Abstract
BRAF mutations occur in 10–15% of colorectal cancers (CRCs) and confer adverse outcome. While RAF inhibitors such as vemurafenib (PLX4032) have proven effective in BRAF mutant melanoma, they are surprisingly ineffective in BRAF mutant CRCs, and the reason for this disparity remains unclear. Compared to BRAF mutant melanoma cells, BRAF mutant CRC cells were less sensitive to vemurafenib, and P-ERK suppression was not sustained in response to treatment. Although transient inhibition of phospho-ERK by vemurafenib was observed in CRC, rapid ERK re-activation occurred through EGFR-mediated activation of RAS and CRAF. BRAF mutant CRCs expressed higher levels of phospho-EGFR than BRAF mutant melanomas, suggesting that CRCs are specifically poised for EGFR-mediated resistance. Combined RAF and EGFR inhibition blocked reactivation of MAPK signaling in BRAF mutant CRC cells and markedly improved efficacy in vitro and in vivo. These findings support evaluation of combined RAF and EGFR inhibition in BRAF mutant CRC patients.
Keywords: BRAF, vemurafenib, EGFR, colorectal cancer, melanoma
Introduction
Mutations in valine-600 (V600) of the BRAF oncogene occur in ~7% of all human cancers, including 10–15% of CRCs and 50–60% of melanomas (1). BRAF belongs to the RAF family of kinases, which also includes ARAF and CRAF. RAF kinases normally function to activate the MAPK signaling pathway in response to signals from activated, GTP-bound RAS. RAF kinases phosphorylate and activate MEK kinases (MEK1 and MEK2), which in turn phosphorylate and activate ERK kinases (ERK1 and ERK2). ERK kinases phosphorylate a number of cellular substrates with key roles in cell proliferation and survival (2,3). BRAF V600 mutations lead to constitutive BRAF kinase activity, phosphorylation of MEK and ERK kinases, and sustained MAPK pathway signaling.
In CRC, BRAF mutations are associated with adverse clinical outcome. Indeed, patients with metastatic CRC harboring BRAF V600 mutations exhibit a ~70% increase in mortality when compared to BRAF wildtype patients (4,5). Furthermore, some studies have suggested that the presence of BRAF mutation predicts lack of response to monoclonal antibodies against the epidermal growth factor receptor (EGFR), such as cetuximab (6). Therefore, novel therapeutic strategies for patients with BRAF mutant CRCs are critically needed.
Recently, the selective RAF inhibitor vemurafenib (PLX4032) was approved by the FDA for the treatment of metastatic melanomas harboring BRAF V600 mutations. While RAF inhibitors such as vemurafenib have produced impressive response rates of ~60–80% in BRAF mutant melanoma patients (7,8), vemurafenib demonstrated disappointing results in BRAF mutant CRC patients, producing only a single partial response (overall response rate of ~5%) in 19 evaluable patients (9). The reason for the difference in efficacy of vemurafenib between BRAF mutant CRCs and melanomas remains unclear. However, elucidating the mechanism of vemurafenib resistance in BRAF mutant CRC may lead to new therapeutic strategies for this lethal subtype of CRC.
Here, we evaluated BRAF CRC and melanoma cell lines harboring BRAF V600 mutations for differences in sensitivity and signal transduction response to RAF inhibition. We found that rapid EGFR-mediated re-activation of the MAPK pathway contributes to the relative insensitivity of BRAF mutant CRC cells to vemurafenib. We also observed that concomitant inhibition of RAF and EGFR in BRAF mutant CRCs leads to sustained suppression of MAPK signaling and to markedly increased therapeutic efficacy in vitro and in tumor xenografts. Together, our results suggest that combined RAF and EGFR inhibition may be a promising therapeutic strategy for patients with BRAF mutant CRC.
Results
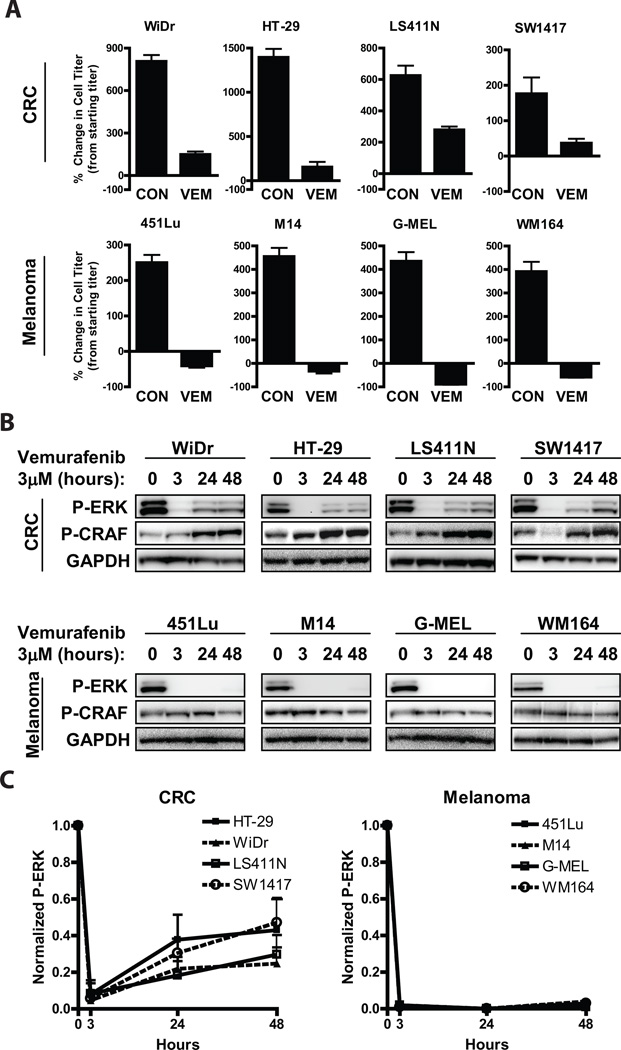
To explore the difference in sensitivity to RAF inhibition between BRAF mutant CRC and BRAF mutant melanomas, we evaluated the effects of vemurafenib treatment on CRC and melanoma cell lines that harbor BRAF V600 mutations (Table S1). Mirroring the disparity in clinical responsiveness to vemurafenib of BRAF mutant CRC and melanoma, CRC cell lines showed decreased sensitivity to vemurafenib in vitro (Fig 1A). Vemurafenib led to a decrease in viable cell numbers relative to pre-treatment starting cell number in BRAF mutant melanoma cell lines. Conversely, although vemurafenib slowed the growth of BRAF mutant CRC cells relative to untreated control, vemurafenib treatment failed to decrease cell number compared to pre-treatment starting cell number in the BRAF mutant CRC cell lines. Consistent with these findings, vemurafenib led to sustained suppression of P-ERK in all melanoma cell lines (Figs. 1B,C). In contrast, vemurafenib treatment transiently suppressed P-ERK in CRC cell lines, but re-accumulation of P-ERK (to ~25–50% of initial levels) was observed by 24 hours, indicating re-activation of the MAPK pathway. This incomplete suppression of P-ERK may underlie the relative insensitivity of BRAF mutant CRC cells to vemurafenib, as a recent study demonstrated that near-complete inhibition of P-ERK is required for tumor responses to vemurafenib in BRAF mutant melanomas (10).
Figure 1. Incomplete suppression of P-ERK in BRAF mutant CRCs is associated with decreased sensitivity to vemurafenib.
(A.) BRAF mutant melanoma and CRC cell lines were treated with (VEM) or without (CON) 3µM vemurafenib for 72h, and viable cell titer was determined by Cell TiterGlo assay. Values represent the change in viable cell titer relative to the starting cell titer immediately prior to treatment.
(B.) BRAF mutant cell lines from (A) were treated with 3µM vemurafenib for the indicated times, and lysates were probed with the indicated antibodies.
(C.) Chemiluminescent quantifications of normalized P-ERK levels from western blots as in (B) are illustrated graphically. Values represent mean of three independent experiments.
The rebound in P-ERK following treatment of BRAF mutant CRC cells with vemurafenib was associated with the induction of CRAF phosphorylation at S338, indicative of activation of the CRAF kinase (Fig. 1B). The rebound in P-ERK after RAF inhibition could still be blocked by the addition of the MEK inhibitor AZD6244 (selumetinib), indicating that P-ERK re-accumulation was still MEK-dependent (Fig. S1). Taken together, these results suggest that incomplete MAPK pathway inhibition may underlie the decreased sensitivity of BRAF mutant CRC to vemurafenib.
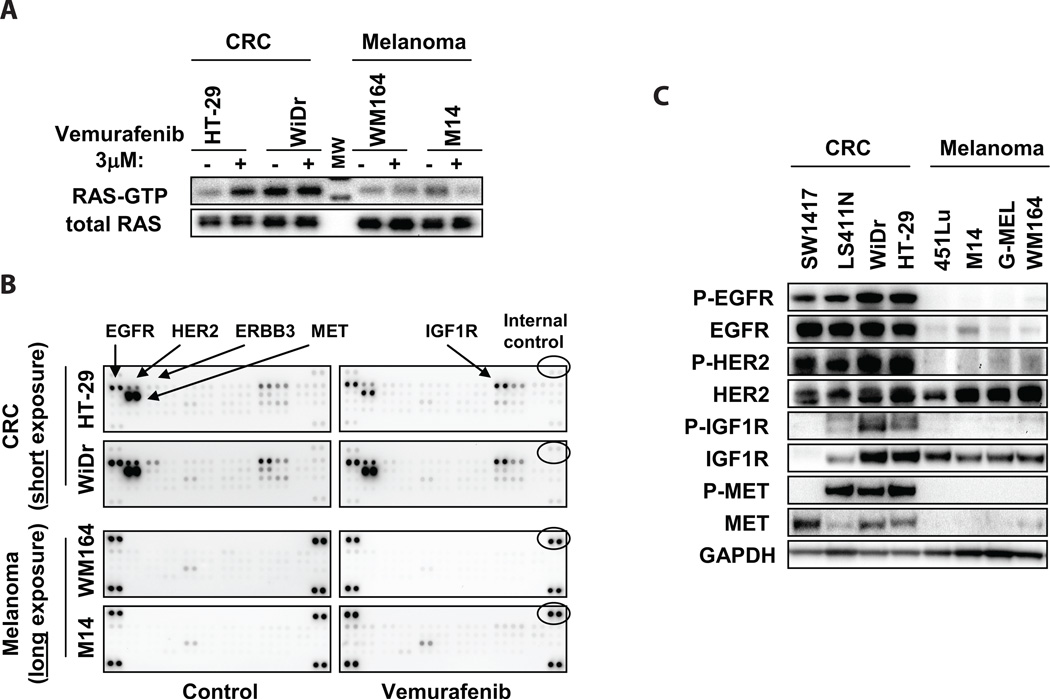
Because CRAF phosphorylation was induced by vemurafenib in BRAF mutant CRC cells, we investigated whether activation of RAS could account for the re-activation of MAPK signaling observed after vemurafenib treatment. RAS can not only activate CRAF directly, but activated RAS can also induce transactivation of BRAF-CRAF heterodimers in the presence of RAF inhibitors such as vemurafenib, leading to paradoxical activation of ERK (11–13). Consistent with this hypothesis, we found that the absolute levels of activated GTP-bound RAS were far higher following vemurafenib treatment in BRAF mutant CRC compared to melanoma cell lines (Fig. 2A).
Figure 2. Increased RTK activation in BRAF mutant CRC.
(A.) Levels of active GTP-bound RAS were determined by RAS-GTP pulldown assay in the indicated cell lines treated with or without 3µM vemurafenib for 24h. (MW = molecular weight marker).
(B.) Cells were treated with or without 3µM vemurafenib for 24h, and cell lysates were evaluated for levels of phosphorylated RTKs using phospho-RTK arrays. Short exposure is shown for BRAF mutant CRC cells, and long exposure is shown for BRAF mutant melanomas cells. Internal controls (indicated) allow comparison of absolute phospho-RTK levels between arrays. Key RTKs are indicated.
(C.) Lysates from BRAF mutant CRC and melanoma cell lines were evaluated by western blot to determine total and phosphorylated protein levels of the RTKs identified in (B).
To determine whether activation of receptor tyrosine kinase (RTK) signaling might account for the observed differences in RAS activation, we evaluated global RTK phosphorylation in BRAF mutant CRC and melanoma cell lines in the presence or absence of vemurafenib using phospho-RTK arrays. Interestingly, we found that RTK phosphorylation was universally low in BRAF mutant melanoma cells, before and after vermurafenib treatment (Fig. 2B). By contrast, BRAF mutant CRC cells displayed high basal levels of several phosphorylated RTKs, including EGFR, HER2, MET, and IGF1R. Notably, with the exception of IGF1R, vemurafenib treatment did not induce phosphorylation of any of these RTKs. Elevated levels of phospho-EGFR (P-EGFR), phospho-HER2 (P-HER2), phospho-MET (P-MET), and phospho-IGF1R (P-IGF1R) in BRAF mutant CRC cells were confirmed by western blot (Fig. 2C). Protein expression levels of EGFR and MET were also elevated in CRC cells relative to melanoma cells. However, only EGFR showed elevated total protein levels and elevated levels of phosphorylation in all BRAF mutant CRC cell lines.
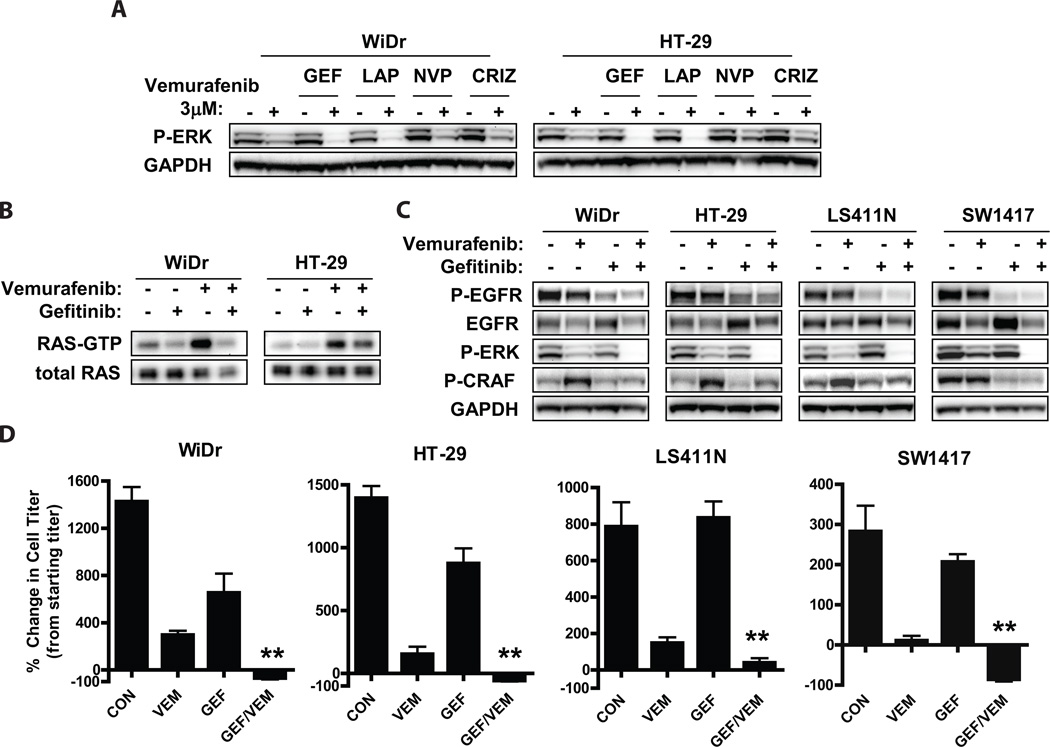
To determine whether a specific RTK might predominantly lead to activation of RAS and re-activation of MAPK signaling in BRAF mutant CRC cells treated with vermurafenib, BRAF mutant CRC cells were treated with small molecule kinase inhibitors of the above RTKs in the presence or absence of vemurafenib. Inhibition of IGF1R (with NVP-AEW541, a selective small molecule inhibitor of IGF1R (14)) or MET (with crizotinib) failed to maintain P-ERK suppression in the presence of vemurafenib (Fig. 3A), even though target RTK inhibition was achieved at the inhibitor concentration used (Figs. S2A–D). However, treatment with the EGFR inhibitor gefitinib or with the dual EGFR/HER2 inhibitor lapatinib led to more complete suppression of P-ERK upon vemurafenib treatment. Since similar suppression of P-ERK in the presence of vemurafenib was observed with gefitinib and lapatinib, it is likely that EGFR, and not HER2, is the predominant mediator of MAPK reactivation upon RAF inhibition (though a potential role for HER2 in BRAF mutant CRC is still possible). More complete suppression of P-ERK was also observed in cells treated with vemurafenib and the EGFR inhibitor erlotinib and in cells transfected with siRNA directed against EGFR, supporting the importance of EGFR in the reactivation of ERK signaling (Figs. S2D, E).
Figure 3. Combined inhibition of EGFR and RAF leads to sustained suppression of P-ERK and increased sensitivity in BRAF mutant CRC cells.
(A.) BRAF mutant CRC cells were treated for 24h with or without 3µM vemurafenib in the presence or absence of gefitinib (GEF, 2 µM), lapatinib (LAP, 1µM), NVP-AEW541 (NVP, 1µM), or crizotinib (CRIZ, 1µM). Lysates were probed with the indicated antibodies.
(B.) Cells were treated for 24h with the indicated inhibitors (vemurafenib 3µM, gefitinib 2µM) and levels of active GTP-bound RAS were determined by RAS-GTP pulldown assay.
(C.) Cells were treated as indicated (vemurafenib 3µM, gefitinib 2µM) for 48h, and lysates were evaluated by western blot.
(D.) BRAF mutant CRC cell lines were treated with 3µM vemurafenib or gefitinib 2µM, alone or in combination for 72h, and viable cell titer was determined by Cell TiterGlo assay. Values represent the change in viable cell titer relative to the starting cell titer immediately prior to treatment. Asterisks represent p <0.01.
Inhibition of EGFR with gefitinib abrogated the induction of activated RAS (RAS-GTP) by vemurafenib in BRAF mutant CRC cell lines (Fig. 3B), supporting a role for EGFR as the major activator of RAS in these cells. Accordingly, gefitinib treatment also abrogated the induction of P-CRAF in vemurafenib-treated BRAF mutant CRC cells (Fig. 3C). Interestingly, P-EGFR levels did not clearly increase after vemurafenib treatment at any time point tested between 0 and 48 hours, even though MAPK activity appeared to recover as early as 3–6 hours after vemurafenib treatment (Figs. 3C, S1, S3). These results suggest that EGFR activation does not increase upon treatment with the vemurafinib, but that EGFR is able to more effectively engage downstream signaling pathways following vemurafenib treatment.
Consistent with the sustained P-ERK suppression achieved in BRAF mutant CRC cells treated with gefitinib and vemurafenib, improved in vitro efficacy was observed with this inhibitor combination (Fig. 3D). Greater inhibition of viable cell number compared to vemurafenib alone was observed in all BRAF mutant cell lines, and all but one cell line showed an absolute decrease in viable cell number relative to pre-treatment starting cell number. The decrease in cell viability achieved with combined vemurafenib and gefitinib was significantly greater than that achieved with vemurafenib in combination with other inhibitors (NVP-AEW541 and crizotinib) that did not lead to improved suppression of P-ERK (Fig. S4 and Fig. 3A). Taken together, these data suggest that EGFR-mediated RAS activation leads to re-activation of MAPK signaling in many BRAF mutant CRCs, and that combined inhibition of RAF and EGFR may lead to improved efficacy in these cancers.
Vemurafenib also led to induction of P-AKT, an important signaling component of the PI3K pathway (Fig. S5). Induction of PI3K-AKT pathway signaling has previously been associated with decreased sensitivity to MAPK inhibition (15). Notably, inhibition of EGFR did not block P-AKT induction by vemurafenib (Fig. S5), despite the profound effect of this combination on cell viability. Previous work from our laboratory has implicated IGF1R as the predominant regulator of PI3K signaling in CRC, including BRAF mutant CRC (16). Accordingly, we found that induction of P-AKT by vemurafenib was associated with an increase in P-IGF1R, and that co-treatment with a small molecule inhibitor of IGF1R could abrogate induction of P-AKT (Fig S5). IGF1R inhibition blocked the induction of P-AKT completely (>90%) in WiDr cells and by ~50% in HT-29 cells. However, even though IGF1R inhibition limited the induction of P-AKT by vemurafenib, this combination was still less effective than vemurafenib and gefitinib (Fig. S4). The failure of IGF1R inhibition to improve suppression of P-ERK by vemurafenib (Fig. 3A, S5) likely accounts for the increased sensitivity of BRAF mutant CRC cells to combined EGFR/RAF inhibition than to combined IGF1R/RAF inhibition and supports the notion that these BRAF mutant cancer cells are highly dependent on MEK-ERK signaling.
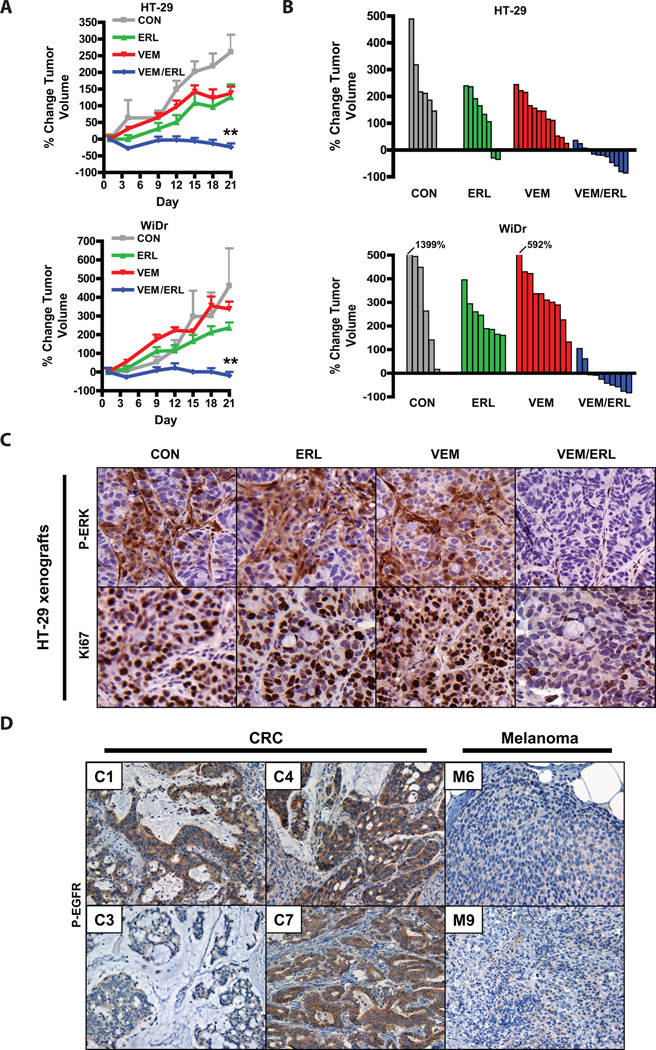
Given the sustained suppression of P-ERK signaling and improved in vitro efficacy of combined RAF and EGFR inhibition, we next tested whether this inhibitor combination strategy was effective in vivo using BRAF mutant CRC xenografts. Relative to vehicle-treated controls, treatment with vemurafenib alone (at a dose previously determined to be optimal for in vivo mouse studies (17)) or with the EGFR inhibitor erlotinib alone led to only modest inhibition of tumor growth in HT-29 xenografts and no significant tumor inhibition in WiDr xenografts (Fig. 4A). However, the combination of vemurafenib and erlotinib led to dramatic tumor inhibition and caused regressions in most tumors (Fig. 4A,B). Mice tolerated the combined treatment well (Fig. S6). Combined treatment with vemurafenib and erlotinib also led to improved inhibition of P-ERK relative to either treatment alone and to improved inhibition of tumor cell proliferation as assessed by Ki67 staining (Figs. 4C, S7A,B). These results support the notion that combined inhibition of RAF and EGFR may be a promising therapeutic strategy for BRAF mutant CRC.
Figure 4. Combined RAF and EGFR inhibition leads to improved in vivo efficacy in BRAF mutant CRC.
(A.) BRAF mutant CRC xenografts derived from HT-29 and WiDr cells were treated with vehicle only (CON), vemurafenib only (VEM, 75mpk twice daily), erlotinib (ERL, 100mpk daily), or both inhibitors (VEM/ERL) in combination for 21d. Average percent change in tumor volume relative to initial tumor volume is shown. Error bars represent SEM. Asterisks represent p <0.001 for combined vemurafenib/erlotinib vs. all other treatment groups.
(B.) Waterfall plots showing the percent change in volume (relative to initial tumor volume) for the individual tumors in each treatment group.
(C.) Tumor tissue from HT-29 xenografts treated for 3d as indicated was evaluated by IHC for P-ERK and a marker of cell proliferation (Ki67). Tumors were harvested 4h after dosing on day 3.
(D.) Levels of P-EGFR were assessed in human BRAF mutant CRCs and melanomas by IHC. Representative examples are shown. CRC cases with the lowest (C3) and highest (C7) P-EGFR levels are shown. 60% of BRAF mutant CRCs (n=10) exhibited high levels of P-EGFR, whereas only 18% of BRAF mutant melanomas (n=11) exhibited high levels of P-EGFR (p<0.05).
To explore whether EGFR might play a role in the insensitivity of human BRAF mutant CRCs to vemurafenib, we evaluated P-EGFR levels in BRAF mutant human CRCs. P-EGFR was detected in all cases of BRAF mutant CRC examined (Fig. 4D). When compared to BRAF mutant melanomas, BRAF mutant CRCs exhibited significantly higher levels of P-EGFR (Fig. 4D), consistent with our findings in cell lines (Fig. 2C) and supporting that human BRAF mutant CRCs may be more poised to exhibit EGFR-mediated resistance than BRAF mutant melanomas. Interestingly, 60% of BRAF mutant CRC cases (n=10) expressed particularly high levels of P-EGFR (scored as 2 or 3, as described in Materials and Methods, compared to only 18% of melanoma cases with similarly elevated expression (n=11), p<0.05), raising the possibility that levels of P-EGFR could predict which BRAF mutant CRCs might be most likely to develop EGFR-mediated resistance to RAF inhibition.
Discussion
Although selective RAF inhibitors like vemurafenib have produced dramatic responses in BRAF V600 mutant melanomas, CRCs harboring identical BRAF V600 mutations have failed to respond (7–9). Here, we present evidence that EGFR-mediated re-activation of MAPK signaling in BRAF mutant CRC leads to incomplete P-ERK suppression to vemurafenib, resulting in reduced sensitivity. This resistance mechanism appears to involve activation of RAS by EGFR, leading to higher levels of activated RAS and P-CRAF induction in BRAF mutant CRCs than in BRAF mutant melanomas. Recent studies have elegantly shown that activated RAS can cause MAPK pathway activation through direct activation of CRAF, or by the transactivation of BRAF-CRAF heterodimers in the presence of vemurafenib (11–13), or possibly through a combination of both mechanisms. Indeed, introduction of an activated RAS mutant into HT-29 cells led to sustained P-ERK levels and resistance to vemurafenib (11). We found that inhibition of EGFR abrogated RAS activation, P-CRAF induction, and P-ERK re-activation upon vemurafenib treatment in BRAF mutant CRC cells (Figs. 3A–C), suggesting that vemurafenib can produce sustained inhibition of mutant BRAF activity and suppression of ERK phosphorylation in the absence of EGFR-mediated feedback signals. Notably, we found that the sustained suppression of P-ERK achieved by combined RAF and EGFR inhibition leads to increased sensitivity in vitro and to tumor regressions in vivo (Figs. 3D, 4A,B). These findings suggest that BRAF mutant CRCs, like their melanoma counterparts, retain a strong dependency on MAPK signaling and that tumor responses are possible if the MAPK pathway is adequately inhibited in these cancers.
Interestingly, although EGFR appeared to mediate re-activation of MAPK signaling in response to vemurafenib, we did not observe evidence of increased EGFR activation per se following vemurafenib treatment, as might be expected in a classical feedback loop. Indeed, P-EGFR levels did not increase after vemurafenib treatment at any time point tested between 0 and 48 hours, even though MAPK activity appeared to recover as early as 3–6 hours after vemurafenib treatment (Figs. 3C, S1, S3). In fact, if anything, a slight decrease in P-EGFR and total EGFR levels was observed at later timepoints. These findings suggest that EGFR is active in BRAF mutant CRC cells prior to vemurafenib treatment, but that EGFR transmits its signal to activate RAS and CRAF only upon vemurafenib treatment (Fig. S8). One possible explanation for this observation may involve Sprouty proteins, which are important MAPK pathway feedback mediators that are transcribed in an ERK-dependent manner. Sprouty proteins can block RTK-mediated activation of RAS (18). Consistent with this hypothesis, we observed that Spouty4 (Spry4) levels decreased after treatment with vemurafenib, and this decrease coincided with induction of P-CRAF and P-ERK (Fig. S9). Still, further studies are necessary to determine whether Sprouty proteins are involved in this de-repression of EGFR-dependent activation of downstream signaling.
BRAF mutant CRC cell lines expressed higher levels of EGFR and P-EGFR than BRAF mutant melanoma cell lines, and human BRAF mutant CRCs exhibited significantly higher levels of P-EGFR than BRAF mutant melanomas (Figs. 2C, 4D). These observations may explain why BRAF mutant CRCs are more susceptible to EGFR-mediated RAF inhibitor resistance through incomplete MAPK suppression. Interestingly, while BRAF mutant melanoma cells had globally low levels of phosphorylated RTKs (perhaps explaining their exquisite sensitivity to single-agent RAF inhibitors), BRAF mutant CRC cells exhibited high levels of several phosphorylated RTKs. This finding raises the possibility that other RTKs in addition to EGFR (e.g. HER2, MET, IGF1R) could mediate resistance to RAF inhibitors through activation of RAS and the MAPK pathway. Importantly, however, in our CRC cell line models we observed that EGFR appeared to exert dominant control over RAS and the MAPK pathway, despite the presence of these additional phosphorylated RTKs (Figs. 3A–C). Still, it remains possible that some BRAF mutant CRCs may depend on RTKs other than EGFR. Interestingly, while we detected the presence of P-EGFR in all cases of BRAF mutant CRC evaluated, we observed that a subset of these cancers (60%) exhibited particularly high P-EGFR levels (Fig. 4D). Future studies will determine whether P-EGFR levels can predict which patients might benefit most from combined RAF/EGFR inhibition, and which might benefit from an alternative approach (e.g. combined RAF/MEK inhibition (Fig. S1), currently in clinical trials for BRAF mutant CRC (19)). In summary, the improved suppression of MAPK signaling and the substantial tumor regressions observed in our xenograft studies support the evaluation of combined RAF/EGFR inhibition in clinical trials for patients with BRAF mutant CRC.
Methods
Detailed methods are included in Supplemental Material.
Cell Lines, Reagents, and Patient Samples
All cell lines were grown in DMEM/F12 (GIBCO) with 10% FBS and assayed in DMEM/F12 with 5% FBS and were obtained from the Massachusetts General Hospital Center for Molecular Therapeutics, which performs routine cell line authentication testing by SNP and STR analysis. Genotype data was obtained from the Sanger Cancer Genome Project (www.sanger.ac.uk/genetics/CGP). Chemical inhibitors from the following sources were dissolved in DMSO for in vitro studies: vemurafenib (Active Biochem); gefitinib, erlotinib, and lapatinib (LC Laboratories), NVP-AEW541 (Selleck Chemicals), crizotinib (ChemieTek), and AZD6244 (Otava Chemicals). Human tumor specimens were obtained from the Massachusetts General Hospital under institutional review board-approved studies. All patients provided written, informed consent. BRAF mutation status was determined by the Massachusetts General Hospital Clinical Laboratory and Department of Pathology.
Xenograft Studies
HT-29 or WiDr cells were injected (5×106 cells per injection) into the flanks of male athymic nude mice (Charles River Laboratories). Once tumors reached an average volume of ~100–200mm3, mice were randomized into treatment arms and tumor volume was assessed by caliper measurements over a 21 day period. For pharmacodynamic studies, tumor tissue was harvested and formalin-fixed 4h after the morning doses of drug on the third day of treatment. Vemurafenib and erlotinib for in vivo studies were obtained from the MGH Pharmacy. Vemurafenib was formulated in 5% DMSO, 1% methylcellulose and dosed at 75mg/kg twice daily by oral gavage. Erlotinib was formulated in polysorbate and dosed at 100mg/kg daily. Animal care and treatment was performed in accordance with institutional guidelines.
Immunohistochemistry
IHC on formalin-fixed paraffin-embedded tissue was performed for P-ERK as previously described (20). IHC for P-EGFR was performed using P-EGFR Y1068 antibody (Cell Signaling #3777, 1:800 dilution in SignalStain Antibody Diluent) according to the manufacturer’s protocol. IHC for Ki67 was performed using Ki67 antibody (Novocastra/Leica NCL-Ki67p at 1:1000 dilution in PBS/3% BSA) and developed using Dako Envision+ system-HRP (DAB). P-EGFR IHC intensity scoring of all human CRC and melanoma specimens was performed by the same pathologists (A.P. and M.S.). Intensities of 0 (no staining), 1 (low staining), 2 (intermediate staining), and 3 (high staining), using P-EGFR staining in normal colonic crypts as a standard for a score of 3.
Significance.
BRAF V600 mutations occur in 10–15% of CRC, yet these tumors show a surprisingly low clinical response rate (~5%) to selective RAF inhibitors like vemurafenib, which have produced dramatic response rates (60–80%) in melanomas harboring the identical BRAF V600 mutation. We found that EGFR-mediated MAPK pathway re-activation leads to resistance to vemurafenib in BRAF mutant CRC and that combined RAF and EGFR inhibition can lead to sustained MAPK pathway suppression and improved efficacy in vitro and in tumor xenografts.
Supplementary Material
Acknowledgments
This study is supported by grants from the National Institutes of Health Gastrointestinal Cancer SPORE P50 CA127003 (J.A.E.), K08 grant CA120060-01 (JAE), R01CA137008-01 (J.A.E.), R01CA140594 (J.A.E), 1U01CA141457-01 (J.A.E.), National Cancer Institute Lung SPORE P50CA090578 (J.A.E.), DF/HCC the American Association for Cancer Research (J.A.E.), the V Foundation (J.A.E.), American Cancer Society RSG-06-102-01-CCE (J.A.E.), and the Ellison Foundation Scholar (J.A.E.).
References
- 1.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 2.Montagut C, Settleman J. Targeting the RAF-MEK-ERK pathway in cancer therapy. Cancer Lett. 2009;283:125–134. doi: 10.1016/j.canlet.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 3.Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24:21–44. doi: 10.1080/02699050500284218. [DOI] [PubMed] [Google Scholar]
- 4.Hutchins G, Southward K, Handley K, Magill L, Beaumont C, Stahlschmidt J, et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol. 2011;29:1261–1270. doi: 10.1200/JCO.2010.30.1366. [DOI] [PubMed] [Google Scholar]
- 5.Van Cutsem E, Kohne CH, Lang I, Folprecht G, Nowacki MP, Cascinu S, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–2019. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 6.Di Nicolantonio F, Martini M, Molinari M, Molinari F, Sartore-Bianchi A, Arena S, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26:5705–5712. doi: 10.1200/JCO.2008.18.0786. [DOI] [PubMed] [Google Scholar]
- 7.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kefford R, Arkenau H, Brown MP, Millward M, Infante JR, Long GV, et al. Phase I/II study of GSK2118436, a selective inhibitor of oncogenic mutant BRAF kinase, in patients with metastatic melanoma and other solid tumors. J Clin Oncol. 2010;28:15s. (suppl; abstr 8503) [Google Scholar]
- 9.Kopetz S, Desai J, Chan E, Hecht JR, O'Dwyer PJ, Lee RJ, et al. PLX4032 in metastatic colorectal cancer patients with mutant BRAF tumors. J Clin Oncol. 2010;28:15s. (suppl; abstr 3534) [Google Scholar]
- 10.Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–599. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–430. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431–435. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 13.Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209–221. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Echeverria C, Pearson MA, Marti A, Meyer T, Mestan J, Zimmermann J, et al. In vivo antitumor activity of NVP-AEW541-A novel, potent, and selective inhibitor of the IGF-IR kinase. Cancer Cell. 2004;5:231–239. doi: 10.1016/s1535-6108(04)00051-0. [DOI] [PubMed] [Google Scholar]
- 15.Gopal YN, Deng W, Woodman SE, Komurov K, Ram P, Smith PD, et al. Basal and treatment-induced activation of AKT mediates resistance to cell death by AZD6244 (ARRY-142886) in Braf-mutant human cutaneous melanoma cells. Cancer Res. 2010;70:8736–8747. doi: 10.1158/0008-5472.CAN-10-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ebi H, Corcoran RB, Singh A, Chen Z, Song Y, Lifshits E, et al. Receptor tyrosine kinases exert dominant control over PI3K signaling in human KRAS mutant colorectal cancers. J Clin Invest. 2011;121:4311–4321. doi: 10.1172/JCI57909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang H, Higgins B, Kolinsky K, Packman K, Go Z, Iyer R, et al. RG7204 (PLX4932), a selective BRAFV600E inhibitor, displays potent antitumor activity in preclinical melanoma models. Cancer Res. 2010;70:5518–5527. doi: 10.1158/0008-5472.CAN-10-0646. [DOI] [PubMed] [Google Scholar]
- 18.Edwin F, Anderson K, Ying C, Patel TB. Intermolecular interactions of Sprouty proteins and their implications in development and disease. Mol Pharmacol. 2009;76:679–691. doi: 10.1124/mol.109.055848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corcoran RB, Dias-Santagata D, Bergethon K, Iafrate AJ, Settleman J, Engelman JA. BRAF gene amplification can promote acquired resistance to MEK inhibitors in cancer cells harboring the BRAF V600E mutation. Sci Signal. 2010;3:ra84. doi: 10.1126/scisignal.2001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engelman JA, Chen L, Tan X, Crosby K, Guimares AR, Upadhyay R, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–1356. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.