Abstract
Background
S100A12 is a calcium-binding protein predominantly expressed in neutrophil granulocytes in response to infections or inflammation. Acute otitis media (AOM) is a local infection mainly caused by Streptococcus pneumoniae (Spn), nontypeable Haemophilus influenzae (NTHi) and/or Moraxella catarrhalis (Mcat).
Methods
To study if S100A12 values could serve as a diagnostic marker, serum S100A12 concentrations were tested in young children before, at onset and after recovery from AOM.
Results
Among 116 children with AOM we found that serum S100A12 concentrations were significantly increased at onset of AOM compared to before infection (p=0.0001), and returned to normal levels when the children recovered from the infection (p=0.01). Elevation of S100A12 correlated with the presence of Spn (p=0.004) or NTHi (p=0.04) in the middle ear, but not with Mcat or upper respiratory viral infection. Change in serum value of S100A12 at onset of AOM were not related to the frequency of occurrence of AOM or the age of the child.
Conclusion
S100A12 may be a useful biomarker for onset of AOM due to Spn and NTHi; and for following children with AOM.
Keywords: S100A12, acute otitis media, biomarker, young children
INTRODUCTION
Acute otitis media (AOM) is one of the most frequent diseases of childhood. AOM episodes can impair hearing in early childhood, which may result in long-term consequences for speech and language development (1). AOM is a local infection of the middle ear and the common pathogens are Streptococcus pneumoniae (Spn) (25% to 50%), nontypeable Haemophilus influenzae (NTHi) (25% to 50%), and Moraxella catarrhalis (Mcat) (3% to 20%) (2). At onset of infection, infiltration of neutrophils and monocytes/macrophages to the middle ear occurs and many pro-inflammatory cytokines are induced, which promote inflammation and cause significant tissue damage. Identification of biomarkers that could indicate the presence of bacterial AOM infection in the context of a viral upper respiratory infection (URI) could benefit in diagnosis, assessment of infection outcome, monitoring populations at higher risk and evaluation of vaccine candidates.
Recently, S100 proteins which originate from phagocytic cytosol have been shown to bear an excellent potential as serum and local markers of inflammation. These pro-inflammatory molecules are activated during the innate immune response in several infectious diseases (3–5). No information on measurement of S100A12 in acute otitis media (AOM) has been previously reported.
Here we report serum S100A12 changes from a pre-infection, healthy state compared with the time of onset of clinical AOM (pathogen-confirmed by tympanocentesis) and then after clinical recovery from AOM has occurred. Serum value of S100A12 were analyzed according to specific otopathogens (Spn, NTHi, Mcat) and compared with levels during viral URI. The concentrations of S100A12 at onset of AOM in otitis prone (OP) and non-otitis prone (NOP) children and children at various ages were also studied. The findings support our hypothesis that serum S100A12 is a potential biomarker for bacterial AOM caused by Spn and NTHi.
MATERIALS AND METHODS
Subjects
The human samples evaluated in this study were collected as part of a prospective, longitudinal study funded by the National Institute for Deafness and Communication Disorders that commenced in June 2006. All the children had samples of serum and nasopharyngeal (NP) and oropharyngeal secretions collected at 6, 9, 12, 15, 18, 21, 24, and 30 months of age. At some of those visits children had a clinical viral URI as previously defined (6). When children developed symptoms of AOM they were brought to the study physicians for diagnosis and treatment. The diagnosis of AOM was made based on symptoms of fever, irritability or ear ache, and physical signs of inflammation of the tympanic membrane (bulging) with the presence of middle ear fluid (pus-laden fluid) documented by tympanocentesis. After being diagnosed with AOM, children received amoxicillin/clavulanate as the primary antibiotic treatment and returned for a follow-up visit three weeks later. Informed consent was obtained at enrollment from the parents or guardians. The sample collection was approved by the IRB at Rochester General Hospital.
For the population studied, the following criteria for subject selection were adopted: (1) Age: all the children were between 6–33 months old. (2) AOM patients: bacterial culture from MEF was positive and pathogen species was verified. (3) Pre-infection healthy stage: the samples were collected within 3 months before the AOM and without any other infections or diseases. (4) Convalescent stage: the samples were collected after successful treatment of AOM at 3 weeks and the child was in a healthy condition. (5) Virus infected samples: the patients had common cold symptoms such as fever and runny nose. (6) Otitis prone (OP) children: AOM occurred 3 times in 6 months or 4 times in 12 months. (7) Non otitis prone (NOP) children: AOM occurred only one time. Any subject with another disease or infection was excluded.
Specimen collection
The method of collection of NP and OP samples, peripheral blood mononuclear cells (PBMCs), serum and the tympanocentesis procedure have been previously described (2).
Identification of otopathogens
Identification of Spn, NTHi and Mcat was performed as described previously (2). Whenever the pathogen was questionable, verification was performed by multiplex PCR, as described previously (7).
Measurement of S100A12 mRNA
Total RNA was extracted from PBMCs using a QIAamp RNA blood Mini Kit (Qiagen, Maryland, USA) according to the manufacturer’s instructions. Total RNA was reverse transcribedto cDNA using an RT2 first strand kit (SABiosciences). Quantitative real-time reverse transcriptase PCR (qRT-PCR) was performedusing a CFX 96 thermocycler (Bio-Rad) with RT2 Profiler human custom kit. The threshold and baseline were set automatically using the pcr/arrayanalysis method according to the manufacturer’s instructions (SABiosciences). CT data were uploaded into the data analysis template on the manufacturer’s website (http://www.sabiosciences.com/pcr/arrayanalysis.php). The relative expression of genes compared with the expression in control samples was calculated on the website using the ΔΔCT method with five housekeeping genes as controls.
Measurement of S100A12 protein
ELISA was performed with the CircuLex S100A12 ELISA Kit. In this assay, a monoclonal antibody specific for S100A12 was pre-coated onto a 96-well microplate. 100ul of diluted serum was pipetted into the wells and the immobilized antibody bound any S100A12 present. After washing, an HRP conjugated polyclonal antibody specific for S100A12 was added to the wells and incubated at room temperature for 1h. Following a wash, the conjugate was reacted with the substrate H2O2-tetramethylbenzidine. The absorbance of the resulting product was measured at 450 nm. A standard curve was constructed by plotting absorbance values versus S100A12 concentrations of calibrators, and concentrations of unknown samples were determined with the standard curve.
Virology
Virology studies were done by the Rochester General Hospital Clinical Laboratories. Influenza A, Influenza B, and RSV were detected by rapid EIA or recovered in culture, all other respiratory viruses were recovered in culture. Due to the methodology, rhinoviruses were not detected. In 2006 to 2007 samples were inoculated onto RMK tube cultures while from 2008 to 2010 they were inoculated onto shell vial cultures.
Statistical analysis
Data from ELISA were analyzed using non-parametric test with two-sided rank analysis. For pathogen specific analysis, data was analyzed by comparing children prior to AOM (healthystate) at the time of AOM and 3 weeks after AOM diagnosis (convalescent state) with paired t-tests. For age difference analysis an ANOVA test was performed. P values < 0.05 (two-tailed) were considered statistically significant.
RESULTS
Serum concentrations of S100A12 at onset of AOM
To study the concentration change of serum S100A12, the sera from 116 children with AOM caused by bacterial infection (bacteria present in MEF and clinical AOM) were analyzed by ELISA. We had sera available from 69 of those children shortly before (<3 months prior) AOM. The mean sera S100A12 value from children with AOM was significantly elevated (36.7 +/− 72.4 ng/ml) compared that before infection (12.1+/−19.8 ng/ml, p=0.0001).
Serum concentration of S100A12 returns to normal after recovery from AOM
To determine if the serum concentration of S100A12 reflected a successful clinical outcome from infection, we had sera from 74 children available to test from the convalescent stage (bacteria presumed eradicated from MEF after pathogen-directed antibiotic treatment because the child had no AOM symptoms or signs). It was found that serum concentrations of S100A12 dropped to pre-infection values; (13.5 +/− 22.1 ng/ml), a significant change compared to the onset of AOM (P= 0.01).
Serum concentration change of S100A12 in children with AOM caused by Spn, NTHi and Mcat
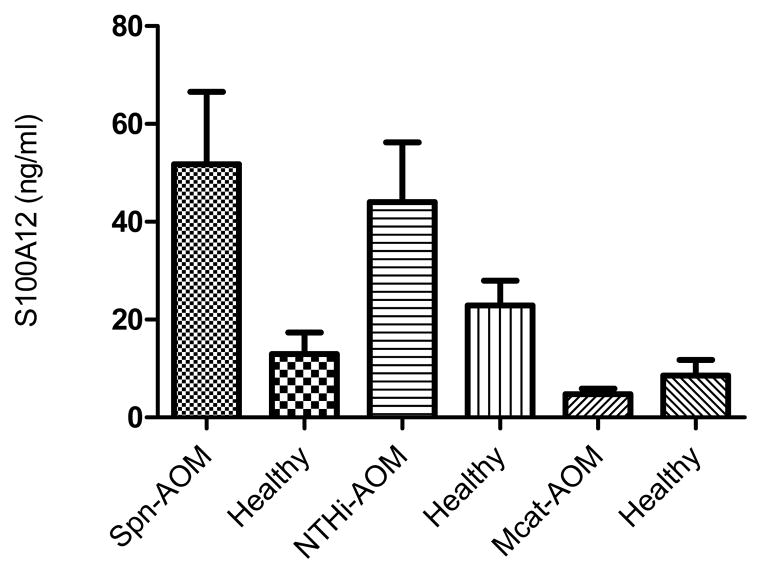
A pair-wise comparison of serum S100A12 concentration was performed for 41 children with AOM caused by Spn between the onset of AOM state and the pre-infection, healthy state; a significant difference was observed (P = 0.004) (Fig. 1). The pair-wise comparison for 29 children with NTHi-induced AOM was also significant (44.1+/−65.4 ng/ml versus 22.9+/−27.1 ng/ml; p=0.044) (Fig. 1). In 17 children with Mcat-induced AOM, serum S100A12 levels were not different at onset of AOM compared with the pre-infection, healthy state (P=0.18) (Fig. 1).
Fig. 1. Serum S100A12 level change in AOM caused by Spn, NTHi and Mcat.
AOM caused by Spn (N=41), p=0.004; by NTHi infection (N=29), p=0.04; and by Mcat (N=17) p=0.18. Values shown are mean +/− standard error.
Serum concentration of S100A12 protein in children during viral upper respiratory infection
It is well known that AOM occurs conconcurrently with viral URIs (6). To study if viral URIs increase serum concentrations of S100A12, S100A12 were tested in 34 sera collected from children infected by respiratory viruses (laboratory confirmed) but without AOM symptoms and signs (9 RSV, 12 influenzae A, 5 influenzae B, 6 parainfluenzae 1 or 2 and 5 adenovirus). The level of S100A12 in those 34 children was not different from 74 samples from children who recovered from AOM (p=0.08).
Serum levels of S100A12 in healthy children of various ages
We assessed differences in S100A12 serum levels in 111 children of different ages at onset of AOM: children were 6–9 months (n = 30), 9–12 months (22), 12–15 months (n= 20), 15–18 months (n = 14), 18–24 months (n = 13), and 24–33 months (n=9), respectively. There was no significant difference in levels among age cohorts (P=0.5), (data not shown).
Serum values of S100A12 in otitis prone and non-otitis prone children
Serum concentrations of S100A12 were analyzed to determine if they would differ among non-otitis prone (n-84; 13.53+/−23.07 ng/ml) and otitis prone (n=28; 14.02+/−15.72 ng/ml) children at onset of AOM, no difference was found (p=0.2).
DISCUSSION
The present study shows that serum concentrations of S100A12 are significantly increased in children at onset of AOM caused by Spn or NTHi. The concentrations of S100A12 were not significantly affected by Mcat, upper respiratory viral infection, child age at onset of AOM or frequency of AOM episodes. Our study suggests that serum S100A12 may be a useful biomarker for onset of AOM caused by Spn or NTHi and for monitoring resolution after infection.
So far, reference values for S100A12 in serum specimens in children have not been reported and normal or reference serum values of S100A12 in adults are uncertain. With a monoclonal sandwich ELISA, several groups found that mean serum S100A12 levels of healthy adults ranged from 10.7 to 75.0 ng/mL (3, 8, 9, 10). However, when using other methods, like mass spectrometry, mean values of S100A12 below 10 ng/mL for healthy adults were obtained (11). The current study showed that the mean serum concentration of S100A12 in normal healthy children was 12.1 ng/mL. With recovery, S100A12 serum levels had a mean of 13.5 ng/mL. This observation provides the first reference for future study of S100A12 in children.
Broides et al (12) found that white blood cell (WBC) counts were higher in the MEF of patients with culture-positive AOM caused by Spn than in those with culture-negative AOM (12). Qvarnberg et al. (13) also found a higher number of neutrophils in AOM caused by Spn than in AOM cases in which no pathogens were isolated. The significant increase of S100A12 serum concentrations in children with AOM may be related to the activation, proliferation and recruitment of neutrophils and other inflammatory cells to the middle ear site.
We found that serum S100A12 value varied among otopathogens. In Spn-induced AOM, S100A12 was elevated more than in NTHi-induced AOM; and in Mcat- induced AOM, no significant change of S100A12 was found. This observation may be related to the pathogen-stimulated activation of neutrophils. Previous clinical work has shown that AOM caused by Spn is associated with significantly more symptoms and signs of inflammation than AOM caused by NTHi or Mcat (14–16). The number of WBC counted in the MEF of patients with AOM caused by Spn has been shown to be significantly higher than the number of white blood cells found in the MEF of patients with AOM caused by NTHi (12).
Respiratory syncytial virus, rhinovirus, influenza or parainfluenza viruses are frequently detected in the NP and sometimes in the middle ear fluid of children with AOM (6). To assess whether the elevated serum levels of S100A12 at onset of AOM were a consequence of a viral URI, we evaluated serum levels of S100A12 in such children and in healthy, virus negative children. As expected no differences were found between the two groups, because secretion of S100A12 is an indicator of activated neutrophils, and many upper respiratory viral infections, including those caused by influenza and parainfluenza, decrease the neutrophil count (17, 18). The finding that serum S100A12 levels and responses were not different among different age cohorts or among NOP and OP children suggests neutrophil activation is not affected by age in the range we studied (6–33 months) or by the OP state.
Acknowledgments
Work supported by R01DC08671 and the Thrasher Research Fund (to MEP). This study would not have been possible without the help and dedication of Dr. Janet Casey at Legacy Pediatrics and her study coordinator Sally Thomas, LPN, CCRC; Dr. Robert Osgood and Dr. Linlin Chen from the Rochester Institute of Technology. We also thank the collaborating pediatricians from Long Pond Pediatrics, Genesis Pediatrics, Rainbow Pediatrics and Lewis Pediatrics, and the parents who consented to this challenging study.
Footnotes
AUTHORSHIP CONTRIBUTIONS AND DISCLOSURE OF CONFLICTS OF INTEREST
The authors declare that a patent has been filed for use of S100A12 as a biomarker of AOM infection.
References
- 1.Teele DW, Klein JO, Chase C, Menyuk P, Rosner BA. Otitis media in infancy and intellectual ability, school achievement, speech, and language at age 7 years. Greater Boston Otitis Media Study Group. J Infect Dis. 1990;162:685–694. doi: 10.1093/infdis/162.3.685. [DOI] [PubMed] [Google Scholar]
- 2.Casey JR, Adlowitz DG, Pichichero ME. New Patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2010 Apr;29(4):304–9. doi: 10.1097/INF.0b013e3181c1bc48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foell D, Seeliger S, Vogl T, Koch HG, Maschek H, Harms E, Sorg C, Roth J. Expression of S100A12 (EN-RAGE) in cystic fibrosis. Thorax. 2003c;58:613–617. doi: 10.1136/thorax.58.7.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim MH, Choi YW, Choi HY, Myung KB, Cho SN. The expression of RAGE and EN-RAGE in leprosy. Br J Dermatol. 2006;154:594–601. doi: 10.1111/j.1365-2133.2005.07112.x. [DOI] [PubMed] [Google Scholar]
- 5.Buhimschi IA, Zhao G, Pettker CM, Bahtiyar MO, Magloire LK, Thung S, Fairchild T, Buhimschi CS. The receptor for advanced glycation end products (RAGE) system in women with intraamniotic infection and inflammation. Am J Obstet Gynecol. 2007;196:181. doi: 10.1016/j.ajog.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Kalu SU, Ataya RS, McCormick DP, Patel JA, Revai K, Chonmaitree T. Clinical spectrum of acute otitis media complicating upper respiratory tract viral infection. Pediatr Infect Dis J. 2011 Feb;30(2):95–99. doi: 10.1097/INF.0b013e3181f253d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Q, Kaur R, Casey JR, Adlowitz DG, Pichichero ME, Zeng M. Identification of Streptococcus pneumoniae and Haemophilus influenzae in culture-negative middle ear fluids from children with acute otitis media by combination of multiplex PCR and multi-locus sequencing typing. Int J Pediatr Otorhinolaryngol. 2011 Feb;75(2):239–44. doi: 10.1016/j.ijporl.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kosaki A, Hasegawa T, Kimura T, Iida K, Hitomi J, Matsubara H, Mori Y, Okigaki M, Toyoda N, Masaki H, Inoue-Shibata M, Nishikawa M, Iwasaka T. Increased plasma S100A12 (EN-RAGE) levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2004;89:5423–5428. doi: 10.1210/jc.2003-032223. [DOI] [PubMed] [Google Scholar]
- 9.Ye F, Foell D, Hirono KI, Vogl T, Rui C, Yu X, Watanabe S, Watanabe K, Uese K, Hashimoto I, Roth J, Ichida F, Miyawaki T. Neutrophil-derived S100A12 is profoundly upregulated in the early stage of acute Kawasaki disease. Am J Cardiol. 2004;94:840–844. doi: 10.1016/j.amjcard.2004.05.076. [DOI] [PubMed] [Google Scholar]
- 10.Uchiyama-Tanaka Y, Mori Y, Kosaki A, Kimura T, Moriishi M, Kawanishi H, Matsubara H. Plasma S100A12 concentrations in peritoneal dialysis patients and subclinical chronic inflammatory disease. Ther Apher Dial. 2008;12:28–32. doi: 10.1111/j.1744-9987.2007.00537.x. [DOI] [PubMed] [Google Scholar]
- 11.Liao H, Wu J, Kuhn E, Chin W, Chang B, Jones MD, O’Neil S, Clauser KR, Karl J, Hasler F, Roubenoff R, Zolg W, Guild BC. Use of mass spectrometry to identify protein biomarkers of disease severity in the synovial fluid and serum of patients with rheumatoid arthritis. Arthritis Rheum. 2004;50:3792–3803. doi: 10.1002/art.20720. [DOI] [PubMed] [Google Scholar]
- 12.Broides Arnon, Leibovitz Eugene, Dagan Ron, Press Joseph, Raiz Simon, Kafka Michael, Leiberman Albrebo, Yermiahu Tikva. Cytology of middle ear fluid during acute otitis media. Pediat Infect Dis J. 2002;21(1):57–60. doi: 10.1097/00006454-200201000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Qvarnberg Y, Holopainen E, Palua T. Aspiration cytology in acute otitis media. Acta Otolaryngol (Stockh) 1984;97:443–9. doi: 10.3109/00016488409132919. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez WJ, Schwartz RH. Streptococcus pneumoniae causes otitis media with higher fever and more redness of tympanic membranes than Haemophilus influenzae or Moraxella catarrhalis. Pediatr Infect Dis J. 1999;18:942–4. doi: 10.1097/00006454-199910000-00023. [DOI] [PubMed] [Google Scholar]
- 15.Palmu AA, Herva E, Savolainen H, Karma P, Mäkelä PH, Kilpi TM. Association of clinical signs and symptoms with bacterial findings in acute otitis media. Clin Infect Dis. 2004 Jan 15;38(2):234–42. doi: 10.1086/380642. [DOI] [PubMed] [Google Scholar]
- 16.Leibovitz E, Jacobs MR, Dagan R. Haemophilus influenzae: a significant pathogen in acute otitis media. Pediatr Infect Dis J. 2004 Dec;23(12):1142–52. [PubMed] [Google Scholar]
- 17.Abramson JS, Giebink GS, Quie PG. Influenza A virus induced polymorphonuclear leukocyte dysfunction in the pathogenesis of experimental pneumococcal otitis media. Infect Immun. 1982;36:289–296. doi: 10.1128/iai.36.1.289-296.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abramson JS, Wheeler JG. Virus-induced neutrophil dysfunction: role in the pathogenesis of bacterial infections. Pediatr Infect Dis J. 1994;13:643–652. [PubMed] [Google Scholar]