Abstract
Type 2 diabetes is often associated with obesity, dyslipidemia, and cardiovascular anomalies and is a major health problem approaching global epidemic proportions. Insulin resistance, a prediabetic condition, precedes the onset of frank type 2 diabetes and offers potential avenues for early intervention to treat the disease. Although lifestyle modifications and exercise can reduce the incidence of diabetes, compliance has proved to be difficult, warranting pharmacological interventions. However, most of the currently available drugs that improve insulin sensitivity have adverse effects. Therefore, attractive strategies to alleviate insulin resistance include dietary supplements. One such supplement is chromium, which has been shown reduce insulin resistance in some, but not all, studies. Furthermore, the molecular mechanisms of chromium in alleviating insulin resistance remain elusive. This review examines emerging reports on the effect of chromium, as well as molecular and cellular mechanisms by which chromium may provide beneficial effects in alleviating insulin resistance.
Keywords: Chromium, insulin resistance, type 2 diabetes
1. Introduction
Type 2 diabetes mellitus is an emerging worldwide health problem, with the number of global cases of type 2 diabetes projected to double to 350 million by the year 2030 [1]. Diabetes is an independent risk-factor for cardiovascular disease [2, 3] and the leading cause of morbidity and mortality in the developed world [4–6]. A major cause of the increased prevalence of type-2 diabetes is the growing epidemic of obesity [7]. In the USA alone, about two-thirds of the population is either overweight or obese [8] and the number of children and adolescents with obesity and type-2 diabetes is on the rise [9]. Chronic over-nutrition, sedentary lifestyles, and the lack of physical activity have contributed to the increasing prevalence of diabetes [10].
Insulin resistance, defined as an impaired responsiveness of the body to insulin, is a pre-diabetic stage in the transition from obesity to full-blown type 2 diabetes [11]. Studies have shown that insulin resistance is observed long before the development of diabetes. In 2010, the prevalence of insulin resistance among adults 20 years or older was 35% and was 50% for those aged 65 or older; this amounts to an estimated 79 million of Americans aged 20 or older with pre-diabetes [12]. In addition, several studies have documented that hyperglycemia at prediabetic levels is an independent risk factor for cardiovascular disease [13]. Early identification and treatment of persons with prediabetes can delay the progression to full-blown diabetes and related cardiovascular disease [14]. Therefore, insulin resistance is an attractive target for therapeutic intervention well ahead of the development of diabetes and its cardiovascular complications. However, pharmacological agents that improve the sensitivity of insulin and thereby help manage insulin resistance are somewhat limited. Diet and exercise can alleviate insulin resistance, but these measures are often difficult to achieve. Although current medications such as thiazolidinediones and biguanides can improve peripheral insulin-sensitivity, these drugs can cause serious adverse effects. Therefore, additional efforts are needed to identify and characterize pharmacological agents that augment insulin sensitivity and thereby overcome the complications of diabetes and cardiovascular disease. In this context, the micronutrient chromium, which is gaining popularity as a dietary supplement to improve the actions of insulin under insulin resistant conditions, merits attention.
The potential role of chromium in regulating blood sugar was first indicated in the late fifties by Mertz and Schwarz [15]. The ‘essentiality’ of chromium in human nutrition was suggested when it was found that chromium supplementation reversed glucose-intolerance in hospitalized patients receiving long-term total parenteral nutrition [16, 17]. However, the notion of ‘essentiality’ of chromium has been questioned owing to the ubiquitous nature of chromium and its low dietary requirement [18]. Nonetheless, several clinical studies have documented a beneficial effect of chromium in insulin resistance and type-2 diabetes. This review will focus on the proposed emerging molecular mechanisms of chromium in alleviating insulin resistance. For a broad overview of the biological actions of chromium and details on the various clinical trials, readers are directed to excellent recent reviews of the subject [19–22].
2. Clinical trials
Clinical studies on chromium in diabetic subjects have yielded mixed results and fueled the controversy surrounding the purported therapeutic benefits chromium. Chromium has shown beneficial effects in improving insulin resistance in some clinical trials, but not others. A recent systematic review conducted by Balk and coworkers [23] involving 41-randomized controlled clinical trials concluded that chromium supplementation significantly improved glycemia among patients with diabetes. However, the authors expressed caution regarding these conclusions, given that about half the clinical trials examined in their review were of poor quality and also stated that well-designed studies are necessary to provide additional support for beneficial effects of chromium.
It is possible that ethnic or genetic factors impact clinical effects of chromium. The above review by Balk and colleagues [23] included trials conducted in Indian, Chinese, and Western populations. In contrast, Kleefstra and co-workers [24] refute the claims of beneficial effects of chromium, citing their randomized, placebo-controlled, double-blind study that failed to show a difference in hemoglobin A1C in subjects from a Western population with moderate glycemic control when placed on chromium [25]. Cefalu and co-workers who have extensively performed both human and animal studies on chromium argue that the discrepancies associated with the benefits of chromium supplementation may be explained by “subject phenotype” that includes several factors such as baseline insulin resistance, alteration in chromium metabolism, and genetic makeup of individuals receiving the supplement [26].
Other factors complicate the analysis of chromium studies. The lack of good analytical tools to assess blood levels of chromium is a major hindrance to understanding the exact role of chromium in these diseases. An additional problem inherent in many such clinical trials is that the subjects were already on a variety of antidiabetic drugs which may contribute to masking or negating any observed insulin-potentiating properties of chromium. In addition, these clinical studies were conducted in subjects with full-blown diabetes. It is likely that chromium may be augmenting insulin actions in pre-diabetic patients. An incremental effect of chromium may be missed in patients with more advanced type-2 diabetes, at which point subtle modifications might be clinically undetectable. These factors may, at least in part, explain the fact that clinical trials on chromium supplementation have failed to demonstrate conclusive evidence for its beneficial effects in human subjects. In contrast to human studies, animal studies using both genetic and nutritional models of diabetes have often demonstrated a beneficial effect of chromium in alleviating insulin resistance, diabetes and lipid anomalies. The absence of other antidiabetic agents and the relatively higher doses of chromium used in the animal experiments may have contributed to some of the discrepancy in results between human and animal studies on chromium supplementation.
3. Chromium and insulin signaling
3.1. Insulin signaling cascade
Despite the controversial results in humans, in the more controlled settings of cell- based and animal studies of insulin resistance and diabetes, chromium has been shown to improve insulin sensitivity and alleviate cardiovascular functions, thereby giving credence to the argument that chromium may possess beneficial effects in countering these conditions. Most of these studies have focused on the role of chromium in insulin signaling.
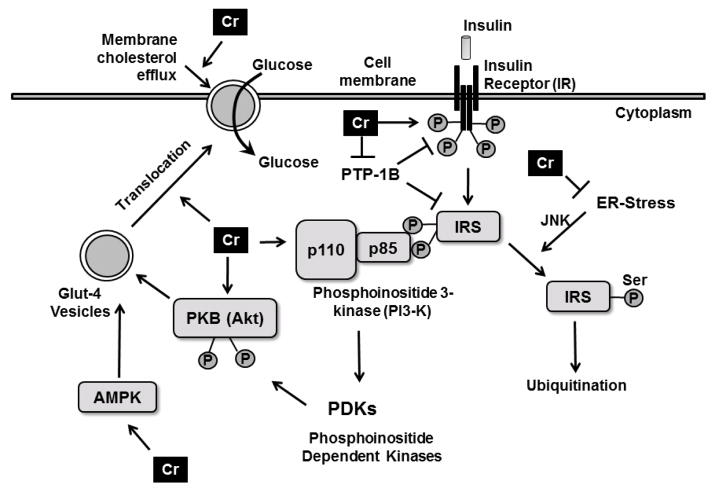
Insulin is a pleiotropic hormone with metabolic, mitogenic functions [27]. By virtue of its well-known metabolic functions, insulin regulates blood glucose levels. In skeletal muscles, insulin promotes glucose uptake by stimulating a cascade of signaling processes initiated by the binding of insulin to extracellular α-subunit of the insulin receptor (IR) on the cellular membrane (Figure 1) [28]. Insulin binding to the α-subunit elicits autophosphorylation of the intracellular β-subunit of IR, thereby activating the intracellular tyrosine kinase domain of the IR. Once activated, the insulin receptor catalytically phosphorylates multiple tyrosine residues on the downstream docking proteins insulin receptor substrate 1 and 2 (IRS-1 and IRS-2, respectively). Tyrosine phosphorylation of IRS-1 and IRS-2 promotes their binding to the Src-homology 2 domains, leading to the association between IRS-1 and the p85 regulatory subunit of phosphatidylinositol 2-kinase (PI3K), resulting in the recruitment of the p110 catalytic subunit of PI3K to the plasma membrane and causing the conversion of phosphatidylinositol-4,5-bisphosphate to phosphatidylinositol-3,4,5-triphosphate. These events lead to the phosphorylation of protein kinase B (Akt) (at Thr308 and serine473) by the involvement of PI3-dependent kinases (PDKs). Activated Akt in turn phosphorylates multiple downstream effectors, including Rab-GTPase activating protein, eventually leading to the translocation of glucose transporter-4 (Glut4)-vesicles from the cytoplasm to the cell surface, regulating cellular glucose uptake. Akt also phosphorylates glycogen synthase kinase-3 to stimulate glycogen formation.
Figure 1.
Putative mechanisms by which chromium augments cellular glucose uptake. Chromium has been shown to enhance the kinase activity of insulin receptor β, to increase the activity of downstream effectors of insulin signaling pI3-kinase and Akt and to enhance Glut4 translocation to the cell surface. Chromium also down-regulates PTP-1B, the negative regulator of insulin signaling and alleviates ER-stress within the cells, rescuing IRS from JNK-mediated serine phosphorylation and subsequent ubiquitination. Transient upregulation of AMPK by chromium leads to increased glucose uptake. Chromium mediates cholesterol efflux from the membranes causing glut4 translocation and glucose uptake.
3.2. Effect of chromium on insulin receptor phosphorylation and kinase activity
A number of studies have focused on molecular mechanisms by which chromium may alter proximal insulin signaling. These studies used cell-based or cell-free systems at basal or insulin-stimulated levels. The model systems were exposed to chromium salts or chromium delivery systems designed to improve chromium availability, including potassium chromate, the popular dietary supplement chromium picolinate, and investigational compounds such as the recently synthesized triphenylalaninate chromium complex [Cr(phe)3]. In addition to the different ligands used, these studies vary with respect to dose and methods used, which may explain some of the variabilities in the outcome.
The first indication of a direct effect of chromium on insulin signaling came from the lab of Yoshimoto and coworkers who investigated the role of chromium in glucose metabolism. Low-molecular-mass chromium-binding oligopeptide (LMCr), first identified in the cytosol of liver cells of mice injected with potassium chromate [29], augmented insulin-mediated metabolism of glucose to carbon dioxide in rat epididymal adipocytes [30]. Vincent and coworkers [31] demonstrated that the LMCr oligopeptide caused a 3–8 fold-stimulation of insulin-dependent tyrosine kinase activity in rat adipocyte membrane without altering the basal (in the absence of insulin) kinase activity. Furthermore, the fact that the apo-oligopeptide devoid of chromium did not activate insulin-dependent tyrosine kinase activity [31] and the kinase activity was proportional to the amount of chromium in the oligopeptide [32] underscored the importance of chromium and its interaction with the oligopeptide in augmenting kinase activity. Other metal ions complexes such as Mn, Fe, and Co failed to potentiate IR activation, furthering the importance of chromium in the process [32].
This LMWCr (molecular weight ~1500Dda) oligopeptide was composed of amino acids, glycine, cysteine, glutamate, and aspartate. Because it required the binding of chromium to exhibit the insulin kinase potentiating activity, the LMWCr was called chromodulin after the calcium binding protein calmodulin [33]. Based on extensive studies with the complex, Vincent and coworkers proposed a model in which LMWCr, augments insulin signaling by binding to and enhancing the kinase activity of insulin receptor [34, 35]. This led to a number of studies designed to isolate and characterize endogenous LMWCr and synthetic molecular mimics of LMWCr . A recently published report claims to have characterized the structure of the apoLMWCr which may pave way for future studies in understanding the role of the polypeptide in the management of diabetes [36].
Wang and coworkers treated CHO-IR cells with chromium picolinate, a popular chromium supplement, and observed that chromium enhanced the tyrosine phosphorylation of insulin receptors in addition to enhancing the insulin receptor tyrosine kinase activity in the membranes fraction of the chromium-treated cells [37] (see Figure 1.) However, in a cell-free system including a recombinant IR domain chromium failed to enhance the auto-tyrosine phosphorylation of the recombinant protein. This lack of effect in the cell-free system may be attributed to the fact that these studies were performed under basal conditions in the absence of insulin stimulation. It would be of interest to know how this experiment would have been affected by the presence of insulin.
3.3. Effect of chromium on IRS, Akt, and PI3-kinase
Studies from our lab are consistent with some, but not all, of the above results: chromium did not upregulate the phosphorylation of the IR at basal levels, but neither did it upregulate IR phosphorylation following insulin stimulation [38, 39]. However, chromium was capable of up-regulating insulin-stimulated insulin signal transduction via affecting effector molecules downstream of the IR, as evidenced by enhanced levels of tyrosine phosphorylation of IRS-1, elevated thr308 and ser473 phosphorylation of Akt and increased PI3-kinase activity in a variety of cellular and animal models of insulin resistance [40]. In obese, insulin-resistant JCR:LA-cp rats, supplementation with chromium picolinate enhances skeletal muscle insulin signaling which was manifested as an increase in insulin-stimulated tyrosine phosphorylation of IRS-1 and PI-3-kinase activity, without altering the total protein levels of any of these molecules [40]. A synthesized triphenylalaninate chromium complex [Cr(phe)3] augmented insulin-stimulated glucose uptake in cultured adipocytes via enhancing insulin-stimulated phosphorylation of Akt in a time- and concentration-dependent manner [39]. Furthermore, oral supplementation of Cr(phe)3 to leptin-deficient obese (ob/ob) mice improved glucose tolerance, reconciled lipid abnormalities, and augmented insulin-stimulated insulin receptor phosphorylation in the liver and muscle [39, 41, 42].
Chromium has been tested in a range of in vivo and in vitro nutritional models of diabetes. Conditions modeling hyperglycemia, hyperinsulinemia, and/or hyperlipidemia alter insulin signaling by, for example, blunting Akt phosphorylation or attenuating PI3kinase activity. In a nutritional model of insulin resistance in which the mice were fed a high-sucrose diet [43] or a high-fat diet [44], chromium treatment reconciled the blunted Akt phosphorylation and the attenuated PI3-kinase activity, consistent with the studies by Cefalu and coworkers. To understand whether this was a direct effect of chromium on insulin signaling, cultured adipocytes were rendered insulin resistant by chronic treatment with insulin and glucose (hyperinsulinemic, hyperglycemic conditions) resulted in a blunting of insulin-stimulated Akt phosphorylation and cellular glucose uptake. Both these effects were inhibited by pretreating the cells with the chromium complex, results consistent with a direct action of chromium on insulin signaling [43].
3.4. Effect of chromium on glucose transporters (Glut)
The effects of chromium on proximal insulin signaling molecules affected distal functional events such as the normal insulin-stimulated translocation of Glut4 vesicles to the cell membrane. In skeletal muscle of high-sucrose-diet-fed mice, insulin-stimulated membrane-associated Glut4 levels were significantly attenuated compared to those from lean mice [43]. Supplementation with Cr(phe)3 reverted the membrane levels of Glut4 to those observed in the lean counterparts. Similar effects were observed by Cefalu and coworkers in diabetic rats that were treated with chromium picolinate [45]. In addition to the effect on skeletal muscle, it has also been reported that chromium increases Glut4 membrane translocation in myocardial tissues [46] and increases Glut-2 levels diabetic rat liver [47, 48].
In an effort to understand the mechanism of chromium-mediated upregulation of Glut4, Elmendorf and coworkers used cultured adipocytes to further investigated the effects of chromium chloride and chromium picolinate on insulin-stimulated Glu4 translocation to the plasma membrane [49]. They found that treatment of adipocytes with chromium resulted in an elevation of Glut4 at the plasma membrane, together with an enhancement of insulin-stimulated glucose transport across the cell membrane. Interestingly, however, in contrast to in-vivo studies, the in-vitro studies suggest that regulation of Glut4 translocation by chromium may be independent of the insulin signaling proteins such as the IR, IRS-1, PI3-kinase, or Akt. Instead, chromium has been observed to increase the fluidity of the membrane by decreasing the membrane cholesterol [49]. Chromium has also been shown to cause an upregulation of sterol regulatory element-binding protein, a membrane-bound transcription factor responsible for controlling cellular cholesterol balance [49]. A reciprocal decrease in the cholesterol efflux protein ABCA1 has also been shown, suggesting an intricate link between chromium and cholesterol homeostasis [50, 51].
Taken together, regardless of the exact mechanisms, chromium both in-vivo and in-vitro augments insulin-dependent Glut4 membrane translocation under insulin resistant conditions that may partly explain the beneficial effects attributed to chromium.
3.5. Effect of chromium on the negative regulators of insulin signaling: PTP-1B, JNK, IRS-1- serine phosphorylation
Whereas chromium augmented insulin signaling under insulin-resistant conditions in animal models and cultured cells, chromium did not affect insulin signaling in lean/control mice, suggesting that under basal conditions, chromium does not affect the normal insulin signaling [38, 39]. Consistent with the observations in experimental systems, in human studies, baseline insulin sensitivity (assessed with the hyperinsulinemic-euglycemic clinical response to chromium) was also more likely in insulin-resistant subjects who had elevated fasting glucose and hemoglobin A1C levels [26, 52].
Because insulin-resistant conditions dictate the activity of chromium, the following section describes the effect of chromium on the negative regulators of insulin signaling that are responsible for insulin resistance. Phosphorylation of tyrosine residues in proteins in response to stimulation by growth factors is governed by reciprocal activities of protein tyrosine phosphatases (PTPs) and protein tyrosine kinases (PTKs), and insulin receptor is no exception [53]. PTP-1B is a phosphatase that has been implicated as a negative regulator of insulin signaling by virtue of its relative specificity towards the tyrosine phosphorylation sites on IR and IRS-1 [54, 55]. Binding of insulin to the IR results in a transient release of hydrogen peroxide; this reversibly oxidizes a critical cystine residue on the catalytic site of PTP-1B, inactivating PTP-1B, thus allowing normal downstream insulin signaling [56, 57]. Consequently, selective inhibitors of PTP-1B have been sought as potential antidiabetic agents [58]. Metals such as vanadate have been thought to mediate insulin potentiating activity via reversibly oxidizing the critical cysteine residue in the active site of PTP-1B [59]. Therefore, it was logical to hypothesize that chromium may work in a similar manner. Early studies by Vincent’s group found that LMWCr enhanced PTP (not PTP-1B) activity in adipocyte membranes [60]. In contrast, they found that in rat hepatoma cells, chromium treatment resulted in a 21–33% reduction in the activity of PTP-1B [61]. Other studies, however, did not observe an effect of Cr(phe)3 or chromium picolinate on the protein levels or specific activity of PTP-1B in cultured cells or in insulin-resistant animals (Yang, X and Sreejayan N, unpublished studies). Wang and coworkers recently reported that chromium did not effectively inhibit recombinant human PTP-1B, nor did it alter the reversible redox regulation of PTP-1B [37]. However, Cefalu’s group found that administration of chromium picolinate attenuated both the protein levels and specific activity of PTP-1B in the skeletal muscle of obese rats [40].
Whereas tyrosine phosphorylation of the insulin receptor propagates insulin signaling, phosphorylation of the serine residue on the amino acid 307 within IRS-1 has been shown to significantly attenuate insulin signaling [62]. Serine phosphorylation of IRS-1 prevents the association of its protein tyrosine binding domain with IR-β subunit, preventing IRS-1 binding to the receptor and insulin-dependent activation of PI3-kinase [63]. Additionally, Ser307 phosphorylation of IRS-1 accelerates the degradation of IRS-1 by an ubiquitin-proteosome-mediated process, thus reducing the protein levels of IRS-1 and further blunting insulin signaling [64]. IRS-1 Ser307 is a major target for Jun NH(2)-terminal kinase (JNK) mediated phosphorylation [62, 65]. Studies with chromium have found that obese insulin resistant mice had elevated hepatic levels of phospho-c-Jun and IRS-1-phospho-Ser307 compared to lean mice. Supplementation with chromium suppressed tyrosine phosphorylation of c-Jun and IRS-1 phospho-Ser307 in the livers of ob/ob mice [42]. Chen and coworkers arrived at similar conclusions by studying IRS-1 serine and c-Jun protein levels in the skeletal muscles of generically obese insulin resistant KK/HIH mice [66]. These observations were further confirmed in-vitro in cultured myotubes rendered insulin-resistant by treating them with palmitic acid [44] or hyperinsulinemic and hyperglycemic conditions [67] that caused elevation in JNK-phosphorylation and reciprocal inhibition of insulin-stimulated phosphorylation of Akt and glucose uptake. Interestingly, chromium successfully mitigated the aforementioned effects of palmitic acid in cultured myotubes, suggesting that chromium may indeed downregulate the proteins involved in insulin resistance.
3.6. Chromium and ER-stress
Emerging evidence suggests that endoplasmic reticulum (ER) stress may play a pivotal role in the development of insulin resistance and may therefore represent a unifying mechanism in understanding the pathophysiology of type-2 diabetes [68, 69]. Elevated ER-stress has been documented in adipocytes and liver cells of genetically obese and high-fat fed mice [70]. ER stress has also been shown to activate c-Jun N-terminal kinase (JNK), which, as mentioned above, induces serine phosphorylation of IRS, leading to the suppression of insulin signaling [70]. Consequently, pharmacological agents that attenuate ER stress (ER-chaperones) have been shown to alleviate the symptoms of diabetes [71, 72]. A recent study found that the protein levels of ER-stress markers viz., phosphorylated pancreatic ER kinase (PERK), α-subunit of translation initiation factor 2 (eIF2α) and inositol-requiring enzyme-1 (IRE-1), were all significantly elevated in ob/ob mice compared to their lean counterparts [42]. Oral supplementation with chromium significantly attenuated the levels of these ER-stress markers. In addition to these in-vivo studies, ER stress was induced in-vitro by treating cultured, differentiated myotubes with thapsigargin, a well known inducer of ER-stress. Pretreatment of the myotubes with chromium resulted in a concentration-dependent inhibition of thapsigargin-induced ER stress. Together, the findings suggest that chromium alleviates ER-stress in the cells, although the exact mechanism by which it does so is unclear. It would be interesting to investigate whether chromium functions as a co-factor in enhancing chaperone like activity in insulin-resistant cells.
3.7. Chromium, oxidative stress, and inflammation
The role of oxidative stress and inflammation in diabetes and its comorbidities is well established, and the potential effect of chromium in regulating oxidative stress and inflammation has been addressed [73]. Chromium attenuates oxidative stress in cultured monocytes and isolated human mononuclear cells subjected to hyperglycemic conditions [74, 75]. Together with reducing oxidative stress, chromium treatment inhibits the release of pro-inflammatory cytokines from monocytes exposed to hyperglycemic conditions. Chromium was found to inhibit protein glycosylation and lipid peroxidation in erythrocytes exposed to high levels of glucose [76]. In addition to these in-vitro studies, it has also been shown that chromium supplementation caused a lowering of proinflammatory cytokines (TNF-alpha, IL-6, CRP), oxidative stress, and lipids levels in streptozotocin-induced diabetic rats [77].
Hazane-Puch and coworkers used a cDNA array to investigate the modulation of gene expression by chromium complexes in human keratinocytes [78]. The array was composed mainly of sequence tags representing antioxidant and DNA-repair genes. An upregulation of the transcripts of glutathione synthetase, heme oxygenase-2, and peroxiredoxin was observed in cells treated with chromium. In addition, chromium inhibited hydrogen peroxide-mediated oxidation of thiols and lipid peroxidation [78]. Chromium supplementation in obese mice significantly attenuated the obesity-associated elevated ratio of oxidized glutathione to reduced glutathione and the formation of protein carbonyl [79]. Similar effects of chromium on elevated oxidative stress in diabetic subjects have been reported from randomized clinical trials in which serum thiobarbituric acid reactive substances were used as a marker for the end-product of lipid peroxidation and total serum antioxidative status and glutathione peroxidase activity were used to assess oxidative stress [80, 81].
3.8. Effect of chromium on AMP-activated protein kinase (AMPK)
AMPK is a serine-threonine kinase that has gained attention for its regulatory role in cellular energy homeostasis [82, 83]. AMPK, which is activated by increases in the AMP:ATP ratio, was originally discovered as a switch that regulates fatty acid oxidation in the heart [84] and skeletal muscle [85]. It has recently emerged as an important regulator of glucose metabolism [86].
It has been shown that in H9c2 myoblasts and isolated mouse cardiomyocytes, Cr(phe)3 stimulated the phosphorylation of the α-catalytic subunit of AMPK at Thr172, as well the downstream targets of AMPK including acetyl-CoA carboxylase (at Ser212) and eNOS (at Ser1177). Chromium also stimulated glucose-uptake in these cells [87]. Both glucose uptake and AMPK phosphorylation mediated by chromium were inhibited by the AMPK inhibitor compound C. In support of in-vitro studies, acute injection of mice with the chromium complex resulted in increased levels of AMP and a substantial decrease in mitochondrial membrane potential in the cardiac tissues. Thus increase of cardiac AMP concentration and the decrease of mitochondrial membrane potential may contribute to the activation of AMPK induced by chromium complexes [87]. (It is interesting to note that a recent paper suggests that chromium normalizes hyperketonemia-induced decrease in mitochondrial membrane potential in monocytes [88]). Although both wortmannin (PI3-kinase inhibitor) and compound C attenuated chromium-induced glucose uptake in cardiomyocytes, neither of these compounds by itself completely blocked the glucose-uptake at the highest possible concentrations tested. However, when both these inhibitors were used in combination, chromium-induced cardiomyocyte glucose uptake was completely abrogated, suggesting that both the insulin signaling pathway and AMPK pathways are affected by chromium [87].
Maulik and coworkers [46] investigated the effect of a niacin-bound chromium complex in a model of ischemia/reperfusion (IR) injury in streptozotocin-induced diabetic rats. These authors found a significant increase in left ventricular functions and a significant reduction in infarct size and increased cardiomyocyte apoptosis in the diabetic mice that were significantly attenuated by the chromium complex. The chromium complex attenuated the streptozotocin-induced left ventricular dysfunction, infarct size and cardiomyocyte apoptosis. Chromium also improved cardiac contractile functions and cardiomyocyte calcium handling in obese mice [79]. In addition, chromium treatment resulted in an increased Glut-4 translocation to the lipid raft fractions, reduced Cav-1, and increased Cav-3 expression, together with phosphorylation of Akt, eNOS and AMPK. These findings further strengthen the notion that both insulin and the AMPK signaling pathways may represent potential targets of chromium.
4. Conclusion
The nutritional supplement chromium (trivalent) has been credited with alleviating insulin resistance and lipid abnormalities although clinical trials have not shown conclusive evidence for its beneficial effect in human subjects. In contrast, chromium improved insulin sensitivity and glucose handling in a number of animal models of type-2 diabetes. However, despite the controversies surrounding the clinical benefits of chromium in type-2 diabetes, it has gained popularity as a nutritional supplement and is a component of many multivitamin/mineral formulations, fortified food, and energy drinks. The molecular mechanisms by which chromium mediates its beneficial effects are unclear. This review addresses some of the recent findings which shed light on the potential cellular pathways that are affected by chromium. Chromium supplementation to animals that were rendered insulin resistant either by genetic or nutritional methods indicate that chromium potentiates the actions of insulin, augments the insulin signaling pathway, blunts the negative-regulators of insulin signaling, enhances AMPK activity, up-regulating cellular glucose uptake, and attenuates oxidative stress. Figure 1 summarizes the pathways described in this review, highlighting only those molecules that have been shown to be affected by chromium both in-vivo and in-vitro. These beneficial effects of chromium, together with its wide safety profile, may justify its use as an adjunct therapy in the management of insulin resistance and type-2 diabetes.
Acknowledgments
This work was supported by grants from ADA (AMDIAB47595), NCRR and the Wyoming INBRE (P20RR016474). The authors are grateful to Drs. Xiaoping Yang, Feng Dong, Machender Reddy, Julia Dolence, Ajaya Kumara Sankara Warrier, Palanichamy Kamalakannan, Allyn Ontko, Min Du and M.N.A. Rao for their assistance at various stages during the research. The authors thank Ms. Virginia Cole for editing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Engelgau MM, Geiss LS, Saaddine JB, Boyle JP, Benjamin SM, Gregg EW, Tierney EF, Rios-Burrows N, Mokdad AH, Ford ES, Imperatore G, Narayan KM. The evolving diabetes burden in the United States. Ann Intern Med. 2004;140:945–50. doi: 10.7326/0003-4819-140-11-200406010-00035. [DOI] [PubMed] [Google Scholar]
- 3.Eckel RH, Kahn R, Robertson RM, Rizza RA. Preventing cardiovascular disease and diabetes: a call to action from the American Diabetes Association and the American Heart Association. Circulation. 2006;113:2943–6. doi: 10.1161/CIRCULATIONAHA.106.176583. [DOI] [PubMed] [Google Scholar]
- 4.Flier JS. Obesity wars: molecular progress confronts an expanding epidemic. Cell. 2004;116:337–50. doi: 10.1016/s0092-8674(03)01081-x. [DOI] [PubMed] [Google Scholar]
- 5.Hogan P, Dall T, Nikolov P. Economic costs of diabetes in the US in 2002. Diabetes Care. 2003;26:917–32. doi: 10.2337/diacare.26.3.917. [DOI] [PubMed] [Google Scholar]
- 6.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–9. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 7.Popkin BM. Recent dynamics suggest selected countries catching up to US obesity. Am J Clin Nutr. 2010;91:284S–288S. doi: 10.3945/ajcn.2009.28473C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 9.Type 2 diabetes in children and adolescents. American Diabetes Association Diabetes Care. 2000;23:381–9. doi: 10.2337/diacare.23.3.381. [DOI] [PubMed] [Google Scholar]
- 10.Koh-Banerjee P, Wang Y, Hu FB, Spiegelman D, Willett WC, Rimm EB. Changes in body weight and body fat distribution as risk factors for clinical diabetes in US men. Am J Epidemiol. 2004;159:1150–9. doi: 10.1093/aje/kwh167. [DOI] [PubMed] [Google Scholar]
- 11.Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:193–205. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]
- 12. [accessed on July 21, 2011]; http://diabetes.niddk.nih.gov/dm/pubs/statistics/#Pre-diabetesY20.
- 13.Haffner SM, Stern MP, Hazuda HP, Mitchell BD, Patterson JK. Cardiovascular risk factors in confirmed prediabetic individuals. Does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA. 1990;263:2893–8. doi: 10.1001/jama.263.21.2893. [DOI] [PubMed] [Google Scholar]
- 14.Gillies CL, Abrams KR, Lambert PC, Cooper NJ, Sutton AJ, Hsu RT, Khunti K. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ. 2007;334:299. doi: 10.1136/bmj.39063.689375.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mertz W, Schwarz K. Relationship of glucose tolerance to impaired intravenous glucose tolerance of rats on stock diets. Am J Physiol. 1959;196:614–618. doi: 10.1152/ajplegacy.1959.196.3.614. [DOI] [PubMed] [Google Scholar]
- 16.Jeejeebhoy KN, Chu RC, Marliss EB, Greenberg GR, Bruce-Robertson A. Chromium deficiency, glucose intolerance, and neuropathy reversed by chromium supplementation, in a patient receiving long-term total parenteral nutrition. Am J Clin Nutr. 1977;30:531–8. doi: 10.1093/ajcn/30.4.531. [DOI] [PubMed] [Google Scholar]
- 17.Freund H, Atamian S, Fischer JE. Chromium deficiency during total parenteral nutrition. JAMA. 1979;241:496–8. [PubMed] [Google Scholar]
- 18.Di Bona KR, Love S, Rhodes NR, McAdory D, Sinha SH, Kern N, Kent J, Strickland J, Wilson A, Beaird J, Ramage J, Rasco JF, Vincent JB. Chromium is not an essential trace element for mammals: effects of a “low-chromium” diet. J Biol Inorg Chem. 2011;16:381–90. doi: 10.1007/s00775-010-0734-y. [DOI] [PubMed] [Google Scholar]
- 19.Wang ZQ, Cefalu WT. Current concepts about chromium supplementation in type 2 diabetes and insulin resistance. Curr Diab Rep. 2010;10:145–51. doi: 10.1007/s11892-010-0097-3. [DOI] [PubMed] [Google Scholar]
- 20.Hummel M, Standl E, Schnell O. Chromium in metabolic and cardiovascular disease. Horm Metab Res. 2007;39:743–51. doi: 10.1055/s-2007-985847. [DOI] [PubMed] [Google Scholar]
- 21.Vincent JB. Chromium: celebrating 50 years as an essential element? Dalton Trans. 2010;39:3787–94. doi: 10.1039/b920480f. [DOI] [PubMed] [Google Scholar]
- 22.Lau FC, Bagchi M, Sen CK, Bagchi D. Nutrigenomic basis of beneficial effects of chromium(III) on obesity and diabetes. Mol Cell Biochem. 2008;317:1–10. doi: 10.1007/s11010-008-9744-2. [DOI] [PubMed] [Google Scholar]
- 23.Balk EM, Tatsioni A, Lichtenstein AH, Lau J, Pittas AG. Effect of chromium supplementation on glucose metabolism and lipids: a systematic review of randomized controlled trials. Diabetes Care. 2007;30:2154–63. doi: 10.2337/dc06-0996. [DOI] [PubMed] [Google Scholar]
- 24.Kleefstra N, Houweling ST, Bilo HJ. Effect of chromium supplementation on glucose metabolism and lipids: a systematic review of randomized controlled trials. Diabetes Care. 2007;30:e102. doi: 10.2337/dc07-1015. author reply e103. [DOI] [PubMed] [Google Scholar]
- 25.Kleefstra N, Houweling ST, Jansman FG, Groenier KH, Gans RO, Meyboom-de Jong B, Bakker SJ, Bilo HJ. Chromium treatment has no effect in patients with poorly controlled, insulin-treated type 2 diabetes in an obese Western population: a randomized, double-blind, placebo-controlled trial. Diabetes Care. 2006;29:521–5. doi: 10.2337/diacare.29.03.06.dc05-1453. [DOI] [PubMed] [Google Scholar]
- 26.Wang ZQ, Qin J, Martin J, Zhang XH, Sereda O, Anderson RA, Pinsonat P, Cefalu WT. Phenotype of subjects with type 2 diabetes mellitus may determine clinical response to chromium supplementation. Metabolism. 2007;56:1652–5. doi: 10.1016/j.metabol.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 28.Myers MG, Jr, White MF. The new elements of insulin signaling. Insulin receptor substrate-1 and proteins with SH2 domains. Diabetes. 1993;42:643–50. doi: 10.2337/diab.42.5.643. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto A, Wada O, Ono T. A low-molecular-weight, chromium-binding substance in mammals. Toxicol Appl Pharmacol. 1981;59:515–23. doi: 10.1016/0041-008x(81)90305-7. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto A, Wada O, Ono T. Isolation of a biologically active low-molecular-mass chromium compound from rabbit liver. Eur J Biochem. 1987;165:627–31. doi: 10.1111/j.1432-1033.1987.tb11486.x. [DOI] [PubMed] [Google Scholar]
- 31.Davis CM, Vincent JB. Chromium oligopeptide activates insulin receptor tyrosine kinase activity. Biochemistry. 1997;36:4382–5. doi: 10.1021/bi963154t. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto A, Wada O, Manabe S. Evidence that chromium is an essential factor for biological activity of low-molecular-weight, chromium-binding substance. Biochem Biophys Res Commun. 1989;163:189–93. doi: 10.1016/0006-291x(89)92119-0. [DOI] [PubMed] [Google Scholar]
- 33.Vincent JB. Quest for the molecular mechanism of chromium action and its relationship to diabetes. Nutr Rev. 2000;58:67–72. doi: 10.1111/j.1753-4887.2000.tb01841.x. [DOI] [PubMed] [Google Scholar]
- 34.Vincent JB. Recent advances in the nutritional biochemistry of trivalent chromium. Proc Nutr Soc. 2004;63:41–7. doi: 10.1079/PNS2003315. [DOI] [PubMed] [Google Scholar]
- 35.Vincent JB. The biochemistry of chromium. J Nutr. 2000;130:715–8. doi: 10.1093/jn/130.4.715. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y, Watson HM, Gao J, Sinha SH, Cassady CJ, Vincent JB. Characterization of the organic component of low-molecular-weight chromium-binding substance and its binding of chromium. J Nutr. 2011;141:1225–32. doi: 10.3945/jn.111.139147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang H, Kruszewski A, Brautigan DL. Cellular chromium enhances activation of insulin receptor kinase. Biochemistry. 2005;44:8167–75. doi: 10.1021/bi0473152. [DOI] [PubMed] [Google Scholar]
- 38.Mackowiak P, Krejpcio Z, Sassek M, Kaczmarek P, Hertig I, Chmielewska J, Wojciechowicz T, Szczepankiewicz D, Wieczorek D, Szymusiak H, Nowak KW. Evaluation of insulin binding and signaling activity of newly synthesized chromium(III) complexes in vitro. Mol Med Report. 2010;3:347–53. doi: 10.3892/mmr_00000264. [DOI] [PubMed] [Google Scholar]
- 39.Yang X, Li SY, Dong F, Ren J, Sreejayan N. Insulin-sensitizing and dcholesterol-lowering effects of chromium (D-Phenylalanine)3. J Inorg Biochem. 2006;100:1187–93. doi: 10.1016/j.jinorgbio.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 40.Wang ZQ, Zhang XH, Russell JC, Hulver M, Cefalu WT. Chromium picolinate enhances skeletal muscle cellular insulin signaling in vivo in obese, insulin-resistant JCR:LA-cp rats. J Nutr. 2006;136:415–20. doi: 10.1093/jn/136.2.415. [DOI] [PubMed] [Google Scholar]
- 41.Yang X, Palanichamy K, Ontko AC, Rao MN, Fang CX, Ren J, Sreejayan N. A newly synthetic chromium complex--chromium(phenylalanine)3 improves insulin responsiveness and reduces whole body glucose tolerance. FEBS Lett. 2005;579:1458–64. doi: 10.1016/j.febslet.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 42.Sreejayan N, Dong F, Kandadi MR, Yang X, Ren J. Chromium alleviates glucose intolerance, insulin resistance, and hepatic ER stress in obese mice. Obesity (Silver Spring) 2008;16:1331–7. doi: 10.1038/oby.2008.217. [DOI] [PubMed] [Google Scholar]
- 43.Dong F, Kandadi MR, Ren J, Sreejayan N. Chromium (D-phenylalanine)3 supplementation alters glucose disposal, insulin signaling, and glucose transporter-4 membrane translocation in insulin-resistant mice. J Nutr. 2008;138:1846–51. doi: 10.1093/jn/138.10.1846. [DOI] [PubMed] [Google Scholar]
- 44.Kandadi MR, Unnikrishnan MK, Warrier AK, Du M, Ren J, Sreejayan N. Chromium (D-phenylalanine)3 alleviates high fat-induced insulin resistance and lipid abnormalities. J Inorg Biochem. 2011;105:58–62. doi: 10.1016/j.jinorgbio.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cefalu WT, Wang ZQ, Zhang XH, Baldor LC, Russell JC. Oral chromium picolinate improves carbohydrate and lipid metabolism and enhances skeletal muscle Glut-4 translocation in obese, hyperinsulinemic (JCR-LA corpulent) rats. J Nutr. 2002;132:1107–14. doi: 10.1093/jn/132.6.1107. [DOI] [PubMed] [Google Scholar]
- 46.Penumathsa SV, Thirunavukkarasu M, Samuel SM, Zhan L, Maulik G, Bagchi M, Bagchi D, Maulik N. Niacin bound chromium treatment induces myocardial Glut-4 translocation and caveolar interaction via Akt, AMPK and eNOS phosphorylation in streptozotocin induced diabetic rats after ischemia-reperfusion injury. Biochim Biophys Acta. 2009;1792:39–48. doi: 10.1016/j.bbadis.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 47.Jain SK, Croad JL, Velusamy T, Rains JL, Bull R. Chromium dinicocysteinate supplementation can lower blood glucose, CRP, MCP-1, ICAM-1, creatinine, apparently mediated by elevated blood vitamin C and adiponectin and inhibition of NFkappaB, Akt, and Glut-2 in livers of zucker diabetic fatty rats. Mol Nutr Food Res. 2011;54:1371–80. doi: 10.1002/mnfr.200900177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tuzcu M, Sahin N, Orhan C, Agca CA, Akdemir F, Tuzcu Z, Komorowski J, Sahin K. Impact of chromium histidinate on high fat diet induced obesity in rats. Nutr Metab (Lond) 2011;8:28. doi: 10.1186/1743-7075-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen G, Liu P, Pattar GR, Tackett L, Bhonagiri P, Strawbridge AB, Elmendorf JS. Chromium activates glucose transporter 4 trafficking and enhances insulin-stimulated glucose transport in 3T3-L1 adipocytes via a cholesterol-dependent mechanism. Mol Endocrinol. 2006;20:857–70. doi: 10.1210/me.2005-0255. [DOI] [PubMed] [Google Scholar]
- 50.Pattar GR, Tackett L, Liu P, Elmendorf JS. Chromium picolinate positively influences the glucose transporter system via affecting cholesterol homeostasis in adipocytes cultured under hyperglycemic diabetic conditions. Mutat Res. 2006;610:93–100. doi: 10.1016/j.mrgentox.2006.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sealls W, Penque BA, Elmendorf JS. Evidence that chromium modulates cellular cholesterol homeostasis and ABCA1 functionality impaired by hyperinsulinemia--brief report. Arterioscler Thromb Vasc Biol. 2011;31:1139–40. doi: 10.1161/ATVBAHA.110.222158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cefalu WT, Rood J, Pinsonat P, Qin J, Sereda O, Levitan L, Anderson RA, Zhang XH, Martin JM, Martin CK, Wang ZQ, Newcomer B. Characterization of the metabolic and physiologic response to chromium supplementation in subjects with type 2 diabetes mellitus. Metabolism. 2010;59:755–62. doi: 10.1016/j.metabol.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–3. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 54.Goldstein BJ. Protein-tyrosine phosphatases: emerging targets for therapeutic intervention in type 2 diabetes and related states of insulin resistance. J Clin Endocrinol Metab. 2002;87:2474–80. doi: 10.1210/jcem.87.6.8641. [DOI] [PubMed] [Google Scholar]
- 55.Sreejayan N, Lin Y, Hassid A. NO attenuates insulin signaling and motility in aortic smooth muscle cells via protein tyrosine phosphatase 1B-mediated mechanism. Arterioscler Thromb Vasc Biol. 2002;22:1086–92. doi: 10.1161/01.atv.0000020550.65963.e9. [DOI] [PubMed] [Google Scholar]
- 56.Mahadev K, Zilbering A, Zhu L, Goldstein BJ. Insulin-stimulated hydrogen peroxide reversibly inhibits protein-tyrosine phosphatase 1b in vivo and enhances the early insulin action cascade. J Biol Chem. 2001;276:21938–42. doi: 10.1074/jbc.C100109200. [DOI] [PubMed] [Google Scholar]
- 57.van Montfort RL, Congreve M, Tisi D, Carr R, Jhoti H. Oxidation state of the active-site cysteine in protein tyrosine phosphatase 1B. Nature. 2003;423:773–7. doi: 10.1038/nature01681. [DOI] [PubMed] [Google Scholar]
- 58.Barr AJ. Protein tyrosine phosphatases as drug targets: strategies and challenges of inhibitor development. Future Med Chem. 2010;2:1563–76. doi: 10.4155/fmc.10.241. [DOI] [PubMed] [Google Scholar]
- 59.Peters KG, Davis MG, Howard BW, Pokross M, Rastogi V, Diven C, Greis KD, Eby-Wilkens E, Maier M, Evdokimov A, Soper S, Genbauffe F. Mechanism of insulin sensitization by BMOV (bis maltolato oxo vanadium); unliganded vanadium (VO4) as the active component. J Inorg Biochem. 2003;96:321–30. doi: 10.1016/s0162-0134(03)00236-8. [DOI] [PubMed] [Google Scholar]
- 60.Davis CM, Sumrall KH, Vincent JB. A biologically active form of chromium may activate a membrane phosphotyrosine phosphatase (PTP) Biochemistry. 1996;35:12963–9. doi: 10.1021/bi960328y. [DOI] [PubMed] [Google Scholar]
- 61.Goldstein BJ, Zhu L, Hager R, Zilbering A, Sun Y, vincent JB. Enhancement of post-receptor insulin signaling by trivalent chromium in hepatoma cells is associated with differential inhibition of specific protein-tyrosine phosphatases. J Trace Elem Exp Med. 2001;14:393–401. [Google Scholar]
- 62.Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307) J Biol Chem. 2000;275:9047–54. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 63.Sykiotis GP, Papavassiliou AG. Serine phosphorylation of insulin receptor substrate-1: a novel target for the reversal of insulin resistance. Mol Endocrinol. 2001;15:1864–9. doi: 10.1210/mend.15.11.0725. [DOI] [PubMed] [Google Scholar]
- 64.Rui L, Fisher TL, Thomas J, White MF. Regulation of insulin/insulin-like growth factor-1 signaling by proteasome-mediated degradation of insulin receptor substrate-2. J Biol Chem. 2001;276:40362–7. doi: 10.1074/jbc.M105332200. [DOI] [PubMed] [Google Scholar]
- 65.Solinas G, Naugler W, Galimi F, Lee MS, Karin M. Saturated fatty acids inhibit induction of insulin gene transcription by JNK-mediated phosphorylation of insulin-receptor substrates. Proc Natl Acad Sci U S A. 2006;103:16454–9. doi: 10.1073/pnas.0607626103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen WY, Chen CJ, Liu CH, Mao FC. Chromium supplementation enhances insulin signalling in skeletal muscle of obese KK/HlJ diabetic mice. Diabetes Obes Metab. 2009;11:293–303. doi: 10.1111/j.1463-1326.2008.00936.x. [DOI] [PubMed] [Google Scholar]
- 67.Wang YQ, Yao MH. Effects of chromium picolinate on glucose uptake in insulin-resistant 3T3-L1 adipocytes involve activation of p38 MAPK. J Nutr Biochem. 2009;20:982–91. doi: 10.1016/j.jnutbio.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 68.Nakatani Y, Kaneto H, Kawamori D, Yoshiuchi K, Hatazaki M, Matsuoka TA, Ozawa K, Ogawa S, Hori M, Yamasaki Y, Matsuhisa M. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J Biol Chem. 2005;280:847–51. doi: 10.1074/jbc.M411860200. [DOI] [PubMed] [Google Scholar]
- 69.Hotamisligil GS. Role of endoplasmic reticulum stress and c-Jun NH2-terminal kinase pathways in inflammation and origin of obesity and diabetes. Diabetes. 2005;54(Suppl 2):S73–8. doi: 10.2337/diabetes.54.suppl_2.s73. [DOI] [PubMed] [Google Scholar]
- 70.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–61. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 71.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–40. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Engin F, Hotamisligil GS. Restoring endoplasmic reticulum function by chemical chaperones: an emerging therapeutic approach for metabolic diseases. Diabetes Obes Metab. 2010;12(Suppl 2):108–15. doi: 10.1111/j.1463-1326.2010.01282.x. [DOI] [PubMed] [Google Scholar]
- 73.Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 2011;50:567–75. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jain SK, Kannan K. Chromium chloride inhibits oxidative stress and TNF-alpha secretion caused by exposure to high glucose in cultured U937 monocytes. Biochem Biophys Res Commun. 2001;289:687–91. doi: 10.1006/bbrc.2001.6026. [DOI] [PubMed] [Google Scholar]
- 75.Jain SK, Lim G. Chromium chloride inhibits TNFalpha and IL-6 secretion in isolated human blood mononuclear cells exposed to high glucose. Horm Metab Res. 2006;38:60–2. doi: 10.1055/s-2006-924981. [DOI] [PubMed] [Google Scholar]
- 76.Jain SK, Patel P, Rogier K. Trivalent chromium inhibits protein glycosylation and lipid peroxidation in high glucose-treated erythrocytes. Antioxid Redox Signal. 2006;8:238–41. doi: 10.1089/ars.2006.8.238. [DOI] [PubMed] [Google Scholar]
- 77.Jain SK, Rains JL, Croad JL. Effect of chromium niacinate and chromium picolinate supplementation on lipid peroxidation, TNF-alpha, IL-6, CRP, glycated hemoglobin, triglycerides, and cholesterol levels in blood of streptozotocin-treated diabetic rats. Free Radic Biol Med. 2007;43:1124–31. doi: 10.1016/j.freeradbiomed.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hazane-Puch F, Benaraba R, Valenti K, Osman M, Laporte F, Favier A, Anderson RA, Roussel AM, Hininger-Favier I. Chromium III histidinate exposure modulates gene expression in HaCaT human keratinocytes exposed to oxidative stress. Biol Trace Elem Res. 2010;137:23–39. doi: 10.1007/s12011-009-8557-9. [DOI] [PubMed] [Google Scholar]
- 79.Dong F, Yang X, Sreejayan N, Ren J. Chromium (D-phenylalanine)3 improves obesity-induced cardiac contractile defect in ob/ob mice. Obesity (Silver Spring) 2007;15:2699–711. doi: 10.1038/oby.2007.322. [DOI] [PubMed] [Google Scholar]
- 80.Lai MH. Antioxidant effects and insulin resistance improvement of chromium combined with vitamin C and e supplementation for type 2 diabetes mellitus. J Clin Biochem Nutr. 2008;43:191–8. doi: 10.3164/jcbn.2008064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cheng HH, Lai MH, Hou WC, Huang CL. Antioxidant effects of chromium supplementation with type 2 diabetes mellitus and euglycemic subjects. J Agric Food Chem. 2004;52:1385–9. doi: 10.1021/jf035074j. [DOI] [PubMed] [Google Scholar]
- 82.Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003;546:113–20. doi: 10.1016/s0014-5793(03)00560-x. [DOI] [PubMed] [Google Scholar]
- 83.Kemp BE, Stapleton D, Campbell DJ, Chen ZP, Murthy S, Walter M, Gupta A, Adams JJ, Katsis F, van Denderen B, Jennings IG, Iseli T, Michell BJ, Witters LA. AMP-activated protein kinase, super metabolic regulator. Biochem Soc Trans. 2003;31:162–8. doi: 10.1042/bst0310162. [DOI] [PubMed] [Google Scholar]
- 84.Kudo N, Barr AJ, Barr RL, Desai S, Lopaschuk GD. High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5′-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J Biol Chem. 1995;270:17513–20. doi: 10.1074/jbc.270.29.17513. [DOI] [PubMed] [Google Scholar]
- 85.Winder WW, Hardie DG. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol. 1996;270:E299–304. doi: 10.1152/ajpendo.1996.270.2.E299. [DOI] [PubMed] [Google Scholar]
- 86.Rutter GA, Da Silva Xavier G, Leclerc I. Roles of 5′-AMP-activated protein kinase (AMPK) in mammalian glucose homoeostasis. Biochem J. 2003;375:1–16. doi: 10.1042/BJ20030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao P, Wang J, Ma H, Xiao Y, He L, Tong C, Wang Z, Zheng Q, Dolence EK, Nair S, Ren J, Li J. A newly synthetic chromium complex-chromium (D- phenylalanine)3 activates AMP-activated protein kinase and stimulates glucose transport. Biochem Pharmacol. 2009;77:1002–10. doi: 10.1016/j.bcp.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 88.Rains JL, Jain SK. Hyperketonemia decreases mitochondrial membrane potential and its normalization with chromium (III) supplementation in monocytes. Mol Cell Biochem. 2011;349:77–82. doi: 10.1007/s11010-010-0662-8. [DOI] [PubMed] [Google Scholar]