Abstract
Rationale
Previous workers have demonstrated that controlled mechanical ventilation (CMV) results in diaphragm inactivity and elicits a rapid development of diaphragm weakness due to both contractile dysfunction and fiber atrophy. Limited data exist regarding the impact of pressure support ventilation (PSV),a commonly used mode of mechanical ventilation--that permits partial mechanical activity of the diaphragm—on diaphragm structure and function.
Objectives
We carried out the present study to test the hypothesis that high level PSV decreases the diaphragm pathology associated with CMV.
Methods
Sprague-Dawley rats were randomly assigned to one of the following five groups:1) control (no mechanical ventilation); 2) 12 hours of CMV (12CMV); 3) 18 hours of CMV (18CMV); 4) 12 hours of PSV (12PSV); or 5) 18 hours of PSV (18PSV).
Measurements and main results
We carried out the following measurements on diaphragm specimens: 4-hydroxynonenal (HNE)—a marker of oxidative stress, active caspase-3 (casp-3),active calpain-1 (calp-1), fiber type cross-sectional area (CSA), and specific force (sp F). Compared to control, both 12PSV and 18PSV promoted a significant decrement in diaphragmatic specific force production, but to a lesser degree than 12CMV and 18CMV. Further, 12CMV, 18PSV, and 18CMV resulted in significant atrophy in all diaphragm fiber types, as well as significant increases in a biomarker of oxidative stress (4-HNE) and increased proteolytic activity (20S proteasome, calpain-1, and caspase-3). Further, while no inspiratory effort occurs during CMV, it was observed that PSV resulted in large decrement, ~96%, in inspiratory effort compared to spontaneous breathing animals.
Conclusion
High levels of prolonged PSV promotes diaphragmatic atrophy and contractile dysfunction. Further, similar to CMV, PSV-induced diaphragmatic atrophy and weakness is associated with both diaphragmatic oxidative stress and protease activation. .
Keywords: disuse muscle atrophy, controlled mechanical ventilation, pressure support ventilation, assist ventilation, atrophy
INTRODUCTION
During controlled mechanical ventilation (CMV) the diaphragm is rendered completely inactive, resulting in a rapid onset of diaphragmatic atrophy and contractile dysfunction in both animals and humans (1–5). Indeed, as few as 12–18 hours of CMV results in significant diaphragm atrophy and contractile dysfunction in both laboratory animals and humans, and 25% of all patients admitted to the adult ICU receive mechanical ventilation (MV) for over 12 hours (2–4, 6–10). Strong evidence now indicates a causallink between CMV and diaphragm pathology (2, 4, 5) and diaphragmatic weakness is thought to be an important contributor to the difficulties in weaning patients from mechanical ventilation (2). At the present time, weaning accounts for up to 40% of the total time spent on the ventilator and this prolongation of time on the ventilator is associated with increased morbidity, mortality, and large increases in healthcare costs (9, 11). Therefore, developing strategies to protect the diaphragm from ventilator-induced weakness is imperative.
CMV-induced diaphragmatic atrophy and weakness occurs primarily due to accelerated protein degradation (2, 12–14). During CMV, diaphragmatic protein breakdown occurs through the degradation of myofibrillar proteins via the ATP-dependent ubiquitin-proteasomepathway (10, 15, 16). However, the ubiquitin-proteasome cannot release and degrade intact actomyosin complexes, and thus myofilaments must be released from the sarcomere to bedegraded by the proteasome system (17–19). This actomyosin release from the sarcomere is achieved by cleavage of structural proteins by the activation of both calpain and caspase-3. Indeed, our work indicates that CMV-induced activation of calpain and caspase-3 is essential, and possibly the rate limiting step, for CMV-induced diaphragmatic atrophy (12, 13, 17, 20). Additionally, recent evidence from our laboratory demonstrates that oxidative stress is an upstream trigger required for CMV-induced activation of calpain and caspase-3 in the diaphragm (6).
Pressure support ventilation (PSV) is a commonly used mode of partial ventilatory support in countries around the world (9, 21). During PSV the patient triggers each breath and the ventilator delivers a flow up to a preset pressure limit (22–24). Therefore, unlike CMV which renders the diaphragm completely inactive, PSV can permit inspiratory muscle activation which is lower than spontaneous breathing (22). Although many studies have investigated the impact of CMV on diaphragmatic proteolysis and weakness, few data exist regarding the impact of other commonly used modes of MV on diaphragmatic oxidative stress, protease activation, and contractile function. To address this gap in our knowledge, we tested the hypothesis that compared to CMV, animals exposed to high levels of prolonged PSV will exhibit less diaphragmatic oxidative stress, protease activation, atrophy, and contractile dysfunction.
METHODS
Animals
These experiments were approved by the University of Florida (Gainesville, FL) Animal Use Committee. Sprague-Dawley rats(4 months old) were randomly assigned to one of five groups, (n=8 per group): 1) an acutely anesthetized control group (CON) with no MV, 2) 12-hours controlled MV group (12CMV), 3) 18-hours controlled mechanical ventilation group (18CMV), 4) 12-hours pressure support ventilation group (12PSV), 5) 18-hours pressure support ventilation group (18PSV).
Acutely Anaesthetized Controls
Control animals were subjected to an acute plane of surgical anesthesia with an IP injection of sodium pentobarbital (60 mg/kg body weight).
Mechanical Ventilation Protocol
All surgical procedures were performed using aseptic techniques. Animals in both the CMV and PSV groups were anesthetized with an intraperitoneal (IP) injection of sodium pentobarbital (60 mg/kg body weight), tracheostomized, and mechanically ventilated (Servo Ventilator 300, Siemens) for 12 or 18 hours. During CMV animals were ventilated with time-triggered, pressure-limited ventilation. Upper airway pressure limit did not exceed 12 cmH2O above PEEP and was typically set at ~8 cmH2O above PEEP to maintain blood gas homeostasis and tidal volumes from 5–8 mL/kg. Respiratory rate was set at 80 bpm, inspiratory rise time was set at 5%, and PEEP was 1 cmH2O. During PSV, animals were ventilated with pressure-triggered, pressure-limited ventilation using identical ventilator settings as the animals in the CMV group.
Upon completion of MV, the diaphragm was quickly removed and a strip of the medial costal diaphragm was used for in vitro contractile measurements and the remaining portion was frozen in liquid nitrogen and stored at −80°C for
Measurement of diaphragmatic time-tension index (TTI)
To evaluate the level of support applied by the ventilator during PSV we measured the time-tension index (TTI) of the diaphragm in both spontaneous breathing rats and animals undergoing PSV. In this regard, the TTI has been used to estimate both the mechanical load and energy utilization of the diaphragm during a variety of experimental conditions (25). Briefly, we measured the inspiratory pressure generated and the temporal components of the breathing cycle in four rats that were switched back and forth between spontaneous breathing and PSV. This was achieved using a device described by Mortola and colleagues (26) that was connected to a non-breathing valve (Hans Rudolf). The generated inspiratory pressures in the upper airway were measured using a pressure transducer (Auto Tran, USA); these values reflect the peak negative inspiratory pressure generated prior to triggering the ventilator. Using mean values for 30–50 breathing cycles the TTI of the diaphragm was computed as (Ti/Ttot)x(Pneg/Pimax) where Ti is the inspiratory time, Ttot is the total duration of the respiratory cycle, Pneg is inspiratory pressure and Pmax is the estimated maximal inspiratory pressure generated by the respiratory system in rats (i.e., −80 cmH2O) (27).
Biochemical Measures
Plasma Cytokines
It is well-established that circulating cytokines contribute to muscle wasting in diseases such as cancer-mediated cachexia (28–31). Further, it appears feasible that mechanical ventilation-induced lung injury could result in increased cytokine production in the lung, and subsequently promote an increase in circulating cytokines during prolonged MV (32–35). At present, it is currently unknown if either PSV and/or CMV result in increased circulating cytokines. Therefore, we measured the levels of circulating cytokines (i.e., KC, IL-6, IL-1β, TNF-α, and IFN-γ) in the plasma of CON rats and in animals following 18PSV and 18CMV. Plasma analysis was performed by the Procarta® Cytokine Assay Service (Affymetrix, Santa Clara, CA).
Western blot analysis
4-Hydroxynonenal (4-HNE; Abcam) was probed as abiomarker of oxidative stress in the diaphragm, while proteolyticactivity was assessed by analysis of cleaved (active) calpain-1(Cell Signaling) and cleaved (active) caspase-3 (Cell Signaling).
20S Proteasome Activity
The in vitro chymotrypsin-like activity of the 20S proteasomewas measured fluorometrically, using techniques described by Stein et al. (36).
Measurement of In Vitro Diaphragmatic Contractile Properties
Upon sacrifice, a diaphragm muscle strip, including the tendinous attachments at the central tendon and rib cage was dissected from the midcostal region. The muscle strip was suspended vertically with one end connected to an isometric force transducer (model FT-03, Grass Instruments, Quincy, MA) within a jacketed tissue bath and diaphragm skeletal muscle contractile properties were measured (4).
Histological Measures
Myofiber Cross-Sectional Area
Sections from frozen diaphragm samples were cut at 10 microns using a cryotome (Shandon Inc., Pittsburgh, PA) and stained as described previously (12). CSA was determined using Scion software (NIH).
Statistical Analysis
Comparisons between groups for each dependent variable weremade by a one-way ANOVA, and, when appropriate, Tukey's honestlysignificant difference test was performed post hoc. Significancewas established at P < 0.05. Data are presented as means± SE.
RESULTS
Systemic, biological, and cytokine response to CMV and high level PSV
There were no significant differences in body weight betweenthe groups (0.297 ± 0.005, 0.299 ± 0.004, 0.304 ± 0.005, 0.304 ± 0.004, and 0.300 ± 0.002 kg) for CON, 12PSV, 12CMV, 18PSV, and 18CMV respectively)before initiation of MV. Heart rate (300–420beats/min), systolic blood pressure (70–130 mmHg), andcolonic (body) temperature (36–37°C) were maintainedrelatively constant during both the 12 and 18 hours of CMV and PSV. In addition, PaO2 and PaCO2 were maintained relatively constantduring CMV and PSV: PaO2 ranged from 60–89 Torr, and PaCO2 rangedfrom 34–46 Torr. Importantly, therewere no significant differences between experimental groupsin any of these measures at the completion of 12 or 18 hours of CMV or PSV. Further, there were no differences in plasma levels of KC, IL-6, IL-1β, TNF-α, or IFN-γ between CON, 18PSV, and 18CMV (Table 1).
Table 1.
Levels of plasma cytokines for CON (n=12), 18PSV (n=5), and 18CMV (n=7).
| Parameter (pg mL−1) | CON | 18PSV | 18CMV |
|---|---|---|---|
| KC | 276.2 ± 16.14 | 243.5 ± 23.42 | 230.1 ± 19.67 |
| IL-6 | 44.76 ± 7.909 | 46.09 ± 22.25 | 46.91± 8.985 |
| IL-1β | 191.6 ± 21.35 | 104.0 ± 28.38 | 158.9 ± 25.95 |
| TNF-α | 25.66 ± 2.187 | 16.10 ± 1.659 | 20.74 ± 3.301 |
| IFN-γ | 54.69 ± 6.935 | 46.10 ± 9.446 | 56.49 ± 12.38 |
Values are mean ± SEM.
Significantly different (P<0.05)
Assessment of diaphragmatic activity during high level PSV using TTI
To estimate the level of diaphragmatic activation in our experiments, we measured the TTI in both animals undergoing PSV and spontaneously breathing animals (Table 2). Note that the observed TTI in the spontaneous breathing animals is comparable to values reported by others (27). Also, notice that compared to PSV animals, the TTI was significantly higher in the spontaneously breathing animals.
Table 2.
Respiratory measurements from a sample of PSV and spontaneous breathing animals (n=4).
| PSV | Spontaneous Breathing | |
|---|---|---|
| Inspiratory Pressure (cmH2O) | −0.22 ± 0.025 | −1.36 ± 0.24 |
| Inspiratory Time (msec) | 120 ± 13 | 570 ± 49 |
| Cycle Time (msec) | 1180 ± 14.4 | 840 ± 57.8 |
| Time Tension Index | 0.0003 ± 0.000063 | 0.008 ± 0.0008 |
Values are mean ± SEM.
Significantly different (P<0.05)
Oxidative Stress
We measured 4-HNE as a biomarker of oxidative damage in the diaphragm. 4-HNE is the primary adduct formed during the lipid peroxidation cascade and 4-HNE has been reported to be a reliable marker used to assess oxidative stress and damage in tissue (37). Diaphragmatic 4-HNE was significantly increased in12CMV (39%), 18PSV (29%), and 18CMV (43%) compared to control (Figure 1). Further, both 12CMV and 18CMV produced a significant increase in 4-HNE modified proteins compared to 12PSV (Figure 1).
Figure 1.
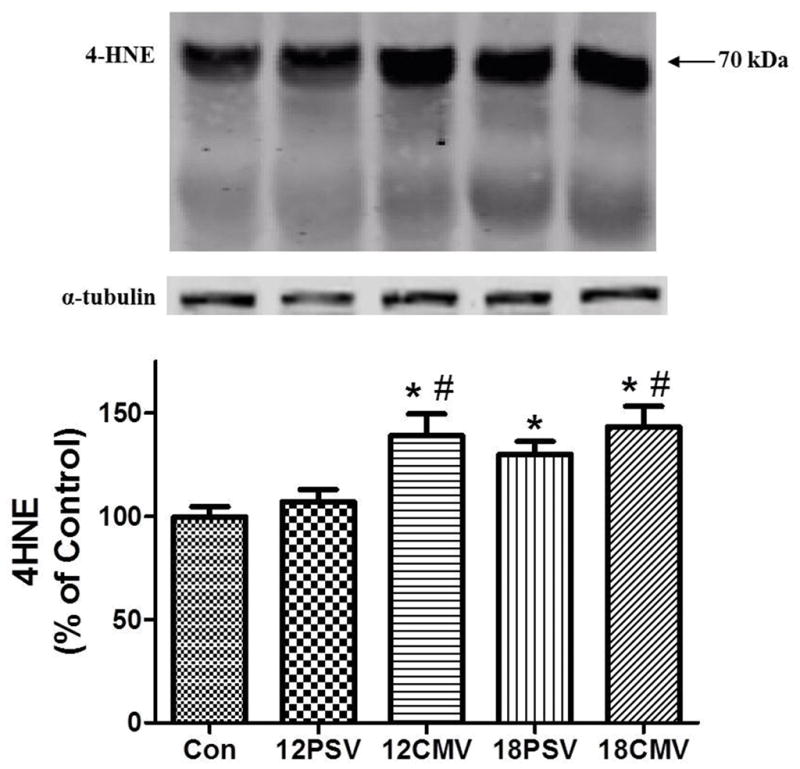
4-HNE accumulation (percentage of control) in diaphragm samples. Values are means ± SE. *Significantly different vs. CON (P < 0.05). #Significantly different vs. 12PSV (P < 0.05).
20S proteasome activity
We measured 20S proteasome activity as a marker of proteolysis. Chymotryspin-like 20S proteasome activity was significantly increased in 12CMV (36%), 18PSV (73%), and 18CMV (73%) (Figure 2). Further, chymotryspin-like 20S proteasome activity was significantly increased with longer durations of both pressure support ventilation (18PSV) and prolonged controlled mechanical ventilation (18CMV) compared to 12PSV (Figure 2).
Figure 2.
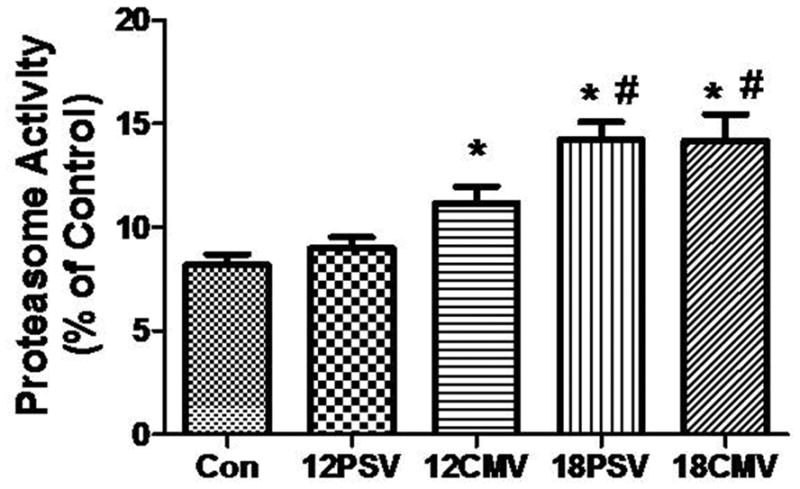
Chymotryspin-like 20S proteasome activity in diaphragm samples. Values are means ± SE. *Significantly different vs. CON (P < 0.05). #Significantly different vs. 12PSV (P < 0.05).
Calpain and Caspase-3 Activation
The dissociation of actomyosin complexes occurs through activated proteases cleaving structural myofilament proteins (17–19). Recent work indicates that oxidative stress is required for MV-induced protease activation in the diaphragm (6). Thus, because of the MV-induced increases in diaphragmatic 4-HNE levels, we also measured the levels of calpain and caspase-3 activity. This was achieved via Western blotting by assessment of the cleaved and active bands ofcalpain-1 and caspase-3. Calpain-1 activity in the diaphragm was significantly increased in 12CMV (59%), 18PSV (52%), and 18CMV (75%) compared to control (Figure 3). Additionally, calpain-1 was significantly elevated in 12CMV and 18CMV compared to 12PSV (Figure 3).
Figure 3.
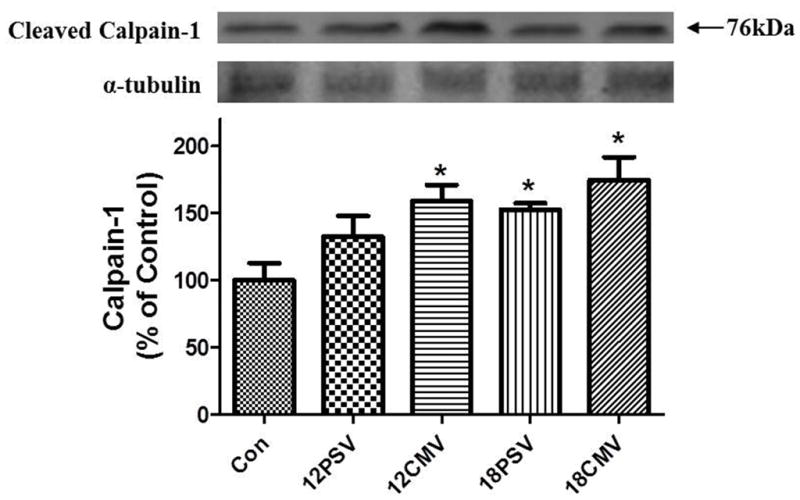
Active calpain 1 protein levels in diaphragm muscle expressed as percentage of control. Values are means ± SE. *Significantly different vs. CON (P < 0.05).
Interestingly, compared to control, calpain-1 is activated in the diaphragm after 12 hours of CMV but longer durations (e.g., 18 hours) are required for calpain-1 activation in the diaphragm during PSV. Caspase-3 is activated through the cleavage of procaspase-3 and a significant increase in the 19-kDa cleaved (active) caspase-3 protein was observed in the diaphragm of 12CMV (37%), 18PSV (75%), and 18CMV (119%) animals compared to control(P < 0.05). Further, prolonged PSV (18PSV) resulted in a significant increase in active caspase-3 in the diaphragm compared to 12PSV, and prolonged CMV (18CMV) resulted in a significant increase in active caspase-3 compared to both 12PSV and 12CMV (Figure 4).
Figure 4.
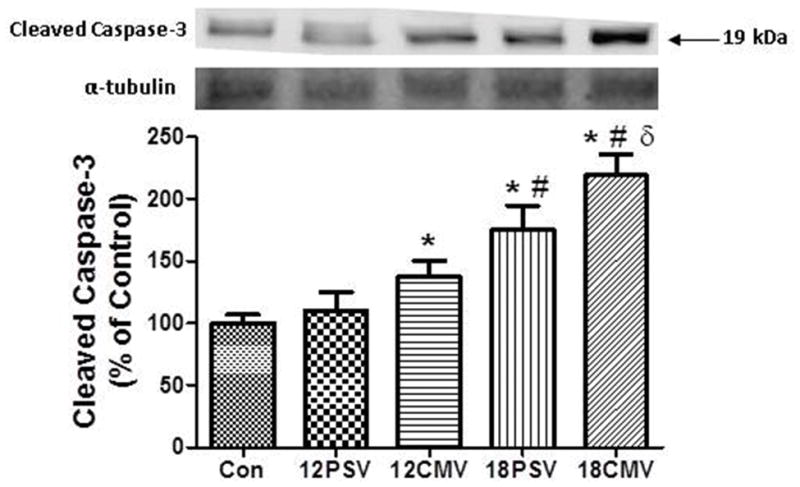
Active caspase-3 protein levels in diaphragm muscle expressed as percentage of control. Values are means ± SE. *Significantly different vs. CON (P < 0.05). #Significantly different vs. 12PSV (P < 0.05). δSignificantly different vs. 12CMV.
Diaphragmatic Myofiber Atrophy
Increased proteolysis and decreased rates of protein synthesis during MV results in atrophy of diaphragm myofibers (3, 38, 39). In the current experiments, diaphragm myofiber CSA was determined in all groups (Figure 5). Compared to control, 12PSV resulted in significant diaphragmatic atrophy only in type IIx/IIb diaphragm myofibers. In contrast, 12CMV, 18PSV, and 18CMV promoted atrophy of type I, type IIa, andtype IIx/IIb diaphragm myofibers. However, note that compared to control, the relative proportions of fiber types did not change due to CMV or PSV. Therefore, although prolonged CMV and PSV both promote diaphragmatic atrophy, the speed of PSV-induced diaphragmatic atrophy occurs at a slower rate than CMV.
Figure 5.
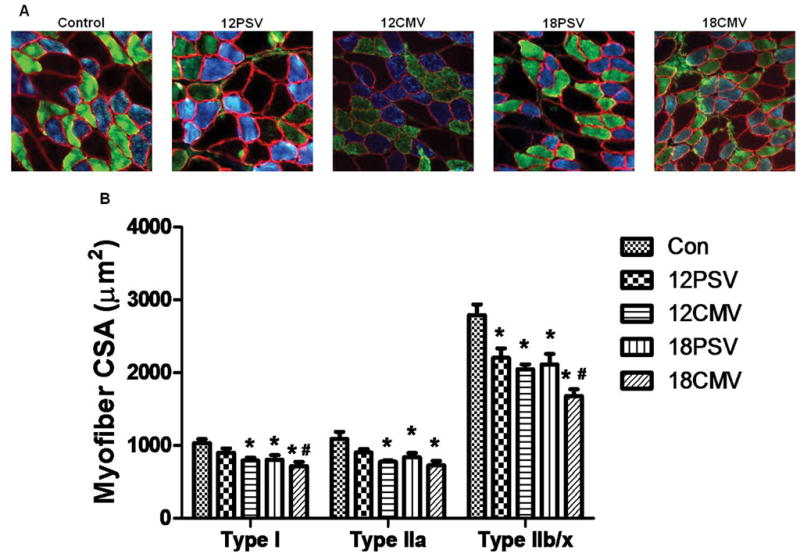
Fiber cross-sectional area (CSA) in diaphragm skeletal muscle myofibers expressing myosin heavy chain (MHC) I (type I), MHC IIa (type IIa), and MHC IIx/IIb (type IIx/IIb). A: representative fluorescent staining of MHC I (DAPI filter/blue), MHC IIa (FITC filter/green), and dystrophin (rhodamine filter/red) proteins in diaphragm samples from CON, 12PSV, 12CMV, 18PSV, and 18CMV. B: type I, type IIa, and type IIx/IIb fiber CSA. Values are means ± SE. *Significantly decreased vs. CON (P < 0.05). #Significantly decreased vs. 12PSV (P < 0.05).
Diaphragmatic Contractile Dysfunction
In addition to diaphragmatic myofiber atrophy, the protease-mediated cleavage of intact myofilaments and dissociation of actomyosin complexes in the diaphragm can negatively impact contractile function. Therefore, we also evaluated the impact of prolonged PSV and CMV on diaphragmatic contractile performance. Compared to control, diaphragmatic contractile performance was significantly depressed at most stimulation frequencies in the12PSV, 18PSV, 12CMV, and 18CMV groups (Figure 6). Also, note that maximal isometrictwitch force was significantly reduced by 9.8% following 12PSV and 16.7% following 18PSV, while significant twitch decreases of 26.9% and 28.5% occurred following 12CMV and 18CMV, respectively.
Figure 6.
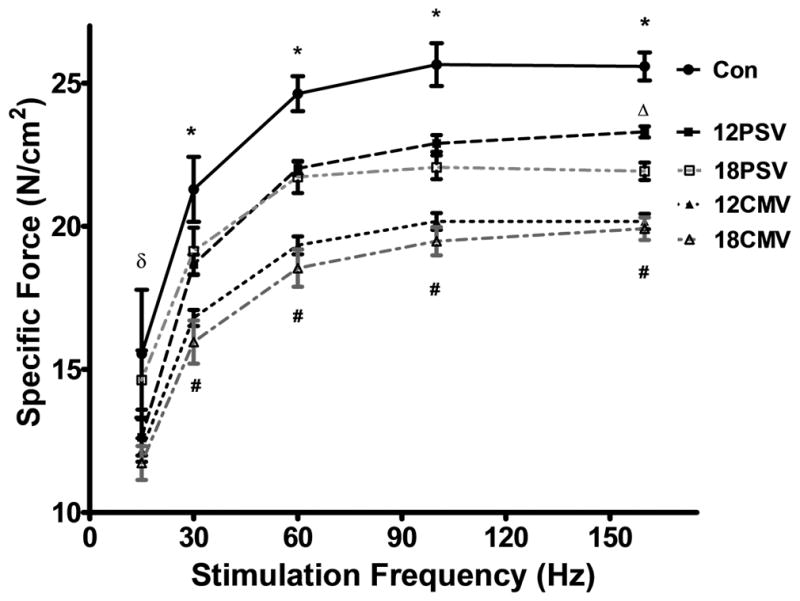
Diaphragmatic force-frequency response (in vitro) of diaphragm samples. Values are mean ± SE. *12PSV, 18PSV, 12CMV, and 18CMV significantly decreased vs. CON (P < 0.05). #12CMV and 18CMV significantly decreased vs. 12PSV and 18PSV (P<0.05). δ18PSV, 12CMV, 18CMV significantly decreased vs. control and 12PSV (P<0.05). Δ18PSV significantly decreased vs. 12PSV.
DISCUSSION
Overview of Principal Findings
It is well established that prolonged CMV results in a rapid onsetof proteolysis that contributes to diaphragmatic atrophy and weakness. However, little is known about the effects of other commonly used modes of MV on diaphragmatic atrophy and weakness. The current study provides important new and important information regarding the effects of a commonly used mode of partial ventilatory support (i.e., PSV). Although Futier et al. have previously examined the impact of PSV on ventilator-induced diaphragm proteolysis and protein synthesis, this is the first study to demonstrate that high levels of prolonged PSV results in significant diaphragmatic atrophy and contractile dysfunction (41). Similar to CMV, high level PSV-induced diaphragmatic atrophy is accompanied by an increase in both oxidative stress and protease activation, with no change in plasma cytokines. This is significant because oxidative stress and protease activation (e.g., calpain and caspase-3) in the diaphragm are both requirements for MV-induced diaphragmatic weakness (6, 10, 13, 16, 42, 43). Hence, the current experiments reveal that the mechanisms involved in PSV-induced diaphragmatic atrophy appear similar to those previously reported for CMV.. A detailed discussion of these findings and other important results follow.
Both CMV and high level PSV promote diaphragmatic atrophy
Numerous studies indicate that prolonged CMV leads to rapid diaphragmatic atrophy in all fiber types of both animals and humans (3, 6–8, 44). Similarly, in the current experiments, as few as 12 hours of CMV resulted in a significant reduction in the CSA of all diaphragm fiber types (Fig. 5). In contrast, 12 hours of PSV resulted in atrophy of only diaphragmatic type IIx/b fibers. Compared to control, both 18 hours of PSV and CMV result in significant diaphragmatic muscle fiber atrophy in all fiber types compared (Fig.5).
High level PSV promotes diaphragmatic contractile dysfunction but at a slower rate than CMV
Many studies indicate that prolonged CMV results in diaphragmatic contractile dysfunction in both humans and other animals (3, 6, 7, 45–49). However, to our knowledge, this study is the first study to demonstrate that PSV also promotes diaphragmatic contractile dysfunction. Interestingly, compared to control animals, both 12 and 18 hours of high level PSV promote significant diaphragmatic contractile dysfunction over a wide range of stimulation frequencies (Fig. 6). However, when compared to the same duration of CMV, the magnitude of the diaphragmatic contractile dysfunction observed during PSV was significantly lower at all stimulation frequencies. For example, compared to control, 18 hours of PSV resulted in an ~16 percent decrease in diaphragmatic maximal tetanic specific force production whereas 18 hours of CMV resulted in an ~24 percent reduction in maximal diaphragmatic specific force production (Fig. 6).
To date, only one published report has documented the impact of a partial support mode of mechanical ventilation on diaphragmatic contractile dysfunction. Specifically, Sassoon et al. reported that 72 hours of assist control MV in rabbits results in a small but insignificant decrease in diaphragmatic contractile dysfunction (46). The explanation for the divergent results between the present study and the Sassoon et al. report is not clear but could be due to differing levels of diaphragmatic contractile activity between the two studies. Indeed, current evidence suggests that the magnitude of MV-induced diaphragmatic weakness is largely dependent upon the level of diaphragmatic inactivity during prolonged MV. For example, Gayan-Ramirez et al. demonstrated that short periods (i.e., 5-min per hour) of intermittent spontaneous breathing during prolonged CMV can partially attenuate CMV-induced contractile dysfunction (50). Thus, diaphragmatic contractile activity during MV appears to be inversely related to the level of MV-induced diaphragmatic weakness. To determine how much PSV reduced diaphragmatic activation below the level of activity during spontaneous breathing, we estimated the magnitude of diaphragmatic activity during both PSV and spontaneous breathing by calculating the TTI (27). Compared to the TTI of spontaneous breathing animals, the TTI of PSV animals was reduced by ~96 percent (Table 2). These findings indicate that the pressure-triggered pressure support settings used in the current study resulted in large reductions in diaphragmatic activity compared to spontaneous breathing. Currently it is unclear in the literature as to the precise effort of breathing in patients being mechanically ventilated with PSV. The possibility exists that a 96% reduction in TTI is not representative of the majority patients ventilated with PSV, and could potentially represent a “high level” of pressure support. However, due to a lack of clinical studies in the area it is difficult to clearly identify what “level” of pressure support the present study utilized. Nonetheless, the present study followed the mechanical ventilator manufacturer’s (Siemens) guidelines for setting and adjusting trigger sensitivity, and thus likely represents the settings commonly used clinically. However, it should be noted that that ‘lower’ levels of PSV (ie. the patient performs more of the inspiratory effort) likely results in larger differences in levels of diaphragm dysfunction and atrophy between PSV and CMV.
Both CMV and high level PSV are associated with diaphragmatic oxidative stress and protease activation
Prolonged CMV promotes a rapid onset of diaphragmatic oxidative damage resulting in oxidation of numerous cellular components including contractile proteins (3, 6, 7, 42, 51, 52). This is important because prevention of MV-induced oxidative stress via antioxidants rescues the diaphragm from protease activation (i.e., calpain and caspase-3), atrophy, and contractile dysfunction (6, 10, 43). Indeed, MV-induced oxidative stress is a required upstream signal to activate proteases and promote the loss of diaphragm muscle protein (6, 10, 43). Therefore, to determine if the magnitude of diaphragmatic oxidative damage differs between prolonged bouts of PSV and CMV, we assessed diaphragmatic levels of 4-HNE-conjugated proteins as a biomarker of oxidative stress. Our findings reveal that compared to control, diaphragmatic levels of 4-HNE were significantly elevated in all of the mechanical ventilation groups except those animals exposed to 12 hours of PSV (Fig. 1). Similarly, diaphragmatic protease activities for animals ventilated for 12 hours using PSV did not differ from control (Fig. 2–4). In contrast, diaphragmatic activities of calpin-1, caspase-3, and the 20S proteasome were significantly higher in the 12CMV, 18PSV, and 18CMV groups compared to control (Fig. 2–4). Collectively, these findings are consistent with the notion that oxidative stress is a requirement for MV-induced protease activation in the diaphragm. However, it is interesting that while 12 hours of PSV was not associated with diaphragmatic oxidative damage or protease activation, 12 hours of PSV did promote diaphragmatic atrophy and contractile dysfunction. While our data does not provide a definitive mechanism to explain these findings, several possibilities exist. First, it is possible that 12 hours of PSV did result in disturbed diaphragmatic redox balance but the measurement of 4-HNE-protein adducts were not sufficient to detect small changes in diaphragmatic redox balance. Further, the possibility exists that small increases in protease activity occurred in the diaphragms of animals exposed to 12 hours of PSV but our measurements of protease activation were not sensitive enough to detect these changes. In support of both of these possibilities, note that compared to control, the mean diaphragmatic levels of 4HNE and calpain I activity tended to be higher in the animals exposed to 12 hours of PSV. Finally, the possibility also exists that PSV resulted in decreased diaphragm protein synthesis without an increase in proteolysis and this imbalance in protein turnover was responsible for the myofiber atrophy that occurred in the type IIx/b fibers in the diaphragm of animals exposed to 12 hours of PSV.
Conclusions
The present experiments provide new and important information regarding the impact of different modes of mechanical ventilation on diaphragmatic weakness and reveal that prolonged high level PSV promotes diaphragmatic atrophy and contractile dysfunction. Further, similar to CMV, high level PSV-induced diaphragmatic atrophy is associated with both diaphragmatic oxidative stress and protease activation.
Supplementary Material
Acknowledgments
This study was funded, in part, by the National Institutes of Health.
This work was supported by the National Institutes of Health awarded to S.K. Powers (RO1 HL780839) and Deutsche Forschungsgemeinschaft (BR 3998/1-1) to CS Bruells
Footnotes
The authors have not disclosed any potential conflicts of interest.
References
- 1.Jaber S, Petrof BJ, Jung B, et al. Rapidly Progressive Diaphragmatic Weakness and Injury during Mechanical Ventilation in Humans. Am J Respir Crit Care Med. doi: 10.1164/rccm.201004-0670OC. [DOI] [PubMed] [Google Scholar]
- 2.Levine S, Nguyen T, Taylor N, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358(13):1327–1335. doi: 10.1056/NEJMoa070447. [DOI] [PubMed] [Google Scholar]
- 3.Powers SK, Kavazis AN, Levine S. Prolonged mechanical ventilation alters diaphragmatic structure and function. Crit Care Med. 2009;37(10 Suppl):S347–353. doi: 10.1097/CCM.0b013e3181b6e760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powers SK, Shanely RA, Coombes JS, et al. Mechanical ventilation results in progressive contractile dysfunction in the diaphragm. J Appl Physiol. 2002;92(5):1851–1858. doi: 10.1152/japplphysiol.00881.2001. [DOI] [PubMed] [Google Scholar]
- 5.Shanely RA, Zergeroglu MA, Lennon SL, et al. Mechanical ventilation-induced diaphragmatic atrophy is associated with oxidative injury and increased proteolytic activity. Am J Respir Crit Care Med. 2002;166(10):1369–1374. doi: 10.1164/rccm.200202-088OC. [DOI] [PubMed] [Google Scholar]
- 6.Whidden MA, Smuder AJ, Wu M, et al. Oxidative stress is required for mechanical ventilation-induced protease activation in the diaphragm. J Appl Physiol. 2010;108(5):1376–1382. doi: 10.1152/japplphysiol.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whidden MA, McClung JM, Falk DJ, et al. Xanthine oxidase contributes to mechanical ventilation-induced diaphragmatic oxidative stress and contractile dysfunction. J Appl Physiol. 2009;106(2):385–394. doi: 10.1152/japplphysiol.91106.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gayan-Ramirez G, Decramer M. Effects of mechanical ventilation on diaphragm function and biology. Eur Respir J. 2002;20(6):1579–1586. doi: 10.1183/09031936.02.00063102. [DOI] [PubMed] [Google Scholar]
- 9.Esteban A, Ferguson ND, Meade MO, et al. Evolution of mechanical ventilation in response to clinical research. Am J Respir Crit Care Med. 2008;177(2):170–177. doi: 10.1164/rccm.200706-893OC. [DOI] [PubMed] [Google Scholar]
- 10.Betters JL, Criswell DS, Shanely RA, et al. Trolox attenuates mechanical ventilation-induced diaphragmatic dysfunction and proteolysis. Am J Respir Crit Care Med. 2004;170(11):1179–1184. doi: 10.1164/rccm.200407-939OC. [DOI] [PubMed] [Google Scholar]
- 11.Dasta JF, McLaughlin TP, Mody SH, et al. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med. 2005;33(6):1266–1271. doi: 10.1097/01.ccm.0000164543.14619.00. [DOI] [PubMed] [Google Scholar]
- 12.McClung JM, Kavazis AN, DeRuisseau KC, et al. Caspase-3 regulation of diaphragm myonuclear domain during mechanical ventilation-induced atrophy. Am J Respir Crit Care Med. 2007;175(2):150–159. doi: 10.1164/rccm.200601-142OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maes K, Testelmans D, Powers S, et al. Leupeptin inhibits ventilator-induced diaphragm dysfunction in rats. Am J Respir Crit Care Med. 2007;175(11):1134–1138. doi: 10.1164/rccm.200609-1342OC. [DOI] [PubMed] [Google Scholar]
- 14.Shanely RA, Van Gammeren D, Deruisseau KC, et al. Mechanical ventilation depresses protein synthesis in the rat diaphragm. Am J Respir Crit Care Med. 2004;170(9):994–999. doi: 10.1164/rccm.200304-575OC. [DOI] [PubMed] [Google Scholar]
- 15.DeRuisseau KC, Kavazis AN, Deering MA, et al. Mechanical ventilation induces alterations of the ubiquitin-proteasome pathway in the diaphragm. J Appl Physiol. 2005;98(4):1314–1321. doi: 10.1152/japplphysiol.00993.2004. [DOI] [PubMed] [Google Scholar]
- 16.McClung JM, Whidden MA, Kavazis AN, et al. Redox regulation of diaphragm proteolysis during mechanical ventilation. Am J Physiol Regul Integr Comp Physiol. 2008 doi: 10.1152/ajpregu.00044.2008. [DOI] [PubMed] [Google Scholar]
- 17.Goll DE, Thompson VF, Li H, et al. The calpain system. Physiol Rev. 2003;83(3):731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 18.Tidball JG, Spencer MJ. Expression of a calpastatin transgene slows muscle wasting and obviates changes in myosin isoform expression during murine muscle disuse. J Physiol. 2002;545(Pt 3):819–828. doi: 10.1113/jphysiol.2002.024935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams AB, Decourten-Myers GM, Fischer JE, et al. Sepsis stimulates release of myofilaments in skeletal muscle by a calcium-dependent mechanism. Faseb J. 1999;13(11):1435–1443. doi: 10.1096/fasebj.13.11.1435. [DOI] [PubMed] [Google Scholar]
- 20.Du J, Wang X, Miereles C, et al. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest. 2004;113(1):115–123. doi: 10.1172/JCI200418330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esteban A, Anzueto A, Alia I, et al. How is mechanical ventilation employed in the intensive care unit? An international utilization review. Am J Respir Crit Care Med. 2000;161(5):1450–1458. doi: 10.1164/ajrccm.161.5.9902018. [DOI] [PubMed] [Google Scholar]
- 22.Jolliet P, Tassaux D. Clinical review: patient-ventilator interaction in chronic obstructive pulmonary disease. Crit Care. 2006;10(6):236. doi: 10.1186/cc5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanders RC, Jr, Thurman TL, Holt SJ, et al. Work of breathing associated with pressure support ventilation in two different ventilators. Pediatr Pulmonol. 2001;32(1):62–70. doi: 10.1002/ppul.1090. [DOI] [PubMed] [Google Scholar]
- 24.Baar K, Nader G, Bodine S. Resistance exercise, muscle loading/unloading and the control of muscle mass. Essays Biochem. 2006;42:61–74. doi: 10.1042/bse0420061. [DOI] [PubMed] [Google Scholar]
- 25.Klawitter PF, Clanton TL. Tension-time index, fatigue, and energetics in isolated rat diaphragm: a new experimental model. J Appl Physiol. 2004;96(1):89–95. doi: 10.1152/japplphysiol.00237.2003. [DOI] [PubMed] [Google Scholar]
- 26.Mortola JP, Noworaj A. Two-sidearm tracheal cannula for respiratory airflow measurements in small animals. J Appl Physiol. 1983;55(1 Pt 1):250–253. doi: 10.1152/jappl.1983.55.1.250. [DOI] [PubMed] [Google Scholar]
- 27.Rollier H, Bisschop A, Gayan-Ramirez G, et al. Low load inspiratory muscle training increases diaphragmatic fiber dimensions in rats. Am J Respir Crit Care Med. 1998;157(3 Pt 1):833–839. doi: 10.1164/ajrccm.157.3.9512103. [DOI] [PubMed] [Google Scholar]
- 28.Argilés JM, Busquets S, López-Soriano FJ. The pivotal role of cytokines in muscle wasting during cancer. Int J Biochem Cell Biol. 2005;37(10):2036–2046. doi: 10.1016/j.biocel.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 29.Callahan LA, Supinski GS. Sepsis-induced myopathy. Crit Care Med. 2009;37(10 Suppl):S354–367. doi: 10.1097/CCM.0b013e3181b6e439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deans C, Wigmore SJ. Systemic inflammation, cachexia and prognosis in patients with cancer. Curr Opin Clin Nutr Metab Care. 2005;8(3):265–269. doi: 10.1097/01.mco.0000165004.93707.88. [DOI] [PubMed] [Google Scholar]
- 31.Dodson S, Baracos VE, Jatoi A, et al. Muscle wasting in cancer cachexia: clinical implications, diagnosis, and emerging treatment strategies. Annu Rev Med. 2011;62:265–279. doi: 10.1146/annurev-med-061509-131248. [DOI] [PubMed] [Google Scholar]
- 32.Adams AB, Simonson DA, Dries DJ. Ventilator-induced lung injury. Respir Care Clin N Am. 2003;9(3):343–362. doi: 10.1016/s1078-5337(03)00042-x. [DOI] [PubMed] [Google Scholar]
- 33.Dani C, Bertini G, Pezzati M, et al. Effects of pressure support ventilation plus volume guarantee vs. high-frequency oscillatory ventilation on lung inflammation in preterm infants. Pediatr Pulmonol. 2006;41(3):242–249. doi: 10.1002/ppul.20350. [DOI] [PubMed] [Google Scholar]
- 34.Dhanireddy S, Altemeier WA, Matute-Bello G, et al. Mechanical ventilation induces inflammation, lung injury, and extra-pulmonary organ dysfunction in experimental pneumonia. Lab Invest. 2006;86(8):790–799. doi: 10.1038/labinvest.3700440. [DOI] [PubMed] [Google Scholar]
- 35.Villar J, Herrera-Abreu MT, Valladares F, et al. Experimental ventilator-induced lung injury: exacerbation by positive end-expiratory pressure. Anesthesiology. 2009;110(6):1341–1347. doi: 10.1097/ALN.0b013e31819fcba9. [DOI] [PubMed] [Google Scholar]
- 36.Stein RL, Melandri F, Dick L. Kinetic characterization of the chymotryptic activity of the 20S proteasome. Biochemistry. 1996;35(13):3899–3908. doi: 10.1021/bi952262x. [DOI] [PubMed] [Google Scholar]
- 37.POWERS S, SMUDER A, KAVAZIS A, et al. Experimental Guidelines for Studies Designed to Investigate the Impact of Antioxidant Supplementation on Exercise Performance. International Journal of Sport Nutrition and Exercise Metabolism. 2010;20(1):2–14. doi: 10.1123/ijsnem.20.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deruisseau KC, Kavazis AN, Powers SK. Selective downregulation of ubiquitin conjugation cascade mRNA occurs in the senescent rat soleus muscle. Exp Gerontol. 2005;40(6):526–531. doi: 10.1016/j.exger.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 39.Ventadour S, Attaix D. Mechanisms of skeletal muscle atrophy. Curr Opin Rheumatol. 2006;18(6):631–635. doi: 10.1097/01.bor.0000245731.25383.de. [DOI] [PubMed] [Google Scholar]
- 40.Reid MB. Invited Review: redox modulation of skeletal muscle contraction: what we know and what we don't. J Appl Physiol. 2001;90(2):724–731. doi: 10.1152/jappl.2001.90.2.724. [DOI] [PubMed] [Google Scholar]
- 41.Futier E, Constantin JM, Combaret L, et al. Pressure support ventilation attenuates ventilator-induced protein modifications in the diaphragm. Crit Care. 2008;12(5):R116. doi: 10.1186/cc7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Powers SK, Kavazis AN, McClung JM. Oxidative stress and disuse muscle atrophy. J Appl Physiol. 2007;102(6):2389–2397. doi: 10.1152/japplphysiol.01202.2006. [DOI] [PubMed] [Google Scholar]
- 43.McClung JM, Kavazis AN, Whidden MA, et al. Antioxidant administration attenuates mechanical ventilation-induced rat diaphragm muscle atrophy independent of protein kinase B (PKB Akt) signalling. J Physiol. 2007;585(Pt 1):203–215. doi: 10.1113/jphysiol.2007.141119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McClung JM, Van Gammeren D, Whidden MA, et al. Apocynin attenuates diaphragm oxidative stress and protease activation during prolonged mechanical ventilation. Crit Care Med. 2009;37(4):1373–1379. doi: 10.1097/CCM.0b013e31819cef63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jaber S, Sebbane M, Koechlin C, et al. Effects of short vs. prolonged mechanical ventilation on antioxidant systems in piglet diaphragm. Intensive Care Med. 2005;31(10):1427–1433. doi: 10.1007/s00134-005-2694-1. [DOI] [PubMed] [Google Scholar]
- 46.Sassoon CS, Zhu E, Caiozzo VJ. Assist-control mechanical ventilation attenuates ventilator-induced diaphragmatic dysfunction. Am J Respir Crit Care Med. 2004;170(6):626–632. doi: 10.1164/rccm.200401-042OC. [DOI] [PubMed] [Google Scholar]
- 47.Van Gammeren D, Falk DJ, DeRuisseau KC, et al. Reloading the diaphragm following mechanical ventilation does not promote injury. Chest. 2005;127(6):2204–2210. doi: 10.1378/chest.127.6.2204. [DOI] [PubMed] [Google Scholar]
- 48.Anzueto A, Peters JI, Tobin MJ, et al. Effects of prolonged controlled mechanical ventilation on diaphragmatic function in healthy adult baboons. Crit Care Med. 1997;25(7):1187–1190. doi: 10.1097/00003246-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 49.Radell PJ, Remahl S, Nichols DG, et al. Effects of prolonged mechanical ventilation and inactivity on piglet diaphragm function. Intensive Care Med. 2002;28(3):358–364. doi: 10.1007/s00134-002-1207-8. [DOI] [PubMed] [Google Scholar]
- 50.Gayan-Ramirez G, Testelmans D, Maes K, et al. Intermittent spontaneous breathing protects the rat diaphragm from mechanical ventilation effects. Crit Care Med. 2005;33(12):2804–2809. doi: 10.1097/01.ccm.0000191250.32988.a3. [DOI] [PubMed] [Google Scholar]
- 51.Powers SK, Kavazis AN, DeRuisseau KC. Mechanisms of disuse muscle atrophy: role of oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2005;288(2):R337–344. doi: 10.1152/ajpregu.00469.2004. [DOI] [PubMed] [Google Scholar]
- 52.Zergeroglu MA, McKenzie MJ, Shanely RA, et al. Mechanical ventilation-induced oxidative stress in the diaphragm. J Appl Physiol. 2003;95(3):1116–1124. doi: 10.1152/japplphysiol.00824.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


