Abstract
Background
The American Joint Committee on Cancer staging system for hilar cholangiocarcinoma may be inaccurate because the bile duct lacks discrete tissue boundaries.
Objectives
To examine the accuracy of the American Joint Committee on Cancer staging schemes and to determine the prognostic implications of tumor depth.
Design, Setting, and Patients
From January 1, 1987, through December 31, 2009, there were 106 patients who underwent resection of hilar cholangiocarcinoma who had pathologic slides available for re-review.
Main Outcome Measures
Tumor depth and overall survival.
Results
Overall median survival was 19.9 months. The 6th and 7th editions of the T-classification criteria were unable to discriminate among T1, T2, and T3 lesions (P>.05 for all). Median survival was associated with the invasion depth of the tumor (≥5 mm vs <5 mm): 18 months vs 30 months (P=.01). On multivariate analysis, tumor depth remained predictive of disease-specific death (hazard ratio,1.70; P=.03).
Conclusions
The American Joint Committee on Cancer T-classification criteria did not stratify patients with regard to prognosis. Depth of tumor invasion is a better predictor of long-term outcome.
Hilar Cholangiocar-cinoma, sometimes referred to as a Klatskin tumor, is a rare neoplasm arising proximal to the confluence of the cystic and common hepatic ducts.1,2 In the United States, hilar cholangiocarcinoma has a reported annual incidence of 1 to 2 persons per 100 000 population.3,4 Although a subset of patients may have a hilar tumor characterized as slow growing and late to metastasize,1,2 most patients with untreated cholangiocarcinoma have a median survival of 6 months.5,6 Although surgical resection is the only curative option, 5-year survival following resection of hilar cholangiocarcinoma has been reported to range from 20% to 40%.7–10
Various staging systems have been proposed to better stratify patients with regard to long-term survival following resection of hilar cholangiocarcinoma.11–17 Currently, the American Joint Committee on Cancer (AJCC) TNM staging system11,12 is the most commonly used method to stage cholangiocarcinoma. The prognostic accuracy of the AJCC staging scheme has, however, been questioned. Although some authors have reported that AJCC stage is predictive of survival,3,18 other investigators have noted its inaccuracy.5,19 Recently, in the new 7th edition of the AJCC Cancer Staging Manual,12 extrahepatic bile duct tumors have been separated into hilar and distal groups with separate staging classifications defined for each. Categorization to one of the T subgroups still, however, depends on whether the tumor is confined to the bile duct, as well as its relationship to surrounding anatomy. This may be problematic because the bile duct lacks discrete tissue boundaries and is not uniformly concentric along its length.20 The objective of the current study was to assess the prognostic accuracy of the 6th and 7th editions of the AJCC staging systems for hilar cholangiocarcinoma. In addition, we sought to identify prognostic factors associated with survival following resection of hilar cholangiocarcinoma, with a particular emphasis on assessing the prognostic importance of depth of tumor invasion.
METHODS
From January 1, 1987, through December 31, 2009, there were 315 patients who underwent surgical resection for hilar cholangiocarcinoma at The Johns Hopkins Hospital (Baltimore, Maryland). Only patients with documented hilar cholangiocarcinoma who had at least 6 months of follow-up were included in this study. Cases with an insufficient number of slide sections containing cholangiocarcinoma were excluded for reevaluation and measurement of invasion depth. Moreover, patients who had undergone an R2-resection (ie, macroscopically positive margins) were excluded. A total of 106 patients had a sufficient number of pathologic slide sections available for reevaluation and measurement of depth of tumor invasion, and they constitute the cohort for the current study.
Data were collected on demographic characteristics; operative details; resection margin status; tumor size and grade; histologic subtype; lymph node status; presence of perineural and/or microscopic vascular invasion; invasion of adjacent liver, gallbladder, and/or duodenum; and survival status. In addition, maximal depth of tumor invasion was assessed. One pathologist (S.-M.H.), who was masked to the clinical and outcome data, re-reviewed each case to measure the depth of tumor invasion. Depth of tumor invasion was defined as the area of deepest infiltration from the mucosal surface (ie, distance from the basal lamina of the adjacent normal epithelium to the most deeply advanced tumor cells).20 Representative images of measurement of invasion depth are depicted in Figure 1. On the basis of sensitivity analyses, the maximum depth of tumor invasion was categorized into 2 groups: >5 mm and ≥5 mm.
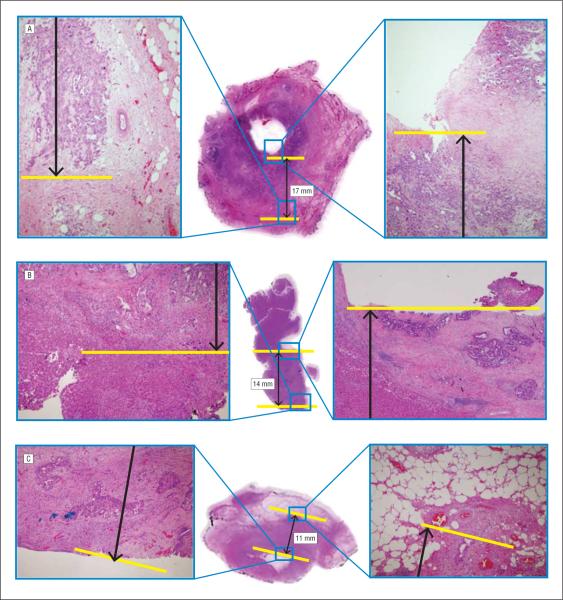
Figure 1.
Pathologic slides (hematoxylin-eosin, original magnification ×1; images in insets are original magnification ×4) of 3 hilar cholangiocarcinomas included in the current study, illustrating the discordance between the American Joint Committee on Cancer 6th and 7th edition T-classification staging schemes and invasion depth of the tumor. A, Invasion depth of 17 mm and classified as a T2 tumor in both editions. B, A T3 tumor in the 6th edition but a T2 tumor in the 7th edition (invasion depth of 14 mm). C, A T2 tumor in the 6th edition whereas a T3 tumor in the 7th edition (maximum depth of tumor invasion, 11 mm).
Summary statistics were obtained using established methods and presented as percentages or median values. Survival time was estimated using the nonparametric product limit method (Kaplan-Meier).21 Differences in survival were examined using the log-rank test. Factors associated with survival were examined using univariate and, where applicable, multivariate Cox proportional hazards regression analyses. The hazard ratio and the 95% confidence intervals were estimated, and a P value less than .05 was considered significant. All statistical analyses were performed using SPSS, version 17.0 (SPSS, Inc, Chicago, Illinois), and Stata, version 10 (StataCorp LP, College Station, Texas).
RESULTS
PATIENT AND TUMOR CHARACTERISTICS
Table 1 shows the clinicopathologic characteristics of the 106 patients. Most patients were male (60 [56.6%]), with the median age of the entire cohort being 60 years (range, 23–84 years). At the time of surgery, 77 patients (72.6%) underwent a bile duct resection alone, and the remaining patients (29 [27.4%]) underwent a bile duct resection and a concomitant hepatectomy. The surgical approach changed over time, with an increasing number of patients undergoing a bile duct resection and concomitant hepatectomy after 1990 (P=.01). On final pathologic analysis, overall median size of the tumor was 2.5 cm (range, 0.4–8.0 cm). Most patients (58 [54.7%]) had a moderately differentiated tumor. The histologic diagnosis was adenocarcinoma in most patients (96 [90.6%]); a small subset of patients had a papillary carcinoma (7 [6.6%]) or other histologic subtypes (3 [2.8%]). On pathologic analysis, perineural invasion was present in 61 patients (57.5%), and microscopic vascular invasion was present in 18 (17.0%). Invasion into the gallbladder or adjacent liver was present in 8.5% and 22.6% of patients, respectively. The margin had microscopic disease present (R1) in 43 patients (40.6%) and was free of microscopic disease (R0) in 63 (59.4%).
Table 1.
Patient and Tumor Characteristics of 106 Patients With Hilar Cholangiocarcinomaa
| Variable | Value |
|---|---|
| Patient characteristic | |
| Age, mean (SD),y | 64 (12) |
| Male sex | 60 (56.6) |
| Tumor characteristics | |
| Tumor size, median (range), cm | 2.5 (0.4–8.0) |
| Histologic type | |
| Adenocarcinoma | 96 (90.6) |
| Papillary carcinoma | 7 (6.6) |
| Other | 3 (2.8) |
| Differentiation | |
| Well | 25 (23.6) |
| Moderately | 58 (54.7) |
| Poorly | 23 (21.7) |
| 6th edition of the AJCC T classification | |
| T1 | 25 (23.6) |
| T2 | 49 (46.2) |
| T3 | 32 (30.2) |
| 7th edition of the AJCC T classification | |
| T1 | 25 (23.6) |
| T2a and T2b | 62 (58.5) |
| T3 | 19 (17.9) |
| Node-positive disease | 14 (13.2) |
| Hepatic invasion, present | 24 (22.6) |
| Pancreatic invasion, present | 2 (1.9) |
| Perineural invasion, present | 61 (57.5) |
| Microscopic vascular invasion, present | 18 (17.0) |
| Depth of tumor invasion, mm | |
| <5 | 32 (30.2) |
| ≥5 | 74 (69.8) |
Abbreviation: AJCC, American Joint Commission on Cancer.
Data are given as number (percentage) of patients unless otherwise indicated.
Patients were classified with regard to the extent of local disease using both the 6th and 7th editions of the AJCC staging schemes. Using the 6th edition T-classification criteria,11 most lesions were categorized as T2 (49 [46.2%]), with fewer patients having T3 (32 [30.2%]) or T1 (25 [23.6%]) tumors. On the basis of the new 7th edition T-classification criteria,12 more patients (62 [58.5%]) were categorized with T2 lesions; in turn, fewer tumors were categorized as T3 (19 [17.9%]) or T1 (25 [23.6%]). No patient had T4 disease, which is defined as invasion into the main portal vein/hepatic artery and therefore is almost always inoperable. When cases were analyzed according to depth of tumor invasion, the median depth of tumor invasion was 7 mm (range, 1–35 mm). Most patients (74 [69.8%]) had tumors with a depth of invasion of 5 mm or greater. Depth of tumor invasion was associated with extent of hepatectomy; patients who had a depth of invasion of 5 mm or greater were more likely to have undergone a concomitant hepatectomy (25 of 74 [33.8%]) compared with the likelihood of patients who had a depth of tumor invasion less than 5 mm (4 of 32 [12.5%]) (P=.02). In contrast, depth of tumor invasion was not associated with R margin status. Of note, depth of tumor invasion was also not necessarily concordant with AJCC T-stage (Figure 1).
FACTORS ASSOCIATED WITH SURVIVAL
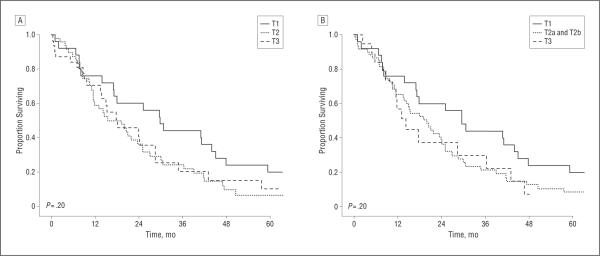
Overall median survival following resection was 19.9 months, with an estimated 3-year survival of 27.8%. On univariate analysis, perineural/microscopic vascular invasion, type of surgical resection, and margin status were not associated with survival (P>.05). In contrast, the presence of gallbladder invasion as well as tumor differentiation were associated with survival (Table 2). Median survival for patients with poor histologic differentiation was 13.7 months, whereas it was 21.8 months for patients with moderately differentiated tumors and 26.9 months for patients with well-differentiated tumors (P<.05 for both). Of note, AJCC T classification was not associated with survival. Specifically, the median survival of patients stratified according to the 6th edition of the AJCC T-classification criteria was T1, 29.7 months vs T2, 15.4 months vs T3, 18.0 months (P>.05) (Figure 2A). The 7th edition of the AJCC T-classification criteria was equally poor at stratifying patients in the various T classifications with regard to survival (T1, 29.7 months vs T2, 19.9 months vs T3, 14.2 months; P>.05) (Figure 2B). In contrast, increasing depth of tumor invasion was associated with a worse outcome. Specifically, patients with a tumor depth of less than 5 mm had a median survival of 29.7 months compared with 17.7 months for patients with a tumor depth of 5 mm or greater. Similarly, 3-year survival was worse among patients with tumors of greater depth(>5mm, 45.5% vs≥5mm, 20.0%; P=.01) (Figure 3).
Table 2.
Univariate and Multivariate Analyses of Factors Associated With Survival in Patients With Hilar Cholangiocarcinoma
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| Prognostic Factor | Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value |
| Male sex | 1.14 (0.75–1.73) | .54 | … | … |
| Age | 1.00 (0.99–1.03) | .54 | … | … |
| Differentiation | ||||
| Well | 1 [Reference] | … | 1 [Reference] | … |
| Moderately | 1.54 (0.90–2.63) | .11 | 1.41 (0.83–2.43) | .20 |
| Poorly | 2.41 (1.26–4.64) | .008 | 2.21 (1.14–4.29) | .02 |
| Tumor size, continuous | 0.86 (0.71–1.05) | .13 | … | … |
| 6th edition of the AJCC T classification | ||||
| T1 | 1 [Reference] | … | … | … |
| T2 | 1.59 (0.95–2.66) | .08 | … | … |
| T3 | 1.42 (0.79–2.54) | .24 | … | … |
| 7th edition of the AJCC T classification | ||||
| T1 | 1 [Reference] | … | … | … |
| T2 | 1.49 (0.91–2.44) | .12 | … | … |
| T3 | 1.68 (0.87–2.27) | .12 | … | … |
| Node-positive disease | 1.27 (0.63–2.54) | .51 | … | … |
| Hepatic invasion, present | 0.77 (0.44–1.33) | .33 | … | … |
| Gallbladder invasion, present | 2.60 (1.23–5.50) | .01 | 1.87 (0.86–4.04) | .11 |
| Perineural invasion, present | 1.25 (0.82–1.91) | .30 | … | … |
| Microscopic vascular invasion, present | 1.58 (0.90–2.79) | .11 | … | … |
| Depth of tumor invasion, mm | ||||
| <5 | 1 [Reference] | … | 1 [Reference] | … |
| ≥5 | 1.82 (1.14–2.90) | .01 | 1.70 (1.05–2.43) | .03 |
Abbreviations: AJCC, American Joint Commission on Cancer; CI, confidence interval. Ellipses indicate that only those factors significant (P<.05) on univariate analyses were included in the multivariate model.
Figure 2.
Overall Kaplan-Meier survival stratified according to the American Joint Committee on Cancer 6th Edition (A) and 7th edition (B) T-classification staging scheme.
Figure 3.
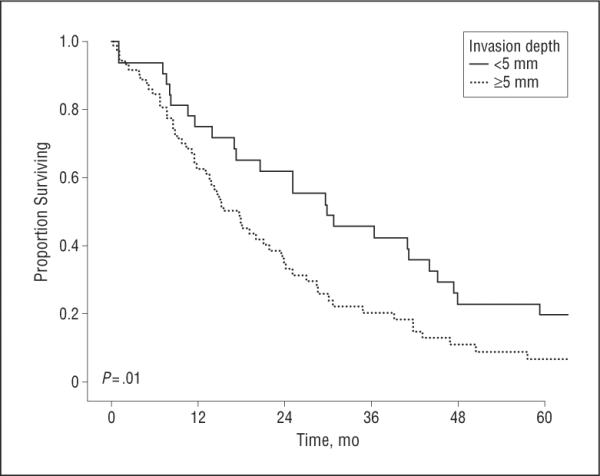
Overall Kaplan-Meier survival stratified according to the depth of tumor invasion (>5 mm vs ≥5 mm).
Increasing depth of tumor invasion was associated with an increased likelihood of certain adverse clinicopathologic factors, including lymph node metastasis, perineural invasion, and invasion of the adjacent liver (P≤.05 for all) (Table 3). After controlling for competing risk factors on multivariate analysis, depth of tumor invasion remained associated with an increased risk of death following surgery (≥5 mm vs <5 mm: hazard ratio, 1.70 [95% confidence interval, 1.05–2.43]; P=.03) (Table 2).
Table 3.
Association Between Presence of Certain Adverse Clinicopathologic Factors and Depth of Tumor Invasiona
| Variable | Depth of Tumor Invasion |
P Value | |
|---|---|---|---|
| <5 mm (n=32) | ≥5 mm (n=74) | ||
| Node-positive disease | 0 | 14 (18.9) | .009 |
| Presence of perineural invasion | 9 (28.1) | 52 (70.3) | <.001 |
| Presence of microscopic vascular invasion | 3 (9.4) | 15 (20.3) | .26 |
| Presence of gallbladder invasion | 0 | 9 (12.2) | .05 |
| Presence of hepatic invasion | 1 (3.1) | 23 (31.1) | <.001 |
Data are given as number (percentage) of patients.
COMMENT
Although a relatively rare tumor, hilar cholangiocarcinoma comprises up to 50% to 70% of all cases of bile duct carcinomas.7,22 Hilar bile duct tumors have been traditionally staged collectively as extrahepatic bile duct tumors in the AJCC Cancer Staging Manual. There has been a growing recognition that hilar vs distal cholangiocarcinomas have a distinct biologic behavior and natural history, as well as therapeutic treatment strategy.3,23,24 In turn, this finding has led the latest (7th) edition of the AJCC Cancer Staging Manual to divide perihilar and distal cholangiocarcinoma into distinct staging subgroups.11,12 Despite the creation of a novel staging system for perihilar bile duct cancers, the criteria for the T-classification subgroups have remained largely unchanged, with a reliance on whether the tumor is confined to the bile duct. As we and others have previously noted, however, these criteria are problematic.17,20,25 Specifically, defining the extent of disease on the basis of whether the tumor is confined to the bile duct histologically may be inaccurate because the bile duct wall is not uniformly concentric along its length. Rather, the bile duct wall is characterized by varying amounts of loose connective and fibrous tissue.26 As such, our group had previously proposed using depth of tumor invasion as a more accurate predictor of prognosis following surgical resection of cholangiocarcinoma.20 Although our previous work suggested that depth of tumor invasion may better predict outcome, the association of tumor depth with outcome had not been examined in a cohort of patients solely with hilar cholangiocarcinoma. The current study is important because, to our knowledge, it is one of the first to attempt validation of the new AJCC 7th edition staging of perihilar cholangiocarcinoma. In addition to identifying which clinicopathologic determinants were associated with survival after resection of hilar cholangiocarcinoma, we showed that depth of tumor invasion is a better method to determine prognosis compared with the AJCC T-classification criteria.
In the 6th edition of the AJCC Cancer Staging Manual, hilar and distal cholangiocarcinomas were staged using the same scheme. Specifically, tumor location within the bile duct was used to categorize patients into different prognostic groups. For example, T classifications were, in part, dependent on whether the tumor invaded through the bile duct and into adjacent structures, such as the liver or pancreas. In a cohort of 87 patients with cholangiocarcinoma, Burke et al15 reported that the 6th edition of the AJCC scheme was not a robust predictor of prognosis. Specifically, in this series, the difference in survival between patients with T1 and T3 lesions was marginal (P=.05), and there were no differences in long-term outcome among patients with T3 and T4 lesions. In a separate study of 42 patients with cholangiocarcinoma, Zervos et al5 noted that the 6th edition of the AJCC T subgroups was not associated with survival following resection (P>.05 for all). In the current study, with a cohort of patients who had hilar cholangiocarcinoma exclusively, we similarly noted that the 6th edition of the AJCC T-classification criteria failed to stratify patients with regard to prognosis following re-section (Figure 2A). However, unlike other studies, we were also able to examine the new 7th edition of the AJCC staging system for hilar cholangiocarcinoma. Although the establishment of a separate, dedicated staging system for hilar cholangiocarcinoma in the 7th edition of the AJCC Cancer Staging Manual was a needed improvement, our data strongly suggest that the new staging system fails to perform any better (Figure 2B).
Several groups, including our own, have suggested that depth of tumor invasion may be a much better predictor of survival among patients undergoing resection of cholangiocarcinoma.20,25,27–29 In a small series of 37 patients with hilar cholangiocarcinoma, Hidaka28 noted that a greater histologic depth of invasion tended to be associated with a worse long-term outcome. In a separate study, Nagahashi et al29 reported that long-term outcome after resection of extrahepatic cholangiocarcinoma was associated with depth of tumor invasion. Specifically, patients with mucosal tumors had a better prognosis compared with that of patients with fibromuscular layer-invasive tumors. We similarly noted that depth of tumor invasion was an important predictor of survival. Whereas the median survival was 29.7 months for patients with a tumor depth of less than 5 mm, the median survival of patients with a tumor depth of 5 mm or greater was only 17.7 months (Figure 3). Not only does the current study serve to independently confirm depth of tumor invasion as an important prognostic factor, but we were able to examine its effect on survival more rigorously. Because previous data were limited by small sample size and lack of statistical power, previous authors were not able to assess depth of tumor invasion relative to other competing risk factors such as tumor grade, microscopic vascular invasion, or invasion into adjacent structures. In the current study, however, multivariate analyses were performed that controlled for these possible confounders, which in turn showed depth of tumor invasion to remain as a strong and independent prognostic factor (Table 2).
The reasons that depth of tumor invasion may be a better predictor of survival compared with the AJCC staging are probably multifactorial. It is important to note that neither the 6th nor the 7th edition of the AJCC T-classification subgroups necessarily correlated with depth of tumor invasion. In fact, some T2 tumors were noted to have a greater depth of tumor invasion compared with that of certain T3 tumors (Figure 1). Some have argued that a cholangiocarcinoma characterized by less depth of tumor invasion but involvement of the adjacent liver (T3) may have a better biologic behavior than tumors of greater tumor depth that lack adjacent liver invasion.20 Invasion of the liver may be more of an anatomic determinant, whereas depth of tumor invasion may be more of a biologic determinant. Our data seem to support this theory because greater depth of tumor invasion was associated with an increased likelihood of having other adverse pathologic factors (Table 3). Patients with a greater depth of tumor invasion were more likely to have lymph node metastasis, perineural invasion, and invasion of the adjacent liver. Although greater depth of tumor invasion was associated with adverse biologic factors and thus, not surprisingly, a worse overall survival, even after controlling for these factors, tumor depth remained one of the best predictors of survival.
The current study has several limitations. Because only patients with adequate follow-up and who had slides available for re-review were included in the study, there may have been a selection bias. There is no reason, however, to suspect that selection of such patients would have resulted in a systematic selection bias that would have affected our primary analysis of tumor depth and survival. Despite our hospital's having one of the largest hepatobiliary experiences in the country, only 106 patients were included in the current study. The lack of association between perineural/microscopic vascular invasion and margin status on outcome may therefore be related to a lack of statistical power. In addition, although the relatively small number of patients in the current study may have been unable to detect small differences in survival among the AJCC T-classification subgroups, the fact remains that depth of tumor invasion was a better predictor of prognosis. Of note, the incidence of R1 resection in the current series was higher than that reported by many other hepatobiliary centers.16,30,31 The reason for this finding is probably multifactorial but is likely due to our past institutional approach of performing only an extrahepatic bile duct resection for hilar cholangiocarcinoma. More recently we have adopted routine liver resection for hilar cholangiocarcinoma, which has been shown to improve R0 resection rates.8,31–33 Interpretation of our data needs to be considered in light of this outcome. Finally, we did not analyze other less frequently used staging systems for hilar cholangiocarcinoma, such as the Blumgart/Memorial Sloan Kettering Cancer Center staging system.15–17 Rather, in the current study we sought to examine the much more widely used AJCC staging system, with a particular emphasis on assessing the new 7th edition relative to depth of tumor invasion.
In conclusion, both the 6th and the newer 7th editions of the AJCC T classification criteria poorly stratified patient survival following resection of hilar cholangiocarcinoma. Depth of tumor invasion was a better predictor of long-term outcome and was associated with other adverse biologic tumor characteristics. Even after controlling for these other competing risk factors, depth of tumor invasion remained a strong predictor of survival. Therefore, depth of bile duct carcinoma invasion appears better than the current AJCC staging of hilar cholangiocarcinoma.
Acknowledgments
Funding/Support: This work was supported by grants P50CA62924 and R01CA120432 (Dr Goggins) and R01DK080736 and R01DK081417 (Dr Anders) from the National Institutes of Health.
Footnotes
Drs de Jong and Hong contributed equally to this work.
Author Contributions: Study concept and design: de Jong, Hong, Goggins, Hirose, Anders, and Pawlik. Acquisition of data: de Jong, Hong, Augustine, Schulick, Anders, and Pawlik. Analysis and interpretation of data: de Jong, Hong, Wolfgang, Schulick, Choti, Anders, and Pawlik. Drafting of the manuscript: de Jong, Hong, Augustine, and Pawlik. Critical revision of the manuscript for important intellectual content: Goggins, Wolfgang, Hirose, Schulick, Choti, Anders, and Pawlik. Statistical analysis: de Jong, Hong, and Pawlik. Obtained funding: Anders. Administrative, technical, and material support: Hong, Schulick, Anders, and Pawlik. Study supervision: Goggins, Wolfgang, Hirose, Schulick, Choti, Anders, and Pawlik.
Financial Disclosure: None reported.
REFERENCES
- 1.Klatskin G. Adenocarcinoma of the hepatic duct at its bifurcation within the porta hepatis: an unusual tumor with distinctive clinical and pathological features. Am J Med. 1965;38:241–256. doi: 10.1016/0002-9343(65)90178-6. [DOI] [PubMed] [Google Scholar]
- 2.Altemeier WA, Gall EA, Zinninger MM, Hoxworth PI. Sclerosing carcinoma of the major intrahepatic bile ducts. AMA Arch Surg. 1957;75(3):450–460. doi: 10.1001/archsurg.1957.01280150140015. [DOI] [PubMed] [Google Scholar]
- 3.Ito F, Cho CS, Rikkers LF, Weber SM. Hilar cholangiocarcinoma: current management. Ann Surg. 2009;250(2):210–218. doi: 10.1097/SLA.0b013e3181afe0ab. [DOI] [PubMed] [Google Scholar]
- 4.Jarnagin WR. Cholangiocarcinoma of the extrahepatic bile ducts. Semin Surg Oncol. 2000;19(2):156–176. doi: 10.1002/1098-2388(200009)19:2<156::aid-ssu8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 5.Zervos EE, Osborne D, Goldin SB, et al. Stage does not predict survival after resection of hilar cholangiocarcinomas promoting an aggressive operative approach. Am J Surg. 2005;190(5):810–815. doi: 10.1016/j.amjsurg.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 6.Nordback IH, Pitt HA, Coleman J, et al. Unresectable hilar cholangiocarcinoma: percutaneous versus operative palliation. Surgery. 1994;115(5):597–603. [PubMed] [Google Scholar]
- 7.Jarnagin WR, Shoup M. Surgical management of cholangiocarcinoma. Semin Liver Dis. 2004;24(2):189–199. doi: 10.1055/s-2004-828895. [DOI] [PubMed] [Google Scholar]
- 8.Seyama Y, Kubota K, Sano K, et al. Long-term outcome of extended hemihepatectomy for hilar bile duct cancer with no mortality and high survival rate. Ann Surg. 2003;238(1):73–83. doi: 10.1097/01.SLA.0000074960.55004.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Unno M, Katayose Y, Rikiyama T, et al. Major hepatectomy for perihilar cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2010;17(4):463–469. doi: 10.1007/s00534-009-0206-3. [DOI] [PubMed] [Google Scholar]
- 10.Young AL, Prasad KR, Toogood GJ, Lodge JP. Surgical treatment of hilar cholangiocarcinoma in a new era: comparison among leading Eastern and Western centers, Leeds. J Hepatobiliary Pancreat Sci. 2010;17(4):497–504. doi: 10.1007/s00534-009-0203-6. [DOI] [PubMed] [Google Scholar]
- 11.Greene F, Page D, Fleming I, et al. AJCC Cancer Staging Manual. 6th ed. Springer; Chicago, IL: 2002. [Google Scholar]
- 12.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th ed. Springer; Chicago, IL: 2009. [Google Scholar]
- 13.Bismuth H, Corlette MB. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet. 1975;140(2):170–178. [PubMed] [Google Scholar]
- 14.Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg. 1992;215(1):31–38. doi: 10.1097/00000658-199201000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burke EC, Jarnagin WR, Hochwald SN, Pisters PW, Fong Y, Blumgart LH. Hilar cholangiocarcinoma: patterns of spread, the importance of hepatic resection for curative operation, and a presurgical clinical staging system. Ann Surg. 1998;228(3):385–394. doi: 10.1097/00000658-199809000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234(4):507–517. doi: 10.1097/00000658-200110000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chamberlain RS, Blumgart LH. Hilar cholangiocarcinoma: a review and commentary. Ann Surg Oncol. 2000;7(1):55–66. doi: 10.1007/s10434-000-0055-4. [DOI] [PubMed] [Google Scholar]
- 18.Hidalgo E, Asthana S, Nishio H, et al. Surgery for hilar cholangiocarcinoma: the Leeds experience. Eur J Surg Oncol. 2008;34(7):787–794. doi: 10.1016/j.ejso.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Klempnauer J, Ridder GJ, von Wasielewski R, Werner M, Weimann A, Pichlmayr R. Resectional surgery of hilar cholangiocarcinoma: a multivariate analysis of prognostic factors. J Clin Oncol. 1997;15(3):947–954. doi: 10.1200/JCO.1997.15.3.947. [DOI] [PubMed] [Google Scholar]
- 20.Hong SM, Pawlik TM, Cho H, et al. Depth of tumor invasion better predicts prognosis than the current American Joint Committee on Cancer T classification for distal bile duct carcinoma. Surgery. 2009;146(2):250–257. doi: 10.1016/j.surg.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 22.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24(2):115–125. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 23.DeOliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245(5):755–762. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma: a spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224(4):463–473. doi: 10.1097/00000658-199610000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong SM, Kim MJ, Pi DY, et al. Analysis of extrahepatic bile duct carcinomas according to the New American Joint Committee on Cancer staging system focused on tumor classification problems in 222 patients. Cancer. 2005;104(4):802–810. doi: 10.1002/cncr.21236. [DOI] [PubMed] [Google Scholar]
- 26.Hong SM, Kang GH, Lee HY, Ro JY. Smooth muscle distribution in the extrahepatic bile duct: histologic and immunohistochemical studies of 122 cases. Am J Surg Pathol. 2000;24(5):660–667. doi: 10.1097/00000478-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Hong SM, Cho H, Moskaluk CA, Yu E. Measurement of the invasion depth of extrahepatic bile duct carcinoma: an alternative method overcoming the current T classification problems of the AJCC staging system. Am J Surg Pathol. 2007;31(2):199–206. doi: 10.1097/01.pas.0000213384.25042.86. [DOI] [PubMed] [Google Scholar]
- 28.Hidaka A. Clinicopathological study of patients undergoing resection of hilar cholangiocarcinoma. Kurume Med J. 2007;54(1–2):41–49. doi: 10.2739/kurumemedj.54.41. [DOI] [PubMed] [Google Scholar]
- 29.Nagahashi M, Shirai Y, Wakai T, et al. Depth of invasion determines the postresectional prognosis for patients with T1 extrahepatic cholangiocarcinoma. Cancer. 2010;116(2):400–405. doi: 10.1002/cncr.24766. [DOI] [PubMed] [Google Scholar]
- 30.Rocha FG, Matsuo K, Blumgart LH, Jarnagin WR. Hilar cholangiocarcinoma: the Memorial Sloan-Kettering Cancer Center experience. J Hepatobiliary Pancreat Sci. 2010;17(4):490–496. doi: 10.1007/s00534-009-0205-4. [DOI] [PubMed] [Google Scholar]
- 31.Ito F, Agni R, Rettammel RJ, et al. Resection of hilar cholangiocarcinoma: concomitant liver resection decreases hepatic recurrence. Ann Surg. 2008;248(2):273–279. doi: 10.1097/SLA.0b013e31817f2bfd. [DOI] [PubMed] [Google Scholar]
- 32.Kosuge T, Yamamoto J, Shimada K, Yamasaki S, Makuuchi M. Improved surgical results for hilar cholangiocarcinoma with procedures including major hepatic resection. Ann Surg. 1999;230(5):663–671. doi: 10.1097/00000658-199911000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neuhaus P, Jonas S, Bechstein WO, et al. Extended resections for hilar cholangiocarcinoma. Ann Surg. 1999;230(6):808–819. doi: 10.1097/00000658-199912000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]