Abstract
Interest in empirically derived dietary patterns has increased over the past decade. However, relatively few studies have evaluated dietary patterns using different dietary methods, or in young populations. We quantitatively compared dietary patterns from a food frequency questionnaire (FFQ) with those from a 3-day food record (FR) in a cohort of adolescents. Subjects from the Western Australian Pregnancy Cohort (Raine) Study completed a semi-quantitative FFQ and a 3-day FR at 14 y of age (n=783). Major dietary patterns were identified using exploratory factor analysis on 38 food groups. Dietary pattern z-scores were compared using 95% limits of agreement (LOA) and Spearman’s r. Two major dietary patterns were identified in the FFQ and FR. A ‘Healthy’ pattern was high in fresh fruit, vegetables, whole grains and grilled or canned fish. A ‘Western’ pattern was high in takeaway foods, confectionery, soft drinks, crisps and fried potato. The nutrient profiles of these dietary patterns were similar when estimated by the FFQ and FR. The LOA between dietary pattern scores from the FFQ and FR were -1.69 to 1.75 (‘Healthy’) and -1.89 to 1.82 (‘Western’). Minor differences in agreement were observed when boys and girls were analysed separately. Spearman’s correlation coefficients between the FFQ and FR were r=0.45 (‘Healthy’) and r=0.36 (‘Western’). Comparable dietary patterns may be obtained from a FFQ and FR using exploratory factor analysis. This supports the use of major dietary patterns identified using a FFQ in this adolescent cohort.
Introduction
The analysis of empirical dietary patterns derived using factor analysis has become increasingly popular in nutritional epidemiology. Factor analysis is a data reduction method, whereby many variables are reduced into a small number of factors that explain underlying constructs or patterns in the original data. This is an attractive method for nutritional epidemiologists, as dietary assessments usually result in a very large number of correlated nutrition variables. While these create a rich data source, they also provide complex analytical challenges. The advantages of factor analysis have been discussed widely (1, 2) but arguably the most attractive feature is that it considers the whole diet and the potentially synergistic effects of foods and nutrients, rather than attempting to isolate the effects of individual nutrients or foods, which may be too small to detect (2). Furthermore, focusing on a small number of major dietary patterns in diet-disease analyses requires fewer statistical tests and leads to a reduced likelihood of chance findings.
Despite their limitations (3) food frequency questionnaires (FFQ) currently remain the most popular dietary assessment tool for large scale epidemiological studies. The majority of studies reporting empirical dietary patterns have used FFQ data, yet relatively few studies have reported their relative validity, i.e. how well dietary patterns in a FFQ compare with dietary patterns using a more precise dietary method, such as a food record (FR). Those that have are largely limited to adult populations (4-8) and very little is known about the relative validity of empirical dietary patterns in child or adolescent populations. Exploring dietary patterns during childhood and adolescence is important for understanding how they can affect short- and long-term health, including adult health. There is also a lack of information on how empirical dietary patterns track from childhood into adulthood (9) and this kind of information could assist in designing interventions to improve dietary intake. To explore the relative validity of empirically-derived dietary patterns in young people, we compared dietary patterns using factor analysis in a FFQ with those identified in a 3-day food record in a cohort of Australian adolescents.
Subjects and Methods
Study Population
Participants were sourced from the West Australian Pregnancy Cohort (Raine) Study, which has been described previously (10). Briefly, the Raine Study commenced with 2,900 women recruited at 16 to 20 weeks gestation between 1989 and 1991. There were 2,868 live births and these children have been followed up at regular intervals from gestation onwards. This study uses data collected at the 14 y follow-up.
Food Frequency Questionnaires
The first complete dietary assessment in this cohort was conducted at the 14 y follow-up which occurred from 2003 to 2006 (mean age 14 y, SD=0.2). All 2,337 adolescents eligible for follow-up, i.e. not deceased or withdrawn from the study at 14 y of age, were sent a semi-quantitative FFQ developed by the Commonwealth Scientific and Industrial Research Organization (CSIRO) in Adelaide, South Australia (11). The FFQ assessed usual dietary intake over the previous year, collecting information on the frequency of consumption of 212 individual foods, mixed dishes and beverages, and their usual serving sizes in relation to a standard serving size (in household units). Questions on fruit and vegetable intakes were asked separately for summer and winter. Nutrient intakes estimated by this FFQ have been evaluated against a 3-day food record in this cohort at 14 y of age (12). Because of the age of respondents and their potential difficulty completing the FFQ, the primary caregiver was asked to complete the FFQ in association with the study adolescent. All FFQs were checked and missing data clarified with the adolescent. Data entry, data verification and the estimation of average daily food and nutrient intakes were carried out by CSIRO. Intakes of all 212 foods were collapsed into 38 food groups devised a priori (Appendix 1) (13). A description of subjects who completed the FFQ has been published elsewhere (13).
Food Records
All subjects eligible for follow-up at 14 y of age were invited to attend the Telethon Institute for Child Health Research, Perth, Western Australia for physical examinations. Those who presented were asked to complete a 3-day FR. The FR was designed to be completed by the adolescents with parental assistance if required. Written instructions and metric measuring cups and spoons were provided to assist with serving size estimations. Adolescents were also asked to record if any of the 3 days in their FR were, in their opinion, not representative of their usual eating habits. This was verified by a dietician who checked all returned FR and followed up queries by telephone (14). Of the 1,286 subjects who agreed, 962 returned a FR (response fraction=75%). Of these, 858 completed a 3-day FR, 35 completed a 1- or 2-day FR, 44 returned an incomplete FR (no full days recorded) and 25 returned a blank FR.
All FR were entered by a dietician into the FoodWorks diet analysis package (15). Over 4,000 foods and beverages were recorded in total. Each item recorded in the FR was matched to a FFQ item where possible and coded to the corresponding food group in Appendix 1. A small number of mixed dishes (n=26) were recorded in the FR without recipe details. These mixed dishes were separated into main components that could be assigned to a FFQ item. For example, a proportion of beef lasagne could be matched with ‘pasta’ and with ‘beef mince-based pasta sauce’ in the FFQ, which then contributed to the ‘refined grains’ and ‘red meat’ food groups, respectively. There were 354 foods that did not match a FFQ item, including various condiments, sauces, beverage bases, artificial sweeteners, gums and vegetables not listed in the FFQ (e.g. eggplant, fennel, artichoke). These foods were coded to the most appropriate food group, with the exception of artificial sweeteners, beverage bases, coconut products, oils and gum, as these did not match any of the a priori food groups. Total intakes for each food group (grams per day) were calculated by summing food intakes within each of the 38 food groups. Average daily nutrient intakes were estimated using Australian Food Composition Tables (16).
Biochemistry
Fasting blood samples were collected at the 14 year follow up by a phlebotomist at the adolescent’s home. Biochemical data included fasting serum glucose, total cholesterol, HDL-cholesterol, calculated LDL-cholesterol and triglycerides. Assays were conducted at PathWest Laboratories, Royal Perth Hospital, using standardised methodologies published elsewhere (17). Erythrocytes were isolated and fatty acids determined using gas chromatography as previously described (18).
Ethics
The Raine Study was conducted according to the guidelines laid down in the Declaration of Helsinki and was approved by the ethics committees of King Edward Memorial Hospital for Women and Princess Margaret Hospital for Children, Perth Western Australia. Informed consent was obtained from the adolescent and their primary caregiver at each follow up.
Statistics
Daily energy intakes <3,000 or >20,000 kJ/day were considered implausible, as in our previous analyses (13). Only complete 3-day FR were considered for this analysis, as a FR completed for less than 3-day may be less likely to reflect usual intake in adolescents (19).
To identify dietary patterns, we conducted separate factor analyses (maximum likelihood method) for the FFQ (13) and the FR, using all 38 food group intakes. All subjects who completed the relevant dietary assessment were included in each factor analysis to maximize the factor solution. Using PROC FACTOR in SAS (20), the factor solutions were initially limited to factors with an eigen value >1 and scree plots were used to confirm the maximum number of factors to retain. For the FR factor analysis it was necessary to downwardly adjust the minimum eigen value in order to retain factors explaining the most variance, as has been suggested for factor analysis (21). The factor solutions were rotated using the varimax option to improve interpretation and render independent dietary patterns. The final rotated factor solution provided factor loadings for each factor or dietary pattern. Food groups with very low loadings (<|0.10|) on all factors were excluded from the final factor solution (i.e. tea, coffee, soy milk, unsaturated and saturated spreads), but all food groups included in the final factor solution were used in calculating dietary pattern scores. All subjects received a z-score for each dietary pattern identified in the FFQ and FR and as such, mean dietary pattern scores for the sample were zero. There were no major differences in the factor solutions for boys and girls, therefore the factor loadings presented are for boys and girls combined.
Three methods were used to examine the relative validity of the dietary patterns. Firstly, Spearman’s rank correlation coefficients were calculated between dietary pattern scores from each dietary method, and between dietary pattern scores and biomarkers. Secondly, agreement between dietary pattern scores was determined according to 1) mean agreement, or the mean of differences between scores e.g. ∑{Western score FFQ – Western score FR}/ n, and 2) the 95% limits of agreement (LOA), calculated as mean agreement ± 1.96 (SD of differences), which shows the range in which 95% of individual differences in dietary pattern scores fall and the spread of overall agreement for the sample (22). Finally, we calculated Spearman’s correlation coefficients between dietary pattern scores and average daily nutrient intakes estimated by the FR, which were adjusted for total energy intake using the residual method (23). This enabled a comparison of dietary pattern scores according to nutrient profile using the FR as a standard. An alpha level of 0.05 was used for all statistical tests.
Results
A total of 1,631 subjects completed the FFQ at the 14 y follow-up (response fraction=70%) and of these, 18 had implausible kJ intakes. Of the 858 3-day FR, 822 were coded ‘representative of usual intake’ and were included in this analysis. None of the 822 subjects who completed a 3-day FR reported implausible kJ intakes. Chi-square tests showed that compared with other cohort members, subjects who completed both dietary assessments (n=783) were more likely to have mothers with ≥ 10 years’ education (66% vs. 58%, p<.0001) and were less likely to be overweight (8% vs. 12% having a BMI >85th percentile for gender and age (24), p=0.03) or have a very low family income (16% vs. 22% with ≤ $30,000 AUD/yr; p=0.03).
Dietary Patterns
Two major dietary patterns were identified in the FFQ. We subjectively named these patterns ‘Healthy’ and ‘Western’ (Table 1). The ‘Healthy’ pattern was positively correlated with intakes of all vegetable types, fresh fruit, legumes, fish (steamed, grilled or canned), whole grains, low fat dairy and mineral water, and negatively correlated with takeaway foods, chips and crisps. The ‘Western’ pattern was positively correlated with intakes of takeaway foods, confectionery, soft drinks, crisps, refined grains, red meats, processed meats, fried potato (chips), potato (not fried), high fat dairy, sauces and dressings, cakes and biscuits, added sugar, fried fish and poultry.
Table 1.
Factor loadings for dietary patterns in the FFQ and 3-day food record
| ‘ Healthy’ Pattern | ‘Western’ Pattern | |||
|---|---|---|---|---|
| FFQ | FR | FFQ | FR | |
| N subjects | 1613 | 822 | 1613 | 882 |
| Yellow, red vegetables | 0.56 * | 0.19 | 0.12 | −0.19 |
| Leafy green vegetables | 0.49 * | 0.34 * | 0.00 | −0.05 |
| Tomato | 0.49 * | 0.49 * | 0.00 | −0.02 |
| Cruciferous vegetables | 0.48 * | 0.01 | 0.27 * | −0.12 |
| Other vegetables | 0.66 * | 0.30 * | 0.22 * | −0.18 |
| Fresh fruit | 0.48 * | 0.40 * | −0.02 | −0.21 * |
| Legumes | 0.43 * | 0.09 | 0.19 | −0.07 |
| Whole grains | 0.39 * | 0.33 * | −0.12 | −0.28 * |
| Fish, steamed, grilled, canned | 0.33 * | 0.20 * | 0.05 | −0.02 |
| Canned fruit | 0.26 * | −0.11 | 0.11 | −0.08 |
| Meat dishes | 0.26 * | −0.15 | 0.15 | −0.10 |
| Soups | 0.26 * | −0.06 | 0.26 * | −0.11 |
| Mineral water | 0.23 * | 0.23 * | −0.05 | −0.30 * |
| Dried fruit | 0.23 * | −0.03 | 0.00 | −0.16 |
| Low fat dairy | 0.22 * | 0.21 * | −0.10 | −0.21 * |
| Potato (not fried) | 0.21 * | −0.01 | 0.34 * | 0.00 |
| Eggs | 0.20 * | 0.03 | 0.24 * | 0.02 * |
| Takeaway foods | −0.20 * | −0.10 | 0.53 * | 0.40 * |
| Red meat | 0.14 | 0.18 | 0.46 * | 0.09 |
| Confectionery | −0.14 | −0.01 | 0.46 * | 0.25 * |
| Refined grains | 0.03 | 0.00 | 0.42 * | −0.13 |
| Processed meats | −0.02 | 0.08 | 0.41 * | 0.00 |
| Crisps | −0.22 * | 0.00 | 0.39 * | 0.21 * |
| Fried potatoes, chips | −0.25 * | −0.12 | 0.39 * | 0.30 * |
| Soft drinks | −0.18 | −0.12 | 0.37 * | 0.55 * |
| Added sugar | 0.13 | −0.01 | 0.21 * | −0.05 |
| Sauces, dressings | 0.13 | 0.07 | 0.34 * | 0.06 |
| Cakes, biscuits | 0.10 | 0.08 | 0.34 * | −0.15 |
| High fat dairy | 0.00 | 0.00 | 0.30 * | 0.09 |
| Milk dishes | 0.13 | −0.16 | 0.20 * | −0.06 |
| Poultry | 0.01 | −0.02 | 0.29 * | −0.14 |
| Fish, fried or battered | 0.02 | 0.02 | 0.23 * | 0.04 |
| Juices | 0.19 | 0.17 | −0.02 | 0.01 |
| Nuts | 0.17 | 0.07 | −0.02 | −0.08 |
| % Total variance | 50 | 25 | 34 | 28 |
| Min Score | −2.13 | −2.51 | −2.06 | −1.38 |
| Max Score | 5.01 | 5.10 | 4.74 | 4.18 |
Factor loadings ≥ |0.20|
Two similar major dietary patterns were identified in the FR, although their factor loadings were generally weaker than those for the FFQ (Table 1). A ‘Healthy’ pattern similar to that in the FFQ was positively correlated with whole grains, most vegetable types, fresh fruit, fish (steamed, grilled or canned), mineral water and low fat dairy. The second major dietary pattern in the FR was positively correlated with several foods key to the FFQ ‘Western’ pattern: takeaway foods, fried potato (chips), soft drinks, crisps, and confectionery. In addition, this pattern was negatively correlated with whole grains, fresh fruit, low fat dairy, and mineral water. We observed very similar patterns when the factor analysis was restricted to those foods in the FR that directly matched FFQ foods. In both the FFQ and FR factor analyses other factors in addition to the ‘Healthy’ and ‘Western’ patterns were identified. However, these were minor; they explained small amounts of variance and loaded with few foods. We therefore excluded minor factors from our comparisons, as others have done (4).
Correlations
There were modest correlations between dietary pattern scores from the FFQ and FR (‘Healthy’ r=0.43 and ‘Western’ r=0.27) however, these improved after adjustment for total energy intake (‘Healthy’ r=0.45 and ‘Western’ r=0.36) (Table 2). Correlations between the ‘Healthy’ pattern were stronger, with r=0.47 for boys and r=0.42 for girls (energy-adjusted) whereas slightly weaker correlations were observed for the ‘Western’ pattern (r=0.34 for boys and r=0.38 for girls).
Table 2.
Correlation coefficients, mean agreement and limits of agreement between dietary pattern scores from a FFQ and 3-day FR.
| ‘Healthy’ Pattern |
‘Western’ Pattern |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | r * | Mean Agreement † | 95% LOA ‡ | LOA diff § | r * | Mean Agreement † | 95% LOA ‡ | LOA diff § | |
| All | 783 | 0.43 0.45** |
0.03 | −1.69, 1.75 | 3.44 | 0.27 0.36** |
−0.03 | −1.89, 1.82 | 3.69 |
| Boys | 403 | 0.47 0.47** |
−0.04 | −1.87, 1.79 | 3.66 | 0.22 0.34** |
0.02 | −1.93, 1.97 | 3.90 |
| Girls | 380 | 0.39 0.42** |
0.11 | −1.48, 1.69 | 3.18 | 0.34 0.38** |
−0.09 | −1.82, 1.64 | 3.46 |
LOA, limits of agreement
Spearman’s rank correlation coefficient, all p<0.001
Mean of differences between dietary pattern scores (FFQ-FR)
Limits of agreement: mean agreement ± 1.96(SDdifferences between methods)
Difference between upper and lower 95% LOA
Partial Spearman’s rank correlation coefficient, adjusted for total energy intake, all p<0.001
Erythrocyte omega-3 and VLC n-3 fatty acids correlated with scores from both dietary patterns (p<0.01, Table 3). ‘Healthy’ pattern scores from both the FFQ and FR were positively associated with omega-3 (r=0.17 and r=0.13, respectively) and VLC n-3 (r=0.17 and r=0.12). Whereas, ‘Western’ pattern scores from both the FFQ and FR were negatively correlated with omega-3 (r=-0.09 and r= -0.13, respectively) and total VLC n-3 fatty acids (r= -0.10 and r= -0.15). A negative correlation was suggested between fasting glucose and ‘Healthy’ pattern scores from both the FFQ (r=-0.09) and FR (r=-0.06). Adjustment for gender made little or no difference to these correlations. There were no observed correlations between either dietary pattern and serum lipids (not shown).
Table 3.
Correlations between biomarkers and dietary pattern scores from a FFQ and 3-day FR.
| ‘ Healthy’ Pattern | ‘Western’ Pattern | |||
|---|---|---|---|---|
| FFQ | FR | FFQ | FR | |
| N subjects | 1219 | 722 | 1219 | 722 |
| Erythrocyte Omega-3 † | 0.17 * | 0.13 * | −0.09 * | −0.13 * |
| Erythrocyte VLC n-3 ‡ | 0.17 * | 0.12 * | −0.10 * | −0.15 * |
| Serum Glucose | −0.09 * | −0.06 | 0.04 | 0.03 |
Partial Spearman’s correlation coefficients adjusted for total energy intake
p-value ≤ 0.01 (H0: r = 0)
18:3n3 alpha-linolenic acid, 18:4n3 Parinaric acid, 20:5n3 eicosapentanoic acid, 22:5n3 docosapentanoic acid and 22:6n3 docosahexanoic acid
20:5n3 eicosapentanoic acid, 22:5n3 docosapentanoic acid, 22:6n3 docosahexanoic acid
Agreement
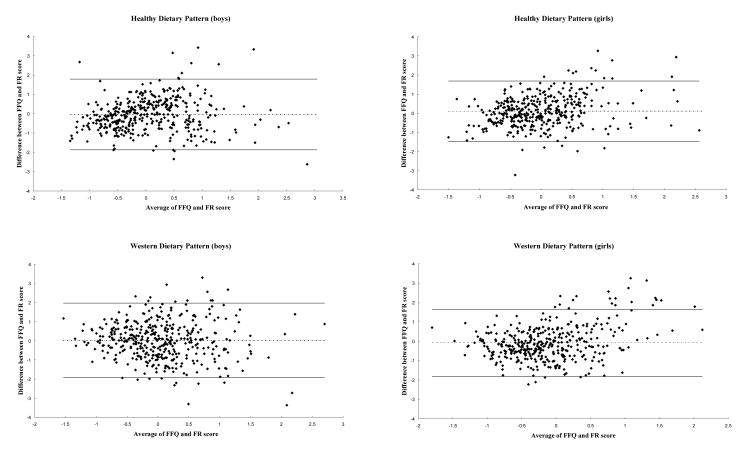
In nearly all comparisons there was acceptable mean agreement between ‘Healthy’ scores and between ‘Western’ scores, i.e. mean agreement was not significantly different from zero (Table 2). However, mean agreement between the ‘Healthy’ pattern in girls (0.11) suggested that ‘Healthy’ pattern scores were on average, slightly higher in the FFQ than the FR. The 95% LOA did not vary considerably between methods, ranging from -1.69 to 1.57 for the ‘Healthy’ pattern and from -1.89 to 1.82 for the ‘Western’ pattern (Table 2). This is illustrated by the Bland-Altman plots for agreement (Figure 1). Although the LOA were slightly narrower for the ‘Healthy’ pattern and for girls (both patterns, Figure 1), these differences were marginal.
Fig 1.
Bland-Altman plots showing mean agreement (-.-.-) and 95% LOA (—) between ‘Healthy’ and ‘Western’ dietary pattern scores in a FFQ and 3-day FR.
Nutrient Profiles
Correlations between nutrient intakes from the FR and dietary pattern scores from the FFQ and the FR are shown in Table 4. As there were no gender differences, a single table presenting the results for boys and girls combined is shown. As expected, the strongest correlations were between FR nutrients and FR dietary pattern scores. ‘Healthy’ pattern scores from both the FFQ and FR correlated positively with FR intakes of protein, folate, fibre, beta carotene, niacin, riboflavin, thiamine, vitamin C, calcium, iron, magnesium, and negatively with total, saturated and monounsaturated fat. ‘Western’ pattern scores from the FFQ and the FR were both positively correlated with FR intakes of total, saturated and monounsaturated fat, and negatively correlated with protein, fibre, folate, beta carotene, riboflavin, thiamine, calcium, iron and magnesium. The majority of statistically significant correlations were consistent for the FFQ and the FR. This suggests that the ‘Healthy’ and ‘Western’ dietary patterns had similar nutrient profiles when identified using the FFQ or the FR.
Table 4.
Correlations between dietary pattern scores and energy-adjusted nutrient intakes from a 3-day food record
| ‘ Healthy’ Pattern | ‘Western’ Pattern | |||
|---|---|---|---|---|
| FFQ | FR | FFQ | FR | |
| N subjects | 783 | 783 | 783 | 783 |
| Total fat | −0.15 * | −0.18 * | 0.16 * | 0.17 * |
| Saturated fat | −0.23 * | −0.26 * | 0.14 * | 0.24 * |
| Polyunsaturated fat | 0.11 * | 0.04 | −0.02 | −0.15 * |
| Monounsaturated fat | −0.10 * | −0.12 * | 0.16 * | 0.18 * |
| Cholesterol | 0.03 | 0.00 | 0.04 | −0.01 |
| Protein | 0.15 * | 0.24 * | −0.09 * | −0.30 * |
| Carbohydrates | 0.00 | −0.03 | −0.05 | 0.07 |
| Sugars | −0.06 | 0.00 | −0.03 | 0.24 * |
| Starch | 0.07 | −0.03 | 0.00 | −0.20 * |
| Dietary fibre | 0.38 * | 0.49 * | −0.26 * | −0.46 * |
| Beta carotene equivalents | 0.24 * | 0.31 * | −0.10 * | −0.30 * |
| Retinol | −0.02 | −0.08 * | −0.02 | −0.06 |
| Folate | 0.26 * | 0.37 * | −0.29 * | −0.34 * |
| Niacin | 0.16 * | 0.24 * | −0.07 | −0.17 * |
| Riboflavin | 0.11 * | 0.13 * | −0.23 * | −0.26 * |
| Thiamine | 0.13 * | 0.18 * | −0.19 * | −0.23 * |
| Vitamin C | 0.18 * | 0.32 * | −0.04 | −0.09 * |
| Sodium | 0.06 | 0.01 | 0.10 * | −0.05 |
| Calcium | 0.17 * | 0.24 * | −0.30 * | −0.42 * |
| Iron | 0.17 * | 0.30 * | −0.21 * | −0.33 * |
| Magnesium | 0.40 * | 0.52 * | −0.37 * | −0.65 * |
| Zinc | 0.08 * | 0.21 * | −0.04 | −0.20 * |
Partial Spearman’s correlation coefficients adjusted for total energy intake in the FFQ
p-value <0.05 (H0: r = 0)
Discussion
We identified two major dietary patterns in this cohort of adolescents; a ‘Healthy’ and ‘Western’ pattern, which are qualitatively similar to ‘Healthy’ or ‘Prudent’, and ‘Western’ patterns described in other studies (4, 5, 7). Having compared these two dietary patterns in a FFQ and 3-day FR, we conclude that they are relatively valid, based on the similarities in nutrient profiles, the mean agreement and the 95% LOA.
To our knowledge, no studies on the relative validity of dietary patterns in adolescents have been published to date. However, studies in adults have compared FFQ dietary patterns similar to ours (‘Healthy’/’Prudent’ and ‘Western’) with those in a FR (4, 5, 7). In these studies, the factor loading matrices were largely similar across dietary methods, but they were not identical (4, 7), as we observed in our study. For example, in the study by Hu et al (4), potatoes (excluding French fries) were positively loaded (0.40) onto a ‘Prudent’ pattern in a FR, but not in a ‘Prudent’ pattern seen in a FFQ. There were also inconsistencies for sweets and desserts (including cakes and biscuits), soups, poultry, fish and other seafood. Similarly, in the study by Crozier et al, boiled potatoes, puddings and processed meats had moderate factor loadings in a ‘Western’ pattern in the FFQ but not in a FR (7). Our study also showed inconsistencies in the factor loadings for these foods.
We noted that factor loadings in our FR dietary patterns were generally weaker compared with the FFQ and as a result, we observed fewer foods with moderate loadings. This most likely reflects the methodological differences between the FR and FFQ. The FFQ collected information on usual dietary intake over the past year, while the FR measured food eaten over a 3-day period, therefore a smaller range of foods are likely to be reported in an individual’s FR and across all FR because there were fewer subjects who completed these. In addition, factor analysis solutions depend on the correlation matrix of food intakes. Therefore, some differences would be expected between factor loading matrices from different dietary assessments, or from repeated dietary assessments based on different numbers of subjects.
The correlations between dietary pattern scores from a FFQ and FR in adult studies range from 0.34 - 0.67 for ‘Healthy’/’Prudent’ patterns and 0.35 - 0.51 for ‘Western’ patterns (4, 5, 7), which are comparable with those observed in our study. However, correlation coefficients can be misleading, whereas mean agreement and 95% LOA are better indicators of how well two measurements compare (25). The 95% LOA between our dietary patterns were acceptable, but slighter narrower for the ‘Healthy’ pattern. Few studies have used LOA, but similar findings were reported in a study of pregnant women where the 95% LOA between a FFQ and FR were -1.58 to 1.58 for a ‘Prudent’ pattern and -2.22 to 2.22 for a ‘Western’ pattern (7). Although we observed some differences between boys and girls, these were relatively minor. Mean agreement suggested that on average, girls had higher ‘Healthy’ pattern scores in their FFQ. This may reflect real differences in eating patterns, or alternatively, girls may be more concerned about body image and be more conscientious about reporting their diet, which could lead to overestimation of ‘healthy food’ intakes. We have previously reported poorer agreement between nutrient intakes in the FFQ and FR among girls (12).
Examining nutrient profiles is a useful way to compare dietary patterns from different dietary methods. Nutrient profiles are informative because they describe the product of a dietary pattern, i.e. nutrient intake. So far we have seen only one other study, that of Hu et al (4), that has compared the nutrient profiles of dietary patterns. They too reported that the nutrient profiles for ‘Prudent’ and ‘Western’ dietary patterns were similar in a FFQ and FR (4). As seen for our ‘Healthy’ pattern, their ‘Prudent’ pattern was positively correlated with fibre, folate, calcium, carotene and magnesium intakes and negatively correlated with total fat and saturated fat intakes in both a FFQ and FR. Their ‘Western’ pattern was also positively correlated with total fat and saturated fat and negatively correlated with fibre, folate, calcium, carotene and magnesium intakes.
When comparing the dietary patterns to biomarkers, we observed statistically significant correlations for omega-3 and VLC n-3 fatty acids. These showed consistent associations with the ‘Healthy’ (positive) and ‘Western’ (negative) patterns in both the FFQ and FR. This consistency further supports the similarity in ‘Healthy’ and ‘Western’ dietary patterns observed using these two dietary methods. We have previously reported that fish and dairy products were the main sources of omega-3 and VLC n-3 fatty acids respectively, in this cohort at 14 years of age (26), and these foods feature in the ‘Healthy’ dietary pattern. Interestingly, despite having a high factor loading for fried fish, the ‘Western’ dietary pattern was negatively associated with erythrocyte omega-3 and VLC n-3 fatty acids. This corresponds with data from a US population-based cohort study showing that n-3 fatty acid intake correlated with intakes of grilled and baked fish, but not with intakes of fried fish (27).
This study benefits from a large sample size (n=783) and good response fractions (70 and 75% for FFQ and FR respectively). Together, the observed ‘Healthy’ and ‘Western’ dietary patterns accounted for a large proportion of the variation in food intakes (53–84%). However, there are several potential limitations in this study. A 3-day FR has been shown to be an appropriate method of assessing usual diet in children (28). However, adolescents may show high levels of variation in their recorded food intake, and 3 days may have been insufficient to capture usual food intake in this cohort (19). It should also be acknowledged that the FR is not an error-free comparison method. Under-reporting in FR is common, particularly among adolescents and children (19). However, we attempted to minimize under-reporting by checking FR for representativeness and by following up incomplete or ambiguous information directly with respondents. We also found that subjects in this reliability study differed in some ways to the rest of the cohort, which may limit the application of these findings. Finally, factor analysis is subjective; the number of factors to retain in the factor solution are arbitrary. However, the significant advantage of dietary patterns is that they take account of the whole diet, and can provide an overall picture of total dietary exposure. While dietary patterns analysis is unlikely to replace the reductionist approach of analysing individual nutrients and foods, it serves as a useful complementary method for diet-disease analyses.
In conclusion, ‘Healthy’ and ‘Western’ dietary patterns identified using a FFQ in this cohort of adolescents are relatively valid in comparison with a 3-day FR. These findings support the use of dietary patterns identified using factor analysis and FFQ data to describe usual dietary intake in this adolescent population. We intend to examine longitudinal relationships between these dietary patterns and various health outcomes in this cohort using data collected at the 17 year follow up and later.
Acknowledgements
We are extremely grateful to all the families who took part in this study and the whole Raine Study team, which includes data collectors, cohort managers, data managers, clerical staff, research scientists and volunteers. We also wish to acknowledge the Commonwealth Scientific and Industrial Research Organization.
This work was supported in part by the Raine Medical Research Foundation at The University of Western Australia, the National Health and Medical Research Council (NHMRC) of Australia (Program Grant ID 003209), the Telstra Foundation, the Western Australian Health Promotion Foundation, the Australian Rotary Health Research Fund, the Heart Foundation of Australia and Beyond Blue, the Telethon Institute for Child Health Research and the UK Medical Research Council (MRC). WHO was supported by an NHMRC Career Development Award (ID 323204).
GLA had the idea for the analysis and wrote the manuscript. NdK, LJB and WHO designed and conducted the original research. TAO’S and GLA cleaned the dietary data. TAM was responsible for all biochemical assays and interpretation of biochemical data. GLA and NDK analyzed the data. All authors were responsible for critical reviews and final approval of the manuscript. GLA and WHO had primary responsibility for the final content.
Appendix 1.
Food groups used in factor analyses
| Food Group | Components |
|---|---|
| Whole grains | Wholemeal, mixed grain or high-fibre sliced bread, oatmeal muesli, bran, wheat germ, other wholegrain breakfast cereals |
| Refined grains | White bread or rolls, refined breakfast cereals, crumpets, muffins, crisp bread, crackers, salted biscuits, rice, noodles, pasta |
| Poultry | Roast or boiled chicken |
| Red meats | Beef, lamb, pork, pureed meat dishes, schnitzel, offal, mince dishes, hamburger patty (without bun) |
| Processed meat | Sausages, frankfurters, bacon, ham, fritz-devon, salami |
| Meat-based mixed dishes | Stew, casserole, Chinese meat and vegetables, curry, goulash |
| Take away foods | Hamburger with bun, pizza, fried or crumbed chicken, sausage roll, meat pie, savoury-filled pastry |
| Fried fish | Fried or battered fish |
| Other fish | Steamed, grilled or canned fish, other seafood |
| Fried potatoes | Hot chips (French fries), potato gems, croquettes or pommes noisettes |
| Potato | Boiled, mashed, roasted, canned or dried potato, potato salad |
| Yellow or red vegetables | Carrots, pumpkin, capsicum |
| Cruciferous vegetables | Cabbage, Brussels sprouts, broccoli, cauliflower, coleslaw |
| Leafy green vegetables | Silver beet, lettuce |
| Other vegetables | Beetroot, zucchini, sweet corn, mushrooms, olives, celery, turnip, swede, onion, cucumber, mixed vegetables |
| Tomato | Fresh and cooked tomato |
| Legumes | Haricot, lima, broad or green beans, peas, baked beans, lentils |
| Fresh fruit | Orange, apple, banana, fruit salad, berries, melons, peach, plum nectarine, apricot, grapes, pineapple, avocado |
| Canned fruit | Fruit canned in syrup or juice |
| Dried fruit | Sultanas, raisins, currants, other dried fruit |
| Low fat dairy products | Reduced fat milk, skim milk, flavoured milk, Sustagen, low fat yoghurt, low fat cheese, cottage cheese |
| Food Group | FFQ Foods |
|---|---|
| Full fat dairy products | Whole milk, cream, ice-cream, full fat yoghurt, full fat cheese, thick shakes |
| Soy milk | Soy milk |
| Milk-based dishes | Milk pudding, mornay dishes, custard |
| Cakes, biscuits, sweet pastries |
Fruit loaf, sweet bun, doughnut, croissant, biscuits, cake, fruit pie or pastry, steamed pudding |
| Confectionery | Chocolate, chocolate covered bars, sweets, toffees, icy poles |
| Added sugar | Honey, jam, marmalade, spooned sugar |
| Crisps | Crisps, corn chips |
| Nuts | Peanuts, other nuts (salted and unsalted) |
| Sauces | Mayonnaise, salad cream, thick sauces e.g. brown sauce |
| Soups | Canned soup, packet soup, homemade soup |
| Eggs | Fried, boiled, scrambled egg, omelette |
| Tea, coffee | Tea, herbal tea, coffee, coffee substitute, decaffeinated coffee |
| Soft drinks | Coca cola, mineral water, other soft drinks, cordial, fruit drink (<=35% fruit juice) |
| Mineral water (plain) | Spring water |
| Juice | Pure fruit juice, vegetable juice |
| Saturated spreads | Butter, butter/margarine blend, lard, table margarine |
| Unsaturated spreads | Canola or other monounsaturated fat margarine, polyunsaturated margarine, low fat spreads |
Reproduced with permission (13)
Footnotes
All authors declare no conflicts of interest.
References
- 1.Newby PK, Tucker KL. Empirically derived eating patterns using factor or cluster analysis: a review. Nutr Rev. 2004;62:177–203. doi: 10.1301/nr.2004.may.177-203. [DOI] [PubMed] [Google Scholar]
- 2.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13:3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Sempos C. Invited commentary: Some limitations of semi-quantitative food frequency questionnaires. Am J Epidemiol. 1992;135:1127–1132. [Google Scholar]
- 4.Hu FB, Rimm E, Smith-Warner SA, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr. 1999;69:243–249. doi: 10.1093/ajcn/69.2.243. [DOI] [PubMed] [Google Scholar]
- 5.Khani BR, Ye W, Terry P, et al. Reproducibility and validity of major dietary patterns among swedish women assessed with a food-frequency questionnaire. J Nutr. 2004;134:1541–1545. doi: 10.1093/jn/134.6.1541. [DOI] [PubMed] [Google Scholar]
- 6.Togo P, Heitmann BL, Sorensen TIA, et al. Consistency of food intake factors by different dietary assessment methods and population groups. Br J Nutr. 2003;90:667–678. doi: 10.1079/bjn2003943. [DOI] [PubMed] [Google Scholar]
- 7.Crozier SR, Inskip HM, Godfrey KM, et al. Dietary patterns in pregnant women: a comparison of food-frequency questionnaires and 4 d prospective diaries. Br J Nutr. 2008;99:869–875. doi: 10.1017/S0007114507831746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okubo H, Murakami K, Sasaki S, et al. Relative validity of dietary patterns derived from a self-administered diet history questionnaire using factor analysis among Japanese adults. Public Health Nutr. 2010;13:1080–1089. doi: 10.1017/S1368980009993211. [DOI] [PubMed] [Google Scholar]
- 9.Mikkilä V, Räsänen L, Raitakari OT, et al. Consistent dietary patterns identified from childhood to adulthood: The cardiovascular risk in Young Finns study. Br J Nutr. 2005;93:923–931. doi: 10.1079/bjn20051418. [DOI] [PubMed] [Google Scholar]
- 10.Newnham JP, Evans SF, Michael CA, et al. Effects of frequent ultrasound during pregnancy - a randomised controlled trial. Lancet. 1993;342:887–891. doi: 10.1016/0140-6736(93)91944-h. [DOI] [PubMed] [Google Scholar]
- 11.Baghurst KI, Record SJ. A computerised dietary analysis system for use with diaries or food frequency questionnaires. Community Health Stud. 1984;8:11–18. doi: 10.1111/j.1753-6405.1984.tb00419.x. [DOI] [PubMed] [Google Scholar]
- 12.Ambrosini GL, de Klerk NH, O’Sullivan TA, et al. The reliability of a food frequency questionnaire for use among adolescents. Eur J Clin Nutr. 2009;63:1251–1259. doi: 10.1038/ejcn.2009.44. [DOI] [PubMed] [Google Scholar]
- 13.Ambrosini GL, Oddy WH, Robinson M, et al. Adolescent dietary patterns are associated with lifestyle and family psycho-social factors. Public Health Nutr. 2009;12:1807–1815. doi: 10.1017/S1368980008004618. [DOI] [PubMed] [Google Scholar]
- 14.Di Candilo KG, Oddy WH, Miller M, et al. Follow-up phone-calls increase nutrient intake estimated by three-day food diaries in 13 year old participants of the raine study. Nutr Dietetics. 2007;64:165–171. [Google Scholar]
- 15.Xyris Software . Foodworks Professional. 4.0 ed Xyris Software; Brisbane, Australia: 2007. [Google Scholar]
- 16.Food Standards Australia New Zealand NUTTAB. 2006 Cited 5 July 2010. Available from: http://www.foodstandards.gov.au/monitoringandsurveillance/foodcompositionprogram/
- 17.Huang R-C, Mori TA, Burke V, et al. Synergy between adiposity, insulin resistance, metabolic risk factors and inflammation in adolescents. Diabetes Care. 2009;32:695–701. doi: 10.2337/dc08-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy KJ, Meyer BJ, Mori TA, et al. Impact of foods enriched with n-3 long-chain polyunsaturated fatty acids on erythrocyte n-3 levels and cardiovascular risk factors. Br J Nutr. 2007;97:749–757. doi: 10.1017/S000711450747252X. [DOI] [PubMed] [Google Scholar]
- 19.Livingstone MBE, Robson PJ, Wallace JMW. Issues in dietary intake assessment of children and adolescents. Br J Nutr. 2004;92:S213–S222. doi: 10.1079/bjn20041169. [DOI] [PubMed] [Google Scholar]
- 20.SAS Institute Incorporated . SAS for Windows. 9.1.3 ed SAS Institute Incorporated; Cary, NC, USA: 2002-2003. [Google Scholar]
- 21.UCLA: Academic Technology Services Statistical Consulting Group Factor analysis using SAS Proc Factor. 1995 Cited 7 July 2010. Available from: http://www.ats.ucla.edu/stat/sas/library/factor_ut.htm.
- 22.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 23.Willett WC, Stampfer M. Implications of total energy intake for epidemiological analyses. In: Willett W, editor. Nutritional epidemiology. 2nd ed Oxford University Press; New York: 1998. pp. 273–298. [Google Scholar]
- 24.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC Growth Charts: United States. Advance Data. 2000:28. [PubMed] [Google Scholar]
- 25.Bland MJ, Altman DG. Statistical methods for assessing agreement between two methods of clinical assessment. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 26.O’Sullivan TA, Ambrosini G, Beilin LJ, et al. Dietary intake and food sources of fatty acids in Australian adolescents. Nutrition. doi: 10.1016/j.nut.2009.11.019. in press. published online, 24 March 2010. [DOI] [PubMed] [Google Scholar]
- 27.Mozaffarian D, Lemaitre RN, Kuller LH, et al. Cardiac benefits of fish consumption may depend on the type of fish meal consumed: The Cardiovascular Health Study. Circulation. 2003;107:1372–1377. doi: 10.1161/01.cir.0000055315.79177.16. [DOI] [PubMed] [Google Scholar]
- 28.Crawford P, Obarzanek E, Morrison J. Comparative advantage of 3-day food records over 24-hour recall and 5-day food frequency validated by observation of 9-and 10-year-old girls. J Am Dietetic Assoc. 1994;94:626–630. doi: 10.1016/0002-8223(94)90158-9. [DOI] [PubMed] [Google Scholar]