Abstract
G protein-coupled receptors (GPCRs) represent the largest and most versatile family of signaling receptors. Their actions are highly regulated, both under physiological conditions and in response to clinically relevant drugs. A key element in this regulation is control of the number of functional receptors at the cell surface. Major processes that mediate this regulation are endocytosis and recycling of receptors. These trafficking events involve a concerted series of steps, and it is increasingly clear that some of these steps can occur on a rapid timescale comparable to functional signaling itself. Here, we discuss and describe an optical imaging approach, based on evanescent field or total internal reflection fluorescence microscopy (TIR-FM), to investigate receptor endocytosis and recycling at the level of discrete membrane fission and fusion events. TIR-FM facilitates the study of receptor trafficking events near the plasma membrane with much greater spatial and temporal resolution than afforded by traditional methods. TIR-FM has already provided new insight to GPCR regulation, and we believe that this method has great potential for addressing a variety of questions in GPCR biology.
Keywords: Fluorescence microscopy, live imaging, total internal reflection, trafficking, endocytosis, recycling, receptors
1. Introduction
Membrane trafficking of signaling receptors is critical to many aspects of animal physiology. Rapid internalization of surface receptors is often stimulated by agonist-induced activation of receptors, and is thought to control signaling both from the plasma membrane and intracellular compartments(1). The functional importance of endocytic trafficking has been well established for members of the GPCR superfamily. Many physiological responses mediated by GPCRs, particularly in the nervous system, occur on a relatively rapid time scale (seconds to minutes). This time scale is significantly shorter than the kinetics of most receptor trafficking events estimated using traditional methods. It is increasingly clear that certain GPCR trafficking events, particular those occurring in the endocytic pathway, can occur with kinetics that are in a similar range as acute signaling. Further, there is increasing evidence that endocytic trafficking contributes to the regulation of receptor number in particular domains of complex cells, such as in controlling receptor number near spatially separated chemical synapses(2). These realizations have motivated increased interest in methods for examining GPCR trafficking with higher temporal and spatial resolution than afforded by traditional methods. Developments in methods and reagents for live fluorescence imaging have greatly facilitated progress in this direction.
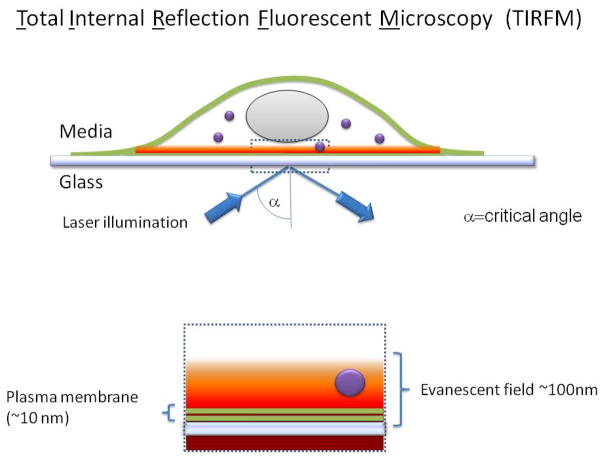
We will focus on the application of total internal reflection-fluorescence microscopy (TIR-FM) to study GPCR trafficking in the endocytic pathway. Reflection of light at a refractive interface generates an evanescent field that diminishes exponentially with distance from the interface. The evanescent field creates a shallow field of illumination, extending in practice ≤100 nm from the reflective surface. If this surface is a coverslip supporting dissociated cells in a culture preparation, the evanescent field of illumination is useful for selectively exciting fluorescent probes located in the basal plasma membrane and extending a short distance into the cytoplasm (Figure 1). TIR-FM facilitates observation of events occurring in the plasma membrane, and in a shallow region of cytoplasm immediately adjacent to the plasma membrane, with high signal-to-background ratio because fluorescent molecules located deeper within cells or in the culture medium are not excited (3–5). Combined with recent improvements in electronic sensor technology, such as the widespread availability of high sensitivity CCD cameras, TIR-FM is capable of investigating membrane events involving small numbers of receptors and with practical time resolution on the order of tens of milliseconds. Newly developed automated systems help to maintain a steady focal plane during acquisition, which is essential for quantitative imaging over prolonged intervals and at physiological temperatures. This combination of technological advances, once in the domain of only highly specialized laboratories, is increasingly available and provides a highly useful platform with which to study surface receptor trafficking at the single cell level.
Figure 1. Schematic view showing the main features of a TIR-FM imaging system.
A standard wide field microscope is used. The evanescent illumination field is generated by total internal reflection at the cover slip/sample interface. This requires illuminating the cover slip with a collimated light source at the critical angle, and is achieved in a typical “through-the-objective” system by focusing a laser beam near the edge of the back focal plane of a high numerical aperture objective. The evanescent field generated at the reflective interface falls off rapidly with distance, selectively exciting fluorophores located at or near the plasma membrane. This results in a signal-to-background ratio that is substantially higher than can be achieved in wide field imaging using standard epifluorescence illumination, and generally higher than that obtainable using confocal fluorescence microscopy.
The availability of a wide range of biologically compatible fluorescent probes, including genetically encoded fluorescent proteins, enable molecular specificity combined with spatio-temporal resolution that is useful for analyzing surface receptor dynamics. These tags have been widely applied in cell biology and are extensively reviewed elsewhere (6–8). Here we focus on imaging a pH sensitive variant of the green fluorescent protein called superecliptic phluorin (SpH or SEP) (9, 10) fused to the amino-terminal extracellular domain of the human beta-2 adrenergic receptor (SEP- β2AR). SEP- β2AR is highly fluorescent at the neutral pH of the extracellular media, but its fluorescence is rapidly and reversibly quenched in the mildly acidic environment of the endocytic and recycling pathways. This property of SEP- β2AR facilitates the detection of discrete endocytic and exocytic events mediating surface receptor removal and insertion.
2. Materials
2.1 Cell culture
HEK-293 cells passage 20–50 (ATCC:CRL-1573)
35 mm disposable MatTek glass bottom dishes (http://www.glass-bottom-dishes.com/)
Dulbecco’s Modified Eagle’s Medium-high glucose (DMEM) supplemented with 10% fetal bovine serum (Sigma)
Lipofectamine 2000 (Invitrogen)
Opti-MEM imaging buffer supplemented with 20 mM HEPES (Invitrogen)
Poly-D-Lysine (Sigma P0899)
Isoproterenol (Sigma I6504 (−))
2.2. Imaging equipment and settings
Inverted fluorescence microscope (Nikon TE2000E) with Perfect Focus and TIRF objectives: 60x/1.45 Oil - Plan Apo TIRF; 100x/1.49 Oil - Plan Apo TIRF. Nikon TIRF system with 440 nm, 488 nm, 514 nm, 561 nm lasers.
EM-CCD cameras Photometrics Quant EMCCD (www.photomet.com ) or iXonEM+ EMCCD 897 Camera from Andor (www.andor.com)
Objective and Petri dish heaters with temperature controller to maintain media temperature at 37°C (Bioscience Tools)
Excitation and emission settings for TIRF: GFP = 488 nm laser excitation (2 mWatt) mCherry = 561 nm laser excitation (2–4 mWatt) 525/50 band pass, 527/21 nm and 645/24 nm dual bandpass emission filter.
Exposure time: continuous 100 msec. exposure for receptor recycling, camera EM gain is set constant to obtain comparable results: X299, binning: 1×1, image: 512×512, pre-amp-gain=4.90, horizontal readout=10 vertical readout time=3.3, temperature= −75. BitDepth=14 bits for Andor iXonEM+.
3. Methods
3.1 Cell Prepration
Dissolve Poly-D-Lysine in sterile water (50 μg/ml) and place 2ml overnight at room temperature. Wash PDL with sterile water (3 washes) and dry the culture dishes.
Seed HEK-293 cells onto the coated dishes.
Transfect with SEP- β2AR (11) construct (1 μg per dish) using lipofectamine 2000 following manufacturer protocol 72 hours prior to imaging.
The day of the imaging, replace incubation media 15 to 30 minutes before experiments with Opti-MEM or a low fluorescence media and return cells to the incubator. Remove phenol red, serum, folic acid and riboflavin and other possible interference from the imaging media. EGFP Photostability should also taken into account during media selection (12).
3.2 Live-cell imaging
-
5
Start by initializing microscope, lasers, camera and temperature control devices 30 to 45 minutes before any data acquisition.
-
6
Select the proper TIRF objective and add a small amount of immersion oil (TYPE DF from Cargille) on the objective and fit the glass bottom dish on the stage of the microscope and to the heating ring element. NOTE: temperature of the imaging media must be monitored and kept constant when dishes are imaged, changes in temperature will affect trafficking kinetics.
-
7
First, find cells using transmission light to get them into focus minimizing photobleaching. Second, illuminate cells in epifluorescent mode to find cells expressing tagged receptors and then switch to TIRF illumination. Move the laser away from the center of the optical path and continue to achieve total internal reflection. Find the plasma membrane by adjusting the focal plane. NOTE: Finding the exact angle for TIRF is the most critical step in this protocol. Cells illuminated in TIRF will present sharp edges and a increased signal to noise ratio when compared with out of TIRF or oblique illumination (Compared fig 2A versus 2B)
-
8
Find cells in the ideal fluorescence range for your experiments and begin data acquisition. NOTE: TIR-FM is intrinsically very sensitive, due to the low level of background fluorescence. We generally strive for the lowest expression level and illumination intensity that is sufficient for later analysis. In our experiments, using an Andor iXonEM+, we have found that an intensity signal of ~2000 (out of a maximum of 16384 in the 14-bit readout mode) to be more than adequate. We recommend using a polyclonal cell line that stably expresses your receptor of interest, to allow rapid identification of cells in a suitably range of fluorescence intensity.
-
9
Acquisition settings for imaging agonist-induced clustering and endocytosis of receptors: Intermittent illumination and acquisition of100msec exposures every 3 seconds. Total time: 10 minutes.
-
10
Initiate data acquisition and acquire 10 to 30 frames before agonist addition.
-
11
Add agonist (10μM isoproterenol) with minimum disturbance to the cell either by an automated perfusion system or by careful addition of the agonist diluted in pre-warmed imaging media. Manual agonist addition should not be performed directly on top of the imaging area/cells but outside of the imaging area.
-
12
For resolving discrete fusion events mediating SEP- β2AR recycling, cells are exposed to the presence of 10μM isoproterenol for 10 minutes in the incubator. This step induces receptor internalization and loads the endocytic pathway inducing extensive recycling events that can be subsequently observed.
-
13
Acquisition settings for observing discrete recycling events: Continuous illumination and acquisition of serial 100msec exposures, using the CCD in frame-transfer readout mode. Total imaging time: 60 seconds.
-
14
Save acquired data. Note: careful consideration must be given to file management and storage. Tags, metadata and thorough indexing will help future data retrieval and analysis. See http://www.openmicroscopy.org for open source tools to support data management.
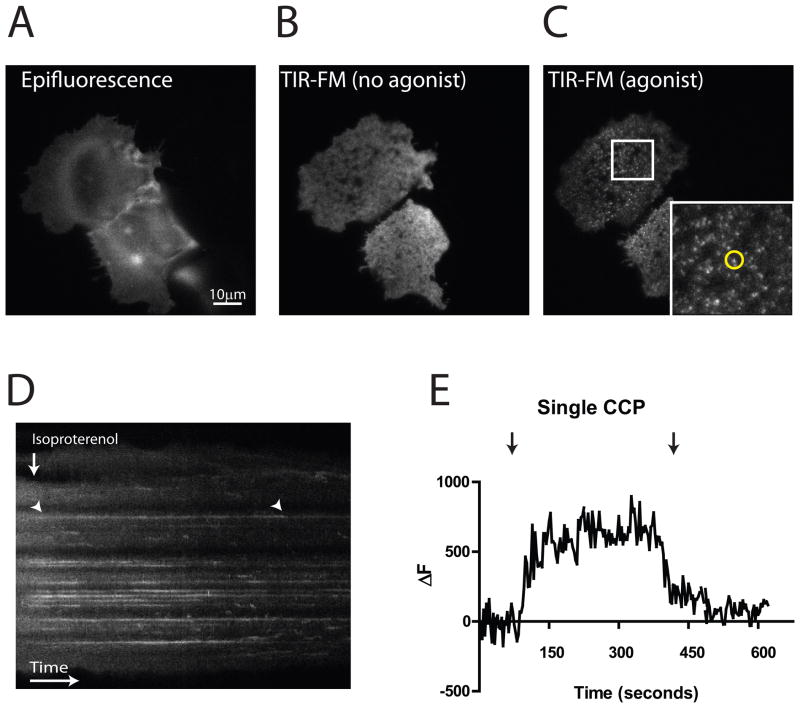
Figure 2. Examples of GPCR localization observed by TIR-FM.
A. Example of SEP- β2AR –expressing HEK293 cells imaged using epifluorescence illumination. Two adjacent cells are shown. B. TIR-FM view of the same field, showing the distinct footprints of each cell on the cover slip. C. TIR-FM view of the same field acquired 1 min after adding agonist (1 μM isoproterenol) to the imaging bath. The region outlined by the white square is show at higher magnification in the inset. The fluorescent spot surrounded by the yellow circle represents a clathrin-coated pit containing SEP- β2ARs. D. Kymograph showing SEP- β2AR dynamics in these representative cells, with increasing time going from left to right in the image. The vertical arrow indicates the addition of isoproterenol to the culture medium. The SEP- β2AR fluorescence intensity pattern shifts from a diffuse appearance to defined horizontal lines, representing receptor clustering into clathrin-coated pits. An example is indicated by the arrowhead at left. The lines disappear shortly after endocytic scission of coated pits, as the SEP- β2AR –containing endocytic vesicles produced by this scission event move rapidly out of the evanescent illumination field. An example is indicated by the arrowhead at right. E. Plot of the time course of maximum fluorescence intensity measured in the circled region indicated in panel C, called ΔF because the value measured in an adjacent (non-clustering) region of the plasma membrane is subtracted. Left arrow indicates the time at which isoproterenol was added, showing the time course of SEP- β2AR concentration in the coated pit. Right arrow indicates the time at which the spot of SEP- β2AR fluorescence disappears from the evanescent illumination field following endocytic scission.
3.3 Analysis
Data management and analysis are critical steps in live cell microscopy. Detailed discussion of image analysis methods is beyond the present scope and is addressed elsewhere (5, 13, 14). Examples include orthogonal views of image series as kymographs, useful for visually representing the time dependence of trafficking events (figure 2D), and intensity-versus-time measurements to follow the dynamics of individual events (Figure 2E). Additional examples can be found in the recent literature; e.g.,(11, 15–17). Practical image analysis has been greatly aided by the development of computer software specifically intended for this application. We typically use ImageJ, an excellent open source program developed by the NIH, which is supported by additional code written by an extensive user base and is available to the scientific community free of charge (rsbweb.nih.gov/ij/). We also recommend Micromanager (www.micro-manager.org) for controlling the microscope and peripheral devices during image acquisition. Micromanager is an open source program that is remarkably powerful and flexible, so is readily adapted to a variety of microscope systems, and it runs as an integrated plug-in linked to ImageJ.
Acknowledgments
The authors thank members of the von Zastrow laboratory and Dr. Kurt Thorn, Director of the UCSF/Nikon Imaging Center, for valuable discussion. The work discussed was supported by research grants from the NIH (DA023444 to G.A.Y., DA010711 to M.v.Z.).
References
- 1.Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10(9):609–22. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shcherbakova OG, Hurt CM, Xiang Y, et al. Organization of beta-adrenoceptor signaling compartments by sympathetic innervation of cardiac myocytes. J Cell Biol. 2007;176(4):521–33. doi: 10.1083/jcb.200604167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steyer JA, Almers W. A real-time view of life within 100 nm of the plasma membrane. Nat Rev Mol Cell Biol. 2001;2(4):268–75. doi: 10.1038/35067069. [DOI] [PubMed] [Google Scholar]
- 4.Schmoranzer J, Goulian M, Axelrod D, Simon SM. Imaging Constitutive Exocytosis with Total Internal Reflection Fluorescence Microscopy 10.1083/jcb.149.1.23. J Cell Biol. 2000;149(1):23–32. doi: 10.1083/jcb.149.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldman RD, Spector DL. Live cell imaging: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 2005. [Google Scholar]
- 6.Lippincott-Schwartz J, Altan-Bonnet N, Patterson GH. Photobleaching and photoactivation: following protein dynamics in living cells. Nat Cell Biol. 2003;(Suppl):S7–14. [PubMed] [Google Scholar]
- 7.Miyawaki A. Innovations in the imaging of brain functions using fluorescent proteins. Neuron. 2005;48(2):189–99. doi: 10.1016/j.neuron.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Giepmans BN, Adams SR, Ellisman MH, Tsien RY. The fluorescent toolbox for assessing protein location and function. Science. 2006;312(5771):217–24. doi: 10.1126/science.1124618. [DOI] [PubMed] [Google Scholar]
- 9.Miesenbock G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394(6689):192–5. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 10.Sankaranarayanan S, De Angelis D, Rothman JE, Ryan TA. The use of pHluorins for optical measurements of presynaptic activity. Biophys J. 2000;79(4):2199–208. doi: 10.1016/S0006-3495(00)76468-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yudowski GA, Puthenveedu MA, von Zastrow M. Distinct modes of regulated receptor insertion to the somatodendritic plasma membrane. Nat Neurosci. 2006;9(5):622–7. doi: 10.1038/nn1679. [DOI] [PubMed] [Google Scholar]
- 12.Bogdanov AM, Bogdanova EA, Chudakov DM, Gorodnicheva TV, Lukyanov S, Lukyanov KA. Cell culture medium affects GFP photostability: a solution. 2009;6(12):859–60. doi: 10.1038/nmeth1209-859. [DOI] [PubMed] [Google Scholar]
- 13.Waters JC. Accuracy and precision in quantitative fluorescence microscopy 10.1083/jcb.200903097. J Cell Biol. 2009;185(7):1135–48. doi: 10.1083/jcb.200903097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.BOLTE S, CORDELIÈRES FP. A guided tour into subcellular colocalization analysis in light microscopy. Journal of Microscopy. 2006;224(3):213–32. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- 15.Yudowski GA, Puthenveedu MA, Leonoudakis D, et al. Real-time imaging of discrete exocytic events mediating surface delivery of AMPA receptors. J Neurosci. 2007;27(41):11112–21. doi: 10.1523/JNEUROSCI.2465-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puthenveedu MA, von Zastrow M. Cargo regulates clathrin-coated pit dynamics. Cell. 2006;127(1):113–24. doi: 10.1016/j.cell.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 17.Pucadyil TJ, Schmid SL. Real-time visualization of dynamin-catalyzed membrane fission and vesicle release. Cell. 2008;135(7):1263–75. doi: 10.1016/j.cell.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]