Abstract
Purpose
The natural flavonoid fisetin was recently identified as a lead compound that stabilizes endothelial cell microtubules. In this study we investigated the antiproliferative and antiangiogenic properties of fisetin in vitro and in vivo.
Methods
Fisetin cytotoxicity was evaluated using Lewis lung carcinoma cells (LLC), endothelial cells and NIH 3T3 cells. Endothelial cell (EC) migration and capillary-like structure formation were evaluated using EAhy 926 cells. In vivo tumour growth inhibition studies were performed using LLC bearing mice treated with fisetin and/or cyclophosphamide (CPA).
Results
The fisetin IC50 was 59 μM for LLC and 77 μM for EC cells, compared to 210 μM for normal NIH 3T3 cells (24 h). Fisetin inhibited EC migration and capillary-like structure formation at non-cytotoxic concentrations (22–44 μM). In mice, fisetin inhibited angiogenesis assessed using the Matrigel plug assay. In LLC bearing mice, fisetin produced a 67% tumour growth inhibition (223 mg/kg, intraperitoneal), similar to the 66% produced by low dose CPA (30 mg/kg, subcutaneous). When fisetin and CPA were combined, however, a marked improvement in antitumour activity was observed (92% tumour growth inhibition), with low systemic toxicity. Tumour histology showed decreased microvessel density with either fisetin or CPA alone, and a dramatic decrease after the fisetin/CPA combination.
Conclusions
We have shown that fisetin not only displays in vitro and in vivo antiangiogenic properties, but that it can also markedly improve the in vivo antitumour effect of CPA. We propose that this drug combination associating a non-toxic dietary flavonoid with a cytotoxic agent could advantageously be used in the treatment of solid tumours.
Keywords: Angiogenesis Inhibitors; administration & dosage; adverse effects; pharmacology; therapeutic use; Animals; Antineoplastic Agents, Alkylating; administration & dosage; adverse effects; pharmacology; therapeutic use; Antineoplastic Agents, Phytogenic; administration & dosage; adverse effects; pharmacology; therapeutic use; Antineoplastic Combined Chemotherapy Protocols; administration & dosage; adverse effects; pharmacology; therapeutic use; Carcinoma, Lewis Lung; drug therapy; pathology; Cell Cycle; drug effects; Cell Line; Cell Movement; drug effects; Cell Proliferation; drug effects; Cell Survival; drug effects; Cyclophosphamide; administration & dosage; adverse effects; pharmacology; therapeutic use; Endothelial Cells; cytology; drug effects; Female; Flavonoids; administration & dosage; adverse effects; pharmacology; therapeutic use; Humans; Mice; Mice, Inbred C57BL; NIH 3T3 Cells; Neovascularization, Pathologic; drug therapy; Tubulin Modulators; administration & dosage; adverse effects; pharmacology; therapeutic use; Tumor Burden; drug effects
Keywords: flavonoid, fisetin, cyclophosphamide, Lewis lung carcinoma, EA.hy 926, endothelial cells, angiogenesis, cytotoxicity, antitumour activity
Background
Tumour vasculature is an attractive target for cancer therapy because a single vessel provides oxygen and nutrients to numerous tumour cells and is the main route for metastatic dissemination of cancer cells (reviewed in [1]). Tumour angiogenesis is the result of an imbalance between pro-angiogenic factors, e.g., vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), and endogenous antiangiogenic factors, such as angiostatin and endostatin [2–4]. Tumour vasculature can be targeted at the angiogenesis level to prevent the formation of new vessels using antiangiogenic agents, or at the vascular level using vascular disrupting agents on already formed vessels [5,6]. The anti-angiogenesis approach has already proven its clinical effectiveness in colon, breast, and non-small-cell lung cancer using VEGF antibody in combination with cytotoxic drugs [7–9].
Several phytochemicals, or compounds derived from edible plants, have been linked to the chemoprevention of cancer [10]. Among these compounds, the natural flavonoids have been shown to display pharmacological properties of interest in the prevention and treatment of cancer, as cytotoxic and/or as antiangiogenic agents [11–14].
In a program aimed at finding novel antiangiogenic agents, we recently identified the natural flavonoid fisetin (3,3′,4′,7-tetrahydroxyflavone) as an interesting lead that can stabilize endothelial cells in vitro at non cytotoxic concentrations (Figure 1) [15]. Fisetin is present in several fruits, vegetables, nuts and wine [16,17], and displays a variety of biological effects including antioxidant, anti-inflammatory [18,19], anti-carcinogenic and in vitro antiangiogenesis [20]. Fisetin has already been shown to be cytotoxic to various human cancer cell lines including leukaemia (HL60) [21], breast (MCF7) [20], colon (HT29) [22], liver (SK-HEP-1, Caco-2) [22,23], neuroblastoma (SHEP, WAC-2) [20], prostate (LNCaP, PC3) [24], and also to several endothelial cells [20]. Fisetin has been shown to inhibit several molecular targets, including cyclin-dependent kinases [25–27], DNA topoisomerases I and II [28,29], urokinase [30], actin [31], and androgen receptor signalling [32]. It has also recently been found that fisetin induces a forced exit from mitosis by targeting the mitotic spindle checkpoint involving the inhibition of Aurora B activities required for the maintenance of normal spindle checkpoint signalling [33].
Figure 1.
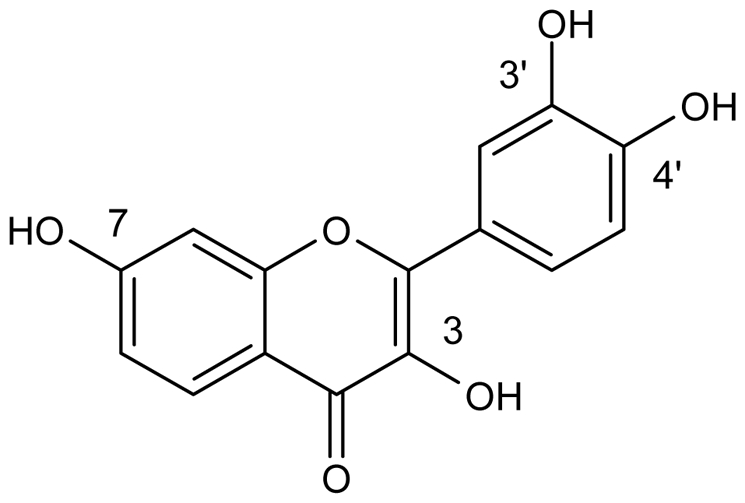
Chemical structure of fisetin (3,3′,4′,7-tetrahydroxyflavone).
In the present study, we further tested fisetin’s in vitro antiangiogenic action and evaluated its in vivo antitumour activity in Lewis lung carcinoma bearing mice. We report here that fisetin displays anti-angiogenic properties in vitro as well as in vivo inhibition of Lewis lung carcinoma tumour growth involving an anti-angiogenic mechanism. In addition, when fisetin was combined with low dose cyclophosphamide, a remarkable improvement in antitumour activity involving an anti-angiogenic mechanism of action was observed. We propose that this relatively non toxic drug combination using a dietary phytochemical with low-dose cyclophosphamide could advantageously be used in the treatment of solid tumours.
Methods
Chemicals
Fisetin (3,3′,4′,7-tetrahydroxyflavone) and cyclophosphamide were purchased from Sigma-Aldrich (Saint-Quentin Fallavier, France). Fisetin stock solution was prepared in dimethylsulfoxide (DMSO) and stored at 4°C in the dark. Cyclophosphamide was dissolved in sterile water.
Cell viability
The murine Lewis lung carcinoma (LLC) cell line, the NIH 3T3 murine fibroblast cell line and the EAhy 926 endothelial cell line (an immortalized human umbilical vein endothelial cell line [34]) were grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 2 mM L-glutamine, 10% foetal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin (37°C, 5% CO2). Exponentially growing cells were plated onto 96-well plates at 5000 cells per well in 200 μl. After 24 hours, cells were exposed to fisetin at the indicated concentrations for an additional 48 h. Viability was assessed using the MTT (1-(4,5-dimethylthiazol-2-yl)-3,5-diphenyltetrazolium) test and absorbance was read at 562 nm using a microplate reader (BioKinetics Reader, EL340). Appropriate controls with DMEM only and MTT were used to determine background absorbance. Experiments were run in quadruplicate and repeated 3 times. Control cells were exposed to 1% DMSO which was not cytotoxic. The results are presented as the inhibitory concentration for 50% of cells (IC50).
Cell cycle analysis
Lewis lung carcinoma and EAhy 926 endothelial cells were seeded in 6-well plates at 105 cells/well. Twenty-four hours later, fisetin was added to the wells at the indicated concentrations and the cells were incubated for 48 h. For each condition, detached and adherent cells were harvested, fixed for at least 30 min in 70% ethanol, and incubated with propidium iodide (50 μg/ml), sodium citrate (1 mg/ml) and RNase A (50 μg/ml) for 30 min in the dark. Nuclei DNA content was determined by flow cytometry (Coulter Epics Beckman, Germany) with red emission (FL-2 channel, 570 nm). After debris exclusion using forward/side scatter gating, 104 nuclei were acquired and analyzed using the WinMDI software. Cells with sub-G1 DNA content were considered to be apoptotic.
Cell migration assay (scratch wound assay)
EAhy 926 endothelial cells were grown to confluence and a wound was introduced by clearing an area of the monolayer using a 100 μl pipette tip. Digital photographs of wounded areas were recorded from each well at a magnification of 100× (time 0 h). Following a change of medium, basic fibroblast growth factor (bFGF, 10 ng/ml, BD Biosciences) and fisetin at the indicated concentrations were added to the medium with 2.5% of FBS. After 24 h incubation, digital photographs of the wound areas were recorded for each well. Migration was evaluated by manually drawing the distance of the wound area (d) at T0h and T24h. Distance values were obtained using the ImageJ software [35]. Results were expressed as a percentage of the controls using the following formula: 100 × [1−(dT0h−dT24h of treated cells)/( dT0h−dT24h of control cells)]. Experiments were performed in triplicate for each concentration and were repeated 3 times.
Formation of capillary-like structures
Fifty μl of gel matrix solution was applied to each well of a 96-well plate and incubated for 30 min at 37°C. EAhy 926 cells (1 × 104) were suspended in 100 μl of medium, plated onto the gel matrix and incubated at 37°C. Adherent cells received bFGF (10 ng/ml) and fisetin at the indicated concentrations. After a 24 h exposure time, in vitro angiogenesis was assessed by counting the number of capillary-like structures in each well at 100× magnification with a light microscope (Zeiss). The in vitro anti-angiogenic effect was calculated using the following formula: 100 × [1−(number of capillary-like tubes in treated cells)/(number of capillary-like tubes in control cells)]. Experiments were performed in duplicate for each condition and repeated 3 times.
Animal experiments
All animal experiments were ethically conducted, according to institutional, French and European guidelines, and were approved by the institutional animal welfare committee.
a. Matrigel plug angiogenesis assay
Fifteen 6-week old C57BL/6J female mice (Janvier, Le Genest Saint Isle, France) were randomly divided into five groups. LLC cells were trypsinized and resuspended at 3 × 107 cells/ml in serum-free medium. Aliquots of cells (0.1 ml, 3 × 106 cells) were mixed with 0.2 ml of phenol red-free Matrigel and injected into the right flank of mice. For the fisetin-treated groups, the cells were injected with four increasing concentrations of fisetin: 12.5, 25, 50 and 100 μg/ml (or 22, 44, 87, 175 and 350 μM). Controls included cells with equal volumes of solvent, whereas the Matrigel mixed with the medium alone was used as a negative control. The Matrigel plugs were removed 14 days after the implantation, weighed and measured for haemoglobin content using the Drabkin’s reagent kit according to the manufacturer’s instructions (Sigma-Aldrich). Haemoglobin concentration was calculated based on a set of haemoglobin standards. The data are presented as mean ± SEM from triplicate experiments.
b. Evaluation of antitumour activity in mice
In preliminary experiments with non tumoured C57BL/6J female mice, fisetin at 223 mg/kg intraperitoneally (i.p.) was found non toxic (based on body weights) when administered for 5 consecutive days in week 1 and for 5 consecutive days in week 2. Fisetin was dissolved in polyethylene glycol 200 (PEG200)/DMSO (7:3; v:v) and injected i.p. in a volume of 0.1 ml. For antitumour evaluation, Lewis lung tumour fragments (about 3 mm3) were injected subcutaneously (s.c.) bilaterally into mouse flanks. Tumour growth was assessed every 2 days using bi-dimensional measurements with a caliper. Tumour volume (mm3) was calculated according to the formula: width2 × length × 0.5 (mm). In the first experiment, fisetin was injected i.p. into 5 tumoured mice at 223 mg/kg on days 5 to 9 and days 12 to 16 post tumour implantation. In a second experiment, 20 mice were randomly divided into 4 groups. Fragments of LLC tumour (3 mm3) were injected bilaterally s.c. into the mouse flanks, and 4 days after tumour implantation, the mice were submitted to the following treatments: mice in the fisetin group were injected i.p. on days 4 to 8, 11, 12, and 14 with 223 mg/kg of fisetin dissolved in a 0.1 ml volume of PEG200/DMSO (7:3; v:v); mice in the cyclophosphamide group were injected s.c. on days 4, 5, 7 and 8 with 30 mg/kg of cyclophosphamide dissolved in water; mice in the combination group were treated with both fisetin and cyclophosphamide as described above; mice in the control group were injected with both vehicles. The 30 mg/kg dose cyclophosphamide was based on previous work that showed that doses of 10–40 mg/kg can be administered daily for prolonged period without undue toxicity [36]. For comparison purposes, the maximum tolerated dose of cyclophosphamide in mice is between 186 to 220 mg/kg when administered as a single dose [37,38] and 170 mg/kg when given every 6 days [39]. Therefore, the 30 mg/kg dose for 4 days used in our experiments can be considered a low cyclophosphamide dose that was not toxic, based on body weight data. Tumour measurements were recorded three times weekly; the mice were euthanized 15 days after tumour inoculation.
Microvessel density evaluation
Tumour tissues were harvested, weighed, frozen in isopentane, immersed in liquid nitrogen, and stored at −70°C until preparation of the histology slides. Ten-micron frozen tissue sections were placed on Superfrost Plus slides. Immunostaining of PECAM-1 (monoclonal rat antibodies anti-PECAM-1 (platelet endothelial cell adhesion molecule 1), clone MEC13.3; BDPharmingen, Le Pont-De-Claix, France) was performed using a three-step procedure as previously described [40]. In brief, the sections were washed three times in 1× phosphate buffered saline (PBS) and incubated for 10 min in 0.3% hydrogen peroxide/PBS. The slides were washed three times with 1× PBS, and incubated with 1% bovine serum albumin (BSA) at room temperature for 30 min. The sections were incubated with the rat primary antibodies anti-PECAM-1 (1:50) in a humidified chamber at 37°C for 1 h. After three washes in PBS, the slides were incubated for 30 min with biotinylated-secondary antibody with goat anti-rat IgG (1:400). After 3 rinses, slides were again incubated with the streptavidin-conjugated peroxidase according to the manufacturer’s instructions (dilution 1/400). The 3,3′-diaminobenzidine (DAB) substrate was then added for 5 to 7 min until a brown precipitate was visible. Sections were rinsed several times in 1× PBS. Sections incubated with BSA instead of the primary antibodies were used as negative controls. Slides were counterstained with Gill’s haematoxylin and treated with a 25% ammonia solution to generate a blue nuclear stain, dehydrated in graded ethanol solutions and xylene, and mounted with Eukitz®. Microvessel density was evaluated under the microscope by counting 3 fields at a magnification of 100× on two different slides. Microvessel density was expressed as the number of PECAM-1 positive microvessels per mm2.
Statistical analyses
Results are expressed as the mean ± SEM of at least 3 independent experiments. Comparisons between means were assessed using the Student t test for unpaired data. If unequal variance was observed, Welch’s correction was applied. Comparisons between several groups were assessed using a one-way analysis of variance (ANOVA) followed by the Dunnett’s multiple comparison test, using an appropriate control group as the reference. The statistical analyses were performed using the GraphPad Prism software. A P value < 0.05 was considered significant.
Results
Fisetin effects on cell viability, cell cycle and apoptosis of Lewis lung carcinoma (LLC) cells, endothelial cells (EAhy 926), and normal cells (NIH 3T3)
Fisetin induced a dose-dependent decreased viability in both Lewis lung carcinoma (LLC) and endothelial cells (EAhy 926), with IC50s of 59 and 77 μM, respectively, for a 24-h exposure time (Table 1). Interestingly, normal NIH 3T3 cells were found 3 times less sensitive to fisetin than either LLC or endothelial cells, with an IC50 of 210 μM (Table 1). When the incubation time was increased to 48 h, the differential sensitivity between normal NIH 3T3 cells and LLC or EAhy 926 cells reached a 5-fold difference.
Table 1. Inhibitory concentration for 50% (IC50) of Lewis lung carcinoma cells (LLC), EAhy 926 endothelial cells and normal NIH 3T3 cells.
Cells were exposed to fisetin at various concentrations for 24 or 48 h, and viability was evaluated by the MTT test. Mean ± SEM of 3 independent experiments each performed in quadruplicate. Statistical significance assessed by one-way analysis of variance followed by the Dunnett’s multiple comparison test, using the IC50 of NIH 3T3 cells as the reference.
| Incubation time (h) | Inhibitory concentration for 50% (IC50) of cells (μM) | ||
|---|---|---|---|
| LLC | EAhy 926 | Normal NIH 3T3 | |
| 24 | 59 ± 9* | 77 ± 9* | 210 ± 14 |
| 48 | 27 ± 0.3* | 28 ± 0.3* | 136 ± 13 |
P < 0.001
To characterize the mechanism of the cytotoxic/antiproliferative effect of fisetin, apoptosis was analyzed on LLC and endothelial cells. Table 2 shows the DNA cell cycle and sub G1 distribution of fisetin-treated cells after 48 h exposure at the indicated concentrations. Fisetin induced apoptosis in a dose-dependent manner in LLC cells, as measured by cells with sub-G1 DNA content. At low concentrations (22 and 44 μM), fisetin induced apoptosis in 5% of LLC compared to 1% in control cells. Higher fisetin concentrations (175–350 μM) induced higher levels of apoptosis (29%). Fisetin also induced a dose-dependent decrease in cells in G1. All tested concentrations of fisetin induced an accumulation of cells in the G2/M phase (25–36%) compared to controls.
Table 2. Cell cycle analysis of Lewis lung carcinoma and EAhy 926 endothelial cells treated with fisetin.
Cells were exposed to the indicated concentrations of fisetin for 48 h. Cells were harvested, fixed, incubated with propidium iodide and analyzed by flow cytometry. Mean ± SEM of 3 independent experiments.
| Cell line | Fisetin Conc (μM) | Percent cell in the indicated phase | |||
|---|---|---|---|---|---|
| SubG1 | G1 | S | G2/M | ||
| Lewis Lung Carcinoma cells | 0 | 1 ± 1 | 70 ± 1 | 14 ± 0.4 | 15 ± 1 |
| 22 | 5 ± 1 | 51 ± 1 | 19 ± 0.3 | 25 ± 1 | |
| 44 | 5 ± 0.1 | 39 ± 1 | 22 ± 1 | 34 ± 1 | |
| 87 | 12 ± 0.1 | 26 ± 1 | 26 ± 1 | 36 ± 1 | |
| 175 | 29 ± 1 | 13 ± 1 | 31 ± 0.3 | 27 ± 2 | |
| 350 | 29 ± 1 | 14 ± 1 | 29 ± 0.1 | 28 ± 0.3 | |
| EAhy 926 endothelial cells | 0 | 4 ± 1 | 66 ± 2 | 18 ± 1 | 12 ± 2 |
| 22 | 11 ± 2 | 67 ± 0.6 | 11 ± 1 | 11 ± 2 | |
| 44 | 15 ± 1 | 65 ± 1 | 12 ± 1 | 8 ± 0.3 | |
| 87 | 35 ± 2 | 52 ± 0.4 | 9 ± 1 | 4 ± 1 | |
| 175 | 38 ± 1 | 49 ± 1 | 8 ± 1 | 5 ± 2 | |
| 350 | 60 ± 0.3 | 31 ± 1 | 7 ± 1 | 2 ± 1 | |
The fisetin effects on the cell cycle distribution of EAhy 926 endothelial cells differed markedly from the LLC cell line. Indeed, at low concentrations (22 and 44 μM), fisetin already induced a higher percentage of apoptotic cells (11–15%), and at high fisetin concentrations (175 and 350 μM) high levels of apoptosis were achieved (38% and 60%, respectively). As sub-G1 apoptosis increased, the percent of cells in G1, S and in G2/M phases decreased (Table 2). Contrary to LLC cells, no accumulation of fisetin-treated endothelial cells was observed in the G2/M phase.
Fisetin inhibits angiogenesis in vitro
a. Effect of fisetin on migration of EAhy 926 endothelial cells
We next examined the possible antiangiogenic effects of fisetin on endothelial cell migration by using the scrape wound assay. Figure 2 illustrates that at 24 h post-wounding of confluent EAhy 926 endothelial cells, control cells migrated and totally filled the scraped area. Fisetin exposure at 22 and 44 μM (24 h), however, resulted in a significant dose-dependent decrease in EAhy 926 endothelial cell migration. The calculated IC50 for the anti-migration effect was 45 ± 0.3 μM (mean ± SEM from 3 independent experiments).
Figure 2. Effect of fisetin on cell migration (scratch wound assay).
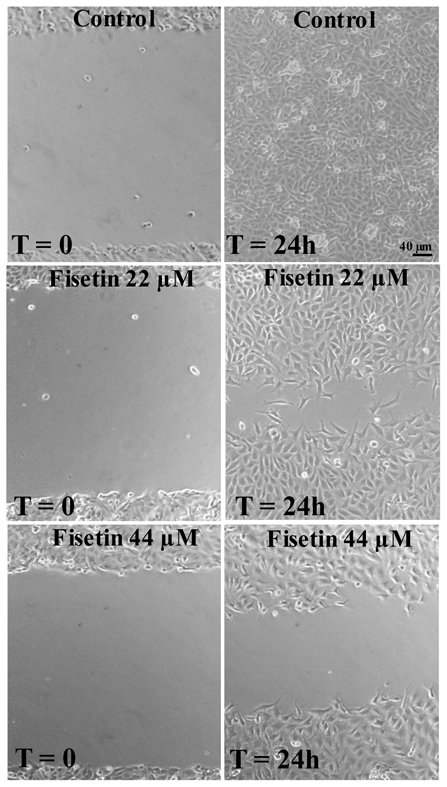
EAhy 926 endothelial cells were grown to confluence and an area was cleared using a 100 μl pipette tip as described in the Methods. Digital photographs were recorded at a magnification of 100 × immediately after the wounding (Time= 0) and at 24 hours (T= 24 h) after the addition of bFGF (10 ng/ml) for the control, or with bFGF plus the indicated concentration of fisetin. Scale bar, 40 μm.
b. Effect of fisetin on capillary-like structure formation on Matrigel
The endothelial cell tube formation assay was used to investigate fisetin anti-angiogenesis effect in vitro. EAhy 926 endothelial cells plated on Matrigel with bFGF formed a capillary-like network within 24 h, as expected (Figure 3). Fisetin at 22, 44 and 87 μM prevented the formation of the capillary-like network in a dose-dependent fashion. The calculated IC50 for the inhibition of capillary-like structure formation was 52 ± 7 μM (mean ± SEM from 3 independent experiments).
Figure 3. Effect of fisetin on capillary-like structure formation.
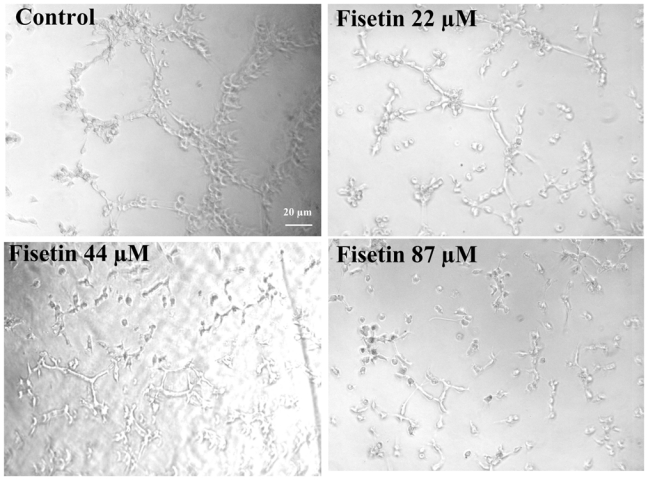
EAhy 926 endothelial cells were grown on Matrigel with bFGF (10 ng/ml) in absence (control), or presence of the indicated fisetin concentrations for a 24-h incubation period. Digital photographs recorded at a magnification of 100 ×. Scale bar, 20 μm.
Fisetin inhibits angiogenesis in vivo
We then investigated fisetin tumour angiogenesis in vivo. LLC cells were mixed with Matrigel with increasing concentrations of fisetin (44 to 350 μM) and injected s.c. into the right flank of mice. Fourteen days later, the mice were sacrificed and the Matrigel plugs removed, weighed, and evaluated for haemoglobin content. The Matrigel plugs were significantly smaller in the fisetin-treated groups compared to the controls. Matrigel plug weights decreased significantly as fisetin concentrations increased (Figure 4-A). To quantify angiogenesis, the haemoglobin content of the Matrigel plugs was assayed. As shown in Figure 4-B, fisetin treatment led to a dose-dependent decrease in Matrigel plug haemoglobin levels, which became significant at 350 μM. These in vivo results indicate that fisetin can decrease tumour angiogenesis.
Figure 4. In vivo Matrigel plug angiogenesis assay.
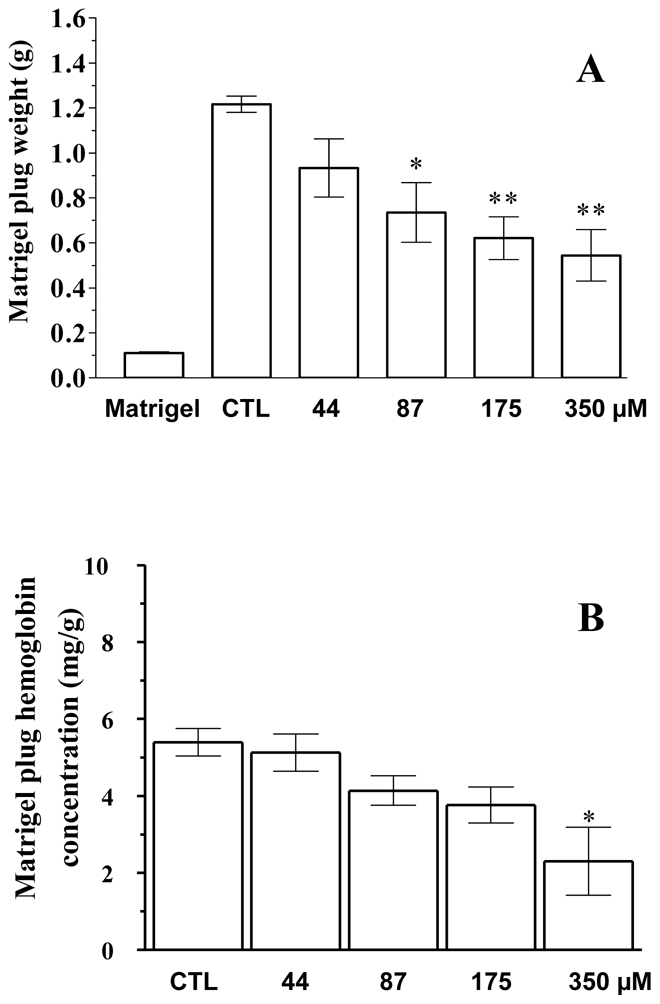
Matrigel plugs containing Lewis lung carcinoma cells were implanted s.c. in mice with solvent (control=CTL) or with the indicated concentrations of fisetin. Fourteen days later, the Matrigel plugs were removed and weighed (A), and the content in haemoglobin was assessed (B), as described in the Methods. * P value < 0.05 and ** P value < 0.01 compared to controls (Dunnett’s t test). The bar indicated “Matrigel” indicates the control without Lewis lung carcinoma cells. Error bars, SEM.
Fisetin antitumour activity in vivo
To determine whether fisetin could inhibit tumour growth in vivo, fisetin was administered to two groups of 5 LLC tumour-bearing mice. Mice in the treated group were injected i.p. with fisetin at 223 mg/kg for 5 consecutive days during week 1, and for another 5 consecutive days in week 2. Mice in the control group received solvent on the same days as the fisetin-treated group. Preliminary experiments showed that fisetin alone was not toxic at this dose level and schedule of administration. On day 15, tumours from the mice treated with fisetin were 50% smaller than control tumours and appeared less vascularized than the controls (data not shown).
In vivo antitumour activity of the combination of fisetin and cyclophosphamide
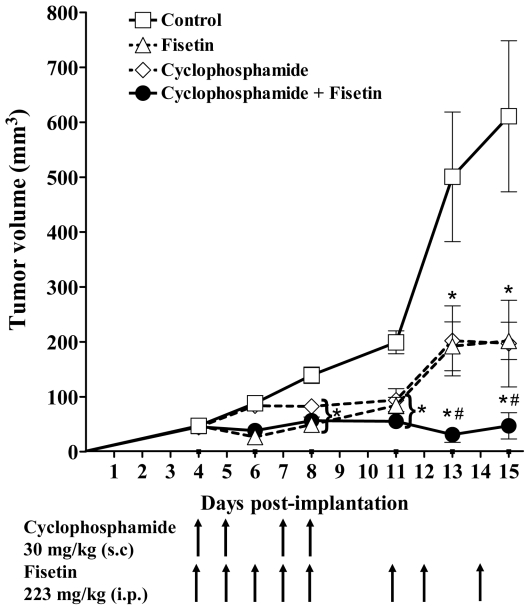
To optimize the in vivo anticancer effect of fisetin found above, fisetin was next combined with low dose cyclophosphamide, a cytotoxic drug reported to possess antiangiogenic properties [39]. Fisetin was administered i.p. at 223 mg/kg daily for 5 days in week 1 (days 4 to 8 post tumour implantation), followed by three injections on days 11, 12 and 14 (Figure 5, triangles). Fisetin treatment led to a 67% tumour growth inhibition compared to the controls (squares). Low dose cyclophosphamide was administered s.c. at 30 mg/kg on four days in week 1 only (days 4, 5, 7, 8), and led to a tumour growth inhibition of 66%, similar to fisetin treatment (Figure 5, diamonds). When fisetin and cyclophosphamide were combined at the same dose levels and schedules as used above, tumour volumes declined dramatically, showing 92% inhibition compared to controls on day 15 (Figure 5, solid circles). Over the two week treatment, this drug combination was not toxic, showing only a 4.6% loss in body weight, similar to that of the fisetin treatment alone (4.3%).
Figure 5. In vivo antitumour activity of the combination of fisetin with cyclophosphamide.
Twenty mice bearing bilateral Lewis lung tumours were randomly assigned to four groups of 5 mice as follows: control, solvent alone (squares); fisetin, 223 mg/kg i.p. on days 4 to 8 and days 11, 12, 14 (triangles); cyclophosphamide, 30 mg/kg s.c., on days 4, 5, 7, 8 (diamonds); and, the combination of cyclophosphamide and fisetin (solid circles), both administered at the same dose and schedule when used alone. Tumour volumes were determined as described in Materials and Methods. Mean ± SEM. The * indicates a significant difference (P<0.05) with the Control group, and the # indicates a significant difference with the Fisetin or the Cyclophosphamide group (ANOVA and Dunnett’s multiple comparison test).
In vivo fisetin antiangiogenic effect
To verify if the in vivo tumour growth inhibition was due to an antiangiogenic effect, tumour sections were stained using PECAM-1 antibodies. PECAM-1 was mainly expressed in endothelial cell membranes of microvessels, as expected (Figure 6-A, Control). The number of microvessels expressing PECAM-1 in the fisetin- and in the cyclophosphamide-treated tumours was significantly diminished compared to those in the control (Figures 6-A and 6-B). However, the treatment with the fisetin and cyclophosphamide drug combination led to an impressive and significant decrease in microvessel density, as depicted in Figures 6-A and 6-B.
Figure 6. Evaluation of microvessel density in Lewis lung carcinoma tumours.
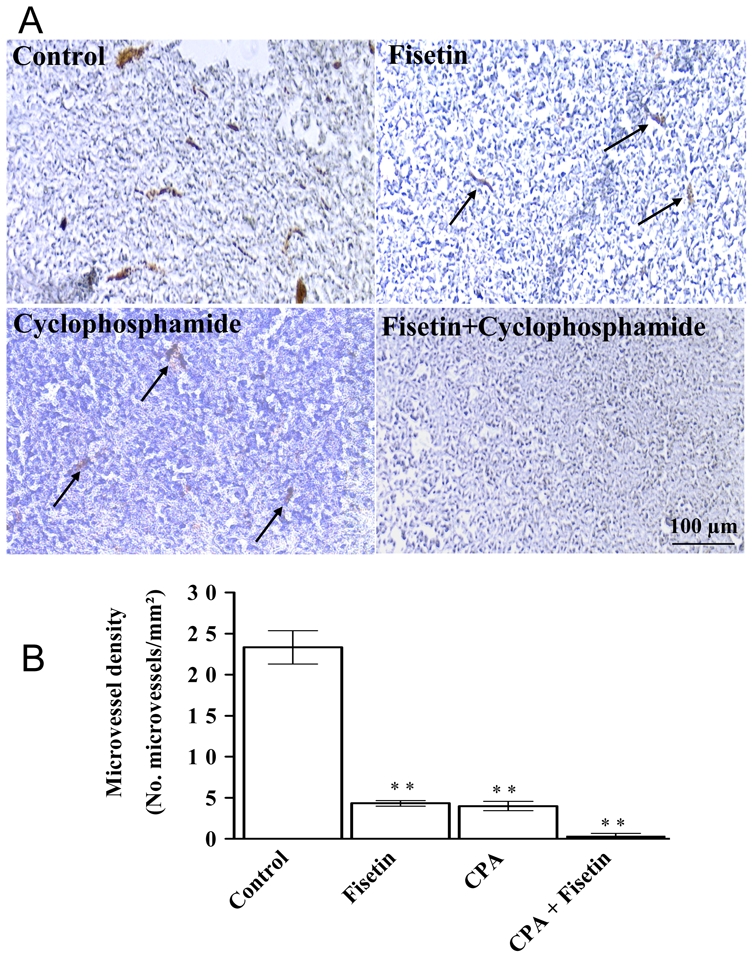
A) Immunohistochemical evaluation of microvessels in Lewis lung tumours using antibodies to PECAM-1 as described in the Methods section. The tumours were treated in vivo with fisetin or cyclophosphamide alone, and with the combination of cyclophosphamide and fisetin. Scale bar, 100 μm. B) Microvessel density (number of vessels per mm2) in tumours after in vivo treatment with the solvent alone (control), with fisetin or cyclophosphamide (CPA) alone, or with the combination of fisetin and cyclophosphamide (CPA). Mean ± SEM. The asterisks indicate a P value < 0.05 compared to controls (Dunnett’s multiple comparison test).
Discussion
Although several phytochemicals have been shown to possess pharmacological properties of potential interest in cancer prevention and/or therapy, their activity in the tumour angiogenic process is presently not well understood [10–14,20]. Because we recently identified the dietary flavonoid fisetin as an interesting lead that can stabilize the cytoskeleton of endothelial cells in vitro at non cytotoxic concentrations [15], we were therefore interested to evaluate the in vivo antiangiogenic activity of this compound.
The fisetin antiproliferative/cytotoxic activity determined in this study on LLC and endothelial cells confirmed its cytotoxic activity reported on other cancer cell lines, e.g., in prostate [24], liver [23], colon [25], and leukaemia cells [21]. In this study, normal NIH 3T3 cells were also found to be about 3-fold less sensitive to fisetin than LLC or endothelial cells. It is of interest that the fisetin relative selectivity towards cancer and endothelial cells, compared to normal cells, has also been observed in other studies [20], and this selectivity was also observed on prostate cancer cells that were shown to be more vulnerable to fisetin compared to normal prostate cells [24]. This relatively non frequent cancer cell selectivity could therefore confer a valuable advantage of this compound for in vivo treatment.
We also observed that fisetin could block LLC cells in the G2/M phase at low concentrations, and could induce apoptosis in endothelial cells also at low concentrations. These observations would suggest that fisetin could first act in vivo on endothelial cells forming the tumour vasculature and then cause apoptosis of cancer cells in the vicinity of the blood vessel. Fisetin-induced G2/M cell accumulation has been previously reported along with decreased activity of several cyclin-dependent kinases [21,23–25]. The signal transduction pathways involved in apoptosis include caspase 3 and increased p53 protein [23].
Our data clearly show that fisetin possesses in vitro antiangiogenic effects, preventing both the migration of endothelial cells and the formation of capillary-like structures at low micromolar concentrations. Previous work on in vitro antiangiogenic effects of fisetin has reported this effect at similar concentrations [20].
With regard to the relevance of the in vitro fisetin concentrations used in our experiments, the fisetin plasma concentrations achieved in mice are in the range of 10 μM after intraperitoneal administration of a dose of 223 mg/kg (Touil YS and Chabot GG, unpublished data). In rats, free fisetin plasma concentrations of 50 μM can also be achieved after an i.v. dose of 10 mg/kg [41]. These plasma concentrations are therefore in the range of the concentration used in vitro to show the antiangiogenic effects with the aglycone (free fisetin). In addition, it should be mentioned that because of the presence of 4 OH substituents on the fisetin molecule, glucuronide and sulphate conjugates are also present at high concentrations in plasma [41] (Touil YS and Chabot GG, unpublished data), and these metabolites could also play a role in the overall antiangiogenic effects observed in vivo in mice. It is of interest that flavonoid sulphates and/or glucuronides of closely related flavonoids (e.g., morin and quercetin) have recently been shown to display superior bioactivities compared to their aglycones (free forms) [42]. It should also be mentioned that mouse tumours usually have a high beta-glucuronidase and sulfatase activities that could hydrolyze locally the conjugates to release the aglycone within the tumour, and therefore contribute to the local antitumour effect [43].
We next investigated if these in vitro antiangiogenic effects could be translated in vivo using Lewis lung carcinoma bearing mice. Fisetin was found to cause significant tumour growth inhibition when used as a single agent at non toxic doses. The mechanism of action involved in the in vivo fisetin antitumour activity most likely involves an antiangiogenic effect, as evidenced by a significant reduction in microvessel density. Although fisetin’s antiangiogenic activity has been previously reported in vivo in rabbit eyes, it should be mentioned that it was by direct application of an emulsion containing fisetin on the cornea [44] and not by systemic administration, as in the present study. To our knowledge this report is the first describing the fisetin’s in vivo antiangiogenic activities after systemic administration in mice.
In an attempt to improve fisetin’s in vivo antitumour effects, we next combined this flavonoid with low dose cyclophosphamide, because this cytotoxic agent has already been shown to improve antiangiogenic therapy [39,45]. This drug combination clearly led to an impressive improvement in antitumour effect with a 92% tumour inhibition at non toxic dosages of both agents. Although the fisetin-CPA drug combination is leading to a greater effect than either drug used alone, the magnitude of this effect could not be analyzed using the Chou and Talalay’s method because measurements made with single doses of either drug in a combination can never alone determine synergism since the sigmoidicity of dose-effect curves and the exclusivity of drug effects cannot be determined from such measurements [46]. Because our data present only one dose level of either drug, we therefore cannot claim synergism, although there was a marked improvement in the anticancer activity of either drug at the single dose level used, as evidenced by tumour growth curves. Moreover, the histological examination of the treated tumours clearly showed that the microvessel density was significantly reduced in the tumours of mice that received the drug combination, thus showing that an antiangiogenic effect was indeed involved in this impressive antitumour activity.
The precise molecular mechanism of action of the increased antitumour and antiangiogenic activity observed with the fisetin-cyclophosphamide drug combination is not precisely known for the moment. Although the antiangiogenic action of each compound alone is probably playing a major role in the improved activity of the combination, other factors could also be involved. For instance, a pharmacokinetic interaction would be possible, as was shown for the drug combination involving thalidomide and cyclophosphamide [37]. However, it should be mentioned that such a pharmacokinetic interaction was observed at a high dose of cyclophosphamide (220 mg/kg) [37], which is a 7-fold higher dose that the one used in the present study (30 mg/kg). Further studies will have to address this issue.
In addition to the stabilization of endothelial cells cytoskeleton [15], the antiangiogenic effect of fisetin and consequent antitumour activity could also involve the inhibition of urokinase plasminogen activator (uPA) in endothelial cells, as was recently reported [30]. UPA is over expressed in tumour vessels and is involved in extracellular matrix degradation responsible for endothelial cell migration and formation of new tumour blood vessels [47]. In addition to the Lewis lung carcinoma model, it is of interest to note that fisetin has recently been reported to be active in prostate cancer xenografts in nude mice through the inhibition of androgen receptor signalling, although angiogenesis was not investigated in this study [32]. Other potential mechanisms of action could involve the direct or indirect inhibition of other factors involved in the complex tumour angiogenic process.
Conclusions
The data reported here provide the first evidence that the dietary flavonoid fisetin can display antiangiogenic and anticancer activities in vivo in mice bearing Lewis lung carcinoma. In addition, the remarkable improvement in the anticancer and antiangiogenic activities of the combination of fisetin with low dose cyclophosphamide deserves further studies given the fact that cyclophosphamide is used in several anticancer drug regimens. Optimization of this drug combination by improved scheduling and/or pharmaceutical formulations is therefore warranted. It is proposed that the relatively non toxic drug combination studied in this work, associating a natural compound and a cytotoxic agent, could be useful in the treatment of solid tumours.
Acknowledgments
This research was supported by the Institut national de la santé et de la recherche médicale (INSERM), by the Centre national de la recherche scientifique (CNRS), by the Institut Fédératif de Recherche (IFR-71) of the Université Paris Descartes, and by a grant from the Institut National du Cancer (National Cancer Institute of France, F-92513 Boulogne-Billancourt Cedex, France).
References
- 1.Kerbel RS. Molecular origins of cancer: Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 4.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 5.Taraboletti G, Margosio B. Antiangiogenic and antivascular therapy for cancer. Curr Opin Pharmacol. 2001;1:378–384. doi: 10.1016/s1471-4892(01)00065-0. [DOI] [PubMed] [Google Scholar]
- 6.Tozer GM, Kanthou C, Baguley BC. Disrupting tumour blood vessels. Nat Rev Cancer. 2005;5:423–435. doi: 10.1038/nrc1628. [DOI] [PubMed] [Google Scholar]
- 7.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 8.Kabbinavar FF, Schulz J, McCleod M, Patel T, Hamm JT, Hecht JR, Mass R, Perrou B, Nelson B, Novotny WF. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol. 2005;23:3697–3705. doi: 10.1200/JCO.2005.05.112. [DOI] [PubMed] [Google Scholar]
- 9.Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L, Dickler M, Overmoyer BA, Reimann JD, Sing AP, Langmuir V, Rugo HS. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 10.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 11.Lopez-Lazaro M. Flavonoids as anticancer agents: structure-activity relationship study. Curr Med Chem Anticancer Agents. 2002;2:691–714. doi: 10.2174/1568011023353714. [DOI] [PubMed] [Google Scholar]
- 12.Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 13.Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacol Ther. 2002;96:67–202. doi: 10.1016/s0163-7258(02)00298-x. [DOI] [PubMed] [Google Scholar]
- 14.Hill S, Williams KB, Denekamp J. Vascular collapse after flavone acetic acid: a possible mechanism of its anti-tumour action. Eur J Cancer Clin Oncol. 1989;25:1419–1424. doi: 10.1016/0277-5379(89)90099-0. [DOI] [PubMed] [Google Scholar]
- 15.Touil YS, Fellous A, Scherman D, Chabot GG. Flavonoid-induced morphological modifications of endothelial cells through microtubule stabilization. Nutr Cancer. 2009;61:310–321. doi: 10.1080/01635580802521346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arai Y, Watanabe S, Kimira M, Shimoi K, Mochizuki R, Kinae N. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J Nutr. 2000;130:2243–2250. doi: 10.1093/jn/130.9.2243. [DOI] [PubMed] [Google Scholar]
- 17.Kimira M, Arai Y, Shimoi K, Watanabe S. Japanese intake of flavonoids and isoflavonoids from foods. J Epidemiol. 1998;8:168–175. doi: 10.2188/jea.8.168. [DOI] [PubMed] [Google Scholar]
- 18.Woodman OL, Chan EC. Vascular and anti-oxidant actions of flavonols and flavones. Clin Exp Pharmacol Physiol. 2004;31:786–790. doi: 10.1111/j.1440-1681.2004.04072.x. [DOI] [PubMed] [Google Scholar]
- 19.Park HH, Lee S, Oh JM, Lee MS, Yoon KH, Park BH, Kim JW, Song H, Kim SH. Anti-inflammatory activity of fisetin in human mast cells (HMC-1) Pharmacological Research. 2007;55:31–37. doi: 10.1016/j.phrs.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Fotsis T, Pepper MS, Aktas E, Breit S, Rasku S, Adlercreutz H, Wahala K, Montesano R, Schweigerer L. Flavonoids, dietary-derived inhibitors of cell proliferation and in vitro angiogenesis. Cancer Res. 1997;57:2916–2921. [PubMed] [Google Scholar]
- 21.Lee WR, Shen SC, Lin HY, Hou WC, Yang LL, Chen YC. Wogonin and fisetin induce apoptosis in human promyeloleukemic cells, accompanied by a decrease of reactive oxygen species, and activation of caspase 3 and Ca2+-dependent endonuclease. Biochemical Pharmacology. 2002;63:225–236. doi: 10.1016/s0006-2952(01)00876-0. [DOI] [PubMed] [Google Scholar]
- 22.Kuntz S, Wenzel U, Daniel H. Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur J Nutr. 1999;38:133–142. doi: 10.1007/s003940050054. [DOI] [PubMed] [Google Scholar]
- 23.Chen YC, Shen SC, Lee WR, Lin HY, Ko CH, Shih CM, Yang LL. Wogonin and fisetin induction of apoptosis through activation of caspase 3 cascade and alternative expression of p21 protein in hepatocellular carcinoma cells SK-HEP-1. Arch Toxicol. 2002;76:351–359. doi: 10.1007/s00204-002-0346-6. [DOI] [PubMed] [Google Scholar]
- 24.Haddad AQ, Venkateswaran V, Viswanathan L, Teahan SJ, Fleshner NE, Klotz LH. Novel antiproliferative flavonoids induce cell cycle arrest in human prostate cancer cell lines. Prostate Cancer Prostatic Dis. 2006;9:68–76. doi: 10.1038/sj.pcan.4500845. [DOI] [PubMed] [Google Scholar]
- 25.Lu X, Jung J, Cho HJ, Lim DY, Lee HS, Chun HS, Kwon DY, Park JH. Fisetin inhibits the activities of cyclin-dependent kinases leading to cell cycle arrest in HT-29 human colon cancer cells. J Nutr. 2005;135:2884–2890. doi: 10.1093/jn/135.12.2884. [DOI] [PubMed] [Google Scholar]
- 26.Sung B, Pandey MK, Aggarwal BB. Fisetin, an inhibitor of cyclin-dependent kinase 6, down-regulates nuclear factor-kappaB-regulated cell proliferation, antiapoptotic and metastatic gene products through the suppression of TAK-1 and receptor-interacting protein-regulated IkappaBalpha kinase activation. Mol Pharmacol. 2007;71:1703–1714. doi: 10.1124/mol.107.034512. [DOI] [PubMed] [Google Scholar]
- 27.Lu H, Chang DJ, Baratte B, Meijer L, Schulze-Gahmen U. Crystal structure of a human cyclin-dependent kinase 6 complex with a flavonol inhibitor, fisetin. J Med Chem. 2005;48:737–743. doi: 10.1021/jm049353p. [DOI] [PubMed] [Google Scholar]
- 28.Olaharski AJ, Mondrala ST, Eastmond DA. Chromosomal malsegregation and micronucleus induction in vitro by the DNA topoisomerase II inhibitor fisetin. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2005;582:79–86. doi: 10.1016/j.mrgentox.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Constantinou A, Mehta R, Runyan C, Rao K, Vaughan A, Moon R. Flavonoids as DNA topoisomerase antagonists and poisons: structure-activity relationships. J Nat Prod. 1995;58:217–225. doi: 10.1021/np50116a009. [DOI] [PubMed] [Google Scholar]
- 30.Jankun J, Selman SH, Aniola J, Skrzypczak-Jankun E. Nutraceutical inhibitors of urokinase: potential applications in prostate cancer prevention and treatment. Oncol Rep. 2006;16:341–346. [PubMed] [Google Scholar]
- 31.Böhl M, Tietze S, Sokoll A, Madathil S, Pfennig F, Apostolakis J, Fahmy K, Gutzeit HO. Flavonoids affect actin functions in cytoplasm and nucleus. Biophys J. 2007;93:2767–2780. doi: 10.1529/biophysj.107.107813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan N, Asim M, Afaq F, Abu ZM, Mukhtar H. A novel dietary flavonoid fisetin inhibits androgen receptor signaling and tumor growth in athymic nude mice. Cancer Res. 2008;68:8555–8563. doi: 10.1158/0008-5472.CAN-08-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salmela AL, Pouwels J, Varis A, Kukkonen AM, Toivonen P, Halonen PK, Perala M, Kallioniemi O, Gorbsky GJ, Kallio MJ. Dietary flavonoid fisetin induces a forced exit from mitosis by targeting the mitotic spindle checkpoint. Carcinogenesis. 2009;30:1032–1040. doi: 10.1093/carcin/bgp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edgell CJ, McDonald CC, Graham JB. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci U S A. 1983;80:3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- 36.Man S, Bocci G, Francia G, Green SK, Jothy S, Hanahan D, Bohlen P, Hicklin DJ, Bergers G, Kerbel RS. Antitumor effects in mice of low-dose (metronomic) cyclophosphamide administered continuously through the drinking water. Cancer Res. 2002;62:2731–2735. [PubMed] [Google Scholar]
- 37.Ding Q, Kestell P, Baguley BC, Palmer BD, Paxton JW, Muller G, Ching LM. Potentiation of the antitumour effect of cyclophosphamide in mice by thalidomide. Cancer Chemother Pharmacol. 2002;50:186–192. doi: 10.1007/s00280-002-0482-y. [DOI] [PubMed] [Google Scholar]
- 38.Siim BG, Lee AE, Shalal-Zwain S, Pruijn FB, McKeage MJ, Wilson WR. Marked potentiation of the antitumour activity of chemotherapeutic drugs by the antivascular agent 5,6-dimethylxanthenone-4-acetic acid (DMXAA) Cancer Chemother Pharmacol. 2003;51:43–52. doi: 10.1007/s00280-002-0529-0. [DOI] [PubMed] [Google Scholar]
- 39.Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O’Reilly MS, Folkman J. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–1886. [PubMed] [Google Scholar]
- 40.Kraling BM, Razon MJ, Boon LM, Zurakowski D, Seachord C, Darveau RP, Mulliken JB, Corless CL, Bischoff J. E-selectin is present in proliferating endothelial cells in human hemangiomas. Am J Pathol. 1996;148:1181–1191. [PMC free article] [PubMed] [Google Scholar]
- 41.Shia CS, Tsai SY, Kuo SC, Hou YC, Chao PD. Metabolism and pharmacokinetics of 3,3′,4′,7-tetrahydroxyflavone (fisetin), 5-hydroxyflavone, and 7-hydroxyflavone and antihemolysis effects of fisetin and its serum metabolites. J Agric Food Chem. 2009;57:83–89. doi: 10.1021/jf802378q. [DOI] [PubMed] [Google Scholar]
- 42.Fang SH, Hou YC, Chang WC, Hsiu SL, Chao PD, Chiang BL. Morin sulfates/glucuronides exert anti-inflammatory activity on activated macrophages and decreased the incidence of septic shock. Life Sci. 2003;74:743–756. doi: 10.1016/j.lfs.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 43.Massaad L, de Waziers I, Ribrag V, Janot F, Beaune PH, Morizet J, Gouyette A, Chabot GG. Comparison of mouse and human colon tumors with regard to phase I and phase II drug-metabolizing enzyme systems. Cancer Res. 1992;52:6567–6575. [PubMed] [Google Scholar]
- 44.Joussen AM, Rohrschneider K, Reichling J, Kirchhof B, Kruse FE. Treatment of corneal neovascularization with dietary isoflavonoids and flavonoids. Exp Eye Res. 2000;71:483–487. doi: 10.1006/exer.2000.0900. [DOI] [PubMed] [Google Scholar]
- 45.Kakeji Y, Teicher BA. Preclinical studies of the combination of angiogenic inhibitors with cytotoxic agents. Invest New Drugs. 1997;15:39–48. doi: 10.1023/a:1005718628223. [DOI] [PubMed] [Google Scholar]
- 46.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 47.Pepper MS, Sappino AP, Stocklin R, Montesano R, Orci L, Vassalli JD. Upregulation of urokinase receptor expression on migrating endothelial cells. J Cell Biol. 1993;122:673–684. doi: 10.1083/jcb.122.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]