Summary
While a great public heath success, vaccines provide suboptimal protection in some patient populations and are not available to protect against many infectious diseases. Insights from innate immunity research have led to a better understanding of how existing vaccines work and informed vaccine development. New adjuvants and delivery systems are being designed based upon their capacity to stimulate innate immune sensors and target antigens to dendritic cells, the cells responsible for initiating adaptive immune responses. Incorporating these adjuvants and delivery systems in vaccines can beneficially alter the quantitative and qualitative nature of the adaptive immune response resulting in enhanced protection.
Introduction
A strong argument can be made that vaccines have had a greater impact on public health than any other advance in the history of medicine. The word “vaccine” is derived from variola vaccinae, the cow pox virus that was used by Edward Jenner in the late 18th century to provide protection against smallpox. In the 20th century, smallpox was estimated to be responsible for over 300 million deaths; many of the survivors were left blind and/or scarred. However, intensive worldwide vaccination and quarantine programs resulted in the eradication of natural smallpox, with the last indigenous case occurring in 1977. Other dreaded infectious diseases that were once commonplace just a few generations ago have now been largely eliminated as a result of effective vaccines and vaccination programs.
Despite these gains, effective vaccines remain elusive for many infectious diseases, including some of the major killers such as tuberculosis, HIV and malaria. Many vaccines, such as the smallpox vaccine, use live, attenuated organisms. Such vaccines often confer long-lasting immunity similar to that seen in natural infection (Coffman et al., 2010). However, disadvantages include a generally higher frequency of adverse events, including the potential to cause disease. This is especially a problem for the ever growing population of immunocompromised individuals, in whom live vaccines are contraindicated because of the risk of disseminated infections. Moreover, for some infections, the ability of the causative pathogen to undergo antigenic variation has made traditional live, attenuated vaccines not practical. Vaccines that employ killed whole organisms or purified antigens (“subunit vaccines”) are intrinsically safer. The sequencing of the genomes of nearly all major pathogens has enabled in silico searching for putative protective antigens, a technique that has been termed “reverse vaccinology” (Sette and Rappuoli, 2010). In addition, the rational design of candidate vaccine antigens has been facilitated by advances in protein structure analysis and engineering (Scarselli et al., 2011). Still, purified antigens administered alone tend to not elicit robust immune responses. For this reason, as discussed in more detail below, killed whole organisms and subunit vaccines are administered with adjuvants (from the Latin, adjuvare, meaning to help), loosely defined as molecules that augment the adaptive immune response and help generate immunological memory (McKee et al., 2007). In addition, chemical conjugation of proteins to carbohydrate capsules has been exploited as a way to overcome the poor immunogenicity of carbohydrate antigens. This has led to some remarkable successes, such as the near elimination of Haemophilus influenzae type B infections in vaccinated children, although this tactic is not tenable in situations where the capsule has a chemical composition also found on human cells. Other strategies to increase the immunogenicity of subunit vaccines include developing new vaccine platforms that can serve as delivery systems. Ideal platforms are thought to target antigen to dendritic cells (DCs), the professional antigen-presenting cells of the immune system most responsible for initiating immune responses, and also have intrinsic adjuvant properties.
Most vaccines in clinical use today protect by eliciting antibody responses. Antibodies provide protection by neutralizing viruses, fixing complement, enabling opsonophagocytosis and/or promoting antibody-dependent cellular cytotoxicity (Pulendran and Ahmed, 2011). However, for some infections, specific antibodies afford little to no protection and antigen-specific CD4+ or CD8+ T cell responses are thought to be required. It is important to keep in mind that the arms of the immune system required for protection against natural infection may differ from those needed for vaccine-mediated protection (Wuthrich et al., 2003). This concept is especially relevant in immunocompromised patients, in whom vaccines should ideally be targeted to the parts of the immune system that are relatively intact. A largely unmet challenge has been the design of safe and efficacious subunit T cell vaccines. This task is made complicated by three major factors. First, the diversity in the population of the human leukocyte antigens (HLA) that make up the major histocompatibility complex (MHC) class I and MHC class II proteins necessary for presentation of foreign antigens to CD8+ T cells and CD4+ T cells, respectively. As antigen-specific T cell responses require peptides from foreign antigens to be presented in an MHC-restricted manner, response rates and autoimmune reactions can vary as a function of HLA type. Second, for subunit vaccines to induce effective CD8+ cytotoxic T cell responses, which are felt to be particularly important in eradicating intracellular infections and neoplasms, activation of the cross-presentation pathway in DCs is required. Third, the traditional adjuvant used in most licensed vaccines, alum, does not stimulate broad T cell responses (Marrack et al., 2009).
Although there probably are more vaccines that will be developed using existing licensed technologies, much of the “low hanging fruit” appears to have already been picked. In addition to novel approaches to antigen selection facilitated by advances in reverse vaccinology, structural design and carbohydrate chemistry, it is likely that the next generation of vaccines against the major infectious diseases that plague mankind will feature novel adjuvants and platforms. Indeed, there is room for improvement even for many of the most successful vaccines. For example, high risk patients, such as the elderly, immunocompromised and those with renal failure, often do not develop protective antibody titers following vaccination. Herein, we review how insights emerging from innate immunity research over the past decade have led to a better understanding of how existing vaccines work and have informed vaccine research and development. The focus will be on preventive vaccines against infectious diseases.
Major features of innate immunity relevant to vaccinology
The innate immune system provides rapid defense against invading pathogens, principally by recognizing and responding to evolutionarily conserved microbial structures termed pathogen-associated molecular patterns (PAMPs). Detection of PAMPs by the host occurs via soluble and cell-associated germline-encoded pattern recognition receptors (PRRs). Many PRRs are highly expressed on DCs, where they can be located on the cell surface, endocytic compartments or the cytoplasm. Upon recognition of foreign antigen, particularly in the presence of PAMPs, DCs can initiate an adaptive immune response which consists of somatically generated antigen-specific B cell (antibody) and T cell responses. Adaptive responses take days to weeks to develop but last considerably longer than innate responses. The generation of memory B and T cells, a goal of most vaccines, can extend the duration of the adaptive response up to the lifetime of the individual. Importantly for adjuvant development, the nature of the immune response will be greatly influenced by the specific PRR or combination of PRRs that are stimulated (Hajishengallis and Lambris, 2011).
Based on their molecular structure, PRRs can be divided into multiple families, including Toll-like receptors (TLRs), C-type lectin receptors (CLRs), NOD-like receptors (NLRs) and RIG-I-like receptors (RLRs) (O'Neill and Bowie, 2010) (figure 1). The TLRs recognize a remarkably diverse number of PAMPs expressed by viral, bacterial, fungal and parasitic pathogen (Akira et al., 2006). TLR1, TLR2, TLR4, TLR5 and TLR6 are primarily expressed on the plasma membrane where they sense specific molecules on the surface of microbes. In contrast, TLR3, TLR7, TLR8 and TLR9 traffic from the endoplasmic reticulum to endolysosomal compartments where they recognize RNA and DNA (Brinkmann et al., 2007; Latz et al., 2004). TLRs initiate signaling pathways through interactions with adaptor proteins, including MyD88 and TRIF. This results in activation of mitogen-associated protein kinases (MAPKs), nuclear factor kB (NF-kB) and interferon regulatory factor (IRF)-responsive genes.
Figure. PRRs targeted by vaccine adjuvants.
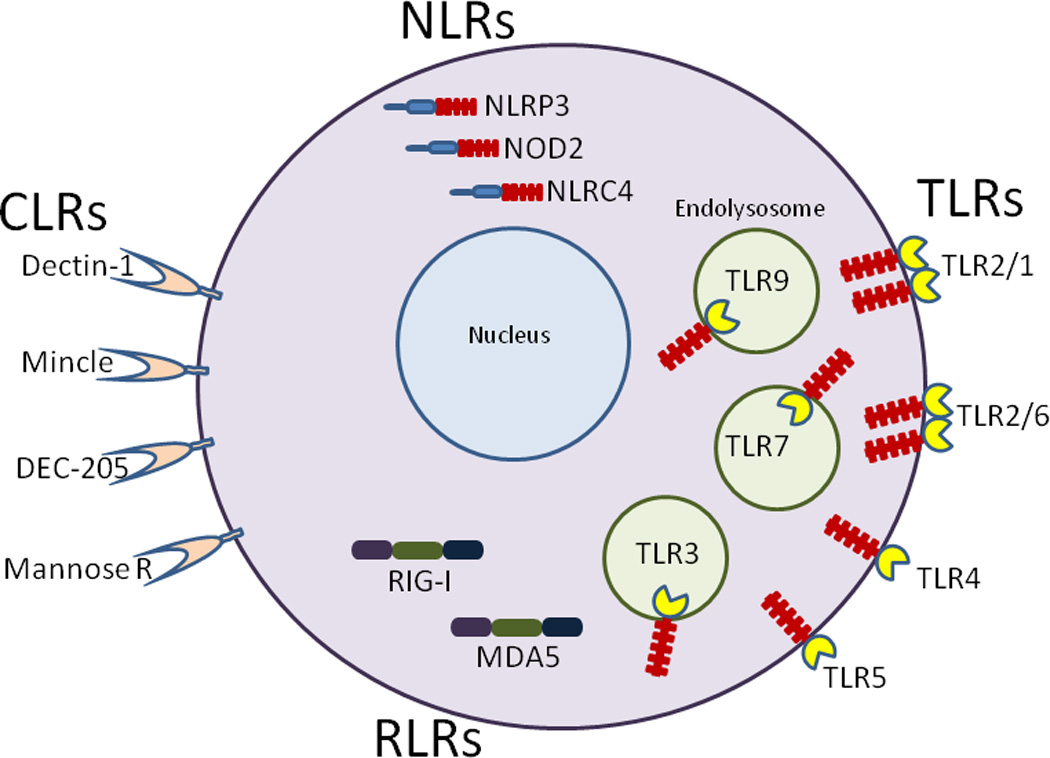
TLR1, TLR2, TLR4, TLR5 and TLR6 are located on the cell surface. TLR2 forms a heterodimer with TLR1 or TLR6. TLR3, TLR7, and TLR9 translocate from the endoplasmic reticulum to endolysosomal compartments following cell stimulation. The CLRs are cell surface receptors whereas the NLRs and RLRs reside in cytoplasm. The interaction of a PRR with its cognate ligand results in initiation of a signaling cascade leading to inflammatory responses.
CLRs consist of a diverse group of at least 17 transmembrane proteins, many of which are preferentially expressed on DCs and function as PRRs for pathogen-associated glycans such as β-glucans and exposed mannoses (Osorio and Reis e Sousa, 2011). Some CLRs recognize endogenous (self) ligands in addition to PAMPs and thus may not be ideal receptors to target. As opposed to the TLRs and CLRs, the NLRs and RLRs lack transmembrane domains and function as cytoplasmic sensors. Over 20 NLRs are predicted based upon the human genome (Elinav et al., 2011). All NLRs contain a nucleotide-binding oligomerization domain and a leucine-rich repeat (LRR). LRRs are also present on TLRs and are responsible for ligand recognition. The NLRs can be subdivided based upon whether they signal through a caspase recruitment domain (CARD) or pyrin domain. Sensing of PAMPs by many NLRs leads to formation of a proteolytically active multiprotein complex termed the inflammasome which matures the cytokines IL-1β and IL-18. The RLRs include the dsRNA sensors, RIG-I and MDA5, which interact with the adapter protein, MAVS, to stimulate NF-kB and IRF3/7 signal transduction pathways (O'Neill and Bowie, 2010). Stimulation of RIG-I also leads to inflammasome activation. Finally, at least six cytoplasmic DNA sensors have been described, each of which engage distinct signaling complexes (Hornung et al., 2009; Hornung and Latz, 2010; Sharma et al., 2011).
Aluminum salts
The use of precipitated aluminum salts as an adjuvant was first described in 1926 (Glenny et al., 1926). Following up on the observation that toxins precipitated with antitoxin were considerably more immunogenic than soluble toxin, the investigators found that adsorption of toxoid to precipitated aluminum salts increased its antigenicity, as measured by antibody titers in injected guinea pigs. For these studies, aluminum potassium sulfate was used. However, because of manufacturing problems with this salt, most clinical vaccines in current use contain aluminum hydroxide and/or aluminum phosphate (Marrack et al., 2009). A commercially available suspension of aluminum hydroxide and magnesium hydroxide (Imject® Alum), although not licensed for clinical use, has been tested in many experimental studies. Although originally used to describe aluminum potassium sulfate, the term “alum” is commonly used in a generic sense to refer to any aluminum salt used as an adjuvant. It should be appreciated though that the salts differ in some of their pharmacological and immunological properties (Clapp et al., 2011).
Adjuvants not only increase the quantity of an immune response but also direct the quality of the response (Coffman et al., 2010). The type of immune response observed following immunization with alum is generally polarized towards a Th2 T cell response with a predominance of Th2-associated antibody subtypes (Didierlaurent et al., 2009). Despite having been in clinical use for over 80 years, the mechanistic basis for the adjuvanticity of alum and its propensity to skew immune responses is controversial (Table 1). It was originally postulated that the slow rate of release of antigen from alum allowed for extended stimulation of the immune system (Glenny et al., 1926). However, subsequent studies cast doubts on the “depot theory” by demonstrating that B cell blasts were found in draining lymph nodes at early time points and that excision of the injection site nodule two weeks after immunization did not affect antibody titers (Marrack et al., 2009).
Table 1.
Proposed hypotheses for the adjuvanticity of alum.
| Hypothesis | Mechanism |
|---|---|
| Depot theory | Slow rate of antigen release from alum |
| Inflammasome activation | Stimulation of the NLRP3 inflammasome |
| Prostaglandin production | Stimulation of PGE2 by a Syk- and p38 MAP kinase-dependent pathway |
| Endogenous danger signals | Release of uric acid and DNA following cell death |
| Membrane lipids | Binding to membrane lipids triggers signaling pathways and antigen uptake |
| B cell priming | Induction of IL-4 |
The importance of the innate immune response to alum’s adjuvanticity was first suggested by studies demonstrating that uptake by antigen-presenting cells is enhanced when soluble antigen is adsorbed to particulate alum and that uptake is accompanied by a brisk proinflammatory response (Mannhalter et al., 1985). Subsequent studies using mice genetically deficient in both MyD88 and TRIF demonstrated that TLR signaling is dispensable for the antibody-enhancing effects of alum (Gavin et al., 2006). Phagocytosis of alum activates the NLRP3 inflammasome in primed macrophages and DCs in vitro by a mechanism that depends upon phagosomal acidification followed by lysosomal damage and cathepsin B release (Hornung et al., 2008; Kool et al., 2008). While inflammasome activation is required for alum-induced IL-1β release, there are conflicting reports regarding whether induction of antibodies by alum is dependent upon the NLRP3 inflammasome (Eisenbarth et al., 2008; Franchi and Nunez, 2008). Recently, it was shown that alum stimulates the production of the prostaglandin PGE2 by the Syk and p38 MAP kinase pathway in an inflammasome-independent manner (Kuroda et al., 2011). Moreover, production of antigen-specific IgE, an immunoglobulin associated with Th2 responses, was markedly reduced in PGE synthase-deficient mice. There is also evidence that Th2-biased responses result from alum-induced cell death leading to the release of endogenous danger signals, including uric acid and DNA (Kool et al., 2011; Marichal et al., 2011).
While these data provide a potential explanation for why alum preferentially induces Th2-type responses, an alum-specific cell surface receptor has not been identified. It has been proposed that alum targets antigen to DCs by binding to membrane lipids in such a manner as to trigger intracellular signaling pathways and antigen uptake (Flach et al., 2011). Interestingly, while atomic force microscopy studies demonstrated that DCs had a selectively strong affinity for alum, this led to an abortive phagocytic response that nevertheless was accompanied by endocytosis of antigen. Moreover, DCs activated by alum exhibited strong and stable binding to CD4+ T cells, but not B cells. However, there is evidence that alum primes naïve B cells in vivo by inducing a population of myeloid cells that produce IL-4 (Jordan et al., 2004).
It is difficult to reconcile all the studies on alum, many of which are seemingly contradictory, and devise a unifying mechanism for the adjuvanticity of alum. Some of the disparate results are likely a result of differences in experimental conditions, such as the antigen and alum preparations used to formulate the vaccine, the chosen route of immunization and the genetic background of the mice (Marrack et al., 2009). In addition, many of the studies require confirmation in humans who generally have minimal inflammatory responses following injection with alum yet still mount robust antibody responses to antigens adsorbed on alum. It is clear though that alum has pleiotropic effects on the immune system and after 80 years of clinical use, we are getting closer to unraveling the mysteries of alum’s adjuvanticity.
Other licensed adjuvants
Besides alum, the only other licensed adjuvants are adjuvant system 04 (AS04), MF59 and AS03. AS04, which consists of 3-O-desacyl-42-monophosphoryl lipid A (MPL) adsorbed onto aluminum hydroxide, is used as an adjuvant in licensed vaccines against human papillomavirus (HPV, Cervarix®) and hepatitis B virus (FENDrix®). MPL is a derivative of Salmonella minnesota LPS which lacks some acyl chains, polysaccharide side groups and phosphates. MPL has approximately 1000-fold less toxicity than its parent molecule, lipid A. This attenuated potency is, in all likelihood, because the 1-phosphate of lipid A (which is missing on MPL), ordinarily coordinates with several positively charged residues on TLR4 (K362, R264 and K341), enhancing the assembly of the active TLR4/MD-2 dimer:dimer. The ability of the toxified lipid A to nevertheless enhance the immune response may be, at least in part, due to MPL preferentially signaling via the TRIF-TRAM pathway, whereas LPS uses both the MyD88-Mal and TRIF-TRAM pathways (Mata-Haro et al., 2007). The basis of the selectivity of MPL for the TRIF pathway has, of yet, no obvious explanation, but may involve enhanced trafficking of MPL via CD14 into a subcellular compartment that is selectively capable of engaging TRIF-TRAM. Other explanations for the lower toxicity of MPL have been offered, including induction of higher levels of the anti-inflammatory cytokine, IL-10, and inefficient activation of the inflammasome-associated enzyme, caspase-1 (Casella and Mitchell, 2008). In clinical studies, the toxicity of AS04 was similar to that of alum alone. However, the AS04-adjuvanted vaccine formulation induced superior antibody and memory B cell responses (Giannini et al., 2006). In addition to the TLR4 agonist, MPL (discussed above), which is already in clinical use, synthetic TLR4 ligands are in development for use as adjuvants (Coler et al., 2011).
MF59 and AS03 are trade names for squalene-based oil-in-water emulsions used as vaccine adjuvants. Although a specific receptor has not been identified, squalene is internalized by DCs (Dupuis et al., 1998). Following intramuscular injection, MF59 induced more changes in gene expression, stronger cytokine production and a greater influx of immune cells compared with alum and the TLR9 agonist, CpG (Mosca et al., 2008). In mice, the adjuvanticity of MF59, as measured by high level antibody responses to a Neisseria meningitides antigen, was dependent on MyD88 but not the NLRP3 inflammasome (Seubert et al., 2011). However, in vitro, MF59 does not activate TLRs suggesting that the requirement for MyD88 is dependent upon its function as an adapter protein for either the IL-1 receptor or TACI (He et al., 2010). AS03 differs from MF59 in that it contains α-tocopherol, a form of vitamin E. The presence of α-tocopherol modulates the cytokine response at the injection site and cell recruitment to the draining lymph nodes, ultimately resulting in enhanced antibody responses (Morel et al., 2011).
AS03 and MF59-adjuvanted influenza vaccines have been approved for use in Europe and Canada, but as of this writing, not the United States. Compared with nonadjuvanted influenza vaccines, recipients of vaccines adjuvanted with MF59 and AS03 have superior antibody responses but also increased reactions (most of which are mild) at the injection site (Pellegrini et al., 2009). Both adjuvants allow for antigen dose sparing, an important factor particularly during pandemics when the capacity to manufacture antigen may be limited (Mbow et al., 2010).
Live, attenuated vaccines
Live attenuated vaccines have species-specific PAMPs with the potential to stimulate their cognate PRRs. Progress has been made defining the contribution of these receptor-ligand interactions to vaccine-mediated protection. Impaired cytokine production was observed when DCs from TLR2-, TLR7- and TLR9-deficient mice were stimulated with the live yellow fever vaccine, YF-17D (Querec et al., 2006). In contrast, TLR4 did not appear to play a role. Consistent with this diversified stimulation, YF-17D induced a mixed Th1/Th2 CD4+ T cell response in wild-type mice in vivo. However, Th1 responses were diminished in MyD88−/− mice and greatly enhanced in TLR2−/− mice, suggesting that distinct TLRs differentially control the quality of the T cell response. Additional studies used a systems biology approach to identify innate immune “genomic signatures” that predict neutralizing antibody and CD8+ T cell responses to YF-17D vaccination (Querec et al., 2009).
Other live, attenuated vaccines in clinical use have been less successful, notably bacillus Calmette-Guérin (BCG), which only affords modest protection against tuberculosis. Interestingly though, BCG, presumably by virtue of its ability to stimulate innate immune responses, appears to reduce the mortality from infections other than tuberculosis, such as neonatal sepsis (Shann, 2011). Similar effects have been seen with other live vaccines, although in some instances an increase in mortality has been observed. To improve the safety profile of live, attenuated vaccines while retaining efficacy, ongoing effects have been directed at retaining their immunostimulatory capacity while decreasing their potential for untoward side effects. Recombinant BCG constructs and subunit vaccine booster strategies directed at altering innate and acquired immune responses to the vaccine are also being pursued. For example, a strain of BCG was engineered to express the pore-forming toxin, listeriolysin and was deleted of genes which inhibit phagosomal acidification (Kaufmann, 2010). The former boosted CD8+ T cell responses by promoting antigen release into the cytosol. The latter promoted apoptosis of host cells with the resultant formation of antigen-laden apoptotic vesicles that got taken up by DCs. In fact, it is becoming increasingly apparent that microbe-induced apoptosis triggers innate immune responses and resultant adaptive responses (Brereton and Blander, 2011). Many pathogens have evolved mechanisms to prevent infected cells from undergoing apoptosis. The effect of deleting genes encoding for apoptosis inhibitors is being tested for many live vaccine candidates, as ways to both increase attenuation and immunogenicity (Eitz Ferrer et al., 2011; Hinchey et al., 2011). Other strategies for improved attenuated vaccines include genetically engineering Salmonella strains to produce predominantly 1-dephosphorylated lipid A (similar to MPL adjuvant, discussed above). This results in retention of immunogenicity despite a five log decrease in virulence in mice (Kong et al., 2011).
Experimental adjuvants that stimulate TLRs
The finding that the major classes of PRRs are found on DCs and respond to microbial infection by triggering adaptive immune responses imparts a strong rationale for the use of PRR agonists as vaccine adjuvants. The physical association of antigen and agonist may be important as DCs have been shown to preferentially process and present antigen from compartments that also contain TLR ligands (Blander and Medzhitov, 2006). It has also become increasingly apparent that the qualitative nature of the adaptive immune response may vary depending on which TLR is stimulated (Querec et al., 2006). Moreover, combinations of TLR agonists can have synergistic effects when used as adjuvants, resulting in greater and more durable responses to antigens as well as dose sparing (Kasturi et al., 2011; Zhu et al., 2008).
Immunostimulatory sequences (ISS) comprised of DNA designed to stimulate TLR9 responses have been developed as vaccine adjuvants. In general, strong antibody and Th1-biased T cell responses have been found with TLR9 agonists. The translational value of murine studies using DNA ISS has been questioned because the cellular distribution of TLR9 is much more limited in the human compared to the mouse. However, human plasmacytoid DCs express TLR9 and make T cell-activating cytokines, including IFNα and IL-12, when stimulated with DNA ISS. Heplisav®, a vaccine combining a CpG-rich TLR9 agonist (1018 ISS) with rHBsAg (Barry and Cooper, 2007), has been tested in phase III clinical trials in adults with chronic renal disease. Imiquimod, a TLR7/8 agonist approved for the topical treatment of certain skin diseases, and related compounds such as resiquimod, are also being studied as vaccine adjuvants. The wider cellular distribution in humans of TLR7, compared to TLR9, make these compounds attractive as adjuvants. However, immune dysfunction, which may be caused at least in part by TLR-independent mechanisms, have limited the systemic use of these compounds (Gunzer et al., 2005). More specific TLR7/8-activating ISS RNAs show greater promise as adjuvants, although they must be complexed so that they are preferentially delivered to endosomal compartments rather than degraded by extracellular RNases. In one study, the adjuvanticity of a ssRNA TLR7 agonist required IFNα production by pDC (Rajagopal et al., 2010). Finally, poly (I):poly (C12U), which is structurally similar to dsRNA and acts as a TLR3 and MDA5 agonist, promotes protective mucosal and systemic antibody responses in mice following intranasal administration with influenza antigens (Ichinohe et al., 2007).
In vitro, TLR2 agonists stimulate robust IL-12 production in DCs and thus would be predicted to promote Th1-type responses (Brightbill et al., 1999). In vivo, this has been the case with some, but not all studies. For example, animals vaccinated with a promiscuous peptide from M. tuberculosis conjugated to the TLR2 ligand, Pam2Cys, mounted Th1-type responses and were protected against mycobacterial challenge (Gowthaman et al., 2011). However, the neisserial outer membrane protein PorB, which was originally used as a carrier protein in vaccines but was later found to have TLR2-dependent adjuvanticity, was found to induce a Th2-type profile (Burke et al., 2007). Interestingly, eosinophilic recall responses were observed, which could be beneficial for vaccines against helminth infections. The outer-surface lipoprotein (OspA) of Borrelia burgdorferi, the causative agent of Lyme disease, is sensed by a heterodimer composed of TLR2 and TLR1. Low antibody titers to recombinant OspA, the major component of a withdrawn vaccine against Lyme disease, were associated with defects in the TLR2/1 signaling pathway (Alexopoulou et al., 2002). In addition to the TLR4 agonist, MPL (discussed above), which is already in clinical use, synthetic TLR4 ligands are in development for use as adjuvants (Coler et al., 2011).
Flagellin, the major structural protein of flagella on gram negative bacteria, has received extensive study as an adjuvant. Extracellular flagellin is sensed via TLR5 whereas intracellular flagellin stimulates the NLRC4 inflammasome by a process requiring direct recognition by the NOD-like receptor protein, NAIP5 (Mizel and Bates, 2010; Zhao et al., 2011). TLR5 signaling generally appears to be more important for adjuvanticity because most flagellin-formulated vaccines are designed to stimulate DCs extracellularly. However, a Listeria monocytogenes strain engineered to express flagellin in the host cell cytoplasm was attenuated because of its ability to hyperactivate the NLRC4 inflammasome yet able to confer protective immunity against virulent L. monocytogenes (Warren et al., 2011). An advantage of flagellin as an adjuvant is it can be incorporated as a fusion protein in recombinant vaccines, either within its hypervariable region or at its N-terminus (Mizel and Bates, 2010). In general, flagellin-adjuvanted vaccines stimulate robust antibody and CD4+ T cell responses. In phase I and phase II trials, a recombinant hemagglutinin influenza-flagellin fusion vaccine was safe and induced seroprotective titers, even in the elderly (Taylor et al., 2011).
TLR-independent adjuvants
Recognition of fungal cell wall β-1,3-D-glucans by the CLR, Dectin-1, activates Syk- and Raf1-dependent signal transduction pathways resulting in Th1 and Th17-skewed T cell responses (LeibundGut-Landmann et al., 2007). Accordingly, vaccination of mice with glucan particles containing entrapped antigen leads to robust antigen-specific Th1 and Th17 CD4+ T cell and antibody responses (Huang et al., 2010). A less selective approach, which is being tested in clinical trials as a therapeutic vaccine, is the use of killed Saccharomyces cerevisiae yeast recombinantly expressing antigens (Stubbs et al., 2001). Another CLR, Mincle, recognizes and mediates the adjuvanticity of the mycobacterial glycolipid, trehalose-6,6’-dimycolate (Schoenen et al., 2010). Other CLRs distinguish exposed mannose residues and targeting these receptors by mannosylating antigens has led to enhanced T cell responses even in the absence of adjuvants (Lam et al., 2005).
NLRs, RLRs and cytoplasmic DNA sensors are attractive targets for adjuvant development because they are generally expressed at high levels in DCs and when activated stimulate NF-kB and IRF-dependent proinflammatory programs. Adjuvants that stimulate these receptors must be able to reach their cytoplasmic location, although membrane transport systems exist for at least for some NLR agonists (Elinav et al., 2011). As cytoplasmic sensors of RNA, the RLRs are critical for mounting innate immune responses to RNA viruses (Loo and Gale, 2011). A DNA vaccine encoding for influenza virus hemagglutinin and a RIG-I agonist stimulated enhanced type-I IFN responses in vitro and increased antibody titers in immunized mice (Luke et al., 2011). RIG-I may also be important in the response to some RNA vaccines. Two single nucleotide polymorphisms (SNPs) in the RIG-I gene were associated with altered antibody titers following administration of the rubella vaccine (a live attenuated ssRNA virus) to children (Ovsyannikova et al., 2010). Genome wide association studies have identified SNPs in other immune genes that contribute to heterogeneous vaccine responses. Indeed, as immunogenetics becomes more sophisticated, it may become possible to predict those individuals who are likely to have suboptimal or adverse vaccine responses and thus could benefit from alternative formulations.
Cytoplasmic DNA is also recognized by the host via the RIG-I pathway when dsDNA is transcribed by RNA polymerase III into immunostimulatory RNA. As noted above, direct mechanisms of dsDNA sensing also exist and DNA vaccines have been shown to possess intrinsic TLR9-independent adjuvanticity via a pathway dependent upon the signaling molecule TANK-binding kinase 1 (TBK1) (Ishii et al., 2008). The transmembrane protein STING, which is upstream of TBK1, was recently shown to directly bind cyclic dinucleotides (Burdette et al., 2011). Cyclic dinucleotides are conserved signaling molecules in bacteria which have adjuvancity (Chen et al., 2010).
Alum and some experimental particulate adjuvants, including chitosan, activate the NLRP3 inflammasome (Bueter et al., 2011), although as noted above, a causal relationship between inflammasome stimulation and adjuvanticity has not been definitively established. Toxicity of NOD1 and NOD2 ligands has generally limited their use as adjuvants, although efforts to develop less toxic agonists of NOD1 and NOD2 that retain their adjuvanticity are ongoing. Complete Freund’s adjuvant (CFA), which consists of inactivated Mycobacteria in a water-in-oil emulsion, contains peptidoglycan and trehalose dimycolate which stimulate NOD2 and Mincle, respectively (Coffman et al., 2010). Additionally, CFA has ligands for TLR2, TLR4, and TLR9. CFA stimulates potent antibody, Th1 and Th17 responses but is generally too toxic to use in humans because of inflammatory side effects.
Liposomes consist of phospholipid bilayers with an aqueous core. A variety of technologies have been developed to incorporate antigens into liposomes, and liposomal influenza and hepatitis A vaccines are licensed in some countries for clinical use. In addition to acting as a delivery system, liposomal vaccines have intrinsic adjuvant effects which can be increased by incorporating PAMPs (Demento et al., 2011). A malaria vaccine adjuvanted with AS01, a liposomal preparation containing MPL and the plant saponin Quillaja saponaria 21 (QS21), demonstrated safety and efficacy in phase 2 and 3 trials in Africa (Asante et al., 2011; The RTS, 2011). Vaccines adjuvanted with QS21 and another saponin-containing formulation, ISCOMATRIX, appear to be particularly potent at inducing CD8+ T cell responses (Duewell et al., 2011).
Dendritic cell vaccines
Immunotherapeutic vaccination with DCs generated ex vivo holds promise for individuals suffering from chronic infectious diseases and neoplasms. One such vaccine was licensed after it was shown to prolong survival in patients with metastatic prostate cancer (Kantoff et al., 2010). Given the expense of isolation, antigen loading and reinjection, routine preventive vaccination with DCs generated ex vivo is unlikely to be tenable. In vivo targeting of antigen to DCs circumvents these problems and also permits delivery to specific DCs subsets based on expression of cell surface markers (Tacken and Figdor, 2011). For example, mice inoculated with an antigen conjugated to a monoclonal antibody directed at DEC-205, an endocytic receptor which is expressed at high levels on lymphoid tissue DCs, had potent stimulation of CD4+ and CD8+ T cell responses (Bonifaz et al., 2004). A phase I clinical trial of a vaccine containing an anti-DEC-205 human monoclonal antibody fused to the HIV gag p24 protein is ongoing.
As ongoing inflammation is deleterious to the host, it is not surprising that following the induction of an inflammatory response, mechanisms exist to shut off inflammation. A novel approach to DC vaccination consists of using RNAi to silence negative regulators of inflammation. Suppressor of cytokine signaling 1 (SOCS1) targets JAK2 for degradation, leading to the inhibition of STAT. Silencing SOCS1 in DCs by coimmunization of HIV DNA with SOCSS1 siRNA expressor DNA resulted in enhanced HIV-specific antibody and T cell responses (Song et al., 2006). Similar salutary efforts were observed when A20, a ubiquitin-modifying enzyme that is required for terminating the NF-kB activation signal, was silenced (Song et al., 2008).
Practical considerations and challenges for the next decade
A remarkable number of PRRs have been discovered in the past decade which in turn has prompted the rational development of candidate adjuvants based upon their ability to stimulate these innate immune sensors. A major challenge for the next decade will be to translate these findings into protective human vaccines that have an acceptable safety profile. In addition to phases I–IV clinical safety evaluations, European and US licensing authorities recommend that novel adjuvants should be evaluated in nonclinical toxicology studies, both as separate entities and as part of the final vaccine formulation (Ahmed et al., 2011; Garcon et al., 2011). This greatly increases the expense of clinical introduction of new adjuvants but is justified as vaccines need to have a very low risk/benefit ratio, particularly preventive vaccines given to predominantly healthy people. Given the association between autoimmunity and dysregulated innate immune responses, a theoretical risk of all vaccines containing immunoenhancing adjuvants is the development of autoimmunity (Ahmed et al., 2011).
Public acceptance and trust needs to be high. An efficacious vaccine against Lyme disease was withdrawn by the manufacturer because of public apprehension (and the resulting litigation) that the vaccine was causing autoimmune side effects. This, even though the FDA and CDC concluded there was no sound evidence of a link (Abbott, 2006). Fears that childhood vaccines can cause autism has caused many parents to refuse to vaccinate their children despite the report linking autism to the MMR vaccine being retracted after it was found to be fraudulent. An ongoing challenge is finding more effective ways to educate the public about the benefits of vaccination. While serious adverse events are always the major concern, side effects such as redness and soreness at the injection site can limit compliance amongst patients and use by vaccines providers. Adjuvants that stimulate potent innate immune responses may also stimulate untoward inflammatory responses, thus precluding some experimental adjuvants from introduction into the clinical arena.
The changing demographics of the world’s population bring with it a growing need for vaccines that target and protect specific groups (Rappuoli et al., 2011). The challenge is two-fold in groups such as the elderly and immunocompromised who have poor responses to traditional vaccines and are susceptible to a variety of infections not commonly seen in the general population. Unique problems exist in developing regions of the world where the health care infrastructure is often underfunded and malnutrition may decrease vaccination responses. The thermal stability of vaccines assumes great importance in areas that lack cold chain distribution systems (Clapp et al., 2011). Political and religious obstacles to vaccination may need to be overcome. For example, a rumor that the polio vaccine was a Western plot to sterilize children and spread AIDS led to low vaccination rates and a polio outbreak that paralyzed over 1,500 children in Nigeria and other countries (Jegede, 2007). Economic considerations have also played a role as vaccines that target infections predominantly afflicting the world’s poorest people are unlikely to be major revenue generators for pharmaceutical companies. Nevertheless, well funded private-public partnerships have been formed to develop vaccines against many of the world’s “neglected diseases”.
In conclusion, insights in the past decade arising from advances in our understanding of innate immunity have provided a clearer idea of how existing, empirically designed adjuvants and live attenuated vaccines work. New vaccines formulations incorporating adjuvants selected because of their ability to specifically signal innate immune pathways have been or are being developed. A major challenge for the next decade will be to continue to bring these advances to the myriad and diverse at risk populations that could benefit from new vaccines.
Acknowledgements
SML is supported by National Institutes of Health grants AI025780 and AI093302-01 and DTG is supported by AI052455, AI07293 and GM54060. The authors thank George Deepe for his critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott A. Lyme disease: Uphill Struggle. Nature. 2006;439:524–525. doi: 10.1038/439524a. [DOI] [PubMed] [Google Scholar]
- Ahmed SS, Plotkin SA, Black S, Coffman RL. Assessing the Safety of Adjuvanted Vaccines. Science Translational Medicine. 2011;3 doi: 10.1126/scitranslmed.3002302. 93rv92. [DOI] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L, Thomas V, Schnare M, Lobet Y, Anguita J, Schoen RT, Medzhitov R, Fikrig E, Flavell RA. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat Med. 2002;8:878–884. doi: 10.1038/nm732. [DOI] [PubMed] [Google Scholar]
- Asante KP, Abdulla S, Agnandji S, Lyimo J, Vekemans J, Soulanoudjingar S, Owusu R, Shomari M, Leach A, Jongert E, et al. Safety and efficacy of the RTS,S/AS01(E) candidate malaria vaccine given with expanded-programme-on-immunisation vaccines-19 month follow-up of a randomised, open-label, phase 2 trial. Lancet Infect Dis. 2011;11:741–749. doi: 10.1016/S1473-3099(11)70100-1. [DOI] [PubMed] [Google Scholar]
- Barry M, Cooper C. Review of hepatitis B surface antigen-1018 ISS adjuvant-containing vaccine safety and efficacy. Expert Opin Biol Ther. 2007;7:1731–1737. doi: 10.1517/14712598.7.11.1731. [DOI] [PubMed] [Google Scholar]
- Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440:808–812. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, Soares H, Brimnes MK, Moltedo B, Moran TM, Steinman RM. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199:815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brereton CF, Blander JM. The unexpected link between infection-induced apoptosis and a Th17 immune response. Journal of Leukocyte Biology. 2011;89:565–576. doi: 10.1189/jlb.0710421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brightbill HD, Libraty DH, Krutzik SR, Yang R-B, Belisle JT, Bleharski JR, Maitland M, Norgard MV, Plevy SE, Smale ST, et al. Host Defense Mechanisms Triggered by Microbial Lipoproteins Through Toll-Like Receptors. Science. 1999;285:732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- Brinkmann MM, Spooner E, Hoebe K, Beutler B, Ploegh HL, Kim Y-M. The interaction between the ER membrane protein UNC93B and TLR3, 7, and 9 is crucial for TLR signaling. The Journal of Cell Biology. 2007;177:265–275. doi: 10.1083/jcb.200612056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueter CL, Lee CK, Rathinam VA, Healy GJ, Taron CH, Specht CA, Levitz SM. Chitosan but not chitin activates the inflammasome by a mechanism dependent upon phagocytosis. J Biol Chem. 2011;286:35447–35455. doi: 10.1074/jbc.M111.274936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, Hayakawa Y, Vance RE. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011;478:515–518. doi: 10.1038/nature10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JM, Ganley-Leal LM, Khatri A, Wetzler LM. Neisseria meningitidis PorB, a TLR2 Ligand, Induces an Antigen-Specific Eosinophil Recall Response: Potential Adjuvant for Helminth Vaccines? The Journal of Immunology. 2007;179:3222–3230. doi: 10.4049/jimmunol.179.5.3222. [DOI] [PubMed] [Google Scholar]
- Casella C, Mitchell T. Putting endotoxin to work for us: Monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cellular and Molecular Life Sciences. 2008;65:3231–3240. doi: 10.1007/s00018-008-8228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Kuolee R, Yan H. The potential of 3',5'-cyclic diguanylic acid (c-di-GMP) as an effective vaccine adjuvant. Vaccine. 2010;28:3080–3085. doi: 10.1016/j.vaccine.2010.02.081. [DOI] [PubMed] [Google Scholar]
- Clapp T, Siebert P, Chen D, Jones Braun L. Vaccines with aluminum-containing adjuvants: Optimizing vaccine efficacy and thermal stability. Journal of Pharmaceutical Sciences. 2011;100:388–401. doi: 10.1002/jps.22284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman RL, Sher A, Seder RA. Vaccine Adjuvants: Putting Innate Immunity to Work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coler RN, Bertholet S, Moutaftsi M, Guderian JA, Windish HP, Baldwin SL, Laughlin EM, Duthie MS, Fox CB, Carter D, et al. Development and Characterization of Synthetic Glucopyranosyl Lipid Adjuvant System as a Vaccine Adjuvant. PLoS ONE. 2011;6:e16333. doi: 10.1371/journal.pone.0016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demento SL, Siefert AL, Bandyopadhyay A, Sharp FA, Fahmy TM. Pathogen-associated molecular patterns on biomaterials: a paradigm for engineering new vaccines. Trends in Biotechnology. 2011;29:294–306. doi: 10.1016/j.tibtech.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H, Kielland A, Vosters O, Vanderheyde N, Schiavetti F, et al. AS04, an Aluminum Salt- and TLR4 Agonist-Based Adjuvant System, Induces a Transient Localized Innate Immune Response Leading to Enhanced Adaptive Immunity. The Journal of Immunology. 2009;183:6186–6197. doi: 10.4049/jimmunol.0901474. [DOI] [PubMed] [Google Scholar]
- Duewell P, Kisser U, Heckelsmiller K, Hoves S, Stoitzner P, Koernig S, Morelli AB, Clausen BE, Dauer M, Eigler A, et al. ISCOMATRIX Adjuvant Combines Immune Activation with Antigen Delivery to Dendritic Cells In Vivo Leading to Effective Cross-Priming of CD8+ T Cells. The Journal of Immunology. 2011;187:55–63. doi: 10.4049/jimmunol.1004114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis M, Murphy TJ, Higgins D, Ugozzoli M, van Nest G, Ott G, McDonald DM. Dendritic Cells Internalize Vaccine Adjuvant after Intramuscular Injection. Cellular Immunology. 1998;186:18–27. doi: 10.1006/cimm.1998.1283. [DOI] [PubMed] [Google Scholar]
- Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitz Ferrer P, Potthoff S, Kirschnek S, Gasteiger G, Kastenmuller W, Ludwig H, Paschen SA, Villunger A, Sutter G, Drexler I, Hacker G. Induction of Noxa-Mediated Apoptosis by Modified Vaccinia Virus Ankara Depends on Viral Recognition by Cytosolic Helicases, Leading to IRF-3/IFN-Î2-Dependent Induction of Pro-Apoptotic Noxa. PLoS Pathog. 2011;7:e1002083. doi: 10.1371/journal.ppat.1002083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinav E, Strowig T, Henao-Mejia J, Flavell Richard A. Regulation of the Antimicrobial Response by NLR Proteins. Immunity. 2011;34:665–679. doi: 10.1016/j.immuni.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Flach TL, Ng G, Hari A, Desrosiers MD, Zhang P, Ward SM, Seamone ME, Vilaysane A, Mucsi AD, Fong Y, et al. Alum interaction with dendritic cell membrane lipids is essential for its adjuvanticity. Nat Med. 2011;17:479–487. doi: 10.1038/nm.2306. [DOI] [PubMed] [Google Scholar]
- Franchi L, Nunez G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1beta secretion but dispensable for adjuvant activity. Eur J Immunol. 2008;38:2085–2089. doi: 10.1002/eji.200838549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcon N, Segal L, Tavares F, Van Mechelen M. The safety evaluation of adjuvants during vaccine development: the AS04 experience. Vaccine. 2011;29:4453–4459. doi: 10.1016/j.vaccine.2011.04.046. [DOI] [PubMed] [Google Scholar]
- Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, Nemazee D. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini SL, Hanon E, Moris P, Van Mechelen M, Morel S, Dessy F, Fourneau MA, Colau B, Suzich J, Losonksy G, et al. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine. 2006;24:5937–5949. doi: 10.1016/j.vaccine.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Glenny AT, Pope CG, Waddington H, Wallace U. Immunological notes. XVII-XXIV The Journal of Pathology and Bacteriology. 1926;29:31–40. [Google Scholar]
- Gowthaman U, Singh V, Zeng W, Jain S, Siddiqui KF, Chodisetti SB, Gurram RK, Parihar P, Gupta P, Gupta UD, et al. Promiscuous Peptide of 16 kDa Antigen Linked to Pam2Cys Protects Against Mycobacterium tuberculosis by Evoking Enduring Memory T-Cell Response. J Infect Dis. 2011 doi: 10.1093/infdis/jir548. [DOI] [PubMed] [Google Scholar]
- Gunzer M, Riemann H, Basoglu Y, Hillmer A, Weishaupt C, Balkow S, Benninghoff B, Ernst B, Steinert M, Scholzen T, et al. Systemic administration of a TLR7 ligand leads to transient immune incompetence due to peripheral-blood leukocyte depletion. Blood. 2005;106:2424–2432. doi: 10.1182/blood-2005-01-0342. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G, Lambris JD. Microbial manipulation of receptor crosstalk in innate immunity. Nat Rev Immunol. 2011;11:187–200. doi: 10.1038/nri2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Santamaria R, Xu W, Cols M, Chen K, Puga I, Shan M, Xiong H, Bussel JB, Chiu A, et al. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nat Immunol. 2010;11:836–845. doi: 10.1038/ni.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchey J, Jeon BY, Alley H, Chen B, Goldberg M, Derrick S, Morris S, Jacobs WR, Jr., Porcelli SA, Lee S. Lysine Auxotrophy Combined with Deletion of theSecA2 Gene Results in a Safe and Highly Immunogenic Candidate Live Attenuated Vaccine for Tuberculosis. PLoS ONE. 2011;6:e15857. doi: 10.1371/journal.pone.0015857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Latz E. Intracellular DNA recognition. Nat Rev Immunol. 2010;10:123–130. doi: 10.1038/nri2690. [DOI] [PubMed] [Google Scholar]
- Huang H, Ostroff GR, Lee CK, Specht CA, Levitz SM. Robust Stimulation of Humoral and Cellular Immune Responses following Vaccination with Antigen-Loaded beta-Glucan Particles. MBio. 2010;1 doi: 10.1128/mBio.00164-10. e00164-00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinohe T, Tamura S-i, Kawaguchi A, Ninomiya A, Imai M, Itamura S, Odagiri T, Tashiro M, Takahashi H, Sawa H, et al. Cross-Protection against H5N1 Influenza Virus Infection Is Afforded by Intranasal Inoculation with Seasonal Trivalent Inactivated Influenza Vaccine. Journal of Infectious Diseases. 2007;196:1313–1320. doi: 10.1086/521304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii KJ, Kawagoe T, Koyama S, Matsui K, Kumar H, Kawai T, Uematsu S, Takeuchi O, Takeshita F, Coban C, Akira S. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature. 2008;451:725–729. doi: 10.1038/nature06537. [DOI] [PubMed] [Google Scholar]
- Jegede AS. What Led to the Nigerian Boycott of the Polio Vaccination Campaign? PLoS Med. 2007;4:e73. doi: 10.1371/journal.pmed.0040073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan MB, Mills DM, Kappler J, Marrack P, Cambier JC. Promotion of B cell immune responses via an alum-induced myeloid cell population. Science. 2004;304:1808–1810. doi: 10.1126/science.1089926. [DOI] [PubMed] [Google Scholar]
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470:543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann SHE. Future Vaccination Strategies against Tuberculosis: Thinking outside the Box. Immunity. 2010;33:567–577. doi: 10.1016/j.immuni.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Kong Q, Six DA, Roland KL, Liu Q, Gu L, Reynolds CM, Wang X, Raetz CRH, Curtiss R. Salmonella Synthesizing 1-Dephosphorylated Lipopolysaccharide Exhibits Low Endotoxic Activity while Retaining Its Immunogenicity. The Journal of Immunology. 2011;187:412–423. doi: 10.4049/jimmunol.1100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool M, Petrilli V, De Smedt T, Rolaz A, Hammad H, van Nimwegen M, Bergen IM, Castillo R, Lambrecht BN, Tschopp J. Cutting Edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008;181:3755–3759. doi: 10.4049/jimmunol.181.6.3755. [DOI] [PubMed] [Google Scholar]
- Kool M, Willart MA, van Nimwegen M, Bergen I, Pouliot P, Virchow JC, Rogers N, Osorio F, Reis ESC, Hammad H, Lambrecht BN. An unexpected role for uric acid as an inducer of T helper 2 cell immunity to inhaled antigens and inflammatory mediator of allergic asthma. Immunity. 2011;34:527–540. doi: 10.1016/j.immuni.2011.03.015. [DOI] [PubMed] [Google Scholar]
- Kuroda E, Ishii Ken J, Uematsu S, Ohata K, Coban C, Akira S, Aritake K, Urade Y, Morimoto Y. Silica Crystals and Aluminum Salts Regulate the Production of Prostaglandin in Macrophages via NALP3 Inflammasome-Independent Mechanisms. Immunity. 2011;34:514–526. doi: 10.1016/j.immuni.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Lam JS, Mansour MK, Specht CA, Levitz SM. A model vaccine exploiting fungal mannosylation to increase antigen immunogenicity. J Immunol. 2005;175:7496–7503. doi: 10.4049/jimmunol.175.11.7496. [DOI] [PubMed] [Google Scholar]
- Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, Lien E, Nilsen NJ, Espevik T, Golenbock DT. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5:190–198. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, Reis e Sousa C. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- Loo Y-M, Gale M. Immune Signaling by RIG-I-like Receptors. Immunity. 2011;34:680–692. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke JM, Simon GG, Soderholm J, Errett JS, August JT, Gale M, Jr., Hodgson CP, Williams JA. Coexpressed RIG-I Agonist Enhances Humoral Immune Response to Influenza Virus DNA Vaccine. J Virol. 2011;85:1370–1383. doi: 10.1128/JVI.01250-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannhalter JW, Neychev HO, Zlabinger GJ, Ahmad R, Eibl MM. Modulation of the human immune response by the non-toxic and non-pyrogenic adjuvant aluminium hydroxide: effect on antigen uptake and antigen presentation. Clin Exp Immunol. 1985;61:143–151. [PMC free article] [PubMed] [Google Scholar]
- Marichal T, Ohata K, Bedoret D, Mesnil C, Sabatel C, Kobiyama K, Lekeux P, Coban C, Akira S, Ishii KJ, et al. DNA released from dying host cells mediates aluminum adjuvant activity. Nat Med. 2011;17:996–1002. doi: 10.1038/nm.2403. [DOI] [PubMed] [Google Scholar]
- Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9:287–293. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata-Haro Vn, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The Vaccine Adjuvant Monophosphoryl Lipid A as a TRIF-Biased Agonist of TLR4. Science. 2007;316:1628–1632. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- Mbow ML, De Gregorio E, Valiante NM, Rappuoli R. New adjuvants for human vaccines. Curr Opin Immunol. 2010;22:411–416. doi: 10.1016/j.coi.2010.04.004. [DOI] [PubMed] [Google Scholar]
- McKee AS, Munks MW, Marrack P. How Do Adjuvants Work? Important Considerations for New Generation Adjuvants. Immunity. 2007;27:687–690. doi: 10.1016/j.immuni.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Mizel SB, Bates JT. Flagellin as an Adjuvant: Cellular Mechanisms and Potential. The Journal of Immunology. 2010;185:5677–5682. doi: 10.4049/jimmunol.1002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel S, Didierlaurent A, Bourguignon P, Delhaye S, Baras B, Jacob V, Planty C, Elouahabi A, Harvengt P, Carlsen H, et al. Adjuvant System AS03 containing α-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine. 2011;29:2461–2473. doi: 10.1016/j.vaccine.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Mosca F, Tritto E, Muzzi A, Monaci E, Bagnoli F, Iavarone C, O'Hagan D, Rappuoli R, De Gregorio E. Molecular and cellular signatures of human vaccine adjuvants. Proceedings of the National Academy of Sciences. 2008;105:10501–10506. doi: 10.1073/pnas.0804699105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill LA, Bowie AG. Sensing and signaling in antiviral innate immunity. Curr Biol. 2010;20:R328–R333. doi: 10.1016/j.cub.2010.01.044. [DOI] [PubMed] [Google Scholar]
- Osorio F, Reis e Sousa C. Myeloid C-type Lectin Receptors in Pathogen Recognition and Host Defense. Immunity. 2011;34:651–664. doi: 10.1016/j.immuni.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Ovsyannikova IG, Haralambieva IH, Dhiman N, O'Byrne MM, Pankratz VS, Jacobson RM, Poland GA. Polymorphisms in the Vitamin A Receptor and Innate Immunity Genes Influence the Antibody Response to Rubella Vaccination. Journal of Infectious Diseases. 2010;201:207–213. doi: 10.1086/649588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini M, Nicolay U, Lindert K, Groth N, Della Cioppa G. MF59-adjuvanted versus non-adjuvanted influenza vaccines: Integrated analysis from a large safety database. Vaccine. 2009;27:6959–6965. doi: 10.1016/j.vaccine.2009.08.101. [DOI] [PubMed] [Google Scholar]
- Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat Immunol. 2011;12:509–517. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querec T, Bennouna S, Alkan S, Laouar Y, Gorden K, Flavell R, Akira S, Ahmed R, Pulendran B. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7 8, and 9 to stimulate polyvalent immunity. J Exp Med. 2006;203:413–424. doi: 10.1084/jem.20051720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querec TD, Akondy RS, Lee EK, Cao W, Nakaya HI, Teuwen D, Pirani A, Gernert K, Deng J, Marzolf B, et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009;10:116–125. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal D, Paturel C, Morel Y, Uematsu S, Akira S, Diebold SS. Plasmacytoid dendritic cell-derived type I interferon is crucial for the adjuvant activity of Toll-like receptor 7 agonists. Blood. 2010;115:1949–1957. doi: 10.1182/blood-2009-08-238543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappuoli R, Mandl CW, Black S, De Gregorio E. Vaccines for the twenty-first century society. Nat Rev Immunol. 2011;11:865–872. doi: 10.1038/nri3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarselli M, Arico B, Brunelli B, Savino S, Di Marcello F, Palumbo E, Veggi D, Ciucchi L, Cartocci E, Bottomley MJ, et al. Rational Design of a Meningococcal Antigen Inducing Broad Protective Immunity. Science Translational Medicine. 2011;3 doi: 10.1126/scitranslmed.3002234. 91ra62. [DOI] [PubMed] [Google Scholar]
- Schoenen H, Bodendorfer B, Hitchens K, Manzanero S, Werninghaus K, Nimmerjahn F, Agger EM, Stenger S, Andersen P, Ruland J, et al. Cutting Edge: Mincle Is Essential for Recognition and Adjuvanticity of the Mycobacterial Cord Factor and its Synthetic Analog Trehalose-Dibehenate. The Journal of Immunology. 2010;184:2756–2760. doi: 10.4049/jimmunol.0904013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sette A, Rappuoli R. Reverse Vaccinology: Developing Vaccines in the Era of Genomics. Immunity. 2010;33:530–541. doi: 10.1016/j.immuni.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seubert A, Calabro S, Santini L, Galli B, Genovese A, Valentini S, Aprea S, Colaprico A, D'Oro U, Giuliani MM, et al. Adjuvanticity of the oil-in-water emulsion MF59 is independent of Nlrp3 inflammasome but requires the adaptor protein MyD88. Proceedings of the National Academy of Sciences. 2011;108:11169–11174. doi: 10.1073/pnas.1107941108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shann F. The nonspecific effects of vaccines and the expanded program on immunization. J Infect Dis. 2011;204:182–184. doi: 10.1093/infdis/jir244. [DOI] [PubMed] [Google Scholar]
- Sharma S, DeOliveira Rosane B, Kalantari P, Parroche P, Goutagny N, Jiang Z, Chan J, Bartholomeu Daniella C, Lauw F, Hall JP, et al. Innate Immune Recognition of an AT-Rich Stem-Loop DNA Motif in the Plasmodium falciparum Genome. Immunity. 2011;35:194–207. doi: 10.1016/j.immuni.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XT, Evel-Kabler K, Rollins L, Aldrich M, Gao F, Huang XF, Chen SY. An alternative and effective HIV vaccination approach based on inhibition of antigen presentation attenuators in dendritic cells. PLoS Med. 2006;3:e11. doi: 10.1371/journal.pmed.0030011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XT, Evel-Kabler K, Shen L, Rollins L, Huang XF, Chen SY. A20 is an antigen presentation attenuator, and its inhibition overcomes regulatory T cell-mediated suppression. Nat Med. 2008;14:258–265. doi: 10.1038/nm1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs AC, Martin KS, Coeshott C, Skaates SV, Kuritzkes DR, Bellgrau D, Franzusoff A, Duke RC, Wilson CC. Whole recombinant yeast vaccine activates dendritic cells and elicits protective cell-mediated immunity. Nat Med. 2001;7:625–629. doi: 10.1038/87974. [DOI] [PubMed] [Google Scholar]
- Tacken PJ, Figdor CG. Targeted antigen delivery and activation of dendritic cells in vivo: Steps towards cost effective vaccines. Seminars in Immunology. 2011;23:12–20. doi: 10.1016/j.smim.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Taylor DN, Treanor JJ, Strout C, Johnson C, Fitzgerald T, Kavita U, Ozer K, Tussey L, Shaw A. Induction of a potent immune response in the elderly using the TLR-5 agonist, flagellin, with a recombinant hemagglutinin influenza-flagellin fusion vaccine (VAX125, STF2.HA1 SI) Vaccine. 2011;29:4897–4902. doi: 10.1016/j.vaccine.2011.05.001. [DOI] [PubMed] [Google Scholar]
- The RTS SCTP. First Results of Phase 3 Trial of RTS,S/AS01 Malaria Vaccine in African Children. New England Journal of Medicine. 2011 doi: 10.1056/NEJMoa1102287. 0, null. [DOI] [PubMed] [Google Scholar]
- Warren SE, Duong H, Mao DP, Armstrong A, Rajan J, Miao EA, Aderem A. Generation of a Listeria vaccine strain by enhanced caspase-1 activation. European Journal of Immunology. 2011;41:1934–1940. doi: 10.1002/eji.201041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuthrich M, Filutowicz HI, Warner T, Deepe GS, Jr., Klein BS. Vaccine immunity to pathogenic fungi overcomes the requirement for CD4 help in exogenous antigen presentation to CD8+ T cells: implications for vaccine development in immune-deficient hosts. J Exp Med. 2003;197:1405–1416. doi: 10.1084/jem.20030109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Yang J, Shi J, Gong Y-N, Lu Q, Xu H, Liu L, Shao F. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477:596–600. doi: 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Egelston C, Vivekanandhan A, Uematsu S, Akira S, Klinman DM, Belyakov IM, Berzofsky JA. Toll-like receptor ligands synergize through distinct dendritic cell pathways to induce T cell responses: implications for vaccines. Proc Natl Acad Sci U S A. 2008;105:16260–16265. doi: 10.1073/pnas.0805325105. [DOI] [PMC free article] [PubMed] [Google Scholar]


