Abstract
OBJECTIVE
Emerging data implicate activation of the complement cascade in the pathogenesis of type 2 diabetes. The objective of the current study was to evaluate the relationships between components of the complement system, metabolic risk factors, and family history of type 2 diabetes in healthy South Asians.
RESEARCH DESIGN AND METHODS
We recruited 119 healthy, first-degree relatives of South Asian subjects with type 2 diabetes (SARs) and 119 age- and sex-matched, healthy South Asian control subjects (SACs). Fasting blood samples were taken for measurement of complement factors and standard metabolic risk factors.
RESULTS
SARs were characterized by significantly higher properdin (mean concentration 12.6 [95% CI 12.2–13.1] mg/L vs. SACs 10.1 [9.7–10.5] mg/L, P < 0.0001), factor B (187.4 [180.1–195.0] mg/L vs. SACs 165.0 [158.0–172.2] mg/L, P < 0.0001), and SC5b-9 (92.0 [86.1–98.3] ng/mL vs. SACs 75.3 [71.9–78.9] ng/mL, P < 0.0001) and increased homeostasis model assessment of insulin resistance (2.86 [2.61–3.13] vs. SACs 2.31 [2.05–2.61], P = 0.007). C-reactive protein did not differ between SARs and SACs (P = 0.17). In subgroup analysis of 25 SARs and 25 SACs with normal oral glucose tolerance tests, properdin, factor B, and SC5b-9 remained significantly elevated in SARs.
CONCLUSIONS
Increased properdin and complement activation are associated with a family history of type 2 diabetes in South Asians independent of insulin resistance, and predate the development of impaired fasting glucose and impaired glucose tolerance. Properdin and SC5b-9 may be novel biomarkers for future risk of type 2 diabetes in this high-risk population and warrant further investigation.
Clinical studies indicate that elevated plasma C3 is associated with type 2 diabetes, and C3 levels correlate with metabolic risk factors, including measures of obesity, insulin resistance, and dyslipidemia (1,2). A functional role for the complement system in the pathogenesis of insulin resistance and type 2 diabetes is supported by a variety of in vitro and in vivo studies suggesting pleiotropic effects of complement components on adipocyte, endothelial, and inflammatory cell function (3).
South Asians living in the U.K. are at particularly high risk for development of type 2 diabetes, which is partly attributed to increased insulin resistance and associated risk factor clustering (4,5). We and others have shown that C3 is elevated in healthy South Asian subjects compared with healthy Caucasian counterparts (6,7). In addition, Siezenga et al. (7) demonstrated increased SC5b-9 (a marker of complement activation) in healthy South Asians compared with Caucasians. These data therefore suggest that complement activation may contribute to the increased risk for type 2 diabetes in South Asians.
The first-degree relatives of patients with type 2 diabetes are at increased risk for the development of both type 2 diabetes and cardiovascular disease, and this is associated with clustering of insulin resistance and other metabolic risk factors (8,9). The relationship between complement activation and family history of type 2 diabetes in South Asians is unknown. The aim of the current study was, therefore, to evaluate the relationships between components of the complement system, metabolic risk factors, and family history of type 2 diabetes in healthy South Asians.
RESEARCH DESIGN AND METHODS
Subjects
First-degree relatives (n = 119; mother, father, or sibling) of South Asian subjects with confirmed type 2 diabetes (SARs) were enrolled into the study through the Diabetes Unit at the Leeds General Infirmary (St. James’s University Hospital, Leeds, U.K.) and the Bradford Royal Infirmary (Bradford, U.K.) as well as directly from within the community through local mosques, temples, and community centers. Only one relative for each patient with type 2 diabetes was recruited. A further 119 unrelated South Asian control subjects (SACs) who were free from a personal or family history of type 2 diabetes were recruited from the same local community networks. All participants were 18 years or older, of South Asian origin (India, Pakistan, or Bangladesh, with their four grandparents originating from one of these countries), and free from a personal history of diabetes (fasting plasma glucose <7.0 mmol/L and no antidiabetes therapy), malignancy, chronic inflammatory condition, or recent infection. Subjects with mixed parentage were excluded from the study. All subjects gave written informed consent according to a protocol approved by The United Leeds Teaching Hospitals Trust and Bradford Hospitals Trust research ethics committees.
Anthropometric and clinical risk factors
BMI was calculated from weight in kilograms divided by the square of height in meters. Waist-to-hip ratio (WHR) was calculated by taking the measurement (to the nearest 0.5 cm) at minimum abdominal girth divided by the measurement at maximal protrusion of the hips. Systolic and diastolic blood pressures were calculated from the mean of three separate readings measured to the nearest 2 mmHg with subjects in the sitting position after a 15-min period of rest. Subjects were classified as nonsmokers, ex-smokers, or current smokers.
Blood sampling
After a 10-h overnight fast, 50 mL of blood was taken from an antecubital vein without stasis using a 19-gauge needle. Blood was taken into lithium heparin on ice for assay of insulin; 0.109 mol/L trisodium citrate on ice for assay of C3, C-reactive protein (CRP), factor B, properdin, and factor H; and EDTA on ice for measurement of C3adesArg, SC5b-9, and Bb fragment. Samples were centrifuged at 2,560 g at 4°C for 30 min, and 0.5 mL aliquots of plasma were snap frozen in liquid nitrogen for storage at −80°C until assay. Blood was also collected into lithium fluoride for plasma glucose estimation, lithium heparin for lipid fraction analysis, and EDTA for glycosylated hemoglobin (HbA1c). A subgroup of participants (n = 33 controls; n = 48 relatives) also underwent a standard oral glucose tolerance test (OGTT).
Analysis of standard biochemical risk factors
Standard biochemical risk factors were analyzed by the Department of Chemical Pathology at the Leeds General Infirmary. Measurements of plasma glucose (by a glucose oxidase method), cholesterol, and triglyceride were made with a Hitachi 747 autoanalyzer (Boehringer Mannheim). HDL was measured by a Hitachi 717 autoanalyzer (Boehringer Mannheim) after removal of chylomicrons, LDL, and VLDL by precipitation with phosphotungstic acid and magnesium chloride. LDL was calculated by the Friedewald equation. HbA1c was measured by a Glycomat autoanalyzer (Ciba Corning). Subjects were classified into those with and without the metabolic syndrome (MetS), in accordance with the International Diabetes Federation definition, using ethnicity-specific cut points for waist circumference (≥90 cm in males and ≥80 cm in females) (10). Insulin resistance was estimated by use of the homeostasis model assessment of insulin resistance (HOMA-IR). Impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) were classified according to World Health Organization criteria.
Analysis of inflammatory factors
In-house enzyme-linked immunosorbent assays (ELISAs) were established using commercially available antibodies for the measurement of CRP (DAKO), C3 (DAKO), factor B (Quidel), properdin (AntibodyShop), and factor H (Quidel). Commercially available ELISA kits were used for the measurement of fragment Bb (Quidel), C3adesArg (Quidel), SC5b-9 (Quidel), and fasting insulin (BioSource). The interassay coefficients of variation were <10% and the intra-assay coefficients of variation were <5% for each ELISA.
Statistical analysis
C3, factor H, waist circumference, and LDL were normally distributed; all other variables were log10 transformed to achieve normal distribution. Differences in normally distributed variables between groups were assessed by Student t test or one-way ANOVA, and data were presented as mean or geometric mean and 95% CIs. Differences in categorical data between groups were assessed by χ2 test. Partial correlation coefficients between complement components and other cardiovascular risk factors were determined after adjustment for age and sex. Linear regression analysis was carried out to identify independent predictors of individual complement components, and general linear model analysis was carried out to determine whether differences in complement components between relatives and control subjects were independent of covariates. Logistic regression analysis was used to identify factors independently associated with family history of diabetes. Statistical significance was taken as P < 0.05. All statistical analyses were performed using PASW Statistics 18 for Windows (SPSS Inc.).
RESULTS
Characteristics of SARs and SACs
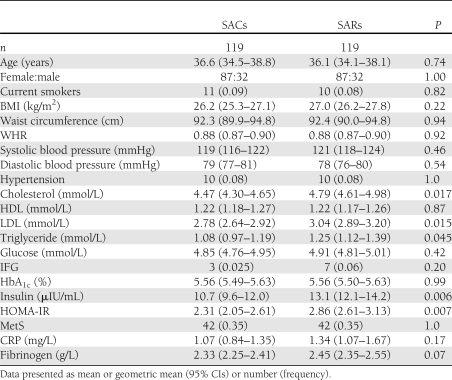
The characteristics of healthy South Asian subjects with and without a family history of type 2 diabetes are presented in Table 1. The SARs and SACs were closely matched for age, sex, and current smoking and had similar BMI, WHR, waist circumference, fasting glucose, IFG, HbA1c, and history of hypertension. Total cholesterol, LDL, triglycerides, insulin, and HOMA-IR were significantly higher in the relatives compared with control subjects, although there was no difference in the prevalence of the MetS between relatives and control subjects (Table 1). The acute phase reactants CRP and fibrinogen were not significantly different between relatives and control subjects (Table 1). There were no significant differences in drug therapies between the two groups (data not shown). In the subgroup of 33 SACs and 48 SARs who underwent OGTT, 5 SACs and 1 SAR had IGT (P = 0.027).
Table 1.
Clinical and biochemical characteristics of SARs and SACs
Relationships between complement components and family history of type 2 diabetes in healthy South Asians
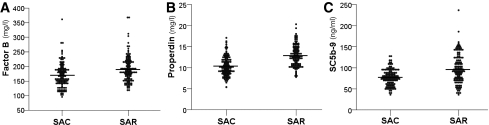
The alternative complement components factor B and properdin and the terminal complement activation component SC5b-9 were significantly higher in SARs compared with SACs, as shown in Fig. 1. In contrast, no significant differences in C3 (SACs 1.23 [95% CI 1.19–1.28] g/L vs. SARs 1.26 [1.21–1.31] g/L, P = 0.40), Bb (SACs 0.73 [0.70–0.76] μg/mL vs. SARs 0.70 [0.67–0.73] μg/mL, P = 0.17), C3adesArg (SACs 121.9 [111.4–133.3] ng/mL vs. SARs 109.3 [101.3–117.9] ng/mL, P = 0.071), or factor H (SACs 214.8 [205.9–223.7] μg/mL vs. SARs 219.9 [210.0–229.7] μg/mL, P = 0.46) were identified.
Figure 1.
Factor B, properdin, and SC5b-9 in SARs of patients with type 2 diabetes and SACs. Factor B (A), properdin (B), and SC5b-9 (C) were significantly higher in SARs compared with SACs. P < 0.0001 for each comparison.
In logistic regression analysis, the factors independently associated with a family history of type 2 diabetes were properdin (odds ratio [OR] for one SD increase in log10properdin 5.26 [95% CI 3.02–9.14], P < 0.0001), SC5b-9 (OR for one SD increase in log10SC5b-9 2.39 [1.55–3.68], P < 0.0001), factor B (OR for one SD increase in log10factorB 1.75 [1.06–2.89], P = 0.030), HOMA-IR (OR for one SD increase in log10HOMA-IR 1.59 [1.04–2.45], P = 0.034), C3adesArg (OR for one SD increase in log10C3adesArg 0.62 [0.40–0.88], P = 0.041), and C3 (OR for one SD increase in C3 0.50 [0.25–0.99], P = 0.047).
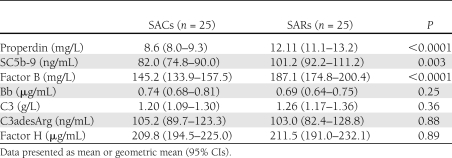
To exclude the potential impact of IFG and IGT, we evaluated a subgroup of 25 SACs and SARs with normal glucose tolerance on OGTT. The characteristics of this subgroup of SACs and SARs are presented in Supplementary Table 1 and were similar to the characteristics of the whole study population. In this subgroup, properdin, SC5b-9, and factor B were significantly higher in SARs compared with SACs, whereas C3, C3adesArg, Bb, and factor H were not significantly different between the groups, as shown in Table 2.
Table 2.
Complement components in a subgroup of SARs and SACs with normal glucose tolerance
Relationships between complement components and metabolic risk factors in South Asians
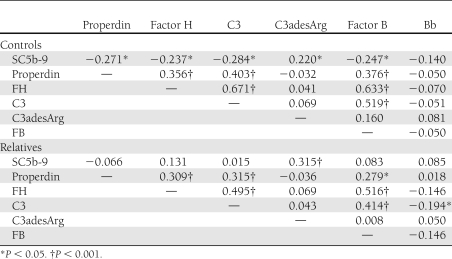
The age- and sex-adjusted correlations between complement components are presented in Table 3. C3, factor B, properdin, and factor H were all significantly correlated with each other in SARs and SACs. For the complement activation markers, C3adesArg was only significantly correlated with SC5b-9 in both SARs and SACs. Factor Bb was significantly inversely correlated with C3 in SARs only, and SC5b-9 was significantly inversely correlated with properdin, factor H, C3, and factor B only in SACs. Complement components were correlated with a variety of cardiometabolic risk factors, as shown in Supplementary Table 2.
Table 3.
Age- and sex-adjusted correlation coefficients between complement components in SARs of subjects with type 2 diabetes and healthy SACs
In linear regression analyses adjusting for age and sex, independent predictors of properdin in SACs were C3, factor B, and HOMA-IR (accounting for 15.9, 3.9, and 3.0% of the variance, respectively), and in SARs were BMI, HOMA-IR, cholesterol, and CRP (14.8, 5.1, 3.9, and 3.3% of the variance, respectively). Independent predictors of SC5b-9 in SACs were C3 and C3adesArg (7.5 and 5.2% of the variance, respectively), and in SARs were C3adesArg and triglyceride (8.8 and 3.0% of the variance, respectively). Independent predictors of factor B in SACs were factor H, CRP, and cholesterol (39.7, 10.5, and 4.1% of the variance, respectively), and in SARs were CRP and factor H (26.4 and 8.3% of the variance, respectively). Age and sex were the only determinants of Bb in SACs (5.4% of the variance), and in SARs, HOMA-IR was the only independent determinant of Bb (10.9% of the variance). Independent predictors of C3 in SACs were factor H, CRP, cholesterol, HOMA-IR, and properdin (45.5, 6.0, 4.0, 2.3, and 1.6% of the variance, respectively), and in SARs were factor H, triglyceride, and CRP (25.1, 12.4, and 4.2% of variance, respectively). Independent determinants of C3adesArg in SACs were hypertension, SC5b-9, and CRP (10.3, 3.7, and 3.3% of the variance, respectively). In SARs, independent determinants were SC5b-9 and CRP (9.0 and 4.3% of variance, respectively). Independent determinants of factor H in SACs were C3, factor B, and BMI (44.1, 10.9, and 1.8% of the variance, respectively), and in SARs were factor B, C3, and CRP (25.5, 9.2, and 3.6% of variance, respectively).
We evaluated the relationships between inflammatory factors, HOMA-IR, and MetS, as an indicator of risk factor clustering. As shown in Supplementary Table 3, there were no significant differences in complement factors between SACs with MetS compared with SACs without MetS. Properdin and C3 were the only complement factors that were significantly higher in SARs with MetS compared with SARs without MetS.
CONCLUSIONS
Insulin resistance is prevalent in South Asians and contributes to increased risk for type 2 diabetes. A role for complement-mediated inflammation is supported by increased C3 and SC5b-9 in South Asians compared with Caucasians (6,7), and may suggest a genetic predisposition. In the current study, SARs had significantly higher insulin and HOMA-IR and displayed dyslipidemia compared with SACs, consistent with previous studies in Caucasians (9,11). SARs and SACs were well matched for age, sex, measures of adiposity, smoking, and prescribed drugs, suggesting these factors are unlikely to account for observed differences.
The associations between elevated properdin, factor B, and SC5b-9 and family history of type 2 diabetes, independent of insulin resistance, represent the key novel findings of the current study. Properdin, factor B, and SC5b-9 were also significantly higher in SARs in a matched subgroup with normal glucose tolerance on OGTT, suggesting these complement factors are elevated preceding the development of IFG and IGT. Factor B, properdin, and SC5b-9 may therefore be specifically related to the processes underpinning development of type 2 diabetes rather than reflecting more generalized inflammatory and metabolic processes.
The results of the current study are consistent with those of Peake et al. (12), who found elevated factor B, but similar Bb, in Caucasian relatives of patients with type 2 diabetes. Factor B is essential for alternative C3 convertase formation (C3bBb) (13) and is an acute phase reactant synthesized by liver and adipose tissue (14). Elevated levels in SARs could reflect generalized inflammation; however, this is not supported by the similarity in CRP, fibrinogen, and C3 between SARs and SACs, consistent with results in Caucasians (15). Plasma factor B is elevated in obesity and decreases with weight loss, suggesting adipose tissue contributes significantly to the plasma pool. However, given the similarity in adiposity between SARs and SACs, it seems unlikely that adiposity per se accounts for the increased factor B in SARs.
Properdin stabilizes the alternative C3 convertase (C3bBbP) and anchors it to activating surfaces, leading to enhanced C3 cleavage (16), suggesting that elevated properdin and factor B in SARs contribute to complement activation and the observed increase in SC5b-9. Plasma properdin mainly arises from secretion by bone marrow–derived cells, particularly cells of the myeloid lineage (17). A proportion of plasma properdin may also be derived from vascular endothelial cells, which synthesize and secrete properdin especially in response to turbulent flow (18). Increased properdin in SARs may therefore reflect ongoing in vivo activation of inflammatory and endothelial cells, leading to enhanced complement activation through stabilization of alternative C3 convertases. Complement activation ultimately leads to the formation of both the surface-bound C5b-9 and soluble SC5b-9 complexes, and increased SC5b-9 reflects ongoing in vivo complement activation. C3adesArg did not differ between SARs and SACs in the current study, inconsistent with previous studies (3). Since C3adesArg is closely associated with obesity, the similarity between SARs and SACs with respect to measures of adiposity may account for the lack of association. Furthermore, previous studies have suggested that C3adesArg is readily redistributed between intravascular and extravascular compartments and may be misleading as a measure of complement activation (12), whereas SC5b-9 has a longer half-life in vivo and is therefore considered to be a more reliable measure of in vivo complement activation (19).
SC5b-9 could theoretically arise via activation of any of the three complement activation pathways or through impaired inhibition of complement activation. The alternative pathway is essential for amplification of complement activation through the classical and mannose-binding lectin pathways, with the majority of SC5b-9 generated via alternative pathway amplification, as demonstrated by a >80% reduction in SC5b-9 by antifactor D and antiproperdin antibodies (20,21). Therefore, elevated properdin and factor B in SARs is consistent with a major contribution of alternative pathway amplification to the increased SC5b-9 in SARs. Factor H, the major fluid-phase inhibitor of alternative C3 convertases, did not differ between relatives and control subjects, suggesting impaired alternative pathway inhibition does not contribute significantly to increased SC5b-9. CD55 and CD59 are membrane-bound inhibitors of C3/C5 convertases and C5b-9 insertion into cell membranes, respectively, are decreased on peripheral blood leukocytes of patients with type 2 diabetes (22) and in retinal vessels of patients with diabetic retinopathy (23), and decrease in response to elevated glucose in vitro (24). These studies suggest that reduced CD55 and CD59 may arise as a consequence of hyperglycemia and lead to complement-induced vascular complications. In the current study we did not measure CD55 and CD59, therefore we cannot exclude that differences in the membrane-bound inhibitors of complement activation predate development of type 2 diabetes and contribute to increased SC5b-9.
Although the site(s) giving rise to increased factor B, properdin, and SC5b-9 in the current study is unknown, animal and in vitro studies suggest functional roles for complement activation in adipocyte triglyceride synthesis and glucose uptake, macrophage infiltration into adipose tissue, insulin resistance, systemic inflammation, and development and progression of atherosclerosis (25,26). Obesity and obesogenic lifestyle factors, such as diet and exercise, contribute to metabolic and inflammatory abnormalities and oxidative stress leading to insulin resistance and types 2 diabetes. Although SARs and SACs were similar with respect to indirect measures of adiposity, the obesity measures used in the current study do not adequately differentiate between visceral and subcutaneous fat or potential interindividual differences in proinflammatory phenotype, including macrophage infiltration into adipose tissue and ectopic fat deposition in liver and skeletal muscle. Although speculative, it is possible that SARs are characterized by an adverse adipose tissue phenotype, ectopic fat deposition, and systemic inflammation, leading to increased factor B and properdin secretion, complement activation on activated leukocyte and endothelial cell surfaces, and generation of C5b-9. A variety of nonlytic actions of C5b-9 on nucleated cells have been described that may contribute to the development of insulin resistance, including the generation of reactive oxygen species, increased secretion of proinflammatory factors, including interleukin-6, tumor necrosis factor-α, and monocyte chemoattractant protein-1 (27), and increased IGF-1 and IGF receptor expression (28). Therefore, complement activation in SARs may occur in the vasculature and contribute directly to the development of insulin resistance rather than arising secondary to insulin resistance.
Limitations of the study
SACs may have a first-degree relative with undiagnosed diabetes; however, this would be expected to underestimate the relationship between complement factors and family history. No study participant had a known history of myocardial infarction; however, no electrocardiogram was carried out to exclude undiagnosed cardiovascular disease. Since complement factors were not consistently associated with conventional metabolic risk factors or MetS, it seems unlikely that complement factors are elevated secondary to clustering of classical cardiometabolic risk factors. As not all participants agreed to an OGTT, we are unable to exclude IGT in these individuals. However, similar results were obtained in a subgroup analysis of 25 SACs and 25 SARs with normal glucose tolerance on OGTT, suggesting it is unlikely that complement factors are elevated secondary to IFG/IGT.
Summary
The results of the current study indicate that elevated properdin, factor B, and SC5b-9 are associated with family history of type 2 diabetes independent of insulin resistance, IFG, IGT, and classical metabolic risk factor clustering. Increased properdin, factor B, and SC5b-9 levels may therefore predate the development of type 2 diabetes and represent novel biomarkers in South Asians with a family history of type 2 diabetes. These data add to a growing body of evidence supporting a role for complement activation in the metabolic and inflammatory processes underpinning the development of type 2 diabetes, and further studies are warranted to investigate the genetic and environmental determinants of complement activation in South Asians.
Acknowledgments
R.S. was supported by a British Heart Foundation clinical PhD studentship (FS/03/056). V.R.R. is supported by a Medical Research Council DTA PhD studentship.
No potential conflicts of interest relevant to this article were reported.
R.S. and A.M.C. researched the data, contributed to discussion, and cowrote the manuscript. V.R.R., K.F.S., and P.J.G. researched the data, contributed to discussion, and reviewed and edited the manuscript. A.M.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this work were previously presented in abstract form at the 13th European Meeting on Complement in Human Disease, Leiden, the Netherlands, 21–24 August 2011; the XXIII Congress of the International Society on Thrombosis and Haemostasis, Kyoto, Japan, 23–28 July 2011; and the 3rd European Meeting on Vascular Biology and Medicine, Hamburg, Germany, 28–30 September 2005.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1483/-/DC1.
References
- 1.Muscari A, Massarelli G, Bastagli L, et al. Relationship of serum C3 to fasting insulin, risk factors and previous ischaemic events in middle-aged men. Eur Heart J 2000;21:1081–1090 [DOI] [PubMed] [Google Scholar]
- 2.Engström G, Hedblad B, Eriksson KF, Janzon L, Lindgärde F. Complement C3 is a risk factor for the development of diabetes: a population-based cohort study. Diabetes 2005;54:570–575 [DOI] [PubMed] [Google Scholar]
- 3.Cianflone K, Xia Z, Chen LY. Critical review of acylation-stimulating protein physiology in humans and rodents. Biochim Biophys Acta 2003;1609:127–143 [DOI] [PubMed] [Google Scholar]
- 4.Nishtar S. Prevention of coronary heart disease in south Asia. Lancet 2002;360:1015–1018 [DOI] [PubMed] [Google Scholar]
- 5.Murphy C, Kanaganayagam GS, Jiang B, et al. Vascular dysfunction and reduced circulating endothelial progenitor cells in young healthy UK South Asian men. Arterioscler Thromb Vasc Biol 2007;27:936–942 [DOI] [PubMed] [Google Scholar]
- 6.Somani R, Grant PJ, Kain K, Catto AJ, Carter AM. Complement C3 and C-reactive protein are elevated in South Asians independent of a family history of stroke. Stroke 2006;37:2001–2006 [DOI] [PubMed] [Google Scholar]
- 7.Siezenga MA, Chandie Shaw PK, van der Geest RN, et al. Enhanced complement activation is part of the unfavourable cardiovascular risk profile in South Asians. Clin Exp Immunol 2009;157:98–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haffner SM, Stern MP, Hazuda HP, Mitchell BD, Patterson JK. Increased insulin concentrations in nondiabetic offspring of diabetic parents. N Engl J Med 1988;319:1297–1301 [DOI] [PubMed] [Google Scholar]
- 9.Mansfield MW, Heywood DM, Grant PJ. Circulating levels of factor VII, fibrinogen, and von Willebrand factor and features of insulin resistance in first-degree relatives of patients with NIDDM. Circulation 1996;94:2171–2176 [DOI] [PubMed] [Google Scholar]
- 10.Alberti KG, Zimmet P, Shaw J; IDF Epidemiology Task Force Consensus Group The metabolic syndrome—a new worldwide definition. Lancet 2005;366:1059–1062 [DOI] [PubMed] [Google Scholar]
- 11.Laws A, Stefanick ML, Reaven GM. Insulin resistance and hypertriglyceridemia in nondiabetic relatives of patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1989;69:343–347 [DOI] [PubMed] [Google Scholar]
- 12.Peake PW, Kriketos AD, Campbell LV, Charlesworth JA. Response of the alternative complement pathway to an oral fat load in first-degree relatives of subjects with type II diabetes. Int J Obes (Lond) 2005;29:429–435 [DOI] [PubMed] [Google Scholar]
- 13.Peake PW, O’Grady S, Pussell BA, Charlesworth JA. Detection and quantification of the control proteins of the alternative pathway of complement in 3T3-L1 adipocytes. Eur J Clin Invest 1997;27:922–927 [DOI] [PubMed] [Google Scholar]
- 14.Cianflone K, Maslowska M. Differentiation-induced production of ASP in human adipocytes. Eur J Clin Invest 1995;25:817–825 [DOI] [PubMed] [Google Scholar]
- 15.Kriketos AD, Greenfield JR, Peake PW, et al. Inflammation, insulin resistance, and adiposity: a study of first-degree relatives of type 2 diabetic subjects. Diabetes Care 2004;27:2033–2040 [DOI] [PubMed] [Google Scholar]
- 16.Kemper C, Atkinson JP, Hourcade DE. Properdin: emerging roles of a pattern-recognition molecule. Annu Rev Immunol 2010;28:131–155 [DOI] [PubMed] [Google Scholar]
- 17.Ishigami T, Umemura S, Iwamoto T, et al. Molecular variant of angiotensinogen gene is associated with coronary atherosclerosis. Circulation 1995;91:951–954 [DOI] [PubMed] [Google Scholar]
- 18.Bongrazio M, Pries AR, Zakrzewicz A. The endothelium as physiological source of properdin: role of wall shear stress. Mol Immunol 2003;39:669–675 [DOI] [PubMed] [Google Scholar]
- 19.Würzner R. Immunochemical measurement of complement components and activation products. Methods Mol Biol 2000;150:103–112 [DOI] [PubMed] [Google Scholar]
- 20.Harboe M, Ulvund G, Vien L, Fung M, Mollnes TE. The quantitative role of alternative pathway amplification in classical pathway induced terminal complement activation. Clin Exp Immunol 2004;138:439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harboe M, Garred P, Karlstrøm E, Lindstad JK, Stahl GL, Mollnes TE. The down-stream effects of mannan-induced lectin complement pathway activation depend quantitatively on alternative pathway amplification. Mol Immunol 2009;47:373–380 [DOI] [PubMed] [Google Scholar]
- 22.Ma XW, Chang ZW, Qin MZ, Sun Y, Huang HL, He Y. Decreased expression of complement regulatory proteins, CD55 and CD59, on peripheral blood leucocytes in patients with type 2 diabetes and macrovascular diseases. Chin Med J (Engl) 2009;122:2123–2128 [PubMed] [Google Scholar]
- 23.Zhang J, Gerhardinger C, Lorenzi M. Early complement activation and decreased levels of glycosylphosphatidylinositol-anchored complement inhibitors in human and experimental diabetic retinopathy. Diabetes 2002;51:3499–3504 [DOI] [PubMed] [Google Scholar]
- 24.Accardo-Palumbo A, Triolo G, Colonna-Romano G, et al. Glucose-induced loss of glycosyl-phosphatidylinositol-anchored membrane regulators of complement activation (CD59, CD55) by in vitro cultured human umbilical vein endothelial cells. Diabetologia 2000;43:1039–1047 [DOI] [PubMed] [Google Scholar]
- 25.Mamane Y, Chung Chan C, Lavallee G, et al. The C3a anaphylatoxin receptor is a key mediator of insulin resistance and functions by modulating adipose tissue macrophage infiltration and activation. Diabetes 2009;58:2006–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu G, Hu W, Shahsafaei A, et al. Complement regulator CD59 protects against atherosclerosis by restricting the formation of complement membrane attack complex. Circ Res 2009;104:550–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cole DS, Morgan BP. Beyond lysis: how complement influences cell fate. Clin Sci (Lond) 2003;104:455–466 [DOI] [PubMed] [Google Scholar]
- 28.Zwaka TP, Torzewski J, Hoeflich A, et al. The terminal complement complex inhibits apoptosis in vascular smooth muscle cells by activating an autocrine IGF-1 loop. FASEB J 2003;17:1346–1348 [DOI] [PubMed] [Google Scholar]