Abstract
OBJECTIVE
The activation of the complement system may be involved in the pathology of myocardial infarction (MI) and type 2 diabetes. To explore their potential as prognostic markers, we characterized two factors in the complement cascade, the end product sC5b-9 and the mannose-binding lectin–associated Ser protease-2 (MASP-2), in type 2 diabetic patients with suspected MI.
RESEARCH DESIGN AND METHODS
Plasma sC5b-9 and MASP-2 were determined in patients with MI and type 2 diabetes (n = 397; median age 70; male 68%). The adjudicated end points were cardiovascular events (CVEs), including cardiovascular mortality and nonfatal MI or stroke.
RESULTS
The median sC5b-9 was 134 μg/L (interquartile range [IQR] 101–190 μg/L) and the median MASP-2 was 333 μg/L (IQR 235–463 μg/L), with no significant correlation between them. Women had higher sC5b-9 than men (median 152 vs. 130 μg/L; P = 0.02). Both sC5b-9 and MASP-2 were correlated to age and creatinine clearance, while MASP-2 was also correlated to BMI. During a median follow-up of 2.4 years, CVEs occurred in 141 patients (36%). Both sC5b-9 (hazard ratio 1.37 [95% CI 1.13–1.65]; P < 0.01) and MASP-2 (0.68 [0.51–0.92]; P = 0.01) predicted CVEs in unadjusted analyses. After multiple adjustments, the predictive capacity remained for sC5b-9 (1.30 [1.02–1.66]; P = 0.04) but not for MASP-2.
CONCLUSIONS
In type 2 diabetic patients with MI, high levels of sC5b-9 predict future CVE. This indicates that the complement system may play a significant role in the pathology of the subsequent myocardial damage and that the pathways leading to complement activation warrant further exploration as potential therapeutic targets to improve the prognosis for these patients.
Patients with diabetes are prone to develop cardiovascular complications, and despite therapeutic improvements, the presence of glucose abnormalities is associated with a poorer outcome in patients with coronary artery disease (1,2). The activation of inflammatory processes triggers several factors contributing to the poor prognosis (3,4).
The complement system is part of the innate immune system and a central feature of inflammation. The activation of the complement cascade helps to clear invading pathogens, but it may also produce negative cardiovascular effects, including atherosclerosis and the aggravation of ischemia/reperfusion injury (4–6). The role of complement activation in diabetes and cardiovascular disease has not been fully explored.
Mannose-binding lectin (MBL) is a pattern-recognition molecule that activates the complement cascade (4). The information relating to a potential link between MBL and cardiovascular complications in patients with and without diabetes is contradictory. In a substudy of the Diabetes Mellitus Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI 2) trial, the combination of a low-coding MBL genotype with a low serum MBL level appeared prognostically unfavorable. This association, however, was blunted by traditional risk factors (7). Together with the three ficolins (H-ficolin, L-ficolin, and M-ficolin), MBL is an upstream activator of the so-called lectin pathway of the complement cascade, and the degree of complement activation may not be directly correlated to measurable amounts of MBL alone. When MBLs or ficolins recognize a fitting pattern on, for example, a microorganism, they are all able to activate the enzyme MBL-associated Ser protease 2 (MASP-2), subsequently initiating the rest of the cascade (6). In the present report, we analyze the levels of MASP-2 in patient samples because this could possibly reflect the ability of all four proteins to initiate complement activation via the lectin pathway.
A potentially more effective way to evaluate the actual degree of complement activation is to measure downstream factors in the complement cascade, such as sC5b-9, the soluble form of the membrane attack complex, which is formed by a number of activated complement factors.
The current study was thus performed with the aim of characterizing sC5b-9 and its relationship to two factors in the lectin pathway of complement activation, MASP-2 and MBL, in patients with type 2 diabetes and myocardial infarction (MI). A secondary aim was to test the hypothesis that sC5b-9 and/or MASP-2 may be useful as prognostic markers in these patients.
RESEARCH DESIGN AND METHODS
DIGAMI 2 compared three different management strategies in 1,253 patients with type 2 diabetes and a suspected acute MI (AMI) (Supplementary Fig. 1). An extensive description of the study has been presented elsewhere (8). There was no significant difference in total mortality or nonfatal MI and stroke between the treatment arms during a median follow-up period of 2.1 years. The present cohort consists of 397 patients participating in a biochemistry substudy of DIGAMI 2 (Supplementary Fig. 1). Assessments of plasma levels of sC5b-9, MASP-2, and MBL were made from samples acquired as soon as possible after hospital admission and 3 months later.
Investigations
In addition to the samples described above, serum creatinine, blood lipids, glucose (mmol/L in whole blood), and HbA1c (upper limit 5.3%) were determined at hospital admission. Creatinine clearance (mL/min) was calculated using the Cockcroft-Gault formula.
Plasma sC5b-9 was analyzed using a novel, sensitive, in-house, time-resolved immunofluorometric assay (9). The limit of detection for the sC5b-9 assay was 1 μg/L. The intra- and interassay coefficients of variation (CVs) were <5 and 12%, respectively. The MASP-2 concentration in plasma was measured using an in-house sandwich assay described in detail elsewhere (10). The limit of detection was 1.6 μg/L, and the intra- and interassay CVs were <10%. As presented previously, the MBL concentration was measured using an in-house time-resolved immunofluorometric assay based on the recognition of a physiologically relevant ligand combined with detection by a specific monoclonal antibody (11).
End points
A composite of time to the first event of cardiovascular death or nonfatal reinfarction or stroke, adjudicated by an independent committee composed of experienced cardiologists, served as the primary end point (8). Secondary end points included all-cause mortality and nonfatal and fatal components of the primary end point analyzed separately.
Statistical methods
Continuous variables are presented as the median and interquartile ranges (IQRs), unless otherwise stated. The Kruskal-Wallis test was used to study differences between groups of patients stratified by dichotomous variables (sex, previous MI, heart failure, and hypertension), while the association between continuous variables (age, BMI, creatinine clearance, B-glucose, and HbA1c at admission) and different parts of the complement system was assessed by means of Spearman rank correlation test (correlation coefficient = rs).
The relationship between sC5b-9, MASP-2, and end points was assessed using Cox proportional hazards regression model to be presented as hazard ratios (HRs) and CIs. Because of a skewed distribution, sC5b-9 and MASP-2 levels were log transformed prior to analysis. Cox proportional hazards regression models were adjusted for age, sex, BMI, previous MI, heart failure, hypertension, creatinine clearance, B-glucose, and HbA1c at admission. These variables were selected because they are known predictors of outcome in the DIGAMI 2 trial or are of clinical importance in the present setting.
Model building using a best subset selection criterion for sC5b-9 and MASP-2 was performed for the primary and secondary end points using the same covariates.
For outcome analyses, sC5b-9 was divided into tertiles. In addition, a separate analysis was performed; it focused on whether the respective levels increased or decreased/remained stable between admission and 3 months. To illustrate time trends for cardiovascular outcome, Kaplan-Meier curves were drawn using sC5b-9 tertiles as strata. A log-rank test for trend was applied to assess differences in event patterns.
Two-tailed statistical tests were used at the 5% significance level. SAS version 9.2 was used for all statistical analyses.
Ethical considerations
The study followed the recommendations of the Helsinki Declaration, and local ethics review boards approved the protocol. Written informed consent was obtained from all patients prior to enrolment.
RESULTS
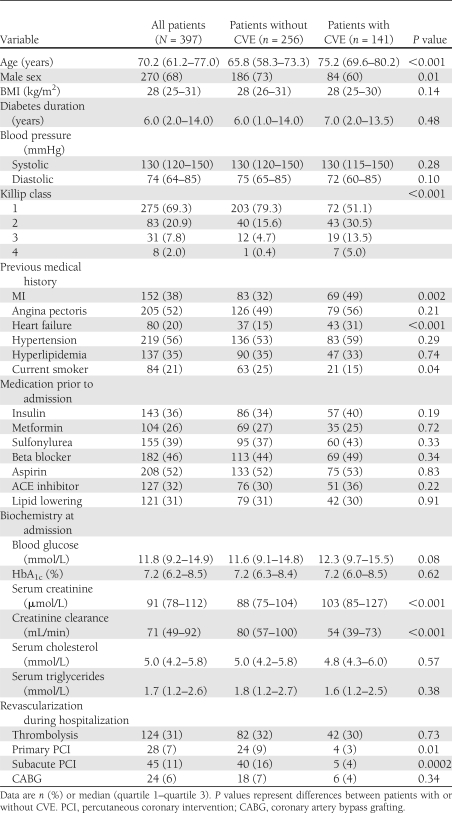
The baseline characteristics of the 397 patients are presented in Table 1.
Table 1.
Baseline characteristics and revascularization procedures during hospitalization for the total cohort of patients with sC5b-9, MASP-2, or MBL levels available at admission and with or without CVEs
The levels of sC5b-9 at admission (n = 391) varied between 17 and 6,944 μg/L (median 134 μg/L, mean 241 μg/L, IQR 101–190 μg/L) and after 3 months (in patients with both admission and 3-month levels measured, n = 298), between 27 and 379 μg/L (median 123 μg/L, mean 129 μg/L, IQR 97–155 μg/L). MASP-2 at admission (n = 387) varied between 50 and 1,356 μg/L (median 333 μg/L, mean 365 μg/L, IQR 235–463 μg/L) and after 3 months (n = 294 patients), between 50 and 1,076 μg/L (median 300 μg/L, mean 322 μg/L, IQR 209–401 μg/L). The levels at admission and after 3 months correlated for all three assessed components of the complement system (sC5b-9: Spearman rank correlation [rs] = 0.56, P < 0.001; MASP-2: rs = 0.78, P < 0.001; and MBL: rs = 0.93, P < 0.001).
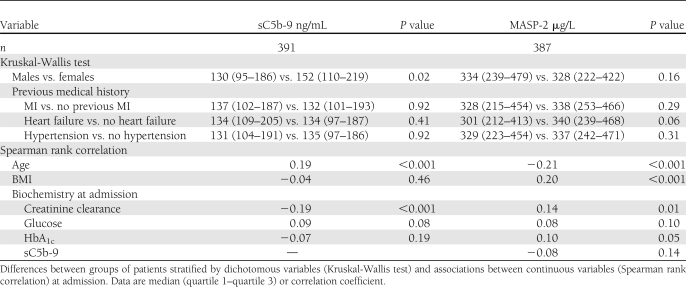
As presented in Table 2, women had significantly higher sC5b-9 at admission than men (median 152 vs. 130 μg/L, respectively; P = 0.02), and there was a significant correlation between sC5b-9, age, and creatinine clearance. When sex differences were assessed separately, women were older than men (74.9 [66.2–80.6] vs. 67.4 [59.1–74.7] years, respectively; P < 0.001) and had a lower creatinine clearance (54.0 [39.0–80.3] vs. 76.9 [56.2–97.5] mL/min, respectively; P < 0.001).
Table 2.
sC5b-9 and MASP-2 levels
The levels of sC5b-9 did not differ between patients with or without a history of MI, heart failure, or hypertension, and sC5b-9 did not correlate to BMI, glucose, or HbA1c at admission.
The results of the Kruskal-Wallis test and Spearman rank correlation test for MASP-2 are outlined in Table 2. The MBL data have recently been presented elsewhere (7).
No significant correlations were found between admission sC5b-9 and MASP-2 or MBL (sC5b-9 and MASP-2: rs = −0.08, P = 0.14; sC5b-9 and MBL: rs = 0.01, P = 0.93).
Mortality and morbidity
During the follow-up period of 2.4 (1.02–3.00) years, 141 (36%) of the 397 patients reached the combined cardiovascular end point, 99 (25%) died (cardiovascular reasons = 81), 60 had a reinfarction, and 25 had a stroke.
sC5b-9
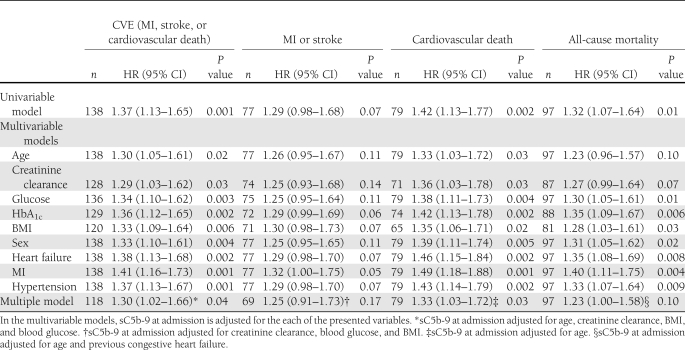
The predictive value of sC5b-9 for the composite end point, nonfatal and fatal components, and all-cause mortality is shown in Table 3. The levels of sC5b-9 at admission predicted cardiovascular events (CVEs) in unadjusted analyses (HR 1.37 [95% CI 1.13–1.65]; P = 0.001) and after univariable adjustments for all covariates. In a multiple Cox regression model adjusting for significant covariates using a best subset selection criterion, sC5b-9 remained as an independent predictor of CVEs (1.30 [1.02–1.66]; P = 0.04). A similar pattern was seen for cardiovascular death but not for the nonfatal subset of the primary end points (Table 3). The prediction of CVEs remained in separate analyses in which sC5b-9 was adjusted for MASP-2 (1.31 [1.08–1.60]; P = 0.007) and for MBL (1.38 [1.13–1.68]; P = 0.002).
Table 3.
Number of events and the predictive value of sC5b-9 at admission (Cox proportional hazards regression models) for respective event
Patients suffering a CVE had higher levels of sC5b9 at admission, 154 (110–219) μg/L vs. 129 (95–177) μg/L (P = 0.003).
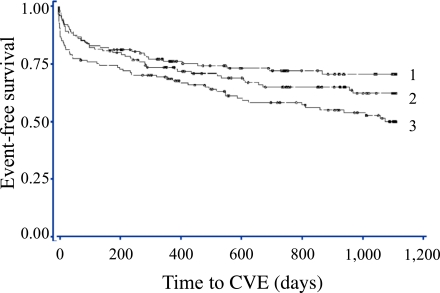
Patients in the highest sC5b-9 tertile ran an increased risk of a CVE (log-rank test P = 0.003) (Fig. 1). Similar findings, with the poorest outcome for patients with high sC5b-9 levels, were demonstrated for all separate end points, except stroke (data not shown).
Figure 1.
Tertiles of sC5b-9 at admission and their impact on CVEs. Log-rank test P = 0.003. 1 = median 85.5 (min 17, max 111) μg/L, 2 = median 134.0 (min 112, max 167) μg/L, 3 = median 232.5 (min 169, max 6,944) μg/L. (A high-quality color representation of this figure is available in the online issue.)
After the 3-month follow-up, the sC5b-9 levels had increased in 141 (47%) and remained stable or decreased in 53% of the patients. Increasing or decreasing sC5b-9 levels did not influence the prognosis (log-rank test for trend P = 0.85).
The levels of sC5b-9 measured in samples taken after 3 months did not predict CVEs (HR 0.81 [0.47–1.41]; P = 0.46). The prediction ability of sC5b-9 measured after 3 months was 0.85 (0.51–1.42; P = 0.53) when studied in all 417 patients with samples available at this time point.
MASP-2
Patients with a CVE had lower MASP-2 levels at admission, 308 (213–423) μg/L, than those without, 350 (243–477) μg/L (P = 0.01). MASP-2 at admission predicted CVEs in unadjusted analyses (HR 0.68 [95% CI 0.51–0.92]; P = 0.01) and after univariable adjustments for glucose, HbA1c, sex, heart failure, MI, and hypertension, but not after adjusting for age, creatinine clearance, or BMI (data not shown). In the final multiple model using a best subset selection criterion, the HR for MASP-2 at admission to predict the composite end point was 0.86 (0.62–1.20; P = 0.37). MASP-2 had no significant impact on the other end points apart from death in univariable analysis (0.67 [0.47–0.95]; P = 0.03). After 3 months, MASP-2 had a significant impact on CVEs (0.66 [0.45–0.98]; P = 0.04), and it remained after adjustments for glucose, HbA1c, BMI, or MI, but not after adjustments for age, creatinine clearance, sex, heart failure, or hypertension or in a multiple model (data not shown).
At admission, MBL did not predict events in the present patient cohort as previously presented (7).
CONCLUSIONS
The main finding was that high admission levels of sC5b-9 predict a poor cardiovascular outcome in patients with type 2 diabetes and MI. The other component of the complement system that was studied, MASP-2, did not independently predict outcome, but the results indicate that in contrast to sC5b-9, patients with low admission levels have a poorer prognosis. This suggests that the activation of the complement system is involved in the pathogenesis of ischemic heart disease in patients with diabetes.
The lectin pathway of the complement cascade is activated when MBL or the ficolins recognize a pathogen-associated molecular pattern of carbohydrates. In all four cases, this activates MASP-2, which in turn induces the cleavage of complement factors C4 and C2. The three pathways of complement activation, the classic, lectin, or alternative pathway, finally merge at the cleavage of C3, resulting in the main effects of complement activation: the recruitment of inflammatory cells, opsonization, and the formation of the C5b-9 complex (an association of C5b, C6, C7, C8, and multiple C9 molecules), also called the membrane attack complex (MAC). The insertion of MAC in membranes leads to swelling and the lysis of pathogens with subsequent cell death. During these processes, a significant amount of the soluble form of the MAC (sC5b-9) is formed in the absence of target membranes. C5b-9 binds to S-protein, which inhibits the membrane-damaging effect and creates a stable nonlytic soluble MAC form (sC5b-9).
The finding that high levels of sC5b-9 at admission predict the combined end point of CVEs and cardiovascular death, even after multiple adjustments, supports the notion that complement activation and subsequent inflammation play a role in cardiovascular disease.
The lack of association between the 3-month levels and CVEs indicates that high sC5b-9 levels during AMI, as a result of complement activation, are predictive of outcome rather than the constitutive level of sC5b-9. This is in line with observations from animal models, as well as clinical studies linking the activation of the complement system (e.g., triggered by ischemia/reperfusion) to the subsequent myocardial damage (4,12–16). Furthermore, complement activation is involved at an early stage of developing atherosclerosis, causing cell proliferation and the release of growth factors (5,17).
It is interesting to note that in contrast to sC5b-9, low levels of MASP-2 appeared to be associated with a poor cardiovascular prognosis, although this did not remain significant after adjustments. While MBL levels vary significantly from person to person based on genetic differences and sC5b-9 levels vary depending on the degree of complement activation, MASP-2 is instead a constitutive protein. As a result, variations in MASP-2 levels are relatively modest and may mirror liver function (18). MASP-2 levels in general are not believed to be the limiting factor in the activation of the lectin pathway (19), and a possible explanation of the association between low MASP-2 and poor outcome could be that liver function and thereby MASP-2 synthesis were lower in the patients who were most ill at admission.
It has been suggested, based primarily on findings in experimental models, that the complement system may play a dual role in the development of atherosclerosis and atherothrombotic events (5,6). Complement activation may act proatherogenically through inflammatory activation. In addition, activation by the alternative pathway and the C3 convertase, with the formation of anaphylatoxins and the MAC, may lead to the onset of acute CVEs by triggering plaque destabilization and rupture (5). On the other hand, the activation of the classical and lectin pathways may protect the vessels by removing immune complexes, cell debris, and apoptotic cells (6). In one of the early studies in this research area, Yasuda et al. (20) found that sC5b-9 in particular was related to myocardial damage in patients with AMI. The hypothesis that different parts of the complement system may play different roles in the setting of cardiovascular disease and diabetes obtains further support from this study, in which there was a lack of correlation between sC5b-9, MASP-2, and MBL.
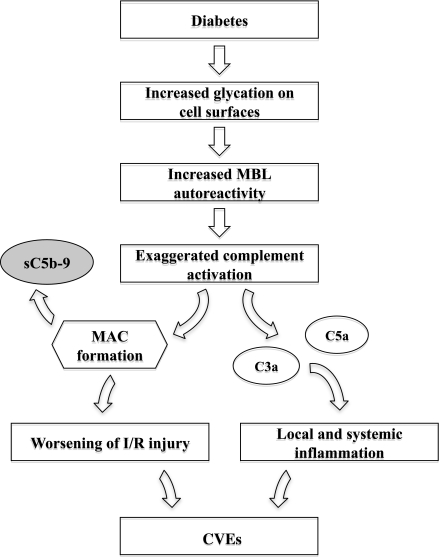
The plasma levels of sC5b-9 in the present cohort are higher (median 134 μg/L at admission) than those reported in a study comprising 193 patients with congestive heart failure (sC5b-9: 88 [69–128] μg/L) and healthy age-matched control subjects (sC5b-9: 98 [72–170] μg/L) (21). The high admission levels may, at least in part, be a result of the activation of the complement cascade due to acute ischemia. However, the sC5b-9 levels remained high after 3 months (median 124 μg/L), with a correlation to the admission levels (rs = 0.56; P < 0.001). In contrast to the expected high correlation between MBL levels at admission and after 3 months, this correlation cannot be explained by genetic factors but may indicate an ongoing increase in complement activation in patients with diabetes and ischemic heart disease. A schematic outline of a potential mechanism behind the involvement of complement activation in the development of CVEs in patients with diabetes and MI is depicted in Fig. 2. In fact, in the above-mentioned heart failure study, sC5b-9 levels were elevated in patients with congestive heart failure due to ischemic heart disease in contrast to patients with congestive heart failure of nonischemic etiology (P < 0.02) (21). Despite differing in absolute quantity (no international standard is available for standardization), the present findings fit with the report by Yasuda et al. (20) of significantly higher plasma sC5b-9 levels in patients with AMI (810 ± 80 μg/L [mean ± SEM]) than with unstable angina (150 ± 50 μg/L), stable angina (90 ± 60 μg/L), or healthy control subjects (60 ± 30 μg/L). The somewhat high 3-month levels in the present analyses indicate that not only the coronary artery disorder but also diabetes per se is associated with complement activation and inflammation. One explanation could be that some of the complement regulatory proteins are affected by glycation. CD59 has been shown to increase the deposition of sC5b-9 (22). In addition, Bjerre et al. (21) proposed a relationship between sC5b-9 and insulin resistance, suggesting that the latter leads to endothelial activation resulting in the activation of the complement system, thereby damaging the heart.
Figure 2.
A potential mechanism behind the involvement of complement activation in the development of CVEs in patients with diabetes and MI. I/R, ischemia/reperfusion.
Women had higher sC5b-9 at admission than men. One possible explanation is that they were older and had lower creatinine clearance, two factors that both correlate to sC5b9 levels. It is possible to speculate that the clearance of sC5b-9 is somewhat compromised in patients with reduced creatinine clearance and that this subsequently leads to higher plasma levels. A positive correlation between age and sC5b-9 and higher levels would then be expected among the women.
If the complement cascade is involved in the development of atherosclerosis and myocardial ischemic damage, not least in patients with diabetes, it is appealing to further explore this system as a possible pharmacological target. The use of agents blocking different parts of the complement cascade (anti-C5 antibody, C1-inhibitor, and soluble complement receptor 1) has been associated with reduced myocardial damage after ischemia and reperfusion in animal models and even in some clinical trials (4,23). The expectations that these drugs will reduce mortality or cardiovascular morbidity, however, have not been met in clinical trials, such as the Assessment of Pexelizumab in Acute Myocardial Infarction (APEX AMI) trial, which randomized 5,745 patients with acute ST-elevation MI to pexelizumab (an anti-C5a antibody) or placebo (24).
The results of the present analyses, with an apparently harmful effect of sC5b-9 and a possible beneficial effect of the lectin pathway, provide some indication of just how complex the impact of the complement system is on cardiovascular disorders and diabetes. It underlines the fact that different parts of the system may have different effects and that this should be taken into consideration when developing new drugs.
Limitations
This is an observational study, albeit prospectively planned, and it is thereby limited to the available subpopulation. For this reason, the findings should be regarded as hypotheses generating and need to be confirmed in prospective trials. In the total biochemical cohort, the number of patients with analyzable samples differed between admission and 3 months for logistical reasons. A larger number had sC5b-9 measured after 3 months (n = 417) than at admission (n = 391). When studying the impact of the 3-month levels, we chose to include only patients for whom both samples were available.
To summarize, patients with AMI and type 2 diabetes have a poor prognosis, and the exaggerated activation of the complement system may be one of the reasons. An improved knowledge of pathophysiological mechanisms is essential to understand the reasons for complications, and it is also a prerequisite for the development of new treatment targets to improve the prognosis for patients with type 2 diabetes and cardiovascular disease.
Acknowledgments
This study was supported by grants from the Swedish Heart-Lung Foundation, the Swedish Research Council (8691), and AFA Insurance. This study was also supported by unconditional grants from Novo Nordisk Denmark. L.G.M. has received research grants from Merck Sharp & Dohme and sanofi-aventis and speakers' honoraria from Merck Sharp & Dohme, sanofi-aventis, Novartis, Bayer-Schering, AstraZeneca, and Lilly. T.K.H. has received speakers\x{2019} honoraria from Schering-Plough, Merck Sharp & Dohme, and AstraZeneca. No other potential conflicts of interest relevant to this article were reported.
L.G.M. and T.K.H. researched and prepared data and wrote and edited the manuscript. M.B. and S.T. performed laboratory analyses, contributed to discussion, and edited the manuscript. L.G.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 47th Annual Meeting of the European Association for the Study of Diabetes, Lisbon, Portugal, 12–16 September 2011.
The authors thank Lars Rydén, Klas Malmberg (Karolinska Institutet), and all the patients and investigators (8) for the opportunity to use the DIGAMI 2 database and John Öhrvik, Karolinska Institutet, for valuable statistical input.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1642/-/DC1.
References
- 1.Norhammar A, Lindbäck J, Rydén L, Wallentin L, Stenestrand U; Register of Information and Knowledge About Swedish Heart Intensive Care Admission (RIKS-HIA) Improved but still high short- and long-term mortality rates after myocardial infarction in patients with diabetes mellitus: a time-trend report from the Swedish Register of Information and Knowledge About Swedish Heart Intensive Care Admission. Heart 2007;93:1577–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lenzen M, Ryden L, Ohrvik J, et al. ; Euro Heart Survey Investigators Diabetes known or newly detected, but not impaired glucose regulation, has a negative influence on 1-year outcome in patients with coronary artery disease: a report from the Euro Heart Survey on diabetes and the heart. Eur Heart J 2006;27:2969–2974 [DOI] [PubMed] [Google Scholar]
- 3.Libby P, Ridker PM, Hansson GK; Leducq Transatlantic Network on Atherothrombosis Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol 2009;54:2129–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjerre M, Hansen TK, Flyvbjerg A. Complement activation and cardiovascular disease. Horm Metab Res 2008;40:626–634 [DOI] [PubMed] [Google Scholar]
- 5.Speidl WS, Kastl SP, Huber K, Wojta J. Complement in atherosclerosis: friend or foe? J Thromb Haemost 2011;9:428–440 [DOI] [PubMed] [Google Scholar]
- 6.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol 2010;11:785–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mellbin LG, Hamsten A, Malmberg K, et al. Mannose-binding lectin genotype and phenotype in patients with type 2 diabetes and myocardial infarction: a report from the DIGAMI 2 trial. Diabetes Care 2010;33:2451–2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malmberg K, Rydén L, Wedel H, et al. ; DIGAMI 2 Investigators Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J 2005;26:650–661 [DOI] [PubMed] [Google Scholar]
- 9.Haahr-Pedersen S, Bjerre M, Flyvbjerg A, et al. Level of complement activity predicts cardiac dysfunction after acute myocardial infarction treated with primary percutaneous coronary intervention. J Invasive Cardiol 2009;21:13–19 [PubMed] [Google Scholar]
- 10.Møller-Kristensen M, Jensenius JC, Jensen L, et al. Levels of mannan-binding lectin-associated serine protease-2 in healthy individuals. J Immunol Methods 2003;282:159–167 [DOI] [PubMed] [Google Scholar]
- 11.Thiel S, Møller-Kristensen M, Jensen L, Jensenius JC. Assays for the functional activity of the mannan-binding lectin pathway of complement activation. Immunobiology 2002;205:446–454 [DOI] [PubMed] [Google Scholar]
- 12.Hill JH, Ward PA. C3 leukotactic factors produced by a tissue protease. J Exp Med 1969;130:505–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imai T, Takase S, Arai M, Fujita T. Serial changes of complement titers in the acute phase of myocardial infarction. Jpn Circ J 1983;47:289–293 [DOI] [PubMed] [Google Scholar]
- 14.Langlois PF, Gawryl MS. Detection of the terminal complement complex in patient plasma following acute myocardial infarction. Atherosclerosis 1988;70:95–105 [DOI] [PubMed] [Google Scholar]
- 15.Schäfer H, Mathey D, Hugo F, Bhakdi S. Deposition of the terminal C5b-9 complement complex in infarcted areas of human myocardium. J Immunol 1986;137:1945–1949 [PubMed] [Google Scholar]
- 16.Väkevä A, Morgan BP, Tikkanen I, Helin K, Laurila P, Meri S. Time course of complement activation and inhibitor expression after ischemic injury of rat myocardium. Am J Pathol 1994;144:1357–1368 [PMC free article] [PubMed] [Google Scholar]
- 17.Niculescu F, Niculescu T, Rus H. C5b-9 terminal complement complex assembly on apoptotic cells in human arterial wall with atherosclerosis. Exp Mol Pathol 2004;76:17–23 [DOI] [PubMed] [Google Scholar]
- 18.Bykov I, Junnikkala S, Pekna M, Lindros KO, Meri S. Effect of chronic ethanol consumption on the expression of complement components and acute-phase proteins in liver. Clin Immunol 2007;124:213–220 [DOI] [PubMed] [Google Scholar]
- 19.Thiel S, Steffensen R, Christensen IJ, et al. Deficiency of mannan-binding lectin associated serine protease-2 due to missense polymorphisms. Genes Immun 2007;8:154–163 [DOI] [PubMed] [Google Scholar]
- 20.Yasuda M, Takeuchi K, Hiruma M, et al. The complement system in ischemic heart disease. Circulation 1990;81:156–163 [DOI] [PubMed] [Google Scholar]
- 21.Bjerre M, Kistorp C, Hansen TK, et al. Complement activation, endothelial dysfunction, insulin resistance and chronic heart failure. Scand Cardiovasc J 2010;44:260–266 [DOI] [PubMed] [Google Scholar]
- 22.Qin X, Goldfine A, Krumrei N, et al. Glycation inactivation of the complement regulatory protein CD59: a possible role in the pathogenesis of the vascular complications of human diabetes. Diabetes 2004;53:2653–2661 [DOI] [PubMed] [Google Scholar]
- 23.Granger CB, Mahaffey KW, Weaver WD, et al. ; COMMA Investigators Pexelizumab, an anti-C5 complement antibody, as adjunctive therapy to primary percutaneous coronary intervention in acute myocardial infarction: the COMplement inhibition in Myocardial infarction treated with Angioplasty (COMMA) trial. Circulation 2003;108:1184–1190 [DOI] [PubMed] [Google Scholar]
- 24.Armstrong PW, Granger CB, Adams PX, et al. ; APEX AMI Investigators Pexelizumab for acute ST-elevation myocardial infarction in patients undergoing primary percutaneous coronary intervention: a randomized controlled trial. JAMA 2007;297:43–51 [DOI] [PubMed] [Google Scholar]