Abstract
Here, we show that oxygen and glucose deprivation (OGD) causes increased small ubiquitin-like modifier (SUMO)-1 and SUMO-2/3 conjugation to substrate proteins in cultured hippocampal neurones. Surprisingly, the SUMO protease SENP-1, which removes SUMO from conjugated proteins, was also increased by OGD, suggesting that the neuronal response to OGD involves a complex interplay between SUMOylation and deSUMOylation. Importantly, decreasing global SUMOylation in cultured hippocampal neurones by overexpression of the catalytic domain of SENP-1 increased neuronal vulnerability to OGD-induced cell death. Taken together, these results suggest a neuroprotective role for neuronal SUMOylation after OGD.
Keywords: ischemia, neuronal culture, oxygen and glucose deprivation (OGD), posttranslational modification, SENP-1, SUMO
Introduction
Posttranslational protein modification by Small Ubiquitin-like MOdifiers (SUMOs) has been postulated as an important signaling pathway in the neuronal response to stroke (Wilkinson et al, 2010). There are three validated SUMO paralogues, SUMO-1–3, with SUMO-2 and SUMO-3 differing in three amino acids. SUMOylation of most proteins is rapidly reversed by SENPs, which also process nascent SUMO to conjugatable SUMO. There are six SENPs (SENP-1–3 and SENP-5–7), varying in cellular distribution, SUMO paralogue specificity, and selectivity for SUMO maturation versus deconjugation. SENP-1 has a broad specificity for SUMO-1 and SUMO-2/3 and acts in both their maturation and deconjugation (Wilkinson and Henley, 2010).
SUMOylation is dramatically increased in hibernating squirrels what led to the hypothesis that it may protect cells from otherwise lethally low levels of oxygen and glucose due to reduced blood flow (Lee et al, 2007). SUMO-1 mRNA is increased by hypoxia (Shao et al, 2004) and SUMOylation is enhanced in several models of ischemia (Cimarosti et al, 2008; Yang et al, 2008a, 2008b). Hypothermia induces SUMO-2/3 conjugation, translocation to the nucleus, and modified gene expression, further suggesting a neuroprotective role (Loftus et al, 2009). Consistent with this, overexpression of UBC9, the sole SUMOylating enzyme, increased tolerance of SHSY5Y cells to oxygen/glucose deprivation (OGD), whereas blocking SUMOylation by expressing its dominant negative enhanced cell death (Lee et al, 2007). Similarly, overexpression of SUMO-1 and SUMO-2 in SHSY5Y cells and cortical neurones increased resistance to OGD, whereas RNAi knockdown of SUMO-1, but not SUMO-2, increased susceptibility (Ja Lee et al, 2009). Recently, it has been shown that SUMO-2/3 knockdown increases vulnerability to OGD (Datwyler et al, 2011), further suggesting SUMO-2/3 conjugation as an endogenous neuroprotective mechanism. Here, we examine the relationship between SUMOylation, SENP-1, and OGD-induced cell death.
Materials and methods
Molecular Biology
The catalytic domain (residues 351 to 644) of wild-type and C603S mutant SENP-1 were subcloned into attenuated Sindbis virus (Kantamneni et al, 2011). We adjusted the titer to achieve ∼90% infection for biochemistry experiments and ∼20% for confocal imaging to allow visualization of individual neurones.
Neuronal Cultures
Hippocampal neurones were prepared from E18 Wistar rats (Martin et al, 2007). On the second day, to inhibit glial growth and generate cultures with <5% glia, the culture medium composed of Neurobasal (Gibco, Paisley, UK), horse serum 10%, B27 (Gibco) and 2 mmol/L glutamine was substituted by Neurobasal with B27 only.
Oxygen and Glucose Deprivation
At 15 days in vitro, cells in glucose-free medium saturated with N2 were incubated at 37°C in 95% N2, 5% CO2 for 75 minutes then returned to conditioned medium and normal atmosphere for the times indicated. Oxygen and glucose deprivation duration was based on previous studies (Wahl et al, 2009) to elicit significant, but not complete cell death, allowing the assessment of potential neuroprotective strategies. Lactate dehydrogenase (LDH) release assays confirmed that following 75 minutes OGD, there was a very substantial increase in cell death in the OGD-treated neurones at 24 hours compared with non-OGD.
Propidium Iodide/Hoechst Assays
In all, 24 hours post-OGD neurones were stained with propidium iodide (4 μg/mL) and Hoechst (2 μg/mL) for 1 hour before imaging. The proportion of Hoechst-positive nuclei that were propidium iodide positive was counted across three fields of view. For each experiment, the mean of at least 20 images was calculated per condition.
Immunoblotting and Densitometry
Cells were lysed in Tris-HCl 50 mmol/L (pH 7.5), NaCl 150 mmol/L, EDTA 10 mmol/L, Triton X-100 1%, sodium dodecyl sulfate 0.1%, protease inhibitor 1%, and NEM 20 mmol/L (Martin et al, 2007). Protein concentrations were determined and the samples were boiled for 5 minutes at 95°C with 5% β-mercaptoethanol and 2% glycerol. Proteins were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis using 4% to 20% gradient gels and immunoblotted using rabbit polyclonal anti-SUMO-1 (Cell Signaling, Hitchin, UK; 1:1000), anti-SUMO-2/3 (Zymed, Paisley, UK; 1:250), anti-SENP-1 (Imgenex, San Diego, CA, USA; 1:1,000), anti-UBC9 (Santa Cruz, Wembley, UK; 1:250), and mouse monoclonal anti-β-actin (Sigma, Dorset, UK; 1:10,000). Blots were scanned and analyzed using ImageJ (NIH, Bethesda, MD, USA). The average optical density for the non-OGD neurones (control lane) was designated as 100%. For analysis of SUMOylation, the entire lane was sampled for proteins between 25 and 250 kDa.
Quantitative Reverse Transcriptase Polymerase Chain Reaction
RNA was extracted from neurones using RNeasy kit (Qiagen, West Sussex, UK), DNAse-treated and reverse transcribed (Retroscript, Ambion, Warrington, UK). SUMO-1, SUMO-2, and SENP-1 mRNAs were measured using TaqMan (Applied Biosystems, Warrington, UK) and normalized to 18S rRNA using multiplexing on an Mx3000P system (Stratagene, Stockport, UK).
Data Handling
Values are expressed as mean values±s.e.m. Student's t-test or analysis of variance followed by Duncan's multiple-range method was applied to determine significance.
Ethics Statement
All animal experiments were performed according to the University of Bristol, UK, and EU good practice guidelines and regulations.
Results
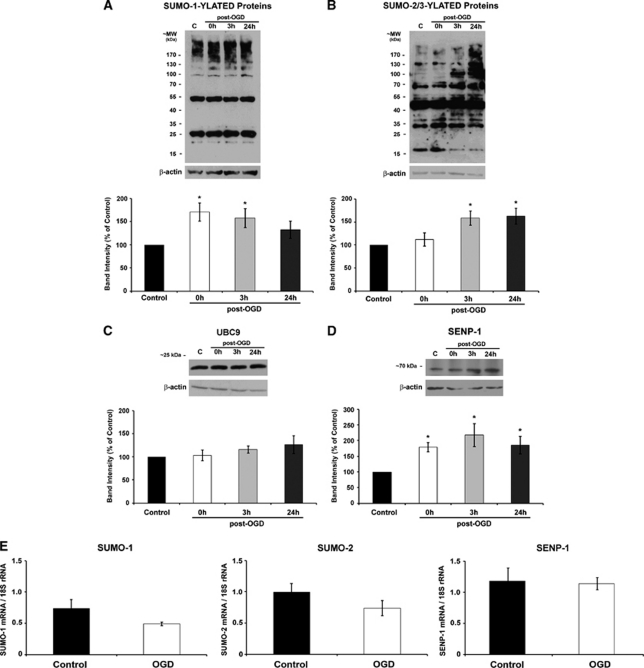
Global SUMOylation by both SUMO-1 and SUMO-2/3 in hippocampal neurones increased markedly following OGD. Increased SUMO-1 conjugation was observed at 0 and 3 hours (Figure 1A) and at 3 and 24 hours post-OGD for SUMO-2/3 (Figure 1B). We reasoned that the increased SUMOylation could be due to either increased UBC9-mediated conjugation or reduced SENP-mediated deconjugation, so we investigated the levels of these enzymes following OGD. Levels of UBC9 were not altered at any time point post-OGD (Figure 1C). Unexpectedly, however, the levels of SENP-1 were increased at all time points post-OGD (Figure 1D). To further characterize the post-OGD changes in protein levels, we used quantitative reverse transcriptase polymerase chain reaction to examine SUMO-1, SUMO-2, and SENP-1 mRNAs before and immediately after OGD. We found that these mRNAs were unaltered following OGD, suggesting that the enhanced SUMOylation and SENP-1 protein levels are not attributable to increased gene expression during OGD (Figure 1E).
Figure 1.
Effects of oxygen and glucose deprivation (OGD) on global protein SUMOylation, UBC9, and SENP-1 levels in hippocampal neurons. (A, B) Representative patterns of SUMO-1 and SUMO-2/3 immunoreactive bands in total cell lysates from control and 75 minutes OGD-treated hippocampal neurons. (C, D) Representative UBC9 and SENP-1 blots. In all cases, the data were quantified from separate immunoblots using cells from three to five different cultures. The results are presented as percentage of control±s.e.m.; * indicates significant difference compared with control P<0.05. (E) The OGD-induced increase in SUMO-1 and SUMO-2/3 conjugation and SENP-1 protein levels is not due to increased transcription. quantitative polymerase chain reaction (qPCR) of RNA extracted from control and OGD-treated hippocampal neurons immediately after OGD (0 hours) revealed no changes in SUMO-1, SUMO-2, or SENP-1 mRNA levels. Graphs showing quantified data from duplicates of five experiments. The results are presented as mean±s.e.m.; no significant difference compared with control P>0.05. SUMO, small ubiquitin-like modifiers.
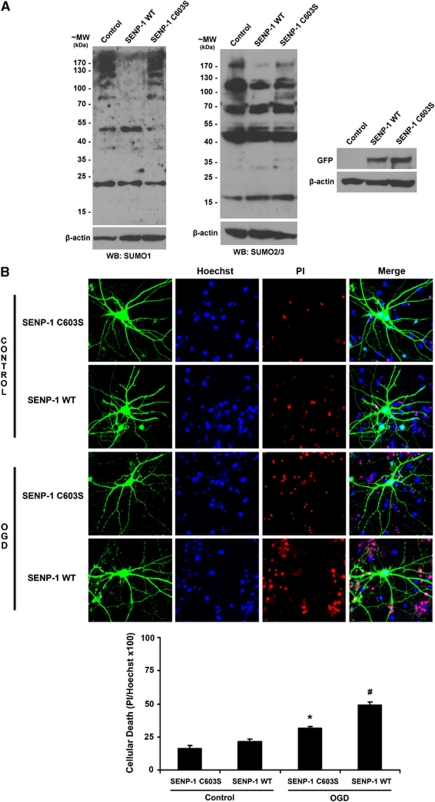
Given the increased SUMOylation observed post-OGD, we sought to determine whether this represented a neuroprotective response. Therefore, we overexpressed green fluorescent protein (GFP)-tagged SENP-1 catalytic domain or the inactive SENP-1 C603S mutant before OGD to downregulate SUMOylation. Virally expressed SENP-1 effectively decreased SUMO-1 and SUMO-2/3 conjugation in neurones (Figure 2A; Kantamneni et al, 2011). We next assessed cell death as a percentage of the total number of cells, defined by Hoechst, that also stain for propidium iodide. Consistent with a protective role for SUMOylation, SENP-1-mediated reduction of SUMOylation significantly increased neuronal death compared with SENP-1 C603S post-OGD but not in control conditions (Figure 2B).
Figure 2.
Decreasing global protein SUMOylation increases vulnerability of hippocampal neurons to oxygen and glucose deprivation (OGD). (A) Overexpression of the GFP-tagged catalytic domain of SENP-1 wild type (WT), but not the catalytically inactive SENP-1 C603S, globally downregulates SUMO-1 (left panel) and SUMO-2/3 (middle panel) conjugation to target proteins. GFP blots show effective viral expression (right panel) (B) Representative images showing the effects of overexpressing SENP-1 on OGD-induced cell death. Hippocampal neurones were infected 15 hours before OGD with Sindbis virus expressing either SENP-1 WT or SENP-1 C603S. After 75 minutes of OGD, cells were washed, returned to normal conditioned growth medium, and incubated for a further 24 hours. They were then stained with Hoechst and propidium iodide (PI), and the proportion of nuclei stained with PI was counted. Graph showing quantified data from four separate experiments. Overexpression of SENP-1 WT significantly increases cellular death in the cultures exposed to OGD. * and # indicate significant differences compared with all groups, P<0.05. SUMO, small ubiquitin-like modifiers.
Discussion
Global SUMOylation is increased in cortical neurones subjected to OGD (Loftus et al, 2009) and overexpression of SUMO-1 and SUMO-2 has been reported to reduce OGD-induced neuronal death (Ja Lee et al, 2009) whereas knockdown of SUMO-2/3 increases OGD-induced neuronal death (Datwyler et al, 2011). Here, we show that SUMOylation by SUMO-1 and SUMO-2/3 is increased in hippocampal neurones following OGD and that counteracting this through overexpression of the catalytic domain of SENP-1 increases OGD-induced neuronal death, suggesting that the post-OGD SUMOylation is neuroprotective.
To investigate the mechanism of the OGD-induced increase in SUMO-1 and SUMO-2/3 conjugation, we assessed the levels of the SUMOylating, UBC9, and the deSUMOylating, SENP-1, enzymes. Unexpectedly, SENP-1 protein levels were increased post-OGD whereas UBC9 was not altered, although it should be noted that these experiments do not address possible changes in enzymatic activity. It has been reported that both SUMO-1 and SENP-1 mRNA levels increase following OGD and hypoxia (Shao et al, 2004; Xu et al, 2010). We did not detect any change in mRNA levels of SUMO-1 or SUMO-2, suggesting that the raised SUMOylation was likely due to the conjugation of preexisting SUMO proteins. Despite observing an increase in SENP-1 protein, we did not observe any change in SENP-1 mRNA, suggesting that this increase may result from reduced SENP-1 degradation post-OGD. It is likely that these variations are attributable to differences in the cell types, experimental challenges, and/or the exposure times used between studies.
Consistent with previous studies, we observed no free SUMO-1 but abundant free SUMO-2/3, indicating that the levels of free SUMO proteins are tightly regulated (Wilkinson and Henley, 2010; Wilkinson et al, 2010). SENP-1 overexpression did not lead to detectable levels of free SUMO-1 or changes in levels of free SUMO-2/3 (Figure 2). We attribute this to compensatory cellular mechanisms that act to regulate free SUMO levels. We did, however, observe changes in free SUMO-2/3 following OGD (Figure 1), suggesting that ischemic insult can compromise the mechanisms regulating SUMO-2/3 levels.
Our results indicate different response times for SUMO-1 and SUMO-2/3 conjugation following stress: SUMO-1 increases immediately after and is still increased at 3 hours before returning to control levels at 24 hours, whereas SUMO-2/3 remains stable immediately after, but is increased at 3 and 24 hours post-OGD. Many SUMO substrates can be modified by both SUMO-1 and SUMO-2/3, and in most cases, the functional differences are unclear (Wilkinson and Henley, 2010). Nonetheless, the fact that SUMO-1 and SUMO-2/3 differ in their conjugation dynamics in response to OGD indicates specific regulation of these paralogues.
We demonstrate that reducing SUMOylation through overexpression of the catalytic domain of SENP-1 impaired neuronal survival, suggesting that post-OGD SUMOylation is neuroprotective. At first, the increased levels of SENP-1 post-OGD appear inconsistent with both increased SUMOylation and neuroprotection, and also with viral overexpression causing deSUMOylation. It is important to note, however, that overexpression of the SENP-1 catalytic domain, used here as a tool to reduce SUMOylation, bypasses normal cellular regulation, targeting to compartments and may not display the same substrate specificity as endogenous SENP-1. Thus, overexpression of the SENP-1 catalytic domain to reduce SUMOylation differs from the subtle but significant increase in endogenous SENP-1 that presumably contributes to the neuronal response to OGD. Therefore, while our data indicate that SUMOylation represents a neuroprotective mechanism post-OGD, there also appears to be a physiologically relevant increase in SENP-1.
The role of the SENP-1 increase is unclear but it may be involved in the maturation of precursor SUMO proteins into conjugation-competent forms. While post-OGD SUMO-1 or SUMO-2 mRNA did not increase, there may be cellular reserves of pro-SUMO proteins, which require SENP-mediated cleavage to initiate conjugation. Thus, we propose that the OGD-induced increase in SENP-1 may contribute to the OGD-induced increase in SUMOylation as shown here and by others (Shao et al, 2004). Although we detected global increases in SUMOylation post-OGD, the neuronal response to OGD is likely to be subject to subtle and complex bidirectional regulation. Indeed, it has been shown that while a global increase in SUMOylation occurs in HeLa cells in response to heat stress or proteasome inhibition, the SUMOylation of some substrates remains unchanged and of others decreases (Golebiowski et al, 2009; Tatham et al, 2011). Thus, it seems that targeted SENP activity is required for an appropriate response to cellular stress. It is currently unclear how the maturation versus deconjugation activities of SENPs are regulated, but our findings suggest that the post-OGD increase in SENP-1 is somehow targeted to enhance SUMOylation through maturation of SUMO and/or to deSUMOylate specific subsets of substrates. Taken together, our results suggest that regulation of SENP-1 has a largely unexplored, but nonetheless critical role in the neuronal response to ischemic stress.
Acknowledgments
The authors thank S Goldstein for SENP-1 constructs, M Usowicz for use of TaqMan, S Martin and S Jacobs for assistance, and P Tidball for technical support.
The authors declare no conflict of interest.
Footnotes
This study was supported by grants from Wellcome Trust, BBSRC, MRC, and ERC.
References
- Cimarosti H, Lindberg C, Bomholt SF, Ronn LC, Henley JM. Increased protein SUMOylation following focal cerebral ischemia. Neuropharmacology. 2008;54:280–289. doi: 10.1016/j.neuropharm.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Datwyler AL, Lättig-Tünnemann G, Yang W, Paschen W, Lee SL, Dirnagl U, Endres M, Harms C.2011SUMO2/3 conjugation is an endogenous neuroprotective mechanism J Cereb Blood Flow MetabIn press. doi: 10.1038/jcbfm.2011.112 [DOI] [PMC free article] [PubMed]
- Golebiowski F, Matic I, Tatham MH, Cole C, Yin Y, Nakamura A, Cox J, Barton GJ, Mann M, Hay RT. System-wide changes to SUMO modifications in response to heat shock. Sci Signal. 2009;2:ra24. doi: 10.1126/scisignal.2000282. [DOI] [PubMed] [Google Scholar]
- Ja Lee Y, Castri P, Bembry J, Maric D, Auh S, Hallenbeck JM. SUMOylation participates in induction of ischemic tolerance. J Neurochem. 2009;109:257–267. doi: 10.1111/j.1471-4159.2009.05957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantamneni S, Wilkinson KA, Jaafari N, Ashikaga E, Rocca D, Rubin P, Jacobs S, Nishimune A, Henley JM. Activity dependent SUMOylation of the brain specific scaffolding protein GISP. Biochem Biophys Res Commun. 2011;409:657–662. doi: 10.1016/j.bbrc.2011.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Miyake S, Wakita H, McMullen DC, Azuma Y, Auh S, Hallenbeck JM. Protein SUMOylation is massively increased in hibernation torpor and is critical for the cytoprotection provided by ischemic preconditioning and hypothermia in SHSY5Y cells. J Cereb Blood Flow Metab. 2007;27:950–962. doi: 10.1038/sj.jcbfm.9600395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus LT, Gala R, Yang T, Jessick VJ, Ashley MD, Ordonez AN, Thompson SJ, Simon RP, Meller R. Sumo-2/3-ylation following in vitro modeled ischemia is reduced in delayed ischemic tolerance. Brain Res. 2009;1272:71–80. doi: 10.1016/j.brainres.2009.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Nishimune A, Mellor JR, Henley JM. SUMOylation regulates kainate-receptor-mediated synaptic transmission. Nature. 2007;447:321–325. doi: 10.1038/nature05736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao R, Zhang FP, Tian F, Anders Friberg P, Wang X, Sjoland H, Billig H. Increase of SUMO-1 expression in response to hypoxia: direct interaction with HIF-1alpha in adult mouse brain and heart in vivo. FEBS Lett. 2004;569:293–300. doi: 10.1016/j.febslet.2004.05.079. [DOI] [PubMed] [Google Scholar]
- Tatham MH, Matic I, Mann M, Hay RT. Comparative Proteomic analysis identifies a role for SUMO in protein quality control. Sci Signal. 2011;4:rs4. doi: 10.1126/scisignal.2001484. [DOI] [PubMed] [Google Scholar]
- Wahl AS, Buchthal B, Rode F, Bomholt SF, Freitag HE, Hardingham GE, Ronn LC, Bading H. Hypoxic/ischemic conditions induce expression of the putative pro-death gene Clca1 via activation of extrasynaptic N-methyl-D-aspartate receptors. Neuroscience. 2009;158:344–352. doi: 10.1016/j.neuroscience.2008.06.018. [DOI] [PubMed] [Google Scholar]
- Wilkinson KA, Henley JM. Mechanisms, regulation and consequences of protein SUMOylation. Biochem J. 2010;428:133–145. doi: 10.1042/BJ20100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson KA, Nakamura Y, Henley JM. Targets and consequences of protein SUMOylation in neurons. Brain Res Rev. 2010;64:195–212. doi: 10.1016/j.brainresrev.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Vatsyayan J, Gao C, Bakkenist CJ, Hu J. Sumoylation of eIF4E activates mRNA translation. EMBO Rep. 2010;11:299–304. doi: 10.1038/embor.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Sheng H, Warner DS, Paschen W. Transient global cerebral ischemia induces a massive increase in protein sumoylation. J Cereb Blood Flow Metab. 2008a;28:269–279. doi: 10.1038/sj.jcbfm.9600523. [DOI] [PubMed] [Google Scholar]
- Yang W, Sheng H, Warner DS, Paschen W. Transient focal cerebral ischemia induces a dramatic activation of small ubiquitin-like modifier conjugation. J Cereb Blood Flow Metab. 2008b;28:892–896. doi: 10.1038/sj.jcbfm.9600601. [DOI] [PubMed] [Google Scholar]