiling technology have ignited interest in using this technique for assessment of human IBD. Current approaches include not only traditional proteomics, but also subproteomics, which analyze differences in cellular compartments, organelles and biological fluids. In the inflammatory bowel diseases, proteomic approaches have shown promise with regard to identifying active disease, differentiating between CD and UC, predicting response to anti-TNFα therapy and providing insight into disease pathogenesis.(134–137) Available reviews summarize recent studies and future directions for use of these techniques in greater detail.(138, 139)
CONCLUSIONS
Diagnosis and ongoing care of patients with CD and UC currently depends on clinical, endoscopic and histologic assessment. Radiology and standard laboratories are additive. Several established and reliable biomarkers are currently used in clinical care to aid in diagnosing IBD, differentiating between CD and UC, assessing disease activity and predicting relapse. Existing biomarkers assess stool and blood for acute phase reactants, leukocyte markers and antibody markers. Available biomarkers are limited in their ability to predict longer-range disease course. Table 1 summarizes the currently available data supporting the uses of available biomarkers in the diagnosis and management of IBD. Improvements in genomic, proteomic and metabolomic array based and mass processing technology are facilitating biomarker discovery in IBD. Rapid advances are concurrently being made in understanding of IBD etiopathogenesis. Effective bidirectional information sharing between these fields of investigation will ultimately lead to improved care of patients with IBD.
Table 1.
General summary of data supporting the roles of biomarkers in IBD
| Biomarker | IBD vs non IBD | CD vs UC | Disease activity | Mucosal healing | Prediction of clinical relapse | Prognosis | CD Phenotype |
|---|---|---|---|---|---|---|---|
| CRP/ESR | Useful | Not used | Useful | Possibly | Possibly | Possibly | Not used |
| Fecal biomarkers | Useful | Not used | Useful | Useful | Possibly | Not used | Not used |
| Serologic markers | Useful | Useful | Not used | Not used | Not used | Useful | Useful |
Acknowledgments
Acknowledgments/Funding: This work was supported in part by the National Institutes of Health Grants DK089016 and L30 RR030244 (MAC) as well as the Division of Gastroenterology’s 5T32DK007130-38 (HNI). We thank Alexandra Gutierrez MD for her input.
Abbreviations
- IBD
Inflammatory bowel diseases
- IBS
Irritable Bowel Syndrome
- CRP
C-reactive protein
- ESR
erythrocyte sedimentation rate
- ASCA
anti-Saccharomyces cerevisiae antibodies
- pANCA
perinuclear anti-neutrophil cytoplasmic antibodies
- CD
Crohn’s disease
- UC
ulcerative colitis
- CDAI
Crohn’s disease activity index
- TNFα
tumor necrosis factor – alpha
- IL
interleukin
- Anti-OmpC
antibody to outer membrane porin
- Anti-I2
Pseudomonas flourescens -associated sequence I–2
- ALCA
antilaminaribioside carbohydrate IgG
- ACCA
antichitobioside carbohydrate IgA
- AΣMA or AMCA
anti-synthetic mannoside antibodies
- GI
gastrointestinal
- ELISA
enzyme linked immunosorbent assay
- IDO1
indoleamine 2,3 dioxygenase-1
- K/T
Kynurenine/tryptophan ratio
Footnotes
Disclosures:
The authors have read the journal’s policy on disclosure of potential conflicts of interest and have no relevant conflicts of interest to disclose with regard to this manuscript. No sources of editorial support were used in the preparation of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- 1.Loftus CG, Loftus EV, Jr, Harmsen WS, et al. Update on the incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota, 1940–2000. Inflamm Bowel Dis. 2007 Mar;13(3):254–61. doi: 10.1002/ibd.20029. [DOI] [PubMed] [Google Scholar]
- 2.Longobardi T, Bernstein CN. Utilization of health-care resources by patients with IBD in Manitoba: a profile of time since diagnosis. Am J Gastroenterol. 2007 Aug;102(8):1683–91. doi: 10.1111/j.1572-0241.2007.01232.x. [DOI] [PubMed] [Google Scholar]
- 3.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009 Nov 19;361(21):2066–78. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sartor RB. Genetics and environmental interactions shape the intestinal microbiome to promote inflammatory bowel disease versus mucosal homeostasis. Gastroenterology. 2010 Dec;139(6):1816–9. doi: 10.1053/j.gastro.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 5.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007 Jul 26;448(7152):427–34. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 6.Best WR, Becktel JM, Singleton JW, Kern F., Jr Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976 Mar;70(3):439–44. [PubMed] [Google Scholar]
- 7.Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980 Mar 8;1(8167):514. doi: 10.1016/s0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- 8.D’Haens G, Sandborn WJ, Feagan BG, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007 Feb;132(2):763–86. doi: 10.1053/j.gastro.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 9.Irvine EJ, Zhou Q, Thompson AK. The Short Inflammatory Bowel Disease Questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn’s Relapse Prevention Trial. Am J Gastroenterol. 1996 Aug;91(8):1571–8. [PubMed] [Google Scholar]
- 10.Sands BE. The placebo response rate in irritable bowel syndrome and inflammatory bowel disease. Dig Dis. 2009;27( Suppl 1):68–75. doi: 10.1159/000268123. [DOI] [PubMed] [Google Scholar]
- 11.Feagan BG, Fedorak RN, Irvine EJ, et al. A comparison of methotrexate with placebo for the maintenance of remission in Crohn’s disease. North American Crohn’s Study Group Investigators. N Engl J Med. 2000 Jun 1;342(22):1627–32. doi: 10.1056/NEJM200006013422202. [DOI] [PubMed] [Google Scholar]
- 12.Sandborn WJ, Schreiber S, Feagan BG, et al. Certolizumab pegol for active Crohn’s disease: a placebo-controlled, randomized trial. Clin Gastroenterol Hepatol. 2011 Aug;9(8):670–8. e3. doi: 10.1016/j.cgh.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 13.Stidham RW, Higgins PD. Value of mucosal assessment and biomarkers in inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2010 Jun;4(3):285–91. doi: 10.1586/egh.10.22. [DOI] [PubMed] [Google Scholar]
- 14.Casellas F, Rodrigo L, Nino P, Pantiga C, Riestra S, Malagelada JR. Sustained improvement of health-related quality of life in Crohn’s disease patients treated with infliximab and azathioprine for 4 years. Inflamm Bowel Dis. 2007 Nov;13(11):1395–400. doi: 10.1002/ibd.20205. [DOI] [PubMed] [Google Scholar]
- 15.Cosnes J, Cattan S, Blain A, et al. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis. 2002 Jul;8(4):244–50. doi: 10.1097/00054725-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Darlington GJ, Wilson DR, Lachman LB. Monocyte-conditioned medium, interleukin-1, and tumor necrosis factor stimulate the acute phase response in human hepatoma cells in vitro. J Cell Biol. 1986 Sep;103(3):787–93. doi: 10.1083/jcb.103.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. Journal of Clinical Investigation [Article] 2003 Jun;111(12):1805–12. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vigushin DM, Pepys MB, Hawkins PN. Metabolic and scintigraphic studies of radioiodinated human C-reactive protein in health and disease. J Clin Invest. 1993 Apr;91(4):1351–7. doi: 10.1172/JCI116336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saverymuttu SH, Hodgson HJ, Chadwick VS, Pepys MB. Differing acute phase responses in Crohn’s disease and ulcerative colitis. Gut. 1986 Jul;27(7):809–13. doi: 10.1136/gut.27.7.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gross V, Andus T, Caesar I, Roth M, Scholmerich J. Evidence for continuous stimulation of interleukin-6 production in Crohn’s disease. Gastroenterology. 1992 Feb;102(2):514–9. doi: 10.1016/0016-5085(92)90098-j. [DOI] [PubMed] [Google Scholar]
- 21.Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006 Mar;55(3):426–31. doi: 10.1136/gut.2005.069476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Florin TH, Paterson EW, Fowler EV, Radford-Smith GL. Clinically active Crohn’s disease in the presence of a low C-reactive protein. Scand J Gastroenterol. 2006 Mar;41(3):306–11. doi: 10.1080/00365520500217118. [DOI] [PubMed] [Google Scholar]
- 23.Henriksen M, Jahnsen J, Lygren I, et al. C-reactive protein: a predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Gut. 2008 Nov;57(11):1518–23. doi: 10.1136/gut.2007.146357. [DOI] [PubMed] [Google Scholar]
- 24.Carlson CS, Aldred SF, Lee PK, et al. Polymorphisms within the C-reactive protein (CRP) promoter region are associated with plasma CRP levels. Am J Hum Genet. 2005 Jul;77(1):64–77. doi: 10.1086/431366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendoza JL, Abreu MT. Biological markers in inflammatory bowel disease: practical consideration for clinicians. Gastroenterol Clin Biol. 2009 Jun;33( Suppl 3):S158–73. doi: 10.1016/S0399-8320(09)73151-3. [DOI] [PubMed] [Google Scholar]
- 26.Ripoche J. Blood platelets and inflammation: their relationship with liver and digestive diseases. Clin Res Hepatol Gastroenterol. 2011 May;35(5):353–7. doi: 10.1016/j.clinre.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 27.Danese S, Motte Cd Cde L, Fiocchi C. Platelets in inflammatory bowel disease: clinical, pathogenic, and therapeutic implications. Am J Gastroenterol. 2004 May;99(5):938–45. doi: 10.1111/j.1572-0241.2004.04129.x. [DOI] [PubMed] [Google Scholar]
- 28.Rutella S, Vetrano S, Correale C, et al. Enhanced platelet adhesion induces angiogenesis in intestinal inflammation and inflammatory bowel disease microvasculature. J Cell Mol Med. 2011 Mar;15(3):625–34. doi: 10.1111/j.1582-4934.2010.01033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harries AD, Beeching NJ, Rogerson SJ, Nye FJ. The platelet count as a simple measure to distinguish inflammatory bowel disease from infective diarrhoea. J Infect. 1991 May;22(3):247–50. doi: 10.1016/s0163-4453(05)80006-4. [DOI] [PubMed] [Google Scholar]
- 30.Andre C, Descos L, Landais P, Fermanian J. Assessment of appropriate laboratory measurements to supplement the Crohn’s disease activity index. Gut. 1981 Jul;22(7):571–4. doi: 10.1136/gut.22.7.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poullis AP, Zar S, Sundaram KK, et al. A new, highly sensitive assay for C-reactive protein can aid the differentiation of inflammatory bowel disorders from constipation- and diarrhoea-predominant functional bowel disorders. Eur J Gastroenterol Hepatol. 2002 Apr;14(4):409–12. doi: 10.1097/00042737-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Lewis JD. The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterology. 2011 May;140(6):1817–26. e2. doi: 10.1053/j.gastro.2010.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solem CA, Loftus EV, Jr, Tremaine WJ, Harmsen WS, Zinsmeister AR, Sandborn WJ. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis. 2005 Aug;11(8):707–12. doi: 10.1097/01.mib.0000173271.18319.53. [DOI] [PubMed] [Google Scholar]
- 34.Colombel JF, Solem CA, Sandborn WJ, et al. Quantitative measurement and visual assessment of ileal Crohn’s disease activity by computed tomography enterography: correlation with endoscopic severity and C reactive protein. Gut. 2006 Nov;55(11):1561–7. doi: 10.1136/gut.2005.084301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oussalah A, Chevaux JB, Fay R, Sandborn WJ, Bigard MA, Peyrin-Biroulet L. Predictors of infliximab failure after azathioprine withdrawal in Crohn’s disease treated with combination therapy. Am J Gastroenterol. 2010 May;105(5):1142–9. doi: 10.1038/ajg.2010.158. [DOI] [PubMed] [Google Scholar]
- 36.Papi C, Festa V, Leandro G, et al. Long-term outcome of Crohn’s disease following corticosteroid-induced remission. Am J Gastroenterol. 2007 Apr;102(4):814–9. doi: 10.1111/j.1572-0241.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- 37.Bitton A, Dobkin PL, Edwardes MD, et al. Predicting relapse in Crohn’s disease: a biopsychosocial model. Gut. 2008 Oct;57(10):1386–92. doi: 10.1136/gut.2007.134817. [DOI] [PubMed] [Google Scholar]
- 38.Boirivant M, Leoni M, Tariciotti D, Fais S, Squarcia O, Pallone F. The clinical significance of serum C reactive protein levels in Crohn’s disease. Results of a prospective longitudinal study. J Clin Gastroenterol. 1988 Aug;10(4):401–5. doi: 10.1097/00004836-198808000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Jurgens M, Mahachie John JM, Cleynen I, et al. Levels of C-reactive protein are associated with response to infliximab therapy in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2011 May;9(5):421–7. e1. doi: 10.1016/j.cgh.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 40.Brignola C, Campieri M, Bazzocchi G, Farruggia P, Tragnone A, Lanfranchi GA. A laboratory index for predicting relapse in asymptomatic patients with Crohn’s disease. Gastroenterology. 1986 Dec;91(6):1490–4. doi: 10.1016/0016-5085(86)90206-4. [DOI] [PubMed] [Google Scholar]
- 41.Peyrin-Biroulet L, Standaert-Vitse A, Branche J, Chamaillard M. IBD serological panels: facts and perspectives. Inflamm Bowel Dis. 2007 Dec;13(12):1561–6. doi: 10.1002/ibd.20226. [DOI] [PubMed] [Google Scholar]
- 42.McKenzie H, Main J, Pennington CR, Parratt D. Antibody to selected strains of Saccharomyces cerevisiae (baker’s and brewer’s yeast) and Candida albicans in Crohn’s disease. Gut. 1990 May;31(5):536–8. doi: 10.1136/gut.31.5.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reumaux D, Sendid B, Poulain D, Duthilleul P, Dewit O, Colombel JF. Serological markers in inflammatory bowel diseases. Best Pract Res Clin Gastroenterol. 2003 Feb;17(1):19–35. doi: 10.1053/bega.2002.0347. [DOI] [PubMed] [Google Scholar]
- 44.Vernier G, Sendid B, Poulain D, Colombel JF. Relevance of serologic studies in inflammatory bowel disease. Curr Gastroenterol Rep. 2004 Dec;6(6):482–7. doi: 10.1007/s11894-004-0070-x. [DOI] [PubMed] [Google Scholar]
- 45.Barnich N, Darfeuille-Michaud A. Adherent-invasive Escherichia coli and Crohn’s disease. Curr Opin Gastroenterol. 2007 Jan;23(1):16–20. doi: 10.1097/MOG.0b013e3280105a38. [DOI] [PubMed] [Google Scholar]
- 46.Barnich N, Carvalho FA, Glasser AL, et al. CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn disease. J Clin Invest. 2007 Jun;117(6):1566–74. doi: 10.1172/JCI30504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lodes MJ, Cong Y, Elson CO, et al. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004 May;113(9):1296–306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sutton CL, Kim J, Yamane A, et al. Identification of a novel bacterial sequence associated with Crohn’s disease. Gastroenterology. 2000 Jul;119(1):23–31. doi: 10.1053/gast.2000.8519. [DOI] [PubMed] [Google Scholar]
- 49.Wei B, Huang T, Dalwadi H, Sutton CL, Bruckner D, Braun J. Pseudomonas fluorescens encodes the Crohn’s disease-associated I2 sequence and T-cell superantigen. Infect Immun. 2002 Dec;70(12):6567–75. doi: 10.1128/IAI.70.12.6567-6575.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Landers CJ, Cohavy O, Misra R, et al. Selected loss of tolerance evidenced by Crohn’s disease-associated immune responses to auto- and microbial antigens. Gastroenterology. 2002 Sep;123(3):689–99. doi: 10.1053/gast.2002.35379. [DOI] [PubMed] [Google Scholar]
- 51.Papp M, Norman GL, Altorjay I, Lakatos PL. Utility of serological markers in inflammatory bowel diseases: gadget or magic? World J Gastroenterol. 2007 Apr 14;13(14):2028–36. doi: 10.3748/wjg.v13.i14.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schoepfer AM, Schaffer T, Mueller S, et al. Phenotypic associations of Crohn’s disease with antibodies to flagellins A4-Fla2 and Fla-X, ASCA, p-ANCA, PAB, and NOD2 mutations in a Swiss Cohort. Inflamm Bowel Dis. 2009 Sep;15(9):1358–67. doi: 10.1002/ibd.20892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schoepfer AM, Schaffer T, Seibold-Schmid B, Muller S, Seibold F. Antibodies to flagellin indicate reactivity to bacterial antigens in IBS patients. Neurogastroenterol Motil. 2008 Oct;20(10):1110–8. doi: 10.1111/j.1365-2982.2008.01166.x. [DOI] [PubMed] [Google Scholar]
- 54.Dotan I, Fishman S, Dgani Y, et al. Antibodies against laminaribioside and chitobioside are novel serologic markers in Crohn’s disease. Gastroenterology. 2006 Aug;131(2):366–78. doi: 10.1053/j.gastro.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 55.Simondi D, Mengozzi G, Betteto S, et al. Antiglycan antibodies as serological markers in the differential diagnosis of inflammatory bowel disease. Inflamm Bowel Dis. 2008 May;14(5):645–51. doi: 10.1002/ibd.20368. [DOI] [PubMed] [Google Scholar]
- 56.Vandewalle-El Khoury P, Colombel JF, Joossens S, et al. Detection of antisynthetic mannoside antibodies (ASigmaMA) reveals heterogeneity in the ASCA response of Crohn’s disease patients and contributes to differential diagnosis, stratification, and prediction. Am J Gastroenterol. 2008 Apr;103(4):949–57. doi: 10.1111/j.1572-0241.2007.01648.x. [DOI] [PubMed] [Google Scholar]
- 57.Rieder F, Schleder S, Wolf A, et al. Association of the novel serologic anti-glycan antibodies anti-laminarin and anti-chitin with complicated Crohn’s disease behavior. Inflamm Bowel Dis. 2010 Feb;16(2):263–74. doi: 10.1002/ibd.21046. [DOI] [PubMed] [Google Scholar]
- 58.Lakatos PL, Papp M, Rieder F. Serologic antiglycan antibodies in inflammatory bowel disease. Am J Gastroenterol. 2011 Mar;106(3):406–12. doi: 10.1038/ajg.2010.505. [DOI] [PubMed] [Google Scholar]
- 59.Reese GE, Constantinides VA, Simillis C, et al. Diagnostic precision of anti-Saccharomyces cerevisiae antibodies and perinuclear antineutrophil cytoplasmic antibodies in inflammatory bowel disease. Am J Gastroenterol. 2006 Oct;101(10):2410–22. doi: 10.1111/j.1572-0241.2006.00840.x. [DOI] [PubMed] [Google Scholar]
- 60.Ruemmele FM, Targan SR, Levy G, Dubinsky M, Braun J, Seidman EG. Diagnostic accuracy of serological assays in pediatric inflammatory bowel disease. Gastroenterology. 1998 Oct;115(4):822–9. doi: 10.1016/s0016-5085(98)70252-5. [DOI] [PubMed] [Google Scholar]
- 61.Benor S, Russell GH, Silver M, Israel EJ, Yuan Q, Winter HS. Shortcomings of the inflammatory bowel disease Serology 7 panel. Pediatrics. 2010 Jun;125(6):1230–6. doi: 10.1542/peds.2009-1936. [DOI] [PubMed] [Google Scholar]
- 62.Abreu MT. Serologies in Crohn’s disease: can we change the gray zone to black and white? Gastroenterology. 2006 Aug;131(2):664–7. doi: 10.1053/j.gastro.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 63.sgiDiagnostic. Available from: www.prometheuslabs.com.
- 64.Ferrante M, Henckaerts L, Joossens M, et al. New serological markers in inflammatory bowel disease are associated with complicated disease behaviour. Gut. 2007 Oct;56(10):1394–403. doi: 10.1136/gut.2006.108043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Joossens S, Reinisch W, Vermeire S, et al. The value of serologic markers in indeterminate colitis: a prospective follow-up study. Gastroenterology. 2002 May;122(5):1242–7. doi: 10.1053/gast.2002.32980. [DOI] [PubMed] [Google Scholar]
- 66.Joossens S, Colombel JF, Landers C, et al. Anti-outer membrane of porin C and anti-I2 antibodies in indeterminate colitis. Gut. 2006 Nov;55(11):1667–9. doi: 10.1136/gut.2005.089623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thia KT, Sandborn WJ, Harmsen WS, Zinsmeister AR, Loftus EV., Jr Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology. 2010 Oct;139(4):1147–55. doi: 10.1053/j.gastro.2010.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mow WS, Vasiliauskas EA, Lin YC, et al. Association of antibody responses to microbial antigens and complications of small bowel Crohn’s disease. Gastroenterology. 2004 Feb;126(2):414–24. doi: 10.1053/j.gastro.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 69.Targan SR, Landers CJ, Yang H, et al. Antibodies to CBir1 flagellin define a unique response that is associated independently with complicated Crohn’s disease. Gastroenterology. 2005 Jun;128(7):2020–8. doi: 10.1053/j.gastro.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 70.Dubinsky MC, Lin YC, Dutridge D, et al. Serum immune responses predict rapid disease progression among children with Crohn’s disease: immune responses predict disease progression. Am J Gastroenterol. 2006 Feb;101(2):360–7. doi: 10.1111/j.1572-0241.2006.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dubinsky MC, Kugathasan S, Mei L, et al. Increased immune reactivity predicts aggressive complicating Crohn’s disease in children. Clin Gastroenterol Hepatol. 2008 Oct;6(10):1105–11. doi: 10.1016/j.cgh.2008.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saverymuttu SH, Peters AM, Crofton ME, et al. 111Indium autologous granulocytes in the detection of inflammatory bowel disease. Gut. 1985 Sep;26(9):955–60. doi: 10.1136/gut.26.9.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaiser T, Langhorst J, Wittkowski H, et al. Faecal S100A12 as a non-invasive marker distinguishing inflammatory bowel disease from irritable bowel syndrome. Gut. 2007 Dec;56(12):1706–13. doi: 10.1136/gut.2006.113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Desai D, Faubion WA, Sandborn WJ. Review article: biological activity markers in inflammatory bowel disease. Aliment Pharmacol Ther. 2007 Feb 1;25(3):247–55. doi: 10.1111/j.1365-2036.2006.03184.x. [DOI] [PubMed] [Google Scholar]
- 75.Roseth AG, Fagerhol MK, Aadland E, Schjonsby H. Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scand J Gastroenterol. 1992 Sep;27(9):793–8. doi: 10.3109/00365529209011186. [DOI] [PubMed] [Google Scholar]
- 76.Konikoff MR, Denson LA. Role of fecal calprotectin as a biomarker of intestinal inflammation in inflammatory bowel disease. Inflamm Bowel Dis. 2006 Jun;12(6):524–34. doi: 10.1097/00054725-200606000-00013. [DOI] [PubMed] [Google Scholar]
- 77.Baveye S, Elass E, Mazurier J, Spik G, Legrand D. Lactoferrin: a multifunctional glycoprotein involved in the modulation of the inflammatory process. Clin Chem Lab Med. 1999 Mar;37(3):281–6. doi: 10.1515/CCLM.1999.049. [DOI] [PubMed] [Google Scholar]
- 78.Kane SV, Sandborn WJ, Rufo PA, et al. Fecal lactoferrin is a sensitive and specific marker in identifying intestinal inflammation. Am J Gastroenterol. 2003 Jun;98(6):1309–14. doi: 10.1111/j.1572-0241.2003.07458.x. [DOI] [PubMed] [Google Scholar]
- 79.Manolakis AC, Kapsoritakis AN, Georgoulias P, et al. Moderate performance of serum S100A12, in distinguishing inflammatory bowel disease from irritable bowel syndrome. BMC Gastroenterol. 2010;10:118. doi: 10.1186/1471-230X-10-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van der Sluys Veer A, Biemond I, Verspaget HW, Lamers CB. Faecal parameters in the assessment of activity in inflammatory bowel disease. Scand J Gastroenterol Suppl. 1999;230:106–10. doi: 10.1080/003655299750025624. [DOI] [PubMed] [Google Scholar]
- 81.Judd TA, Day AS, Lemberg DA, Turner D, Leach ST. Update of fecal markers of inflammation in inflammatory bowel disease. J Gastroenterol Hepatol. 2011 Oct;26(10):1493–9. doi: 10.1111/j.1440-1746.2011.06846.x. [DOI] [PubMed] [Google Scholar]
- 82.von Roon AC, Karamountzos L, Purkayastha S, et al. Diagnostic precision of fecal calprotectin for inflammatory bowel disease and colorectal malignancy. Am J Gastroenterol. 2007 Apr;102(4):803–13. doi: 10.1111/j.1572-0241.2007.01126.x. [DOI] [PubMed] [Google Scholar]
- 83.van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ. 2010;341:c3369. doi: 10.1136/bmj.c3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gisbert JP, McNicholl AG, Gomollon F. Questions and answers on the role of fecal lactoferrin as a biological marker in inflammatory bowel disease. Inflamm Bowel Dis. 2009 Nov;15(11):1746–54. doi: 10.1002/ibd.20920. [DOI] [PubMed] [Google Scholar]
- 85.Langhorst J, Elsenbruch S, Koelzer J, Rueffer A, Michalsen A, Dobos GJ. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol. 2008 Jan;103(1):162–9. doi: 10.1111/j.1572-0241.2007.01556.x. [DOI] [PubMed] [Google Scholar]
- 86.Sipponen T, Karkkainen P, Savilahti E, et al. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn’s disease and histological findings. Aliment Pharmacol Ther. 2008 Nov 15;28(10):1221–9. doi: 10.1111/j.1365-2036.2008.03835.x. [DOI] [PubMed] [Google Scholar]
- 87.Roseth AG, Aadland E, Jahnsen J, Raknerud N. Assessment of disease activity in ulcerative colitis by faecal calprotectin, a novel granulocyte marker protein. Digestion. 1997;58(2):176–80. doi: 10.1159/000201441. [DOI] [PubMed] [Google Scholar]
- 88.Roseth AG, Aadland E, Grzyb K. Normalization of faecal calprotectin: a predictor of mucosal healing in patients with inflammatory bowel disease. Scand J Gastroenterol. 2004 Oct;39(10):1017–20. doi: 10.1080/00365520410007971. [DOI] [PubMed] [Google Scholar]
- 89.Buderus S, Boone J, Lyerly D, Lentze MJ. Fecal lactoferrin: a new parameter to monitor infliximab therapy. Dig Dis Sci. 2004 Jun;49(6):1036–9. doi: 10.1023/b:ddas.0000034568.69407.47. [DOI] [PubMed] [Google Scholar]
- 90.Sipponen T, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Farkkila M. Crohn’s disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn’s disease activity index and endoscopic findings. Inflamm Bowel Dis. 2008 Jan;14(1):40–6. doi: 10.1002/ibd.20312. [DOI] [PubMed] [Google Scholar]
- 91.Sipponen T, Bjorkesten CG, Farkkila M, Nuutinen H, Savilahti E, Kolho KL. Faecal calprotectin and lactoferrin are reliable surrogate markers of endoscopic response during Crohn’s disease treatment. Scand J Gastroenterol. 2010 Mar;45(3):325–31. doi: 10.3109/00365520903483650. [DOI] [PubMed] [Google Scholar]
- 92.Scarpa M, D’Inca R, Basso D, et al. Fecal lactoferrin and calprotectin after ileocolonic resection for Crohn’s disease. Dis Colon Rectum. 2007 Jun;50(6):861–9. doi: 10.1007/s10350-007-0225-6. [DOI] [PubMed] [Google Scholar]
- 93.Lamb CA, Mohiuddin MK, Gicquel J, et al. Faecal calprotectin or lactoferrin can identify postoperative recurrence in Crohn’s disease. Br J Surg. 2009 Jun;96(6):663–74. doi: 10.1002/bjs.6593. [DOI] [PubMed] [Google Scholar]
- 94.Costa F, Mumolo MG, Ceccarelli L, et al. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut. 2005 Mar;54(3):364–8. doi: 10.1136/gut.2004.043406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tibble JA, Sigthorsson G, Bridger S, Fagerhol MK, Bjarnason I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. 2000 Jul;119(1):15–22. doi: 10.1053/gast.2000.8523. [DOI] [PubMed] [Google Scholar]
- 96.D’Inca R, Dal Pont E, Di Leo V, et al. Can calprotectin predict relapse risk in inflammatory bowel disease? Am J Gastroenterol. 2008 Aug;103(8):2007–14. doi: 10.1111/j.1572-0241.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- 97.Sipponen T, Kolho KL. Faecal calprotectin in children with clinically quiescent inflammatory bowel disease. Scand J Gastroenterol. 2010 Aug;45(7–8):872–7. doi: 10.3109/00365521003782389. [DOI] [PubMed] [Google Scholar]
- 98.Pepe MS, Etzioni R, Feng Z, et al. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001 Jul 18;93(14):1054–61. doi: 10.1093/jnci/93.14.1054. [DOI] [PubMed] [Google Scholar]
- 99.Pineton de Chambrun G, Peyrin-Biroulet L, Lemann M, Colombel JF. Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol. 2010 Jan;7(1):15–29. doi: 10.1038/nrgastro.2009.203. [DOI] [PubMed] [Google Scholar]
- 100.Froslie KF, Jahnsen J, Moum BA, Vatn MH. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007 Aug;133(2):412–22. doi: 10.1053/j.gastro.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 101.Lin HM, Helsby NA, Rowan DD, Ferguson LR. Using metabolomic analysis to understand inflammatory bowel diseases. Inflamm Bowel Dis. 2011 Apr;17(4):1021–9. doi: 10.1002/ibd.21426. [DOI] [PubMed] [Google Scholar]
- 102.Gupta NK, Thaker AI, Kanuri N, et al. Serum analysis of tryptophan catabolism pathway: Correlation with Crohn’s disease activity. Inflamm Bowel Dis. 2011 Aug 5; doi: 10.1002/ibd.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004 Oct;4(10):762–74. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 104.Ferdinande L, Demetter P, Perez-Novo C, et al. Inflamed intestinal mucosa features a specific epithelial expression pattern of indoleamine 2,3-dioxygenase. Int J Immunopathol Pharmacol. 2008 Apr-Jun;21(2):289–95. doi: 10.1177/039463200802100205. [DOI] [PubMed] [Google Scholar]
- 105.Dieckgraefe BK, Stenson WF, Korzenik JR, Swanson PE, Harrington CA. Analysis of mucosal gene expression in inflammatory bowel disease by parallel oligonucleotide arrays. Physiol Genomics. 2000 Nov 9;4(1):1–11. doi: 10.1152/physiolgenomics.2000.4.1.1. [DOI] [PubMed] [Google Scholar]
- 106.Barcelo-Batllori S, Andre M, Servis C, et al. Proteomic analysis of cytokine induced proteins in human intestinal epithelial cells: implications for inflammatory bowel diseases. Proteomics. 2002 May;2(5):551–60. doi: 10.1002/1615-9861(200205)2:5<551::AID-PROT551>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 107.Hansen JJ, Holt L, Sartor RB. Gene expression patterns in experimental colitis in IL-10-deficient mice. Inflamm Bowel Dis. 2009 Jan 9; doi: 10.1002/ibd.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brown SL, Riehl TE, Walker MR, et al. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. J Clin Invest. 2007 Jan;117(1):258–69. doi: 10.1172/JCI29159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ciorba MA, Bettonville EE, McDonald KG, et al. Induction of IDO-1 by immunostimulatory DNA limits severity of experimental colitis. J Immunol. 2010 Apr 1;184(7):3907–16. doi: 10.4049/jimmunol.0900291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gurtner GJ, Newberry RD, Schloemann SR, McDonald KG, Stenson WF. Inhibition of indoleamine 2,3-dioxygenase augments trinitrobenzene sulfonic acid colitis in mice. Gastroenterology. 2003 Dec;125(6):1762–73. doi: 10.1053/j.gastro.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 111.Matteoli G, Mazzini E, Iliev ID, et al. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut. 2010 May;59(5):595–604. doi: 10.1136/gut.2009.185108. [DOI] [PubMed] [Google Scholar]
- 112.Beeken WL. Serum tryptophan in Crohn’s disease. Scand J Gastroenterol. 1976;11(7):735–40. [PubMed] [Google Scholar]
- 113.Forrest CM, Gould SR, Darlington LG, Stone TW. Levels of purine, kynurenine and lipid peroxidation products in patients with inflammatory bowel disease. Adv Exp Med Biol. 2003;527:395–400. doi: 10.1007/978-1-4615-0135-0_46. [DOI] [PubMed] [Google Scholar]
- 114.Forrest CM, Youd P, Kennedy A, Gould SR, Darlington LG, Stone TW. Purine, kynurenine, neopterin and lipid peroxidation levels in inflammatory bowel disease. J Biomed Sci. 2002 Sep-Oct;9(5):436–42. doi: 10.1007/BF02256538. [DOI] [PubMed] [Google Scholar]
- 115.Lin HM, Barnett MP, Roy NC, et al. Metabolomic analysis identifies inflammatory and noninflammatory metabolic effects of genetic modification in a mouse model of Crohn’s disease. J Proteome Res. 2010 Apr 5;9(4):1965–75. doi: 10.1021/pr901130s. [DOI] [PubMed] [Google Scholar]
- 116.Lin HM, Edmunds SI, Helsby NA, Ferguson LR, Rowan DD. Nontargeted urinary metabolite profiling of a mouse model of Crohn’s disease. J Proteome Res. 2009 Apr;8(4):2045–57. doi: 10.1021/pr800999t. [DOI] [PubMed] [Google Scholar]
- 117.Otter D, Cao M, Lin HM, et al. Identification of urinary biomarkers of colon inflammation in IL10-/- mice using Short-Column LCMS metabolomics. J Biomed Biotechnol. 2011;2011:974701. doi: 10.1155/2011/974701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shiomi Y, Nishiumi S, Ooi M, et al. GCMS-based metabolomic study in mice with colitis induced by dextran sulfate sodium. Inflamm Bowel Dis. 2011 Nov;17(11):2261–74. doi: 10.1002/ibd.21616. [DOI] [PubMed] [Google Scholar]
- 119.Schrocksnadel K, Wirleitner B, Winkler C, Fuchs D. Monitoring tryptophan metabolism in chronic immune activation. Clin Chim Acta. 2006 Feb;364(1–2):82–90. doi: 10.1016/j.cca.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 120.Schroecksnadel K, Winkler C, Duftner C, Wirleitner B, Schirmer M, Fuchs D. Tryptophan degradation increases with stage in patients with rheumatoid arthritis. Clin Rheumatol. 2006 May;25(3):334–7. doi: 10.1007/s10067-005-0056-6. [DOI] [PubMed] [Google Scholar]
- 121.Widner B, Sepp N, Kowald E, et al. Enhanced tryptophan degradation in systemic lupus erythematosus. Immunobiology. 2000 Apr;201(5):621–30. doi: 10.1016/S0171-2985(00)80079-0. [DOI] [PubMed] [Google Scholar]
- 122.Wolf AM, Wolf D, Rumpold H, et al. Overexpression of indoleamine 2,3-dioxygenase in human inflammatory bowel disease. Clin Immunol. 2004 Oct;113(1):47–55. doi: 10.1016/j.clim.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 123.Williams HR, Cox IJ, Walker DG, et al. Characterization of inflammatory bowel disease with urinary metabolic profiling. Am J Gastroenterol. 2009 Jun;104(6):1435–44. doi: 10.1038/ajg.2009.175. [DOI] [PubMed] [Google Scholar]
- 124.Hong SK, Maltz BE, Coburn LA, et al. Increased serum levels of L-arginine in ulcerative colitis and correlation with disease severity. Inflamm Bowel Dis. 2010 Jan;16(1):105–11. doi: 10.1002/ibd.21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Balasubramanian K, Kumar S, Singh RR, et al. Metabolism of the colonic mucosa in patients with inflammatory bowel diseases: an in vitro proton magnetic resonance spectroscopy study. Magn Reson Imaging. 2009 Jan;27(1):79–86. doi: 10.1016/j.mri.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 126.Martin FP, Rezzi S, Philippe D, et al. Metabolic assessment of gradual development of moderate experimental colitis in IL-10 deficient mice. J Proteome Res. 2009 May;8(5):2376–87. doi: 10.1021/pr801006e. [DOI] [PubMed] [Google Scholar]
- 127.Gobert AP, Cheng Y, Akhtar M, et al. Protective role of arginase in a mouse model of colitis. J Immunol. 2004 Aug 1;173(3):2109–17. doi: 10.4049/jimmunol.173.3.2109. [DOI] [PubMed] [Google Scholar]
- 128.IBDChip. Available from: http://www.progenika.com.
- 129.Arijs I, Li K, Toedter G, et al. Mucosal gene signatures to predict response to infliximab in patients with ulcerative colitis. Gut. 2009 Dec;58(12):1612–9. doi: 10.1136/gut.2009.178665. [DOI] [PubMed] [Google Scholar]
- 130.Arijs I, Quintens R, Van Lommel L, et al. Predictive value of epithelial gene expression profiles for response to infliximab in Crohn’s disease. Inflamm Bowel Dis. 2010 Dec;16(12):2090–8. doi: 10.1002/ibd.21301. [DOI] [PubMed] [Google Scholar]
- 131.Burakoff R, Chao S, Perencevich M, et al. Blood-based biomarkers can differentiate ulcerative colitis from crohn’s disease and noninflammatory diarrhea. Inflamm Bowel Dis. 2011 Aug;17(8):1719–25. doi: 10.1002/ibd.21574. [DOI] [PubMed] [Google Scholar]
- 132.McKinney EF, Lyons PA, Carr EJ, et al. A CD8+ T cell transcription signature predicts prognosis in autoimmune disease. Nat Med. 2010 May;16(5):586–91. doi: 10.1038/nm.2130. 1p following 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee JC, Lyons PA, McKinney EF, et al. Gene expression profiling of CD8+ T cells predicts prognosis in patients with Crohn disease and ulcerative colitis. J Clin Invest. 2011 Oct 3;121(10):4170–9. doi: 10.1172/JCI59255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nanni P, Parisi D, Roda G, et al. Serum protein profiling in patients with inflammatory bowel diseases using selective solid-phase bulk extraction, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and chemometric data analysis. Rapid Commun Mass Spectrom. 2007;21(24):4142–8. doi: 10.1002/rcm.3323. [DOI] [PubMed] [Google Scholar]
- 135.M’Koma AE, Seeley EH, Washington MK, et al. Proteomic profiling of mucosal and submucosal colonic tissues yields protein signatures that differentiate the inflammatory colitides. Inflamm Bowel Dis. 2011 Apr;17(4):875–83. doi: 10.1002/ibd.21442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Meuwis MA, Fillet M, Lutteri L, et al. Proteomics for prediction and characterization of response to infliximab in Crohn’s disease: a pilot study. Clin Biochem. 2008 Aug;41(12):960–7. doi: 10.1016/j.clinbiochem.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 137.Meuwis MA, Fillet M, Geurts P, et al. Biomarker discovery for inflammatory bowel disease, using proteomic serum profiling. Biochem Pharmacol. 2007 May 1;73(9):1422–33. doi: 10.1016/j.bcp.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 138.Roda G, Caponi A, Benevento M, et al. New proteomic approaches for biomarker discovery in inflammatory bowel disease. Inflamm Bowel Dis. 2010 Jul;16(7):1239–46. doi: 10.1002/ibd.21212. [DOI] [PubMed] [Google Scholar]
- 139.Alex P, Gucek M, Li X. Applications of proteomics in the study of inflammatory bowel diseases: Current status and future directions with available technologies. Inflamm Bowel Dis. 2009 Apr;15(4):616–29. doi: 10.1002/ibd.20652. [DOI] [PMC free article] [PubMed] [Google Scholar]