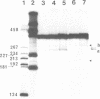
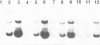
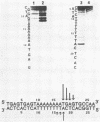
Abstract
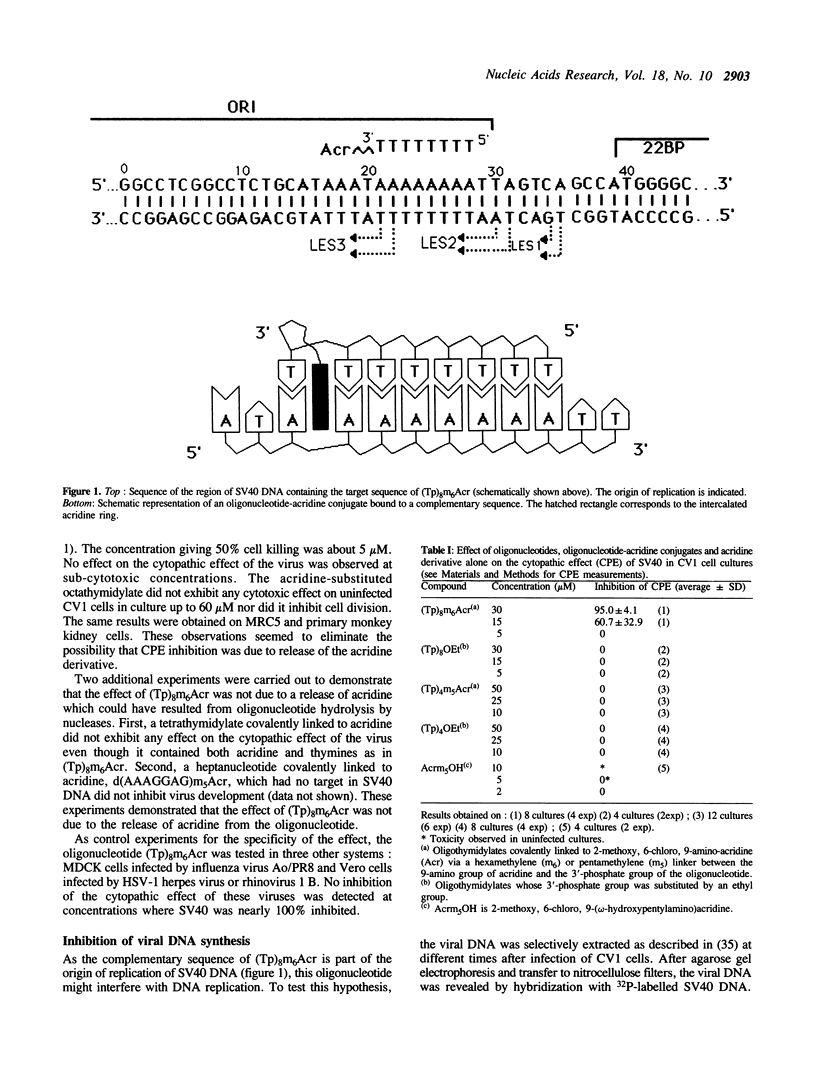
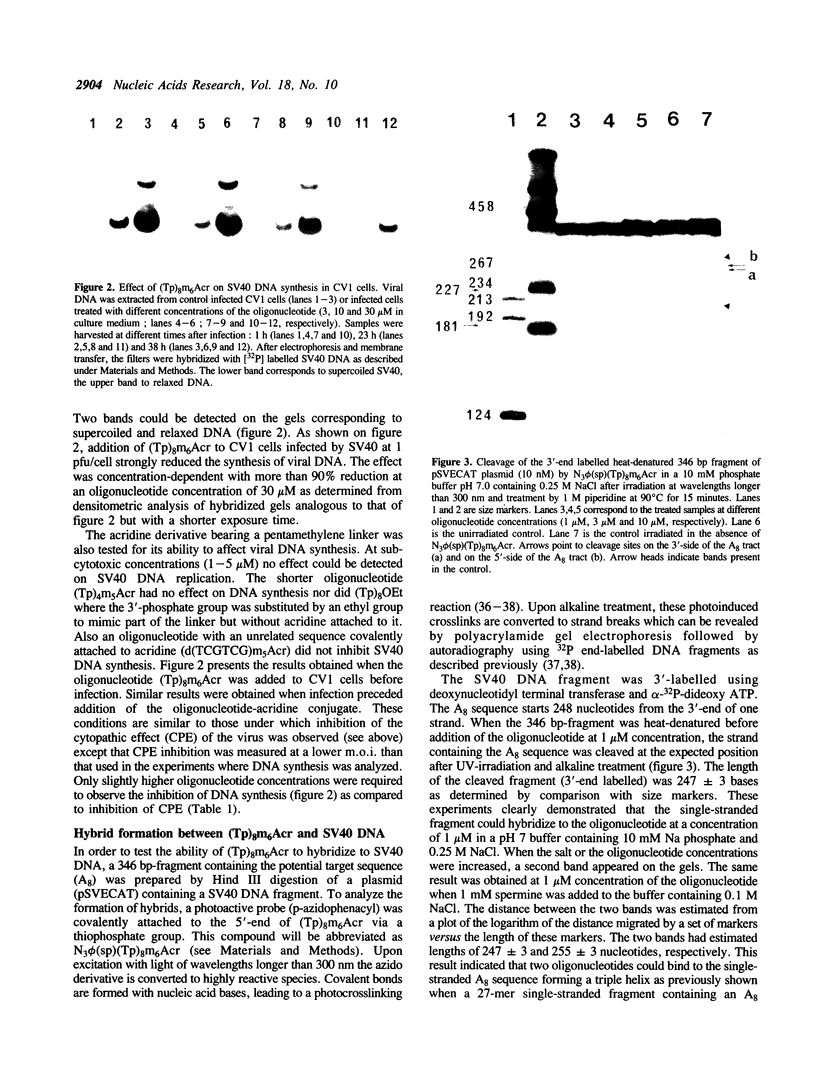
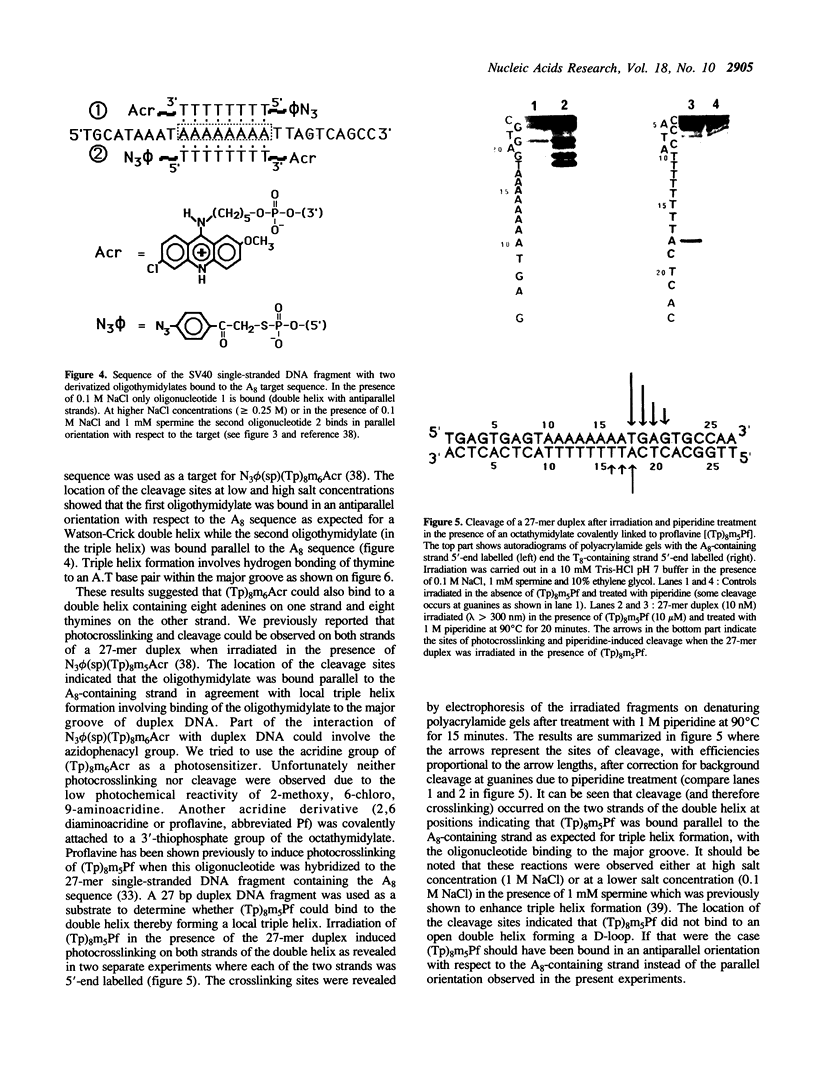
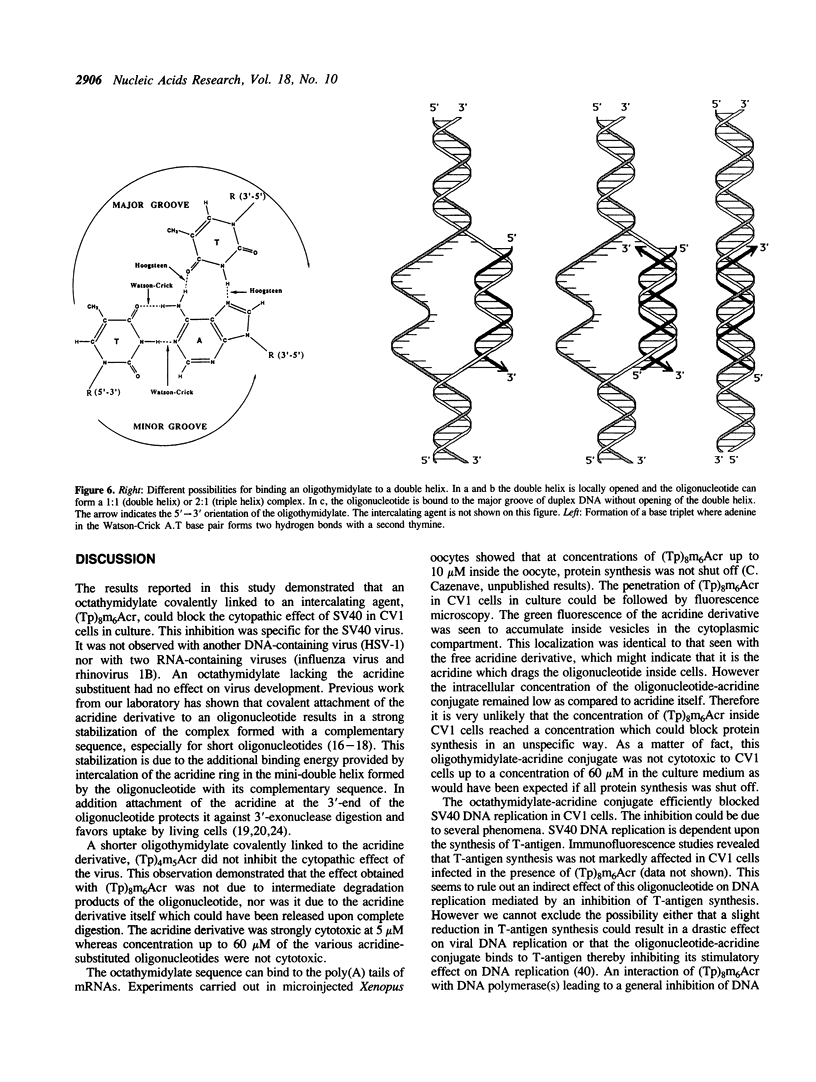
An octathymidylate covalently linked via its 3'-end to an acridine derivative inhibited the cytopathic effect of Simian Virus SV40 on CV-1 cells in culture. Control experiments revealed that this effect was virus-specific and did not arise as a result of oligonucleotide degradation by nucleases. A photoactive probe was covalently attached to the 5'-end of the oligonucleotide-acridine conjugate. Upon UV-irradiation, photocrosslinking was shown to occur at the A. T-rich region within the viral origin of replication. A local triple helix can form at moderate salt concentrations with two octathymidylate-acridine conjugates bound to the octaadenylate sequence. Alternatively the octathymidylate-acridine conjugate can bind to the major groove of duplex DNA forming a local triple helix. Different mechanisms are discussed to explain the inhibition of viral DNA replication.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asseline U., Delarue M., Lancelot G., Toulmé F., Thuong N. T., Montenay-Garestier T., Hélène C. Nucleic acid-binding molecules with high affinity and base sequence specificity: intercalating agents covalently linked to oligodeoxynucleotides. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3297–3301. doi: 10.1073/pnas.81.11.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asseline U., Toulme F., Thuong N. T., Delarue M., Montenay-Garestier T., Hélène C. Oligodeoxynucleotides covalently linked to intercalating dyes as base sequence-specific ligands. Influence of dye attachment site. EMBO J. 1984 Apr;3(4):795–800. doi: 10.1002/j.1460-2075.1984.tb01887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake K. R., Murakami A., Miller P. S. Inhibition of rabbit globin mRNA translation by sequence-specific oligodeoxyribonucleotides. Biochemistry. 1985 Oct 22;24(22):6132–6138. doi: 10.1021/bi00343a015. [DOI] [PubMed] [Google Scholar]
- Blake K. R., Murakami A., Spitz S. A., Glave S. A., Reddy M. P., Ts'o P. O., Miller P. S. Hybridization arrest of globin synthesis in rabbit reticulocyte lysates and cells by oligodeoxyribonucleoside methylphosphonates. Biochemistry. 1985 Oct 22;24(22):6139–6145. doi: 10.1021/bi00343a016. [DOI] [PubMed] [Google Scholar]
- Cazenave C., Chevrier M., Nguyen T. T., Hélène C. Rate of degradation of [alpha]- and [beta]-oligodeoxynucleotides in Xenopus oocytes. Implications for anti-messenger strategies. Nucleic Acids Res. 1987 Dec 23;15(24):10507–10521. doi: 10.1093/nar/15.24.10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazenave C., Loreau N., Thuong N. T., Toulmé J. J., Hélène C. Enzymatic amplification of translation inhibition of rabbit beta-globin mRNA mediated by anti-messenger oligodeoxynucleotides covalently linked to intercalating agents. Nucleic Acids Res. 1987 Jun 25;15(12):4717–4736. doi: 10.1093/nar/15.12.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazenave C., Loreau N., Toulmé J. J., Hélène C. Anti-messenger oligodeoxynucleotides: specific inhibition of rabbit beta-globin synthesis in wheat germ extracts and Xenopus oocytes. Biochimie. 1986 Sep;68(9):1063–1069. doi: 10.1016/s0300-9084(86)80180-8. [DOI] [PubMed] [Google Scholar]
- Cazenave C., Stein C. A., Loreau N., Thuong N. T., Neckers L. M., Subasinghe C., Hélène C., Cohen J. S., Toulmé J. J. Comparative inhibition of rabbit globin mRNA translation by modified antisense oligodeoxynucleotides. Nucleic Acids Res. 1989 Jun 12;17(11):4255–4273. doi: 10.1093/nar/17.11.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb S., DeLucia A. L., Koff A., Tsui S., Tegtmeyer P. The adenine-thymine domain of the simian virus 40 core origin directs DNA bending and coordinately regulates DNA replication. Mol Cell Biol. 1986 Dec;6(12):4578–4584. doi: 10.1128/mcb.6.12.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson M., Dean F. B., Bullock P., Echols H., Hurwitz J. Unwinding of duplex DNA from the SV40 origin of replication by T antigen. Science. 1987 Nov 13;238(4829):964–967. doi: 10.1126/science.2823389. [DOI] [PubMed] [Google Scholar]
- Evans T., Schon E., Gora-Maslak G., Patterson J., Efstratiadis A. S1-hypersensitive sites in eukaryotic promoter regions. Nucleic Acids Res. 1984 Nov 12;12(21):8043–8058. doi: 10.1093/nar/12.21.8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnor C., Bertrand J. R., Thenet S., Lemaître M., Morvan F., Rayner B., Malvy C., Lebleu B., Imbach J. L., Paoletti C. alpha-DNA. VI: Comparative study of alpha- and beta-anomeric oligodeoxyribonucleotides in hybridization to mRNA and in cell free translation inhibition. Nucleic Acids Res. 1987 Dec 23;15(24):10419–10436. doi: 10.1093/nar/15.24.10419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacherio D., Hager L. P. A poly(dT)-stimulated ATPase activity associated with simian virus 40 large T antigen. J Biol Chem. 1979 Sep 10;254(17):8113–8116. [PubMed] [Google Scholar]
- Green P. J., Pines O., Inouye M. The role of antisense RNA in gene regulation. Annu Rev Biochem. 1986;55:569–597. doi: 10.1146/annurev.bi.55.070186.003033. [DOI] [PubMed] [Google Scholar]
- Haeuptle M. T., Frank R., Dobberstein B. Translation arrest by oligodeoxynucleotides complementary to mRNA coding sequences yields polypeptides of predetermined length. Nucleic Acids Res. 1986 Feb 11;14(3):1427–1448. doi: 10.1093/nar/14.3.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna M. M., Meares C. F. Synthesis of a cleavable dinucleotide photoaffinity probe of ribonucleic acid polymerase: application to trinucleotide labeling of an Escherichia coli transcription complex. Biochemistry. 1983 Jul 19;22(15):3546–3551. doi: 10.1021/bi00284a002. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hélène C., Lancelot G. Interactions between functional groups in protein-nucleic acid associations. Prog Biophys Mol Biol. 1982;39(1):1–68. doi: 10.1016/0079-6107(83)90013-5. [DOI] [PubMed] [Google Scholar]
- Hélène C., Montenay-Garestier T., Saison T., Takasugi M., Toulmé J. J., Asseline U., Lancelot G., Maurizot J. C., Toulmé F., Thuong N. T. Oligodeoxynucleotides covalently linked to intercalating agents: a new class of gene regulatory substances. Biochimie. 1985 Jul-Aug;67(7-8):777–783. doi: 10.1016/s0300-9084(85)80167-x. [DOI] [PubMed] [Google Scholar]
- Kawasaki E. S. Quantitative hybridization-arrest of mRNA in Xenopus oocytes using single-stranded complementary DNA or oligonucleotide probes. Nucleic Acids Res. 1985 Jul 11;13(13):4991–5004. doi: 10.1093/nar/13.13.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. J., Kelly T. J. Simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6973–6977. doi: 10.1073/pnas.81.22.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthman H., Nilsson M. G., Magnusson G. Non-contiguous segments of the polyoma genome required in cis for DNA replication. J Mol Biol. 1982 Nov 15;161(4):533–550. doi: 10.1016/0022-2836(82)90406-5. [DOI] [PubMed] [Google Scholar]
- Minshull J., Hunt T. The use of single-stranded DNA and RNase H to promote quantitative 'hybrid arrest of translation' of mRNA/DNA hybrids in reticulocyte lysate cell-free translations. Nucleic Acids Res. 1986 Aug 26;14(16):6433–6451. doi: 10.1093/nar/14.16.6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morvan F., Rayner B., Imbach J. L., Thenet S., Bertrand J. R., Paoletti J., Malvy C., Paoletti C. alpha-DNA II. Synthesis of unnatural alpha-anomeric oligodeoxyribonucleotides containing the four usual bases and study of their substrate activities for nucleases. Nucleic Acids Res. 1987 Apr 24;15(8):3421–3437. doi: 10.1093/nar/15.8.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser H. E., Dervan P. B. Sequence-specific cleavage of double helical DNA by triple helix formation. Science. 1987 Oct 30;238(4827):645–650. doi: 10.1126/science.3118463. [DOI] [PubMed] [Google Scholar]
- Praseuth D., Chassignol M., Takasugi M., Le Doan T., Thuong N. T., Hélène C. Double helices with parallel strands are formed by nuclease-resistant oligo-[alpha]-deoxynucleotides and oligo-[alpha]-deoxynucleotides covalently linked to an intercalating agent with complementary oligo-[beta]-deoxynucleotides. J Mol Biol. 1987 Aug 20;196(4):939–942. doi: 10.1016/0022-2836(87)90416-5. [DOI] [PubMed] [Google Scholar]
- Praseuth D., Le Doan T., Chassignol M., Decout J. L., Habhoub N., Lhomme J., Thuong N. T., Hélène C. Sequence-targeted photosensitized reactions in nucleic acids by oligo-alpha-deoxynucleotides and oligo-beta-deoxynucleotides covalently linked to proflavin. Biochemistry. 1988 Apr 19;27(8):3031–3038. doi: 10.1021/bi00408a055. [DOI] [PubMed] [Google Scholar]
- Praseuth D., Perrouault L., Le Doan T., Chassignol M., Thuong N., Hélène C. Sequence-specific binding and photocrosslinking of alpha and beta oligodeoxynucleotides to the major groove of DNA via triple-helix formation. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1349–1353. doi: 10.1073/pnas.85.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schellekens H., Stitz L. W. Simple method for measuring growth inhibition by interferon of cells in monolayer. J Virol Methods. 1980;1(4):197–200. doi: 10.1016/0166-0934(80)90058-0. [DOI] [PubMed] [Google Scholar]
- Smith C. C., Aurelian L., Reddy M. P., Miller P. S., Ts'o P. O. Antiviral effect of an oligo(nucleoside methylphosphonate) complementary to the splice junction of herpes simplex virus type 1 immediate early pre-mRNAs 4 and 5. Proc Natl Acad Sci U S A. 1986 May;83(9):2787–2791. doi: 10.1073/pnas.83.9.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stein C. A., Subasinghe C., Shinozuka K., Cohen J. S. Physicochemical properties of phosphorothioate oligodeoxynucleotides. Nucleic Acids Res. 1988 Apr 25;16(8):3209–3221. doi: 10.1093/nar/16.8.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B., Gerard R. D., Guggenheimer R. A., Gluzman Y. T antigen and template requirements for SV40 DNA replication in vitro. EMBO J. 1985 Nov;4(11):2933–2939. doi: 10.1002/j.1460-2075.1985.tb04026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J. S., François J. C., Montenay-Garestier T., Saison-Behmoaras T., Roig V., Thuong N. T., Hélène C. Sequence-specific intercalating agents: intercalation at specific sequences on duplex DNA via major groove recognition by oligonucleotide-intercalator conjugates. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9198–9202. doi: 10.1073/pnas.86.23.9198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuong N. T., Asseline U., Roig V., Takasugi M., Hélène C. Oligo(alpha-deoxynucleotide)s covalently linked to intercalating agents: differential binding to ribo- and deoxyribopolynucleotides and stability towards nuclease digestion. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5129–5133. doi: 10.1073/pnas.84.15.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulmé J. J., Krisch H. M., Loreau N., Thuong N. T., Hélène C. Specific inhibition of mRNA translation by complementary oligonucleotides covalently linked to intercalating agents. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1227–1231. doi: 10.1073/pnas.83.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng B. Y., Prussak C. E. Sequence and structural requirements for primase initiation in the SV40 origin of replication. Nucleic Acids Res. 1989 Mar 11;17(5):1953–1963. doi: 10.1093/nar/17.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verspieren P., Cornelissen A. W., Thuong N. T., Hélène C., Toulmé J. J. An acridine-linked oligodeoxynucleotide targeted to the common 5' end of trypanosome mRNAs kills cultured parasites. Gene. 1987;61(3):307–315. doi: 10.1016/0378-1119(87)90194-6. [DOI] [PubMed] [Google Scholar]
- Zamecnik P. C., Goodchild J., Taguchi Y., Sarin P. S. Inhibition of replication and expression of human T-cell lymphotropic virus type III in cultured cells by exogenous synthetic oligonucleotides complementary to viral RNA. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4143–4146. doi: 10.1073/pnas.83.12.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamecnik P. C., Stephenson M. L. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc Natl Acad Sci U S A. 1978 Jan;75(1):280–284. doi: 10.1073/pnas.75.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial A., Thuong N. T., Hélène C. Selective inhibition of the cytopathic effect of type A influenza viruses by oligodeoxynucleotides covalently linked to an intercalating agent. Nucleic Acids Res. 1987 Dec 10;15(23):9909–9919. doi: 10.1093/nar/15.23.9909. [DOI] [PMC free article] [PubMed] [Google Scholar]