Short abstract

Coal-tar-based sealcoat products, widely used in the central and eastern U.S. on parking lots, driveways, and even playgrounds, are typically 20−35% coal-tar pitch, a known human carcinogen that contains about 200 polycyclic aromatic hydrocarbon (PAH) compounds. Research continues to identify environmental compartments—including stormwater runoff, lake sediment, soil, house dust, and most recently, air—contaminated by PAHs from coal-tar-based sealcoat and to demonstrate potential risks to biological communities and human health. In many cases, the levels of contamination associated with sealed pavement are striking relative to levels near unsealed pavement: PAH concentrations in air over pavement with freshly applied coal-tar-based sealcoat, for example, were hundreds to thousands of times higher than those in air over unsealed pavement. Even a small amount of sealcoated pavement can be the dominant source of PAHs to sediment in stormwater-retention ponds; proper disposal of such PAH-contaminated sediment can be extremely costly. Several local governments, the District of Columbia, and the State of Washington have banned use of these products, and several national and regional hardware and home-improvement retailers have voluntarily ceased selling them.
Abstract

Coal-tar-based sealcoat products, widely used in the central and eastern U.S. on parking lots, driveways, and even playgrounds, are typically 20−35% coal-tar pitch, a known human carcinogen that contains about 200 polycyclic aromatic hydrocarbon (PAH) compounds. Research continues to identify environmental compartments—including stormwater runoff, lake sediment, soil, house dust, and most recently, air—contaminated by PAHs from coal-tar-based sealcoat and to demonstrate potential risks to biological communities and human health. In many cases, the levels of contamination associated with sealed pavement are striking relative to levels near unsealed pavement: PAH concentrations in air over pavement with freshly applied coal-tar-based sealcoat, for example, were hundreds to thousands of times higher than those in air over unsealed pavement. Even a small amount of sealcoated pavement can be the dominant source of PAHs to sediment in stormwater-retention ponds; proper disposal of such PAH-contaminated sediment can be extremely costly. Several local governments, the District of Columbia, and the State of Washington have banned use of these products, and several national and regional hardware and home-improvement retailers have voluntarily ceased selling them.
Introduction
Driveways and parking lots are common features of cities, suburbs, and small towns. Most single-family residences in the U.S. have paved driveways, and we encounter parking lots at multifamily residences, schools, offices, and commercial businesses. Most people in developed countries, when outdoors, probably spend as much time walking on pavement as on any other type of surface.
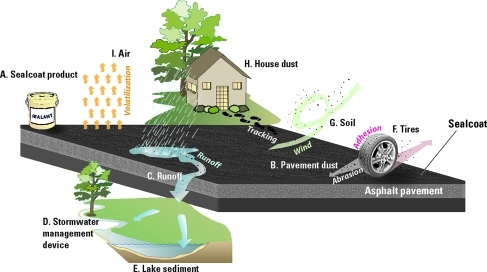
There are differences among paved surfaces, however. Most pavement is concrete or asphalt. The asphalt pavement of many parking lots, driveways, and even some playgrounds in North America is sprayed or painted with a black, shiny coating referred to as “sealcoat,” “pavement sealant,” or “driveway sealer” (Figure 1A). Sealcoat is marketed as improving pavement appearance and increasing pavement longevity.1 In addition to making pavement black, however, one type of commonly used pavement sealcoat contains refined coal tar and is a potent source of polycyclic aromatic hydrocarbons (PAHs).2−8 The contribution of pavement sealcoat to PAH contamination of soils, lakes, and homes has only recently been recognized.4−6
Figure 1.
PAHs from coal-tar-based pavement sealcoat are transported by different pathways to various environmental compartments. Once dry, the sealcoat product (A), which contains high concentrations of PAHs, is abraded into a powder and becomes part of the dust on the pavement (B). That dust is transported by storm runoff (C) to stormwater management devices (D) or to receiving streams and lakes (E). Parking lot dust also adheres to tires (F) that track it onto unsealed pavement, and wind and runoff transport the dust to nearby soils (G). Dust particles also are tracked on shoes into residences, where they become incorporated into house dust (H). Volatile PAHs in coal-tar-based sealcoat are released into the air (I). PAH concentrations associated with each compartment and literature sources are provided in Table 1.
Coal-Tar-Based Sealcoat: A Newly Identified Source of PAHs
The two primary sealcoat product types on the market are refined coal-tar-pitch emulsion and asphalt emulsion. Coal-tar pitch, a known (Group 1) human carcinogen,9 is the residue remaining after the distillation of crude coal tar (a byproduct of the coking of coal), and contains about 200 PAH compounds.10 Most coal-tar-based sealcoat products consist of 20–35% coal-tar pitch as the binder. Asphalt is the residue remaining after the distillation of crude oil and is the binder in asphalt-based sealcoat products. Although the two sealcoat product types are similar in appearance, PAH concentrations in coal-tar-based sealcoat are about 1000 times higher than those in asphalt-based sealcoat11 (Table 1).
Table 1. Concentrations of PAHs as Reported in the Literature for Environmental Compartments Shown in Figure 1, and Definitions of PAH Summations Used.
| environmental compartment (Figure 1) | medium | PAH concentration (median or mean) in coal-tar-based sealcoat or affected medium | PAH concentration (median or mean) in asphalt sealcoat, affected medium, or associated with unsealed pavement | summationa | units | reference |
|---|---|---|---|---|---|---|
| A | sealcoat products | 66 000 | 50 | ΣPAH16 | mg/kg | (11,22) |
| B | pavement dust | 2200 | 11 | ΣPAH12 | mg/kg | (3) |
| 4760 | 9 | ΣPAH16 | mg/kg | (4) | ||
| 685 | <1 | ΣPAH16 | mg/kg | (5) | ||
| C | runoff, particles | 3500 | 54 | ΣPAH12 | mg/kg | (2) |
| runoff, unfiltered waterb | 71 | 2 | ΣPAH16 | μg/L | (7) | |
| 52 | 5 | ΣPAH18 | μg/L | (25) | ||
| D | stormwater-management-device sediment | 646 | 2 | ΣPAH16 | mg/kg | (5) |
| E | lake sedimentc | 33 | 0.4 | ΣPAHCMB | mg/kg | (6) |
| F | tires | 1380 | 3 | ΣPAH16 | mg/kg | (5) |
| G | soild | 105 | 2 | ΣPAH16 | mg/kg | (5) |
| H | settled house dust | 129 | 5 | ΣPAH16 | mg/kg | (4) |
| I | air (0.03 m from pavement), 3–8 years after sealing | 1320 | 66 | ΣPAH8 | ng/m3 | (28) |
| air (1.28 m from pavement), 3–8 years after sealing | 138 | 26 | ΣPAH8 | ng/m3 | (28) | |
| air (0.03 m from pavement), 1.6 h after sealing | 297 000 | 66 | ΣPAH8 | ng/m3 | (29) | |
| air (1.28 m from pavement), 1.6 h after sealing | 5680 | 26 | ΣPAH8 | ng/m3 | (29) |
ΣPAH12 is the sum of concentrations of the 12 parent PAH (naphthalene, acenaphthylene, acenaphthene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benz[a]anthracene, chrysene, benzo[a]pyrene, and dibenz[a,h]anthracene), which are those PAHs used in computation of the probable effects concentration (PEC) sediment-quality guideline,41 less 2-methylnaphthalene. ΣPAH16 is the sum of the concentrations of the 16 priority pollutants identified by the U.S. Environmental Protection Agency,42 equal to the sum of ΣPAH12 and concentrations of benzo[b]fluoranthene, benzo[ghi]perylene, benzo[k]fluoranthene, and indeno[1,2,3-cd]pyrene. ΣPAH18 is equal to ΣPAH16 plus concentrations of 1-methylnaphthalene and 2-methylnaphthalene. ΣPAHCMB is the sum of concentrations of phenanthrene, anthracene, fluoranthene, pyrene, benz[a]anthracene, chrysene, benzo[a]pyrene, benzo[b]fluoranthene, benzo[ghi]perylene, benzo[k]fluoranthene, indeno[1,2,3-cd]pyrene, and benzo[e]pyrene. ΣPAH8 is the sum of concentrations of phenanthrene, anthracene, 4,5-methylphenanthrene, 1-methylphenanthrene, fluoranthene, pyrene, chrysene, and benzo[b]fluoranthene. On the basis of PAH data from primarily combustion sources presented in Mahler et al.,4 ΣPAH12 is about 70–75% of ΣPAH16. ΣPAH18 is similar to ΣPAH16, as the additional compounds in the summation either are not detected or are detected at very low concentrations.2,25
Collected >3 months after sealcoat application.
Means for urban lakes with >70% PAH from sealcoat and 0–20% from sealcoat.
Concentration in soil adjacent to a sealed parking lot.
In the U.S., coal-tar-based sealcoat is used primarily east of the Continental Divide, and asphalt-based sealcoat is used primarily west of the Continental Divide.3 Coal-tar-based sealcoat also is used in Canada.12 Geographic differences in use in North America likely are a historical and economic artifact of the location of most coal-tar-distillation plants near steel mills, which historically were (and are) in the central and eastern United States. An estimated 85 million gallons (320 million liters) of coal-tar-based sealcoat are used annually in the United States.11
What Are Polycyclic Aromatic Hydrocarbons (PAHs)?
PAHs are a large group of organic compounds composed of two or more fused benzene rings arranged in various configurations. Those with a low molecular weight (two or three benzene rings) tend to be more volatile, soluble, and biodegradable than those with a higher molecular weight (four or more benzene rings). PAHs occur naturally in coal and petroleum products and are formed by the incomplete combustion of organic matter, from fossil fuels to wood to cigarettes. PAHs have many urban sources, including used motor oil, automobile exhaust, industrial atmospheric emissions, tire particles, and asphalt.13,14PAHs always occur as a mixture of different PAH compounds, and are ubiquitous in the urban environment. Of all known PAH sources, the highest concentrations are in coal tar and the related compound creosote. Most laboratories analyze only a subset of PAHs, and concentrations of total PAHs are reported as the sum of the subset analyzed as described in Table 1.
The pavement sealcoat issue has been evolving since 2000, when PAH concentrations were discovered to be increasing in many urban lakes across the United States,15 even as concentrations of other contaminants like lead, polychlorinated biphenyls (PCBs), and DDT were decreasing.16,17 This was an apparent reversal from earlier reports that PAH concentrations in the U.S. were decreasing in response to reduced emissions from power plants and industries.18,19 The earlier studies, however, had focused on lakes in undeveloped watersheds, whereas the upward trends in PAHs were in lakes in urban and suburban watersheds. This meant, first, that reductions in PAH emissions caused by changes in home-heating and power-generation technology had been eclipsed in urban areas by some other urban source of PAHs,15 and second, that this other source was specific to urban and suburban areas.
A breakthrough in understanding urban sources of PAHs came in 2003, when staff with the City of Austin, TX, noted elevated PAH concentrations (ΣPAH16 > 1000 mg/kg) in some sediment samples collected from small tributaries and drainages in largely residential areas.20 Concentrations of PAHs this high are typical of contaminated soils at some manufactured gas plant Superfund sites,21 but cannot be accounted for by common urban sources (e.g., tire wear, vehicle emissions, asphalt).2 City of Austin staff connected the dots and hypothesized that the source of the elevated PAHs was particles eroded from parking lots that were coated with coal-tar-based sealcoat.22 Since that time, an understanding has emerged of relations between coal-tar-based pavement sealcoat and PAHs in the environment.
Migration of PAHs from Sealcoated Surfaces into the Environment
Sealcoat doesn't remain on the pavement surface indefinitely, and different applicators recommend reapplication from every 1 to 2 years (e.g., ref (23)) to every 3 to 5 years (e.g., ref (24)). Tires and snowplows, in particular, abrade the friable sealcoat surface into fine particles.5,11 The overall annual loss of sealcoat from parking lots in a warm climate is about 2.4% of total sealcoat applied, with wear being most rapid (about 5% per year) in driving areas.11 Higher wear rates have been noted in a cold-weather climate.7 The mobilized sealcoat particles and associated PAHs are transported to various environmental compartments (Figure 1, Table 1).
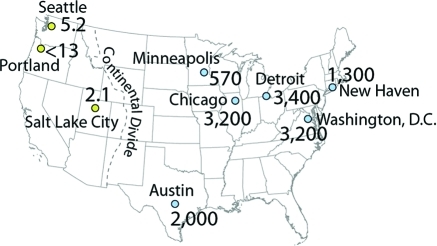
The first compartment is the dust on the pavement surface itself, generated as the sealcoat is abraded from the surface (Figure 1B). Concentrations of PAHs in fine particles (dust) on pavement with coal-tar-based sealcoat are hundreds of times higher than those in dust on concrete pavement or on asphalt pavement that is unsealed or that has asphalt-based sealcoat3−5 (Table 1). PAHs in dust on sealcoated pavement in the central and eastern U.S. are about 1000 times higher than in dust on sealcoated pavement in the western U.S., supporting anecdotal reports of geographic differences in product use3 (Figure 2).
Figure 2.
PAHs in dust swept from sealcoated parking lots show a striking geographic difference. PAH concentrations in dust from parking lots in central and eastern U.S. cities, where coal-tar-based sealcoat is commonly used, are about 1000 times higher than in the western U.S., where asphalt-based sealcoat is more commonly used. Concentrations are the sum of 12 PAHs (ΣPAH12), in mg/kg. (Figure adapted from ref (3), Figures 1 and 2).
Stormwater runoff transports abraded sealcoat particles off sealed pavement (Figure 1C, Table 1). The PAH concentration measured in particles in runoff from parking lots with coal-tar-based sealcoat (3500 mg/kg) was 65 times higher on average than the concentration in particles in runoff from unsealed asphalt and cement lots.2 Concentrations in unfiltered stormwater runoff from coal-tar-sealcoated pavement are particularly elevated during the months following sealcoat application. The mean ΣPAH16 in stormwater runoff from a coal-tar-sealcoated parking lot during the 3 months following sealcoat application was 1357 μg/L and the 3-month mean during the following two years ranged from 17 to 116 μg/L.7 This relatively elevated concentration persists for years—the median ΣPAH18 in stormwater runoff from a parking lot in Madison, WI, 5 years after the last application of coal-tar-based sealcoat, was 52 μg/L.25 That concentration is about 10 times higher than that in runoff from a mixed-use strip mall, arterial street, and unsealed parking lot (4.8–5.7 μg/L), more than 20 times higher than in runoff from a minor arterial street and a commercial rooftop (1.8–2.4 μg/L), and about 1000 times higher than in runoff from a residential feeder street (0.05 μg/L).25
In many communities, the first stop for stormwater runoff is a stormwater-retention pond or other stormwater-management device (Figure 1D), where suspended sediment and associated contaminants settle out. Stormwater ponds are designed to efficiently collect sediment-associated contaminants, which creates an unintended problem for many municipalities because PAHs accumulate in pond sediment. In 5 of 10 ponds sampled in the Minneapolis-St. Paul, MN, metropolitan area, concentrations of PAHs in sediment exceeded Minnesota’s Level 2 Soil Reference Value of 3 mg/kg benzo[a]pyrene equivalents (BaPeq), greatly increasing the cost for disposal.26 Even a small amount of sealcoated pavement can be the dominant source of PAHs to sediment that collects in stormwater-management devices, as demonstrated at the University of New Hampshire Stormwater Center.5 Sediment collected from a stormwater-management device receiving runoff from a parking lot with coal-tar-based sealcoat contained ΣPAH16 of 393–1180 mg/kg; sediment in devices receiving mixed runoff (4% sealed pavement and 96% unsealed pavement) contained 61–638 mg/kg ΣPAH16; and sediment in a device in the center of an adjacent unsealed lot contained less than 4 mg/kg ΣPAH16.5
Some sealcoat particles that are not trapped by stormwater ponds or other collection devices are transported down streams and rivers to lakes, where they are deposited in lake sediment (Figure 1E). Do the PAHs associated with the particles constitute a majority of PAHs in urban lake sediments, and might coal-tar-based sealcoat account for many of the upward trends in PAHs reported by Van Metre et al.?15 An initial indication comes from a comparison of PAH ratios, or “fingerprints”, of the dust collected from parking lots in nine U.S. cities to that of PAHs in sediment from lakes in the same watersheds.3 In the central and eastern U.S., PAH fingerprints of lake sediment and dust from sealcoated parking lots were similar, and were different from fingerprints of lake sediment and dust in the western U.S., reflecting regional differences in sealcoat product type used. A more sophisticated source-apportionment method—a statistical approach that quantifies the contribution of sources with known PAH profiles to an environmental receptor—was used to quantify the contribution of identified urban PAH sources to PAHs in bed sediment in 40 U.S. urban lakes.6 Coal-tar-based sealcoat was estimated to contribute about one-half of the PAHs in the lake sediment, when averaged across the 40 lakes; vehicle-related sources and coal combustion also were important contributors. PAH concentrations in lake sediment and the proportion contributed from coal-tar-based sealcoat were greater in the central and eastern U.S. than in the western U.S. Using sediment cores, trends in PAHs were investigated for eight urban lakes; of the six with significant upward trends, source apportionment indicated that coal-tar-based sealcoat was the cause of the trend in all six of them.
Turning our attention back to sealed pavement, dust from pavement with coal-tar-based sealcoat contaminates nearby unsealed pavement, with concentrations decreasing with distance from the sealed pavement.5 A petrographic analysis of dust from unsealed pavement in Fort Worth, TX, found that coal-tar pitch was the dominant (92%) source of PAHs in the dust.8 Particles are transported by adhesion to vehicle tires and by wind from sealed to unsealed surfaces—ΣPAH16 in particles swept from tires driven over sealed lots were 400 times higher than in particles swept from tires driven over unsealed lots5 (Table 1, Figure 1F). Transport of abraded coal-tar-based sealcoat particles by wind and tires might be one reason why PAH concentrations in dust from unsealed parking lots in the central and eastern U.S. (median ΣPAH12 27 mg/kg), where coal-tar-based sealcoat is predominantly used, are significantly higher than those in dust from unsealed parking lots in the western U.S. (median ΣPAH12 0.8 mg/kg), where the asphalt-based product is predominantly used.3
PAHs in particles abraded from coal-tar-based sealcoat also are transported by wind, runoff, and snow removal to nearby soils (Table 1, Figure 1G). ΣPAH16 in surface soil adjacent to coal-tar-sealed lots at the University of New Hampshire was as high as 411 mg/kg, and concentrations decreased with distance from the sealed lot to less than 10 mg/kg.5 The highest concentrations were measured in areas where snow was piled adjacent to the lots during the winter months—snowplows were scraping the sealcoat off with the snow. PAHs in surface soils from commercial areas in Fort Worth, TX, were dominantly (88%) from coal-tar pitch.8
PAHs from pavement sealed with coal-tar-based sealcoat can contaminate the indoor environment (Figure 1H) as well as the outdoor environment. In a study in Austin, TX, apartments with parking lots with coal-tar-based sealcoat had ΣPAH16 in house dust that was 25 times higher, on average, than ΣPAH16 in house dust from apartments with parking lots with other surface types (concrete, unsealed asphalt, or asphalt-based sealcoat)4 (Table 1). The presence or absence of coal-tar-based sealcoat on the apartment complex parking lot was strongly correlated with PAH concentrations in house dust. Although tobacco smoking, candle and incense burning, and barbecue and fireplace use have been suggested to affect PAH concentrations in house dust, Mahler et al.4 found no relation between any of these and PAH concentrations in the house dust. Concentrations of individual PAHs in house dust collected from apartments in Austin adjacent to pavement with coal-tar-sealcoated parking lots were about 140 times higher than those measured in a study of house dust in California.27 Lower concentrations of PAHs in house dust in California are consistent with the very low concentrations of PAHs measured in pavement dust in the western U.S. (Figure 2), where coal-tar-based sealcoat is not commonly used.
In addition to contaminating stormwater, sediment, soil, and house dust, PAHs from coal-tar-based sealcoat contaminate air (Figure 1I). Several of the lower molecular weight PAHs in coal-tar-based sealcoat are volatile, which is why sealed parking lots and driveways frequently give off a strong smell. A recent study28 reported that the flux of ΣPAH8 from in-use parking lots with coal-tar-based sealcoat of various ages (mostly more than 3 years old) was 60 times higher than that from unsealed pavement on average. A second study29 reported that ΣPAH8 in air just after sealcoat application was hundreds to thousands of times higher than that above unsealed parking lots (Table 1), and that one-quarter to one-half of the PAHs in the applied sealcoat were lost to the atmosphere during the first 16 days following application. A mass balance indicated that ΣPAH8 emissions from new applications of coal-tar-based sealant each year are larger than annual vehicle emissions of PAHs for the U.S.29
Biological Concerns
The detrimental effects of PAHs on terrestrial and aquatic ecosystems are well documented.30 For example, when fish are exposed to PAHs, they exhibit chronic effects, including fin erosion, liver abnormalities, cataracts, skin tumors, and immune system impairments leading to increased susceptibility to disease.31 When benthic macroinvertebrates—insects and other organisms that live at the bottom of rivers and lakes and that make up the base of the aquatic food chain—are exposed to PAHs, they are susceptible to a number of detrimental effects, including inhibited reproduction, delayed emergence, sediment avoidance, and mortality.31 The most important mechanism by which acute effects occur in benthic invertebrates is a nonspecific narcosis-like mode of action that results in the degradation of cell membranes.32 Ultraviolet (UV) radiation greatly increases the toxicity of PAHs in a wide variety of aquatic organisms.33−36
As the importance of coal-tar-based sealcoat as a source of PAHs has emerged, several studies have looked at potential biological effects of this particular source of PAHs. When sediment was spiked with coal-tar-based sealcoat to provide a range of environmentally relevant PAH concentrations, frogs (Xenopus laevis) had stunted growth or delayed development at 30 mg/kg ΣPAH16, and complete mortality occurred at the highest treatment of 300 mg/kg ΣPAH16.37 Salamanders (Ambystoma maculatum) and newts (Notophthalmus viridescens) exposed to sediment contaminated with coal-tar-based sealcoat at PAH concentrations similar to the highest treatment in the frog study had stunted growth, difficulty swimming or righting themselves, and liver problems.38,39 These effects were magnified by the addition of UV light.38 At the community level, macroinvertebrate communities exposed to sediment spiked with coal-tar-based sealcoat had significant decreases in species abundance and richness at ΣPAH16 concentrations exceeding 300 mg/kg.40 Similarly, in a study of urban streams, aquatic invertebrate communities downstream from parking lots with coal-tar-based sealcoat suffered losses of abundance and diversity along a gradient of increasing total PAH concentration, starting near the ΣPAH12 probable effects concentration (PEC) value of 22.8 mg/kg.20,41 These studies demonstrate that PAHs in sediment contaminated by coal-tar-based sealcoat are bioavailable and that environmentally relevant concentrations adversely affect amphibians and benthic communities, two robust indicators of aquatic ecosystem health. The finding of adverse biological effects to biota when exposed to sediment with PAH concentrations near the PEC has widespread relevance: Of the 40 U.S. urban lakes investigated by Van Metre and Mahler,6 sediment in the nine lakes with the greatest mass loading of PAHs from coal-tar-based sealcoat had concentrations of PAHs that exceeded the PEC.
Human-Health Concerns
Coal tar and coal-tar pitch are listed as Group 1 (carcinogenic to humans) carcinogens,9 and the U.S. EPA currently classifies seven PAH compounds as probable human carcinogens (Group B2): benz[a]anthracene, benzo[a]pyrene, benzo[b]fluoranthene, benzo[k]fluoranthene, chrysene, dibenz[a,h]anthracene, and indeno[1,2,3-cd]pyrene.42 Coal tar itself is a powerful mutagen: The mutagenicity index for coal tar is about 1000 times that of asphalt cements.43 However, although coal-tar-based sealcoat has been on the market since at least 1960,6 little has been published to date about the contribution of the sealcoat to PAH exposures and the associated potential for adverse human-health outcomes.
The elevated concentrations of PAHs in house dust, soil, air, water, and sediment associated with coal-tar-based sealcoat raise the possibility of several complete exposure pathways for humans. Incidental ingestion of house dust and soil is particularly relevant for small children, who put their hands and objects into their mouths. A recent study44 reported that children living in homes adjacent to pavement with coal-tar-based sealcoat likely are exposed to about 14-fold higher doses of PAHs through ingestion of house dust than are children living in residences adjacent to unsealed pavement, and that exposure from ingestion of PAH-contaminated house dust is estimated to be more than double that from diet, even under conservative assumptions. Ingestion of contaminated soil is another way that children might be exposed to PAHs from coal-tar-based sealcoat, particularly given that ingestion rates of soil typically exceed those of house dust.45 Incidental ingestion of dust directly from sealed pavement also might be important, because the extremely high concentrations of PAHs measured in these materials (Table 1) could translate to substantial doses from miniscule exposures. On a long-term basis, nondietary ingestion of PAH-contaminated house dust and soil likely are the most important routes of exposure, but a complete human-health risk analysis is required before the cancer risk associated with ingestion of these media can be quantified.
Other routes of exposure to coal-tar-based sealcoat, in addition to ingestion, might have implications for human health. Relatively high acute exposures might occur from inhalation of wind-blown particles or fumes that volatilize from sealed parking lots, especially during sealcoat application. Sealcoat applicators, in particular, might be subject to substantial inhalation exposures, but such exposures have not yet been characterized. Other potential routes include skin contact with sealcoat and abraded sealcoat particles and contaminated soil, sediment, dust, and water. Such exposures likely would be relatively infrequent and short-term. However, PAHs are readily absorbed through the skin,46 and circumstances that increase the frequency or magnitude of exposure events, such as daily activity on pavement treated with coal-tar-based sealcoat, might be associated with increased cancer risk.
Regulatory and Retail Actions
Research to date, as documented here, provides a compelling weight-of-evidence that coal-tar-based sealcoat products are an important source of PAHs to our environment. A patchwork of actions has been taken to either ban or restrict the use of coal-tar-based sealcoat in the United States. The first ban was implemented by the City of Austin, TX, in 2006.47 As of January 2012, 15 municipalities and two counties in four states (Minnesota, New York, Texas, and Wisconsin), the District of Columbia, and the State of Washington had enacted some type of ban, affecting nearly 10.4 million people.48 Other local and state jurisdictions have used voluntary or limited-use restrictions for certain groups (e.g., city government) to discourage the use of coal-tar-based sealcoat.48
Minnesota, in particular, has been actively engaged in this issue after municipalities contacted state agencies and the Minnesota Legislature for assistance addressing PAH-contaminated stormwater pond sediment.49 Costs for disposing of this sediment could reach $1 billion if PAHs in sediment in just 10% of the estimated 20 000 municipal stormwater ponds in the Minneapolis-St. Paul, MN, metropolitan area exceed Minnesota’s Level 2 human-health risk-based Soil Reference Value of 3 mg/kg BaPeq50 (Donald Berger, Minnesota Pollution Control Agency, written communication, 2011). The Minnesota Legislature passed a bill in 2009 that provides small grants to local governments for use in treating or disposing of contaminated sediment in stormwater ponds, provided that the governments restrict the use of undiluted coal-tar-based sealcoat.49 As of January 2012, 13 municipalities had passed ordinances and three municipalities have received grants for remediation of stormwater ponds.
Several national and regional hardware and home-improvement retailers have voluntarily ceased selling coal-tar-based driveway-sealer products.48 Some private applicators have chosen to use only asphalt-based sealcoat (e.g., refs (51,52)). Many professional sealcoating companies in areas unaffected by bans or restrictions use coal-tar-based sealcoat, however, and coal-tar-based sealcoat products are readily available online for purchase by homeowners.
No action has been taken at a federal level to restrict the use of coal-tar-based sealcoat. Coke product residues, such as coal tar, are not classified as hazardous waste under the Resource Conservation and Recovery Act if the product is recycled.53 This exemption allows coal-tar pitch to be used in the production of aluminum (∼95% of use), commercial carbon, built-up roofing, and pavement sealcoat.54
Because PAHs are a ubiquitous and persistent class of urban contaminants, a decade or more might be required to assess the effectiveness of bans, restrictions, and/or changes in the retail availability of coal-tar-based sealcoat on reducing PAH concentrations in urban water bodies. Research on trends in the occurrence of PCBs and DDTs supports this concern. Following national bans on use of PCBs and DDT in the 1970s, it was 10–15 years before concentrations in lakes and reservoirs decreased by one-half.17,55 Unlike these chemicals, all sources of PAHs in urban watersheds will not be eliminated by banning coal-tar-based sealcoat. However, reductions in PAH loads over time might be sufficient to provide more options for disposal of dredged material from stormwater ponds and navigation channels and reduce risk to terrestrial and aquatic ecosystems and human health.
Biography
Barbara Mahler, Ph.D., and Peter Van Meter, Ph.D., are Research Hydrologists at the U.S. Geological Survey Texas Water Science Center, where they investigate occurrence of and trends in sediment-associated contaminants. Their recent research has focused on identifying sources of polycyclic aromatic compounds to the environment. Judy Crane, Ph.D., is a research scientist with the Minnesota Pollution Control Agency, where she specializes in contaminated sediments; she led an international PAH Work Group for the SETAC Sediment Advisory Group from 2008–2011. Alison Watts, Ph.D., is a research faculty in the Department of Civil Engineering at the University of New Hampshire; her research interests include fate and transport of contaminants in stormwater runoff and impacts of land use on water quality. Mateo Scoggins is an environmental scientist with the Watershed Protection Department of the City of Austin, TX, where he has investigated urban stream ecology for the past 18 years. E. Spencer Williams, Ph.D., is a member of the research faculty at the Center for Reservoir and Aquatic Systems Research at Baylor University in Waco, TX, where his research focuses on exposure and risk assessment in the contexts of bioaccumulation, water quality and reuse, and airborne and settled particulates.
The authors declare no competing financial interest.
References
- Dubey G.Understanding how sealcoating works ... and how it can save you money; http://pavementpro.org/understanding.htm.
- Mahler B. J.; Van Metre P. C.; Bashara T. J.; Wilson J. T.; Johns D. A. Parking lot sealcoat: An unrecognized source of urban polycyclic aromatic hydrocarbons. Environ. Sci. Technol. 2005, 39, 5560–5566. [DOI] [PubMed] [Google Scholar]
- Van Metre P. C.; Mahler B. J.; Wilson J. T. PAHs underfoot: Contaminated dust from coal-tar sealcoated pavement is widespread in the United States. Environ. Sci. Technol. 2009, 43 (1), 20–25. [DOI] [PubMed] [Google Scholar]
- Mahler B. J.; Van Metre P. C.; Wilson J. T.; Musgrove M.; Burbank T. L.; Ennis T. E.; Bashara T. J. Coal-tar-based parking lot sealcoat: An unrecognized source of PAH to settled house dust. Environ. Sci. Technol. 2010, 44, 894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polycyclic aromatic hydrocarbons released from sealcoated parking lots – A controlled field experiment to determine if sealcoat is a significant source of PAHs in the environment; University of New Hampshire Stormwater Center: Durham, NH, 2010. [Google Scholar]
- Van Metre P. C.; Mahler B. J. Contribution of PAHs from coal-tar pavement sealcoat and other sources to 40 U.S. lakes. Sci. Total Environ. 2010, 409, 334–344. [DOI] [PubMed] [Google Scholar]
- Watts A. W.; Ballestero T. P.; Roseen R. M.; Houle J. P. Polycyclic aromatic hydrocarbons in stormwater runoff from sealcoated pavements. Environ. Sci. Technol. 2010, 44, 8849–8854. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Van Metre P. C.; Mahler B. J.; Wilson J. T.; Ligouis B.; Razzaque M.; Schaeffer C.; Werth C. The influence of coal-tar sealcoat and other carbonaceous materials on polycyclic aromatic hydrocarbon loading in an urban watershed. Environ. Sci. Technol. 2010, 44 (23), 1217–1233. [DOI] [PubMed] [Google Scholar]
- Monographs on the Evaluation of Carcinogenic Risks to Humans Supplement 7: Coal-Tar Pitches (Group 1); International Agency for Research on Cancer: Lyon, France, 1987; http://monographs.iarc.fr/ENG/Monographs/suppl7/Suppl7-57.pdf. [Google Scholar]
- Toxicological profile for wood creosote, coal tar creosote, coal tar, coal tar pitch, and coal tar pitch volatiles; U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry: Atlanta, GA, September 2002; http://www.atsdr.cdc.gov/ToxProfiles/tp85.pdf. [Google Scholar]
- Scoggins M.; Ennis T.; Parker N.; Herrington C. A photographic method for estimating wear of coal tar sealcoat from parking lots. Environ. Sci. Technol. 2009, 43 (13), 4909–4914. [DOI] [PubMed] [Google Scholar]
- Reconnaissance study of coal tar sealcoat application in Toronto and an estimate of related PAH emissions; Diamond Environmental Group, Department of Geography and Department of Chemical Engineering, University of Toronto: Toronto, ON, 2011. Prepared for Environment Canada. [Google Scholar]
- Rogge W. F.; Hildemann L. M.; Mazurek M. A.; Cass G. R.; Simoneit B. R. T. Sources of fine organic aerosol. 3. Road dust, tire debris, and organometallic brake lining dust: roads as sources and sinks. Environ. Sci. Technol. 1993, 27 (9), 1892–1904. [Google Scholar]
- Yunker M. B.; Macdonald R. W.; Vingarzan R.; Mitchell R. H.; Goyette D.; Sylvestre S. PAHs in the Fraser River basin: A critical appraisal of PAH ratios as indicators of PAH source and composition. Org. Geochem. 2002, 33, 489–515. [Google Scholar]
- Van Metre P. C.; Mahler B. J.; Furlong E. T. Urban sprawl leaves its PAH signature. Environ. Sci. Technol. 2000, 34, 4064–4070. [Google Scholar]
- Mahler B. J.; Van Metre P. C.; Callender E. Trends in metals in urban and reference lake sediments across the United States, 1970–2001. Environ. Toxicol. Chem. 2006, 25 (7), 1698–1709. [DOI] [PubMed] [Google Scholar]
- Van Metre P. C.; Mahler B. J. Trends in hydrophobic organic contaminants in urban and reference lake sediments across the United States, 1970–2001. Environ. Sci. Technol. 2005, 39, 5567–5574. [DOI] [PubMed] [Google Scholar]
- Furlong E. T.; Cessar L. R.; Hites R. A. Accumulation of polycyclic aromatic hydrocarbons in acid sensitive lakes. Geochem. Cosmochim. Acta 1987, 51 (11), 2965–2975. [Google Scholar]
- Gschwend P. M.; Hites R. A. Fluxes of polycyclic aromatic hydrocarbons to marine and lacustrine sediments in the northeastern United States. Geochem. Cosmochim. Acta 1981, 45 (12), 2359–2367. [Google Scholar]
- Scoggins M.; McClintock N.; Gosselink L.; Bryer P. Occurrence of polycyclic aromatic hydrocarbons below coal-tar-sealed parking lots and effects on stream benthic macroinvertebrate communities. J. N. Am. Benthol. Soc. 2007, 26 (4), 694–707. [Google Scholar]
- Haeseler F.; Blancher D.; Druelle V.; Werner P.; Vandecasteel J.-P. Ecotoxicological assessment of soils of former manufactured gas plant sites: Bioremediation potential and pollutant mobility. Environ. Sci. Technol. 1999, 33 (24), 4379–4384. [Google Scholar]
- PAHs in Austin, Texas, Sediments and Coal-Tar Based Pavement Sealants, Polycyclic Aromatic Hydrocarbons; City of Austin Watershed Protection and Development Review Department: Austin, TX, 2005; http://www.ci.austin.tx.us/watershed/downloads/coaltar_draft_pah_study.pdf. [Google Scholar]
- The Blacktop Guy - FAQ. http://www.blacktopguy.com/faq.php#_13.
- A-Line Asphalt Products Frequently Asked Questions. http://www.alineasphaltproducts.com/faq.html.
- Selbig W. R.Concentrations of Polycyclic Aromatic Hydrocarbons (PAHs) in Urban Stormwater, Madison, Wisconsin, 2005–08; Open File Report 2009–1077; U.S. Geological Survey: Denver, CO, 2009; http://pubs.usgs.gov/of/2009/1077/pdf/ofr20091077.pdf. [Google Scholar]
- Polta R.; Balogh S.; Craft-Reardon A.. Characterization of stormwater pond sediments. Final project report, EQA Report 06–572; Environmental Quality Assurance Department, Metropolitan Council Environmental Services: St. Paul, MN, 2006; http://www.metrocouncil.org/Environment/sediment/FinalReport.pdf. [Google Scholar]
- Whitehead T.; Metayer C.; Gunier R. B.; Ward M. H.; Nishioka M. G.; Buffler P.; Rappaport S. M.. Determinants of polycyclic aromatic hydrocarbon levels in house dust. J. Expo. Sci. Env. Epid. 2011, 21 (March/April), 123-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Metre P. C.; Majewski M. S.; Mahler B. J.; Foreman W. T.; Braun C. L.; Wilson J. T.; Burbank T.. Volatilization of polycyclic aromatic hydrocarbons from coal-tar-sealed pavement. Chemosphere 2012, http://dx.doi.org/10.1016/j.chemosphere.2011.12.072. [DOI] [PubMed] [Google Scholar]
- Van Metre P. C.; Majewski M. S.; Mahler B. J.; Foreman W. T.; Braun C. L.; Wilson J. T.; Burbank T. PAH volatilization following application of coal-tar-based pavement sealant. Atmos. Environ. 2012, http://dx.doi.org/10.1016/j.atmosenv.2012.01.036.. [Google Scholar]
- Douben P. E. T.PAHs: An Ecotoxicological Perspective; John Wiley & Sons Ltd.: West Sussex, England, 2003. [Google Scholar]
- Toxicological profile for polycyclic aromatic hydrocarbons; http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=122&tid=25. [PubMed]
- Van Brummelen T. A.; et al. Bioavailability and ecotoxicity of PAHs. In PAHs and Related Compounds. The Handbook of Environmental Chemistry; Neilson A.; Hutzinger O., Eds.; Springer Verlag: Berlin, Germany, 1998; Vol. 3, part J. [Google Scholar]
- Ireland D. S.; Burton G. A. Jr.; Hess G. G. In situ toxicity evaluations of turbidity and photoinduction of polycyclic aromatic hydrocarbons. Environ. Toxicol. Chem. 1996, 15, 574–581. [Google Scholar]
- Diamond S. A.; Milroy N. J.; Mattson V. R.; Heinis L. J.; Mount D. R. Photoactivated toxicity in amphipods collected from polycyclic aromatic hydrocarbon-contaminated sites. Environ. Toxicol. Chem. 2003, 22, 2752–2760. [DOI] [PubMed] [Google Scholar]
- Diamond S. A.; Mount D. R.; Burkhard L. P.; Ankley G. T.; Makynen E. A.; Leonard E. N. Effect of irradiance spectra on the photoinduced toxicity of three polycyclic aromatic hydrocarbons. Environ. Toxicol. Chem. 2000, 19, 1389–1396. [Google Scholar]
- Ankley G. T.; et al. Assessing risks from photoactivated toxicity of PAHs to aquatic organisms. In PAHs: An Ecotoxicological Perspective; Douben P. E. T., Ed.; John Wiley & Sons Ltd.: West Sussex, England, 2003; pp 275–296. [Google Scholar]
- Bryer P. J.; Elliott J. N.; Willingham E. J. The effects of coal tar based pavement sealer on amphibian development and metamorphosis. Ecotoxicology 2006, 15, 241–247. [DOI] [PubMed] [Google Scholar]
- Bommarito T.; Sparling D. W.; Halbrook R. S. Toxicity of coal-tar pavement sealants and ultraviolet radiation to Ambystoma maculatum. Ecotoxicology 2010, 19, 1147–1156. [DOI] [PubMed] [Google Scholar]
- Bommarito T.; Sparling D. W.; Halbrook R. S. Toxicity of coal-tar and asphalt sealants to eastern newts, Notophthalmus viridescens. Chemosphere 2010, 81 (2), 187–193. [DOI] [PubMed] [Google Scholar]
- Bryer P. J.; Scoggins M.; McClintock N. L. Coal-tar based pavement sealant toxicity to freshwater macroinvertebrates. Environ. Pollut. 2009, 158 (5), 1932–1937. [DOI] [PubMed] [Google Scholar]
- MacDonald D. D.; Ingersoll C. G.; Berger T. A. Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch. Environ. Contamin. Toxicol. 2000, 39, 20–31. [DOI] [PubMed] [Google Scholar]
- Integrated Risk Information System (IRIS); http://cfpub.epa.gov/ncea/iris/index.cfm.
- Detection of coal tar materials in asphalt pavements using chemical and biological methods; http://www.asphaltinstitute.org/public/engineering/PDFs/Environmental/Detection_Coal_Tar_Materials_Asphalt_Pavements_Using_Chem_Bio_Methods.pdf.
- Williams E. S.; Mahler B. J.; Van Metre P. C. Coal-tar pavement sealants might substantially increase children’s PAH exposures. Environ. Pollut. 2012, http://dx.doi.org/10.1016/j.envpol.2012.01.. [DOI] [PubMed] [Google Scholar]
- Exposure Factors Handbook: 2011 ed.; EPA/600/R-090/052F; U.S. Environmental Protection Agency: Washington, D.C., 2011; http://www.epa.gov/ncea/efh/pdfs/efh-complete.pdf. [Google Scholar]
- Roy T. A.; Krueger A. J.; Taylor B. B.; Mauro D. M.; Goldstein L. S. Studies estimating the dermal bioavailability of polynuclear aromatic hydrocarbons from manufactured gas plant tar-contaminated soils. Environ. Sci. Technol. 1998, 32 (20), 3113–3117. [Google Scholar]
- An ordinance amending the city code to add a new Chapter 6-6 relating to coal tar pavement products, creating offenses, and providing penalties, Ordinance No. 2005117-070, 2005; http://www.ci.austin.tx.us/edims/document.cfm?id=94225.
- Status of actions regarding use of coal tar-based sealants; http://www.pca.state.mn.us/index.php/view-document.html?gid=16180.
- Crane J. L.; Grosenheider K.; Wilson C. B.. Contamination of Stormwater Pond Sediments by Polycyclic Aromatic Hydrocarbons (PAHs) in Minnesota—The Role of Coal Tar-based Sealcoat Products as a Source of PAHs; Minnesota Pollution Control Agency: St. Paul, MN, 2010, document tdr-g1-07; http://www.pca.state.mn.us/index.php/view-document.html?gid=12960. [Google Scholar]
- Stollenwerk J.; Smith J.; Ballavance B.; Rantala J.; Thompson D.; McDonald S.; Schnick E.. Managing dredged materials in the State of Minnesota; Minnesota Pollution Control Agency: St. Paul, MN, 2011, document wq-gen2-01; http://www.pca.state.mn.us/index.php/view-document.html?gid=12959. [Google Scholar]
- South GA Sealcoating and Striping Sealer performance F.A.Q.; http://www.sgasealcoating.com/sealcoating_faq.htm#5.
- A Better Driveway. Driveway sealcoating – now environmentally friendly; http://www.abetterdriveway.com/residential_services.
- Hazardous Waste Recycling Exemptions; http://www.epa.gov/osw/hazard/recycling/regulations.htm.
- Valle S.; Panero M. A.; Shor L.. Pollution prevention and management strategies for polycyclic aromatic hydrocarbons in the New York/New Jersey Harbor; Harbor Consortium of the New York Academy of Sciences: New York, NY, 2007. [Google Scholar]
- Van Metre P. C.; Wilson J. T.; Callender E.; Fuller C. C. Similar rates of decrease of persistent, hydrophobic contaminants in riverine systems. Environ. Sci. Technol. 1998, 32 (21), 3312–3317. [Google Scholar]