Abstract
Milk Thistle contains silybin, which is a potential iron chelator. We aimed to determine whether silybin reduced iron absorption in patients with hereditary haemochromatosis. In this crossover study, on three separate occasions, 10 patients who were homozygous for the C282Y mutation in the HFE gene (and fully treated) consumed a vegetarian meal containing 13.9mg iron with: 200ml water; 200ml water and 140mg silybin (Legalon® Forte); or 200ml tea. Blood was drawn once before, then 0.5, 1, 2, 3 and 4h after the meal. Consumption of silybin with a meal resulted in a reduction in the postprandial increase in serum iron (AUC ±SE) compared with water (silybin 1726.6 ± 346.8 v water 2988.8 ± 167; P<0.05) and tea (silybin 1726.6 ± 346.8 v tea 2099.3 ± 223.3; P<0.05). In conclusion, silybin has the potential to reduce iron absorption, and this deserves further investigation as silybin could be an adjunct in the treatment of haemochromatosis.
Keywords: Hereditary Haemochromatosis, iron absorption, milk thistle, silybin
Introduction and Methods
In HFE-related hereditary haemochromatosis (HH), dietary iron uptake is increased relative to iron requirements. Treatment of HH involves repeated phlebotomy, which effectively reduces iron stores to maintain them at a normal level. Alternative treatments, if used alongside phlebotomy treatment, would reduce the need for maintenance phlebotomy and would improve the management of HH, particularly in patients with more severe phenotypes. Black tea, a source of iron-binding polyphenols, has been shown to cause a small reduction in both iron absorption and the rate of iron re-accumulation in HH (Kaltwasser et al., 1998). The polyphenol silybin is found in Milk Thistle (Silibum marianum), and it is purported to aid liver function. Silybin has a high affinity for ferric iron, forming an iron-silybin complex (Borsari et al., 2001). Therefore, anecdotal reports of reduced requirements for maintenance phlebotomy in HH patients taking Milk Thistle may be attributable to the iron-binding effect of silybin, by reducing non-haem iron absorption. Our objective was to investigate the iron-binding potential of silybin in vivo. This pilot study was approved by King’s College Hospital Local Research Ethics Committee (LREC reference: 06/Q0703/56) and the Medicines and Healthcare Regulatory Authority (Eudract number: 2006-002099-16), and was a registered controlled trial (Current Controlled Trials Ltd. reference: CCT-NAPN-15285). All participants gave their written informed consent to take part in this study. This was a crossover study; all patients received all treatments and in random order. Participants were 10 patients (9 male, 1 female) who were homozygous for the C282Y mutation in HFE, with grade 4 siderosis and fibrosis (n 7) or cirrhosis (n 3) on liver biopsy. Patients received phlebotomy treatment to maintain their serum ferritin within the normal range, but were not phlebotomised during the study. Additional patient characteristics are shown in Table 1. After an overnight fast, on 3 occasions, blood was taken through an indwelling catheter immediately before and at 0.5, 1, 2, 3 and 4 hours after ingestion of a meal. The meal consisted of vegetables in tomato sauce, mashed potato and white bread, and contained 478 kcal energy, 22 g fat, 124 mg vitamin C (Holland et al., 1991), and 13.9 mg non-haem iron (3.9 mg endogenous and 10 mg added in the form of FeCl3 solution). Meals were prepared in one batch and frozen until the evening of the study, then reheated before serving. The FeCl3 solution was added to the meal after reheating and immediately before serving. On each occasion, the meal was consumed with a different treatment: (i) 200 ml water, (ii) 200 ml water and silybin in the form of Legalon Forte® 140 mg (Madaus GmbH, Köln, DE) or (iii) 200 ml black tea infusion containing 170 mg polyphenols expressed as gallic acid equivalents (Conway et al., 2006). After the meal, patients were allowed to consume only water for a period of 4 hours. Quantitative analysis of serum iron was carried out in King’s College Hospital, using the revised method approved by the International Committee for Standardisation in Haematology. Iron absorption, based on the serum iron response following ingestion of the meal, was estimated using a method that had been validated in individuals who were avid for iron (Hoppe et al., 2004; Conway et al., 2006). This method had been previously applied in a study on HH patients (Hutchinson et al., 2007). The appearance and clearance of total serum iron was assessed by calculating the area under the serum iron curve (AUC) over the 4 hour observation period (AUC 0 - 4). A Wilcoxon non-parametric test for related samples was used for comparison of AUC 0 – 4 following: (i) silybin versus water, (ii) tea versus water, and (iii) silybin versus tea.
Table 1.
Demographic data in HH patients (n 10). Total body iron burden was determined by the amount of iron removed to normalise serum ferritin
| Mean (± s.e.) | Range | |
|---|---|---|
| Serum ferritin (μg/L) | 111.7 ± 31 | 28 – 300 |
| Haemoglobin (g/dl) | 14.7 ± 0.4 | 12.6 – 17.5 |
| Age (year) | 56.8 ± 3.1 | 43 – 69 |
| Length of time since diagnosis (year) | 5.7 ± 1.2 | 1-11 |
| Total body iron burden (g) | 11.4 ± 1.6 | 5.8-19 |
Results and Discussion
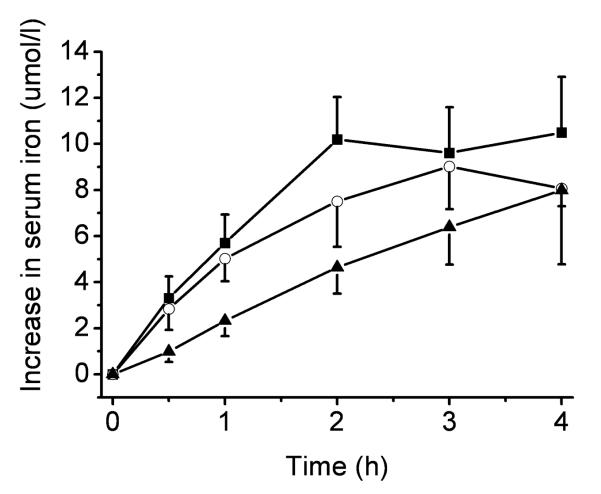
Consumption of silybin with a meal, compared with water, resulted in a significant reduction in the amount of dietary iron absorbed (Figure 1; P<0.05). Based on findings in vitro, in the neutral pH of the duodenum, silybin could form a complex with unchelated iron in the ferric form (Bosari et al., 2001), rendering it unavailable for absorption. Likewise, it is recognised that consuming tea with meals reduces the absorption of non-haem iron in healthy individuals, and the potential use of tea as a means of reducing dietary iron absorption in hereditary haemochromatosis has been previously investigated (Kaltwasser et al., 1998). However, in our study, tea did not reduce the absorption of iron from a single meal compared with water (P=0.203), and our results suggest that silybin is more effective at limiting the post-prandial increase in serum iron compared with tea (P<0.05). The calculated ascorbic acid content of the test meal was 124 mg (Holland et al., 1991). Ascorbic acid is a powerful promoter of the absorption of non-haem iron (Cook and Monsen, 1977), and its presence in a meal has been shown to counteract the iron-binding effect of tea polyphenols (Hurrell et al, 1999); indeed, this may have been the case in this study. In contrast, silybin resulted in a significant reduction in the amount of iron absorbed from a single meal, even in the presence of ascorbic acid. A published study reported that the iron-chelating properties of silybin were responsible for a decrease in body iron stores in patients with chronic hepatitis C who had been taking silybin orally for 12 weeks (Bares et al., 2008), which supports the outcome of our study. Likewise, silybin in conjunction with desferrioxamine may be a more effective means of reducing iron stores in patients with β-thalassaemia, compared with desferrioxamine alone (Gharagozloo et al., 2009). In conclusion, our findings suggest that in HH patients 140 mg silybin taken as a single dose with a meal reduces the absorption of non-haem iron. The small number of patients is a limiting factor and a type I statistical error cannot be ruled out. Nevertheless, this is an interesting preliminary finding that deserves further investigation with a larger group of HH patients, as silybin could be a useful adjunct in the treatment of HH.
Figure 1.
Serum iron curves in HH patients (n 10) following ingestion of a test meal containing 13.1 mg non-haem iron with water (-■-), tea (-○-) or Legalon Forte ® 140 mg (-▲-).Consumption of silybin with a meal resulted in a reduction in the postprandial increase in serum iron, compared with water (Legalon Forte ® 140 mg versus water, P = 0.037) and tea (Legalon Forte ® 140 mg versus tea, P = 0.037). Tea did not reduce the absorption of iron from a single meal compared with water (tea versus water, P = 0.203).
Values are means ± SEM.
Acknowledgements
We thank Janet Fernau and Kit Farrow for inviting members of the Haemochromatosis Society to participate in this study. We are grateful to the Haemochromatosis Society for providing funding for this study, and to King’s College London for providing sponsorship. We also thank Dr Ulrich Mengs for donating Legalon® Forte 140 mg capsules on behalf of Madaus GmbH, Germany, and laboratory staff at the Blood Sciences Laboratory (King’s College Hospital, London) for measuring full blood count and serum ferritin.
Support. We gratefully acknowledge funding from The Haemochromatosis Society. We also thank Dr Ulrich Mengs for donating Legalon® Forte 140 mg capsules on behalf of Madaus GmbH, Germany.
Footnotes
Conflict of Interest The authors do not have financial conflicts of interest of any kind, nor have personal relationships with other people or institutions that could inappropriately influence our work.
References
- Bares JM, Berger J, Nelson JE, Messner DJ, Schildt S, Standish LJ, Kowdley KV. Silybin treatment is associated with reductionin serum ferritin in patients with chronic hepatitis C. J Clin Gastroenterol. 2008;42:937–944. doi: 10.1097/MCG.0b013e31815cff36. [DOI] [PubMed] [Google Scholar]
- Borsari M, Gabbi C, Ghelfi F, Grandi R, Saladini M, Severi S, et al. Silybin, a new iron-chelating agent. J Inorg Biochem. 2001;85:123–9. doi: 10.1016/s0162-0134(01)00198-2. [DOI] [PubMed] [Google Scholar]
- Conway RE, Geissler CA, Hider RC, Thompson RPH, Powell JJ. Measurement of dietary iron bioavailability using serum iron curves. J Nutr. 2006;136:1910–4. doi: 10.1093/jn/136.7.1910. [DOI] [PubMed] [Google Scholar]
- Cook JD, Monsen ER. Vitamin C, the common cold and iron absorption. American Journal of Clinical Nutrition. 1977;30:235–241. doi: 10.1093/ajcn/30.2.235. [DOI] [PubMed] [Google Scholar]
- Holland B, Welch AA, Unwin ID, Buss DH, Paul AA, Southgate DAT. McCance and Widdowson’s The Composition of Foods. fifth edition The Royal Society of Chemistry and Ministry of Agriculture, Fisheries and Foods; London: 1991. p. 462. [Google Scholar]
- Hoppe M, Hulthen L, Hallberg L. The validation of using serum iron increase to measure iron absorption in human subjects. Br J Nutr. 2004;92:485–488. doi: 10.1079/bjn20041207. [DOI] [PubMed] [Google Scholar]
- Hurrell RF, Reddy M, Cook JD. Inhibition of non-haem iron absorption in man by polyphenolic-containing beverages. The British Journal of Nutrition. 1999;81:289–295. [PubMed] [Google Scholar]
- Hutchinson C, Geissler CA, Powell JJ, Bomford A. Proton pump inhibitors suppress absorption of dietary non-haem iron in hereditary haemochromatosis. Gut. 2007;56:1291–5. doi: 10.1136/gut.2006.108613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharagozloo M, Moayedi B, Zakerinia M, Hamidi M, Karimi M, Maracy M, Amirghofran Z. Combined therapy of silymarin and desferrioxamine in patients with β-thalassaemia major: a randomised double-blind clinical trial. Fundamental and Clinical Pharmacology. 2009;23:359–365. doi: 10.1111/j.1472-8206.2009.00681.x. [DOI] [PubMed] [Google Scholar]
- Kaltwasser JP, Werner E, Schalk K, Hansen C, Gottschalk R, Seidl C. Clinical trial on the effect of regular tea drinking on iron accumulation in genetic haemochromatosis. Gut. 1998;43:699–704. doi: 10.1136/gut.43.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]